Abstract
Generation of reactive-oxygen species (ROS) has been suggested as a mechanism of fetal membrane (FM) weakening leading to rupture, particularly with preterm premature rupture of the fetal membranes (preterm PROM). In vitro, FM incubation with Tumor necrosis factor (TNF) mimics physiological FM weakening, concomitant with generation of ROS and collagen remodeling. Proinflammatory cytokines are also postulated to have a role in the development of the FM physiological weak zone where rupture normally initiates in term gestations. We hypothesized that antioxidant treatment may block ROS development and resultant FM weakening. Two studies examining antioxidant effects upon FM strength were conducted, one in vivo and the other in vitro. FM of patients enrolled in a multicenter placebo-controlled trial to determine the effect of Vitamin C (1 gm/day) and Vitamin E (400IU/day) upon complications of preeclampsia were examined for FM biomechanical properties and biochemical remodeling at birth. Separately, biomechanics and biochemical markers of remodeling were determined in FM fragments incubated with TNF with or without Vitamin C pre-incubation. Supplemental dietary Vitamin C in combination with Vitamin E did not modify rupture strength, work to rupture, or MMP9 (protein or activity) either within or outside the term FM physiological weak zone. In vitro, TNF decreased FM rupture strength by 50% while increasing MMP9 protein. Vitamin C did not inhibit these TNF-induced effects. Vitamin C alone had a weakening effect on FM in vitro. We speculate that vitamin C supplementation during pregnancy will not be useful in the prevention of preterm PROM.
Keywords: fetal membrane rupture, Vitamin C, amnion, matrix-metalloproteinase, MMP9
INTRODUCTION
Preterm premature rupture of the fetal membranes (preterm PROM) is the initiating event in approximately one third of preterm births resulting in significant infant mortality and morbidity (1, 2). The exact mechanisms by which fetal membranes (FM) weaken and rupture in term and preterm gestations are unknown. However, it has been hypothesized that FM weaken and ultimately rupture as the result of collagen remodeling and apoptosis (3–5).
We have previously described a localized region of the FM, overlying the cervix, which is mechanically weak relative to other regions of the FM, and exhibits biochemical and histological characteristics suggestive of increased collagen remodeling. This para-cervical physiological weak zone is present in term FM of both vaginally delivered infants and infants delivered by Caesarian section with no labor (6, 7). Other investigators have also reported distinctive biochemical and histological differences in the para-cervical region of the FM suggestive of increased tissue remodeling. Specifically, increased MMP9, increased apoptosis, and differences in the cellular density have been described within the component membranes (amnion and choriodecidua) of this para-cervical weak zone (8–14). As the rupture tear line consistently passes through the weak zone, we have presumed that FM rupture initiates within this region (5, 12). Recently we reported that FM fragments incubated in vitro with TNF weaken with concomitant changes in biochemical markers of remodeling, mimicking the characteristics of the natural weak zone (15).
Generation of reactive-oxygen species (ROS) has been strongly associated with tissue remodeling, particularly in inflammatory processes initiated by TNF and IL1β (16–19). Furthermore, ROS have been associated with the induction of MMP9 and prostaglandins (20). Antioxidants have therefore been proposed as potential inhibitors of premature FM remodeling and preterm PROM. Given the importance of Vitamin C in collagen synthesis, its widespread dietary use by pregnant and non-pregnant individuals, and its purported minimal toxicity even in large doses, the anti-oxidant Vitamin C could have a potential use in pregnancy to neutralize ROS and thereby prevent aberrant fetal membrane weakening, preterm PROM, and resultant preterm birth. Vitamin E is a lipid-soluble antioxidant that may inhibit the potentially membrane-damaging effects of ROS-induced lipid peroxidation, and has also been proposed as a potential modulator of the risk of fetal membrane rupture. (21). Use of Vitamin C and Vitamin E have been investigated in in vitro studies and in clinical trials (22–25). Because of the postulated benefits of Vitamin C and E upon rupture of membranes, the effect of this anti-oxidant regimen on PROM and preterm PROM was also determined in these studies.
In this single-center, ancillary study performed within an observational component of a multicenter, placebo-controlled trial of Vitamin C and E supplementation for prevention of significant maternal and fetal morbidities associated with pregnancy associated hypertension, we examined FM from participating subjects to determine the effect of the combination of Vitamin C and E given from mid-gestation to delivery upon FM biomechanics, remodeling and the development of the physiological weak zone. Separately, the effect of in-vitro pre-incubation of Vitamin C upon cytokine-induced FM weakening and remodeling was also evaluated.
MATERIALS AND METHODS
Materials
Reagents were from Sigma Chemical Company (St. Louis, MO) unless stated otherwise.
Biological Samples
The protocols for both the in vivo and in vitro studies were approved by the Institutional Review Board of the MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio.
In vivo study
All patients in this study were part of an observational sub-study within a multicenter, placebo controlled investigation of the effect of a combination of Vitamin C (1000 mg/day) and Vitamin E (400 IU/day) given from the second trimester until delivery as part of a trial to prevent significant maternal and fetal morbidities associated with pregnancy associated hypertension (CAPPS trial). The multicenter study was conducted by the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Maternal-Fetal Medicine Units Network. Recruitment for the parent study was from 2007–2009; recruitment for the sub-study was from 2008–2009. Additional consent was obtained from participants for participation in this single-center study. Details of the parent trial are reported in the primary analysis (26). Briefly, women with a singleton pregnancy between 9 weeks 0 days and 16 weeks 6 days of gestation were eligible if they had experienced no prior pregnancy lasting beyond 19 weeks 6 days, did not have hypertension or proteinuria, and were not taking anti-hypertensive medications or more than 150 mg Vitamin C or more than 75 IU Vitamin E daily. Other exclusion criteria were: pregestational diabetes, treatment with antiplatelet drugs or nonsteroidal anti-inflammatory agents, uterine bleeding within the week prior to recruitment, uterine malformation, serious medical complication, known chromosomal or fetal anomalies, in vitro fertilization resulting in the current pregnancy, or illicit drug or alcohol abuse. After an initial compliance trial, eligible and consenting women were randomly assigned to receive two capsules of a combination of 500 mg vitamin C (ascorbic acid) and 200 IU of vitamin E (RRR alpha tocopherol acetate) or a matching placebo (both supplied by Strides, Inc. (Somerset, NJ) daily until delivery. Certified research nurses collected baseline and pregnancy outcome information for each study participant at monthly prenatal study visits and at hospitalization for delivery. Data from all women were analyzed according to the group to which they were randomized, regardless of whether they took the study capsules. FM were collected by the research nurses at delivery and brought immediately to the laboratory. FM biomechanical testing was performed within 1 hour of delivery and tissue samples were taken for later biochemical analysis as described below.
