Abstract
Circadian pacemaking requires the orderly synthesis, posttranslational modification, and degradation of clock proteins. In mammals, mutations in casein kinase 1 (CK1) ε or δ can alter the circadian period, but the particular functions of the WT isoforms within the pacemaker remain unclear. We selectively targeted WT CK1ε and CK1δ using pharmacological inhibitors (PF-4800567 and PF-670462, respectively) alongside genetic knockout and knockdown to reveal that CK1 activity is essential to molecular pacemaking. Moreover, CK1δ is the principal regulator of the clock period: pharmacological inhibition of CK1δ, but not CK1ε, significantly lengthened circadian rhythms in locomotor activity in vivo and molecular oscillations in the suprachiasmatic nucleus (SCN) and peripheral tissue slices in vitro. Period lengthening mediated by CK1δ inhibition was accompanied by nuclear retention of PER2 protein both in vitro and in vivo. Furthermore, phase mapping of the molecular clockwork in vitro showed that PF-670462 treatment lengthened the period in a phase-specific manner, selectively extending the duration of PER2-mediated transcriptional feedback. These findings suggested that CK1δ inhibition might be effective in increasing the amplitude and synchronization of disrupted circadian oscillators. This was tested using arrhythmic SCN slices derived from Vipr2−/− mice, in which PF-670462 treatment transiently restored robust circadian rhythms of PER2::Luc bioluminescence. Moreover, in mice rendered behaviorally arrhythmic by the Vipr2−/− mutation or by constant light, daily treatment with PF-670462 elicited robust 24-h activity cycles that persisted throughout treatment. Accordingly, selective pharmacological targeting of the endogenous circadian regulator CK1δ offers an avenue for therapeutic modulation of perturbed circadian behavior.
Keywords: circadian clock, suprachiasmatic nucleus, period protein, Tau mutation, pacemaking
In mammals, circadian rhythms of behavior and physiology are driven by a molecular clockwork in which transcriptional activators CLOCK and BMAL1 drive the expression of repressors period (Per) and cryptochrome (Cry). As PER and CRY proteins accumulate, they complex with BMAL1–CLOCK to repress their own transcription (1, 2), and thus a subsequent cycle of circadian gene expression can only commence once PER and CRY proteins are cleared from the nucleus and degraded. Posttranslational modification of clock proteins imparts temporal control over the clock feedback loops by modulating protein–protein interactions, nuclear entry and export, and degradation (3–5). A dominant role for casein kinase (CK)-mediated protein phosphorylation in circadian timing is now well established. In Drosophila, mutations of doubletime (dbt, an ortholog of mammalian CK1) result in either short (dbts) or long (dbtl) circadian rhythms (6, 7). In mammals, both CK1ε and CK1δ can complex with PER and CRY (8) and phosphorylate PER proteins, leading to proteasome-mediated degradation and nuclear translocation (9–11). In humans, familial advanced sleep phase syndrome (FASPS) is associated with a T44A missense mutation in CK1δ, leading to hypophosphorylation of PER2 in vitro (12), although in Syrian hamsters and mice, the CK1εtau mutation results in hyperphosphorylation and subsequent destabilization of its PER targets with significant shortening of behavioral activity cycles to 20 h (13–16). Finally, extensive in vitro screening of pharmacologic compounds shows that the majority of compounds that lengthen the period do so by inhibiting CK1ε/CK1δ-mediated phosphorylation of PER (17).
Although the specific contributions of CK1ε and CK1δ to the regulation of the mammalian clock in vivo remain unclear, differences in the potency of the two isoforms are evident. Ck1ε−/− mice exhibit only a slight effect on the period of free-running locomotor activity cycles (16) or liver cells (18). In contrast, embryonic fibroblast cells (MEFs) derived from Ck1δ−/− mice and liver tissue derived from tissue-specific Ck1δ knockouts exhibit significant period lengthening (18, 19). Nonetheless, rhythmicity persists in these cells and targeting of both CK1ε and CK1δ isoforms is necessary to render MEFs arrhythmic (19). RNA interference targeting of either isoform results in significant period lengthening in a U2OS cell line, but significantly greater effects are observed with CK1δ-directed RNAi (17). These studies suggest that, although both isoforms are likely regulators of the circadian clock, accurate cellular circadian timing can persist in the absence of CK1ε. No studies have yet detailed, however, the impact of targeting the isoforms of CK1 on behavior in vivo or examined the cellular consequences of manipulating CK1 activity in the brain's central pacemaker, the suprachiasmatic nucleus (SCN).
Here, we used isoform-selective pharmacological inhibitors of CK1ε and CK1δ both in vivo and in vitro and revealed CK1δ to be a dominant endogenous regulator of PER-mediated circadian pacemaking in the brain and in behavioral rhythms. We further demonstrate that CK1δ-selective inhibition can be used to synchronize and restore circadian rhythms in arrhythmic animals, highlighting this enzyme as an important therapeutic target for regulation of disturbed sleep and other circadian disorders.
Results
CK1δ Inhibition Lengthens Period in SCN and Peripheral Oscillators in Vitro.
