Abstract
The hypothalamic hormone gonadotropin-releasing hormone (GnRH) stimulates the synthesis and release of the pituitary gonadotropins. GnRH acts through a plasma membrane receptor that is a member of the G protein-coupled receptor (GPCR) family. These receptors interact with heterotrimeric G proteins to initiate downstream signaling. In this study, we have investigated which G proteins are involved in GnRH receptor-mediated signaling in LβT2 pituitary gonadotrope cells. We have shown previously that GnRH activates ERK and induces the c-fos and LHβ genes in these cells. Signaling via the Gi subfamily of G proteins was excluded, as neither ERK activation nor c-Fos and LHβ induction was impaired by treatment with pertussis toxin or a cell-permeable peptide that sequesters Gβγ-subunits. GnRH signaling was partially mimicked by adenoviral expression of a constitutively active mutant of Gαq (Q209L) and was blocked by a cell-permeable peptide that uncouples Gαq from GPCRs. Furthermore, chronic activation of Gαq signaling induced a state of GnRH resistance. A cell-permeable peptide that uncouples Gαs from receptors was also able to inhibit ERK, c-Fos, and LHβ, indicating that both Gq/11 and Gs proteins are involved in signaling. Consistent with this, GnRH caused GTP loading on Gs and Gq/11 and increased intracellular cAMP. Artificial elevation of cAMP with forskolin activated ERK and caused a partial induction of c-Fos. Finally, treatment of Gαq (Q209L)-infected cells with forskolin enhanced the induction of c-Fos showing that the two pathways are independent and additive. Taken together, these results indicate that the GnRH receptor activates both Gq and Gs signaling to regulate gene expression in LβT2 cells.
The family of G protein-coupled receptors is the largest and most complex group of integral membrane proteins involved in signal transduction. These receptors can be activated by a diverse array of external stimuli, including growth factors, neurotransmitters, peptide, and protein hormones, chemokines, and other ligands. Agonist binding to a specific receptor on the cell surface causes a conformational change in the receptor that allows it to interact with its cognate G protein, stimulating guanine nucleotide exchange on the α-subunit of the G protein. The release of the GTP-bound α-subunit and βγ-subunits from the receptor-G protein complex initiates a broad range of intracellular signaling events, including the activation of classical effectors such as phospholipase C, adenylate cyclases, and ion channels, and regulation of the intracellular level of inositol phosphates, calcium, cyclic AMP, and other second messengers (for reviews see Refs. 1–8).
Gonadotropin-releasing hormone (GnRH)1 is a hypothalamic decapeptide, which serves as a key regulator of the reproductive system. In the pituitary, GnRH signals are transmitted via a specific cell surface receptor, which is a member of the G protein-coupled receptor superfamily. When GnRH binds to its receptor, it induces interaction of the receptor with heterotrimeric G proteins. This interaction then initiates a variety of intracellular signaling events, including an increase in phosphoinositide turnover, which results in a rise in intracellular diacylglycerol and calcium levels, and an increase in intracellular cAMP levels (9–13). These second messengers then activate downstream kinases including protein kinase C, calcium-dependent kinases such as Pyk2 and calmodulin-dependent kinase IV, and the cAMP-dependent protein kinase PKA.
In dispersed pituitary cell cultures, treatment with pertussis toxin (PTX) results in decreased inositol phosphate (IP) turnover in response to GnRH, suggesting that a PTX-sensitive G protein (such as Gi/o) couples the receptor to IP turnover (14, 15). In human reproductive tract tumors, the GnRH receptor also couples to Gi (16). However, in G-GH3 cells, which are GH3 somatomammotropes transfected with the rat GnRH-receptor, GnRH evoked IP turnover is insensitive to PTX (17), indicating that a different G protein may be involved in signal transduction in these cells.
Studies using immuno-depletion and G protein labeling showed that the GnRH receptor is coupled to Gq/11 in αT3–1 pituitary cells (18, 19). Similarly, in CHO-K1 and COS-7 cells expressing the human GnRH receptor, GnRH couples exclusively to the Gq/11 family of G proteins (19). However, the GnRH receptor also couples to Gs in primary pituitary cultures and G-GH3 cells. This G protein activates adenylate cyclase, leading to production of cAMP and activation of protein kinase A (20, 21). The promiscuity of the GnRH receptor is underscored by recent studies (22) showing that the GnRH receptor is able to couple to all three subfamilies of G proteins, Gq/11, Gs, and Gi, when overexpressed in rat pituitary cultures and G-GH3 cells. It is evident from all of these studies that cell context is extremely important for coupling of the GnRH receptor to different G proteins and highlights the danger of extrapolating results from one cell type to another.
