Abstract
Dynamic changes in gene positioning contribute to differential expression of virulence-related gene families in protozoan pathogens; however, the role of nuclear architecture in gene expression in the human malaria parasite Plasmodium falciparum remains poorly understood. Here we investigated the developmentally regulated ribosomal RNA (rRNA) gene family in P. falciparum, which, unlike that in most eukaryotes, contains only a few unlinked copies of rRNA genes scattered over the subtelomeric regions of several chromosomes. We show that active and silent members of this gene family cluster in a single perinuclear nucleolus. This rDNA nuclear confinement is DNA sequence dependent, as plasmids carrying rDNA fragments are targeted to the nucleolus. Likewise, insertion of an rDNA sequence into a subtelomere from a chromosome lacking rRNA genes leads to repositioning in the nucleolus. Furthermore, we observed that rDNA spatial organization restricted interchromosomal interactions, as chromosome end-bearing rRNA genes were found to be preferentially juxtaposed, demonstrating nonrandom association of telomeres. Using Br-UTP incorporation, we observed two α-amanitin–resistant nucleolar transcription sites that disappeared when the rDNA cluster broke up in the replicative blood stages. Taken together, our results provide conceptual insights into functionally differentiated nuclear territories and their role in gene expression in malaria parasites.
Keywords: nucleus, nucleolus, telomeres, transcription regulation
Eukaryotic nuclei are highly organized structures in which DNA and proteins are confined to specific subcompartments such as nucleoli, nuclear bodies, nuclear speckles, transcription and replication foci, and chromosome territories. The most prominent intranuclear domain is the nucleolus, the ribosome factory. This subcompartment is formed upon transcription of the ribosomal DNA (rDNA) by RNA polymerase I (Pol I). The formation of single or multiple nucleoli is seemingly linked to the genomic organization of the tandemly repeated rDNA. For example, budding yeast has ∼150 rDNA units clustered on a single chromosome (chromosome 12) and forms one nucleolus, whereas human cells have ∼400 tandem repeats clustered on five chromosomes (chromosomes 13, 14, 15, 21, and 22) and form multiple nucleoli per nucleus (1, 2). In addition, in yeast, the deletion of chromosomal rDNA and replacement by an equivalent number of plasmids bearing single rDNA repeats generates multiple small nucleoli (3), highlighting the importance of the tandem arrangement in nucleolar organization.
In the human malaria parasite Plasmodium falciparum, rDNA is not arranged in tandem arrays; instead, single rDNA units (18S–5.8S–28S) are spread out on different chromosomes (chromosomes 1, 5, 7, 11, and 13, Fig. 1A) (4–6). Moreover, the few rDNA units are slightly different in sequence and are differentially transcribed over the course of the parasite developmental cycle (7–9). In this work, we investigated the nuclear position of active and silent members of this unusual rRNA gene family. Unexpectedly, we found that rDNA units from different chromosomes cluster in a single perinuclear nucleolus, regardless of their transcriptional status. This clustering of rRNA genes was observed only in prereplicative parasite blood stages, and was associated with the presence of two α-amanitin–resistant transcription sites detected by BrUTP incorporation technique, here described for P. falciparum parasites. In replicative and dividing blood stages, rDNA was dispersed in the nucleus and α-amanitin–resistant transcription was not any longer visible, revealing a link between rDNA clustering and transcription. In addition, chromosome ends bearing rDNA units were found to be preferentially associated, suggesting that rDNA clustering may create physical constraints that shape the global genome nuclear organization and consequently the control of malaria gene expression.
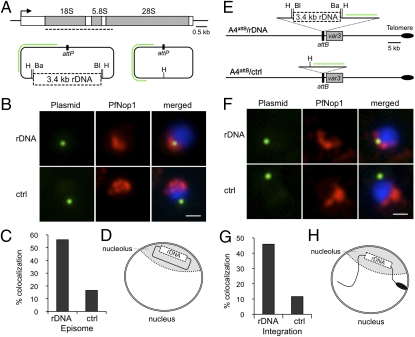
Fig. 1.
Dispersed rRNA genes clustered in a single perinuclear nucleolus. (A) 3D7 P. falciparum linear chromosome map showing the positions of rRNA genes: five rDNA units (circles) located on chromosomes 1, 5, 7, 11, and 13, and a tandem of three 5S rRNA genes (stars) on chromosome 14. rDNA units from chromosomes 1, 5 and 7 correspond to the previously named S1, A2, and A1 genes, respectively (9, 11). rDNA copies from chromosomes 11 and 13 correspond to a duplication of the S2 gene. This duplication was confirmed by bioinformatic analysis and pulse-field gradient gel electrophoresis. A-types are transcribed in asexual blood stages, whereas S-types are predominantly transcribed in sexual stages of the parasite (8, 9, 11). The malaria genome project has identified two additional incomplete units (lacking 18S gene) on both subtelomeres of chromosome 8 (not indicated in map). It is not known whether these units are functionally active (6). Small vertical black bars denote centromere positions on each chromosome. Triangles indicate target locations of FISH probes used in this study (green in this figure and red in Fig. 3). (B) Immunofluorescence analysis using antibodies against the nucleolar protein PfNop1 (red) combined with DNA FISH (green) for individual rRNA genes (A1, A2, S1, S2, and 5S) and a subtelomeric unrelated gene (PFD0110w) on chromosome 4. The percentage of signals that localized in or near the nucleolus and the number of nuclei analyzed are as follows: A1 93.2%, n = 73; A2 90.5%, n = 95; S1 92.3%, n = 91; S2 90.0%, n = 80; 5S 90.6%, n = 128; PFD0110w 24.6%, n = 57. Parasites are in ring stage, and nuclear DNA is stained with DAPI (blue). (Scale bar, 1 μm.)
