Abstract
Overproduction of neuronal nitric oxide synthase (nNOS)-derived NO is detrimental during cerebral ischemia. Normobaric hyperoxia (NBO) has been shown to be neuroprotective, extending the therapeutic time window for ischemic stroke, but the mechanism is not fully understood. In the present study, using a rat model of ischemic stroke, we investigated the effect of early NBO treatment on neuronal NO production. Male Sprague-Dawley rats were given normoxia (30% O2) or NBO (95% O2) during 10, 30, 60 or 90 minutes filament occlusion of the middle cerebral artery. NOx− (nitrite plus nitrate) and 3-nitrotyrosine were measured in the ischemic cortex. Ischemia caused a rapid increase in the production of NOx−, with a peak at 10 minutes after ischemia onset, then gradually declining to the baseline level at 60 minutes. NBO treatment delayed the NOx− production peak to 30 minutes and attenuated the total amount of NOx−. Ischemia also increased 3-nitrotyrosine formation, which was significantly reduced by NBO treatment. Inhibition of nNOS by pre-treatment with 7-nitroindazole had similar effect as NBO treatment on NOx− and 3-nitrotyrosine production, and when combined with NBO, no further reduction in NO production was observed. Furthermore, NBO treatment significantly decreased brain infarct volume. Taken together, our findings demonstrate that delaying and attenuating the early NO release from nNOS may be an important mechanism accounting for NBO’s neuroprotection.
Keywords: cerebral ischemia, nitric oxide, normobaric hyperoxia
1. INTRODUCTION
Excitotoxicity is well established as an important trigger and executioner of tissue damage in focal cerebral ischemia. Excitotoxic mechanisms can cause acute cell death, but can also initiate molecular events that lead to delayed cell death (Dirnagl et al. 1999). Nitric oxide (NO) derived from constitutively expressed neuronal nitric oxide synthase (nNOS) and from the inducible isoform (iNOS) expressed by many cell types in the brain is one of the important mediators in the excitotoxic injury cascades (Keynes and Garthwaite 2004; Moro et al. 2004). Following cerebral ischemia, high concentration of glutamate released from ischemic neurons activates several postsynaptic glutamate receptor/ion channel complexes, most notably the N-methyl-D-aspartate (NMDA) receptor and its Ca2+ channel. Intracellular Ca2+ influx activates several calcium dependent cytodestructive enzymes, including nNOS, leading to high local NO concentrations (Samdani et al. 1997).
Excessive production of NO mediates cellular injury via several different mechanisms. NO directly causes lipid peroxidation and depletes cellular energy via disruption of mitochondrial enzymes and nucleic acids (Christopherson and Bredt 1997). In addition, NO reacts readily with superoxide anion to form peroxynitrite, a reactive species capable of producing extensive cellular and tissue destruction (Reiter et al. 2001). NO also triggers neuronal death via apoptosis (Sugawara et al. 2004). And the magnitude of NO overproduction correlates well with the severity of tissue injury (Matsui et al. 1999). Moreover, pharmacologically selective nNOS inhibitors (Yoshida et al. 1994; Zhang et al. 1996b) or nNOS knockout (Ferriero et al. 1996; Panahian et al. 1996) attenuate the infarction volume after focal cerebral ischemia.
In rodent experiments (Flynn and Auer 2002; Henninger et al. 2007; Henninger et al. 2008; Hou et al. 2007; Kim et al. 2005; Liu et al. 2006; Shin et al. 2007; Singhal et al. 2002a; Singhal et al. 2002b) and several clinical trials (Chiu et al. 2006; Singhal et al. 2005; Singhal et al. 2007), we and others have documented that the delivery of 95% or 100% oxygen at normal atmospheric pressure (normobaric hyperoxia, NBO) during transient focal cerebral ischemia is neuroprotective, without increasing free radical generation (Kim et al. 2005; Liu et al. 2006; Singhal et al. 2002b) or inducing vasoconstriction (Shin et al. 2007). Imaging studies indicate that NBO slows down the process of ischemic cell death (Henninger et al. 2007; Singhal et al. 2002a; Singhal et al. 2007). These promising results have suggested that NBO therapy might be a useful strategy to expand the narrow therapeutic time window or “buy time” until reperfusion can be achieved. However, the neuroprotective mechanisms are not understood clearly. Given the important role of nNOS-derived NO in excitotoxic neuronal cell death at the acute phase of ischemic stroke, we hypothesized that attenuating NO production in the ischemic brain may be an important mechanism via which NBO therapy exerts its neuroprotection. In the present study, we tested this hypothesis by investigating NO production and 3-nitrotyrosine formation in ischemic cortex of normoxic and NBO-treated rats at early stages of ischemia. In order to understand the source of NO, we also carried out parallel experiments in rats pre-treated with 7-nitroindazole (7-NI), an nNOS selective inhibitor (Yoshida et al. 1994; Zhang et al. 1996b).