In vitro study
Eleven FM were collected from uncomplicated patients undergoing prelabor, elective repeat cesarean delivery between 37 and 39 weeks gestation, following written informed consent. None of the patients whose FM were obtained for this in vitro study were part of the CAPPS trial. Membranes were discarded if meconium stained, if infection was suspected from the clinical history, if chorioamnionitis was detected on subsequent pathological review, or if the amnion and choriodecidua were separated at delivery. FM were taken immediately after delivery to the laboratory where they were cut from the placental disc and washed in cold PBS. Regions within 1cm of the placental disc and near the para-cervical weak zone were not utilized. FM were then prepared for explant culture after which biomechanical testing and biochemical analysis was performed as described below.
In vivo study- Quantification of Spontaneous FM Separation
If it was clear by inspection that the FM components had separated (amnion from choriodecidua) in greater than 5% of the total FM surface, biomechanical testing was not done. Instead, the percentage separation of the membrane layers was determined in the manner described in our previous report (27). Briefly, FM were cut from the placental disk, washed with Hanks Balanced Salt Solution, removed of blood clots, and then laid flat with amnion side up. All areas where the amnion was separated from the choriodecidua were then cut out. The separated areas (amnion plus choriodecidua) were weighed together. The remaining adherent membranes were then weighed. The percentage of separated FM was determined by dividing the weight of the separated FM by the total weight of the combined (separated plus adherent) membranes.
In vivo study -Topographical Mapping and Search for a Discrete Region of Weakness
If the FM were adherent, our previously reported membrane cutting/mapping procedures and strategy were used to identify the discrete zone of FM weakness (6, 7). A 10-cm diameter disc was centered on the weakest rupture test points on the 3-dimensional FM map. The region within the disc was designated as the weak zone and was compared to the remaining FM for differences in physical properties and biochemical characteristics. A 10-cm diameter was arbitrarily selected because it is the maximum cervical dilatation and thus the maximum area through which the membranes can balloon out during delivery. This diameter also resulted in at least four rupture test points within the designated weak region (four to seven points in the FM examined), which allowed determination that these were weak zones and not merely an area containing a single weak point. Following this procedure, tissue specimens were put aside from both the weak zone and other regions for biochemical testing (Western blot and gel zymography) as described below.
In vivo study - FM Biomechanical Testing
FM physical properties were determined using our previously reported methodology (7, 15). Briefly, biomechanical testing was performed using modified industrial rupture testing equipment (Com-Ten industries, St Petersburg, FL, USA) by ASTM (American Society for Testing and Materials) standards. A mechanically driven, one-cm diameter, rounded plunger was forced at a speed of 8.4 cm / minute, through membrane pieces supported on a fixture with a 2.5cm diameter orifice. Force (applied to the FM) and resultant FM displacement data were collected continuously and analyzed by data reduction software. Rupture strength and Work to Rupture were determined from generated force/displacement curves.
In vitro Vitamin C treatment: FM Explant Culture
FM fragments were cultured as per previously described protocols (15, 28, 29). FM from six patients were used; one patient FM was utilized for each experiment. In each experiment three FM fragments were used for each of the four treatment groups (12 fragments/experiment). FM fragments were bluntly dissected (8cm × 8cm) and placed in 150mm2 culture dishes containing 20ml Earles Minimum Essential Medium, alpha modification (MEM), antibiotic anti-mycotic solution, 50ug/ml gentamicin sulfate and 0.2% lactalbumin hydrosylate (EMEML). Culture dishes were rocked gently in an atmosphere containing 5% CO2, air and 100% relative humidity at 37°C. After 24h equilibration in EMEML, medium was removed and replaced. Cultures were pre-treated with or without 1.0 mM Vitamin C for 6h, then with or without addition of 50ng/ml TNF for an additional 72h. This dose of TNF was selected as the minimum effective dose to reliably produce FM weakening in two previous studies (15, 29). The dose of Vitamin C and preincubation period were selected based on our previous work in amnion cells and amnion tissue (30, 31) and pilot studies at lower Vitamin C doses showing no effect. Four study conditions were utilized: control, Vitamin C pretreatment, TNF alone, Vitamin C pre-treatment followed by TNF. Following incubation, FM samples were subjected to biomechanical testing, Western blot and gel zymography as outlined below.
In vitro Vitamin C treatment: FM Biomechanical Testing
Treated FM fragments were removed from culture, washed twice in 20ml MEM, maintained in MEM and kept moist for the entirety of biomechanical testing. FM physical properties were determined using the methodology described above for FMs derived from the in-vivo trial.
Gel Zymography
In-gel zymographic analysis was performed following polyacrylaminde gel electrophoresis using 40ug cell lysate:ZB on 10% gels/tris-HCl containing gelatin, according to the BioRad Protocol. Active, human, recombinant, 66KD MMP2 and/or 83KDMMP9 (90% purity by SDS-PAGE:EMD Biosciences, La Jolla, CA) were used as relative markers in some zymograms and western blots (below).
Western Blotting
Denatured samples were electrophoresed on 4–15% gels/tris-HCl and resolved proteins were electrophoretically transferred to polyvinylidene fluoride membrane (GE Healthcare, Piscataway, NJ) according to BioRad Protocols. Membranes were blocked in 5% non-fat dry milk/tris-buffered saline:0.5% Tween-20 (TBST) for 30m then incubated overnight at 10°C with MMP9 antibody (1:100) (Santa Cruz, Santa Cruz, CA). Membranes were washed three times in TBST, incubated with HRP-conjugated secondary antibody (1:10000) in 5% non-fat milk:TBST for 30m, then again washed three times in TBST. Chemiluminescent detection was performed using Lumigen-PS according to the manufacturer’s protocol (GE) and blots were exposed against Super RX film (Fuji, Tokyo, Japan) for equivalent time periods.
Quantitation and statistical analysis
Developed films and stained gels were scanned using an EPSON Perfection 1200U scanner. Densitometry was performed using ImageJ software (NIH, Bethesda, MD). The value for an equivalent scanned blank region within a “dye-only” lane of each blot was subtracted from the value for each lane containing protein. Data points for cumulative western blots and zymograms represent the mean +/− SD for amnion cell lysates obtained from 3 different patients. All in vitro experiments were performed at least three times. FM data were analyzed by ANOVA followed by post hoc pair wise comparisons using Statview Software. Data and results are termed significant when P <0.05.
RESULTS
In Vivo Study - Does Supplemental Dietary Vitamin C affect Normal FM Remodeling and Weakening or the Development of the FM Physiological Weak Zone?
Of the 223 patients recruited to the observational study of the parent randomized controlled trial of combined antioxidant supplementation at MetroHealth Medical Center, 73 patients participated in the single center ancillary study. Of these, 39 had amnion-choriodecidua separation of greater than 5% of the total FM surface area, precluding comprehensive strength testing. There was no difference in the number of vitamin treated and control FM that had amnion-choriodecidua separation (17/34 vitamin treated vs. 22/39 placebo, p=.42). There was also no difference in the mean percent separation (63±28% among those vitamin treated vs. 69±27% placebo, p= 0.30). The clinical characteristics of this group were also not different from those whose FM were strength tested (Table 1).
