Abstract
Estrogen receptor α (ERα) plays an important role in the onset and progression of breast cancer, whereas p53 functions as a major tumor suppressor. We previously reported that ERα binds to p53, resulting in inhibition of transcriptional regulation by p53. Here, we report on the molecular mechanisms by which ERα suppresses p53’s transactivation function. Sequential ChIP assays demonstrated that ERα represses p53-mediated transcriptional activation in human breast cancer cells by recruiting nuclear receptor corepressors (NCoR and SMRT) and histone deacetylase 1 (HDAC1). RNAi-mediated down-regulation of NCoR resulted in increased endogenous expression of the cyclin-dependent kinase (CDK)-inhibitor p21Waf1/Cip1 (CDKN1A) gene, a prototypic transcriptional target of p53. While 17β-estradiol (E2) enhanced ERα binding to p53 and inhibited p21 transcription, antiestrogens decreased ERα recruitment and induced transcription. The effects of estrogen and antiestrogens on p21 transcription were diametrically opposite to their known effects on the conventional ERE-containing ERα target gene, pS2/TFF1. These results suggest that ERα uses dual strategies to promote abnormal cellular proliferation: enhancing the transcription of ERE-containing proproliferative genes and repressing the transcription of p53-responsive antiproliferative genes. Importantly, ERα binds to p53 and inhibits transcriptional activation by p53 in stem/progenitor cell-containing murine mammospheres, suggesting a potential role for the ER–p53 interaction in mammary tissue homeostasis and cancer formation. Furthermore, retrospective studies analyzing response to tamoxifen therapy in a subset of patients with ER-positive breast cancer expressing either wild-type or mutant p53 suggest that the presence of wild-type p53 is an important determinant of positive therapeutic response.
Keywords: nuclear receptor corepressor, mammary epithelial cells, mammospheres, tumor suppressor protein, tamoxifen therapy
Cells integrate multiple pathways to elicit responses to various signals, depending on the cellular context and tissue microenvironment. Estrogen receptor α (ERα) is a ligand-dependent transcriptional regulator that, when bound to estrogen response elements (EREs) on target gene promoters, regulates transcription via estrogen-dependent recruitment of steroid receptor coactivators (SRCs), such as SRC1, SRC2/DRIP1/TIF2/NCoA2/, and SRC3/AIB1/TRAM1/pCIP/RAC3/ACTR, followed by chromatin histone acetylation (1–3). On the other hand, when antiestrogens such as tamoxifen are bound to ERα, various corepressors, including nuclear receptor corepressor (NCoR) and silencing mediator for retinoid and thyroid hormone receptors (SMRT), are recruited. This is followed by recruitment of histone deacetylases (HDACs), leading to transcriptional repression (4, 5). ERα signaling plays an important role in many tissues, including the mammary gland, where its expression is required for normal gland development and the preservation of adult mammary gland function. When deregulated, ERα becomes abnormally proproliferative and greatly contributes to the onset and progression of breast cancer (6, 7).
Similar to ERα, tumor suppressor p53 also plays a central role in many cellular processes, such as cell cycle regulation, apoptosis, senescence, and differentiation (8–10). Although these functions of p53 are essential to maintain normal cellular homeostasis, if left uncontrolled, they can lead to deleterious consequences. As such, mutations in the p53 gene and aberrations in the p53 signaling pathway pave the way to tumorigenesis (11). Extensive research over the past two decades has placed p53 at the hub of a very complex network of signaling pathways that integrate a variety of intracellular and extracellular inputs. It is becoming increasingly clear that the opposing effects of various components of the p53 signaling network must be carefully balanced to correctly regulate p53 function and induce the appropriate cellular response (11, 12). One such modulator of p53 signaling is ERα. We have reported that in ER-positive human breast cancer cells, as well as in a mouse xenograft model, ERα binds to p53 and represses its transcriptional function (13–15), resulting in inhibition of p53-mediated cell cycle arrest (14) and apoptosis (15).
Compared with many other cancer types, p53 mutations are not very frequent in breast cancer. Approximately 80% of breast cancers express wild-type p53. Moreover, 70–80% of breast cancers are also ER-positive, and the majority of these express wild-type p53, albeit functionally debilitated. Interestingly, most ERα-negative breast tumors express mutant p53 (16–18). Therefore, it is likely that abrogation of the p53 signaling pathway is a major event in both ERα-positive and -negative tumors; in the former, wild-type p53 is functionally repressed by ERα, and in the latter, p53 is inactivated by mutations. ERα is the primary target of endocrine therapy in breast cancer. However, a major clinical obstacle is that a large fraction of patients with ERα-positive breast tumors do not respond to tamoxifen therapy (19–21). The presence of wild-type p53 has been reported to have a positive impact on the therapeutic response and prognosis of breast cancer patients (17, 22–24). However, the cellular and molecular mechanisms by which p53 influences endocrine therapy efficacy remain elusive.
Recent progress in the identification and characterization of normal and tumor stem and progenitor cells in murine and human mammary gland tissue has triggered an intense effort to explore the mechanisms that regulate the balance between symmetric and asymmetric division of these cells (25, 26). Disabling p53 increases stem cell production by favoring symmetric division over asymmetric division, suggesting an important role for p53 in mammary tissue homeostasis and cancer formation (25). Importantly, a fraction of mammospheres derived from normal murine and human mammary tissue and breast cancer tissue display stem/progenitor cell characteristics and express ERα (27–29). However, whether ERα disables wild-type p53 in these cells remains to be elucidated.