2. RESULTS
2.1. Effects of NBO treatment on NOx− production in the ischemic cortex
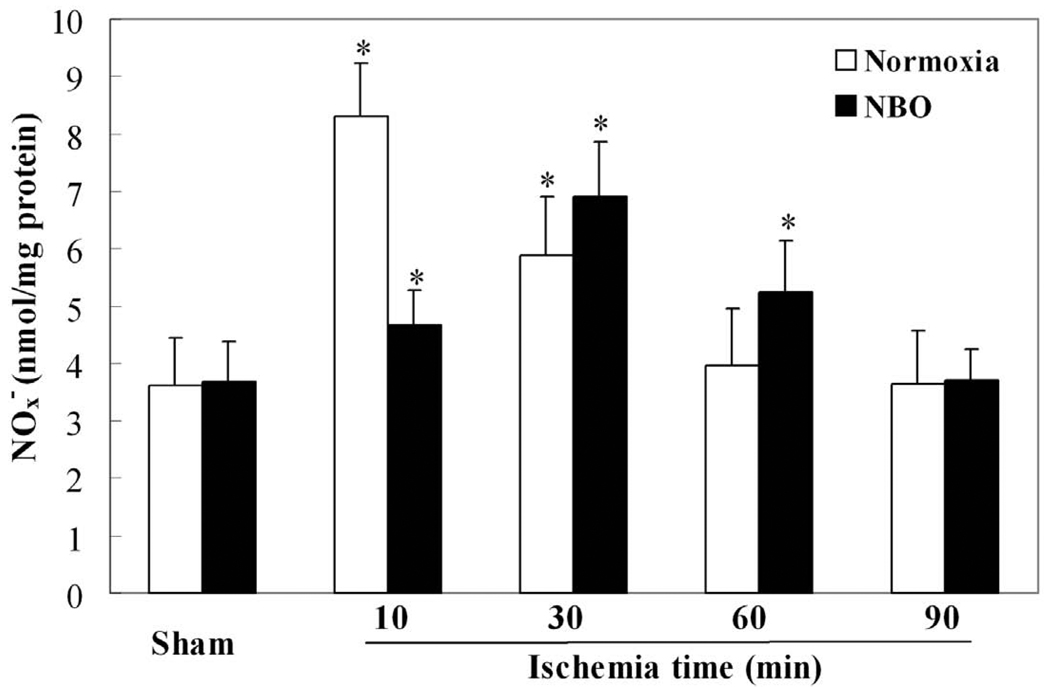
The concentrations of NOx− in the ischemic cortex were measured using the Griess method following 10, 30, 60 and 90 minutes of ischemia. In both normoxic and NBO groups, the production of NOx− was time-dependent. In the normoxic groups, ischemia caused a rapid increase in the production of NOx− in the ischemic cortex (Fig. 1). The concentration of NOx− reached a peak at 10 minutes, which was 2.3-fold of the sham-control level (8.31 ± 0.93 vs. 3.62 ± 0.81 nmol/mg protein, n=5). Thereafter, the production of NOx− gradually declined, and returned to basal level at 60 minutes after the onset of MCAO. NBO treatment both delayed and attenuated the ischemia-induced production of NOx−. The NOx− production peak was shifted from 10 minutes to 30 minutes after ischemia onset, and the peak amount of NOx− was 6.91 ± 0.94 nmol/mg protein, n=5), a decrease of 17% as compared to the normoxic group’s peak level. Additionally, the NOx− level of NBO group returned to basal level at 90 min after the onset of ischemia. Compared with the sham-control, there was no significant difference in the amount of NOx− in the contralateral cortex for both NBO and normoxic groups (data not shown).
Fig. 1.
The time-course of NOx− (nitrate plus nitrite) production in ischemic cortices of MCAO rats. In both normoxic (30% O2) and NBO (95% O2) groups, the production of NOx− was time-dependent. Compared with normoxic groups, NBO treatment delayed and attenuated the production of NOx−. Data were expressed as Mean ± S.D. * P<0.05 versus sham-control level, n=5.
2.2. Effects of NBO treatment on 3-nitrotyrosine formation in the ischemic cortex
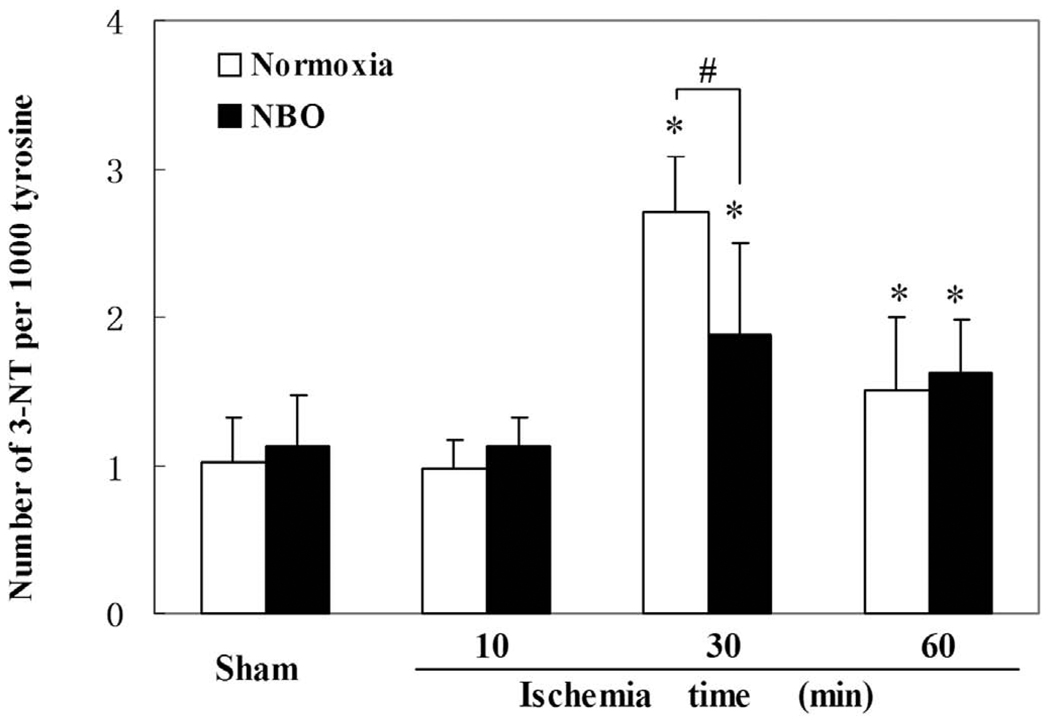
Ischemia-induced NO generation is known to react with superoxide to form peroxynitrite, leading to the formation of 3-notrotyrosine, which has been used as a biomarker for peroxynitrite and as an indicator of NO-mediated brain injury. Using high pressure liquid chromatography/electrochemical detection (HPLC-EC), we determined the formation of 3-nitrotyrosine in ischemic cortex at 10, 30, and 60 minutes after MCAO onset. For both normoxic and NBO groups, as shown in Fig. 2, the formation of 3-nitrotyrosine was significantly increased at 30, and 60 minutes in the ischemic cortices compared to the sham-control level. Moreover, NBO treatment decreased 3-nitrotyrosine formation by 30.5 ± 4.5% (p<0.05, n=5) compared to the normoxic group at 30 minutes after ischemia onset.