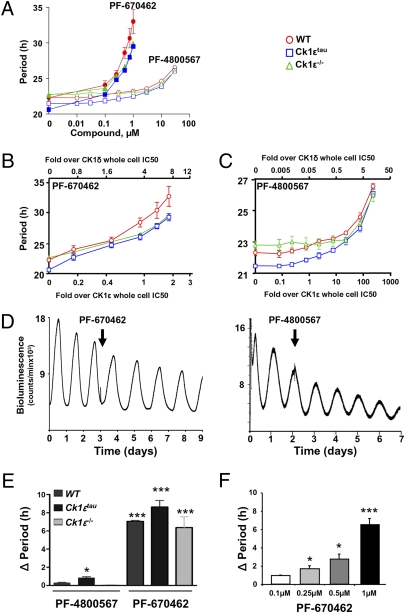
To address the respective contributions of the two isoforms to molecular pacemaking, detailed dose–response curves were generated for CK1δ-selective (PF-670462) and CK1ε-selective (PF-4800567) inhibitors using primary lung fibroblast cells obtained from WT, Ck1εtau mutant, and Ck1ε−/− mice bred on a PER2::Luc reporter background (Fig. 1A and Fig. S1 A and B). Treatment with PF-670462 caused significant dose-dependent lengthening of the circadian period: high-dose PF-670462 (1 μM) extended the period in WT fibroblasts to 33 h (Fig. 1A). In contrast, only slight effects on the period were observed following the highest doses of PF-4800567. To assess the period-lengthening effects of these compounds relative to their ability to inhibit the kinase activity of CK1ε and CK1δ, we replotted these data based on the previously established IC50 (20) of each drug for each CK1 isoform (Fig. 1 B and C). This demonstrated pronounced period lengthening in response to either compound only after CK1δ is engaged (>1-fold CK1δ IC50). A significant lengthening of the period was observed at 1 μM PF-4800567 (approximately 10-fold CK1ε IC50; approximately 0.5-fold CK1δ IC50) in WT (0.9 ± 0.13 h increase in period) but not Ck1ε−/−-derived cells (0.3 ± 0.3 h) (Fig. 1 A–C). This suggests that endogenous CK1ε exerts some influence on circadian clock speed, although inhibition of CK1δ at this dose of PF-4800567 cannot be excluded because a similar degree of lengthening was achieved in WT cells by 0.1 μM PF-670462 (approximately 0.7-fold CK1δ IC50). Increasing PF-670462 dosage to the point of complete inhibition of both CK1 isoforms (2.5 μM and above) rendered the cells arrhythmic, revealing their essential role in pacemaking.
Fig. 1.
Differential effects of selective inhibition of CK1ε and CK1δ on the circadian period of fibroblasts and SCN in vitro. (A) Group data (mean ± SEM) reveal dose-dependent period lengthening by CK1 inhibition in WT, Ck1ε−/−, and Ck1εtau derived fibroblasts. Red, WT; blue, CK1εtau; green, Ck1ε−/−. (B and C) Data in A plotted as fold over whole cell IC50 of CK1δ (upper axis) or CK1ε (lower axis) for PF-670462 or PF-4800567. Note even when it is 10-fold over the IC50 of CK1ε, PF-4800567 is ineffective in lengthening the circadian period. (D) Representative recordings of PER2::Luc bioluminescence from WT SCN treated with CK1 inhibitors (arrow,1 μM). (E) Group data reveal interaction between CK1ε genotype and CK1 inhibitor (1 μM) in slowing SCN pacemaker. Whereas PF-670462 lengthened period in all three genotypes, PF-4800567 was only effective against CK1ε τ mutant (mean ± SEM, *P < 0.05, ***P <0.001, n = 3–5). (F) Group data reveal dose-dependent prolongation of WT SCN circadian period with PF-670462.
The effects of CK1 inhibition were examined in SCN slices using bioluminescence recording. CK1δ-selective inhibition significantly and dose-dependently lengthened the SCN period by up to 8 h in all three genotypes (Fig. 1 D–F). In contrast, the CK1ε-selective inhibitor did not affect the circadian period in the SCN of WT or Ck1ε−/− but did significantly lengthen the period in Ck1εtau SCN slices (Fig. 1E), consistent with the predicted gain-of-function action of this mutation (15, 16). The action of CK1ε inhibition on Ck1εtau was explored further by structural modeling of the enzyme and substrate. The model strongly suggests that the R178C mutation in CK1εtau most likely influences substrate binding and does not directly impact MgATP or ATP-competitive inhibitor binding as previously proposed (14). This was confirmed by in vitro binding assays, which suggest that both inhibitors are capable of binding to either the WT or the CK1εtau enzyme with equivalent affinity, hence excluding the possibility that the effectiveness of PF-4800567 in CK1εtau cells is due to altered binding (Fig. S2). To complement these studies, we determined the impact of gene-specific knockdown of CK1δ in Ck1ε−/− fibroblasts by lentiviral-delivered shRNA against CK1δ. As evidenced by a CK1δ-specific antibody, knockdown reduced CK1δ protein expression (Fig. S1 C–F) and eliminated circadian oscillations within two to three cycles. Taken together, these findings demonstrate that CK1 activity, mediated by either CK1δ or CK1ε, is necessary for the circadian pacemaker to progress.