In the present study, we examined the mechanisms by which ERα binds to p53 on p53 target gene promoters and represses p53-mediated transcriptional activation. We provide evidence that ERα recruits corepressors when bound to p53 and show that the ERα–p53 interaction is relevant to normal mammary stem cell regulation and to breast cancer patient response to antiestrogen therapy.
Results
p53-Bound ERα Recruits Transcriptional Corepressors and HDAC1 to the p21Waf1/Cip1 (CDKN1A) Gene Promoter.
We have previously demonstrated that ERα binds to p53, leading to functional repression of p53 transcriptional activity (13–15). To explore the mechanisms by which ERα inhibits p53-mediated transcriptional activation of the p21 gene, a prototypic p53-target gene, we performed conventional and sequential site-specific ChIP assays to analyze whether ERα recruits corepressors to the p53-binding site of the p21 promoter. For sequential ChIP assays, chromatin from human MCF-7 breast cancer cell extract was immunoprecipitated using anti-p53 antibody, reimmunoprecipitated a second time with anti-ERα antibody, and reimmunoprecipitated a third time with antibodies against NCoR, SMRT, RIP 140, or HDAC1. NCoR and SMRT were found in a complex with ERα and p53 on the p21 promoter; however, the amount of RIP 140 in the complex was very low, reflecting low levels of this corepressor in the complex or a relatively weak antibody (Fig. 1A). Importantly, HDAC1 was present in the p53/ERα/NCoR/SMRT complex on the p21 promoter (Fig. 1A). NCoR, SMRT, and HDAC1 were not recruited to the p21 promoter when ERα was knocked down, suggesting that ERα is required for their recruitment (Fig. 1B). ERα knockdown did not affect NCoR, SMRT, or HDAC1 protein expression levels (Fig. 1C) but, as expected, resulted in the loss of ERα on the promoter (Fig. 1D), further supporting the role of ERα in recruiting the corepressors and HDAC1 to the p21 promoter. Treatment with E2 enhanced the recruitment of HDAC1 and NCoR to the p21 promoter but did not affect SMRT recruitment (Fig. 1E). Furthermore, knocking down NCoR resulted in transcriptional activation of an exogenously transfected p21 promoter plasmid (Fig. 1F) and increased endogenous expression of p21 mRNA and protein (Fig. 1 G and H). Collectively, these data suggest that ERα-mediated inhibition of p53 transcriptional activity is NCoR-dependent. Furthermore, the importance of NCoR in the ERα–p53 repressor complex is strengthened by the finding that, although the ERα-L539A mutant is capable of accessing the p53 target promoter via p53 (Fig. S1B), it is defective in repressing p53 (14), as this mutant is less efficient in recruiting NCoR compared with wild-type ERα (Fig. S1C).
Fig. 1.
ERα recruits transcriptional corepressors to repress p53-mediated transcriptional activation. (A) ChIP and sequential ChIP assays were performed on MCF-7 cells with primers specific to the p53-binding site of the p21 promoter. The primary ChIP was performed with anti-p53 antibody, and the immunoprecipitate was subjected to a second ChIP with anti-ERα antibody. The immunoprecipitate from the ERα ChIP was then subjected to the third ChIP with antibodies against HDAC1, NCoR, SMRT, and RIP140. ChIP with IgG was the negative control. (B) ChIP assay as in A with antibodies against NCoR, SMRT, HDAC1, and IgG in MCF-7 cells transfected with NS or ERα siRNAs. (C) Western analysis of protein expression of NCoR, SMRT, RIP140, HDAC1, and ERα in MCF-7 cells transfected with NS siRNA or ERα siRNA. (D) ChIP assay performed on ERα knockdown MCF-7 cells using antibodies against p53, ERα, or IgG. (E) Recruitment of NCoR, SMRT, and HDAC1 to the p53-binding site of the p21 promoter in MCF-7 cells treated with E2 (10 nM) or vehicle for 3 h, as analyzed by ChIP assay. (F) MCF-7 cells were transfected with −2326 p21-luc reporter and NCoR shRNA plasmid or a nonspecific (NS) shRNA plasmid. Cells were harvested 24 h posttransfection and analyzed for luciferase activity. Data are averages from three samples with SD. (G) Transcription of the endogenous p21 gene in MCF-7 cells treated as in F was assayed by qPCR. Data are averages from three samples with SD. (H) Western analysis of protein expression of NCoR, ERα, and p53 in MCF-7 cells transfected with NS or NCoR siRNAs.
Effects of Estrogen and Antiestrogens Cofactor Recruitment and Transcription of the p53 Target Gene p21 Are Diametrically Opposite to Their Effects on the ERE-Containing ERα Target Gene pS2.