Table 1. Clinical characteristics of CAPPS study patients.
Clinical characteristics of patients whose FM were strength tested and those with greater than 5% separation are shown. Characteristics of preterm patients are separately displayed.
| STRENGTH TESTED | SEPARATED | PRETERM | |||
|---|---|---|---|---|---|
| VITAMIN | PLACEBO | VITAMIN | PLACEBO | TOTAL | |
| Patients (%) |
15 (44.1) | 19 (55.9) | 21 (53.8) | 18 (46.2) | 9 2 Separated |
| Gestational age (weeks)* |
38.6 | 37.6 | 39.7 | 39.1 | 33.9♦ |
| Mean maternal age (years)* |
23.6 | 23.4 | 23.7 | 23.4 | 25.6 |
| AROM (%) |
7 (46.7) | 9 (47.4) | 13 (62) | 13 (72.2) | 5 (55.6) |
| SROM (%) | 8 (53.3) | 10 (52.6) | 8 (38) | 5 (27.8) | 4 (44.4) |
| SVD (%) | 12 (80) | 17 (89.5) | 18 (85.7) | 10 (55.7) | 7 (77.8) |
| C/S (%) | 3 (20) | 2 (10.5) | 3 (14.3) | 8 (44.4) | 2 (22.2) |
| ROM Duration (min)* |
530.6 | 544 | 544 | 750 | 558 |
Abbreviations: AROM – artificial rupture of membranes; SROM – spontaneous rupture of membranes; SVD – spontaneous vaginal delivery; C/S – cesarean section; RO M – rupture of membranes.
(* mean, ♦ gestational age in premature group different from term groups, p < .01)
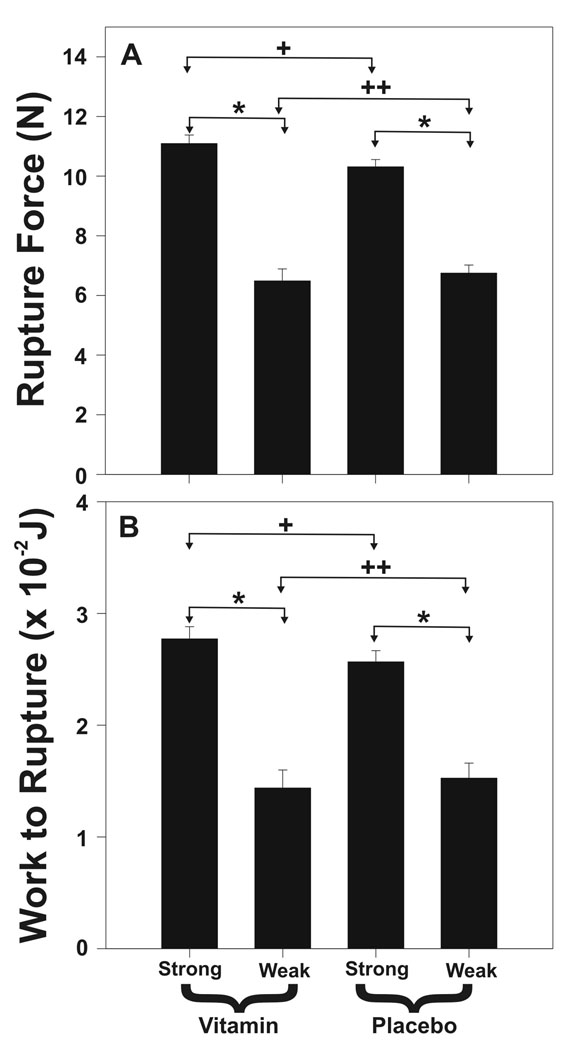
In 27 strength tested term FM (13 vitamin treated and 14 placebo delivered at 37 weeks’ gestation or greater), breaking force and work to rupture were determined in an average of 21.2 locations over the surface of each FM, with 4.3 of those test spots within the weak zone. Weak zones were easily identifiable along the rupture tear lines in both vitamin treated and control groups. Rupture force (6.6±1.5 N vs. 10.7±4.0 N, p=.001) and work to rupture (0.019±.011 joules vs. 0.030±0.014 joules, p=.001) were lower in weak zones compared with other FM regions, respectively (Figure 1). Rupture force (RF) and work to rupture (WR) were not different between vitamin treated and placebo groups when all tested points over the entire FM were considered (RF 10.2±4.4 N vs. 9.6±3.8 N, p=.09 and WR 0.028±0.014 j vs. 0.027±0.013 j, p=.38), when only test points within the weak zones were considered (RF 6.7±2.1 N vs. 6.5±2.9 N, p=.58 and WR 0.019±0.013 j vs. 0.018±0.009 j, p=.91) or when only the weakest point in each FM was considered (RF 4.45±2.0 N vs. 4.2±1.4 N, p=.69 and WR 0.011±0.005 j vs. 0.011±0.004 j, p=.87) (Figure 1).
Figure 1. In Vivo Vitamin C and Vitamin E effect upon FM Strength.
(A) Rupture Force and (B) Work to Rupture are shown for FM of vitamin treated (N = 13) and placebo control (N = 14) patients. Results from FM fragments cut from the weak zone and other areas of each FM are s displayed separately. Data are shown as mean ± SD. (Panel A: + p=.09, ++ p=.71, * p < .001; Panel B: + p=.14, ++ p=.75, * p <.001)
Preterm cases were too few for analysis to be informative (3/34 vitamin treated vs. 6/39 placebo). Two preterm cases had more than 5% separation. Only three preterm cases were remote from term: 30 weeks (placebo), 32 weeks (placebo) and 33 weeks (vitamin treated). Rupture force of these three significantly preterm FM was greater than the term FM (11.4±3.9 N vs. 9.86±4.1 N; p< 0.01) when all test points were considered.