Lengthening of Behavioral Rhythms in Vivo by CK1 Inhibition: Differential Roles of CK1ε and CK1δ
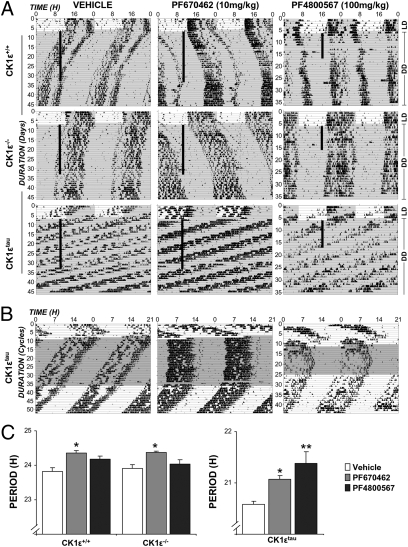
To extend these findings to the in vivo setting, the impact of pharmacological inhibition of CK1ε and δ isoforms was tested in WT, Ck1εtau, and Ck1ε−/− mice. Doses were selected to elicit concentrations in the brain capable of selectively engaging each isoform [PF-4800567 100 mg/kg/d: 3-fold IC50 over CK1ε, 0.2-fold over CK1δ; PF-670462 10 mg/kg/d: 3-fold IC50 over CK1δ, 0.7-fold over CK1ε (20)]. Animals were initially injected at zeitgeber time (ZT) 10 in 12-h light and 12-h dark (LD), and injections in constant darkness (DD) continued at the same external time. Daily treatment of the CK1δ-selective inhibitor significantly and equivalently lengthened the period of activity rhythms in all three genotypes (Fig. 2 A–C) (WT: 0.44 ± 0.05 h, Ck1εtau: 0.39 ± 0.1 h, Ck1ε−/−: 0.42 ± 0.04 h; all P < 0.05 vs. vehicle); after 24 d of treatment, activity onset occurred 10.4 ± 1.6 h after injection time in WT mice. When treatment ceased, mice free-ran from this phase with a normal circadian period. Period lengthening of behavioral rhythms was dose-dependent (0.4 vs. 0.8 h for 10 vs. 30 mg/kg/d, respectively) (Fig. S3). Importantly, in WT and Ck1ε−/− mice, the CK1δ-selective inhibitor caused nearly identical period lengthening, suggesting that the effect on the period is not due to drug action on both CK1 isoforms. In contrast, CK1ε inhibition had no significant effect on the period or phase of behavioral rhythms in WT or Ck1ε−/− mice, even though 30 min after injection, this dose produces a drug level of 455 nM (Fig. 2 A–C). These data thus reveal a strong dependence on endogenous CK1δ for setting the period of behavioral circadian rhythms. In line with cellular and SCN studies, CK1ε inhibition selectively lengthened the period in Ck1εtau mice, demonstrating the biological efficacy of this drug when applied to an appropriate (gain-of-function) genetic model.
Fig. 2.
Differential effects of selective inhibition of CK1ε and CK1δ on the circadian period of locomotor activity in vivo. (A) Representative wheel-running actograms of Ck1ε+/+, Ck1ε−/−, and Ck1εtau mice treated with vehicle (Left), PF-670462 (Center), or PF-4800567 (Right) reveal no effect of daily vehicle injection but significant period lengthening by PF-670462 in both WT, Ck1ε−/−, and Ck1εtau mutant mice. In contrast, selective inhibition of CK1ε by PF-4800567 is only effective in gain-of-function Ck1εtau animals. (B) Actograms from Ck1εtau mice plotted on 21-h time base for ease of inspection. (C) Group data reveal interaction between CK1 inhibition and genotype in setting circadian period in vivo (mean ± SEM, *P < 0.05, **P <0.01 vs. vehicle, two-way ANOVA with Bonferonni's post hoc test) (n = 6–8).
Impact of CK1δ-Selective Inhibition on SCN Rhythms and Nuclear Localization of PER2.