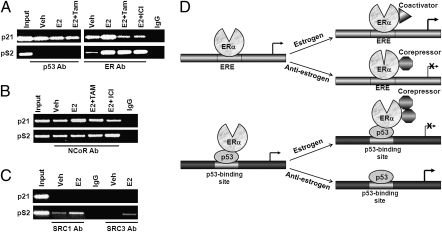
We previously observed that ionizing radiation (known to affect the posttranslational modification and activation status of p53) disrupts the ERα–p53 interaction (13, 14). Therefore, we wanted to explore whether estrogens or antiestrogens, which bind to ERα and modulate ERα function, affect ERα’s ability to bind to p53. Indeed, quantitative ChIP (qChIP) analysis showed that E2 treatment increased ERα binding to the p21 promoter, whereas tamoxifen and fulvestrant (ICI 182780) disrupted the interaction (Fig. 2A). These effects were not caused by changes in p53 or ERα protein levels following E2 and antiestrogen treatment (Fig. 2B). A time-course experiment in MCF-7 cells showed that E2 repressed endogenous p21 transcription, whereas tamoxifen and fulvestrant increased p21 transcription (Fig. 2C). Importantly, when measured in parallel by quantitative real-time PCR (qPCR) in the same cells, both E2 and antiestrogens elicited diametrically opposite effects on transcription of pS2/TFF2, a prototypic ERE-containing ERα target gene (Fig. 2D), compared with their effects on p21 transcription. Therefore, we tested whether these opposite effects on p21 and pS2 gene transcription were caused by differential recruitment of ERα, corepressors, or coactivators to the p21 and pS2 gene promoters. Although p53 recruitment to the p53 binding site of the p21 promoter was not considerably affected by E2 or antiestrogens, ERα recruitment was enhanced by E2 and reduced by antiestrogens (Fig. 3A). However, in the case of the pS2 promoter, E2 enhanced ERα binding to the ERE in both the presence and absence of antiestrogens, as expected (Fig. 3A). Similarly, recruitment of NCoR to the p21 promoter has a pattern different from that of its recruitment to the pS2 promoter. E2 enhanced NCoR recruitment to the p53 binding site of the 21 promoter, whereas tamoxifen and fulvestrant increased NCoR recruitment to the ERE site of the pS2 promoter (Fig. 3B). Interestingly, neither coactivator SRC1 nor SRC2 was recruited to the p21 promoter in the presence of E2; however, as expected, both were recruited to the pS2 promoter (Fig. 3C). These observations suggest that besides regulating ERE-containing target genes, ERα can also effectively regulate p53 target genes by influencing the transcriptional function of p53 (Fig. 3D). Interestingly, repression of p53-mediated transcriptional activation by ERα appears to be gene-selective, as it fails to repress some p53 target genes tested, suggesting the importance of promoter context (Fig. S2).
Fig. 2.
Estrogen enhances and antiestrogens disrupt the ERα–p53 interaction on the p21 promoter, leading to diametrically opposite effects on transcription of p21 compared with that of pS2. (A) qChIP was performed to analyze the ERα–p53 interaction on the p21 promoter in MCF-7 cells. Cells were grown in media with dextran-coated charcoal-treated FBS for 4 d and treated with E2 (10 nM) with or without 1 μM 4-hydroxy tamoxifen or ICI 182780 for 3 h. (B) Western analysis of ERα and p53 protein levels in lysates of MCF-7 cells treated with E2, E2 plus tamoxifen, or E2 plus ICI 182780. (C and D) The effects of estrogen and antiestrogens on p21 and pS2 transcription, respectively, in MCF-7 cells, as assayed by qPCR (value = mean ± SD; *P < 0.05; ***P < 0.001).
Fig. 3.
Differential effects of ERα bound to ERE-containing promoters versus p53 binding site-containing promoters. (A) ChIP using anti-p53 or anti-ERα antibodies to analyze p53 and ERα binding to endogenous p21 and pS2 gene promoters in MCF-7 cells treated with E2, E2 plus tamoxifen, or E2 plus ICI 182780. (B) ChIP assay using anti-NCoR antibody, demonstrating differential recruitment of NCoR to pS2 vs. p21 gene promoters in MCF-7 cells treated as in A. (C) ChIP assay using SRC1 and SRC3 antibodies, demonstrating differential recruitment of SRC1 and SRC3 to pS2 versus p21 gene promoters in MCF-7 cells treated as in A. (D) A model for the dual roles of ERα in promoting proliferation via direct binding to ERE elements and indirect binding to p53 binding sites (by tethering to p53).
ERα–p53 Interaction Exists in Mammary Stem Cells.
Mammary epithelial stem cells (SCs) are considered to be primary targets in the onset and progression of breast cancer, and several recent reports demonstrate that p53 is a key regulator of stem cell division (26). Although activation of wild-type p53 in breast cancer stem cells (CSCs) has been reported to reverse this process (25), the causes of p53 inactivation remain unclear. Mammary epithelial cells form spherical colonies termed “mammospheres” when cultured on nonadherent surfaces, and these mammospheres are enriched in cells with functional characteristics of stem/progenitor cells (30). To test whether ERα suppresses wild-type p53 in mammospheres, we derived mouse mammospheres (25, 31) from mammary epithelial cells isolated from C57BL/6 ERαfl/fl mice (32) (Fig. S3A). Compared with mouse mammary epithelial cells, the mammospheres expressed very high levels of Sca-1, but relatively low levels of cytokeratin 14, cytokeratin 18, E-cadherin, and integrin α 6 (Itga6) (Fig. 4A). The expression pattern of these markers is a characteristic of cultured murine mammospheres capable of repopulating the entire mammary gland (33–35). As the mammospheres also expressed both p53 and ERα (Fig. 4B), we used micro-ChIP assays to analyze whether ERα and p53 cooccupy the p53 binding site of the p21 promoter and whether ERα occupancy suppresses p21 transcription in these cells. Indeed, as is the case with MCF-7 cells, ERα binds to the p53 site of the p21 promoter but not to a nonspecific (NS) site without any p53-binding sequence (Fig. 4C). Subsequent deletion of the floxed ERα gene using Cre-recombinase–expressing lentivirus resulted in a decrease in ERα transcript levels and a considerable increase in p21 levels, suggesting that loss of ERα induces p53 activity in stem cell-containing mammospheres (Fig. 4D).