Fig. 2.
The effect of NBO treatment on 3-nitrotyrosine (3-NT) production in ischemic cortices of MCAO rats. In both the normoxic (30% O2) and the NBO (95% O2) groups, the formation of 3-NT was time-dependent, both peaked at 30 minutes and NBO treatment significantly attenuated the production peak level of 3-NT. Data were expressed as Mean ± S.D. * P<0.05 versus the level of sham-control, # P<0.05 versus the level of normoxic group, n=5.
2.3. Effects of selective nNOS inhibitor on the production of NO and 3-nitrotyrosine in ischemic cortex
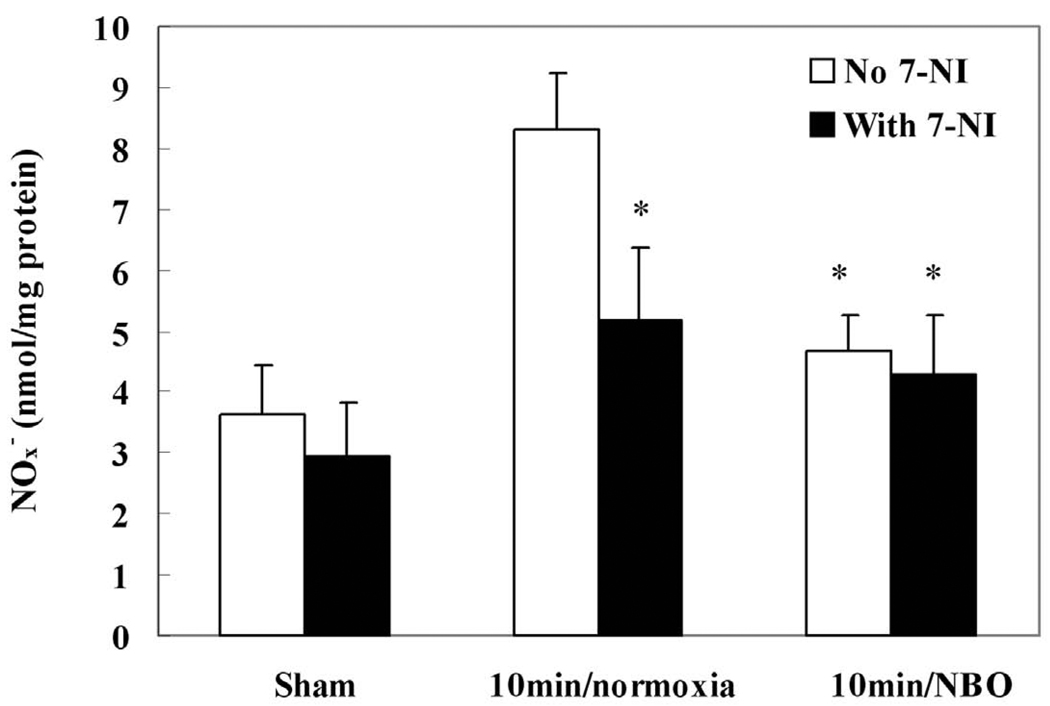
The above experiments demonstrate that NBO can reduce the production of NO, and its nitration product, 3-nitrotyrosine. In order to investigate the source of NO production, we used the nNOS selective inhibitor, 7-NI, and examined its effect on the production of NO and 3-nitrotyrosine. Based on the time-course of NOx− production in normoxic groups, we chose the peak NOx− production time point (10 minutes after ischemia onset) to clarify the source of NO and to compare the effects of NBO and nNOS inhibitor on NO production. As shown in Fig. 3, under normoxic conditions, pre-treatment with 7-NI significantly suppressed NOx− production in the ischemic cortex (p<0.05, n=4), indicating that nNOS is a major source of NOx− production. NBO treatment alone had similar effects as 7-NI on NOx− production. Importantly, when NBO was given to the rats that had been pre-treated with 7-NI, no further reduction was observed for NO production. These results suggest that NBO and 7-NI are likely acting through the same mechanism, i.e., through inhibition of nNOS.
Fig. 3.
The effects of NBO (95% O2) and nNOS inhibitor (7-NI) on the production of NOx− (nitrite plus nitrate) in ischemic cortex at 10 minutes after MCAO onset. 7-NI (25 mg/kg) was intraperitoneally injected 20 minutes before the onset of ischemia. Compared with the normoxic (30% O2) group, both NBO and 7-NI significantly suppressed the production of NOx−. Data were expressed as Mean ± S.D. * P<0.05 versus normoxic group, n=4.