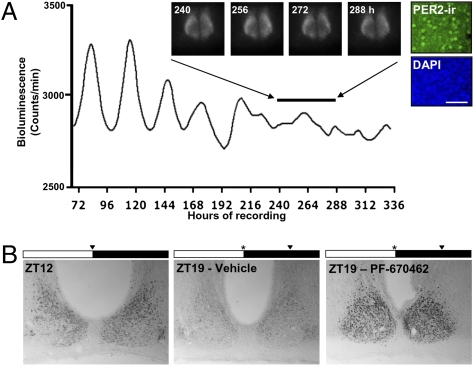
To study the impact of CK1 inhibition on PER subcellular localization, we raised and characterized an anti-PER2 antiserum capable of detecting this protein in subcellular compartments. Specificity of the antibody was confirmed by detection of clear circadian rhythms of immunoreactivity in WT SCN, colocalization with PER2::Luc bioluminescence, and absence from SCN tissue derived from Per2−/− mice (Fig. S4A). When applied to SCN slices at high doses, PF-670462 (10 μM) caused persistent period lengthening with eventual loss of rhythmicity (Fig. 3). Subsequent immunostaining of SCN slices demonstrated that this loss of rhythmicity was accompanied by enhanced nuclear retention of PER2 protein in SCN neurons (Fig. 3A), suggesting that CK1δ inhibition affects PER2 localization. To confirm the impact of CK1δ inhibition on PER2 cellular localization in vivo, WT mice were injected with PF-670462 (100 mg/kg, i.p.) or vehicle at ZT12, and brain tissue was collected at the time of injection or 7 h later (ZT19) (n = 3). Wheel-running records collected from a parallel group of mice demonstrated that this dose and timing of PF-670462 administration caused a 5.18 ± 1.51 h phase delay in activity onset (Fig. S4B), with no effect of vehicle (0.17 ± 0.69 h). As expected, strong PER2 immunoreactivity was observed in the SCN of all of the mice at ZT12 (Fig. 3B) (354 ± 31 cells/30-μm section). By ZT19, PER2 immunoreactivity was greatly reduced in the SCN of vehicle-treated mice (100 ± 13 cells) but was clearly retained in the nuclear compartment in the PF-670462-treated mice (Fig. 3B) (398 ± 90 cells).
Fig. 3.
CK1δ inhibitor increases nuclear retention of PER2 in SCN neurons in vitro and in vivo. (A) Representative recording of PER2::Luc signal from SCN slice treated with PF-670462 from the start of the experiment. Note the ultimate loss of circadian oscillation and sustained expression of PER2::Luc bioluminescence. (Insets) The bioluminescence images of the same slice by CCD camera. Post hoc immunostaining reveals nuclear localization of PER2 (green). Nuclei were counterstained by DAPI (blue). (B) Immunostaining for PER2 in SCN of WT mice sampled at ZT12 or injected with either vehicle or PF-670462 at ZT12 and then sampled at ZT19. Note retention of nuclear PER2 in SCN of drug-treated mouse. Black triangle marks time of sampling. *Marks time of drug/vehicle injection.
High doses of PF-670462 caused a similar (but reversible) loss of bioluminescence rhythms in lung fibroblast cells (Fig. S1A). To examine the corresponding changes in PER2 expression, immunostaining for PER2 was conducted on synchronized WT fibroblast cultures at the peak and trough of the PER2 protein cycle (as predicted by bioluminescence rhythms in parallel-matched cultures of PER2::Luc fibroblasts) (Fig. S4 C–E). In vehicle-treated cells, strong nuclear staining was observed at CT12, whereas staining at CT0 was weak and largely confined to the cytoplasm. In the presence of PF-670462, nuclear and perinuclear localization of PER2 immunoreactivity were clearly visible at both CT12 and CT0. Together, these data demonstrate that inhibition of CK1 stabilizes the nuclear localization of endogenous PER2 in both SCN neurons and peripheral cells.
CK1δ-Selective Inhibition Leads to Altered Internal Phasing of the Molecular Clockwork.
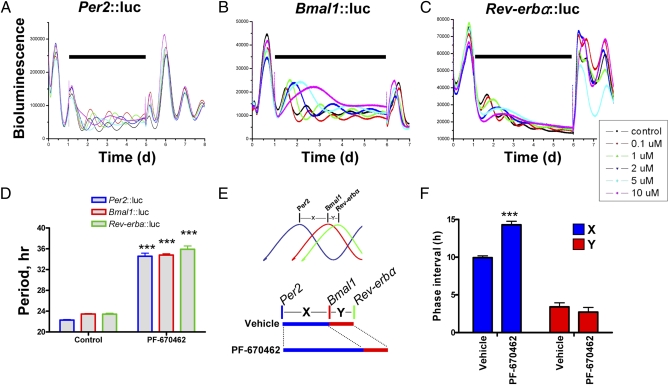
A prediction from our studies is that CK1δ-selective inhibition should prolong PER-dependent E-Box–mediated negative feedback and alter internal phasing of interlocked feedback loops that constitute the molecular pacemaker. To test this, we applied PF-670462 to individual Rat-1 cell lines with bioluminescent reporters for Bmal1, Per2, or Rev-erbα, in which the timing of peak bioluminescence provided by each reporter marks a distinct phase of the circadian cycle (21, 22). PF-670462 lengthened the period in a dose-dependent manner for each of the reporters (Fig. 4 A–D). The relative phasing of these reporters, however, revealed significant asymmetric responses. The interval between the peaks of Per2 and Bmal1 was significantly extended, whereas the interval between peaks of Bmal1 and Rev-erbα was unaltered (Fig. 4 E and F). Thus, CK1δ inhibition changes the internal phasing of the molecular pacemaker, consistent with prolongation of PER-mediated negative feedback.
Fig. 4.