We have demonstrated recently (23) that GnRH activates the ERK, c-Jun N-terminal kinase, and p38 MAPK families in the LβT2 cells. These cells express the mRNAs for the GnRH receptor and hence for the α- and β-subunits of LH and FSH are a good model for pituitary gonadotropes (24, 25). Activation and nuclear localization of ERK occur via a PKC and MEK-dependent but calcium-independent process. GnRH also induces c-Fos and LHβ protein expression. Surprisingly, induction of both of these genes is PKC-independent but calcium- and MEK-dependent in LβT2 cells (23). Both PKC and calcium signaling are activated via the phospholipase C pathway. Activation of phospholipase C would be consistent with coupling to Gαq as this G protein can activate PLCβ1 and β3 (26). However, activation can also be Gαq-independent as Gβγ can activate PLCβ2 (27).
In this study, we address the question of whether multiple G proteins are involved in GnRH receptor signaling in LβT2 cells. By using membrane-permeable TAT peptides designed to uncouple the receptor from the G protein, we show that both Gq/11 and Gs proteins are involved in GnRH receptor signaling in LβT2 cells.
EXPERIMENTAL PROCEDURES
Materials
GnRH was purchased from Sigma. Phorbol 12-myristate 13-acetate (PMA), forskolin, and protein kinase A inhibitor 14–22 (PKI) were from Calbiochem. The rabbit polyclonal anti-active MAPK antibodies raised against the dually phosphorylated form of ERK1 (Thr202/Tyr204) were from Promega (Madison, WI) or Cell Signaling Technologies (Worcester, MA). Rabbit and goat polyclonal anti-c-Fos antibodies (sc-52 and sc-52-G), the Gαq/11 and Gαs C-terminal antibodies, and the horseradish peroxidase-linked anti-rabbit secondary antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The rabbit polyclonal anti-LHβ antibody was kindly provided by Dr. A. F. Parlow at the National Hormone Pituitary Program, NIDDK, National Institutes of Health. TRITC-conjugated anti-rabbit antibodies were purchased from Jackson ImmunoResearch Laboratory, Inc. (West Grove, PA). Recombinant adenoviruses expressing lacZ or wild-type or GTPase-deficient (activated) Q209L mutant Gαq have been described elsewhere (28). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Invitrogen. All other reagents were purchased from either Sigma or Fisher.
Cell Culture
LβT2 cells were maintained in monolayer cultures in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics at 37 °C in a 10% CO2 environment. Cells were starved overnight in serum-free DMEM and then stimulated with GnRH or other agonists. hIRcB cells, which are Rat-1 fibroblasts overexpressing the human insulin receptor, were maintained as described previously (29) in DMEM/Ham’s F-12 medium with 50 units/ml penicillin, 50 µg/ml streptomycin, 10% FBS, 0.5% glutamax, and 500 nm methothrexate at 37 °C in a 5% CO2 environment.
Expression of Fusion Proteins
The βARK-CT fusion protein was purified as a GST fusion protein as described previously (30). For the TAT fusion peptides, oligonucleotides encoding the Gq-CT (QLNLKEYNLV), Gs-CT (RMHLRQYELL), or PLCβ2 (NRSYVISSFTELKAYDLLSK) peptides were cloned into expression vector pTAT-HA (31). Fusion proteins containing the desired peptide fused to hexahistidine and HA tags were expressed in BL-21-SI cells and purified in the denatured state on Ni2+-Sepharose beads using standard protocols. Recombinant protein was eluted in a gradient of imidazole and dialyzed against PBS. Protein concentration was determined using the Bradford assay, and aliquots of the peptides were frozen at −80 °C until use.