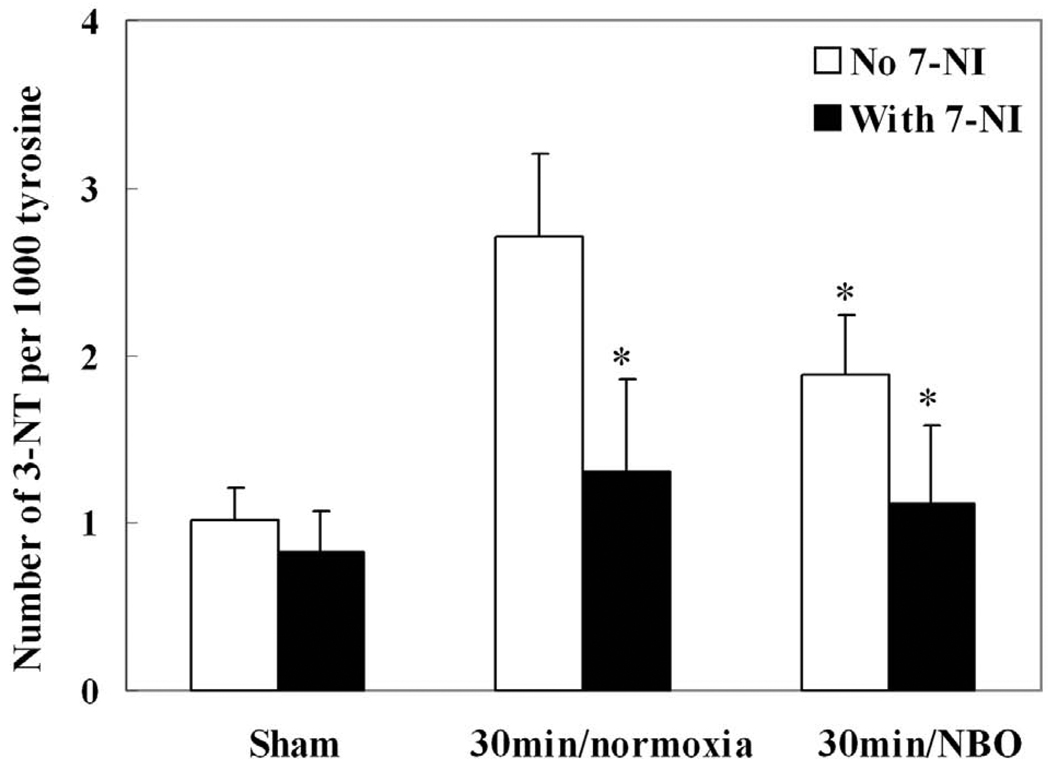
We also studied the effects of NBO and 7-NI on 3-nitrotyrosine formation in the ischemic cortex at its peak production time (30 minutes after ischemia onset). As shown in Fig. 4, 7-NI suppressed the formation of 3-nitrotyrosine in ischemic cortex in both normoxic and NBO group (p<0.05, n=4). Similarly, NBO partly suppressed the formation of 3-nitrotyrosine compared to the normoxic group.
Fig. 4.
The effects of NBO (95% O2) and nNOS inhibitor (7-NI) treatments on the formation of 3-nitrotyrosine (3-NT) in ischemic cortex at 30 minutes after MCAO onset. 7-NI (25 mg/kg) was intraperitoneally injected 20 minutes before the onset of ischemia. Compared with normoxic (30% O2) group, both NBO and 7-NI significantly suppressed the formation of 3-NT. Data were expressed as Mean ± S.D. * P<0.05, versus normoxic group, n=4.
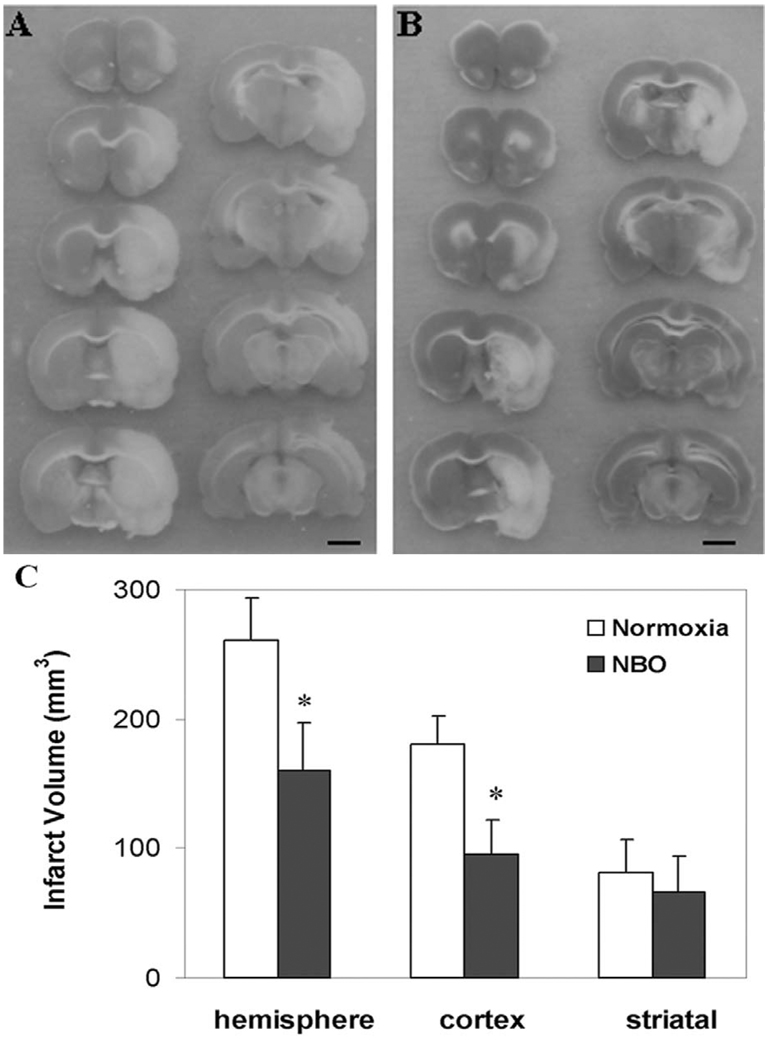
2.4. Effects of NBO treatment on brain tissue infarction
To demonstrate that the delayed and attenuated NO production observed above is associated with improved final tissue outcome, we assessed the infarct volume by TTC staining after 22.5 hrs reperfusion following 90 min ischemia. We found that NBO treatment significantly decreased total (hemispheric) infarct volume (Fig. 5), particularly in the cortical region. Although the infarct volume in the subcortex (striatal) was also reduced, it was not statistically significant.