Inhibition of CK1 by PF-670462 restructures the internal phase relationships of the individual clock components. (A–C) Representative bioluminescence recordings of Per2, Bmal1, and Rev-erbα promoter activity in Rat-1 fibroblasts reveal reversible, dose-dependent period lengthening and ultimate arrhythmia induced by high-dose PF-670462. Note, black horizontal bar indicates exposure of cells to the inhibitor. (D) Group data reveal equivalent PF-670462-induced (1 μM) period lengthening in Rat-1 cells when reported by Per2, Bmal1, or Rev-erbα promoter constructs (mean ± SEM, ***<0.001 vs. vehicle). (E). Schematic plot of internal phase relationships (X and Y) of the various components of the oscillator (Upper) as reported by Per2, Bmal1, and Rev-erbα promoter sequences exposed to vehicle or 1 μM PF-670462. (F) Group data reveal selective lengthening of the X phase, but not the Y phase, by PF-670462 at 1 μM (mean ± SEM, ***<0.001 vs. vehicle).
Critical Role for CK1δ in Stabilizing SCN Cellular Pacemaking in Vitro and Behavioral Rhythms in Vivo.
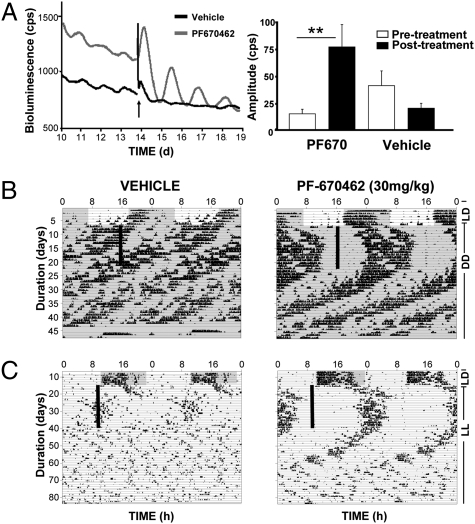
We speculated that enhancement of PER stability with a CK1δ-selective inhibitor could be effective in synchronizing and enhancing circadian oscillations in conditions where the oscillator is known to be weak and of low amplitude. We tested this in the Vipr2−/− mouse model, in which the molecular pacemaking in the SCN is compromised, leading to an arrhythmic or weakly rhythmic circadian phenotype in constant conditions and accompanied by reduced expression of Per mRNA and protein (23, 24). To examine the potential restorative effects of CK1δ inhibition, SCN slices derived from Vipr2−/− mice bred on a PER2::Luc reporter background were treated with vehicle or PF-670462. Before drug treatment, circadian gene expression in Vipr2−/− SCN progressively damped and became disorganized (Fig. 5A). Treatment with PF-670462 resulted in an immediate induction of long-period (mean 32.9 ± 0.7 h; range 31.5–34.1 h; n = 4), high-amplitude (mean % change in amplitude vs. pretreatment: +410.6 ± 55.2%) bioluminescence oscillations (Fig. 5A). In contrast, vehicle-treated slices exhibited significantly lower amplitude circadian oscillations (mean % change in amplitude vs. pretreatment −43.8 ± 14.4%), with no significant effect on the period (mean 25.8 ± 0.9 h; range 24.3–27.3 h; n = 3).
Fig. 5.
Activity rhythms compromised by environmental or genetic perturbation of the SCN can be restored by daily CK1 inhibition in vivo. (A) Representative recordings (Left) of SCN slices derived from Vipr2−/− mice that exhibited damped and disorganized oscillations of the PER2::Luc reporter. Treatment with PF-670462 (arrow; 1 μM) but not vehicle resulted in an immediate induction of high-amplitude and long-period bioluminescent oscillation (**P < 0.01). Group data (Right) (mean ± SEM) reveal significant increase in amplitude of oscillation with PF-670462. (B) Representative traces of Vipr2−/− mice housed in DD and exhibiting arrhythmic or weakly rhythmic short period circadian activity cycles (n = 5). Daily vehicle treatment (Left) had no entraining action, whereas daily injections of PF-670462 (Right) (30 mg/kg) induced robust entrainment. (C) Representative actograms show behavioral activity of WT mice housed under LL (n = 8). Activity patterns of WT mice treated with vehicle (Left) deteriorated rapidly. In contrast, daily injection of PF-670462 (30 mg/kg) produced stable entrainment of locomotor activity in LL with activity onset ∼13 h after time of injection.
Given that inhibition of CK1δ activity transiently reactivated pacemaking in the SCN, we tested whether repeated daily treatment might restore defective circadian behavior of Vipr2−/− mice in vivo. Following 7–10 d in LD conditions, mice were transferred to DD and received daily injections of vehicle or PF-670462 (30 mg/kg/d). Vipr2−/− mice showed no entrainment to the daily vehicle treatment and exhibited either persistent short-period cycles (three of five mice; mean period 22.5 ± 0.03 h) to weak or arrhythmic behavior (two of five mice) (Fig. 5B and Fig. S5). In contrast, daily injections of PF-670462 induced robust entrainment in these mice (Fig. 5B and Fig. S5), which persisted throughout the course of treatment (period: 24.0 ± 0.08 h). Upon release from treatment, four of the five mice exhibited a short period phenotype (22.2 ± 0.1 h), whereas the other exhibited arrhythmic behavior.