Fig. 4.
ERα represses p53 in murine mammary stem cells. (A) qPCR analysis of the expression of Sca1, cytokeratin 14 (Ck14), cytokeratin 18 (Ck18), E-cadherin (E-Cad), and integrin α 6 (Itga6) in murine mammary epithelial cells (normalized to 1) and murine stem cell-containing mammospheres derived from the mammary glands of C57 BL/6 ERαfl/fl mice. Data are averages from three samples with SD. (B) Western analysis of ERα and p53 proteins in murine mammospheres and MCF-7 cells. (C) Micro-ChIP analysis of the ERα–p53 interaction on the p21 gene promoter in murine mammospheres (Upper). Micro-ChIP analysis of a region of the p21 gene (NS) that does not contain any p53-binding sites (negative control) (Lower). (D) PCR analysis of ERα and p21 RNA levels in C57BL/6 ERαfl/fl murine mammospheres with or without infection with Cre-recombinase lentivirus.
Importance of p53 Status in the Response of ER-Positive Breast Cancer Patients to Tamoxifen Therapy.
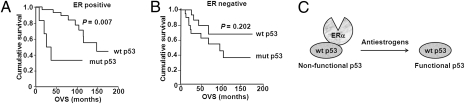
Based on the data obtained from our cell culture models and mouse xenograft studies (13–15), we postulated that one of the determinants for responsiveness to antiestrogen therapy could be the ability of antiestrogens to reactivate p53 by disrupting the ERα–p53 interaction. To test this hypothesis, we analyzed the p53 status of 35 ER-positive tamoxifen-treated breast cancer cases and 36 ER-negative cases (Table S1) with a clinical follow-up of up to 176 mo. Because only mutant p53 protein is usually detectable in clinical samples, we used positive protein detection by immunohistochemical analysis to determine mutant p53 status. Importantly, none of the 17 patients whose overall survival (OVS) was positively impacted by tamoxifen treatment had detectable mutant p53 protein in their tumors (in other words, their tumors expressed wild-type p53), whereas among the 18 ER-positive patients who did not respond to tamoxifen therapy, 7 (39%) had tumors expressing mutant p53. Thus, the OVS of patients with ER-positive breast cancer under tamoxifen treatment was significantly influenced by p53 status (P = 0.007) (Fig. 5A). Multivariate analysis by Cox regression model revealed that p53 is a stable predictor of OVS in ER-positive patients [P = 0.012; relative risk 5.4; 95% confidence interval (CI) 1.45–20.76]. In contrast, mutant p53 was not a predictor for OVS in patients with ER-negative tumors, for whom tamoxifen is not a standard treatment (Fig. 5B).
Fig. 5.
ERα-positive breast cancer patients with tumors expressing wild-type p53 respond better to tamoxifen therapy. (A) Kaplan-Meier analysis of the overall survival of 35 ERα-positive (28 patients had tumors with wild-type p53 and 7 patients had tumors with mutant p53), tamoxifen-treated breast cancer patients as a function of wild-type or mutant p53 expression. (B) Similar analysis as in A of 36 ERα-negative breast cancer patients (17 patients had tumors with wild-type p53 and 19 patients had tumors with mutant p53) who were not treated with tamoxifen. (C) A model for the role of the ERα–p53 interaction in tumor response to antiestrogen therapy.
Discussion
A strong relationship of estrogen signaling and ER with p53 has been documented (16, 18, 36–41), and we have reported that ERα and p53 physically interact, leading to inhibition of p53 function in cell culture and xenograft models (13–15). However, the mechanisms underlying functional repression of p53 by ERα have remained unclear. Our results demonstrate that ERα accesses p53 target gene promoters by directly binding to p53, and ERα represses p53 function by recruiting NCOR, SMRT, and HDAC1. In addition to our earlier observation that p53 is necessary for recruitment of ERα to the p21 gene promoter (14), the data presented here show that ERα is necessary for the recruitment of corepressors and HDAC1, as none of them could be detected on the p53-binding site of the p21 gene promoter when ERα was knocked down. These observations, along with the fact that ERα, NCoR, SMRT, and HDAC1 were detected on the p21 promoter in a sequential ChIP assay starting with p53 antibody, strongly suggest that p53, ERα, NCoR, SMRT, and HDAC1 coexist in a single complex on the p53-binding site(s) of the human p21 promoter. The functional significance of NCoR recruitment was ascertained by the observation that transcription from an exogenously transfected p21 promoter reporter and the endogenous p21 gene were both increased when NCoR was knocked down. Furthermore, the ERα-L539A mutant, although capable of interacting with promoter-bound p53, had reduced ability to recruit NCoR to the ER–p53 complex and, therefore, was less efficient in repressing p53’s transcriptional activity. Notably, ERα-L539A, which is also deficient in recruiting coactivators and incapable of activating transcription of ERE-containing target genes in the presence of E2, was capable of activating the endogenous ERα-target gene pS2 in the presence of antiestrogens (42).