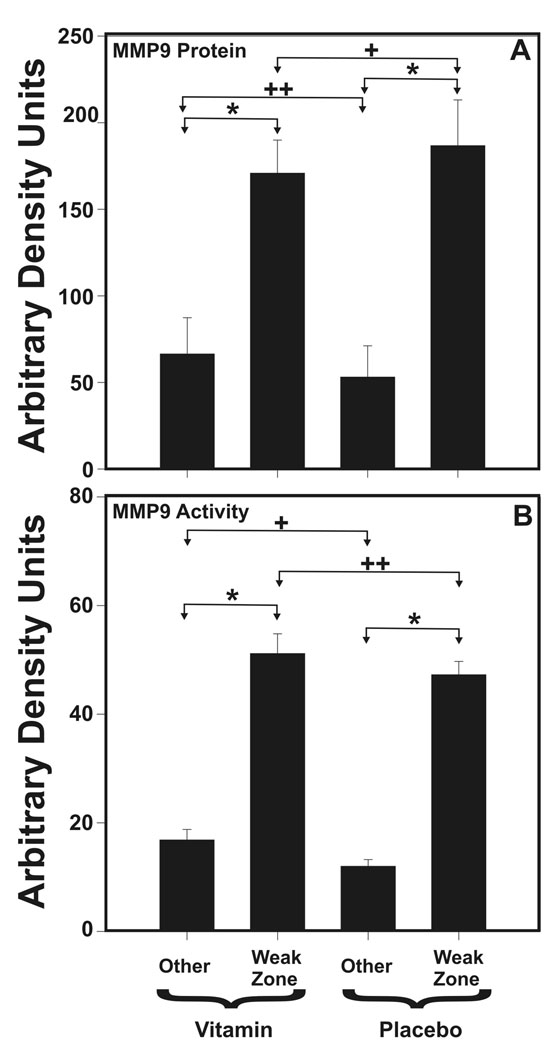
Among term FM, tissue immediately adjacent to the strength tested sites in both vitamin treated and control FM was examined for MMP9 and MMP2 protein and enzymatic activity by densitometric analysis of Western blot and gel zymography, respectively. Consistent with our previous findings, MMP9 protein and activity were increased in the weak zone of each group (both vitamin treated and placebo) when compared with other regions of the same FM (p < 0.001, Figure 2). However, MMP9 protein and activity were not different between the vitamin treated and placebo FM when either weak zones or regions outside the weak zones were compared (Figure 2). MMP2 protein and activity did not differ between weak zone and other FM regions or between vitamin treated and placebo groups (data not shown). Findings for preterm FM were similar (data not shown).
Figure 2. In Vivo Vitamin C and Vitamin E effect upon FM MMP9.
(A) MMP9 immunoreactivity determined by densitometry of Western blots and (B) MMP9 Activity determined by densitometry of Gel zymographs are displayed for FM of vitamin treated (N = 13) and placebo control (N = 14) patients. In each case results from FM fragments cut from the weak zone and other areas of each FM are displayed separately. Data are shown as mean ± SD. (Panel A: + p=.27, ++ p=.17, * p < .001; Panel B: + p=.13, ++ p=.10, * p <.001)
In Vitro Study – Does Vitamin C affect TNF-induced FM remodeling and weakening in vitro?
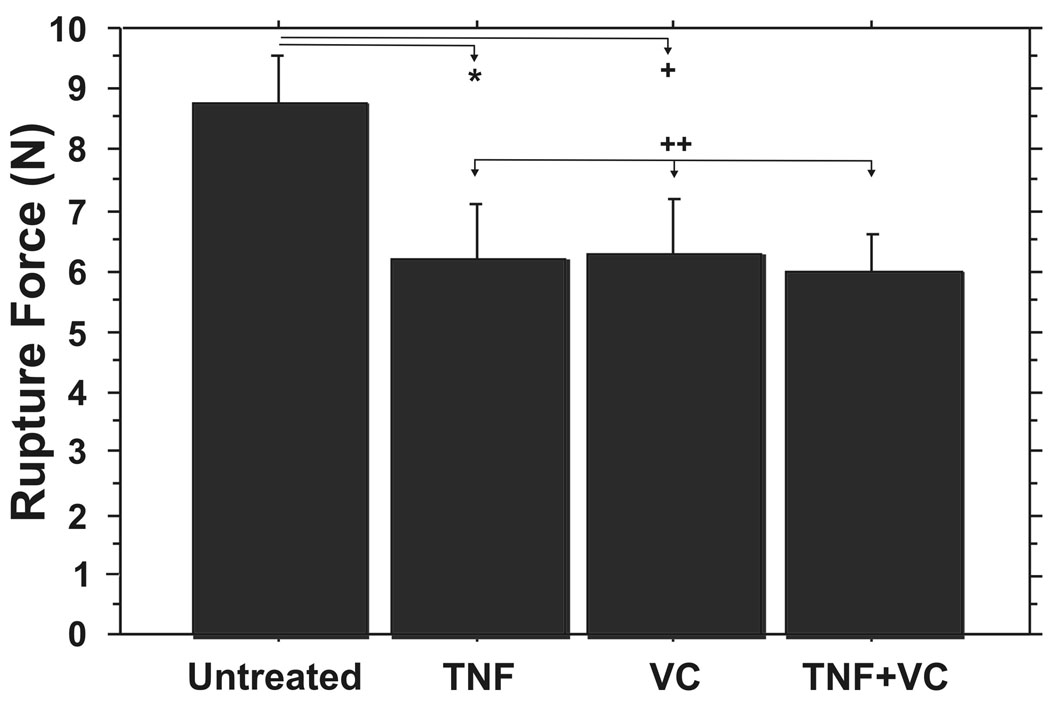
FM fragments incubated with TNF alone exhibited significantly decreased rupture force when compared with controls (6.21±2.47 N vs. 8.76±2.07 N; p=0.04), consistent with our previous reports (15, 29) (Figure 3). Vitamin C pretreatment did not inhibit the TNF-induced weakening (5.97±1.81 N vs. 6.21±2.47 N; p = .86). FM fragments pretreated with Vitamin C and then treated with TNF were the weakest of the four experimental groups (p=0.01 vs. control) (Figure 3). Unexpectedly, FM fragments pretreated with Vitamin C alone showed a significant decrease in rupture force compared with the control group (6.26±2.79 N vs 8.76±2.07 N; p=0.02) (Figure 3).
Figure 3. In Vitro Vitamin C does not inhibit TNF induced FM weakening.