We then tested the restorative effect of CK1δ inhibition in a second model of circadian disruption: exposure to continuous light. Following 7–10 d in LD conditions, WT mice were transferred to constant light (LL), at which time drug administration commenced (Fig. 5C and Fig. S5) (n = 8). Under LL, WT mice treated with vehicle exhibited an initial lengthening in the period of wheel-running activity that then became disrupted after approximately 7 d. In contrast, daily injection of PF-670462 prevented this disruption, and mice exhibited a stable entrainment of locomotor activity (period: 24.1 ± 0.05 h), with the onset of activity occurring 13.3 ± 0.2 h after the time of injection (Fig. 5C and Fig. S5). Upon cessation of PF-670462 treatment, circadian activity cycles free-ran from this phase but progressively lost coherence, with five of eight drug-treated mice becoming arrhythmic by 4 wk posttreatment, which was a pattern similar to that of the vehicle-treated group. Thus, daily treatment with PF-670462 is capable of restoring robust behavioral rhythmicity in two different models (genetic or environmental) of disrupted circadian timing and augments defective circadian pacemaking cells in the SCN in vitro.
Discussion
By using selective pharmacological targeting in combination with genetic manipulation of CK1, our data reveal the essential role of CK1 in circadian clock progression and of CK1δ in particular as an endogenous regulator of the period of circadian rhythms. Critically, we demonstrate the profound impact of CK1δ inhibition on behavioral activity rhythms in vivo and on molecular oscillations in the SCN pacemaker and peripheral cells in vitro, thereby revealing the primacy of the CK1δ axis in physiologically representative models. These studies demonstrate that period lengthening following inhibition of CK1 activity is associated with stabilization and nuclear retention of PER protein and selective extension of the interval of PER-mediated negative feedback within the circadian clockwork. Finally, we show that the pharmacological inhibition of CK1 can be used to rescue defective SCN pacemaking in vitro and disrupted circadian behavior in vivo.
Both the SCN and peripheral cell oscillators were rendered arrhythmic when both CK1ε and CK1δ were inhibited by high dose PF-670462 or by combined genetic and pharmacological disruption. These data complement a recent report showing that CK1δ-deficient fibroblasts remain rhythmic but that a dominant negative CK1ε mutant suppresses circadian rhythmicity in the Ck1δ−/− background (19). Thus, CK1 activity, in the form of either or both CK1δ and CK1ε, is an essential element of the clockwork. Presumably, if PER proteins cannot be processed by CK1 effectively and in a timely fashion, the circadian molecular cycle cannot progress.
In vitro studies in mammalian cell lines and tissues have also implicated both isoforms in setting the period of circadian oscillations (16, 18, 19), but the lethality of the CK1δ-null mutation has precluded an examination of their specific contributions to behaviorally relevant pacemaking in vivo. Our data now show that selective pharmacological suppression of the activity of CK1δ dose-dependently lengthens the period of circadian behavioral rhythms in vivo. This extends reports of the acute effects of single injections on activity onset in mice and primates (25, 26). The apparent extension of the period we observed during repeated daily injection to WT mice in DD may arise either from a tonic increase in the period or represent the additive effects of a series of acute daily phase delays. This issue is difficult to resolve, but the extension of the SCN period following continuous CK1δ inhibition in vitro argues for the former, parametric effect. However, the phase response curve to single PF-670462 injections in rats consists only of phase delays, and, although these are of largest amplitude for injection at CT12 and CT15, injections at all phases can delay the behavioral rhythm (25, 26). Therefore, the apparent period lengthening that we observe in the mice could arise from serial nonparametric phase shifts. Regardless of the underlying nature of the effect, the results demonstrate that CK1δ activity is a central regulator of WT circadian period and that CK1ε plays a relatively minor role in tuning the period, except within the CK1εtau mutant background.
Interestingly, a marked contrast exists between the effects of pharmacological and genetic inhibition of CK1δ activity on pacemaking in vitro, insofar as PF-670462 extended the circadian period in SCN and other tissues by ∼8 h, whereas the null mutation of CK1δ extended the period by ∼1.5 h (18). This difference may reflect the contrasting molecular contexts of acute pharmacological inactivation of an existing enzyme and a genetic deletion where the protein is absent throughout development and tissue is potentially subject to developmental compensatory changes. Indeed, Etchegaray et al. (18) showed that CK1ε is overexpressed in Ck1δ−/− liver and vice versa. Unlike genetic deletion, acute pharmacological inactivation also results in a catalytically inactive protein that nonetheless remains to perform other roles (e.g., complex formation, target protein binding). Thus, null mutations and pharmacological inactivation may generate different phenotypes. Within the current context, maintenance of substrate binding by inactivated CK1δ or CK1ε raises the possibility that pharmacological targeting of one CK1 isoform could indirectly attenuate the activity of the other isoform through competitive binding of PER. Should this be the case, it is possible that the dramatic period-lengthening effects achieved by PF-670462 reflect direct pharmacological inhibition of CK1δ combined with indirect inhibition of CK1ε by a stoichiometrically dominant (yet catalytically inactive) CK1δ. Nevertheless, the dominance (be it functional or stoichiometric) of CK1δ versus CK1ε in setting the circadian period is clear and reinforced particularly by the low efficacy of PF-4800567 in modulating period in SCN slices or in vivo and by the marginal effect of CK1ε-null mutation.