Immunostaining
Immunostaining was performed essentially as described previously (23). For c-Fos and LHβ staining, LβT2 cells were plated on 10-mm acid-washed glass coverslips and stimulated with agonists at 37 °C. Cells were washed with phosphate-buffered saline (PBS) and fixed with 3.7% formaldehyde in PBS for 20 min at room temperature. Following two washes in PBS, the cells were permeabilized and blocked in PBS containing 5% BSA and 0.5% Nonidet P-40 for 10 min. Coverslips were incubated with the rabbit anti-c-Fos antibody (1:400 dilution) or rabbit anti-LHβ antibody (1:1200 dilution) for 60 min at room temperature, washed once in PBS, and then incubated with TRITC-conjugated anti-rabbit IgG antibody (1:100 dilution) in PBS with 5% BSA and 0.5 Nonidet P-40 for 30 min at room temperature. Following a wash with PBS, coverslips were incubated with a DNA intercalating dye (Hoechst 33258, Sigma) diluted 1:250 for 60 min to stain nuclei. Finally, the coverslips were extensively washed with PBS, rinsed with water, and mounted in PBS containing 15% gelvatol (polyvinyl alcohol), 33% glycerol, and 0.1% sodium azide.
For phospho-ERK staining, cells were washed with PBS, fixed in 3.7% formaldehyde in PBS as above, and then washed with TBS-Triton (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 0.1% Triton X-100). The cells were permeabilized in 100% methanol at −20 °C for 10 min, washed with TBS-Triton, then blocked with 5% normal horse serum in TBS-Triton for 60 min at room temperature to reduce nonspecific staining. Coverslips were incubated with the anti-active MAPK antibody at a 1:400 dilution in 5% bovine serum albumin in TBS-Triton overnight at 4 °C. The cells were washed with 0.1% BSA in TBS-Triton and then incubated with a TRITC-conjugated anti-rabbit IgG antibody at a 1:100 dilution in 3% BSA in TBS-Triton for 60 min at room temperature. Coverslips were washed with TBS-Triton, incubated with Hoechst 33258 dye (1:250 dilution) in TBS-Triton for 60 min at room temperature. The coverslips were washed and mounted as described above. Staining was visualized on a Zeiss Axiophot fluorescence microscope and photographed using the ISEE imaging system (Inovision, Raleigh, NC). The percentage of cells showing phospho-ERK, c-Fos, or LHβ immunofluorescence was counted from a minimum of five independent fields of cells per experiment.
Western Blotting
LβT2 or hIRcB cells were grown to confluence in 6- or 24-well plates, washed once with PBS, and incubated in serum-free medium overnight. Cells were stimulated with agonists for various times at 37 °C. Thereafter, cells were washed with ice-cold PBS, lysed on ice in SDS sample buffer (50 mm Tris, 5% glycerol, 2% SDS, 0.005% bromphenol blue, 84 mm dithiothreitol, 100 mm sodium fluoride, 10 mm sodium pyrophosphate, and 2 mm sodium orthovanadate, pH 6.8), boiled for 5 min to denature proteins, and sonicated for 5 min to shear the chromosomal DNA. Equal volumes (30–40 µl) of these lysates were separated by SDS-PAGE on 10% gels, electrotransfered to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, MA). The membranes were blocked with 5% non-fat dried milk in TBS-Tween (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.1% Tween 20). Blots were incubated with primary antibodies in blocking buffer for 60 min at room temperature and then incubated with horseradish peroxidase-linked secondary antibodies followed by chemiluminescent detection. For the phospho-specific antibodies, the polyvinylidene difluoride membranes were immediately stripped by placing the membrane in stripping buffer (0.5 m NaCl and 0.5 m acetic acid) for 10 min at room temperature. The membrane was then washed once for 10 min in TBS-Tween, re-blocked, and blotted with antibodies to the unphosphorylated form of the enzyme to control for equal protein loading.
Adenovirus Infection
LβT2 cells were transduced at a multiplicity of infection (m.o.i.) of 10 plaque-forming units/cell for 16 h with either a control recombinant adenovirus containing the lacZ gene or the recombinant adenoviruses expressing wild-type Gαq (WT-Gαq) or active mutant Gαq (Q209L-Gαq) in DMEM, 2% heated and inactivated FBS. For acute infection studies, medium was changed to serum-free DMEM for 1–24 h following infection, and then the cells were processed for immunofluorescence. For the chronic studies, infected cells were incubated for 60 h at 37 °C under 10% CO2 in high glucose DMEM with 2% heat-inactivated FBS. The efficiency of adenovirus-mediated gene transfer was greater than 90% as measured by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) staining of lacZ-infected cells (data not shown). The survival of LβT2 cells was unaffected by adenoviral infection, because the total amount of cell protein remained the same in infected and uninfected cells.
