Abstract
The monoterpenoid indole alkaloids (MIAs) of Madagascar periwinkle (Catharanthus roseus) continue to be the most important source of natural drugs in chemotherapy treatments for a range of human cancers. These anticancer drugs are derived from the coupling of catharanthine and vindoline to yield powerful dimeric MIAs that prevent cell division. However the precise mechanisms for their assembly within plants remain obscure. Here we report that the complex development-, environment-, organ-, and cell-specific controls involved in expression of MIA pathways are coupled to secretory mechanisms that keep catharanthine and vindoline separated from each other in living plants. Although the entire production of catharanthine and vindoline occurs in young developing leaves, catharanthine accumulates in leaf wax exudates of leaves, whereas vindoline is found within leaf cells. The spatial separation of these two MIAs provides a biological explanation for the low levels of dimeric anticancer drugs found in the plant that result in their high cost of commercial production. The ability of catharanthine to inhibit the growth of fungal zoospores at physiological concentrations found on the leaf surface of Catharanthus leaves, as well as its insect toxicity, provide an additional biological role for its secretion. We anticipate that this discovery will trigger a broad search for plants that secrete alkaloids, the biological mechanisms involved in their secretion to the plant surface, and the ecological roles played by them.
Keywords: catharanthine, Catharanthus roseus, surface secretion, vindoline
The vinca alkaloids are a well known source of drugs derived from the Madagascar periwinkle (Catharanthus roseus). The four major vinca alkaloids used in various cancer chemotherapies are vinblastine, vincristine (or semisynthetic derivatives), vindesine, and vinorelbine (Fig. S1A). In addition, clinical trials are continuing with new semisynthetic derivatives such as vinflunine (Fig. S1A) that may have improved biological properties and may be effective in a broader range of cancers. Although remarkable efforts by chemists have yielded the total synthesis of dimeric alkaloids (1–5), they or their monomeric precursors, catharanthine and vindoline, are still harvested and purified from periwinkle plants (Fig. S1B), and the process of alkaloid extraction and purification produces low yields at very high cost.
The complexity of the vinca alkaloids is also matched by the multistep pathway involved in their assembly. This pathway requires tryptamine and the monoterpene secologanin for alkaloid assembly, and the pathway is highly regulated by development-, environment-, organ-, and cell-specific controls (6) that are poorly understood. Although biosynthesis of complex alkaloids such as tabersonine and catharanthine seem to occur within the protoderm and cortical cells of Catharanthus root tips (7), the pathway appears to be compartmented in multiple cell types in above-ground organs such as stems, leaves, and flowers (8). The 2-C-methyl-d-erythritol-4-phosphate pathway, which supplies the carbon skeletons for secologanin biosynthesis (9) as well as geraniol 10-hydroxylase (G10H) involved in the first committed step in this pathway, have been localized to biochemically specialized internal phloem parenchyma (IPAP) cells (10–12). In contrast, loganic acid O-methyltransferase (LAMT) and secologanin synthase that catalyze the terminal reactions in secologanin biosynthesis are expressed exclusively within the epidermis of young leaves and stems (13). This suggests very strongly that an uncharacterized pathway intermediate is transported between IPAP and epidermal cells to elaborate the secologanin molecule. The epidermis of leaves, stems, and flower buds also express tryptophan decarboxylase (8, 14), strictosidine synthase (8, 14), strictosidine β-glucosidase (14), tabersonine 16-hydroxylase (14) and 16-hydroxytabersonine 16-O-methyltransferase (16-OMT) (14, 15), whereas the N-methyltransferase, dioxygenase, and acetyltransferase (8, 14) responsible for the last three steps in vindoline biosynthesis are expressed within leaf mesophyll cells or in specialized idioblasts and laticifers.
Carborundum abrasion has been used as a unique and complementary approach to obtain epidermis-enriched leaf extracts to measure alkaloid metabolites, enzyme activity, and gene expression levels (14, 15). RNA analysis through random sequencing of the leaf epidermis has shown that this single layer of cells is biochemically rich in a variety of biosynthetic pathways that include those for flavonoid, very long chain fatty acids, pentacyclic triterpenes, and monoterpenoid indole alkaloids (MIAs), respectively (13). This biosynthetic diversity in a single cell type coincides with uncharacterized mechanisms that deliver lipids and triterpenes to the leaf surface to form the cuticle that seals the plant surface (16) and advanced alkaloid precursors to specialized leaf mesophyl idioblasts and laticifers (8, 13, 14) for elaboration into vindoline. The present study shows that directional transport mechanisms are coupled to alkaloid biosynthesis in the leaf epidermis to allow secretion of catharanthine into the surface wax while vindoline accumulates within specialized idioblast/laticifer cells in the leaf. This spatial separation of MIAs provides a clear explanation for the low levels of dimeric anticancer alkaloids found in Catharanthus plants.
