Abstract
Dopamine (DA) replacement therapy with l-DOPA remains the most effective treatment for Parkinson’s disease, but causes dyskinesia (abnormal involuntary movements) in the vast majority of the patients. The basic mechanisms of l-DOPA-induced dyskinesia (LID) have become the object of intense research focusing on neurochemical and molecular adaptations in the striatum. Here we review this vast literature and highlight trends that converge into a unifying pathophysiological interpretation. We propose that the core molecular alteration of striatal neurons in LID consists in an inability to turn down supersensitive signaling responses downstream of DA D1 receptors (where supersensitivity is primarily caused by DA denervation). The sustained activation of intracellular signaling pathways induced by each dose of l-DOPA leads to abnormal cellular plasticity and high bioenergetic expenditure. The over-exploitation of signaling pathways and energy reserves during treatment impairs the ability of striatal neurons to dynamically gate cortically driven motor commands. LID thus exemplifies a disorder where ‘too much’ molecular plasticity leads to plasticity failure in the striatum.
Keywords: Striatonigral, Striatopallidal, Medium spiny neuron, ERK, MAPK, Transcription, Complications
Introduction
Although it is presently recognized that several neuronal systems degenerate in Parkinson’s disease (PD), the characteristic motor symptoms that define this disease are due to the demise of nigrostriatal dopamine (DA) neurons (Fearnley and Lees, 1991 and Morrish et al., 1996). Accordingly, these symptoms are alleviated by the DA precursor, l-DOPA. Following peripheral administration, l-DOPA crosses the blood–brain barrier and is rapidly decarboxylated to DA in the brain. Importantly, the capacity for l-DOPA uptake and its conversion to DA is not compromised by the loss of nigrostriatal DA neurons, because other cell systems in the brain are endowed with the enzyme that converts l-DOPA to DA [aromatic amino acid decarboxylase; reviewed in Cenci and Lundblad (2006)]. However, with the loss of nigrostriatal DA neurons the presynaptic control of DA release and clearance becomes greatly impaired [reviewed in Cenci and Lundblad (2006)], leading to large fluctuations in extracellular levels of DA concomitant with the l-DOPA dosing cycles. These variations are closely associated with the development of abnormal involuntary movements, AIMs (dyskinesia) (Chase, 1998), a very common complication of the pharmacotherapy of PD (Fabbrini et al., 2007). It has been estimated that 10% of PD patients per year develop dyskinesia during the first 7 years of l-DOPA pharmacotherapy (Grandas et al., 1999), but the reported prevalence of dyskinesia varies greatly among PD patient cohorts (Manson and Schrag, 2006). l-DOPA-induced dyskinesia (LID) most typically consists of choreiform movements that affect the limbs, the head and the trunk when plasma and brain levels of l-DOPA are high (‘peak-dose dyskinesia’) (Fabbrini et al., 2007). The appearance of these movements correlates with a large increase in extracellular DA levels in the striatum (de la Fuente-Fernandez et al., 2004 and Pavese et al., 2006). This particular brain structure mediates the dyskinetic effects of PD treatment, as indicated by the results of local drug infusion experiments (Buck et al., 2009 and Carta et al., 2006) and cell transplantation procedures [see Lane et al. (2010), this volume].
Striatal neurons express high levels of DA receptors and undergo profound molecular and structural adaptations following DA depletion [see Surmeier et al. (2010), this volume]. During the past 10 years, evidence has accumulated to indicate that DA replacement therapy, whether pharmacological or cell-based, cannot reset the striatum back to its normal physiological state but introduces novel functional/dysfunctional states that strictly correlate with the profile of treatment-induced motor effects. This knowledge has been gained through studies performed in rodents and non-human primates in which the nigrostriatal DA pathway was severed using specific neurotoxins, thus producing a model of PD in laboratory animals. The most commonly used neurotoxins are 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in non-human primates and 6-hydroxydopamine (6-OHDA) in rats and mice. After DA depletion, animals develop parkinsonian-like motor deficits that correlate with the extent of striatal DA denervation and that can be ameliorated by dopaminergic drugs [reviewed in (Fox and Brotchie, 2010) and (Dunnett, 2010), this volume; see also Cenci et al. (2002)]. When treated with l-DOPA, both rodents and non-human primates can develop AIMs of the limbs, head and trunk with dystonic and hyperkinetic features [reviewed in (Cenci and Ohlin, 2009), (Cenci et al., 2002) and (Jenner, 2003)]. Also in these animal models, the expression of LID correlates temporally and quantitatively with an increase in striatal extracellular DA levels (Lindgren et al., 2010), similarly to the situation reported in dyskinetic PD patients.
An active area of research is addressing the basic mechanisms of LID and the factors accounting for individual differences in the susceptibility to this movement disorder. There is large consensus that dyskinesia is caused by two interacting factors: striatal DA denervation and pulsatile treatment with l-DOPA. These two factors in combination lead to (1) dysregulated DA transmission, (2) secondary changes in non-dopaminergic transmitter systems, (3) abnormal intracellular signaling and synaptic plasticity in striatal neurons and (4) altered activity patterns in the basal ganglia output pathways. We have reviewed the full extent of this pathophysiological cascade in recent articles (Cenci, 2007, Cenci, 2009 and Cenci and Lindgren, 2007). The present chapter will zoom in on the third layer of alterations, namely, molecular changes in striatal neurons. We shall first summarize basic mechanisms of DA receptor-dependent signaling in the intact and DA-denervated striatum. We shall next review intracellular signaling responses and patterns of gene expression that have been found to correlate with LID in animal models. Finally, we shall integrate the main findings into a unifying interpretation of this movement disorder.
1.1 Dopamine receptor signaling in the neurons of the intact striatum: modulation of cyclic AMP/PKA pathways and non-canonical signaling
Before examining the molecular adaptations associated with LID, it is helpful to provide an overview of the signal transduction mechanisms and intracellular events that follow the interaction of DA with its receptors in the intact brain. Molecular cloning has revealed five DA receptors as the products of different genes (Grandy and Civelli, 1992 and Lachowicz and Sibley, 1997). All DA receptors are coupled to G proteins, and pharmacological studies have helped to group these receptors into two main classes: on one hand, D1-like DA receptors (D1, D5) and, on the other hand, D2-like receptors (D2, D3, D4), which are oppositely linked to adenylyl cyclase enzyme(s) and to the production/reduction of cyclic AMP (cAMP) (Sibley and Monsma, 1992). D1-like receptors increase cAMP levels and activate protein kinase A (PKA), whereas D2-like receptors decrease cAMP levels as well as PKA activity (Robinson and Caron, 1997, Sibley et al., 1998 and Vallar and Meldolesi, 1989). D1 and D2 receptors are abundantly expressed in the striatum, whereas the other members of the D1 and D2 subfamilies predominate in mesocorticolimbic structures [reviewed in (Sibley and Monsma, 1992) and (Strange, 1993)].
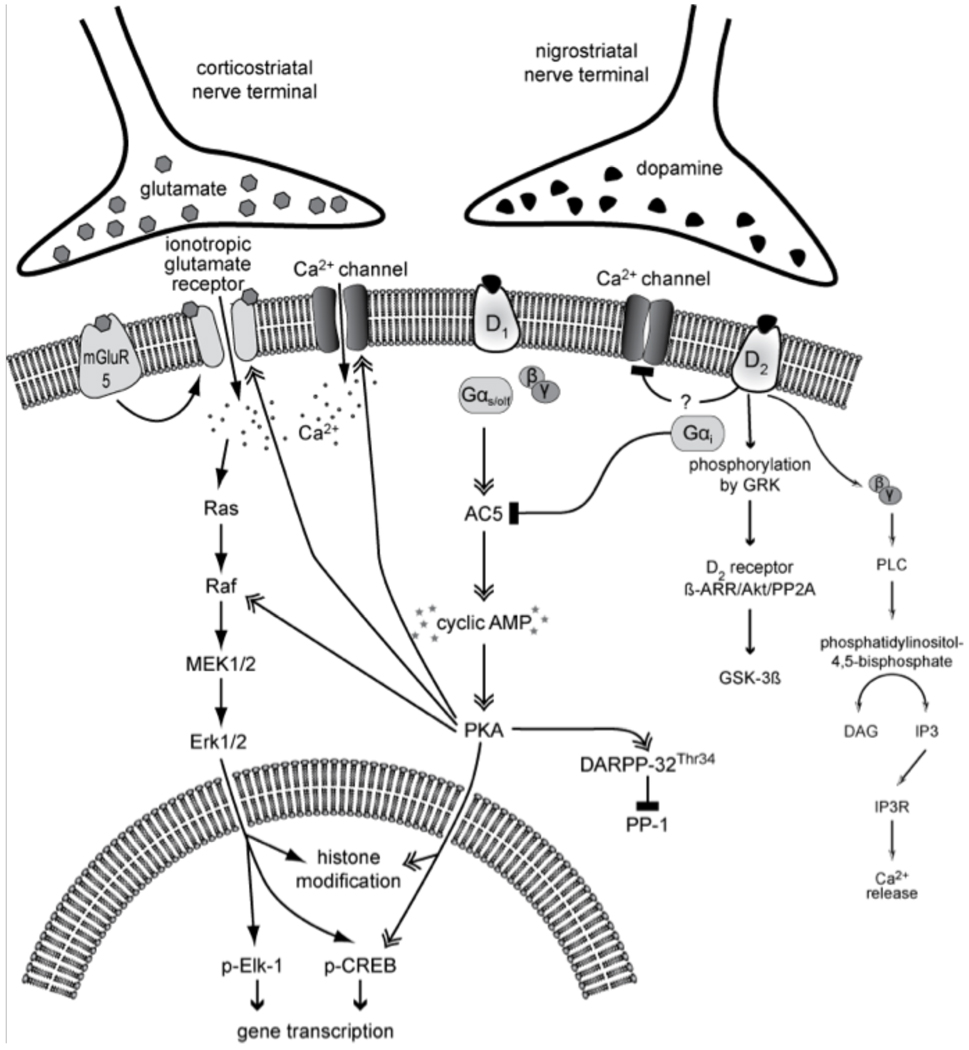
In the striatum, D1 and D2 receptors are largely segregated into the two main classes of efferent neurons (medium-sized spiny neurons, MSN) that give rise to the ‘direct’ and ‘indirect’ striatofugal pathways, respectively (Gerfen, 1992) [see also Surmeier et al. (2010), this volume]. Thus, these two neuronal populations are differently affected by DA, with PKA activity induced in one subset of neurons and repressed in the other (Fig. 1). Activated PKA phosphorylates surrounding proteins that are critical to neuronal plasticity and gene expression (Schulman, 1995). In particular, activation of PKA by D1 receptors has facilitatory effects on N-methyl-d-aspartate (NMDA) receptors, amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)/kainate receptors and l-type Ca2+ channels (Cepeda and Levine, 1998, Cepeda and Levine, 2006, Cepeda et al., 1993, Chao et al., 2002, Dudman et al., 2003, Gray et al., 1998 and Konradi et al., 1996). Calcium entry through NMDA receptors or l-type Ca2+ channels can in turn activate calcium-dependent kinases, such as CaM kinase II and IV (Ghosh et al., 1994 and Ginty, 1997). PKA as well as CaM kinases can phosphorylate the transcription factor cAMP response element-binding protein (CREB) on Serine 133, inducing the expression of immediate early genes (c-fos), and opioid precursor genes, such as prodynorphin (preproenkephalin-B) or preproenkephalin (preproenkephalin-A) (Cole et al., 1995, Dudman et al., 2003, Ghosh et al., 1994 and Rajadhyaksha et al., 1999) (Fig. 1). Because of their cellular location and effect on PKA, D1 receptor agonists induce the expression of immediate early genes and prodynorphin in ‘direct pathway’ MSN. In ‘indirect pathway’ neurons, immediate early genes and opioid precursor genes (preproenkephalin-A) are induced by D2 receptor antagonists (Konradi et al., 1996, Leveque et al., 2000 and Robertson et al., 1992). It can thus be assumed that normal signaling downstream of D1 and D2-like receptors is essential to maintain the distinctive gene expression patterns and physiological responses of ‘direct pathway’ and ‘indirect pathway’ MSN.
Figure 1. Canonical and non-canonical signaling cascades downstream of D1 and D2 dopamine receptors.