Results
Previous studies, using immunofluorescence microscopy and PfNop1 antibodies, have described the nucleolus of blood-stage forms of P. falciparum parasites as a “hat-like” structure (10) very similar to that described in yeast. The presence of a single nucleolus was confirmed here by using antibodies against other conserved nucleolar proteins such as PfPol I (PFE0465c, Fig. S1A) and PfNop5 (PF10_0085, Fig. S1B), and by simultaneous detection of rRNA transcripts by RNA FISH (Fig. S1C).
Given the unusual distribution of active and silent P. falciparum rDNA single units on different chromosomes, we hypothesized that the parasite might use distinct nuclear subcompartments to regulate differential transcription of rRNA genes. We presumed that rDNA units actively transcribed in blood stages [A1 and A2 genes (9, 11)] would be localized to the nucleolus, whereas silent rDNA [S1 and S2 genes (9, 11)] would be elsewhere in the nucleus. To test this hypothesis, we examined the localization of individual rRNA genes relative to the nucleolus using immunofluorescence of PfNop1 combined with DNA FISH (immuno-FISH). To discriminate between individual rRNA genes, we generated FISH probes specific for the upstream noncoding region of each rDNA unit (2–4 kb from transcription initiation site, Fig. 1A). As expected, active rRNA genes were localized within or adjacent to the nucleolus in ∼90% of the nuclei (A1 93.2%, n = 73; A2 90.5%, n = 95; Fig. 1B). Surprisingly, a similar percentage of localization in or near the nucleolus was observed for the silent members (S1 92.3%, n = 91; S2 90.0%, n = 80; Fig. 1B), indicating that nucleolar positioning is independent of transcriptional activity. Similar results were observed when using PfPol I as nucleolar marker. A control gene (PFD0110w) from a subtelomeric position on chromosome 4 (which lacks rRNA genes) was detected apart from the nucleolus (Fig. 1B).
In yeast, Pol III-transcribed 5S rRNA genes are located next to rDNA tandem repeats and are nucleolar by definition (12). In the P. falciparum genome, three small 5S rRNA genes are located on chromosome 14 (Fig. 1A). To determine the nuclear position of P. falciparum 5S rDNA, we performed immuno-FISH using PfNop1 antibodies and a 5S rDNA probe (which includes the three tandem repeated 5S genes and the separating noncoding regions). 5S FISH signals were seen associated or adjacent to the nucleolus in ∼90% of the nuclei (n = 128; Fig. 1B), suggesting that in P. falciparum the nucleolus also concentrates Pol III transcription. This finding also predicts that other P. falciparum Pol III-transcribed genes, such as tRNA genes, may exhibit a similar localization, as seen in yeast (13). Taken together, these data show that all P. falciparum rRNA genes, although dispersed on different chromosomes, are clustered in a single perinuclear subregion. Thus, P. falciparum is the first example showing that tandem repetition is not an absolute requirement for the formation of a single nucleolus.
To gain insight into the factors that determine the clustering of dispersed rDNA, we tested whether the rDNA sequence itself might contain information that determines its location in the nucleolus. We analyzed the spatial position of rDNA-carrying plasmids transfected into P. falciparum parasites. rDNA fragments covering an entire rRNA unit were cloned into a transfection vector derived from pLN-ENR-GFP (14); however, we were able to obtain stable transfected parasites for only one rDNA fragment of 3.4 kb (Fig. 2A). Immuno-FISH analysis of the transfected parasites using PfNop1 antibodies and a probe for the plasmid backbone revealed that no additional nucleolus was formed around the plasmid, as previously seen in yeast (3). By contrast, we observed that plasmids carrying rDNA were associated with the nucleolus (pLN-rDNA 56.19%, n = 105; Fig. 2 B–D), whereas plasmids without the rDNA fragment were generally localized away from the nucleolus (pLN-ctrl 16.51%, n = 109; Fig. 2 B and C). This preferential nucleolar localization implies that the selectable drug gene (Pol II promoter), also present in the plasmid, can be actively transcribed while localized within the nucleolus. Alternatively, the two elements (Pol II promoter versus rDNA fragment) may exert different localization tendencies on the episomes, thereby explaining why we did not observed a higher percentage of plasmids containing rDNA in the nucleolus.