Results
Waxy Surface of C. roseus Contains the Complement of Leaf Catharanthine but Not Vindoline.
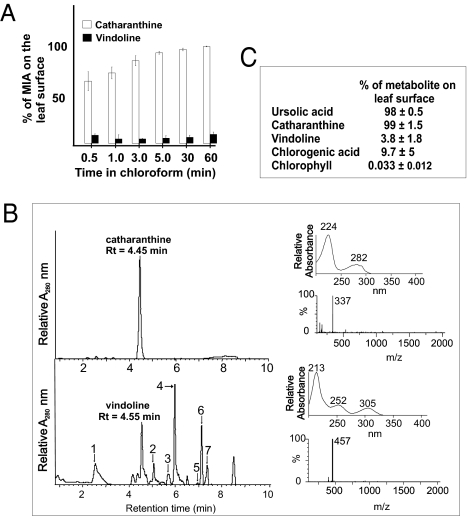
Dipping of leaves in chloroform has been used to remove surface waxes from plant tissues (16) as well as other hydrophobic surface chemicals such as the pentacyclic triterpene ursolic acid that accumulates only on the surface of Catharanthus leaves (13). Analysis of chloroform soluble chemicals also showed that catharanthine occurs exclusively on the surface of these leaves (Fig. 1A). Remarkably, only 2% to 5% of the vindoline could be extracted even if leaves were dipped in chloroform for as long as 1 h (Fig. 1A). The identities and quantities of catharanthine and vindoline were verified by ultra-performance liquid chromatography (UPLC)–diode array detection (DAD)–MS (Fig. 1B) compared with those of authentic catharanthine (m/z 337) and vindoline (m/z 457) standards (Fig. S2).
Fig. 1.
Catharanthine accumulates almost entirely in leaf wax exudates outside of the leaf epidermis, whereas vindoline is found within leaf cells. (A) Measurement of catharanthine and vindoline extracted from the leaf surface was made by dipping them in chloroform for different times. The experiment for each time point was performed in triplicate. The levels of catharanthine and vindoline per whole leaf representing the 100% value were 240 ± 9 μg and 113 ± 6 μg, respectively, in leaf number 3, as seen in Fig. 2. (B) Chloroform-extracted MIAs and methanol-extracted MIAs remaining in leaves after chloroform treatments were measured by UPLC-MS (Upper). MIAs 1 to 7 were tentatively identified by their absorption and mass spectra as serpentine (1, m/z = 349); deacetoxyvindoline (2, m/z = 399); anhydrovinblastine (3, m/z = 794); unknown (4, m/z = 615); 16-methoxytabersonine (5, m/z = 367); unknown (6, m/z = 594); and unknown (7, m/z = 535 and 594). (C) Triplicate measurements of ursolic acid, catharanthine, vindoline, chlorogenic acid, and chlorophyll extracted from the leaf surface by dipping them in chloroform for 1 h. The levels per leaf pair of ursolic acid, catharanthine, vindoline, chlorogenic acid, and chlorophyll representing 100% were 919 ± 90, 271 ± 63, 120 ± 19, 210 ± 18, and 131 ± 17 μg/g fresh weight, respectively.
Analysis of the chloroform-soluble fraction showed that all the leaf catharanthine was on the leaf surface together with ursolic acid (Fig. 1C), which is known to be present in the leaf cuticle (13), whereas only 3.8% of the vindoline appeared to be chloroform-soluble. The extraction of chlorophyll and chlorogenic acid in chloroform was used to estimate if this solvent could extract metabolites from within leaf cells. Virtually no chlorophyll, a marker for leaf mesophyll metabolites, and less than 10% of the chlorogenic acid, a marker for leaf epidermis metabolites, were detected (Fig. 1C). These data, together with the retention of a number of other metabolites within the leaf (Fig. 1B Lower), highlight the secretion of catharanthine to the leaf surface, whereas vindoline may be sequestered within idioblasts and laticifers (8, 13, 14).
The Mediterranean Deep orchid variety of C. roseus accumulates very low levels of vindoline because it lacks tabersonine 16-hydroxylase activity (17). The complement of catharanthine is also found on the surface of this variety when leaves were dipped in chloroform (Fig S3A), whereas UPLC analysis of extracts of dipped leaf showed that only low levels of vindoline can be found within the leaf (Fig. 1B and Fig. S3B). The decrease in vindoline content in this mutant line facilitated the detection of vindorosine by improving the absorption and mass spectra that could be obtained (Fig. S3C). These results confirm that, although a number of alkaloids accumulate within Catharanthus cells (Fig. 1B and Fig. S3B) of these two cultivars, only catharanthine appears to be secreted to the surface.