Being coupled to stimulatory (Gs/olf) and inhibitory (Gi/o) GTP-binding proteins, D1 and D2 receptors have opposite effects on the adenylyl cyclase/cAMP/PKA/DARPP-32 cascade (‘canonical pathway’), which regulates the levels of phosphorylation of multiple cellular and nuclear targets. In particular, the cAMP/PKA/DARPP-32 pathway modulates the activity of MAPK-dependent signaling pathways downstream of glutamate receptors. It has been recently recognized that cAMP-independent pathways are also recruited following D2 receptor stimulation (non-canonical pathways; cf. Section ‘DA receptor signaling in the neurons of the intact striatum: modulation of cAMP/PKA pathways and non-canonical signaling’). Full names of the molecules shown in this drawing are given in the list of abbreviations.
Inactivation of DA receptors is an active process. After interaction with their ligand, DA receptors get phosphorylated by G-protein-coupled receptor kinases (GRKs), which is followed by their binding to arrestin (Claing et al., 2002). Arrestin binding blocks further G-protein-mediated signaling and leads to receptor internalization. Like other G-protein-coupled receptors (GPCRs), DA receptors are subjected to endocytosis (Ariano et al., 1997, Kim et al., 2008, Paspalas et al., 2006, Vargas and Von Zastrow, 2004 and Xiao et al., 2009). An insufficient internalization of DA receptors has recently emerged as an important mechanism in LID and will be further discussed below (see Section ‘Altered intracellular trafficking of DA and glutamate receptors’).
Beside their role in receptor internalization, arrestins are involved in a non-canonical (cAMP/PKA-independent) signaling pathway downstream of D2 receptors. Activation of D2 receptors leads to the formation of a protein complex composed of β-arrestin, protein kinase B (Akt and protein phosphatase 2A (PP2A) (Beaulieu et al., 2005). The formation of this complex results in the dephosphorylation/inactivation of Akt by PP2A and subsequent stimulation of glycogen synthase kinase 3 (GSK3)-mediated signaling (Beaulieu et al., 2005) (Fig. 1). GSK3 is a regulator of many cellular functions, including cell architecture, motility and survival (Jope and Johnson, 2004). Thus, even though DA stimulation of D2 receptors decreases the levels of PKA activity in ‘indirect pathway’ MSN, it can promote DA-dependent adaptations through the activation of this non-canonical signaling pathway, as has been shown to occur following administration of amphetamine and apomorphine (Beaulieu et al., 2007). Inhibition of D2 receptors with anti-psychotic drugs, a situation that is akin to the loss of DA in PD, prevents D2/beta arrestin 2-mediated signaling (Masri et al., 2008). These results warrant future investigations on the role of β-arrestin/Akt/PP2A in the pathophysiology of both parkinsonism and LID. In fact, recent data from a non-human primate model of LID lend support to the hypothesis that striatal Akt/GSK3 signaling may be involved in the development of the movement disorder (Morissette et al., 2010). An additional pathway deserving a closer look is one linking D2 receptors to a mobilization of intracellular calcium stores. The coupling of D2 receptors to Gi/o proteins causes both an inhibition of adenylyl cyclase (through the Gαi subunit) and a release of Gβγ subunits, which are capable of stimulating phospholipase Cβ isoforms, leading to production of diacylglycerol (DAG) and inositol trisphosphate (IP3), followed by mobilization of intracellular Ca2+ stores (Fig. 1) (Hernandez-Lopez et al., 2000). A possible role of this pathway in the cellular adaptations associated with PD and LID has not yet been explored.
DA receptor-mediated changes in gene expression necessitate the re-packaging of histones along the DNA. Tightly packed histones prevent transcription factors from binding to the DNA. Modifications on the DNA (such as methylation of CpG islands) and post-translational modifications of histone proteins affect the histone–DNA interaction and the ability of transcription factors to transactivate a promoter. These epigenetic mechanisms have received much attention in the last couple of years. Histones are subject to a wide variety of post-translational modifications including lysine acetylation, lysine and arginine methylation, serine and threonine phosphorylation, lysine ubiquitination and sumoylation (Vaquero et al., 2003). A host of protein-modifying enzymes such as histone acetyltransferases, histone deacetylases, histone methyltransferases or kinases help to organize the histone structure and contribute to cell-specific gene expression patterns. DA receptor-mediated signal transduction pathways can selectively activate or repress these modifying enzymes and thus trigger chromatin remodelling (Kumar et al., 2005 and Schroeder et al., 2008).
1.2 DA receptor signaling in the DA-denervated striatum
As striatal DA levels decline in PD (Bernheimer et al., 1973 and Hornykiewicz, 1975), allostatic changes take place in the nigrostriatal system. Adaptive mechanisms are employed to maintain stability and functionality including a reduced rate of DA inactivation and increased DA synthesis and turnover in residual DA neurons (Zigmond et al., 1990), as well as changes in post-synaptic DA receptor sensitivity (Mishra et al., 1974 and Staunton et al., 1981). These adaptive mechanisms can compensate for a large decline in DA concentrations, accounting for the fact that manifest parkinsonian motor symptoms only occur when the loss of nigral DA neurons exceeds 50% (Fearnley and Lees, 1991). Post-synaptic adaptations affecting DA receptors and their downstream signaling mechanisms are particularly relevant to this chapter, because denervation-induced supersensitivity of striatal DA receptors has long been regarded as an important determinant of LID (Klawans et al., 1977, Marconi et al., 1994 and Nutt, 1990). This supersensitivity does not necessarily stem from an increased receptor number, because no consistent changes in ligand-binding activities at D1, D2 or D3 receptors have been found in dyskinetic PD patients (Hurley et al., 1996, Rinne et al., 1991 and Turjanski et al., 1997), and findings from animal models have been contradictory, particularly with respect to D1 (cf. Gerfen et al., 1990 and Konradi et al., 2004) and D3 receptors (cf. Bezard et al., 2003, Hurley et al., 1996, Mela et al., 2010 and Quik et al., 2000). Although increased receptor expression at the plasma membrane may contribute to D1 supersensitivity (Guigoni et al., 2007), denervation-induced supersensitivity of DA receptors is generally attributed to altered signal transduction mechanisms in the dopaminoceptive neuron (Gerfen, 2003, Pifl et al., 1992, Prieto et al., 2009 and Zhen et al., 2002). The following alterations have been documented to occur in the DA-denervated striatum: (1) aberrant activation of non-canonical signaling cascades downstream of D1 receptors; (2) exuberant activation of canonical signaling pathways following D1 and D2 receptor stimulation and (3) reduced expression of negative signaling modulators.
The most relevant example of non-canonical signaling pathway activation was provided by Gerfen and collaborators, reporting that treatment with D1 receptor agonists induces mitogen-activated protein kinases (MAPK) in striatonigral neurons in the DA-denervated but not the intact striatum (Gerfen et al., 1990). Because this signaling alteration is particularly relevant to LID, it will be extensively discussed in the following paragraphs.
Hyperactive canonical signaling seems to depend on an increased coupling of both D1 and D2 receptors to their G proteins. The G-protein-coupling efficiency of striatal DA receptors has been studied in both rat and monkey models of PD by measuring DA agonist-induced guanosine 5'-O-(gamma[35S]thio)triphosphate ([35S]GTP-gammaS) binding. These studies have shown that DA denervating lesions result in increased agonist-induced G-protein-binding activity at both D2- and D1-type receptors (Aubert et al., 2005, Cai et al., 2002 and Geurts et al., 1999) and that the G-protein-coupling activity of D1, but not D2 receptors, is further enhanced in LID (Aubert et al., 2005). The increased guanosine triphosphate (GTP)-binding activity of D1 receptors is paralleled by an upregulation of Gα-olf proteins (Corvol et al., 2004 and Herve et al., 1993) and by a pronounced overactivity of the D1 receptor-adenylyl cyclase signal transduction pathway (Mishra et al., 1974 and Pifl et al., 1992).
The duration and extent of DA receptor activation is limited not only by internalization processes (discussed at Section ‘Altered intracellular trafficking of DA and glutamate receptors’) but also by a range of negative signaling modulators, whose expression and efficiency seem to be altered in the DA-denervated striatum (Geurts et al., 2003, Harrison and LaHoste, 2006 and Zhen et al., 2002). Relevant to this chapter are the changes found in a family of proteins named regulators of G-protein signaling (RGS), which promote GTP hydrolysis by the alpha subunit of heterotrimeric G proteins, thereby inactivating the G protein and rapidly switching off GPCR signaling pathways (De Vries et al., 2000). A selective decrease in RGS4 and RGS9 mRNA levels occurs in the rat striatum following DA denervation (Geurts et al., 2003), and this change might predispose to overactive DA receptor signaling, hence to LID. In keeping with this hypothesis, viral vector-mediated overexpression of RGS9-2 in the striatum reduces the severity of l-DOPA-induced AIMs in both rat and monkey models of PD (Gold et al., 2007).
2. Molecular alterations associated with LID
What happens when DA formed from exogenous l-DOPA acts on supersensitive receptors in the parkinsonian striatum? A rapidly growing literature indicates that the molecular responses to DA differ greatly between animals exhibiting dyskinesia and those showing a normal pattern of motor activation. Prominent molecular differences between the two response categories have been found at all levels of investigation, as will be explained below.
2.1 Altered intracellular trafficking of DA and glutamate receptors
Both DA denervation and l-DOPA treatment can alter GPCR desensitization and internalization, and important differences have been observed between dyskinetic and non-dyskinetic subjects in both rodent and non-human primate models of PD. In the striatum of non-human primates treated with MPTP, D1 receptor density at the plasma membrane is increased and becomes further increased in LID (Guigoni et al., 2007), while D2 receptor distribution is only modestly affected. In the rat model of LID, an exaggerated expression of D1 receptors at the cell membrane correlates with the severity of the treatment-induced AIMs (Berthet et al., 2009). Importantly, a recent study has shown that the internalization of D1 receptors in striatal neurons can be enhanced by lentiviral over-expression of GRK6 and that this intervention alleviates LID in both rat and non-human primate models of PD (Ahmed et al., 2010). These observations indicate that therapeutic strategies increasing D1 receptor internalization may have a useful role in the future treatment of LID.
In addition to the D1 receptor, ionotropic glutamate receptors show altered subcellular localization in LID. This phenomenon has been studied extensively with respect to the NMDA receptor complex in both monkey and rat models of LID. In drug-naïve MPTP-treated macaques, NR1 and NR2B subunits were found to be significantly decreased in synaptosomal membranes, while the abundance of NR2A was unaltered (Hallett et al., 2005). Dyskinesiogenic l-DOPA treatment normalized the abundance of NR1 and NR2B and raised NR2A levels significantly above unlesioned control values. These results led to the suggestion that a relative enhancement in the synaptic abundance of NR2A is implicated in LID (Hallett et al., 2005). Studies in 6-OHDA-lesioned rats have emphasized the role of NR2B, showing that the receptor-trafficking alteration most critically associated with LID consists in a re-distribution of NR2B subunits between synaptic and extrasynaptic membranes (Fiorentini et al., 2006 and Gardoni et al., 2006). Protein complexes consisting of D1-NMDA receptor subunits exhibited a similar re-distribution in chronically l-DOPA-treated dyskinetic rats (Fiorentini et al., 2006). The phenomenon was attributed to an altered interaction of NR2B with post-synaptic scaffolding proteins in striatal neurons [see Gardoni et al. (2010), this volume]. Following DA denervation, the synapse-associated scaffolding proteins, PSD-95, SAP-97 and SAP102, appear to be shifted from the post-synaptic membrane into other subcellular compartments (Gardoni et al., 2006 and Nash et al., 2005). Although chronic l-DOPA treatment tends to restore the synaptic levels of these proteins, the restoration is more pronounced in non-dyskinetic animals compared to dyskinetic ones (Gardoni et al., 2006).
Recently, an altered trafficking of AMPA receptor subunits also has been associated with LID. In a study performed on MPTP-lesioned and l-DOPA-treated macaques, dyskinetic animals were found to exhibit a re-distribution of AMPA receptors, and particularly of the GluR2/3 subunit, from the vesicular fraction into the post-synaptic membrane (Silverdale et al., 2010). The increased relative abundance of AMPA receptor subunits in the post-synaptic membrane was suggested to render striatal neurons more sensitive to glutamate (Silverdale et al., 2010), providing a rationale to the use of AMPA receptor antagonists in the treatment of LID. This suggestion is indeed supported by independent behavioural pharmacological studies in both rat and nonhuman primate models of PD (Kobylecki et al., 2010 and Konitsiotis et al., 2000). Further studies are, however, required to clarify how an altered subcellular distribution of ionotropic glutamate receptor subunits affects the intrinsic excitability and synaptic responses of striatal neurons in LID. Moreover, it will be important to identify key common upstream mechanisms and downstream effectors. These studies will guide the choice of the most appropriate target in the design of future anti-dyskinetic therapies.