TNF incubation resulted in significant FM weakening that was not inhibited by pretreatment with Vitamin C. Rupture Force for four treatment groups are displayed: Control, Vitamin C pre-treatment, TNF, Vitamin C followed by TNF (see Methods for details). Data points represent pooled results of six experiments using three FM fragments per treatment group in each experiment (N=18). Data are presented as mean ± SD. (* p=.04, + p=.02, ++ p=.86)
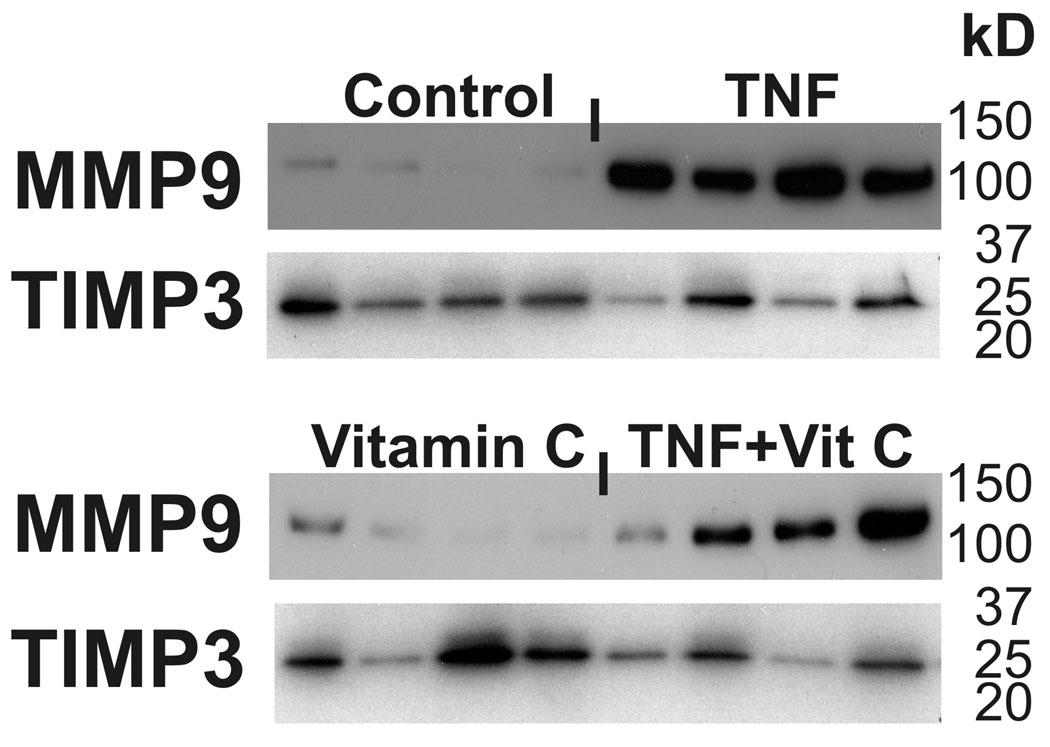
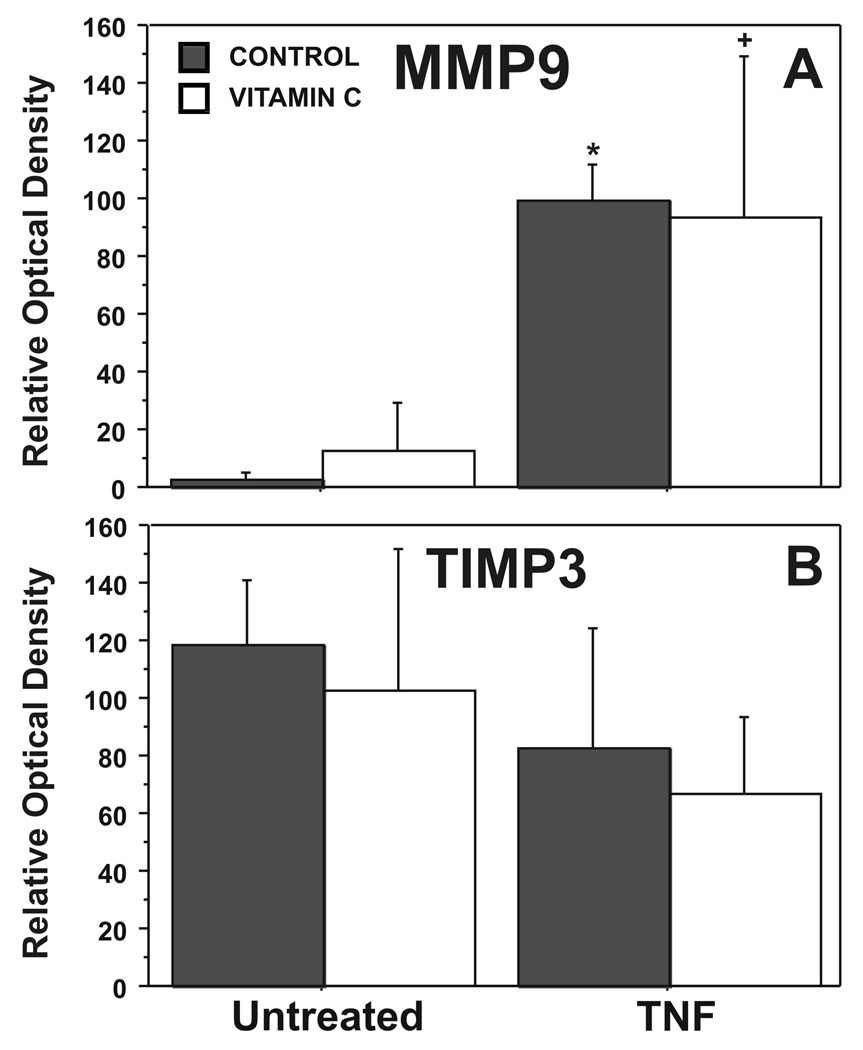
Consistent with our previous in vitro studies of TNF induced FM weakening, Western Blot analysis demonstrated minimal detectable MMP9 protein in the control FM and high levels of MMP-9 protein with TNF treatment (5). Vitamin C did not significantly blunt the TNF induced increases in MMP9 (Figure 4). Densitometer analysis of Western Blots confirmed the differences in MMP9 between the control group and both the TNF treatment group (p<0.001), and the group preincubated with Vitamin C and then treated with TNF (p= 0.03) (Figure 5A). TIMP-3 was not different between control and TNF treated groups (Figure 5B).
Figure 4. In Vitro Vitamin C does not inhibit TNF induced changes in MMP9 and TIMP3.
Western Blots for a representative FM. Treatment groups include: Control, Vitamin C pre-treatment, TNF, Vitamin C followed by TNF (see Methods for details). Data are presented as mean ± SD.
Figure 5. In Vitro Vitamin C does not inhibit TNF induced changes in MMP9 and TIMP3.
Densitometry for pooled results of five experiments using three FM fragments per treatment group in each experiment (N=15) are displayed. A. MMP9; B. TIMP3. Treatment groups include: Control, Vitamin C pre-treatment, TNF, Vitamin C followed by TNF (see Methods for details). (* p <.001, + p < .05, both versus control untreated)
DISCUSSION
The major findings of these studies were: 1) Maternal supplementation with Vitamin C in combination with Vitamin E throughout pregnancy did not change the normal FM remodeling process or the development of the weak zone as manifest at term; 2) The biomechanical and biochemical characteristics of the FM physiological weak zones of vitamin treated and placebo patients who delivered at term were similar; 3) Vitamin C preincubation did not inhibit the ability of TNF to induce FM remodeling and weakening in vitro; and 4) Vitamin C itself had a unexpected weakening effect upon FM in vitro.