Fig. 2.
Nucleolar clustering is rDNA sequence dependent. (A) Schematic representation of Plasmodium rDNA unit composed of 18S-5.8S-28S genes. Dashed line denotes position of 3.4-kb rDNA fragment used to prepare the pLN-rDNA plasmid (Left). The pLN-ctrl control plasmid (Right) is the same plasmid without the rDNA fragment. The enzyme digestion sites (Ba, BamHI; Bl, BlpI; H, HindIII) used for plasmid preparation are indicated. Green line denotes position of plasmid FISH probe. (B) DNA FISH analysis of plasmid (green) and immunofluorescence of PfNop1 (red) in 3D7 parasites transfected with pLN-rDNA (first row) and pLN-ctrl (second row). Parasites are in ring stage, and nuclei are shown by DAPI staining (blue). (Scale bar, 1 μm.) (C) Quantification of colocalization of plasmids with nucleolus (pLN-rDNA n = 105 and pLN-ctrl n = 109). (D) Schematic recapitulating plasmid repositioning in the nucleolus in the presence of the rDNA sequence. (E) Schematic of pLN-rDNA and pLN-ctrl integration into subtelomeric attB locus (adjacent to the IT4var3) from A4attB parasites (15), via homologous recombination and single-site crossover. As inferred from 3D7 genome, IT4var3 locates in subtelomere from chromosome 9, which lacks rRNA genes. PCR analysis confirmed plasmid integration and lack of episomes in recombinant parasites (Fig. S2). (F) Immuno-FISH analysis, as in (B), of integrated plasmid (green) and nucleolus (red) in A4attB/rDNA (first row) and A4attB/ctrl (second row) parasites. (G) Quantification of colocalization of integrated plasmid with nucleolus (A4attB/rDNA n = 181 and A4attB/ctrl n = 164). (H) Schematic recapitulating subtelomere repositioning in nucleolus in presence of rDNA sequence. It is speculated that specific proteins are implicated in recognition and positioning in the nucleolar compartment.
We further examined the importance of the rDNA sequence for nucleolar localization by attempting to insert the same plasmids into an internal and subtelomeric region of the genome, using parasite lines that allow site-specific integration (14, 15). Although integration in the internal region of chromosome 7 (∼670 kb away from the A1 rRNA gene) failed, we successfully inserted the plasmids into the subtelomeric region of chromosome 9 (which lacks rRNA genes) (Fig. 2E and Fig. S2). Immuno-FISH analysis of the nucleolus and plasmid backbone, as described above, showed that the chromosome carrying the 3.4-kb rDNA sequence is more frequently associated with the nucleolus (A4attB/rDNA 45.86%, n = 181; Figs. 2 F–H), than when carrying the control plasmid (A4attB/ctrl 11.59%, n = 164; Fig. 2 F and G). These results suggest that nucleolar positioning is driven by the rDNA sequence, thus explaining the presence of silent and actively transcribed rRNA genes in a common subnuclear compartment.
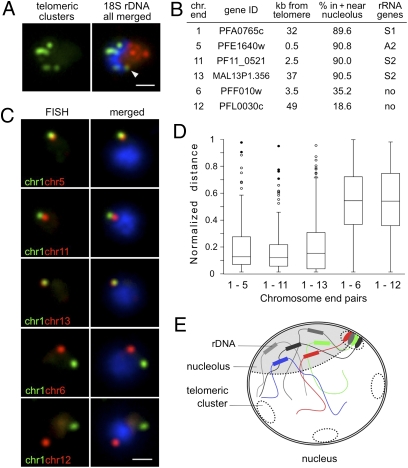
Given the localization of rDNA loci on subtelomeric regions (∼100 kb from the telomere, Fig. 1A), we investigated the impact of rDNA clustering on telomeric cluster organization (16). Two-color DNA FISH analysis using rDNA and telomeric probes demonstrated that the nucleolus localizes to one pole of the nucleus, excluding telomeric clusters (Fig. 3A). It was notable, however, that one telomeric cluster frequently localized adjacent to the nucleolus (56.67%, n = 60, Fig. 3A and Fig. S3A). It was tempting to speculate about the existence of a nucleolus-associated telomeric cluster composed of rDNA-carrying chromosome ends. To study chromosome end interactions adjacent to the nucleolus, we chose four chromosomes bearing rDNA units and prepared FISH probes for the first gene adjoining the noncoding subtelomeric region from each chromosome (chromosomes 1, 5, 11, and 13; Figs. 1A and 3B). Subtelomeric genes from chromosomes lacking rRNA genes were used as controls (chromosomes 6 and 12; Figs. 1A and 3B). We first verified by immuno-FISH that the chosen rDNA-carrying chromosome ends localized in or near the nucleolus, whereas control chromosome ends localized away from the nucleolus (Fig. 3B and Fig. S3B). We next performed two-color DNA FISH and pairwise analysis of chromosome ends by measuring the distance between the centers of the two FISH signals, which was normalized to the nuclear diameter (17). We observed that pairs of chromosome ends sharing rDNA sequences had smaller separating normalized distances (mean ± SD: chr1–chr5 0.21 ± 0.21, n = 112; chr1–chr11 0.19 ± 0.20, n = 101; chr1–chr13 0.23 ± 0.24, n = 118; Fig. 3 C–E) than pairs of chromosome ends with rDNA and unrelated sequences (chr1–chr6 0.57 ± 0.24, n = 111; chr1–chr12 0.58 ± 0.24, n = 93; Fig. 3 C and D), indicating that chromosome ends bearing rRNA genes are closely juxtaposed. These data provide evidence of nonrandom telomeric cluster composition in P. falciparum parasites and implicate rDNA as a genetic element that favors specific chromosomal associations. They further support previous observations in yeast, demonstrating the importance of unique sequence elements to promote telomere pairing (18).