2.2 Altered intracellular signaling
Stimulation of supersensitive DA receptors by l-DOPA would be expected to result in a large activation and inhibition, respectively, of cAMP-dependent signaling pathways in D1- and D2-rich striatal neurons. While the contribution of D2-rich, ‘indirect pathway’ MSN to LID has not yet been elucidated, the overactivity of D1-mediated signaling in ‘direct pathway’ MSN has indeed been shown to play a major role in LID across all animal models so far examined (mouse, Darmopil et al., 2009 and Santini et al., 2007; rat, Lindgren et al., 2010 and Westin et al., 2007; and macaque, Aubert et al., 2005). Chronically l-DOPA-treated, dyskinetic rats and mice show increased striatal phosphorylation of DA- and cAMP-regulated phosphoprotein of 32 KDa (DARPP-32) at the threonine-34 residue (Picconi et al., 2003 and Santini et al., 2007). Phosphorylation of DARPP-32 at threonine 34 is induced by l-DOPA through the stimulation of D1 receptors and PKA (Lebel et al., 2010). Because phospho-Thr34-DARPP32 is a potent inhibitor of protein phosphatase-1 (PP-1) (Svenningsson et al., 2004), it is not surprising that the striatal levels of several phosphorylated substrates are elevated in the ‘dyskinetic’ striatum. Particularly important substrates include subunits of NMDA (Chase and Oh, 2000 and Dunah et al., 2000) and AMPA receptors (Santini et al., 2007), ERK1/2 (Pavon et al., 2006, Santini et al., 2007 and Westin et al., 2007) and the downstream targets of ERK1/2, including molecules involved in the regulation of protein translation (Santini et al., 2009) and gene transcription (Santini et al., 2009 and Westin et al., 2007). As extensively discussed below (see Section ‘Studies of signaling pathway activation in dyskinetic rodents reveal a common pattern of alterations’), the phosphorylated forms of all these molecules are significantly increased in abundance in l-DOPA-treated dyskinetic animals compared to non-dyskinetic cases (see also Fig. 1).
A large body of recent studies has addressed the role of Ras-ERK1/2 signaling in LID, pointing to this pathway as an important target for future anti-dyskinetic treatments. Extracellular signal-regulated kinases 1 and 2 (ERK1/2) belong to the MAPK family of signaling cascades, which share the motif of three serially linked kinases regulating each other by sequential phosphorylation (Seger and Krebs, 1995). The MAPK signaling system was originally discovered as a critical regulator of cell division and differentiation and was later found to be recruited in mature neurons by different types of extracellular stimuli inducing long-term synaptic and behavioural adaptations (Sweatt, 2001). In both rat (Westin et al., 2007) and mouse models of PD (Santini et al., 2007), phosphorylated ERK1/2 is detected in striatal MSN following the administration of l-DOPA, and the levels of this phosphoprotein correlate positively with the l-DOPA-induced AIMs scores. The time course of ERK1/2 phosphorylation in dyskinetic animals indicate that the kinase is activated in striatal MSN by each dose of l-DOPA and remains active for at least 120 min post-dosing (Westin et al., 2007), which is an unusually long interval (cf. Valjent et al., 2000). An involvement of ERK1/2 in LID is demonstrated by the anti-dyskinetic effects of treatments that inhibit upstream components of the Ras-ERK signaling cascade (Lindgren et al., 2010, Santini et al., 2007 and Schuster et al., 2008). Moreover, in a recent comparison of compounds targeting different types of glutamate receptors, we found that the only pharmacological agents exerting significant anti-dyskinetic effects were those capable of reducing l-DOPA-induced phospho-ERK1/2 levels in the striatum (namely, selective antagonists of group I metabotropic glutamate receptors, Rylander et al., 2009). The most potent pharmacological means to suppress l-DOPA-induced phospho-ERK1/2 is represented by D1-like receptor antagonists (Westin et al., 2007), indicating that the activation of ERK1/2 by l-DOPA is mediated by D1 receptors. Unfortunately, D1 antagonists do not have any clinical utility in PD, because they would interfere with the therapeutic action of l-DOPA. It is therefore important to identify non-dopaminergic treatments that can normalize ERK1/2-mediated signaling without compromising the beneficial effects of l-DOPA.
How does Ras-ERK signaling contribute to LID? As a mediator of synaptic and behavioural plasticity, this pathway is assumed to contribute to persistent neural adaptations that maintain the brain in a dyskinesia-prone state (Cenci and Lindgren, 2007). Indeed, Ras-ERK1/2 signaling has been shown to mediate the following, long-lasting cellular adaptations in animal models of LID: (1) nuclear signaling responses in striatal neurons, such as activation of histone kinases, histone modifications and induction of nuclear transcription factors (Santini et al., 2007, Santini et al., 2009 and Westin et al., 2007); (2) striatal regulation of the mammalian target of rapamycin complex 1 (mTORC1), which is in turn involved in the control of protein translation and synaptic plasticity (Santini et al., 2009) and (3) endothelial proliferation and angiogenic activities in the basal ganglia (Lindgren et al., 2009).
In addition to mediating long-lasting effects of l-DOPA treatment, the activation of Ras-ERK1/2 signaling seems to contribute to the acute expression of dyskinesia. Indeed, using a short drug treatment regimen (3 days) in 6-OHDA-lesioned rats, we found that an inhibitor of the ERK1/2 upstream kinase (mitogen-activated protein kinase kinase, MEK) acutely reduced the severity of l-DOPA-induced AIMs (Lindgren et al., 2009). The acute consequences of ERK1/2 activation may rely on the phosphorylation of membrane-bound receptors, ion channels and synaptic proteins (Sweatt, 2001), but the targets involved are as yet unknown. This gap of knowledge is not surprising. While long-term adaptations contributing to the development of LID have been intensely investigated, we know virtually nothing about the molecular and electrophysiological activities mediating the acute expression of dyskinetic movements after each dose of l-DOPA. Further investigations are thus needed to unravel the ‘electrophysiological signature’ of LID in the striatum, ranging from changes in conductances and synaptic responses in D1-versus D2-positive MSN, to activity patterns at the single-unit and neuronal ensemble level.
2.3 Altered expression and regulation of transcription factors
As explained above (see Section ‘DA receptor signaling in the neurons of the intact striatum: modulation of cyclic AMP/PKA pathways and non-canonical signaling’), phosphorylation of the nuclear protein, CREB at Serine 133 mediates changes in gene expression downstream of DA receptors. Phosphorylated CREB binds to the cAMP responsive element (CRE) and to the closely related activator protein-1 (AP-1) site in the promoter region of many genes, including c-fos and the opioid precursor genes, preproenkephalin and prodynorphin (Cole et al., 1995, Konradi et al., 1993 and Konradi et al., 1994). Prodynorphin is of particular interest because its upregulation after chronic l-DOPA treatment is a consistent correlate of LID across species and parkinsonian conditions (Aubert et al., 2007, Cenci et al., 1998, Henry et al., 2003 and Lundblad et al., 2004). The upregulation of prodynorphin mRNA by l-DOPA, which depends on the D1 receptor (St-Hilaire et al., 2005), concurs with reduced levels of opioid receptor binding in the projection targets of ‘direct pathway’ MSN (Aubert et al., 2007 and Johansson et al., 2001), which is indicative of a hyperactive opioid transmission along this pathway. While CREB mediates D1-dependent prodynorphin transcription in the intact striatum (Cole et al., 1995), the same protein is not required for the induction of prodynorphin by l-DOPA in the DA-denervated striatum (Andersson et al., 2001). In the latter situation, CRE/AP-1 elements in the prodynorphin promoter are bound and transactivated by ΔFosB-related proteins and JunD (Andersson et al., 2001). This switch in transcriptional control is associated with a large increase in the striatal levels of ΔFosB-like proteins, which correlates positively with dyskinesia severity (Andersson et al., 1999). Unlike other Fos family proteins, ΔFosB and its post-translationally modified forms are uniquely stable (Carle et al., 2007). Accordingly, the upregulation of ΔFosB-like proteins and prodynorphin mRNA by chronic l-DOPA treatment shows a strikingly protracted time course. The expression of both markers rises gradually during a 2-week course of l-DOPA administration (Andersson et al., 2001 and Valastro et al., 2007), maintaining high levels of expression for up to 1 year (Westin et al., 2001), which is the longest period of l-DOPA administration so far examined. Following discontinuation of l-DOPA treatment, the striatal levels of ΔFosB and prodynorphin mRNA remain significantly elevated for weeks (Andersson et al., 2003). Intrastriatal infusion of anti-sense oligonucleotides targeting fosB/ΔfosB mRNA attenuates the gradual increase in dyskinesia severity induced by repeated l-DOPA administration and the associated upregulation of prodynorphin mRNA (Andersson et al., 1999). These results indicate that ΔFosB-like transcription factors induce and maintain changes in striatal gene expression that favour the expression of dyskinesia. In keeping with this contention, molecular interventions that reduce the transcriptional activity of ΔFosB have been found to attenuate the severity of already established LID in a non-human primate model of PD (Berton et al., 2009).
2.4 Altered plasticity of corticostriatal synapses
The idea that LID is caused by abnormal plasticity of corticostriatal synapses was proposed 10 years ago (Calabresi et al., 2000 and Calon et al., 2000) and has remained very popular ever since (Jenner, 2008). The first evidence of altered activity-dependent plasticity of corticostriatal synapses was provided by Picconi et al. (2003). This study compared the inducibility and reversal of corticostriatal long-term potentiation (LTP) in brain slices from l-DOPA-treated, dyskinetic or non-dyskinetic rats. High-frequency stimulation of cortical afferents was found to induce a normal LTP in both groups, but dyskinetic rats showed a lack of depotentiation upon subsequent low-frequency stimulation of the same afferent pathway (Picconi et al., 2003). This loss of bidirectional synaptic plasticity points to aberrant gating of cortical inputs in the dyskinetic striatum. If corticostriatal LTP cannot be promptly reversed, striatal neurons would be unable to ‘erase’ irrelevant information when processing cortically driven motor commands. The mechanisms accounting for the loss of synaptic depotentiation in dyskinetic animals have not been completely resolved. The alteration was originally attributed to an overactive signaling downstream of D1 receptors and ensuing hyperphosphorylation of DARPP-32, leading to persistent inhibition of intracellular phosphatases (Picconi et al., 2003). Further investigations are, however, required to clarify which phosphorylated substrates, and upstream kinases, impede the reversal of LTP. It will also be important to verify the relative involvement of ‘direct pathway’ versus ‘indirect pathway’ MSN in this synaptic abnormality, an issue that has not been addressed thus far.
2.5 Altered gene expression patterns
The pattern of striatal mRNA expression differentiating l-DOPA-treated, dyskinetic animals from non-dyskinetic ones was investigated by Konradi and collaborators using Affymetrix gene chip arrays (Konradi et al., 2004). In this study, rats with 6-OHDA lesions were given a therapeutic dose of l-DOPA, or saline, for 21 days and killed 18 h after the last injection. l-DOPA-treated rats were classified as dyskinetic or non-dyskinetic based on the AIMs scores recorded during the treatment. The most salient features of the mRNA expression profile associated with dyskinesia indicated increased transcriptional activity of GABAergic neurons, structural and synaptic plasticity, altered calcium homeostasis and calcium-dependent signaling and an imbalance between metabolic demands and capacity for energy production in the striatum. More specifically, genes coding for ion transporters, calcium-dependent ATPases and voltage-gated ion channels were upregulated in the dyskinetic striatum, a pattern indicative of increased neuronal activity and high ATP consumption. Accordingly, dyskinetic rats showed upregulation of the mitochondrial gene, cytochrome oxidase subunit I (CO-I), which is a marker of increased metabolic demands in neurons. At the same time, dyskinetic animals showed significant downregulation of genes encoding two key enzymes of the phosphocreatine pathway, guanidinoacetate methyltransferase and ubiquitous mitochondrial creatine kinase (Mi-CK), a finding that was later confirmed with proteomics methods (Valastro et al., 2007). Dyskinetic rats also had reduced expression of genes coding for glyceraldehyde-3-phosphate dehydrogenase and lactate dehydrogenase, both of which are involved in energy production through the glycolytic pathway. Several genes encoding ribosomal proteins were downregulated in dyskinetic rats, a pattern suggestive of cellular stress (Warner, 1999). These results were the first to indicate that dyskinesiogenic treatment with l-DOPA elevates the metabolic demands of striatal neurons but downregulates pathways involved in energy production in the striatum.