Microinjection
LβT2 cells were grown on glass coverslips to 50% confluency. Cells were starved in serum-free DMEM overnight. Cytoplasmic microinjection of the various reagents was carried out using a semiautomatic Eppendorf microinjection system. All reagents for microinjection were dissolved in microinjection buffer (5 mm sodium phosphate, pH 7.2, 100 mm KCl). Rabbit polyclonal antibodies against the C terminus of Gαq/11, the C terminus of Gαs, preimmune rabbit IgG, or a GST-βARK fusion protein were injected at a concentration of 5 mg/ml. Sheep IgG (5 mg/ml) was co-injected in all cases to allow identification of injected cells. After allowing the cells to recover for 1 h, the cells were stimulated with 100 nm GnRH for a further 1 h. Staining for c-Fos was performed as described above except that a goat polyclonal antibody against c-Fos and a TRITC-labeled anti-goat secondary were used.
Determination of Intracellular cAMP
LβT2 cells were plated in 96-well cell culture plates with a cell concentration of 105 cells/well, incubated in serum-free DMEM overnight, and stimulated with 100 nm GnRH or 10 µm forskolin for various times. The medium was aspirated, and the cells were lysed for 10 min, and a competitive enzyme-linked immunosorbent assay was performed as described by the manufacturer (Amersham Biosciences). Briefly, 100-µl samples were transferred to a 96-well plate; 100 µl of anti-cAMP serum was added and incubated for 2 h at 3–5 °C. The competitor cAMP-peroxidase conjugate was added and incubated at 3–5 °C for a further 1 h. The immune complexes were washed four times with 400 µl of wash buffer, and 150 µl of enzyme substrate was immediately dispensed into wells. The plate was covered and mixed on a microtiter plate shaker for 1 h at room temperature. The reaction was stopped by the addition of 100 µl of 1.0 m sulfuric acid and then read in a plate reader at 450 nm.
Trypsin Sensitivity Assay for G Protein Activation
The trypsin sensitivity assay was performed as described on membranes prepared from LβT2 cells (32). The cells were rinsed twice with ice-cold PBS and scraped in ice-cold lysis buffer containing 10 mm Tris-HCl, pH 7.4, 5 mm EDTA, 10 µg/ml benzamidine, 10 µg/ml soybean trypsin inhibitor (type II-S), and 5 µg/ml leupeptin. The lysate was centrifuged at 45,000 × g for 10 min at 4 °C. The pellet was homogenized in 1% CHAPS in 50 mm HEPES, with a Potter Teflon-glass homogenizer, and stored at −80 °C until use. For the trypsin sensitivity assay, the membranes (50 µg of protein per tube) were incubated in buffer containing 25 mm HEPES, pH 7.5, 1 mm EDTA, 20 mm 2-mercaptoethanol, 25 mm MgCl2, 100 mm NaCl, 0.7% CHAPS, and 10 µm GDP with or without 50 µm GTPγS and in the absence or presence of 100 nm GnRH for 5 min at 30 °C. The membranes were then treated with 100 µg/ml N-tosyl-l-phenylamine chloromethyl ketone (TPCK)-trypsin (1:25 ratio of trypsin to total protein) for 15 min at room temperature. The resulting digested products were separated by SDS-PAGE, and the α-subunits of Gq/11, Gs, and Gi were detected by immunoblotting.