Production of Dimeric MIAs Occurs Only Within Older Catharanthus Leaves.
C. roseus (L.) G. Don cv. Little Delicata leaves of different ages were analyzed for their levels of catharanthine, vindoline, and 3′4′-anhydrovinblastine (Fig. 2). Although the concentrations of vindoline and catharanthine increased with leaf age from leaves 1 to 3, these MIAs leveled off and began to diminish from leaves 4 to 9. In all cases, virtually all the catharanthine was in the chloroform-soluble fraction whereas all the vindoline appeared only in extracts of whole leaves (Fig. 2). The accumulation and secretion of catharanthine in the leaf wax exudate of young leaves strongly suggests that the pathway for catharanthine biosynthesis is also expressed in the leaf epidermis, as it is for 16-methoxytabersonine (13, 15). Despite the fact that catharanthine and vindoline accumulated within separate locations, the levels of 3′4′-anhydrovinblastine did begin to increase within the fully expanded leaves 3 to 6 (Fig. 2), whereas they disappeared in older leaves 7 to 9. The timing of 3′4′-anhydrovinblastine accumulation is consistent with earlier studies (18), suggesting that only older leaves were competent to produce or accumulate these dimers. It is interesting that small amounts of catharanthine were detected within leaves 3 and 4 (Fig. 2), coinciding with the appearance of 3′4′-anhydrovinblastine in older leaves (18, 19). As the biosynthesis of MIAs, including catharanthine is restricted to younger leaves (7, 13) and to protoderm and cortical cells of root tips (8), the source of catharanthine inside older leaves is not known. It is possible that the catharanthine used for production of 3′4′-anhydrovinblastine is transported from root sources, but the transport of catharanthine from this underground source remains to be established. Alternatively the internal sources of catharanthine found in older leaves may be delivered by a switching in the transport of this molecule from the leaf epidermis toward the internal tissues of the leaf by transport mechanisms that remain to be determined.
Fig. 2.
Distribution of catharanthine and vindoline in the leaf wax exudate and within leaf cells in leaves of different ages.
Several Catharanthus Species and Hybrids Secrete Catharanthine to the Leaf Surface.
The 100% surface accumulation of catharanthine was confirmed in leaves of several cultivars of C. roseus (L.) G. Don such as Little Delicata and Pacifica Peach, as well as in different species such as Catharanthus longifolius (Pichon) Pichon, Catharanthus ovalis, and Catharanthus trichophyllus (Baker) Pichon (Fig. S4). These results, together with the idioblast/laticifer–specific expression of the last two steps in vindoline biosynthesis (8, 13) and uncharacterized oriented transport mechanisms, provides a clear biological model for the sequestration of catharanthine and vindoline in separate leaf locations. As approximately 20% of plant species produce alkaloids, this result should trigger a broader search to discover if many more plants secrete alkaloids and to discover their biological/ecological roles on plant surfaces.
Madagascar periwinkle is also an extremely popular annual bedding plant because it blooms continuously throughout the growing season and there are many cultivars that produce a range of interesting colors. In addition, the plant is heat- and drought-tolerant, which makes it a favorite for cultivation in the southern United States. Unfortunately, these cultivars are highly susceptible to fungal infections by Phytopthora nicotianiae that causes aerial blight. This has led to the recent development of the Cora lines of C. roseus that appear to be resistant to this fungal pathogen. The mechanism of resistance in these new cultivars has been attributed to the production and accumulation of dimeric and trimeric MIAs (19). All of the Cora cultivars analyzed (Fig. S4; no. 6, deep lavender; no. 7, white; no. 8, lavender; no. 9, violet; no. 10, apricot; and no. 11, burgundy) also accumulate all of their catharanthine on the leaf surface, whereas vindoline was almost exclusively found within leaves. Remarkably, the Cora cultivars accumulate similar levels of catharanthine and vindoline as in other Catharanthus cultivars (Fig. S4) and detailed UPLC-MS analysis did not reveal the presence of trimeric alkaloids in the Cora cultivars.
Catharanthine Has Antifungal, Antiinsect, and Other Biological Properties.
The spatial separation of vindoline and catharanthine appears to prevent dimer formation, perhaps because they may also interfere with cell division in Catharanthus as in cancer cells. The presence of catharanthine in the wax layer may also have a biological role, as shown with the MIA ibogaine that displays antifungal activity against Candida albicans (20). Incubation of catharanthine with zoospores of P. nicotianiae inhibits both growth of zoospores and the formation of hyphae at a concentration of 10 μg/mL of culture medium (Table 1). The concentration of catharanthine/square centimeter of leaf surface in leaves 1 to 5 (Fig. 2) varied between 23 μg in leaves 1 and 2 and 14 μg in leaf 5, sufficiently high to affect the activities of P. nicotianiae zoospores on the leaf surface of Catharanthus species. In addition, the catharanthine but not the vindoline component of dimeric MIAs has been shown to interact directly with tubulin, and catharanthine alone can induce self-association of tubulin (21). The binding constants to tubulin for vinblastine (4.3 μM), catharanthine (2.8 mM), and vindoline (50 mM) were described by Prakash and Timasheff (21). However all the C. roseus lines that are resistant (Cora lines) or susceptible to this fungal pathogen contain similar levels of catharanthine (Fig. S4). This suggests that, although catharanthine can inhibit the in vitro growth of P. nicotianiae zoospores (Table 1), additional factors are involved in the in vivo disease resistance observed in Cora lines.