A more recent micro-array study focused instead on a comparison between acutely and chronically l-DOPA-treated rats (El Atifi-Borel et al., 2009). Here, the l-DOPA dose was above threshold for the induction of dyskinesia in all animals. Although acute and chronic l-DOPA treatment were found to regulate a common set of genes involved in signal transduction, transcription and synaptic transmission, a threefold larger number of genes were altered in response to repeated drug administration. The difference between acute and chronic l-DOPA treatment was particularly noticeable for genes involved in metabolism, protein biosynthesis (ribosomal genes), neurite outgrowth, synaptogenesis and cell proliferation. This study pointed to a relationship between repeated l-DOPA exposure and structural cellular modifications in the striatum. Although l-DOPA can induce dyskinesia even upon its first administration (Nadjar et al., 2009), chronic treatment with l-DOPA is required to establish a long-lasting predisposition to this movement disorder. In fact, repeated administration of l-DOPA induces a gradual increase in dyskinesia severity over time and also reduces the dyskinetic threshold dose of the drug (Cenci and Lundblad, 2006). This delayed effect, often referred to as the ‘dyskinesia-priming action of l-DOPA’, conceivably relies on structural modifications of cells and synapses in the brain.
3. Towards a unifying molecular interpretation of LID
The literature reviewed above has revealed a large number of molecular alterations that are linked to the expression and development of LID and uncovered therapeutic targets at many possible levels. Further studies are required to shed light on the mechanisms through which these molecular changes alter the function of cells and circuits within the basal ganglia. Based on the current information, it is however possible to outline some general molecular features of LID, and these will be the object of the following paragraphs.
3.1 Studies of signaling pathway activation in dyskinetic rodents reveal a common pattern of alterations
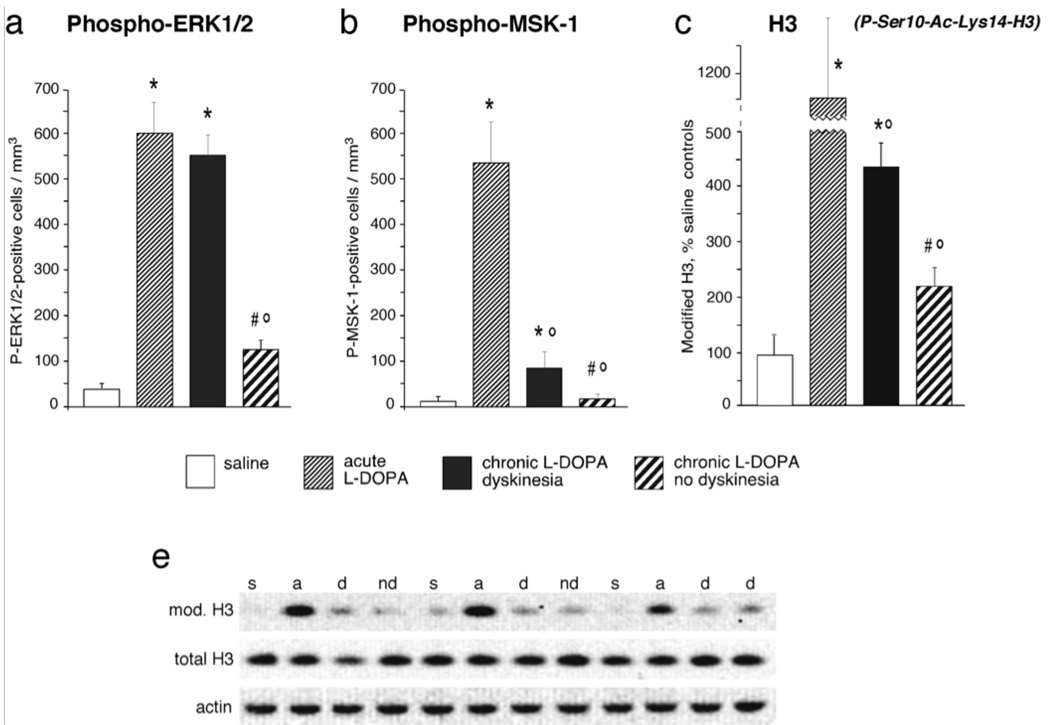
As mentioned above (cf. Sections ‘Altered intracellular signaling’ to ‘Altered expression and regulation of transcription factors’), nuclear signaling responses to l-DOPA are more exuberant in dyskinetic animals compared to non-dyskinetic subjects. How does this difference come about? Important clues have emerged from a comparison between chronically and acutely l-DOPA-treated rats (Andersson et al., 2001, Cenci et al., 1999, Valastro et al., 2007 and Westin et al., 2007), and further unpublished data by Konradi and Cenci (Fig. 2). In these studies, rats with 6-OHDA lesions were given a therapeutic dose of l-DOPA either on one single occasion (acute treatment) or repeatedly for 10–14 days, during which they were monitored on the AIMs scale. As indicators of intracellular signaling activation we examined the levels of phosphorylated (Thr 34) DARPP-32 (not shown), ERK1/2 and mitogen- and stress-activated kinase-1 (MSK-1, nuclear target of ERK), and the histone modification, phospho(Ser10)-Acetyl(Lys14)-histone 3, which has been linked to chromatin remodelling during active gene transcription (Cheung et al., 2000). A common pattern of group differences emerged from the analysis of each of the markers under investigation. Acute l-DOPA treatment caused a large activation of signaling molecules in the DA-denervated striatum in all animals. Chronic administration of l-DOPA abolished this upregulation in animals that had remained free from dyskinesia during the treatment. By contrast, rats that had developed dyskinesia showed overtly sensitized (e.g. ERK1/2) or only partially densensitized responses to the last injection of l-DOPA, with levels of phosphoproteins that were significantly elevated above control values in each of the comparisons. Similar results have been recently reported from a mouse model of LID (Santini et al., 2007). Overall, these data indicate that chronic treatment with l-DOPA would tend to normalize supersensitive signaling responses in DA-denervated striatal neurons, but fails to achieve this effect in dyskinetic subjects. Because upregulation of the above phosphoproteins by l-DOPA relies on the D1 receptor (see Sections ‘Altered intracellular signaling’ and ‘Altered expression and regulation of transcription factors’), we conclude that the core molecular alteration associated with LID consists in the inability of striatal neurons to desensitize adenylyl cyclase-dependent and MAPK-dependent signaling cascades downstream of D1 receptors. The mechanisms underlying such an inability have not been resolved and may be related to a deficient internalization of D1 receptors (Berthet et al., 2009). Surface receptor availability is not, however, the only mechanism regulating the duration of downstream signaling responses, and the possibility that intracellular signaling inhibitors are less efficient in dyskinetic animals ought to be addressed in future studies. In fact, the striatal gene expression profile detected in dyskinetic rats by Konradi and collaborators (Konradi et al., 2004) pointed to an altered expression/activity of intracellular phosphatases, while also revealing upregulation of positive upstream modulators of Ras-ERK signaling. In particular, RAS guanyl nucleotide-releasing protein 1 (RasGRP1) was strongly upregulated in dyskinetic rats relative to non-dyskinetic rats and controls (Konradi et al., 2004). RasGRP1 (also called CalDAG-GEF2) is a DAG-regulated nucleotide exchange factors that activates Ras and the Erk/MAP kinase cascade through the exchange of guanosine diphosphate (GDP) for GTP (Yang and Kazanietz, 2003). A closely related protein, RasGRP2 (CalDAG-GEFI), activates Rap1A and inhibits Ras-dependent activation of the ERK/MAP kinase cascade (Kawasaki et al., 1998). A recent study has addressed the regulation of RasGRP1 and RasGRP2 gene expression in the rat model of LID (Crittenden et al., 2009). In the DA-denervated striatum, l-DOPA treatment produced upregulation of RasGRP1 and downregulation of RasGRP2, and both of these effects correlated significantly with the severity of the AIMs. These data are consistent with an overactivity of Ras-ERK1/2 signaling in the dyskinetic striatum (cf. Section ‘Altered intracellular signaling’) and point to the dysregulation of RasGRP1 (CalDAG-GEF2) and RasGRP2 (CalDAG-GEFI) as being an important upstream mechanism. These data warrant a search for therapeutic strategies targeting striatum-enriched Ras-ERK1/2 signaling modulators. This approach may relieve LID while avoiding the many potential side effects of a complete, systemic blockade of MAPK signaling.
Figure 2. Deficient densensitization of DA receptor-dependent signaling in the dyskinetic striatum.
Acute treatment with l-DOPA results in a supersensitive activation of cAMP- and MAPK-dependent pathways signaling to the nucleus, here exemplified by the phosphorylation of ERK1/2 (on Thr202-Tyr204), MSK-1 (on Ser376) and by a modification of histone 3 that has been linked to chromatin remodelling during active gene transcription (phospho[Ser10]-Acetyl[Lys14]-H3). Chronic l-DOPA administration normalizes these supersensitive responses only in animals that remain free from dyskinesia during the treatment (non-dyskinetic group). Levels of signaling pathway activation remain significantly elevated above control values in dyskinetic animals. Thus, a shift towards normal molecular responses during chronic l-DOPA treatment is associated with a resistance to dyskinesia, while persistent supersensitivity is a correlate of LID. These data show both published and unpublished results obtained from 6-OHDA-lesioned rats, which received chronic (10–12 days) or acute treatment with l-DOPA (l-DOPA methyl ester, 10 mg/kg/dose combined with 15 mg/kg/dose of benserazide), or physiological saline, and were killed 30 minutes post injection. Data in (a) and (b) represent counts of immunoreactive neurons in the lateral part of the DA-denervated caudate–putamen, from animals reported in Westin et al. (2007), and five chronically l-DOPA-treated non-dyskinetic rats from the same study (not reported in the paper). Data in (c) show results from Western immunoblotting analysis. The optical density on specific immunoreactive bands was normalized to the corresponding β-actin bands, and results from each group were expressed as a percentage of saline control values. A Western blot showing bands immunoreactive for phospho[Ser10]-Acetyl[Lys14]-histone 3, total histone 3 and beta-actin is shown in (d). Full descriptions of the experimental procedures can be found in Westin et al. (2007) (for a, b) and Schroeder et al. (2008) (for c, d). Abbreviations in (d): s, saline control; a, acute l-DOPA; d, chronic l-DOPA/dyskinesia; nd, chronic l-DOPA/no dyskinesia). Values indicate group means ± SEM from 3 to 10 animals per group, which were statistically compared using one-factor analysis of variance and post-hoc Newman– Keuls test. P < 0.05 vs. *, saline-treated 6-OHDA-lesioned controls; °, acute l-DOPA; #, chronic l-DOPA with dyskinesia.
3.2 Potential consequences of dysregulated D1 receptor-dependent signaling
In summary, in dyskinetic animals each dose of l-DOPA results in an abnormally large and sustained activation of adenylyl cyclase-dependent and MAPK-dependent signaling cascades in striatal neurons, with downstream changes in epigenetic and transcriptional activities. Some of the latter changes are very long-lasting (See section ‘Altered expression and regulation of transcription factors’) and prone to accumulate upon repeated exposure to l-DOPA (Fig. 3 and Fig. 4). By integrating these findings with other data reported in the literature, it is possible to make some reasonable assumptions on the cellular consequences of such a response pattern.
Figure 3. Levels of FosB/FosB-like immunoreactivity and prodynorphin mRNA are persistently upregulated in the dyskinetic striatum.
(a) ΔFosB-like proteins have very slow elimination kinetics and can therefore accumulate in striatal neurons during the course of chronic l-DOPA treatment. This effect is seen only in animals that develop dyskinesia. Acute l-DOPA treatment also induces the expression of FosB/FosB-like immunoreactivity and prodynorphin mRNA, but this upregulation is transient (Cenci et al., 1999). In the experiments shown here, acutely and chronically l-DOPA-treated animals were killed at 3 hours or 2 days post-injection to better separate the effects of the chronic treatment from those of the last drug injection. (b) Prodynorphin mRNA shows the same expression pattern as FosB/ΔFosB like immunoreactivity (indeed, ΔFosB-like transcription factors mediate the upregulation of prodynorphin mRNA induced by l-DOPA in the DA-denervated striatum; see Section ‘Altered expression and regulation of transcription factors’). Values in (a) represent numbers of FosB/ΔFosB-immunoreactive cells/mm2 measured in the lateral part of the DA-denervated caudate–putamen from animals in previously published studies (Cenci et al., 1999] and Westin et al., 2007]). Data in (b) represent the hybridization signal to prodynorphin mRNA in the DA-denervated caudate–putamen (expressed as a percentage of the values on the contralateral intact side in each group) (data from Cenci et al., 1998] and Mela et al., 2007]). In each data set, values indicate group means ± SEM from 3 to 10 animals per group, P < 0.05 vs. saline-treated 6-OHDA-lesioned controls; # chronic l-DOPA with dyskinesia. Acutely and chronically l-DOPA-treated rats were compared with their own saline control group. (c–e) Cellular levels of FosB/ΔFosB immunostaining are larger in chronically l-DOPA-treated dyskinetic rats compared to acutely l-DOPA-treated animals, and a similar pattern of group differences applies to prodynorphin mRNA (f–h). Photomicrographs in (f–h) show emulsion-coated autoradiographs of striatal sections visualized under dark field optics. The sections had been hybridized with a 35S-labeled oligonucleotide probe complementary to prodynorphin mRNA. Scale bar, 50 µm. Full descriptions of all the experimental procedures can be found in our original publications.