Similar to NCoR, HDAC1 appears to be playing an important role in the p53/ERα/NCoR/SMRT/HDAC1 complex. HDAC1 binds to p53 and down-regulates its transactivation function, and this inactivation is largely dependent on the 30 C-terminal residues of p53 (43). Interestingly, this same region of p53 has been mapped as the domain that interacts with ERα (14). As HDAC1 can directly bind to ERα (44), ERα may be facilitating HDAC1 binding to p53, which in turn could lead to deacetylation and inactivation of p53. In addition to repressing p53 function by recruiting corepressors and HDAC1, the possibility that ERα recruits other HDACs or prevents recruitment of p53 coactivators remains to be tested. Of note, HDAC1 was reported to antagonize p53 via Sp1 in regulating p21 transcription (45). Furthermore, HDAC1 and HDAC2 act in concert to repress the expression of p21 and p57 and promote G1 to S phase progression in mouse embryonic fibroblasts in vitro and B cells in vivo (46).
The effects of E2 and antiestrogens were diametrically opposite on p21 (prototypic p53-target gene) transcription versus pS2 (prototypic ER-target gene) transcription. E2 repressed p21 transcription, whereas E2 increased pS2 transcription, as expected. Consistent with this, E2 enhanced ERα and NCoR recruitment to the p21 promoter, whereas both tamoxifen and fulvestrant decreased ERα and NCoR recruitment. Similar to the p21 case, E2 enhanced ERα recruitment to the pS2 promoter, although the transcriptional response was opposite; both tamoxifen and fulvestrant enhanced p21 transcription but reduced pS2 transcription. These observations led us to propose a model for ERα function (Fig. 3D). We propose that when bound to p53 on p53 target gene promoters, ERα recruits corepressors in the presence of estrogen to repress transcription. Antiestrogens, on the other hand, disrupt ERα-p53 interaction thereby alleviating transcriptional repression.This is in addition to what has been previously reported: when bound to the ERE of ER target genes, ERα recruits coactivators in the presence of E2 to activate transcription, whereas it recruits corepressors in the presence of antiestrogens to repress transcription (47, 48). Collectively, these findings suggest dual roles for ERα in promoting breast tumorigenesis: ERα increases the transcription of ERE-containing proproliferation genes and also counteracts p53’s ability to activate antiproliferative p53 target genes (such as p21, BTG2, and PUMA) and to repress proproliferative p53 target genes (such as survivin). Our previous finding that ERα represses both p53-mediated activation of p21 (14) and p53-mediated repression of survivin (15) is consistent with this model. HDAC1 and HDAC4, but not SRC1 or SRC3, are recruited to the ERα–p53 complex on the survivin promoter, leading to inhibition of p53-mediated transcriptional repression (15).
As repression of p53-mediated transcriptional activation by ERα appears to be gene-selective, promoter context likely determines the formation and/or functional consequences of the ERα–p53 complex. This is consistent with the importance of promoter context in transcriptional outcome in general (49) and in protein–protein interactions involving p53 (50). We observed that ERα antagonizes p53-dependent transcriptional regulation of p53 target genes by physically binding to promoter-bound p53 on p53 response elements; however, it has also been reported that ERα and p53 (wild-type or mutant) bind to EREs and p53 response elements, respectively, to cooperatively enhance FLT1 transcription (40). The combined results of these studies highlight the complexities of ER-mediated regulation of p53 transcriptional activity and suggest that such regulation is highly context-dependent. It is likely that cooperation of ERα with p53 in cis (40) and physical interaction of ERα with p53 (resulting in repression of p53 function) (13–15) are both dependent upon the target genes and signaling context. Moreover, we have not ruled out the possibility that on certain ERE-containing genes and some p53 target genes, p53 may repress ER function. Alternatively, in some cases, physical interaction between ERα and p53 may result in activation of either ER or p53. Another intriguing possibility is a potential role for the ERα–p53 interaction in the “gain of function” by certain p53 mutants. Future experiments should address these plausible and interesting scenarios.
Our micro-ChIP data show that ERα and p53 are expressed in stem cell-containing murine mammospheres and that they interact with one another, resulting in inhibition of p53’s ability to activate p21 transcription. It is likely that normal signaling mechanisms operating to regulate the ERα–p53 interaction in mammary SCs could be disrupted in breast CSCs, favoring predominance of ERα over p53 and symmetric over asymmetric cell division, thereby leading to abnormal proliferation.