Table 1.
Effect of catharanthine concentration on growth of Phytopthora parasitica
| Catharanthine treatment, μg/mL | Day 0 |
Day 2 |
Day 4 |
|||
| Zoospores, ×103 | Presence of hyphae | Zoospores, ×103 | Presence of hyphae | Zoospores, ×103 | Presence of hyphae | |
| 0 | 30 | — | 146 ± 6 | +++ | 1,493 ± 9 | ++++ |
| 1* | 30 | — | 520 | ++ | 400 | ++ |
| 10 | 30 | — | 20 ± 14 | — | 30 ± 14 | — |
| 50 | 30 | — | 20 ± 14 | — | 20 ± 14 | — |
The growth of P. parasitica in the presence of different concentrations of catharanthine was monitored by counting the number of zoospores and by visual measurement of the abundance of hyphal growth after 2 and 4 d of cultivation compared with those growing in the presence of B5 medium.
*All experiments were performed in triplicate apart from those showing zoospore growth in 1 μg/mL of catharanthine, which was a single measurement.
The potential biological role of catharanthine on herbivory was also tested by direct feeding of Catharanthus leaves to Spodoptera littoralis, Spodoptora eridania, Helicoverpa armigera, Phaedon cochleariae, and Bombyx mori (Table S1). Although third instar larvae of S. eridania would not eat Catharanthus leaves, the two Spodoptora species, as well as H. armigera, were generally able to consume various amounts of these leaves without any insects dying during the course of the experiment. In contrast, both P. cochleariae and B. mori refused to eat Catharanthus leaves and died after 5 d without feeding.
It was found that sixth instar larvae of B. mori would consume pulverized Catharanthus leaf extracts when these were incorporated into commercially available Mulberry diet (Table 2). When the larvae were not fed anything, they all died within 2 d, whereas none died when they were fed their regular Mulberry diet over a 7-d period. In contrast, insects being fed mulberry diet mixed with different amounts of Catharanthus leaves died progressively sooner in a dose-responsive manner (Table 2). Similarly, larvae died after 2 d when they were fed Mulberry diets containing catharanthine-enriched leaf surface extracts, whereas they died after 4 d of feeding with Catharanthus leaf extracts obtained after the catharanthine and other surface components were removed by chloroform treatment. The regurgitate, feces, intestinal tracts, and bodies of larvae as well as the remaining Mulberry/Catharanthus diet were collected to analyze the alkaloid content in each component (Table S2). Although no anhydrovinblastine could be found in the regurgitate, feces, intestinal tracts, and bodies of larvae, all contained detectable levels of catharanthine and vindoline, with the highest levels found in the larval body and in the feces. When larvae were fed Mulberry diets containing different amounts of catharanthine, they also died progressively sooner in a dose-responsive manner (Table 2). These results suggest that the presence of catharanthine on the leaf surface could be an important deterrent to insect herbivory by causing decreased feeding or through its toxicity that appears to lead to insect death (Table 2).
Table 2.
Effect of catharanthine concentration on fifth-instar B. mori larvae
| Treatment* | Catharanthine, μg | Days before death |
| No food | 0 | 2 ± 1 |
| Mulberry diet | 0 | 7 |
| Mulberry diet + 2/3 catharanthus leaf | 92.1 ± 8.6 | 5.3 ± 0.6 |
| Mulberry diet + 1 catharanthus leaf | 138.1 ± 12.8 | 4 ± 1 |
| Mulberry diet + 2 catharanthus leaves | 276.3 ± 25.7 | 3.3 ± 0.6 |
| Mulberry diet + chloroform extracts of Leaf surface | 180.5 ± 39.6 | 2 ± 0 |
| Mulberry diet + extracts of chloroform treated leaf | 1.4 ± 0.4 | 4.3 ± 0.6 |
| Mulberry diet + catharanthine | 50 | 4.7 ± 0.6 |
| Mulberry diet + catharanthine | 250 | 2.4 ± 1.5 |
| Mulberry diet + catharanthine | 500 | 1.7 ± 0.6 |
*Fifth-instar B. mori larvae were fed various diets in triplicate for a 1-wk period. Larvae not provided food died within 2 d of initiating the experiment compared with larvae fed a Mulberry diet that did not die during 7 d of observation.