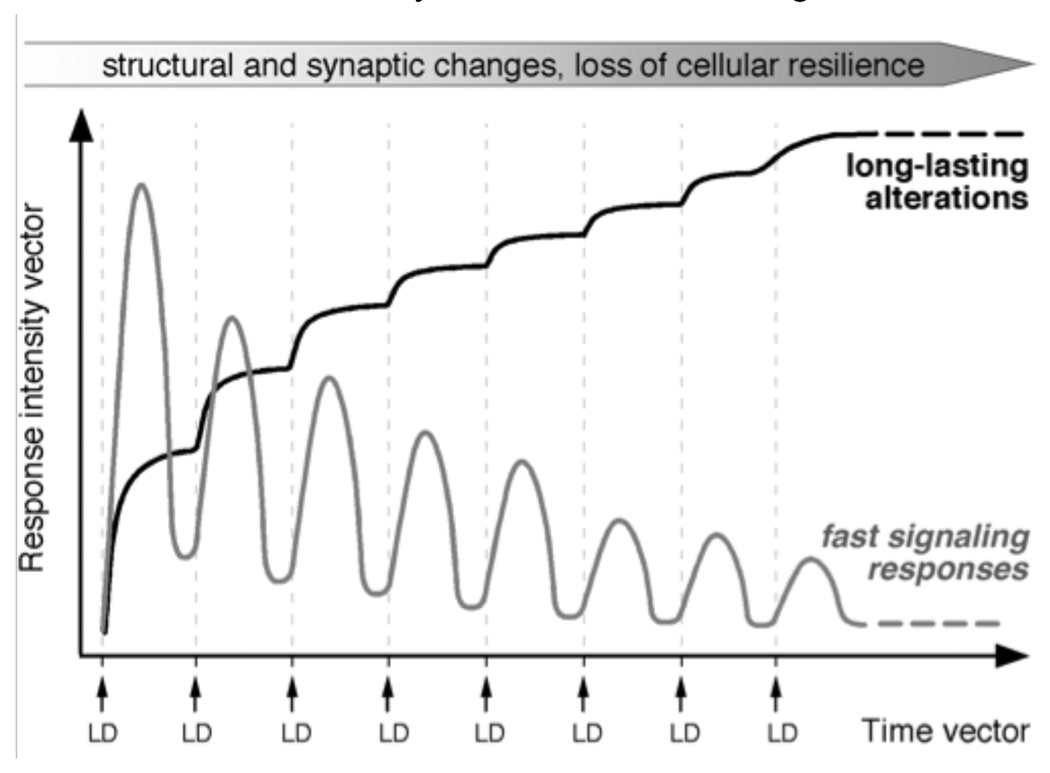
Figure 4. Maladaptive plasticity of striatal neurons in LID.
In animal models of LID, each dose of l-DOPA (LD) causes an abnormally large and prolonged activation of cAMP- and MAPK-dependent signaling pathways in striatal neurons (fast signaling responses). Although these responses would tend to normalize during chronic drug treatment, desensitization processes are inefficient in dyskinetic subjects. Hence, in these subjects, treatment with l-DOPA disrupts the dynamics of signaling networks that are normally under tight spatiotemporal control. Signalling dysregulation has both acute electrophysiological effects and long-term consequences on the function of striatal neurons. Long-term cellular alterations associated with LID have been documented to occur in D1/prodynorphin-positive MSN, including altered regulation of CRE/AP-1-dependent transcription, increased expression of neurotransmitter-related genes and increased synthesis and phosphorylation of cytoskeletal proteins (see Sections ‘Altered expression and regulation of transcription factors’ and ‘Potential consequences of dysregulated D1 receptor-dependent signaling’). This pattern of responses would predict the occurrence of profound structural and synaptic rearrangements in these neurons, associated with a large bioenergetic expenditure. Plastic modifications affecting D2/prepronkephalin MSN in LID are far less understood and will need to be addressed in future studies.
One conceivable consequence is a pronounced structural and synaptic rearrangement of striatal neurons. Beside the data provided by micro-array studies (cf. Section ‘Altered gene expression patterns’), this contention is supported by novel evidence linking the D1 receptor/PKA pathway to upregulation and/or increased phosphorylation of cytoskeletal proteins (Lebel et al., 2009 and Sgambato-Faure et al., 2005). In particular, a recent study in 6-OHDA-lesioned rats has reported a significant increase in the striatal levels of phosphorylated tau protein following chronic pulsatile l-DOPA treatment (Lebel et al., 2010). These data are interesting because abnormal tau phosphorylation can lead to cytoskeletal and synaptic alterations (Mazanetz and Fischer, 2007). Cytoskeletal modifications in LID are also suggested by the striatal upregulation of arc (activity-regulated cytoskeletal-associated gene), since arc participates in cytoskeletal rearrangements during synaptic plasticity (Steward and Worley, 2001). In the rat model of LID, Arc mRNA and protein levels show a sustained upregulation in prodynorphin-positive MSN within the same striatal regions exhibiting high ΔFosB-like immunoreactivity (Sgambato-Faure et al., 2005). This would suggest that ‘direct pathway’ MSN undergo long-term structural and synaptic modifications in LID, a suggestion that needs to be verified by future investigations.
Another consequence of repeated and sustained signaling activation is a large energy expenditure in striatal neurons, a hypothesis supported by the results of gene expression micro-array studies (discussed above) and biochemical investigations (Valastro et al., 2009). A large bioenergetic expenditure is also supported by the findings of endothelial proliferation in the striatum and other basal ganglia nuclei in dyskinetic rats (Westin et al., 2006), because angiogenic responses in the adult brain are usually associated with long-lasting increases in local metabolic demands. A large energy requirement, associated with impairments in certain bioenergetic pathways (Konradi et al., 2004), would be expected to make striatal MSN more vulnerable to glutamate- and DA-mediated toxicity.
Indeed, MSN are very sensitive to bioenergetic deficits. Primary genetic mitochondrial defects can lead to striatal degeneration and associated dystonia and dyskinesia [reviewed in Damiano et al., (2010)]. Insufficient availability of high-energy phosphates resulting in impaired activity of plasma membrane ATPases would alter the response of striatal MSN to excitatory amino acids, lowering the threshold for excitotoxicity (Calabresi et al., 1995). In addition high extracellular DA levels (as induced by a l-DOPA bolus) can prime the striatum for neurodegenerative events. Indeed, DA-dependent degeneration and apoptosis of striatal neurons has been documented to occur in situations associated with high extracellular levels of DA, such as intrastriatal DA injections (Hattori et al., 1998), metamphetamine administration (Schmidt et al., 1985) and genetic ablation of the DA transporter (Cyr et al., 2003). In primary striatal cultures, DA induces neuronal apoptotic death via stimulation of D1 receptors, leading to sustained activation and cytoplasmic retention of ERK1/2 (Chen et al., 2009). Sustained activation of ERK1/2 has been shown to render neurons more vulnerable to glutamate toxicity in some model systems (Luo and DeFranco, 2006).
It is conceivable that the combination of high extracellular DA levels, sustained intracellular signaling and bioenergetic deficits may lead to a progressive deterioration of striatal neurons in LID. Although this possibility is not yet supported by experimental data, only few studies have addressed the integrity of striatal neurons in animal models of LID (Lindgren et al., 2007 and Westin et al., 2006). In these studies, 6-OHDA-lesioned rats were treated with l-DOPA for a relatively short period (2–3 weeks), and the striata were examined using histological methods (total neuronal cell counts and Fluorogold histochemistry) that cannot detect neuronal deterioration preceding/accompanying cell death. Yet, in this animal model, dietary supplementation of creatine can reduce the severity of LID (Valastro et al., 2009), supporting a contribution of bioenergetic deficits to the pathophysiology of this movement disorder. Patients in the complicated phase of PD receive l-DOPA treatment for years despite their dyskinesias. The experimental data reviewed above call for further investigations addressing the long-term consequences of a prolonged, dyskinesiogenic l-DOPA treatment on the integrity and resilience of striatal neurons.
4 “Too much” molecular plasticity leads to plasticity failure
While the overactivation of D1-dependent signaling pathways may cause large morphological and functional rearrangements in striatal neurons (‘too much plasticity’), it also hijacks the molecular machinery by which these neurons normally respond to incoming stimuli (Fig. 4). Striatal neurons integrate convergent cortical and thalamic inputs to modify the output of the basal ganglia, playing a pivotal role in movement selection and adaptive motor control [see Wiecki and Frank (2010), this volume]. Conceivably, dyskinesia represents a disorder of motor selection featuring an inability to suppress excessive, purposeless movements. Dysregulated D1-dependent signaling and sustained potentiation of corticostriatal synapses would be expected to cause abnormal gating of cortically driven motor commands, being key to this movement disorder. Moreover, if corticostriatal synapses are clogged by irrelevant information (Picconi et al., 2003), dyskinesia would be expected to go hand in hand with an impaired capacity for experience-dependent modification of action selection.
This pathophysiological hypothesis is supported by a large number of clinical observations. In the non-complicated stages of PD, treatment with l-DOPA promotes ‘positive’ plasticity, resulting in long-lasting symptomatic benefit [reviewed in Cenci et al. (2009)], improved efficacy of non-invasive cortical stimulation methods (Fierro et al., 2001 and Rodrigues et al., 2008) and modulation of activity-dependent synaptic plasticity in basal ganglia output neurons (Prescott et al., 2009). By contrast, in the advanced, complicated stages of PD, treatment with l-DOPA loses its capacity to induce a long-lasting symptomatic improvement extending beyond the effects of single doses (Nutt and Holford, 1996). In the same disease stages, l-DOPA worsens striatum-dependent learning functions (Cools et al., 2007 and Feigin et al., 2003) and negatively affects cortical plasticity. Motor cortex plasticity has been probed in PD patients using a paired associative stimulation protocol (Morgante et al., 2006). While l-DOPA was found to restore LTP-like cortical plasticity in non-dyskinetic subjects, it failed to achieve an effect in dyskinetic patients. The mechanisms underlying a lack of cortical plasticity in LID are presently unknown, but have been suggested to depend on synchronized neuronal activities that impair information processing in basal ganglia–thalamo–cortical loops (Hammond et al., 2007 and Morgante et al., 2006). Abnormal oscillatory patterns of neural activity have been detected in the subthalamic nucleus and substantia nigra reticulata in dyskinetic PD patients and rat models of LID, respectively (Alonso-Frech et al., 2006 and Meissner et al., 2006). Future studies ought to address the role played by striatal neurons in the genesis of these pathological activity patterns.
Concluding remarks
During the past 10 years, considerable advances have been made in unravelling the molecular mechanisms of LID. This progress has been facilitated by the availability of rodent models of LID, in which improved methods of behavioural analysis have allowed investigators to distinguish dyskinetic animals from those that remained free from dyskinesia despite receiving the same l-DOPA treatment [reviewed in Cenci and Ohlin, (2009)]. A large body of literature using these models has shown that the molecular, neurochemical and cellular effects of l-DOPA are very different depending on whether or not the treatment induces AIMs. As well as revealing novel concepts and therapeutic targets, these studies have raised awareness that plastic modifications of striatal cells and synapses are a major determinant of treatment outcome in PD. In future research, it will be important to define the interactions between DA-dependent adaptations and genetic susceptibility factors [see (Gasser, 2010) and (Martin et al., 2010)] in determining different profiles of treatment response in PD.