Vitamin C, a water soluble agent, is excreted freely in the pregnant women’s urine with increasing doses. Urinary excretion increases with gestation in step with the normal increase in GFR, resulting in a decrease in plasma levels with increasing gestation. Vitamin C reaches the avascular FM largely through the maternal circulation to the decidual side. A small portion also reaches the FM from the amniotic side via crossing the placenta into the fetal circulation, excretion in the fetal urine, and entry into the amniotic fluid. Although amniotic fluid Vitamin C levels decrease with gestation, they are greater with supplementation as demonstrated in studies of maternal – fetal transport in guinea pigs by Das et al (32). The decidual side of the FM sees direct exposure from the maternal circulation. Studies of Casanueva et al demonstrate that although the plasma levels of Vitamin C in supplemented pregnant women (only 100 mg/d) were not greater than those of unsupplemented women and decreased with gestation, leukocyte levels increased markedly with supplementation (25). Tissue levels in the FM would thus also be expected to increase with supplementation.
More than half of the FM in our in vivo study had greater than 5% separation of the amnion from the choriodecidua and therefore were not strength tested because we could not comprehensively compare the rupture strength over the entire FM surface. Separated FM have generally been excluded from biomechanical studies. We and others have recently reported that separation is a component of the normal FM weakening process (11, 27, 33, 34). In this study, we found that the number of FM demonstrating separation, and the percentage of separation, was similar in the vitamin treated and placebo groups.
Normal rupture of term FM is thought to initiate in a weakened para-cervical region which develops late in gestation due to collagen remodeling and apoptosis (2, 5, 11). The findings of this report were unexpected as Vitamin C is reported to counteract these processes. Specifically, Vitamin C modulates collagen mRNA transcription, plays an essential role in the formation and stabilization of the collagen triple helix, is involved in the formation of strengthening collagen cross-links (35), and also protects collagen by shielding TIMP from degradation by ROS. In many studies Vitamin C is also shown to inhibit apoptosis (36). The impetus for clinical trials of Vitamin C to prevent preterm PROM stems from numerous reports of a relationship of increased ROS concentration, decreased antioxidants, and preterm PROM. Lower levels of Vitamin C in serum, leucocytes and amniotic fluid (37–40), and low Vitamin C intake before or during pregnancy have also been associated with an increased risk of preterm PROM (24). These reports were re-enforced by in vitro studies suggesting supplemental anti-oxidant vitamins may be effective in preventing PROM. Plessinger et al demonstrated that in vitro Vitamin C (28.8 mg/ml) and Vitamin E pretreatment prevented hypochlorous acid induced apoptosis in the amnion epithelium and collagen degradation (38). Tannetta et al showed that a combination of Vitamin C and E inhibited apoptosis of trophoblast cells in culture (41). Ahn et al. studied 80 normal-term placentas of patients, half with low levels and the other half with high levels of prenatal Vitamin C. They reported that high levels of prenatal Vitamin C were associated with decreased apoptosis in syncytiotrophoblastic cells and decreased expression of the endothelial scavenger receptor LOX-1. Activation of LOX-1 induces the generation of ROS and decreases nitric oxide release from endothelial cells (42). Finally, in a single study, daily supplementation with Vitamin C after 20 weeks gestation was reported to decrease the incidence of PROM (24).
Other investigators have reported conflicting results. Vitamin C has been reported to induce apoptosis in human articular chondrocytes (43), PC 12 cells (44), and human promyelocytic leukemic HL-60 cells (45). It also has been found to induce decomposition of lipid hydroperoxides to endogenous genotoxins (46), and increase oxidative stress in humans after acute muscle injury in the presence of N-acetyl-cysteine (47). In addition, our laboratory has reported that Vitamin C pre-incubation does not inhibit, and may exacerbate, hydrogen peroxide induced apoptosis in WISH cells (30), amnion epithelial and mesenchymal cells, and amnion tissue (31). It is thus clear, that in in-vitro environments Vitamin C can act either in an anti-oxidant or pro-oxidant manner. Specific evidence is provided by Muhlhofer et al that high intervenous doses of Vitamin C are less likely to have pro-oxidant effects in vivo (48). Nonetheless, Spinnato et al suggested that this vitamin C and E combination may increase the risk of preterm PROM (25). Additionally, the parent trial for this single center ancillary study found no reductions in preterm PROM with vitamin supplementation (26). These latter findings are consistent with our finding that Vitamin C does not strengthen, and can weaken membranes, and our previous finding that Vitamin C can precipitate amnion cell apoptosis (31).
Although the antioxidant Vitamin C did not inhibit TNF induced FM weakening, we have recently reported that another antioxidant, α-lipoic acid, was effective in this regard. The mechanism by which α-lipoic acid inhibits TNF and IL1β effects in full-thickness FM and amnion cells is not certain. TNF and IL1β are known to generate ROS, with subsequent upregulation of nuclear transcription factor nuclear factor-kB (NFkB). Induction and DNA binding of NFkB are known inducers of both PGE2 production and MMP9 (49). Alpha-lipoic acid has previously been shown to inhibit TNF-induced NFkB transcriptional activity and MMP9 expression in vascular smooth muscle cells (50), inhibit bone resorption by suppressing PGE2 synthesis (51), and attenuate LPS-induced NFkB DNA binding in monocytes by activating the PI3K/AKT pathway (52, 53). Thus, the mechanism of α-lipoic acid action may be through blockade of NFkB rather than antioxidant properties.
Although our study had sufficient power to address the effect of Vitamin C and E on the weakening process in term FM, there were not enough FM from preterm deliveries for an adequate investigation of the effect of Vitamin C and E on prematurely delivered FM. Furthermore, the six preterm FM strength tested were all from deliveries at greater than 30 weeks gestation and thus did not include patients in the range of greatest clinical interest. Only two studies describing the biomechanics of FM of prematurely delivered infants exist; neither recognizes that FM are heterogeneous over their surfaces (54, 55). The average breaking force of preterm-delivered FM was greater than that of term-delivered FM, consistent with the results of these previous studies (54, 55). There were not enough preterm FM to compare vitamin treatment versus placebo groups. Future research will be directed toward understanding possible differences in the effect of Vitamin C on preterm versus term FM.
Our study demonstrates that supplemental Vitamin C does not impact the normal FM remodeling process that leads to weakening and rupture at term. Also, Vitamin C does not inhibit the ability of TNF to induce weakness in term FM treated in vitro. Furthermore, because Vitamin C treatment alone caused FM weakness in our in vitro model system, we speculate that Vitamin C, particularly at high doses, may induce collagen degradation and apoptosis. The assumption that supplemental Vitamin C might be beneficial and would, in any case, not be harmful as a prophylaxis regimen for preterm PROM may be erroneous. Caution should be exercised in utilizing Vitamin C supplementation to prevent preterm PROM.
Acknowledgement
The authors wish to thank the MFMU research nurses at MetroHealth Medical Center for their help in collecting the placentas which made this study possible.
Support: The project described was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development HD40544-01 (BMM), HD48476 (JJM); The National Heart, Lung, and Blood Institute; and the National Center for Research Resources (M01 RR00080, UL1 RR024989). Its contents do not necessarily represent the official view of NICHD, NHLBI, NCRR or NIH.