The conventional understanding of the genomic ERα signaling pathway is that antiestrogens block estrogen from binding to ERα, cause ERα to recruit transcriptional corepressors, and lead to transcriptional repression of ER target genes. Here we show that the antiestrogen tamoxifen can also disrupt the ERα–p53 inhibitory complex, resulting in reactivation of p53. This raised the possibility that the latter function of tamoxifen could be one of the determinants of response of ER-positive breast cancer patients to tamoxifen therapy. Indeed, results from our pilot retrospective analysis of clinical OVS data of tamoxifen-treated patients are consistent with studies on other patient cohorts (17, 22–24) and support the idea that ER-positive breast cancer patients whose tumors express wild-type p53 (as opposed to mutant p53) will be more responsive to tamoxifen therapy, as tamoxifen will disrupt the ERα–p53 interaction, thereby reactivating p53. A prospective clinical trial to directly verify this possibility is underway. Based on our results, future studies on the ER–p53 interaction should provide insight into its role not only in normal mammary gland development and breast cancer but also in other tissues and cancers where ER and p53 are expressed and may have preventive and therapeutic implications.
Materials and Methods
Cell Culture.
MCF-7 and Saos2 cells were maintained in DMEM supplemented with 10% FBS (Invitrogen) or in DMEM media containing 10% dextran-coated charcoal-treated FBS at 37 °C under 5% CO2.
Antibodies, Drugs, and Western Analysis.
Antibodies were obtained from the following companies: anti-p53 (DO-1), -ERα (HC-20), -p21, -SMRT, -RIP140, -SRC1, -SRC3, -cytokeratin-14, and -LamininA/C antibodies, and normal rabbit and mouse serum from Santa Cruz; anti-NCoR and -HDAC1 from Upstate Biotechnology; and anti-β-tubulin and β-Actin (A2066) from Sigma-Aldrich. 17β-estradiol and 4-hydroxytamoxifen were purchased from Sigma-Aldrich, and ICI 182780 was purchased from Tocris Bioscience. Cell lysates were analyzed on SDS/PAGE gels, followed by Western blotting with antibodies against various proteins, as noted in the figure legends. Specific proteins were detected by the enhanced chemiluminescence method (Amersham Biosciences).
Plasmids and siRNAs.
The −1265 PCNA-luc has been previously described (14). PRc/CMV hp53 and −2326 p21-luc were generous gifts from A. J. Levine (Institute of Advanced Study, Princeton, NJ), and W. El-Deiry (University of Pennsylvania School of Medicine, Philadelphia, PA), respectively. The pCR3.1-based hERα expression plasmids (ER wild-type; ER L539A) were from C. Smith (Baylor College of Medicine, Houston, TX). The pCR3.1/p53 construct was generated by cloning full-length p53 cDNA (HindIII and XbaI fragment) from the pRc/CMV hp53 plasmid into the pCR3.1 vector. NCoR (pKD-v2) and control (pKD-NegCon-v1) shRNA plasmids were purchased from Upstate Biotechnology. The p53 and ERα siRNAs (SMARTpools) were obtained from Dharmacon.
Luciferase Reporter Assays and Transfection of siRNAs and shRNAs.
Transient transfections of MCF-7 (1.5 × 105) cells with ERα or p53 siRNA were performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. NS siRNA was used as a control. For reporter assays, 0.5 × 105 cells were seeded in 12-well plates and transfected with the following plasmids using Fugene 6 Transfection Reagent (Roche Diagnostics), according to the manufacturer's protocol: for MCF-7 cells, −2326 p21-luc reporter plasmid and NCoR shRNA plasmid or a NS shRNA plasmid; for Saos2 cells, −1265 PCNA-luc reporter plasmid and p53 expression plasmid alone or in combination with ERα wild-type or ER L539A mutant expression plasmid or a molar equivalent of vector (PCR 3.1). The total amount of transfected DNA was kept constant by the addition of empty plasmid. After 24 h, cells were harvested and lysed, and luciferase activity was measured using the Luciferase Reporter Assay System (Promega).
qPCR.
Total cellular RNA was isolated using the Absolutely RNA Miniprep kit (Stratagene). Up to 1 μg of total RNA from individual samples was reverse transcribed in 20 μL of reaction using the iScript cDNA synthesis kit (Bio-Rad). One microliter of the resulting cDNA was used in a total volume of 25 μL of PCR; qPCR was performed on an ABI Prism 7300 sequence detection system with SYBR Green PCR Master Mix (Bio-Rad) using specific primers. Relative mRNA levels or DNA levels (in the case of qChIP) were calculated using the ΔΔCt method. For mRNA analysis, β-actin mRNA was used as an endogenous control. p53 binding to the p21 promoter was used to normalize q-ChIP. Primer sequences for qPCR are described in SI Materials and Methods.
ChIP, qChIP, Sequential ChIP, and Micro-ChIP.
ChIP assays were performed as described previously (14, 15). For qChIP, DNA in the final step was analyzed by qPCR. For sequential ChIP assays, cell lysates were initially incubated with anti-p53 antibody, and the immunocomplexes were eluted at 37 °C for 30 min in rechip buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris-HCl, pH 8.1), followed by incubation with anti-ERα antibody. Secondary immunocomplexes were eluted in rechip buffer and incubated with antibodies against NCoR, SMRT, RIP140, and HDAC1. Final immunocomplexes were eluted in elution buffer (500 μL 1% SDS, 0.1 M NaHCO3) and processed for DNA analysis. Micro-ChIP was performed essentially as described (51) with some modifications. Additional details and primer sequences are described in SI Materials and Methods.