Acknowledgements
The authors’ ongoing projects in this area are supported by grants from the Swedish Research Council, the Michael J. Fox Foundation for Parkinson’s Research, the Swedish Parkinson Foundation, the EU grant contract number 222918 (REPLACES) FP7 (Thematic priority HEALTH) (MAC), and NS48235 (CK). The authors thank Elissa Strome and Joanna Kaczmarska for technical support, Hanna Lindgren and Elisabet Ohlin for helpful discussion.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AC5
adenylyl cyclase type 5
- AIMs
abnormal involuntary movements
- Akt
protein kinase B
- AMPA
amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AP-1
activator protein-1
- Arc
activity-regulated cytoskeletal-associated protein
- cAMP
cyclic AMP
- CRE
cAMP response element
- CREB
cAMP response element-binding protein
- D1,2,3,4,5
dopamine receptors
- DA
dopamine
- DAG
diacylglycerol
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- DAT
dopamine transporter
- Elk-1
Ets like gene1
- oncogene
transcription factor
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- GDP
guanosine diphosphate
- GKS3
glycogen synthase kinase 3
- GPCRs
G-protein-coupled receptors
- G-protein
guanosine triphosphate-binding protein
- GRKs
G-protein-coupled receptor kinases
- GTP
guanosine triphosphate
- IP3
inositol trisphosphate
- IP3R
inositol trisphosphate receptor
- l-DOPA
l-3,4-dihydroxyphenylalanine
- LID
l-DOPA-induced dyskinesia
- LTP
long-term potentiation
- MAPK
mitogen-activated protein kinases
- MEK
mitogen-activated protein kinase kinase
- mGluR
metabotropic glutamate receptor
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MSK-1
mitogen- and stress-activated kinase 1
- MSN
medium-sized spiny neurons
- mTORC1
mammalian target of rapamycin complex 1
- NMDA
N-methyl-d-aspartate
- PD
Parkinson’s disease
- PET
positron emission tomography
- PKA
protein kinase A
- PLC
phospholipase C
- PP-1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- Raf
member of the MAP kinase pathway (rapidly accelerated fibrosarcoma oncogene)
- Ras
oncogene of the MAP kinase pathway (RAt sarcoma oncogene)
- RasGRP1
RAS guanyl nucleotide-releasing protein 1
- RasGRP2
RAS guanyl nucleotide-releasing protein 2
- RGS
regulators of G-protein signaling
- β-ARR
beta arrestin
References
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, et al. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2010;2(28) doi: 10.1126/scitranslmed.3000664. 28ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Frech F, Zamarbide I, Alegre M, Rodríguez-Oroz MC, Guridi J, Manrique M, Valencia M, Artieda J, Obeso JA. Slow oscillatory activity and levodopa-induced dyskinesias in Parkinson's disease. Brain. 2006;129(Pt7):1748–1757. doi: 10.1093/brain/awl103. [DOI] [PubMed] [Google Scholar]
- Andersson M, Hilbertson A, Cenci MA. Striatal fosB expression is causally linked with l-DOPA-induced abnormal involuntary movements and the associated upregulation of striatal prodynorphin mRNA in a rat model of Parkinson's disease. Neurobiol Dis. 1999;6(6):461–474. doi: 10.1006/nbdi.1999.0259. [DOI] [PubMed] [Google Scholar]
- Andersson M, Konradi C, Cenci MA. cAMP response element-binding protein is required for dopamine-dependent gene expression in the intact but not the dopamine-denervated striatum. J Neurosci. 2001;21(24):9930–9943. doi: 10.1523/JNEUROSCI.21-24-09930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Westin JE, Cenci MA. Time course of striatal DeltaFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17(3):661–666. doi: 10.1046/j.1460-9568.2003.02469.x. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Sortwell CE, Ray M, Altemus KL, Sibley DR, Levine MS. Agonist-induced morphologic decrease in cellular D1A dopamine receptor staining. Synapse. 1997;27(4):313–321. doi: 10.1002/(SICI)1098-2396(199712)27:4<313::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Hakansson K, Li Q, Dovero S, Barthe N, et al. Increased D1 dopamine receptor signaling in levodopa-induced dyskinesia. Ann Neurol. 2005;57(1):17–26. doi: 10.1002/ana.20296. [DOI] [PubMed] [Google Scholar]
- Aubert I, Guigoni C, Li Q, Dovero S, Bioulac BH, Gross CE, et al. Enhanced preproenkephalin-B-derived opioid transmission in striatum and subthalamic nucleus converges upon globus pallidus internalis in L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol Psychiatry. 2007;61(7):836–844. doi: 10.1016/j.biopsych.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27(4):881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Berthet A, Porras G, Doudnikoff E, Stark H, Cador M, Bezard E, et al. Pharmacological analysis demonstrates dramatic alteration of D1 dopamine receptor neuronal distribution in the rat analog of L-DOPA-induced dyskinesia. J Neurosci. 2009;29(15):4829–4835. doi: 10.1523/JNEUROSCI.5884-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Guigoni C, Li Q, Bioulac BH, Aubert I, Gross CE, et al. Striatal overexpression of DeltaJunD resets L-DOPA-induced dyskinesia in a primate model of Parkinson disease. Biol Psychiatry. 2009;66(6):554–561. doi: 10.1016/j.biopsych.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Ferry S, Mach U, Stark H, Leriche L, Boraud T, et al. Attenuation of levodopa-induced dyskinesia by normalizing dopamine D3 receptor function. Nat Med. 2003;9(6):762–767. doi: 10.1038/nm875. [DOI] [PubMed] [Google Scholar]
- Buck K, Voehringer P, Ferger B. Site-specific action of l-3,4-dihydroxyphenylalanine in the striatum but not globus pallidus and substantia nigra pars reticulata evokes dyskinetic movements in chronic l-3,4-dihydroxyphenylalanine-treated 6-hydroxydopamine-lesioned rats. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.12.032. [DOI] [PubMed] [Google Scholar]
- Cai G, Wang HY, Friedman E. Increased dopamine receptor signaling and dopamine receptor-G protein coupling in denervated striatum. J Pharmacol Exp Ther. 2002;302(3):1105–1112. doi: 10.1124/jpet.102.036673. [DOI] [PubMed] [Google Scholar]
- Calabresi P, De Murtas M, Pisani A, Stefani A, Sancesario G, Mercuri NB, et al. Vulnerability of medium spiny striatal neurons to glutamate: role of Na+/K+ ATPase. Eur J Neurosci. 1995;7(8):1674–1683. doi: 10.1111/j.1460-9568.1995.tb00689.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Giacomini P, Centonze D, Bernardi G. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol. 2000;47(4) Suppl 1:S60–S68. discussion S68-69. [PubMed] [Google Scholar]
- Calon F, Grondin R, Morissette M, Goulet M, Blanchet PJ, Di Paolo T, et al. Molecular basis of levodopa-induced dyskinesias. Ann Neurol. 2000;47(4) Suppl 1:S70–S78. [PubMed] [Google Scholar]
- Carle TL, Ohnishi YN, Ohnishi YH, Alibhai IN, Wilkinson MB, Kumar A, et al. Proteasome-dependent and -independent mechanisms for FosB destabilization: identification of FosB degron domains and implications for DeltaFosB stability. Eur J Neurosci. 2007;25(10):3009–3019. doi: 10.1111/j.1460-9568.2007.05575.x. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochem. 2006;96(6):1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Dopamine dysregulation of movement control in L-DOPA-induced dyskinesia. Trends Neurosci. 2007;30(5):236–243. doi: 10.1016/j.tins.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cenci MA. Pathophysiology of L-DOPA-Induced Dyskinesia in Parkinson's Disease. In: Dunnett S, Björklund A, Iversen L, Iversen S, editors. Dopamine Handbook. New York, NY: Oxford University Press; 2009. pp. 434–444. [Google Scholar]
- Cenci MA, Lee CS, Bjorklund A. L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10(8):2694–2706. [PubMed] [Google Scholar]
- Cenci MA, Lindgren HS. Advances in understanding L-DOPA-induced dyskinesia. Curr Opin Neurobiol. 2007;17(6):665–671. doi: 10.1016/j.conb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Post- versus presynaptic plasticity in L-DOPA-induced dyskinesia. J Neurochem. 2006;99(2):381–392. doi: 10.1111/j.1471-4159.2006.04124.x. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE. Rodent models of treatment-induced motor complications in Parkinson's disease. Parkinsonism Relat Disord. 2009;15 Suppl 4:S13–S17. doi: 10.1016/S1353-8020(09)70828-4. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE, Rylander D. Plastic effects of L-DOPA treatment in the basal ganglia and their relevance to the development of dyskinesia. Parkinsonism Relat Disord. 2009;15 Suppl 3:S59–S63. doi: 10.1016/S1353-8020(09)70782-5. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Tranberg A, Andersson M, Hilbertson A. Changes in the regional and compartmental distribution of FosB- and JunB-like immunoreactivity induced in the dopamine-denervated rat striatum by acute or chronic L-dopa treatment. Neuroscience. 1999;94(2):515–527. doi: 10.1016/s0306-4522(99)00294-8. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Rev Neurosci. 2002;3(7):574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci U S A. 1993;90(20):9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Dopamine and N-methyl-D-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20(1):1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. Where do you think you are going? The NMDA-D1 receptor trap. Sci STKE. 2006;2006(333):pe20. doi: 10.1126/stke.3332006pe20. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem. 2002;83(3):704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Chase TN. Levodopa therapy: consequences of the nonphysiologic replacement of dopamine. Neurology. 1998;50(5) Suppl 5:S17–S25. doi: 10.1212/wnl.50.5_suppl_5.s17. [DOI] [PubMed] [Google Scholar]
- Chase TN, Oh JD. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000;23(10 Suppl):S86–S91. doi: 10.1016/s1471-1931(00)00018-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Rusnak M, Lombroso PJ, Sidhu A. Dopamine promotes striatal neuronal apoptotic death via ERK signaling cascades. Eur J Neurosci. 2009;29(2):287–306. doi: 10.1111/j.1460-9568.2008.06590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell. 2000;5(6):905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002;66(2):61–79. doi: 10.1016/s0301-0082(01)00023-5. [DOI] [PubMed] [Google Scholar]
- Cole RL, Konradi C, Douglass J, Hyman SE. Neuronal adaptation to amphetamine and dopamine: molecular mechanisms of prodynorphin gene regulation in rat striatum. Neuron. 1995;14(4):813–823. doi: 10.1016/0896-6273(95)90225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Lewis SJ, Clark L, Barker RA, Robbins TW. L-DOPA disrupts activity in the nucleus accumbens during reversal learning in Parkinson's disease. Neuropsychopharmacology. 2007;32(1):180–189. doi: 10.1038/sj.npp.1301153. [DOI] [PubMed] [Google Scholar]
- Corvol JC, Muriel MP, Valjent E, Feger J, Hanoun N, Girault JA, et al. Persistent increase in olfactory type G-protein alpha subunit levels may underlie D1 receptor functional hypersensitivity in Parkinson disease. J Neurosci. 2004;24(31):7007–7014. doi: 10.1523/JNEUROSCI.0676-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Cantuti-Castelvetri I, Saka E, Keller-McGandy CE, Hernandez LF, Kett LR, et al. Dysregulation of CalDAG-GEFI and CalDAG-GEFII predicts the severity of motor side-effects induced by anti-parkinsonian therapy. Proc Natl Acad Sci U S A. 2009;106(8):2892–2896. doi: 10.1073/pnas.0812822106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr M, Beaulieu JM, Laakso A, Sotnikova TD, Yao WD, Bohn LM, et al. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc Natl Acad Sci U S A. 2003;100(19):11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano M, Galvan L, Deglon N, Brouillet E. Mitochondria in Huntington's disease. Biochim Biophys Acta. 2010;1802(1):52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Darmopil S, Martin AB, De Diego IR, Ares S, Moratalla R. Genetic inactivation of dopamine D1 but not D2 receptors inhibits L-DOPA-induced dyskinesia and histone activation. Biol Psychiatry. 2009;66(6):603–613. doi: 10.1016/j.biopsych.2009.04.025. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, et al. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias. Brain. 2004;127(Pt 12):2747–2754. doi: 10.1093/brain/awh290. [DOI] [PubMed] [Google Scholar]
- De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, et al. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87(4):922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah AW, Wang Y, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, et al. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson's disease. Mol Pharmacol. 2000;57(2):342–352. [PubMed] [Google Scholar]
- Dunnett SB. Behavioural analysis of motor and non-motor symptoms in rodent models of Parkinson's disease. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)84003-8. In press. [DOI] [PubMed] [Google Scholar]