Footnotes
Presentations: Data presented in part at Annual Meeting of the Society of Gynecology Investigation, Reno, NV, March 2007, International Federation of Placenta Associations meeting in Kingston, ON, Canada, August, 2007, and MFM meeting February, 2010.
REFERENCES
- 1.Mercer BM. Premature Rupture of the membranes. An expert’s view. Obstet Gynecol. 2003;101:178–193. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 2.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87(6):590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 3.Parry S, Strauss JF., 3rd Premature rupture of the fetal membranes. N Engl J Med. 1998;338(10):663–670. doi: 10.1056/NEJM199803053381006. [DOI] [PubMed] [Google Scholar]
- 4.Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11(7):427–437. doi: 10.1016/j.jsgi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27(11–12):1037–1051. doi: 10.1016/j.placenta.2006.01.002. (Review) [DOI] [PubMed] [Google Scholar]
- 6.El Khwad M, Pandey V, Stetzer B, et al. Fetal membranes from term vaginal deliveries have a zone of weakness exhibiting characteristics of apoptosis and remodeling. J Soc Gynecol Investig. 2006;13(3):191–195. doi: 10.1016/j.jsgi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.El Khwad M, Stetzer B, Moore RM, et al. Term human fetal membranes have a weak zone overlying the lower uterine pole and cervix before onset of labor. Biol Reprod. 2005;72(3):720–726. doi: 10.1095/biolreprod.104.033647. [DOI] [PubMed] [Google Scholar]
- 8.Malak TM, Bell SC. Structural characteristic of term human fetal membranes: A novel zone of extreme morphological alteration within the rupture site. Br J Obstet Gynecol. 1994;101:375–386. doi: 10.1111/j.1471-0528.1994.tb11908.x. [DOI] [PubMed] [Google Scholar]
- 9.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14(1):237–241. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 10.McLaren J, Taylor DJ, Bell SC. Prostaglandin E(2)-dependent production of latent matrix metalloproteinase-9 in cultures of human fetal membranes. Mol Hum Reprod. 2000;6(11):1033–1040. doi: 10.1093/molehr/6.11.1033. [DOI] [PubMed] [Google Scholar]
- 11.Meinert M, Malmström A, Tufvesson E, et al. Labour induces increased concentrations of biglycan and hyaluronan in human fetal membranes. Placenta. 2007;28:482–486. doi: 10.1016/j.placenta.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Reti NG, Lappas M, Riley C, et al. Why do membranes rupture at term? Evidence of increased cellular apoptosis in the supracervical fetal membranes. Am J Obstet Gynecol. 2007;196(5):484.e1–484.e10. doi: 10.1016/j.ajog.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Lappas M, Odumetse TL, Riley C, et al. Pre-labour fetal membranes overlying the cervix display alterations in inflammation and NF-kappaB signalling pathways. Placenta. 2008;29(12):995–1002. doi: 10.1016/j.placenta.2008.09.010. Epub 2008 Oct 25. [DOI] [PubMed] [Google Scholar]
- 14.Lappas M, Riley C, Rice GE, Permezel M. Increased expression of ac-FoxO1 protein in prelabor fetal membranes overlying the cervix: possible role in human fetal membrane rupture. Reprod Sci. 2009;16(7):635–641. doi: 10.1177/1933719109332831. Epub 2009 Mar 16. [DOI] [PubMed] [Google Scholar]
- 15.Kumar D, Fung W, Moore RM, et al. Proinflammatory cytokines found in amniotic fluid induce collagen remodeling, apoptosis, and biophysical weakening of cultured human fetal membranes. Biol Reprod. 2006;74(1):29–34. doi: 10.1095/biolreprod.105.045328. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan M, Yu Z, Ferrans V, Sulciner D, et al. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulciner D, Irani K, Yu Z, Ferrans V, Goldschmidt-Clermonst P, Finkel T. Rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol. 1996;16:7115–7121. doi: 10.1128/mcb.16.12.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowie A, O'Neill LA. Oxidative stress and nuclear factor-kappaB activation: a reassessment of the evidence in the light of recent discoveries. Biochem Pharmacol. 2000;59(1):13–23. doi: 10.1016/s0006-2952(99)00296-8. [DOI] [PubMed] [Google Scholar]
- 19.Liu ZG. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005;15(1):24–27. doi: 10.1038/sj.cr.7290259. [DOI] [PubMed] [Google Scholar]
- 20.Lappas M, Permezel M, Rice GE. N-Acetyl-cysteine inhibits phospholipid metabolism, proinflammatory cytokine release, protease activity and nuclear factor-kappaB deoxyribonucleic acid-binding activity in human fetal membranes in vitro. J Clin Endocrinol Metab. 2003;88(4):1723–1729. doi: 10.1210/jc.2002-021677. [DOI] [PubMed] [Google Scholar]
- 21.Woods JR, Jr, Plessinger MA, Miller RK. Vitamins C and E: missing links in preventing preterm premature rupture of membranes? Am J Obstet Gynecol. 2001;185(1):5–10. doi: 10.1067/mob.2001.115868. [DOI] [PubMed] [Google Scholar]
- 22.Steyn PS, Odentaal HJ, Schoeman J, Stander C, Fanie N, Grove D. A randomized, double-blind placebo-controlled trial of ascorbic acid supplementation for the prevention of preterm labor. J Obste. Gynaecol. 2003;23(2):150–155. doi: 10.1080/014436103000074673. [DOI] [PubMed] [Google Scholar]
- 23.Rumbold A, Crowther CA. Vitamin C supplementation in pregnancy. Cochrane Database of Systematic Reviews. 2005;(Issue 1) doi: 10.1002/14651858.CD004069.pub2. Art. No.: CD004072. DOI: 10.1002/14651858.CD004072.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Casanueva E, Ripoll C, Tolentino M, et al. Vitamin C supplementation to prevent premature rupture of the chorioamniotic membranes: a randomized trial. Am J Clin Nutr. 2005;81(4):859–863. doi: 10.1093/ajcn/81.4.859. [DOI] [PubMed] [Google Scholar]
- 25.Spinnato JA, 2nd, Freire S, Pinto e Silva JL, et al. Antioxidant supplementation and premature rupture of the membranes: a planned secondary analysis. Am J Obstet Gynecol. 2008;199(4):433.e1–433.e8. doi: 10.1016/j.ajog.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts JM, Myatt L, Spong CY, et al. for the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Vitamin C and E do not reduce maternal and fetal/neonatal adverse outcomes related to pregnancy associated hypertension when administered to low risk nulliparous women from early gestation. New Engl J Med. 2010 (In Press) [Google Scholar]