Fig. 3.
Nucleolar clustering of rDNA determines preferential association of rDNA-carrying chromosome ends. (A) Two-color DNA FISH of telomeric clusters (rep20, green) and 18S rDNA (red). The 18S rDNA FISH probe simultaneously detects the five rDNA units, which are clustered at one pole of the nucleus opposing the telomere clusters. White arrow points to the potential nucleolus-associated telomeric cluster. (B) Table presenting relative nuclear position of chromosome-ends used in C determined by immuno-FISH using PfNop1 antibodies (Fig. S3B). Gene ID numbers according to www.plasmodb.org. (C) Two-color DNA FISH analysis of chromosome end pairs. Chromosome end 1 is labeled in green, and chromosome ends 5, 11, 13, 6, and 12 are shown in red. In all images, the parasites are in ring-stage, and nuclei are shown by DAPI staining (blue). (Scale bar, 1 μm.) (D) Box plot representation of the normalized distance distributions. Normalized distance (ND) = separating distance of two signals/nucleus diameter (17). ND ranges from 0 to 1. Upper and lower limits indicate maximum and minimum values. Boxes denote 50% of distances [interquartile range (IQR)], and line within boxes indicates median. Filled circles (●) correspond to outliers (3 × IQR); open circles (○) are suspected outliers (1.5 × IQR). Numbers of nuclei analyzed are as follows: chr1–chr5, n = 112; chr1–chr11, n = 101; chr1–chr13, n = 118; chr1–chr6, n = 111; chr1–chr12, n = 93. Average nucleus diameter (±SD) for these experiments is 2.09 ± 0.22 μm. (E) Schematic recapitulating nucleolar clustering of dispersed rRNA genes and the nucleolus-associated telomeric cluster. We propose that this rDNA cluster impose physical constraints that may shape the global genome nuclear organization, with potential consequences for gene expression in P. falciparum parasites.
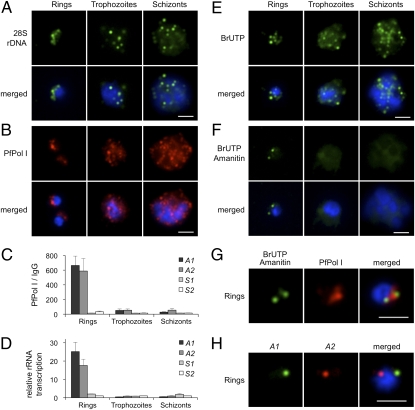
The P. falciparum blood-stage cycle takes ∼48 h to be completed and includes parasite maturation through ring (∼0–18 h), trophozoite (∼18–38 h) and schizont (∼38–48 h) stages. DNA synthesis and multiple mitotic divisions begin ∼30 h after invasion of the red blood cell. To examine whether rDNA clustering is maintained through cell-cycle progression, we collected parasites from a synchronized culture at three time points: ∼12 h, ∼30 h, and ∼40 h within the same cycle. FISH analysis using a 28S probe demonstrated that rDNA was clustered at one pole of the nucleus in ring-stage parasites; however, in replicative and dividing stages, individual rDNA units disintegrated and dispersed to multiple foci in the nucleus (Fig. 4A), demonstrating that rDNA nucleolar clustering is cell cycle dependent. Notably, the disorganization of rDNA in late-stages was linked with the relocation of nucleolar proteins such as PfPol I (Fig. 4B), PfNop1 and PfNop5 (Fig. S4). These proteins were detected in the nucleoplasm and cytoplasm in mature stages. To test whether PfPol I remains associated with the dispersed rDNA units, we carried out ChIP assays coupled to quantification by real-time PCR. In trophozoite and schizont-stages, PfPol I was dissociated from the transcribed region of the four rRNA genes (Fig. 4C). Consistent with the presence of PfPol I in the transcribed region of A1 and A2 rRNA genes only in early stages, levels of 45S precursor rRNA detected by real-time PCR were greatest in the ring stage (Fig. 4D). Chromatin analysis also revealed that activation of rRNA genes is associated with low nucleosome density and changes in histone marks (Fig. S5) previously described for Pol II-transcribed genes in P. falciparum (19).