Discussion
Plants are well known to secrete a range of secondary metabolites onto their surfaces that may offer a chemical barrier to insect herbivores or to attack by fungi and other pathogens. Although these leaf wax exudates may contain unusual fatty acids, triterpenes (13, 22, 23) and other terpenes, hydrophobic flavonoids (24) and other phenols, there is little evidence for the secretion of alkaloids onto the plant surface. Water-soluble pyrolizidine alkaloids were recently reported on the surface of Scenecio jacobea (25), although the levels found were small compared with those within the leaves. The present study shows that MIAs are clearly found on plant leaf surfaces, as the complement of catharanthine in several Catharanthus species accumulates in the leaf wax exudate (Figs. 1 and 2 and Fig. S3). In contrast, vindoline, anhydrovinblastine, and the other MIAs accumulate only within leaf cells.
Secretion of Catharanthine to the Leaf Surface Expands the Leaf Cell Types Involved in the Biosynthesis and Accumulation of MIAs in Catharanthus.
Previous studies have shown that silicon carbide abrasion techniques could be used to preferentially extract metabolites, RNA, or proteins for metabolomic, transcriptomic, or enzymatic analysis (14, 15). This technique is used in the present study to determine the ratios of G10H; LAMT; 16-OMT; 16-methoxy-2,3-dihydro-3 hydroxy tabersonine-N-methyltransferase (NMT) and deacetylvindoline-4-O-acetyltransferase (DAT) enzyme activities in leaf epidermis enriched extracts compared with whole leaf extracts (Fig. 3). As shown by previous studies (14, 15), the specific activities of LAMT and 16-OMT were more than 16-fold higher within leaf epidermis enriched extracts compared with whole leaves, whereas little or no G10H, NMT, or DAT could be detected within leaf epidermis enriched extracts (Fig. 3). These results support the leaf epidermis as a major site of MIA biosynthesis.
Fig. 3.
Model for biosynthesis and secretion of secondary metabolites produced in the epidermis of C. roseus leaves. (A) Enrichment of monoterpenoid indole alkaloid pathway enzyme activities in leaf epidermis enriched protein extracts produced by carborundum abrasion compared with those found in whole leaves. The whole leaf–specific activities used to establish the fold enrichment of enzymes in the leaf epidermis were a follows: G10H (184 ± 14 nmol/μg protein), LAMT (0.69 ± 0.02 pmol/μg protein), 16-OMT (1.89 ± 0.05 pmol/μg protein), NMT (0.47 ± 0.01 pmol/μg protein), and DAT (0.37 ± 0.02 pmol/ μg protein). (B) The leaf epidermis is specialized for biosynthesis of flavonoids, the fatty acid components of waxes, triterpenes, and MIAs. Although the 10-hydroxygeraniol required for biosynthesis of secologanin is made in specialized IPAP cells via the methyl–erythritol pathway (MEP) and G10H, this metabolite or another intermediate is transported to the leaf epidermis, where loganic acid is converted to secologanin via LAMT and secologanin synthase (SLS). Within the leaf epidermis, tryptophan is decarboxylated in the cytoplasm to tryptamine by tryptophan decarboxylase (TDC) and strictosidine is formed by the coupling of tryptamine and secologanin in the leaf epidermal vacuole by strictosidine synthase (STR1). Strictosidine is released into the cytoplasm as a reactive aglycone by cytosolic strictosidine β-glucosidase (SGD), where mostly unknown biochemical transformations lead to the production of the three major—Corynanthe, Iboga, or Aspidosperma—backbones of MIAs. A common intermediate is converted to catharanthine or to tabersonine by uncharacterized biochemical reactions. Virtually all the catharanthine is secreted into the leaf cell surface, together with fatty acid components of waxes and the triterpene, ursolic acid. After tabersonine is converted into 16-methoxytabersonine in the leaf epidermis by tabersonine-16-hydroxylase (T16H) and by 2,3-dihydro-3-hydroxy-N-methyltabersonine (16OMT), this metabolite may be secreted into leaf mesophyl cells, where a further uncharacterized oxidation takes place, followed by a N-methylation (NMT) located in chloroplast thylakoids, hydroxylation (D4H), and O-acetylation (DAT) to yield vindoline. The last two reactions in vindoline biosynthesis appear to occur in specialized leaf idioblast and laticifer cells. It is postulated that wounding or herbivory may bring catharanthine and vindoline together to allow the formation of dimeric anticancer MIAs. The dotted lines represent uncharacterized reactions or unknown mechanisms that remain to be documented.