Fig. 5.
The effect of NBO on brain infarct volume. TTC staining illustrating infarct brain region (pale zones) of normoxic group (A) and NBO group (B) after 22.5 hrs reperfusion following 90 min ischemia, scale bar = 3 mm. (C) NBO treatment (95% O2) significantly decreased the infarct volume. Data were expressed as Mean ± S.D. * P<0.05 versus normoxic group, n=5.
3. DISCUSSION
In the present study, our results demonstrate that ischemia caused a rapid increase in NO end products NOx− (nitrate plus nitrite) in the ischemic cortex, reaching a peak at 10 minutes (2.3-fold of the basal level) after the onset of ischemia, which then returned to baseline level at 60 minutes in the normoxic rats. Consistent with our findings, others have documented similar pattern of NO production during ischemia (Kader et al. 1993; Malinski et al. 1993; Zhang et al. 1995). For the first time, our present study shows that NBO treatment both delayed and attenuated ischemia-induced NO production, with a shift of the peak time from 10 minutes to 30 minutes, and a 17% reduction in its peak level.
NO is produced by three isoforms of nitric oxide synthase (NOS): type I or neuronal NOS (nNOS), type II or inducible NOS (iNOS), and type III or endothelial NOS (eNOS) (Marletta 1994). nNOS is constitutively expressed in neurons, eNOS is constitutively expressed in endothelial cells, and iNOS is found in activated immune cells and in peri-ischemic but not normal brain tissue (Marletta 1994). Enhanced NO production from eNOS improves micro-circulation and ameliorates ischemic damage, whereas increased NO production from nNOS or iNOS can promote ischemic damage via free radical damage, micro-circulatory failure, and tissue inflammation (Iadecola 1997). It has been reported that nNOS activity increases significantly 10 minutes after focal ischemia and returns to normal after 60 minutes (Kader et al. 1993). Both the protein level and catalytic activity of eNOS are upregulated within 1 hour and become maximal at 24 hours after cerebral ischemia (Samdani et al. 1997). On the other hand, iNOS becomes detectable at 6–12 hours and is maximal at 48 hours after cerebral ischemia (Samdani et al. 1997; Zhang et al. 1996a). Thus, upregulation of nNOS and eNOS is likely to be responsible for the increased NO production in the brain at early stages of focal cerebral ischemia. Furthermore, nNOS is likely the major source of NO because nNOS activity is at least 20 times higher than that of eNOS in the brain (Schmidt et al. 1993). In this study, we found that pre-treatment with selective nNOS inhibitor 7-NI nearly abolished the overproduction of NO at 10 minutes of MCAO (Fig. 3), indicating that the increased NO production was mainly derived from nNOS. Together with our observation that combined treatment of 7-NI and NBO did not further decrease NO production, we postulate that NBO delays and attenuates early NO production through inhibition of nNOS, leading to reduced brain injury.
NO is a short-lived and relatively unreactive radical, but NO can combine with superoxide to form the potent oxidant peroxynitrite, ONOO−, which has been shown to play a significant role in NO-mediated ischemic and post-ischemic damage (Eliasson et al. 1999; Gursoy-Ozdemir et al. 2000). Under normal physiological conditions, the concentration of NO and superoxide is relatively low, and so is peroxynitrite production. However, under pathological conditions in which NO production is increased, a significant amount of peroxynitrite is formed. Nitration on the 3-position of tyrosine is a major result of peroxynitrite attack on proteins. Protein nitration may be fundamentally related to, and predictive of, oxidative cell injury (Kamat 2006) and nitrotyrosine itself is toxic. Furthermore, this nitrated residue can also be used as a diagnostic marker for the formation of peroxynitrite and indirectly for superoxide and nitric oxide generation. In the present study, we found that the formation of 3-nitrotyrosine was significantly increased at 30 minutes of ischemia in the normoxic group, which was mostly inhibited by selective nNOS inhibitor 7-NI (Fig. 4), further supporting that nNOS is the major source of NO production at early stages of cerebral ischemia. Our finding that NBO treatment reduced 3-nitrotyrosine production by 30% is consistent with its partial inhibition on NO production (Fig 1).
Since increased brain NO concentration at the early stages of focal ischemia correlates with a larger infarct volume and decreased brain NO concentration at this time is associated with a smaller infarct volume (Chen et al. 2002), it is plausible to speculate that nNOS-derived NO production and the resulting peroxynitrite contribute to early ischemic brain damage. Indeed, earlier studies using pharmacological inhibitors (Yoshida et al. 1994; Zhang et al. 1996b) or nNOS knockout mice (Ferriero et al. 1996; Panahian et al. 1996) have demonstrated a neuroprotective effect of nNOS inhibition in focal cerebral ischemia. We and others have shown that NBO treatment is neuroprotective and several mechanisms have been proposed: NBO improves tissue oxygenation and aerobic metabolism (Liu et al. 2006; Shin et al. 2007; Singhal et al. 2007), inhibits peri-infarct depolarizations that contribute to infarct growth (Shin et al. 2007), preserves blood brain barrier integrity (Liu et al. 2009a; Liu et al. 2009b), and favorably alters hemodynamic parameters such as cerebral blood volume (Shin et al. 2007). Our present study showed that intro-ischemia NBO treatment attenuated the infarction volume (measured at 24 hours post-ischemia) in the ischemic cortex where the production of NO and 3-nitrotyrosine was also inhibited at much earlier stroke stages. These results suggest that NBO may exert its neuroprotection by delaying and attenuating nNOS-derived NO production and the resulting peroxynitrite.