Fig. 4.
Disruption of rDNA nucleolar clustering and decrease of rDNA transcription in replicative and mitotic stages. (A) FISH analysis of 28S rDNA (green) during 48-h blood-stage cycle. In ring stage (0–18 h postinvasion), rDNA is clustered at one pole of the nucleus, whereas in replicative and dividing stages (trophozoite, 18–38 h and schizont, 38–48 h), the individual rDNA units appear dispersed at multiple sites. (B) Immunofluorescence analysis of PfPol I (red). Ring-stage parasites show a half-moon perinuclear nucleolus. In trophozoites, PfPol I shows an additional diffuse pattern in the nucleoplasm and cytoplasm. In schizont segmented forms, PfPol I appears to relocalize at the nuclear periphery (Fig. S4). (C) ChIP analysis of PfPol I occupation on the individual rRNA genes (A1, A2, S1, and S2) during blood-stage cycle (mean ± SEM; n = 4). (D) Relative rRNA gene transcription throughout the blood-stage cycle. S2 values were set to 1 (mean ± SEM; n = 3). (E) Br-UTP labeling of nascent RNA (green, mean ± SD: rings 5.51 ± 1.57 foci/nucleus, n = 69; trophozoites 11.09 ± 3.30, n = 56; schizont-stages >20 foci). (F) Br-UTP labeling of nascent RNA in the presence of α-amanitin (green; rings 2.02 ± 0.69 foci/nucleus, n = 61). (G) Colocalization of α-amanitin–resistant foci (green) with nucleolus (red, PfPol I). (H) Two-color DNA FISH of A1 (green) and A2 (red) rRNA genes in ring-stage parasites (colocalization 23.30%, n = 103). In all images, parasite nuclei are shown by DAPI staining (blue). (Scale bars, 2 μm.)
To further visualize active PfPol I transcription in the nucleus, we labeled nascent RNA with bromo-UTP in the presence of α-amanitin, an inhibitor of Pol II and Pol III (20, 21). In the absence of α-amanitin, several discernible fluorescent foci were detected in P. falciparum nuclei (5.51 ± 1.57 foci in ring, 11.09 ± 3.30 foci in trophozoite and >20 foci in schizont-stages, Fig. 4E). When α-amanitin was included in the transcriptional buffer, the number of transcriptional sites was reduced to two perinuclear foci in early stages (2.02 ± 0.69 foci, Fig. 4F). Simultaneous detection of bromo-UTP RNA and PfPol I confirmed the nucleolar localization of both α-amanitin–resistant transcriptional foci (Fig. 4G), and two-color DNA FISH confirmed that A1 and A2 rRNA genes were dissociated in ∼80% of the nuclei (n = 103, Fig. 4H). These observations suggest the presence of two single-gene Pol I “transcription factories” (22, 23) within the nucleolus (Fig. S6A), which may be adequate for such highly transcribed genes. In late-stages, the α-amanitin–resistant transcriptional sites were barely visible (Fig. 4F). Taken together, these results show an apparent correlation between spatial nuclear organization, rRNA synthesis and the cell cycle.
Discussion
This work shows a remarkable degree of spatial organization in the nucleus of malaria parasites. By developing a variety of tools, we defined different nuclear landmarks in P. falciparum parasites. In addition to the perinuclear virulence gene clusters (telomeric and nontelomeric) (16, 24), we report here previously uncharacterized gene territories: the rRNA genes in the nucleolus and the transcription sites. Notably, these functionally differentiated subnuclear compartments are derived from the clustering of genes located in heterologous chromosomes. The nucleolar compartment is shown here to bring together active and silent rDNA loci from five different chromosomes (Fig. 3E, model). The silent telomeric clusters are composed of four to seven heterologous chromosome ends (16, 25) and the low number of total transcription sites certainly imply the juxtaposition of active genes from different chromosomes (22, 23, 26) (Fig. S6A, model). This scenario of multiple interchromosomal associations makes P. falciparum apparently distinct from the two current models of interphase chromosome organization: the Rabl configuration (18, 27), with centromeres and nucleolus at opposite sites of the nucleus, and the discrete chromosome territories (28).
The question arises of what forces drive these interchromosomal associations? Experimental evidence in this protozoan parasite suggests that DNA sequence and histone modifications might be crucial for nuclear spatial organization. It was shown that P. falciparum subtelomeric repeated sequences (including rep20) are necessary for telomeric cluster formation (29), and plasmids containing rep20 localized together with telomeric clusters (30). It was also reported that trimethylation of K9 on histone H3 is likely involved in promotion of chromosomal loop interactions with the nuclear periphery (24) (Fig. S6B, model). In the present study, we describe rDNA sequence as another genomic element implicated in nuclear positioning. We show that the rDNA sequence can override inherent nuclear positioning of chromosomes or episomes into the nucleolus (Fig. 2). Moreover, rDNA clustering appears to restrict chromosomal interactions by favoring the clustering of subtelomeric rDNA-carrying chromosome ends (Fig. 3). These observations may serve as a basis for a model of spatial nuclear organization for other gene families scattered over the P. falciparum linear genome, as well as for other Aplicomplexa parasites in which single rDNA units are also dispersed on different chromosomes (31).