The present studies have expanded the complexity of MIA biosynthesis in medicinal periwinkle and have provided insights into the leaf epidermal specialization of this pathway to assemble catharanthine and 16-methoxytabersonine (Fig. 3) from the early precursors tryptamine and secologanin. The elaboration of these two metabolites, combined with the secretion of catharanthine into the wax layer (Figs. 1 and 2 and Figs. S3 and S4), the possible secretion of 16-methoxytabersonine or a later intermediate into the mesophyll layer, and leaf mesophyll-specific expression of the last three or four steps in vindoline biosynthesis (6, 8, 13) (Fig. 3), describes the biological mechanism that appears to be required for Catharanthus species to accumulate both end products in the same plant, but in two separate locations. The spatial separation of these two alkaloids also explains why plants do not accumulate higher levels of dimeric anticancer drugs such as anhydrovinblastine. The biological reasons for this may be related to the toxicity of anhydrovinblastine that may also interfere with cell division in Catharanthus as it does in cancer cells. Although this may not occur in fifth instar B. mori larvae (Table 2), it may be hypothesized that catharanthine and vindoline could be part of preformed chemical defenses that are mixed by wounding caused by herbivory (Fig. 3), whereby they could combine in the intestinal tracts of the herbivore, where anhydrovinblastine would be produced in chemical or enzymatic dimerization. The mechanism of dimer formation in older Catharanthus leaves is obscure, but this may be associated with the appearance of the development-specific expression in older leaves of an alkaline class III peroxidase capable of converting catharanthine and vindoline into anhydrovinblastine (26). If such a mechanism is involved, a key question that remains is how catharanthine and vindoline could be placed in the same cellular compartment as this peroxidase. The appearance of low levels of catharanthine within older leaves (Fig. 2) suggests this may be the case, but it is not clear from where the catharanthine is derived.
Conclusions
This study reveals that assembly of MIAs in C. roseus are remarkably dynamic, involving at least three cell types within leaves. Rates of biosynthesis, as well as oriented transport mechanisms, precisely regulate where, when, and how different MIAs accumulate during plant growth and development. The intimate association between cellular specialization and oriented secretion ensures that both catharanthine and vindoline are deposited into separate leaf locations, which explains the need for this unique and complex biological organization. Remarkably, Catharanthus leaves and/or their leaf extracts deterred feeding by Spodoptora littoralis (27) and Spodoptera exigua (28), whereas leaf extracts inhibited growth of Spodoptera litura (29). The discovery of catharanthine on the leaf surfaces of four separate Catharanthus species suggests that many more plant species may actually secrete alkaloids for defensive reasons as well as other functions that remain to be discovered in nature.
Methods
Leaf Sample Preparation.
C. roseus plants were grown in a greenhouse under a 16/8-h light/dark photoperiod at 30 °C. Fresh young leaves from the third developmental stage (Fig. 2) were harvested and their fresh weights were recorded. To obtain surface extracts containing waxes, triterpenes and MIAs, leaves were dipped in 5 mL chloroform (30) in 15-mL conical sterile capped polypropylene tubes (Sarstedt) for different times (30 s to 1 h) at room temperature. The stripped leaves were removed and surface extract samples were evaporated to dryness by vacuum centrifugation in an SPD SpeedVac (Fisher Scientific). Before analysis by UPLC-MS, the dry materials were resuspended in 5 mL methanol. Stripped leaves were air-dried in a fume hood for 60 min to remove chloroform residues before they were extracted for MIAs.
Extraction of MIAs from Leaves.
Intact leaves or chloroform surface stripped leaves were frozen in liquid nitrogen, pulverized with a mortar and pestle, and homogenized with 5 mL methanol or chloroform. As catharanthine and vindoline from stripped or intact leaves were equally well extracted in either solvent system, all extractions were performed with methanol. The extracts were shaken at 100 rpm using an Innova 2000 shaker (New Brunswick Scientific) at room temperature for 1 h. The samples were mixed by vortex (Genie 2 vortex set at 10; Fisher Scientific) and centrifuged at 5,000 × g for 10 min to separate leaf debris and methanol. The methanol extracts were harvested and stored at −20 °C until further analysis. An aliquot of 200 μL of each extract was filtered through (0.22 μm) PALL filter (VWR) before analysis by UPLC/single-quadrupole MS (Waters).
UPLC-MS Analysis of MIAs.