RESULTS
GnRH-mediated Signaling Is Pertussis Toxin-insensitive in LβT2 Cells
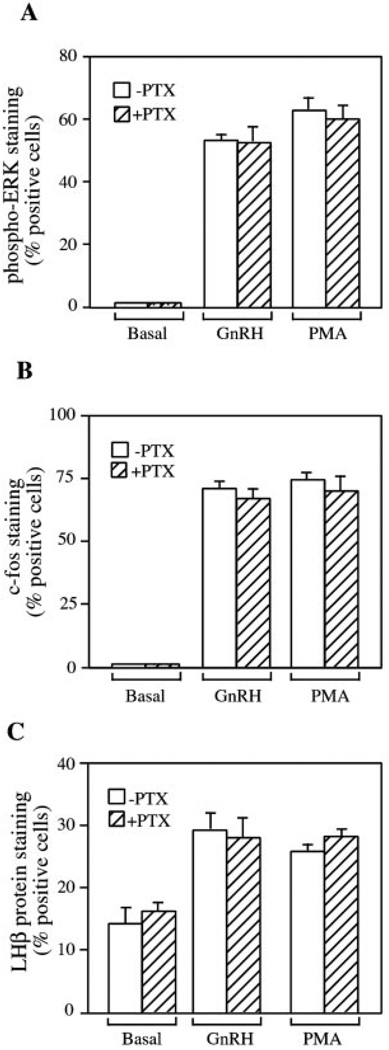
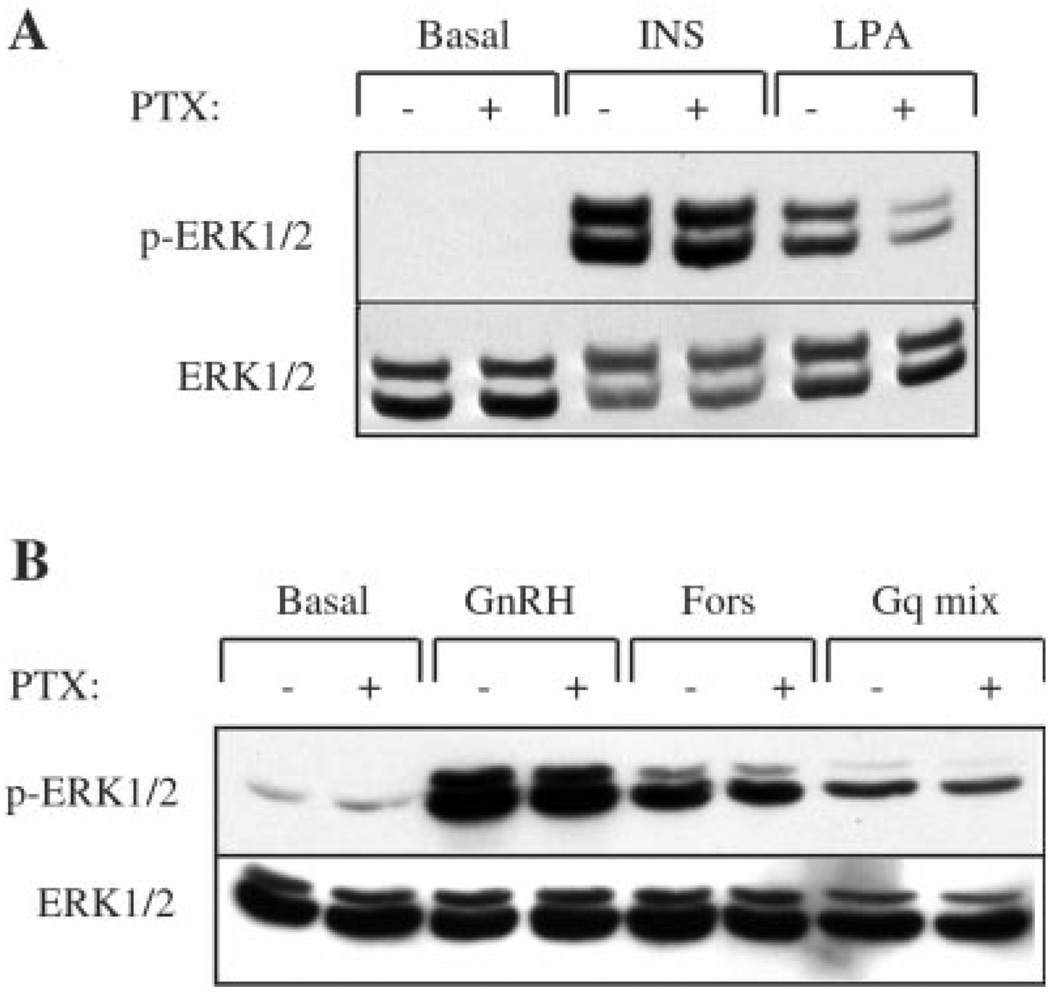
Signaling via the Gi/o family of G proteins can be distinguished by its sensitivity to pertussis toxin. This toxin caused the ADP-ribosylation and inactivation of Gi/o α-subunits. To address the issue of GnRH receptor coupling to Gi/o, LβT2 cells were pretreated with PTX (100 ng/ml) for 16 h and then stimulated with GnRH (100 nm) or PMA (100 nm) for 5 min. ERK activation was measured by immunostaining with an antibody to the active, dually phosphorylated form of ERK (Thr202/Tyr204). We have shown previously (23) that GnRH causes the appearance of staining for phospho-ERK in the nucleus in LβT2 cells. The effect of GnRH can be mimicked by treating cells with the phorbol ester PMA to artificially activate PKC. Pretreatment with PTX alone did not cause activation of ERK in LβT2 cells (Fig. 1A) nor did it impair the ability of GnRH or PMA to stimulate ERK. Similarly, LβT2 cells were pretreated with PTX overnight and then stimulated with GnRH or PMA for 60 min or overnight, and the cells were fixed and stained for c-Fos and LHβ protein expression, respectively. As for ERK activation, pretreatment with PTX did not induce either gene nor reduce GnRH- or PMA-stimulated c-Fos and LHβ protein expression (Fig. 1, B and C). To verify that this dose of PTX will inactivate Gi, we determined whether PTX could block LPA signaling in hIRcB cells. LPA has been shown to signal through the Gi heterotrimeric protein leading to Gβγ-mediated activation of MAPK (30). Pretreatment of cells with PTX reduced ERK activation in response to 10 µm LPA by immunoblotting, whereas activation by insulin was unaffected (Fig. 2A). To confirm the staining results, LβT2 cells were pretreated with PTX (100 ng/ml) overnight and then stimulated with GnRH, forskolin, or a mixture of agonists (33) known to signal via Gαq (Gqmix: 50 nm bombesin, 50 nm bradykinin, and 10 nm endothelin-1). Immunoblotting with antibodies to phospho-ERK showed that PTX did not reduce GnRH-, forskolin-, or Gqmix-induced ERK activation (Fig. 2B). Collectively, these data argue against the participation of PTX-sensitive G proteins in GnRH-evoked signaling in LβT2 cells.
FIG. 1. Effect of PTX on GnRH-induced ERK activation and c-Fos and LHβ protein expression in LβT2 cells.