Period lengthening mediated by CK1δ inhibition was accompanied by retention of PER protein in the nuclear compartment in peripheral cells and in the SCN in vivo. This enhanced nuclear localization may be due to a net increase in overall cellular PER concentrations, as a consequence of the reduced degradation of the PER target by CK1 inhibition (20). CK1-mediated phosphorylation of PER is known to affect nuclear translocation of the protein (9–11) and therefore may also contribute to the nuclear retention we observed.
The daily enhancement of PER nuclear retention and activity mediated by CK1δ inhibition could synchronize weak circadian oscillations. Indeed, the CK1δ-selective inhibitor reestablished circadian rhythms in arrhythmic SCN from Vipr2−/− mice, which are subject to compromised interneuronal circadian synchrony and PER expression (23, 27). In vivo, Vipr2−/− animals exhibit weakly rhythmic or disrupted circadian behavior because of these SCN deficiencies, but this behavioral deficit was ameliorated by daily inhibition of CK1δ, presumably by its action on the SCN. The extent of CK1 inhibition is, however, clearly critical for oscillator function because higher doses cause arrhythmia within WT SCN. Mice rendered arrhythmic by exposure to constant lighting (LL) also showed rapid and stable entrainment to a daily regimen of PF-670462, with an approximate 12-h delay from treatment to activity onset. Continuous illumination leads to desynchrony within the SCN, likely through direct effects on Per gene expression, leading to the behavioral arrhythmia (28). The behavioral rescue we observed may therefore reflect the effect of repeated daily CK1 inhibition in synchronizing rhythmic accumulation (and subsequent decline) of PER protein across SCN neurons, leading to reestablishment of an overt circadian phenotype. This interpretation is also compatible with our observation in Vipr2−/− animals.
In conclusion, our findings clearly indicate that CK1δ-selective inhibition acts as a potent in vivo regulator of the circadian clock and may represent a mechanism for entrainment of disrupted or desynchronized circadian rhythms. In humans, disrupted circadian rhythms are now believed to underpin a large number of disease states (29). Targeting of CK1δ therefore offers a promising therapeutic avenue in conditions that involve circadian disruptions, such as rotational shift workers and circadian sleep disorders.
Materials and Methods
Drugs, Antibodies, and Plasmids.
Both PF-670462 and PF-4800567 were developed and provided by Pfizer Global Research and Development. PF-670462 exhibits 6-fold CK1δ selectivity over CK1ε (25), and PF-4800567 is a selective inhibitor of CK1ε (IC50 = 32 nM), with greater than 20-fold selectivity over CK1δ (20). Both compounds show a high selectivity for CK1 over a panel of other kinases (20). Anti-PER2 antiserum was raised in rabbits against synthetic peptides of PER2 (NGYVDFSPSPTSPTKEP-[C]-amide). Immunostaining was performed as described (16). Monoclonal anti-CK1ε antibody was obtained from BD Transduction Laboratories, polyclonal anti-CK1δ antibody (C-18) was obtained from Santa Cruz, and anti-α-Tubulin (T6199) was from Sigma. The generation of various mouse CK1ε plasmids was described previously (16).
In Vivo Drug Administration.
Ck1εtau and Ck1ε−/− were generated at the University of Manchester as described previously (16) and subsequently bred with the PER2::Luc mice. Eight- to 12-wk-old mice (n = 5–8) were placed under an LD lighting cycle for 7–10 d, before release into DD or LL at the initiation of drug administration. Following 21–28 d of daily administration of vehicle (20% 2-hydroxypropyl β-cyclodextrin; Sigma), PF-670462 (10–30 mg/kg, i.p.), or 10 d of PF-4800567 (100 mg/kg, s.c.), wheel-running activity was recorded in DD or LL for a further 14–28 d. All drugs were administered at ZT10 based on the final day of LD recording. Running wheel activity was recorded and analyzed using ClockLab software as described previously (16). The Per2−/− mice were derived from a line created by Dr. David Weaver (University of Massachusetts) (30). All experiments were conducted under the aegis of the 1986 Home Office Animals (Scientific Procedures) Act (United Kingdom).
Bioluminescence Recording and Imaging.
Bioluminescence emissions from whole slices of PER2::Luc SCN and cell culture dishes were recorded by PMT, Lumicycle, and CCD cameras as described previously (16, 27). For drug treatment, individual dishes containing slice tissue or cells under PMT recording were treated with the CK1 inhibitors 24 h after synchronization. The compounds were left continuously with the samples thereafter, although the luminescence patterns were recorded for at least 6 d. Waveforms of rhythmic bioluminescence emission from whole slices and individual cells were analyzed in BRASS software and RAP algorithm. Circadian peak phases for the three reporters before and after treatment were determined by the Lumicycle analysis software (Actimetrics). Data are presented as photon cpm. Baseline correction was calculated using a 24-h moving average for the shRNA knockdown study.