The analytes were separated using an Aquity UPLC BEH C18 column with a particle size of 1.7 μm and column dimensions of 1.0 × 50 mm. Samples were maintained at 4 °C and 5-μL injections were made into the column. The analytes were detected by photodiode array and MS. The solvent systems for alkaloid analysis were as follows: solvent A, methanol:acetonitrile:5-mM ammonium acetate and 6:14:80; solvent B, methanol:acetonitrile:5-mM ammonium acetate at 25:65:10. The following linear elution gradient was used: 0–0.5 min 99% A, 1% B at 0.3 mL/min; 0.5–0.6 min 99% A, 1% B at 0.4 mL/min; 0.6–7.0 min 1% A, 99% B at 0.4 mL/min; 7.0–8.0 min 1% A, 99% B at 0.4 mL/min; 8.0–8.3 min 99% A, 1% B at 0.4 mL/min; 8.3–8.5 min 99% A, 1% B at 0.3 mL/min; and 8.5–10.0 min 99% A, 1% B at 0.3 mL/min. Alkaloid reference standards (Fig. S2) were also analyzed by using this method and to establish standard curves to quantitate the levels of catharanthine, vindoline, and anhydrovinblastine in the extracts.
The mass spectrometer was operated with a capillary voltage of 2.5 kV, cone voltage of 34 V, cone gas flow of 2 L/h, desolvation gas flow of 460 L/h, desolvation temperature of 400 °C, and a source temperature of 150 °C. Catharanthine was detected at 280 nm and its identity was verified by its diode array profile, its mass (337 m/z), and retention time (4.45 min) compared with authentic standard. Vindoline was detected at 305 nm and its identity was verified by its diode array profile, its mass (457 m/z) and retention time (4.55 min). The 3′,4′-anhydrovinblastine was detected at 270 nm and its identity was verified by its diode array profile, its mass (793 m/z), and its retention time (5.7 min). Chromatographic peaks were integrated and compared with standard curves for catharanthine, vindoline and 3′,4′-anhydrovinblastine to give the total amount of alkaloids in each sample.
UPLC-MS Analysis of Triterpenes.
Triterpenes were extracted and analyzed by UPLC-MS as described previously (13).
Supplementary Material
Acknowledgments
This work was funded by a discovery grant from the National Sciences and Engineering Research Council of Canada and by a Tier 1 Canada Research Chair in Plant Biotechnology (to V.D.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0911451107/-/DCSupplemental.
References
- 1.Mangeney P, Andriamialisoa RZ, Langlois N, Langlois Y, Potier P. Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus species (Apocynaceae) J Am Chem Soc. 1979;101:2243–2245. [Google Scholar]
- 2.Kutney JP, et al. A highly efficient and commercially important synthesis of the antitumor catharanthus alkaloids vinblastine and leurosidine from catharanthine and vindoline. Heterocycles. 1988;27:1845–1853. [Google Scholar]
- 3.Kuehne ME, Matson PA, Bornmann WG. Enantioselective syntheses of vinblastine, leurosidine, vincovaline and 20'-epi-vincovaline. J Org Chem. 1991;56:513–528. [Google Scholar]
- 4.Magnus P, Mendoza JS, Stamford A, Ladlow M, Willis P. Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analog: Solvent effect on the stereochemistry of the crucial C-15/C-18' bond. J Am Chem Soc. 1992;114:10232–10245. [Google Scholar]
- 5.Yokoshima S, et al. Stereocontrolled total synthesis of (+)-vinblastine. J Am Chem Soc. 2002;124:2137–2139. doi: 10.1021/ja0177049. [DOI] [PubMed] [Google Scholar]
- 6.Facchini PJ, De Luca V. Opium poppy and Madagascar periwinkle: Model non-model systems to investigate alkaloid biosynthesis in plants. Plant J. 2008;54:763–784. doi: 10.1111/j.1365-313X.2008.03438.x. [DOI] [PubMed] [Google Scholar]
- 7.Laflamme P, St-Pierre B, De Luca V. Molecular and biochemical analysis of a Madagascar periwinkle root-specific minovincinine-19-hydroxy-O-acetyltransferase. Plant Physiol. 2001;125:189–198. doi: 10.1104/pp.125.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Pierre B, Vazquez-Flota FA, De Luca V. Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell. 1999;11:887–900. doi: 10.1105/tpc.11.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contin A, van der Heijden R, Lefeber AW, Verpoorte R. The iridoid glucoside secologanin is derived from the novel triose phosphate/pyruvate pathway in a Catharanthus roseus cell culture. FEBS Lett. 1998;434:413–416. doi: 10.1016/s0014-5793(98)01022-9. [DOI] [PubMed] [Google Scholar]