Generation of Primary Murine Mammary Epithelial Cells and Mammospheres.
Primary mammary epithelial cells and mammospheres were prepared from 9- to 12-wk-old virgin female mice. Murine mammospheres were generated by adapting previously described methods (25, 31). Details are in SI Materials and Methods.
Lentiviral Transduction of Mammospheres.
Murine mammospheres were transduced with Cre-recombinase lentivirus at 1 × 107 TU/mL or multiplicity of infection (MOI) 1 on day 4 of their growth and harvested on day 6 for subsequent molecular analysis. Cre-recombinase lentivirus was prepared as described in SI Materials and Methods.
Analysis of Clinical Breast Cancer Samples.
Files of patients with breast cancer (surgery performed 1986 through 1988) were retrieved from the surgical pathology archives at the Dr. Margarete Fischer-Bosch-Institute. Seventy-one breast cancer cases were selected for the study. Breast cancer diagnosis was confirmed with hematoxylin and eosin staining of paraffin-embedded tissue sections. Immunohistochemical analyses for ERα and p53 statuses were performed on 3-μm sections using Dako clone 1D5 and clone DO-7 antibodies, respectively, in a DAKO autostainer with ChemMate En Vision K5007 (Dako Cytomation). Slides were evaluated by an expert pathologist who was blinded to the clinical information. Statistical analysis was performed using SPSS software version 12.1. Univariate survival analysis was by the Kaplan-Meier method, and multivariate survival analysis was calculated by the Cox proportional hazard model. Associations between ER status and clinical parameters were assessed by χ2 test. All P values resulted from two-sided tests and were considered significant when P < 0.05.
Supplementary Material
Acknowledgments
We thank A. J. Levine (Institute of Advanced Study, Princeton, NJ), C. L. Smith (Baylor College of Medicine, Houston, TX), and W. S. El-Deiry (University of Pennsylvania School of Medicine, Philadelphia, PA) for generous gifts of plasmids; R. Chakrabarti and S. Sinha (State University of New York at Buffalo, Buffalo, NY) for help in optimization of mouse mammary epithelial cell culture and generation of mammospheres; M. Ip (Roswell Park Cancer Institute, Buffalo, NY) for discussions and critical reading of the manuscript; and S. Bansal, A. Sayeed (Roswell Park Cancer Institute, Buffalo, NY) and D. Singleton (University of Cincinnati College of Medicine, Cincinnati, OH) for discussions and general help. This work was supported by National Cancer Institute Grant CA079911 (to G.M.D.), Susan G. Komen for the Cure Foundation Grant BCTR0600180 (to G.M.D.), The Jayne & Phil Hubbell Family and Roswell Park Alliance Foundation Grant (to G.M.D.), National Institutes of Health Grants T32 CA059268 (to A.E.G.) and 2T32CA009072 (to W.M.S.), Robert Bosch Foundation Stuttgart (to B.A.K., H.B., and F.P.), and National Cancer Institute Cancer Center Support Grant CA016056 (to Roswell Park Cancer Institute).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009575107/-/DCSupplemental.
References
- 1.Glass CK, Rosenfeld MG. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 2.McKenna NJ, O'Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 3.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 4.Perissi V, Jepsen K, Glass CK, Rosenfeld MG. Deconstructing repression: Evolving models of co-repressor action. Nat Rev Genet. 2010;11:109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 5.Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke RB, Anderson E, Howell A. Steroid receptors in human breast cancer. Trends Endocrinol Metab. 2004;15:316–323. doi: 10.1016/j.tem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. doi: 10.1172/JCI27987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 9.Vousden KH, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 11.Oren M, et al. Regulation of p53: Intricate loops and delicate balances. Biochem Pharmacol. 2002;64:865–871. doi: 10.1016/s0006-2952(02)01149-8. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal ML, Taylor WR, Chernov MV, Chernova OB, Stark GR. The p53 network. J Biol Chem. 1998;273:1–4. doi: 10.1074/jbc.273.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Ip MM, Podgorsak MB, Das GM. Disruption of estrogen receptor alpha-p53 interaction in breast tumors: A novel mechanism underlying the anti-tumor effect of radiation therapy. Breast Cancer Res Treat. 2009;115:43–50. doi: 10.1007/s10549-008-0044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, et al. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006;281:9837–9840. doi: 10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 15.Sayeed A, et al. Estrogen receptor alpha inhibits p53-mediated transcriptional repression: Implications for the regulation of apoptosis. Cancer Res. 2007;67:7746–7755. doi: 10.1158/0008-5472.CAN-06-3724. [DOI] [PubMed] [Google Scholar]
- 16.Cattoretti G, Rilke F, Andreola S, D'Amato L, Delia D. P53 expression in breast cancer. Int J Cancer. 1988;41:178–183. doi: 10.1002/ijc.2910410204. [DOI] [PubMed] [Google Scholar]
- 17.Miller LD, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier M, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12:1157–1167. doi: 10.1158/1078-0432.CCR-05-1029. [DOI] [PubMed] [Google Scholar]