Another remarkable feature of P. falciparum nuclear architecture is that it undergoes dynamic changes throughout the 48-h blood-stage cycle. Unlike yeast in which the nucleolus remains intact as the cell progresses through mitosis (2, 32), in P. falciparum we found the nucleolus to disassemble during replicative and dividing stages, the same time at which the telomeric clusters have also been observed to break apart (33). This event is linked with a dramatic decrease in rRNA gene transcription (Fig. 4D). By contrast, the number of the α-amanitin–sensitive transcription sites is strikingly increased at late stages (Fig. 4E), which is consistent with the increase in parasite transcriptional activity observed at this time (34, 35). In conclusion, these observations clearly show a coordinated cascade of spatial nuclear organization linked to the control of gene transcription. Moreover, our work opens avenues into understanding the complex processes that control malaria parasite-mediated virulence and stage-specific gene expression.
Materials and Methods
Parasites, Plasmid Construction, and Transfection.
P. falciparum blood stage parasites were cultured under conditions described previously (36) and synchronized by consecutive plasmagel and sorbitol treatments.
To prepare the pLN-rDNA plasmid, we PCR amplified a ∼3.4-kb rDNA fragment from 3D7 genomic DNA with primers containing restriction sites for the enzymes BamHI and BlpI, and cloned into the attP containing plasmid pLN-ENR-GFP (14). The plasmid control (pLN-ctrl) was obtained by digestion of pLN-rDNA with the enzyme HindIII to remove the rDNA fragment. Plasmids were verified by sequencing before transfection.
Ring-stage parasites were transfected by electroporation as previously published.(37). For episomal parasite lines, we transfected parasites from 3D7 strain and selection was done using 2.5 μg/mL blasticidin hydrochloride. For site-specific plasmid integration, 3D7attB or A4attB parasite lines were cotransfected with the pINT plasmid (14, 15) and selection was done using 2.5 μg/mL blasticidin hydrochloride and 300 μg/mL G418 sulfate. The presence of the plasmids, integrity of the fragment and plasmid integration were confirmed by PCR using different combinations of primers. Sequences of primers used for cloning or PCR analysis are listed in Table S1.
Antibodies.
A rabbit anti-PfPol I antibody was prepared by Sigma-Genosys; rabbits were immunized with a synthetic peptide coupled with a KLH/MBS carrier, N-CYEEGRGYDIDEQND-C. Antibodies against PfNop5 were obtained from Eurogentec; the antibody generation protocol consisted of immunizing rats with two synthetic peptides coupled to a KLH/MBS carrier: N-CFNKRKSDFRFKREAD-C and N-KFQNAFDETNKLMESC-C. Anti-BrdU mouse monoclonal antibody was purchased from Santa Cruz Biotechnology. AlexaFluor 488- or 568-conjugated goat anti-rabbit, anti-rat, or anti-mouse highly cross-absorbed antibodies were purchased from Invitrogen.
Immunofluorescence and FISH.
Immunofluorescence assays were performed on synchronized parasites fixed in suspension as described previously (33). Antibodies dilutions were as follows: rabbit anti-PfPolI 1:500 or 1:1,000, rabbit anti-PfNop1 1:100, rat anti-PfNop1 1:50, rat anti-PfNop5 1:50, and secondary antibodies 1:500.
DNA FISH was performed on parasites fixed in suspension as described previously (33). For immuno-DNA FISH, parasites were first subjected to immunofluorescence, postfixed in 4% paraformaldeyde for 15 min, deposited on microscope slides, permeabilized in 0.1% Triton X-100 and hybridized with FISH probes at 72 °C for 10 min and then at 37 °C overnight. For immuno-RNA FISH, parasites were treated as for immuno-DNA FISH, except that hybridization was performed at 37 °C overnight only. A telomere probe (rep20 or TARE6) was obtained as described elsewhere (33). All other FISH probes were PCR-amplified from 3D7 genomic DNA using the primers listed in Table S1.
Br-UTP Transcription Assay.
Br-UTP incorporation was adapted from a previous protocol (38). Synchronized cultures of parasites were permeabilized with saponin, washed and resuspended in transcriptional buffer (50 mM Hepes, 100 mM KCl, 5 mM MgCl2, 0.5 mM EGTA, 0.5mM PMSF, 5 mM DTT, 100 U/mL RNase inhibitor, 2 mM ATP, 1 mM CTP, 1 mM GTP, and 0.5 mM Br-UTP). After 15–30 min of incubation at 37 °C, parasites were fixed with 4% paraformaldehyde for 15 min. Parasites were then subjected to immunofluorescence assays using an anti-BrdU antibody as described above. rRNA transcription sites were detected by including α-amanitin (200 μg/mL) in the transcriptional buffer.