- 10.Burlat V, Oudin A, Courtois M, Rideau M, St-Pierre B. Co-expression of three MEP pathway genes and geraniol 10-hydroxylase in internal phloem parenchyma of Catharanthus roseus implicates multicellular translocation of intermediates during the biosynthesis of monoterpene indole alkaloids and isoprenoid-derived primary metabolites. Plant J. 2004;38:131–141. doi: 10.1111/j.1365-313X.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- 11.Mahroug S, Burlat V, St-Pierre B. Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev. 2007;6:363–381. [Google Scholar]
- 12.Oudin A, et al. Spatial distribution and hormonal regulation of gene products from methyl erythritol phosphate and monoterpene-secoiridoid pathways in Catharanthus roseus. Plant Mol Biol. 2007;65:13–30. doi: 10.1007/s11103-007-9190-7. [DOI] [PubMed] [Google Scholar]
- 13.Murata J, Roepke J, Gordon H, De Luca V. The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell. 2008;20:524–542. doi: 10.1105/tpc.107.056630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murata J, De Luca V. Localization of tabersonine 16-hydroxylase and 16-OH tabersonine-16-O-methyltransferase to leaf epidermal cells defines them as a major site of precursor biosynthesis in the vindoline pathway in Catharanthus roseus. Plant J. 2005;44:581–594. doi: 10.1111/j.1365-313X.2005.02557.x. [DOI] [PubMed] [Google Scholar]
- 15.Levac D, Murata J, Kim WS, De Luca V. Application of carborundum abrasion for investigating the leaf epidermis: Molecular cloning of Catharanthus roseus 16-hydroxytabersonine-16-O-methyltransferase. Plant J. 2008;53:225–236. doi: 10.1111/j.1365-313X.2007.03337.x. [DOI] [PubMed] [Google Scholar]
- 16.Samuels L, Kunst L, Jetter R. Sealing plant surfaces: Cuticular wax formation by epidermal cells. Annu Rev Plant Biol. 2008;59:683–707. doi: 10.1146/annurev.arplant.59.103006.093219. [DOI] [PubMed] [Google Scholar]
- 17.Magnotta M, Murata J, Chen J, De Luca V. Identification of a low vindoline accumulating cultivar of Catharanthus roseus (L.) G. Don by alkaloid and enzymatic profiling. Phytochemistry. 2006;67:1758–1764. doi: 10.1016/j.phytochem.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Naaranlahti T, Auriola S, Lapinjoki SP. Growth-related dimerization of vindoline and catharanthine in Catharanthus roseus and effect of wounding on the process. Phytochemistry. 1991;30:1451–1453. [Google Scholar]
- 19.Bowman RN. US Patent 5491285. 1996 [Google Scholar]
- 20.Yordanov M, et al. Ibogaine reduces organ colonization in murine systemic and gastrointestinal Candida albicans infections. J Med Microbiol. 2005;54:647–653. doi: 10.1099/jmm.0.45919-0. [DOI] [PubMed] [Google Scholar]
- 21.Prakash V, Timasheff SN. Mechanism of interaction of vinca alkaloids with tubulin: Catharanthine and vindoline. Biochemistry. 1991;30:873–880. doi: 10.1021/bi00217a042. [DOI] [PubMed] [Google Scholar]
- 22.Usia T, Watabe T, Kadota S, Tezuka Y. Cytochrome P450 2D6 (CYP2D6) inhibitory constituents of Catharanthus roseus. Biol Pharm Bull. 2005;28:1021–1024. doi: 10.1248/bpb.28.1021. [DOI] [PubMed] [Google Scholar]
- 23.Kubo I, Matsumoto A. Secreted oleanolic acid on the cuticle Olea europaea (Oleaceae); a chemical barrier to fungal attack. Experientia. 1984;40:937–938. [Google Scholar]
- 24.Valant-Vetschera KM, Brem B. Chemodiversity of exudate flavonoids as highlighted by publications of Eckhard Wollenweber. Nat Prod Comm. 2006;1:921–926. [Google Scholar]
- 25.Vrieling K, Derridj S. Pyrrolizidine alkaloids in and on the leaf surface of Senecio jacobaea L. Phytochemistry. 2003;64:1223–1228. doi: 10.1016/j.phytochem.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Costa MMR, et al. Molecular cloning and characterization of a vacuolar class III peroxidase from Catharanthus roseus. Plant Physiol. 2008;146:403–417. doi: 10.1104/pp.107.107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisner J, Weissenberg M, Palevitch D, Aharonson N. Phagodeterrency induced by leaves and leaf extracts of Catharanthus roseus in the larva of Spodoptera littoralis. J Econ Entomol. 1981;74:131–135. [Google Scholar]
- 28.Luijendijk TJC, van de Meijden E, Verpoorte R. Involvement of strictosidine as a defensive chemical in Catharanthus roseus. J Chem Ecol. 1996;22:1355–1366. doi: 10.1007/BF02027718. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande SG, Joseph M, Sharma RN. Insect growth and development inhibition properties of Catharanthus roseus. Int J Trop Agric. 1988;6:287–290. [Google Scholar]
- 30.Gniwotta F, et al. What do microbes encounter at the plant surface? Chemical composition of pea leaf cuticular waxes. Plant Physiol. 2005;139:519–530. doi: 10.1104/pp.104.053579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.