Gene Knockdown in Cells.
For lentiviral-mediated shRNA gene delivery, viral packaging and transduction of the shRNA were performed as described (31, 32). Concentrated (Vivaspin20; Sartorius Ltd.) lentiviral particles expressing Ck1δ shRNA was applied to CK1ε-null mouse lung fibroblasts. After 48–72 h, cells were subjected to PMT recording. shRNA against mouse Ck1δ was purchased from Sigma (CCGGCCTGAATTTCTGCCGTTCCTTCTCGAGAAGGAACGGCAGAAATTCAGGTTTTT, TRCN0000023769). Mission Nontarget shRNA Control Vector was used as control (Sigma). Western blotting was performed to confirm knockdown efficiency.
Supplementary Material
Acknowledgments
We thank J. A. O'Brien (Medical Research Council, Laboratory of Molecular Biology, Cambridge, UK) O. Jones, H. Piggins, D. Green (University of Manchester, Manchester, UK), A. Kramer (Charite University, Berlin, Germany), D. Weaver (University of Massachusetts Medical School, Worcester, MA) J. Takahashi, (University of Texas Southwestern Medical Center, Dallas, TX), and A. Millar (University of Edinburgh, Edinburgh, Scotland) for technical assistance and provision of research materials. J. Sprouse (Lundbeck) kindly provided the PF-670462 compound. This project was supported by the Biotechnology and Biological Sciences Research Council (Grants EO232231 and EO225531) and Medical Research Council of the United Kingdom.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005101107/-/DCSupplemental.
References
- 1.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mignot E, Takahashi JS. A circadian sleep disorder reveals a complex clock. Cell. 2007;128:22–23. doi: 10.1016/j.cell.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 4.Vanselow K, et al. Differential effects of PER2 phosphorylation: Molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godinho SI, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316:897–900. doi: 10.1126/science.1141138. [DOI] [PubMed] [Google Scholar]
- 6.Kloss B, et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iepsilon. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 7.Price JL, et al. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 9.Camacho F, et al. Human casein kinase Idelta phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001;489:159–165. doi: 10.1016/s0014-5793(00)02434-0. [DOI] [PubMed] [Google Scholar]
- 10.Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells. Mol Cell Biol. 2002;22:1693–1703. doi: 10.1128/MCB.22.6.1693-1703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eide EJ, et al. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- 13.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 14.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallego M, Eide EJ, Woolf MF, Virshup DM, Forger DB. An opposite role for tau in circadian rhythms revealed by mathematical modeling. Proc Natl Acad Sci USA. 2006;103:10618–10623. doi: 10.1073/pnas.0604511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng QJ, et al. Setting clock speed in mammals: The CK1 epsilon tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isojima Y, et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106:15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Etchegaray JP, et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29:3853–3866. doi: 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Chen R, Lee Y, Yoo S, Lee C. Essential roles of CKIdelta and CKIepsilon in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106:21359–21364. doi: 10.1073/pnas.0906651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walton KM, et al. Selective inhibition of casein kinase 1 epsilon minimally alters circadian clock period. J Pharmacol Exp Ther. 2009;330:430–439. doi: 10.1124/jpet.109.151415. [DOI] [PubMed] [Google Scholar]
- 21.Ueda HR, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37:187–192. doi: 10.1038/ng1504. [DOI] [PubMed] [Google Scholar]
- 22.Meng QJ, et al. Ligand modulation of REV-ERBalpha function resets the peripheral circadian clock in a phasic manner. J Cell Sci. 2008;121:3629–3635. doi: 10.1242/jcs.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harmar AJ, et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badura L, et al. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322:730–738. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- 26.Sprouse J, Reynolds L, Swanson TA, Engwall M. Inhibition of casein kinase I epsilon/delta produces phase shifts in the circadian rhythms of Cynomolgus monkeys. Psychopharmacology (Berl) 2009;204:735–742. doi: 10.1007/s00213-009-1503-x. [DOI] [PubMed] [Google Scholar]
- 27.Maywood ES, et al. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 29.Bechtold DA, Gibbs JE, Loudon AS. Circadian dysfunction in disease. Trends Pharmacol Sci. 2010;31:191–198. doi: 10.1016/j.tips.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Bae K, et al. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- 31.Jones VC, McKeown L, Verkhratsky A, Jones OT. LV-pIN-KDEL: A novel lentiviral vector demonstrates the morphology, dynamics and continuity of the endoplasmic reticulum in live neurones. BMC Neurosci. 2008;9:10. doi: 10.1186/1471-2202-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu W, Meng QJ, Tyler NJ, Stokkan KA, Loudon AS. A circadian clock is not required in an arctic mammal. Curr Biol. 2010;20:533–537. doi: 10.1016/j.cub.2010.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.