Image Analysis.
Images were captured using a motorized Nikon Eclipse 80i epifluorescence microscope with a CoolSnap HQ2 camera. NIS Elements 3.0 software was used for acquisition and measurements of distances and nuclear diameter. ImageJ (http://rsbweb.nih.gov/ij/) was used for composition. Chromatic aberrations were corrected in all images by aligning the red, green, and blue channel signals from 0.2 μm TetraSpeck microspheres. Data obtained are 2D images of the 3D parasite nuclei. Thus, accidental overlap of fluorescence signals cannot be ruled out. For all figures, 50–200 nuclei were scored from at least three independent experiments, and colocalization/association was defined as any overlap between two fluorescent signals. For scoring relative position to the nucleolus, we used three categories: association, adjacent, and apart.
Transcript Analysis.
Total RNA was isolated from synchronized cultures using the TRIzol method (39). Extracted RNA was DNase-treated and reverse-transcribed with SuperScript II Reverse Transcriptase using random hexamers. Real-time PCR was performed in duplicate using a Realplex4 EpgradientS thermal cycler. The pairs of primers used are listed in Table S1. The transcription level of each rRNA gene was assessed using the equation obtained through the standard curve.
ChIP Analysis.
The ChIP assay was performed using a previously described protocol (19, 25), with some modifications. Parasite cultures were treated with saponin and cross-linked with 1% formaldehyde for 10 min. Approximately 10 μg of chromatin was incubated at 4 °C with 7.5 μL anti-PfPol I and the respective preimmune serum or rabbit IgG. Input and immunoprecipitated DNA was analyzed by real-time PCR as described above, using the primer pairs listed in Table S1. We calculated the percentage of immunoprecipitation for PfPol I and IgG, and then the ratio PfPolI/IgG. Preimmune serum and rabbit IgG produced similar results.
Supplementary Material
Acknowledgments
We thank J. J. Lopez-Rubio, R. Hernandez-Rivas, and E. Fabre for helpful discussions and critical reading of the manuscript, D. Fidock (Columbia University Medical Center, New York, NY) for providing plasmids (pLN-ENR-GFP and pINT) and parasite lines (3D7attB and A4attB), M. Nunes for helping with the RT-PCR analysis and parasite transfection, and R. Amino for helping with image analysis. This work was supported by the Portuguese Foundation for Science and Technology (fellowship SFRH/BD/11756/2003 to L.M.-S), the French Agency for Research (ANR Blanc 0274-01), and European Research Council Executive Agency Advanced Grant (PlasmoEscape 250320).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001045107/-/DCSupplemental.
References
- 1.McStay B, Grummt I. The epigenetics of rRNA genes: From molecular to chromosome biology. Annu Rev Cell Dev Biol. 2008;24:131–157. doi: 10.1146/annurev.cellbio.24.110707.175259. [DOI] [PubMed] [Google Scholar]
- 2.Carmo-Fonseca M, Mendes-Soares L, Campos I. To be or not to be in the nucleolus. Nat Cell Biol. 2000;2:E107–E112. doi: 10.1038/35014078. [DOI] [PubMed] [Google Scholar]
- 3.Oakes M, et al. Mutational analysis of the structure and localization of the nucleolus in the yeast Saccharomyces cerevisiae. J Cell Biol. 1998;143:23–34. doi: 10.1083/jcb.143.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langsley G, Hyde JE, Goman M, Scaife JG. Cloning and characterisation of the rRNA genes from the human malaria parasite Plasmodium falciparum. Nucleic Acids Res. 1983;11:8703–8717. doi: 10.1093/nar/11.24.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellems TE, et al. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987;49:633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MJ, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunderson JH, et al. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987;238:933–937. doi: 10.1126/science.3672135. [DOI] [PubMed] [Google Scholar]
- 8.Waters AP, Syin C, McCutchan TF. Developmental regulation of stage-specific ribosome populations in Plasmodium. Nature. 1989;342:438–440. doi: 10.1038/342438a0. [DOI] [PubMed] [Google Scholar]
- 9.Fang J, Sullivan M, McCutchan TF. The effects of glucose concentration on the reciprocal regulation of rRNA promoters in Plasmodium falciparum. J Biol Chem. 2004;279:720–725. doi: 10.1074/jbc.M308284200. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo LM, et al. The unusually large Plasmodium telomerase reverse-transcriptase localizes in a discrete compartment associated with the nucleolus. Nucleic Acids Res. 2005;33:1111–1122. doi: 10.1093/nar/gki260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang J, McCutchan TF. Thermoregulation in a parasite's life cycle. Nature. 2002;418:742. doi: 10.1038/418742a. [DOI] [PubMed] [Google Scholar]
- 12.Nomura M, Nogi Y, Oakes M. Transcription of rDNA in the yeast Saccharomyces cerevisiae. Molecular Biology Intelligence Unit. The Nucleolus. 2004:128–153. [Google Scholar]