A, effect of PTX on GnRH-induced ERK activation. LβT2 cells were plated on acid-washed coverslips and incubated in serum-free DMEM overnight with or without 100 ng/ml PTX. Cells were then stimulated with 100 nm GnRH or 100 nm PMA for 5 min at 37 °C, fixed, and processed for immunofluorescence. Active ERK was visualized with an antibody against dually phosphorylated ERK (Thr202/Tyr204) and TRITC-labeled secondary antibody. Nuclei were counterstained with Hoechst 33258 DNA dye. Cells with nuclear fluorescence were scored as positive for ERK activation. B, effect of PTX on GnRH-induced c-Fos expression. LβT2 cells on coverslips were starved overnight in serum-free medium with or without 100 ng/ml PTX. Cells were then stimulated with 100 nm GnRH or 100 nm PMA for 60 min. Nuclear c-Fos expression was visualized using a rabbit anti-c-Fos antibody, followed by a TRITC-conjugated secondary antibody. Nuclei were counterstained with Hoechst 33258 DNA dye. Cells with nuclear c-Fos immunofluorescence were counted as positive for c-Fos expression. C, effect of PTX on GnRH-induced LHβ protein expression. LβT2 cells on coverslips were starved in serum-free DMEM. Cells were stimulated with 100 nm GnRH or 100 nm PMA overnight. LHβ protein expression was visualized using a rabbit anti-LHβ antibody, followed by a TRITC-conjugated secondary antibody. Nuclei were counterstained with Hoechst 33258 DNA dye. Cells with perinuclear LHβ staining were counted as positive for LHβ protein expression. All results are the mean ± S.E. of three experiments and are presented as the percentage of cells positive for immunofluorescence.
FIG. 2. Effect of PTX on ERK activation in response to other agonists.
A, effect of PTX on LPA and insulin-induced ERK activation. hIRcB cells were incubated overnight in serum-free medium in the presence or absence of PTX (100 ng/ml), and cells were then stimulated with 100 ng/ml insulin (INS) or 10 µm LPA for 5 min at 37 °C. Whole-cell lysates were separated by SDS-PAGE and immunoblotted with the antibody against phospho-ERK (Thr202/Tyr204). The blots were then stripped and re-blotted for ERK protein to verify equal loading. B, effect of PTX on Gq-induced ERK activation. LβT2 cells were starved with serum-free DMEM overnight before treatment with 100 nm GnRH, 10 µm forskolin (Fors), or a mixture of Gq agonists (Gqmix: 50 nm bombesin, 50 nm bradykinin, and 10 nm endothelin-1) for 5 min at 37 °C. Whole-cell lysates were separated by SDS-PAGE and immunoblotted as above. Blots were stripped and re-blotted for ERK protein to determine equal total protein loading. Blots are representative of two experiments with similar results.