By delaying and attenuating nNOS-derived NO production and the resulting peroxynitrite, NBO might slow down the evolution of ischemic damage to the neuronal tissue and cerebral vasculature, thus extending the time window for acute stroke treatment. As a fact, Kim et al (Kim et al. 2005) documented that NBO therapy administered shortly after onset of stroke slows down the transition of ischemia to infarction and extends the therapeutic time window from 1 hour to 3 hours. Henninger et al recently showed that early NBO in combination with tPA treatment beyond 3 hrs reduced infarct volumes without increasing hemorrhage volume (Henninger et al. 2008). In a recent study, we also demonstrated that NBO treatment during ischemia reduced tPA-associated neurovascular complications (Liu et al. 2009a). In the present study, we did not perform experiments to further demonstrate whether there is a causal link between the delay in NO production and the slowing down of tissue infarction in NBO-treated rats. However, our results clearly indicate that NBO can both delay and attenuate ischemia-induced NO release, which would thereby ameliorate NO-induced brain damage at the early stages of ischemic stroke.
In summary, our results demonstrate that NBO treatment both delays and attenuates NO production following cerebral ischemia, which may represent an important mechanism contributing toward the observed neuroprotection by NBO treatment.
4. EXPERIMENTAL PROCEDURE
4.1. Animal Handling
The Laboratory Animal Care and Use Committee of the University of New Mexico approved all experimental protocols. Adult male Sprague–Dawley rats (280–320 g; Charles River Laboratories, MA) were used for all experiments. The rats were maintained in a climate-controlled vivarium with a 12 hours light–dark cycle with free access to food and water.
For all surgical procedures, 4.0% isoflurane in 30% O2:70% N2 was used for anesthesia induction, and 1.75% for anesthesia maintenance. Rectal temperature was maintained at 37.5 ± 0.5°C using a heating pad.
4.2. Preparation of Middle Cerebral Artery Occlusion (MCAO) Rat Model
The MCAO model used in this study were performed as described previously (Liu et al. 2006). A silicone rubber coated monofilament nylon suture with a diameter of 0.37 mm was used for the MCAO. Continuous Laser–Doppler flowmetry (LDF; Perimed, North Royalton, OH) was used to monitor regional cerebral perfusion to ensure adequacy of MCAO. Rats that did not demonstrate a significant reduction of LDF values to at least 30% of the base level after MCAO were excluded from this study. The sham-operated rats were subjected to the same surgical procedures, except that the suture was advanced only 10 mm (not 19 mm) above the bifurcation and withdrawn after 10 seconds.
4.3. Normobaric Hyperoxia Therapy
NBO treatment was initiated by switching inspired gas from normoxia gas (30% O2) to NBO (95% O2) for the indicated periods of time, and terminated by switching back to normoxia, as described in detail previously (Liu et al. 2006).
4.4. Experimental protocol and tissue processing
Rats were randomly divided into normoxic and NBO groups, with five rats in each group. The NBO treatment was started one minute after the onset of MCAO, and lasted for 10, 30, 60 or 90 minutes. Rats were sacrificed at 10, 30, 60 or 90 minutes after MCAO onset. Rat heads were quickly removed and immersed in liquid nitrogen for 10 seconds and then the brains were removed. Using a brain-cutting matrix, a 4-mm coronal section was made from a region 3 mm away from the tip of the frontal lobe, which contained the main infarction area according to our earlier studies with TTC staining (Liu et al. 2006; Liu et al. 2004). The meninges were carefully removed, and then a longitudinal cut was made 2 mm away from the midline between two hemispheres on each brain slice to exclude tissue primarily supplied by the anterior cerebral artery. The right and left cortex were collected as ipsilateral cortex (ischemic cortex) and contralateral cortex, respectively, and stored at −80°C until further analysis.
All tissue samples were sonicated in ice-cold PBS (pH 7.4) for 15 seconds in a thermally regulated sonicator (Branson Sonifier, Branson Corp., Danbury, CT). After sonication, samples were centrifuged at 14,000 × g for 10 minutes at 4°C. The supernatants were analyzed for protein contents, NOx− (nitrite plus nitrate), and 3-nitrotyrosine. The protein contents were measured by Bradford Assay (Biorad Laboratories, Richmond, CA). The concentrations of NOx− and 3-nitrotyrosine were assessed as described below.
4.5. NO production measurement
Total amount of NO end production, nitrate plus nitrite (NOx−), was measured using a commercial enzymatic nitric oxide assay kit (Oxford Biomedical Research, Oxford, MI), in which the conversion of nitrate to nitrite by nitrate reductase is assayed using the Griess reagent. Samples of each tissue were sonicated, as described above, and then centrifuged. 5 µl of each supernatant was mixed with 495 µl PBS, and filtered through a 3k MW cut-off membrane (Pall Life Science, East Hills, NY). NOx− was measured spectrophotometrically according to manufacturer’s instructions. In each experimental group, five animals were studied. Data are expressed as nanomole NOx− per milligram protein.
4.6. HPLC-EC detection of 3-nitrotyrosine
Protein extraction and hydrolysis procedures, and HPLC-EC analysis and calculation were performed as described before (Ding et al. 2005). In brief, tissue samples were sonicated and centrifuged. Acetonitrile was added to the supernatant, gently centrifuged, and the pellets were dissolved in 0.1 M NaOAc, pH 7.2 at a final concentration of protein at 4 mg/ml. Twenty mg/ml protease (XIV from Streptomyces griseus, Sigma, St. Louis, MO) dissolved in 0.1 M NaOAc, pH 7.2 were added to samples at a final ratio of 1:5 (w/w). The samples were placed in a 50°C water bath for 18 hours. All hydrolyzed mixtures were then transferred to a 3000 Da micro-filtration tube (Pall Life Science, East Hills, NY) to remove intact protease or undigested protein. Filtrates were then analyzed on an ESA (Chelmsford, MA) CoulArray HPLC instrument equipped with 12 electrochemical channels. The analytical column was a TOSOHAAS (Mongtomeryville, PA) ODS 80-TM C-18 reverse phase column, and the mobile phase was 50 mM sodium citrate/5% methanol (v/v), pH 4.7. Both 3-nitrotyrosine and tyrosine were detected by the EC detector. The level of 3-nitrotyrosine was expressed as the number of 3-nitrotyrosine per 1000 tyrosine.