- 19.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 20.Jordan VC, O'Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25:5815–5824. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 21.Schiff R, Osborne CK. Endocrinology and hormone therapy in breast cancer: New insight into estrogen receptor-alpha function and its implication for endocrine therapy resistance in breast cancer. Breast Cancer Res. 2005;7:205–211. doi: 10.1186/bcr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergh J, Norberg T, Sjögren S, Lindgren A, Holmberg L. Complete sequencing of the p53 gene provides prognostic information in breast cancer patients, particularly in relation to adjuvant systemic therapy and radiotherapy. Nat Med. 1995;1:1029–1034. doi: 10.1038/nm1095-1029. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita H, et al. p53 protein accumulation predicts resistance to endocrine therapy and decreased post-relapse survival in metastatic breast cancer. Breast Cancer Res. 2006;8:R48. doi: 10.1186/bcr1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berns EM, et al. Complete sequencing of TP53 predicts poor response to systemic therapy of advanced breast cancer. Cancer Res. 2000;60:2155–2162. [PubMed] [Google Scholar]
- 25.Cicalese A, et al. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138:1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Krizhanovsky V, Lowe SW. Stem cells: The promises and perils of p53. Nature. 2009;460:1085–1086. doi: 10.1038/4601085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alvi AJ, et al. Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 2003;5:R1–R8. doi: 10.1186/bcr547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke RB, et al. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277:443–456. doi: 10.1016/j.ydbio.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 29.Dontu G, El-Ashry D, Wicha MS. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol Metab. 2004;15:193–197. doi: 10.1016/j.tem.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Dontu G, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pei XH, et al. CDK inhibitor p18(INK4c) is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell. 2009;15:389–401. doi: 10.1016/j.ccr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104:14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao MJ, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67:8131–8138. doi: 10.1158/0008-5472.CAN-06-4493. [DOI] [PubMed] [Google Scholar]
- 34.Welm BE, et al. Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev Biol. 2002;245:42–56. doi: 10.1006/dbio.2002.0625. [DOI] [PubMed] [Google Scholar]
- 35.Pontier SM, Muller WJ. Integrins in mammary-stem-cell biology and breast-cancer progression—a role in cancer stem cells? J Cell Sci. 2009;122:207–214. doi: 10.1242/jcs.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Díaz-Cruz ES, Furth PA. Deregulated estrogen receptor alpha and p53 heterozygosity collaborate in the development of mammary hyperplasia. Cancer Res. 2010;70:3965–3974. doi: 10.1158/0008-5472.CAN-09-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G, Schwartz JA, Brooks SC. Estrogen receptor protects p53 from deactivation by human double minute-2. Cancer Res. 2000;60:1810–1814. [PubMed] [Google Scholar]
- 38.Shirley SH, et al. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009;69:3405–3414. doi: 10.1158/0008-5472.CAN-08-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivaraman L, Conneely OM, Medina D, O'Malley BW. p53 is a potential mediator of pregnancy and hormone-induced resistance to mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:12379–12384. doi: 10.1073/pnas.221459098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menendez D, Inga A, Resnick MA. Estrogen receptor acting in cis enhances WT and mutant p53 transactivation at canonical and noncanonical p53 target sequences. Proc Natl Acad Sci USA. 2010;107:1500–1505. doi: 10.1073/pnas.0909129107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medina D, Kittrell FS. p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res. 2003;63:6140–6143. [PubMed] [Google Scholar]
- 42.Lupien M, et al. Raloxifene and ICI182,780 increase estrogen receptor-alpha association with a nuclear compartment via overlapping sets of hydrophobic amino acids in activation function 2 helix 12. Mol Endocrinol. 2007;21:797–816. doi: 10.1210/me.2006-0074. [DOI] [PubMed] [Google Scholar]
- 43.Juan LJ, et al. Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem. 2000;275:20436–20443. doi: 10.1074/jbc.M000202200. [DOI] [PubMed] [Google Scholar]
- 44.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Lagger G, et al. The tumor suppressor p53 and histone deacetylase 1 are antagonistic regulators of the cyclin-dependent kinase inhibitor p21/WAF1/CIP1 gene. Mol Cell Biol. 2003;23:2669–2679. doi: 10.1128/MCB.23.8.2669-2679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi T, et al. Histone deacetylases 1 and 2 act in concert to promote the G1-to-S progression. Genes Dev. 2010;24:455–469. doi: 10.1101/gad.552310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- 48.Shang Y, Brown M. Molecular determinants for the tissue specificity of SERMs. Science. 2002;295:2465–2468. doi: 10.1126/science.1068537. [DOI] [PubMed] [Google Scholar]
- 49.Das G, Hinkley CS, Herr W. Basal promoter elements as a selective determinant of transcriptional activator function. Nature. 1995;374:657–660. doi: 10.1038/374657a0. [DOI] [PubMed] [Google Scholar]
- 50.Schumm K, Rocha S, Caamano J, Perkins ND. Regulation of p53 tumour suppressor target gene expression by the p52 NF-kappaB subunit. EMBO J. 2006;25:4820–4832. doi: 10.1038/sj.emboj.7601343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dahl JA, Collas P. MicroChIP—a rapid micro chromatin immunoprecipitation assay for small cell samples and biopsies. Nucleic Acids Res. 2008;36:e15. doi: 10.1093/nar/gkm1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.