- 27.Strohl A, Kumar D, Novince R, et al. Decreased Adherence and Spontaneous Separation of Fetal Membrane Layers--Amnion and Choriodecidua—a Possible Part of the Normal Weakening Process. Placenta. 2010;31(1):18–24. doi: 10.1016/j.placenta.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fortunato SJ, Menon R, Swan KF, Lyden TW. Organ culture of amniochorionic membrane in vitro. Am J Reprod Immunol. 1994;32(3):184–187. doi: 10.1111/j.1600-0897.1994.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 29.Moore RM, Novak JB, Kumar D, Mansour JM, Mercer BM, Moore JJ. Alpha-lipoic acid inhibits tumor necrosis factor-induced remodeling and weakening of human fetal membranes. Biol Reprod. 2009;80(4):781–787. doi: 10.1095/biolreprod.108.073205. Epub 2008 Dec 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar D, Lundgren DW, Moore RM, Silver RJ, Moore JJ. Hydrogen peroxide induced apoptosis in amnion-derived WISH cells is not inhibited by vitamin C. Placenta. 2004;25(4):266–272. doi: 10.1016/j.placenta.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kumar D, Moore RM, Elkhwad M, Silver RJ, Moore JJ. Vitamin C exacerbates hydrogen peroxide induced apoptosis and concomitant PGE2 release in amnion epithelial and mesenchymal cells, and in intact amnion. Placenta. 2004;25(6):573–579. doi: 10.1016/j.placenta.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Das S, Powers HJ. The effects of maternal intake and gestational age on materno-fetal transport of vitamin C in the guinea pig. Brit J Nutri. 1998;80:485–491. [PubMed] [Google Scholar]
- 33.Arikat S, Novince RW, Mercer BM, et al. Separation of amnion from choriodecidua is an integral event to the rupture of normal term fetal membranes and constitutes a significant component of the work required. Am J Obstet Gynecol. 2006;194(1):211–217. doi: 10.1016/j.ajog.2005.06.083. [DOI] [PubMed] [Google Scholar]
- 34.Kumar D, Novince R, Strohl A, et al. A new methodology to measure strength of adherence of the fetal membrane components, amnion and the choriodecidua. Placenta. 2009;30(6):560–563. doi: 10.1016/j.placenta.2009.03.014. Epub 2009 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selman C, McLaren JS, Meyer C, et al. Life-long Vitamin C supplementation in combination with cold exposure does not affect oxidative damage or lifespan in mice, but decreases expression of antioxidant protection genes. Mech Ageing Dev. 2006;127(12):897–904. doi: 10.1016/j.mad.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Agostini F, Izzotti A, Balansky R, Bennicelli C, De Flora S. Modulation of apoptosis by cancer chemopreventive agents. Mutation Research. 2005;591:173–186. doi: 10.1016/j.mrfmmm.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Woods JR, Jr, Cavanaugh JL, Norkus EP, Plessinger MA, Miller RK. The effect of labor on maternal and fetal vitamins C and E. Am J Obstet Gynecol. 2002 Nov;187(5):1179–1183. doi: 10.1067/mob.2002.127131. [DOI] [PubMed] [Google Scholar]
- 38.Plessinger MA, Woods JR, Miller RK. Pretreatment of human amnion-chorion with Vitamins C and E prevents hypochlorous acid-induced damage. Am J Obstet Gynecol. 2000;183:979–985. doi: 10.1067/mob.2000.106676. [DOI] [PubMed] [Google Scholar]
- 39.Siega-Riz AM, Promislow JH, Savitz DA, Thorp JM, Jr, McDonald T. Vitamin C intake and the risk of preterm delivery. Am J Obstet Gynecol. 2003;189(2):519–525. doi: 10.1067/s0002-9378(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 40.Barrett BM, Sowell A, Gunter E, Wang M. Potential role of ascorbic acid and beta-carotene in the prevention of preterm rupture of fetal membranes. Int J Vitam Nutr Res. 1994;64(3):192–197. [PubMed] [Google Scholar]
- 41.Tannetta DS, Sargent IL, Linton EA, Redman CWG. Vitamins C and E inhibit apoptosis of cultured human term placenta trophoblast. Placenta. 2008;29:680–690. doi: 10.1016/j.placenta.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Ahn YM, Kim YJ, Park H, Park B, Lee H. Prenatal vitamin C status is associated with placental apoptosis in normal-term human pregnancies. Placenta. 2007;28(1):31–38. doi: 10.1016/j.placenta.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Malicev E, Woyniak G, Knezevic, Radosavljevic D, Jeras M. Vitamin C induced apoptosis in human articular chondrocytes. Pflugers Arch. 2000;440 Suppl:R46–R48. [PubMed] [Google Scholar]
- 44.Si F, Ross GM, Shin SH. Glutathione protects PC 12 cells from ascorbate and dopamine induced apoptosis. Exp Brain Res. 1998;123:263–268. doi: 10.1007/s002210050568. [DOI] [PubMed] [Google Scholar]
- 45.Satoh K, Ida Y, Hosaka M, et al. Induction of apoptosis by cooperative action of vitamins C and E. Anticancer Res. 1998;18:4371–4375. [PubMed] [Google Scholar]
- 46.Lee SH, Oe T, Blair IA. Vitamin C-induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 47.Childs A, Jacobs, Kaminski T, Halliwell B, Leeuwenburgh C. Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med. 2001;31:745–753. doi: 10.1016/s0891-5849(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 48.Muhlhofer A, Mrosek S, Schlegel B, Trommer W, Rozario F, Bohles H, Schremmer D, Zoller WG, Biesalski HK. High-dose intravenous vitamin C is not associated with an increase of prooxidative biomarkers. Eur J Clin Nutrition. 2004;58:1151–1158. doi: 10.1038/sj.ejcn.1601943. [DOI] [PubMed] [Google Scholar]
- 49.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction. 2005;130:569–581. doi: 10.1530/rep.1.00197. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Kim H, Park K, et al. α-Lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-κB transcriptional activity. Exp Mol Med. 2007;39(1):106–113. doi: 10.1038/emm.2007.12. [DOI] [PubMed] [Google Scholar]
- 51.Ha H, Lee J, Kim H, et al. α-Lipoic acid inhibits inflammatory bone resorption by suppressing prostaglandin E2 synthesis. J Immunol. 2005;176:111–117. doi: 10.4049/jimmunol.176.1.111. [DOI] [PubMed] [Google Scholar]
- 52.Zhang W, Frei B. α-Lipoic acid inhibits TNF-α-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Wei H, Frei B. α-Lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. PNAS. 2007;104(10):4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pressman EK, Cavanaugh JL, Woods JR. Physical properties of the chorioamnion throughout gestation. Am J Obstet Gynecol. 2002;187(3):672–675. doi: 10.1067/mob.2002.125742. [DOI] [PubMed] [Google Scholar]
- 55.Chua WK, Oyen ML. Do we know the strength of the chorioamnion? A critical review and analysis. Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S128–S133. doi: 10.1016/j.ejogrb.2009.02.029. Epub 2009 Mar 14. Review. [DOI] [PubMed] [Google Scholar]