- 13.Thompson M, Haeusler RA, Good PD, Engelke DR. Nucleolar clustering of dispersed tRNA genes. Science. 2003;302:1399–1401. doi: 10.1126/science.1089814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nkrumah LJ, et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat Methods. 2006;3:615–621. doi: 10.1038/nmeth904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melcher M, et al. Identification of a role for the PfEMP1 semi-conserved head structure in protein trafficking to the surface of Plasmodium falciparum infected red blood cells. Cell Microbiol. 2010 doi: 10.1111/j.1462-5822.2010.01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freitas-Junior LH, et al. Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature. 2000;407:1018–1022. doi: 10.1038/35039531. [DOI] [PubMed] [Google Scholar]
- 17.Xu N, Tsai CL, Lee JT. Transient homologous chromosome pairing marks the onset of X inactivation. Science. 2006;311:1149–1152. doi: 10.1126/science.1122984. [DOI] [PubMed] [Google Scholar]
- 18.Schober H, et al. Controlled exchange of chromosomal arms reveals principles driving telomere interactions in yeast. Genome Res. 2008;18:261–271. doi: 10.1101/gr.6687808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Rubio JJ, et al. 5′ Flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol. 2007;66:1296–1305. doi: 10.1111/j.1365-2958.2007.06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzer M, de Bruin D, Ravetch JV. Transcription mapping of a 100 kb locus of Plasmodium falciparum identifies an intergenic region in which transcription terminates and reinitiates. EMBO J. 1992;11:1949–1955. doi: 10.1002/j.1460-2075.1992.tb05248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weil PA, Blatti SP. HeLa cell deoxyribonucleic acid dependent RNA polymerases: Function and properties of the class III enzymes. Biochemistry. 1976;15:1500–1509. doi: 10.1021/bi00652a022. [DOI] [PubMed] [Google Scholar]
- 22.Cook PR. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland H, Bickmore WA. Transcription factories: Gene expression in unions? Nat Rev Genet. 2009;10:457–466. doi: 10.1038/nrg2592. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Rubio JJ, Mancio-Silva L, Scherf A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe. 2009;5:179–190. doi: 10.1016/j.chom.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 25.Freitas-Junior LH, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 26.Osborne CS, et al. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet. 2004;36:1065–1071. doi: 10.1038/ng1423. [DOI] [PubMed] [Google Scholar]
- 27.Bystricky K, Laroche T, van Houwe G, Blaszczyk M, Gasser SM. Chromosome looping in yeast: Telomere pairing and coordinated movement reflect anchoring efficiency and territorial organization. J Cell Biol. 2005;168:375–387. doi: 10.1083/jcb.200409091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301. doi: 10.1038/35066075. [DOI] [PubMed] [Google Scholar]
- 29.Figueiredo LM, Freitas-Junior LH, Bottius E, Olivo-Marin JC, Scherf A. A central role for Plasmodium falciparum subtelomeric regions in spatial positioning and telomere length regulation. EMBO J. 2002;21:815–824. doi: 10.1093/emboj/21.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Donnell RA, et al. A genetic screen for improved plasmid segregation reveals a role for Rep20 in the interaction of Plasmodium falciparum chromosomes. EMBO J. 2002;21:1231–1239. doi: 10.1093/emboj/21.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercereau-Puijalon O, Barale JC, Bischoff E. Three multigene families in Plasmodium parasites: Facts and questions. Int J Parasitol. 2002;32:1323–1344. doi: 10.1016/s0020-7519(02)00111-x. [DOI] [PubMed] [Google Scholar]
- 32.Heun P, Taddei A, Gasser SM. From snapshots to moving pictures: New perspectives on nuclear organization. Trends Cell Biol. 2001;11:519–525. doi: 10.1016/s0962-8924(01)02174-2. [DOI] [PubMed] [Google Scholar]
- 33.Mancio-Silva L, Rojas-Meza AP, Vargas M, Scherf A, Hernandez-Rivas R. Differential association of Orc1 and Sir2 proteins to telomeric domains in Plasmodium falciparum. J Cell Sci. 2008;121:2046–2053. doi: 10.1242/jcs.026427. [DOI] [PubMed] [Google Scholar]
- 34.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gritzmacher CA, Reese RT. Protein and nucleic acid synthesis during synchronized growth of Plasmodium falciparum. J Bacteriol. 1984;160:1165–1167. doi: 10.1128/jb.160.3.1165-1167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nunes MC, Goldring JP, Doerig C, Scherf A. A novel protein kinase family in Plasmodium falciparum is differentially transcribed and secreted to various cellular compartments of the host cell. Mol Microbiol. 2007;63:391–403. doi: 10.1111/j.1365-2958.2006.05521.x. [DOI] [PubMed] [Google Scholar]
- 37.Cowman A, et al. Transfection of Plasmodium falciparum. Methods in Malaria Research. 2008;5th edition:281–301. [Google Scholar]
- 38.Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414:759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- 39.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum. Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.