- El Atifi-Borel M, Buggia-Prevot V, Platet N, Benabid AL, Berger F, Sgambato-Faure V. De novo and long-term l-Dopa induce both common and distinct striatal gene profiles in the hemiparkinsonian rat. Neurobiol Dis. 2009;34(2):340–350. doi: 10.1016/j.nbd.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007;22(10):1379–1389. doi: 10.1002/mds.21475. quiz 1523. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Feigin A, Ghilardi MF, Carbon M, Edwards C, Fukuda M, Dhawan V, et al. Effects of levodopa on motor sequence learning in Parkinson's disease. Neurology. 2003;60(11):1744–1749. doi: 10.1212/01.wnl.0000072263.03608.42. [DOI] [PubMed] [Google Scholar]
- Fierro B, Piazza A, Brighina F, La Bua V, Buffa D, Oliveri M. Modulation of intracortical inhibition induced by low- and high-frequency repetitive transcranial magnetic stimulation. Exp Brain Res. 2001;138(4):452–457. doi: 10.1007/s002210100728. [DOI] [PubMed] [Google Scholar]
- Fiorentini C, Rizzetti MC, Busi C, Bontempi S, Collo G, Spano P, et al. Loss of synaptic D1 dopamine/N-methyl-D-aspartate glutamate receptor complexes in L-DOPA-induced dyskinesia in the rat. Mol Pharmacol. 2006;69(3):805–812. doi: 10.1124/mol.105.016667. [DOI] [PubMed] [Google Scholar]
- Fox SH, Brotchie JM. The MPTP-lesioned non-human primate models of Parkinson’s disease. Past, present and future. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)84007-5. In press. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Ghiglieri V, Di Luca M, Calabresi P. Assemblies of glutamate receptor subunits with postsynaptic density proteins and their alterations in Parkinson’s disease. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)83009-2. In press. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Picconi B, Ghiglieri V, Polli F, Bagetta V, Bernardi G, et al. A critical interaction between NR2B and MAGUK in L-DOPA induced dyskinesia. J Neurosci. 2006;26(11):2914–2922. doi: 10.1523/JNEUROSCI.5326-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser T. Identifying PD-causing genes and genetic susceptibility factors: current approaches and future prospects. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)83001-8. In press. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15(4):133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum animal model of Parkinson's disease. Neuroscientist. 2003;9(6):455–462. doi: 10.1177/1073858403255839. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Geurts M, Hermans E, Cumps J, Maloteaux JM. Dopamine receptor-modulated [35S]GTPgammaS binding in striatum of 6-hydroxydopamine-lesioned rats. Brain Res. 1999;841(1–2):135–142. doi: 10.1016/s0006-8993(99)01812-0. [DOI] [PubMed] [Google Scholar]
- Geurts M, Maloteaux JM, Hermans E. Altered expression of regulators of G-protein signaling (RGS) mRNAs in the striatum of rats undergoing dopamine depletion. Biochem Pharmacol. 2003;66(7):1163–1170. doi: 10.1016/s0006-2952(03)00447-7. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Ginty DD, Bading H, Greenberg ME. Calcium regulation of gene expression in neuronal cells. J Neurobiol. 1994;25(3):294–303. doi: 10.1002/neu.480250309. [DOI] [PubMed] [Google Scholar]
- Ginty DD. Calcium regulation of gene expression: isn't that spatial? Neuron. 1997;18(2):183–186. doi: 10.1016/s0896-6273(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Hoang CV, Potts BW, Porras G, Pioli E, Kim KW, et al. RGS9-2 negatively modulates L-3,4-dihydroxyphenylalanine-induced dyskinesia in experimental Parkinson's disease. J Neurosci. 2007;27(52):14338–14348. doi: 10.1523/JNEUROSCI.4223-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandas F, Galiano ML, Tabernero C. Risk factors for levodopa-induced dyskinesias in Parkinson's disease. J Neurol. 1999;246(12):1127–1133. doi: 10.1007/s004150050530. [DOI] [PubMed] [Google Scholar]
- Grandy DK, Civelli O. G-protein-coupled receptors: the new dopamine receptor subtypes. Curr Opin Neurobiol. 1992;2(3):275–281. doi: 10.1016/0959-4388(92)90115-2. [DOI] [PubMed] [Google Scholar]
- Gray PC, Scott JD, Catterall WA. Regulation of ion channels by cAMP-dependent protein kinase and A-kinase anchoring proteins. Curr Opin Neurobiol. 1998;8(3):330–334. doi: 10.1016/s0959-4388(98)80057-3. [DOI] [PubMed] [Google Scholar]
- Guigoni C, Doudnikoff E, Li Q, Bloch B, Bezard E. Altered D(1) dopamine receptor trafficking in parkinsonian and dyskinetic nonhuman primates. Neurobiol Dis. 2007;26(2):452–463. doi: 10.1016/j.nbd.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Hallett PJ, Dunah AW, Ravenscroft P, Zhou S, Bezard E, Crossman AR, et al. Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson's disease. Neuropharmacology. 2005;48(4):503–516. doi: 10.1016/j.neuropharm.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30(7):357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Harrison LM, LaHoste GJ. Rhes, the Ras homolog enriched in striatum, is reduced under conditions of dopamine supersensitivity. Neuroscience. 2006;137(2):483–492. doi: 10.1016/j.neuroscience.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Hattori A, Luo Y, Umegaki H, Munoz J, Roth GS. Intrastriatal injection of dopamine results in DNA damage and apoptosis in rats. Neuroreport. 1998;9(11):2569–2572. doi: 10.1097/00001756-199808030-00026. [DOI] [PubMed] [Google Scholar]
- Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM. Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson's disease. Exp Neurol. 2003;183(2):458–468. doi: 10.1016/s0014-4886(03)00064-5. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, et al. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J Neurosci. 2000;20(24):8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Levi-Strauss M, Marey-Semper I, Verney C, Tassin JP, Glowinski J, et al. G(olf) and Gs in rat basal ganglia: possible involvement of G(olf) in the coupling of dopamine D1 receptor with adenylyl cyclase. J Neurosci. 1993;13(5):2237–2248. doi: 10.1523/JNEUROSCI.13-05-02237.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Brain monoamines and parkinsonism. Natl Inst Drug Abuse Res Monogr Ser. 1975;3:13–21. doi: 10.1037/e472122004-001. [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Jolkkonen J, Stubbs CM, Jenner P, Marsden CD. Dopamine D3 receptors in the basal ganglia of the common marmoset and following MPTP and L-DOPA treatment. Brain Res. 1996;709(2):259–264. doi: 10.1016/0006-8993(95)01309-1. [DOI] [PubMed] [Google Scholar]
- Hurley MJ, Stubbs CM, Jenner P, Marsden CD. D3 receptor expression within the basal ganglia is not affected by Parkinson's disease. Neurosci Lett. 1996;214(2–3):75–78. doi: 10.1016/0304-3940(96)12884-6. [DOI] [PubMed] [Google Scholar]
- Jenner P. The MPTP-treated primate as a model of motor complications in PD: primate model of motor complications. Neurology. 2003;61(6 Suppl 3):S4–S11. doi: 10.1212/wnl.61.6_suppl_3.s4. [DOI] [PubMed] [Google Scholar]
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9(9):665–677. doi: 10.1038/nrn2471. [DOI] [PubMed] [Google Scholar]
- Johansson PA, Andersson M, Andersson KE, Cenci MA. Alterations in cortical and basal ganglia levels of opioid receptor binding in a rat model of l-DOPA-induced dyskinesia. Neurobiol Dis. 2001;8(2):220–239. doi: 10.1006/nbdi.2000.0372. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Toki S, Canales JJ, Harlan P, Blumenstiel JP, et al. A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc Natl Acad Sci U S A. 1998;95(22):13278–13283. doi: 10.1073/pnas.95.22.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim OJ, Ariano MA, Namkung Y, Marinec P, Kim E, Han J, et al. D2 dopamine receptor expression and trafficking is regulated through direct interactions with ZIP. J Neurochem. 2008;106(1):83–95. doi: 10.1111/j.1471-4159.2008.05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klawans HL, Goetz C, Nausieda PA, Weiner WJ. Levodopa-induced dopamine receptor hypersensitivity. Trans Am Neurol Assoc. 1977;102:80–83. [PubMed] [Google Scholar]
- Kobylecki C, Cenci MA, Crossman AR, Ravenscroft P. Calcium-permeable AMPA receptors are involved in the induction and expression of L-DOPA-induced dyskinesia in Parkinson's disease. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2010.06776.x. In press. [DOI] [PubMed] [Google Scholar]
- Konitsiotis S, Blanchet PJ, Verhagen L, Lamers E, Chase TN. AMPA receptor blockade improves levodopa-induced dyskinesia in MPTP monkeys. Neurology. 2000;54(8):1589–1595. doi: 10.1212/wnl.54.8.1589. [DOI] [PubMed] [Google Scholar]
- Konradi C, Cole RL, Heckers S, Hyman SE. Amphetamine regulates gene expression in rat striatum via transcription factor CREB. J Neurosci. 1994;14(9):5623–5634. doi: 10.1523/JNEUROSCI.14-09-05623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Kobierski LA, Nguyen TV, Heckers S, Hyman SE. The cAMP-response-element-binding protein interacts, but Fos protein does not interact, with the proenkephalin enhancer in rat striatum. Proc Natl Acad Sci U S A. 1993;90(15):7005–7009. doi: 10.1073/pnas.90.15.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci. 1996;16(13):4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Westin JE, Carta M, Eaton ME, Kuter K, Dekundy A, et al. Transcriptome analysis in a rat model of L-DOPA-induced dyskinesia. Neurobiol Dis. 2004;17(2):219–236. doi: 10.1016/j.nbd.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48(2):303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Lachowicz JE, Sibley DR. Molecular characteristics of mammalian dopamine receptors. Pharmacol Toxicol. 1997;81(3):105–113. doi: 10.1111/j.1600-0773.1997.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Lane EL, Björklund A, Dunnett SB, Winkler C. Cell replacement therapy: Unraveling the mechanisms underlying graft-induced dyskinesia. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)84015-4. In press. [DOI] [PubMed] [Google Scholar]
- Lebel M, Chagniel L, Bureau G, Cyr M. Striatal inhibition of PKA prevents levodopa-induced behavioural and molecular changes in the hemiparkinsonian rat. Neurobiol Dis. 2010;38(1):59–67. doi: 10.1016/j.nbd.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Lebel M, Patenaude C, Allyson J, Massicotte G, Cyr M. Dopamine D1 receptor activation induces tau phosphorylation via cdk5 and GSK3 signaling pathways. Neuropharmacology. 2009;57(4):392–402. doi: 10.1016/j.neuropharm.2009.06.041. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, et al. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20(11):4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA. l-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson's disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem. 2010 doi: 10.1111/j.1471-4159.2009.06556.x. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Ohlin KE, Cenci MA. Differential Involvement of D1 an D2 dopamine receptors in L-DOPA-induced angiogenic activity in a rat model of Parkinson's Disease. Neuropsychopharmacology. 2009;1–12 doi: 10.1038/npp.2009.74. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Rylander D, Ohlin KE, Lundblad M, Cenci MA. The "motor complication syndrome" in rats with 6-OHDA lesions treated chronically with L-DOPA: relation to dose and route of administration. Behav Brain Res. 2007;177(1):150–159. doi: 10.1016/j.bbr.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16(1):110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J Biol Chem. 2006;281(24):16436–16442. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- Manson A, Schrag A. Levodopa-induced dyskinesias, the Clinical Problem: Clinical Features, Incidence, Risk Factors, Management and Impact on Quality of Life. In: Bezard E, editor. Recent breakthroughs in Basal Ganglia Research. New York, NY: Nova Science Publishers Inc; 2006. pp. 369–380. [Google Scholar]
- Marconi R, Lefebvre-Caparros D, Bonnet AM, Vidailhet M, Dubois B, Agid Y. Levodopa-induced dyskinesias in Parkinson's disease phenomenology and pathophysiology. Mov Disord. 1994;9(1):2–12. doi: 10.1002/mds.870090103. [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. The Impact of Genetic Research on Our Understanding of Parkinson's Disease. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)83002-X. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, et al. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc Natl Acad Sci U S A. 2008;105(36):13656–13661. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanetz MP, Fischer PM. Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases. Nat Rev Drug Discov. 2007;6(6):464–479. doi: 10.1038/nrd2111. [DOI] [PubMed] [Google Scholar]