4.7. Treatment with nNOS inhibitor
We also carried out parallel experiments in animals pre-treated with 7-NI, an nNOS selective inhibitor (Yoshida et al. 1994; Zhang et al. 1996b). The rats received either vehicle (DMSO, n=4) or 7-NI (25 mg/kg, n=4). The drug was intraperitoneally injected 20 minutes before the onset of ischemia, since maximal inhibition of nNOS activity occurred 30 minutes after systemic injection of 7-NI (MacKenzie et al. 1994). 7-NI at 25 mg/kg was selected because this dose has been reported to effectively inhibit ischemia-induced NO production (Jiang et al. 1999). Rats inhaled 30% O2 or 95% O2 one minute after the onset of ischemia, and were sacrificed at 10 minutes or 30 minutes after the onset of ischemia, for measurements of NOx− and 3-nitrotyrosine, respectively.
4.8. Quantification of Brain Infarction
In order to investigate the impact of NBO treatment on brain infarction volume under our experimental conditions, ten rats were randomly divided into normoxic and NBO groups, with five rats in each group. The MCA was occluded for 90 minutes and then reperfused. In the normoxic groups, 30% O2 was administered one minute after MCAO; in the NBO group, a NBO gas mixture of 95% O2 was administered and lasted until the end of the 90-minute ischemia. Rats were sacrificed at 22.5 hours after reperfusion. The brain was rapidly removed, cooled in ice-cold saline for 10 minutes, and cut into 2 mm coronal sections. Brain slices were stained with 2,3,5-triphenyletetrazolium chloride (TTC) for 30 minutes in the dark. Total (hemispheric), cortical, and subcortical (striatal) infarction volumes were measured with Image Pro Plus software.
4.9. Statistics
Statistical analysis of data was carried out using ANOVA. Differences between means were regarded as statistically significant if p<0.05.
Research Highlights
NBO treatment delays and attenuates the production of NOx− and 3-nitrotyrosine.
Early NBO treatment inhibits NO release from nNOS.
Reducing NO production is an important mechanism of neuroprotection by NBO.
Acknowledgement
This work was supported in part by grants from American Heart Association (0555669Z and 0765461Z) and NIH (R01 AG031725 and P20 RR15636).
List of abbreviations
- NBO
Normobaric hyperoxia
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- NOx−
nitrite plus nitrate
- NMDA
N-methyl-D-aspartate
- 7-NI
7-nitroindazole
- LDF
Laser–Doppler flowmetry
- MCAO
middle cerebral artery occlusion
- TTC
2,3,5-triphenyltetrazolium chloride
- HPLC-EC
high pressure liquid chromatography/electrochemical detection
- 3-NT
3-nitrotyrosine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen SH, Fung PC, Cheung RT. Neuropeptide Y-Y1 receptor modulates nitric oxide level during stroke in the rat. Free Radic Biol Med. 2002;32(8):776–784. doi: 10.1016/s0891-5849(02)00774-8. [DOI] [PubMed] [Google Scholar]
- Chiu EH, Liu CS, Tan TY, Chang KC. Venturi mask adjuvant oxygen therapy in severe acute ischemic stroke. Arch Neurol. 2006;63(5):741–744. doi: 10.1001/archneur.63.5.741. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Bredt DS. Nitric oxide in excitable tissues: physiological roles and disease. J Clin Invest. 1997;100(10):2424–2429. doi: 10.1172/JCI119783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Hudson LG, Liu KJ. Inorganic arsenic compounds cause oxidative damage to DNA and protein by inducing ROS and RNS generation in human keratinocytes. Mol Cell Biochem. 2005;279(1–2):105–112. doi: 10.1007/s11010-005-8227-y. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22(9):391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Eliasson MJ, Huang Z, Ferrante RJ, Sasamata M, Molliver ME, Snyder SH, Moskowitz MA. Neuronal nitric oxide synthase activation and peroxynitrite formation in ischemic stroke linked to neural damage. J Neurosci. 1999;19(14):5910–5918. doi: 10.1523/JNEUROSCI.19-14-05910.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferriero DM, Holtzman DM, Black SM, Sheldon RA. Neonatal mice lacking neuronal nitric oxide synthase are less vulnerable to hypoxic-ischemic injury. Neurobiol Dis. 1996;3(1):64–71. doi: 10.1006/nbdi.1996.0006. [DOI] [PubMed] [Google Scholar]
- Flynn EP, Auer RN. Eubaric hyperoxemia and experimental cerebral infarction. Ann Neurol. 2002;52(5):566–572. doi: 10.1002/ana.10322. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31(8):1974–1980. doi: 10.1161/01.str.31.8.1974. discussion 1981. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007;27(9):1632–1642. doi: 10.1038/sj.jcbfm.9600463. [DOI] [PubMed] [Google Scholar]
- Henninger N, Bratane BT, Bastan B, Bouley J, Fisher M. Normobaric hyperoxia and delayed tPA treatment in a rat embolic stroke model. J Cereb Blood Flow Metab. 2009;29(1):119–129. doi: 10.1038/jcbfm.2008.104. [DOI] [PubMed] [Google Scholar]
- Hou H, Grinberg O, Williams B, Grinberg S, Yu H, Alvarenga DL, Wallach H, Buckey J, Swartz HM. The effect of oxygen therapy on brain damage and cerebral pO(2) in transient focal cerebral ischemia in the rat. Physiol Meas. 2007;28(8):963–976. doi: 10.1088/0967-3334/28/8/017. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997;20(3):132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Jiang MH, Kaku T, Hada J, Hayashi Y. 7-Nitroindazole reduces nitric oxide concentration in rat hippocampus after transient forebrain ischemia. Eur J Pharmacol. 1999;380(2–3):117–121. doi: 10.1016/s0014-2999(99)00555-5. [DOI] [PubMed] [Google Scholar]
- Kader A, Frazzini VI, Solomon RA, Trifiletti RR. Nitric oxide production during focal cerebral ischemia in rats. Stroke. 1993;24(11):1709–1716. doi: 10.1161/01.str.24.11.1709. [DOI] [PubMed] [Google Scholar]
- Kamat JP. Peroxynitrite: a potent oxidizing and nitrating agent. Indian J Exp Biol. 2006;44(6):436–447. [PubMed] [Google Scholar]
- Keynes RG, Garthwaite J. Nitric oxide and its role in ischaemic brain injury. Curr Mol Med. 2004;4(2):179–191. doi: 10.2174/1566524043479176. [DOI] [PubMed] [Google Scholar]
- Kim HY, Singhal AB, Lo EH. Normobaric hyperoxia extends the reperfusion window in focal cerebral ischemia. Ann Neurol. 2005;57(4):571–575. doi: 10.1002/ana.20430. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(10):1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(3):343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Liu W, Hendren J, Qin XJ, Liu KJ. Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia. Stroke. 2009a;40(7):2526–2531. doi: 10.1161/STROKEAHA.108.545483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Hendren J, Qin XJ, Shen J, Liu KJ. Normobaric hyperoxia attenuates early blood-brain barrier disruption by inhibiting MMP-9-mediated occludin degradation in focal cerebral ischemia. J Neurochem. 2009b;108(3):811–820. doi: 10.1111/j.1471-4159.2008.05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie GM, Rose S, Bland-Ward PA, Moore PK, Jenner P, Marsden CD. Time course of inhibition of brain nitric oxide synthase by 7-nitro indazole. Neuroreport. 1994;5(15):1993–1996. doi: 10.1097/00001756-199410000-00039. [DOI] [PubMed] [Google Scholar]
- Malinski T, Bailey F, Zhang ZG, Chopp M. Nitric oxide measured by a porphyrinic microsensor in rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1993;13(3):355–358. doi: 10.1038/jcbfm.1993.48. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase: aspects concerning structure and catalysis. Cell. 1994;78(6):927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Matsui T, Nagafuji T, Kumanishi T, Asano T. Role of nitric oxide in pathogenesis underlying ischemic cerebral damage. Cell Mol Neurobiol. 1999;19(1):177–189. doi: 10.1023/A:1006985112459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro MA, Cardenas A, Hurtado O, Leza JC, Lizasoain I. Role of nitric oxide after brain ischaemia. Cell Calcium. 2004;36(3–4):265–275. doi: 10.1016/j.ceca.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Panahian N, Yoshida T, Huang PL, Hedley-Whyte ET, Dalkara T, Fishman MC, Moskowitz MA. Attenuated hippocampal damage after global cerebral ischemia in mice mutant in neuronal nitric oxide synthase. Neuroscience. 1996;72(2):343–354. doi: 10.1016/0306-4522(95)00563-3. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Qi W. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochem Biophys. 2001;34(2):237–256. doi: 10.1385/CBB:34:2:237. [DOI] [PubMed] [Google Scholar]
- Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28(6):1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178(2):153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Shin HK, Dunn AK, Jones PB, Boas DA, Lo EH, Moskowitz MA, Ayata C. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130(Pt 6):1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36(4):797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002a;58(6):945–952. doi: 10.1212/wnl.58.6.945. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Ratai E, Benner T, Vangel M, Lee V, Koroshetz WJ, Schaefer PW, Sorensen AG, Gonzalez RG. Magnetic resonance spectroscopy study of oxygen therapy in ischemic stroke. Stroke. 2007;38(10):2851–2854. doi: 10.1161/STROKEAHA.107.487280. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Wang X, Sumii T, Mori T, Lo EH. Effects of normobaric hyperoxia in a rat model of focal cerebral ischemia-reperfusion. J Cereb Blood Flow Metab. 2002b;22(7):861–868. doi: 10.1097/00004647-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Fujimura M, Noshita N, Kim GW, Saito A, Hayashi T, Narasimhan P, Maier CM, Chan PH. Neuronal death/survival signaling pathways in cerebral ischemia. NeuroRx. 2004;1(1):17–25. doi: 10.1602/neurorx.1.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Limmroth V, Irikura K, Moskowitz MA. The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J Cereb Blood Flow Metab. 1994;14(6):924–929. doi: 10.1038/jcbfm.1994.123. [DOI] [PubMed] [Google Scholar]
- Zhang F, Casey RM, Ross ME, Iadecola C. Aminoguanidine ameliorates and L-arginine worsens brain damage from intraluminal middle cerebral artery occlusion. Stroke. 1996a;27(2):317–323. doi: 10.1161/01.str.27.2.317. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M, Bailey F, Malinski T. Nitric oxide changes in the rat brain after transient middle cerebral artery occlusion. J Neurol Sci. 1995;128(1):22–27. doi: 10.1016/0022-510x(94)00216-b. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Reif D, Macdonald J, Tang WX, Kamp DK, Gentile RJ, Shakespeare WC, Murray RJ, Chopp M. ARL 17477, a potent and selective neuronal NOS inhibitor decreases infarct volume after transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1996b;16(4):599–604. doi: 10.1097/00004647-199607000-00009. [DOI] [PubMed] [Google Scholar]