- Meissner W, Ravenscroft P, Reese R, Harnack D, Morgenstern R, Kupsch A, Klitgaard H, Bioulac B, Gross CE, Bezard E, Boraud T. Increased slow oscillatory activity in substantia nigra pars reticulata triggers abnormal involuntary movements in the 6-OHDA-lesioned rat in the presence of excessive extracellular striatal dopamine. Neurobiol Disease. 2006;22(3):586–598. doi: 10.1016/j.nbd.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Mela F, Marti M, Dekundy A, Danysz W, Morari M, Cenci MA. Antagonism of metabotropic glutamate receptor type 5 attenuates l-DOPA-induced dyskinesia and its molecular and neurochemical correlates in a rat model of Parkinson's disease. J Neurochem. 2007;101(2):483–497. doi: 10.1111/j.1471-4159.2007.04456.x. [DOI] [PubMed] [Google Scholar]
- Mela F, Millan MJ, Brocco M, Morari M. The selective D(3) receptor antagonist, S33084, improves parkinsonian-like motor dysfunction but does not affect L-DOPA-induced dyskinesia in 6-hydroxydopamine hemi-lesioned rats. Neuropharmacology. 2010;58(2):528–536. doi: 10.1016/j.neuropharm.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Mishra RK, Gardner EL, Katzman R, Makman MH. Enhancement of dopamine-stimulated adenylate cyclase activity in rat caudate after lesions in substantia nigra: evidence for denervation supersensitivity. Proc Natl Acad Sci U S A. 1974;71(10):3883–3887. doi: 10.1073/pnas.71.10.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R. Motor cortex plasticity in Parkinson's disease and levodopa-induced dyskinesias. Brain. 2006;129(Pt 4):1059–1069. doi: 10.1093/brain/awl031. [DOI] [PubMed] [Google Scholar]
- Morissette M, Samadi P, Hadj Tahar A, Belanger N, Di Paolo T. Striatal Akt/GSK3 signaling pathway in the development of L-Dopa-induced dyskinesias in MPTP monkeys. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(3):446–454. doi: 10.1016/j.pnpbp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- Nadjar A, Gerfen CR, Bezard E. Priming for l-dopa-induced dyskinesia in Parkinson's disease: a feature inherent to the treatment or the disease? Prog Neurobiol. 2009;87(1):1–9. doi: 10.1016/j.pneurobio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JE, Johnston TH, Collingridge GL, Garner CC, Brotchie JM. Subcellular redistribution of the synapse-associated proteins PSD-95 and SAP97 in animal models of Parkinson's disease and L-DOPA-induced dyskinesia. Faseb J. 2005;19(6):583–585. doi: 10.1096/fj.04-1854fje. [DOI] [PubMed] [Google Scholar]
- Nutt JG. Levodopa-induced dyskinesia: review, observations, and speculations. Neurology. 1990;40(2):340–345. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Holford NH. The response to levodopa in Parkinson's disease: imposing pharmacological law and order. Ann Neurol. 1996;39(5):561–573. doi: 10.1002/ana.410390504. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Rakic P, Goldman-Rakic PS. Internalization of D2 dopamine receptors is clathrin-dependent and select to dendroaxonic appositions in primate prefrontal cortex. Eur J Neurosci. 2006;24(5):1395–1403. doi: 10.1111/j.1460-9568.2006.05023.x. [DOI] [PubMed] [Google Scholar]
- Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, et al. Clinical correlates of levodopa-induced dopamine release in Parkinson disease: a PET study. Neurology. 2006;67(9):1612–1617. doi: 10.1212/01.wnl.0000242888.30755.5d. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martin AB, Mendialdua A, Moratalla R. ERK phosphorylation and FosB expression are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry. 2006;59(1):64–74. doi: 10.1016/j.biopsych.2005.05.044. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Hakansson K, Bernardi G, Greengard P, Fisone G, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Pifl C, Nanoff C, Schingnitz G, Schutz W, Hornykiewicz O. Sensitization of dopamine-stimulated adenylyl cyclase in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated rhesus monkeys and patients with idiopathic Parkinson's disease. J Neurochem. 1992;58(6):1997–2004. doi: 10.1111/j.1471-4159.1992.tb10939.x. [DOI] [PubMed] [Google Scholar]
- Prescott IA, Dostrovsky JO, Moro E, Hodaie M, Lozano AM, Hutchison WD. Levodopa enhances synaptic plasticity in the substantia nigra pars reticulata of Parkinson's disease patients. Brain. 2009;132(Pt 2):309–318. doi: 10.1093/brain/awn322. [DOI] [PubMed] [Google Scholar]
- Prieto GA, Perez-Burgos A, Fiordelisio T, Salgado H, Galarraga E, Drucker-Colin R, et al. Dopamine D(2)-class receptor supersensitivity as reflected in Ca2+ current modulation in neostriatal neurons. Neuroscience. 2009;164(2):345–350. doi: 10.1016/j.neuroscience.2009.08.030. [DOI] [PubMed] [Google Scholar]
- Quik M, Police S, He L, Di Monte DA, Langston JW. Expression of D(3) receptor messenger RNA and binding sites in monkey striatum and substantia nigra after nigrostriatal degeneration: effect of levodopa treatment. Neuroscience. 2000;98(2):263–273. doi: 10.1016/s0306-4522(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C. L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J Neurosci. 1999;19(15):6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinne JO, Laihinen A, Lonnberg P, Marjamaki P, Rinne UK. A post-mortem study on striatal dopamine receptors in Parkinson's disease. Brain Res. 1991;556(1):117–122. doi: 10.1016/0006-8993(91)90554-9. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49(2):285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Caron MG. Selective inhibition of adenylyl cyclase type V by the dopamine D3 receptor. Mol Pharmacol. 1997;52(3):508–514. doi: 10.1124/mol.52.3.508. [DOI] [PubMed] [Google Scholar]
- Rodrigues JP, Walters SE, Stell R, Mastaglia FL, Thickbroom GW. Spike-timing-related plasticity is preserved in Parkinson's disease and is enhanced by dopamine: evidence from transcranial magnetic stimulation. Neurosci Lett. 2008;448(1):29–32. doi: 10.1016/j.neulet.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Rylander D, Recchia A, Mela F, Dekundy A, Danysz W, Cenci MA. Pharmacological modulation of glutamate transmission in a rat model of L-DOPA-induced dyskinesia: effects on motor behavior and striatal nuclear signaling. J Pharmacol Exp Ther. 2009;330(1):227–235. doi: 10.1124/jpet.108.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Alcacer C, Cacciatore S, Heiman M, Herve D, Greengard P, et al. L-DOPA activates ERK signaling and phosphorylates histone H3 in the striatonigral medium spiny neurons of hemiparkinsonian mice. J Neurochem. 2009;108(3):621–633. doi: 10.1111/j.1471-4159.2008.05831.x. [DOI] [PubMed] [Google Scholar]
- Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson's disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2(80):ra36. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27(26):6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233(3):539–544. [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, Tapper AR, et al. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33(12):2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman H. Protein phosphorylation in neuronal plasticity and gene expression. Curr Opin Neurobiol. 1995;5(3):375–381. doi: 10.1016/0959-4388(95)80051-4. [DOI] [PubMed] [Google Scholar]
- Schuster S, Nadjar A, Guo JT, Li Q, Ittrich C, Hengerer B, et al. The 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor lovastatin reduces severity of L-DOPA-induced abnormal involuntary movements in experimental Parkinson's disease. J Neurosci. 2008;28(17):4311–4316. doi: 10.1523/JNEUROSCI.4720-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- Sgambato-Faure V, Buggia V, Gilbert F, Levesque D, Benabid AL, Berger F. Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64(11):936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Monsma FJ., Jr Molecular biology of dopamine receptors. Trends Pharmacol Sci. 1992;13(2):61–69. doi: 10.1016/0165-6147(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Sibley DR, Ventura AL, Jiang D, Mak C. Regulation of the D1 dopamine receptor through cAMP-mediated pathways. Adv Pharmacol. 1998;42:447–450. doi: 10.1016/s1054-3589(08)60784-x. [DOI] [PubMed] [Google Scholar]
- Silverdale MA, Kobylecki C, Hallett PJ, Li Q, Dunah AW, Ravenscroft P, et al. Synaptic recruitment of AMPA glutamate receptor subunits in levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate. Synapse. 2010;64(2):177–180. doi: 10.1002/syn.20739. [DOI] [PubMed] [Google Scholar]
- St-Hilaire M, Landry E, Levesque D, Rouillard C. Denervation and repeated L-DOPA induce complex regulatory changes in neurochemical phenotypes of striatal neurons: implication of a dopamine D1-dependent mechanism. Neurobiol Dis. 2005;20(2):450–460. doi: 10.1016/j.nbd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Staunton DA, Wolfe BB, Groves PM, Molinoff PB. Dopamine receptor changes following destruction of the nigrostriatal pathway: lack of a relationship to rotational behavior. Brain Res. 1981;211(2):315–327. doi: 10.1016/0006-8993(81)90704-6. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30(1):227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Strange PG. New insights into dopamine receptors in the central nervous system. Neurochem Int. 1993;22(3):223–236. doi: 10.1016/0197-0186(93)90050-f. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, et al. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)83008-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76(1):1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Turjanski N, Lees AJ, Brooks DJ. In vivo studies on striatal dopamine D1 and D2 site binding in L-dopa-treated Parkinson's disease patients with and without dyskinesias. Neurology. 1997;49(3):717–723. doi: 10.1212/wnl.49.3.717. [DOI] [PubMed] [Google Scholar]
- Valastro B, Andersson M, Lindgren HS, Cenci MA. Expression pattern of JunD after acute or chronic L-DOPA treatment: comparison with deltaFosB. Neuroscience. 2007;144(1):198–207. doi: 10.1016/j.neuroscience.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Valastro B, Dekundy A, Danysz W, Quack G. Oral creatine supplementation attenuates L-DOPA-induced dyskinesia in 6-hydroxydopamine-lesioned rats. Behav Brain Res. 2009;197(1):90–96. doi: 10.1016/j.bbr.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Valastro B, Dekundy A, Krogh M, Lundblad M, James P, Danysz W, et al. Proteomic analysis of striatal proteins in the rat model of L-DOPA-induced dyskinesia. J Neurochem. 2007;102(4):1395–1409. doi: 10.1111/j.1471-4159.2007.04655.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallar L, Meldolesi J. Mechanisms of signal transduction at the dopamine D2 receptor. Trends Pharmacol Sci. 1989;10(2):74–77. doi: 10.1016/0165-6147(89)90082-5. [DOI] [PubMed] [Google Scholar]
- Vaquero A, Loyola A, Reinberg D. The constantly changing face of chromatin. Sci Aging Knowledge Environ. 2003;2003(14):RE4. doi: 10.1126/sageke.2003.14.re4. [DOI] [PubMed] [Google Scholar]
- Vargas GA, Von Zastrow M. Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J Biol Chem. 2004;279(36):37461–37469. doi: 10.1074/jbc.M401034200. [DOI] [PubMed] [Google Scholar]
- Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci. 1999;24(11):437–440. doi: 10.1016/s0968-0004(99)01460-7. [DOI] [PubMed] [Google Scholar]
- Westin JE, Andersson M, Lundblad M, Cenci MA. Persistent changes in striatal gene expression induced by long-term L-DOPA treatment in a rat model of Parkinson's disease. Eur J Neurosci. 2001;14(7):1171–1176. doi: 10.1046/j.0953-816x.2001.01743.x. [DOI] [PubMed] [Google Scholar]
- Westin JE, Lindgren HS, Gardi J, Nyengaard JR, Brundin P, Mohapel P, et al. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J Neurosci. 2006;26(37):9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin JE, Vercammen L, Strome EM, Konradi C, Cenci MA. Spatiotemporal pattern of striatal ERK1/2 phosphorylation in a rat model of L-DOPA-induced dyskinesia and the role of dopamine D1 receptors. Biol Psychiatry. 2007;62(7):800–810. doi: 10.1016/j.biopsych.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ, et al. Neurocomputational models of motor and cognitive deficits in Parkinson's Disease. Prog Brain Res. 2010 doi: 10.1016/S0079-6123(10)83014-6. In press. [DOI] [PubMed] [Google Scholar]
- Xiao MF, Xu JC, Tereshchenko Y, Novak D, Schachner M, Kleene R. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J Neurosci. 2009;29(47):14752–14763. doi: 10.1523/JNEUROSCI.4860-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Kazanietz MG. Divergence and complexities in DAG signaling: looking beyond PKC. Trends Pharmacol Sci. 2003;24(11):602–608. doi: 10.1016/j.tips.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Zhen X, Torres C, Cai G, Friedman E. Inhibition of protein tyrosine/mitogen-activated protein kinase phosphatase activity is associated with D2 dopamine receptor supersensitivity in a rat model of Parkinson's disease. Mol Pharmacol. 2002;62(6):1356–1363. doi: 10.1124/mol.62.6.1356. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13(7):290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]