A Constitutively Active Gαq Mutant Induces c-Fos and LHβ Protein Expression Acutely in LβT2 Cells
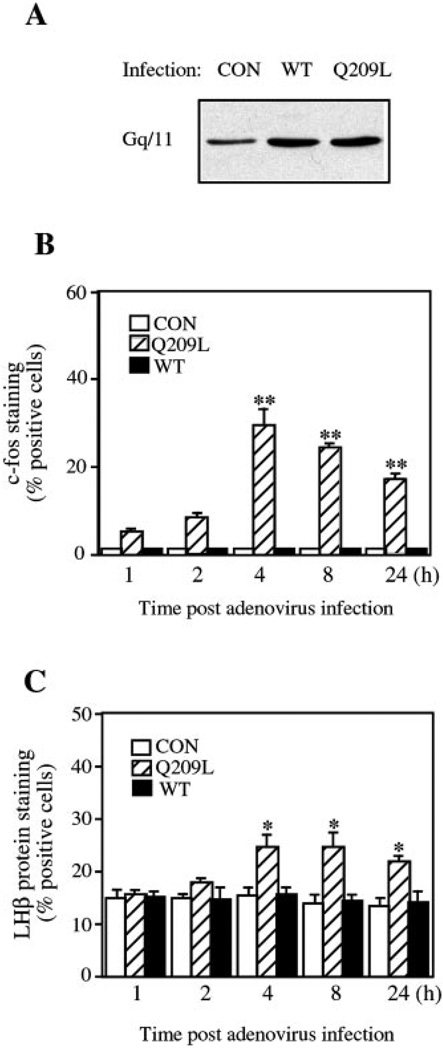
Previous studies have implicated the Gq/11 proteins in GnRH signaling (18, 19). To explore further the functional importance of Gαq in GnRH induced-ERK activation, c-Fos and LHβ protein expression in LβT2 cells, we used recombinant adenovirus vectors expressing either wild-type (WT) or a constitutively active mutant (Q209L) Gαq, or a control virus expressing β-galactosidase. To demonstrate protein expression, LβT2 cells were infected with these adenovirus vectors at a multiplicity of infection of 10. Whole-cell lysates were immunoblotted with an anti-Gαq/11 antibody recognizing the C terminus of the protein. Infection with either WT or mutant Q209L-Gαq adenoviruses caused a 3-fold increase in Gαq compared with infection with the β-galactosidase control adenovirus (Fig. 3A). We then assessed the effects of WT and Q209L-Gαq expression on the activation of ERK and induction of c-Fos and LHβ protein expression. After infection, Q209L-Gαq induced c-Fos and LHβ protein expression acutely, reaching a maximum at 4–8 h post-infection (Fig. 3, B and C). In contrast, control- or WT-expressing adenovirus did not stimulate c-Fos and LHβ protein expression (Fig. 3, B and C). However, we were unable to detect activation of ERK by Q209L-Gαq. This may be related to the transient activation of ERK in LβT2 cells. We have shown previously (23) that ERK is activated within 1–5 min of GnRH treatment and decreases over the course of 2 h.
FIG. 3. Expression and effect of Gαq on c-Fos and LHβ protein expression in LβT2 cells.
A, LβT2 cells were infected with recombinant adenoviruses expressing wild-type Gαq (WT), Q209L-Gαq (Q209L), or lacZ control (CON) at an m.o.i. of 10. After infection for 16 h, whole-cell lysates were analyzed by Western blotting with an anti-Gαq/11 C-terminal antibody. B and C, effect of Gαq expression on c-Fos and LHβ protein expression in LβT2 cells. LβT2 cells on acid-washed coverslips were infected with the adenoviruses expressing wild-type (WT), Q209L-Gαq (Q209L), or lacZ control (CON) at an m.o.i. of 10. After infection for 16 h, the medium was changed, and cells were allowed to express the viral protein for 1, 2, 4, 8, or 24 h. The cells were then fixed and processed for immunofluorescence as Fig. 1. Data are mean ± S.E. of three experiments and are presented as percentage of cells positive for c-Fos or LHβ expression. Asterisks indicate statistical significance relative to 1 h group (*, p < 0.05; **, p < 0.01).
Chronic Expression of a Constitutively Active Gαq Causes GnRH Resistance
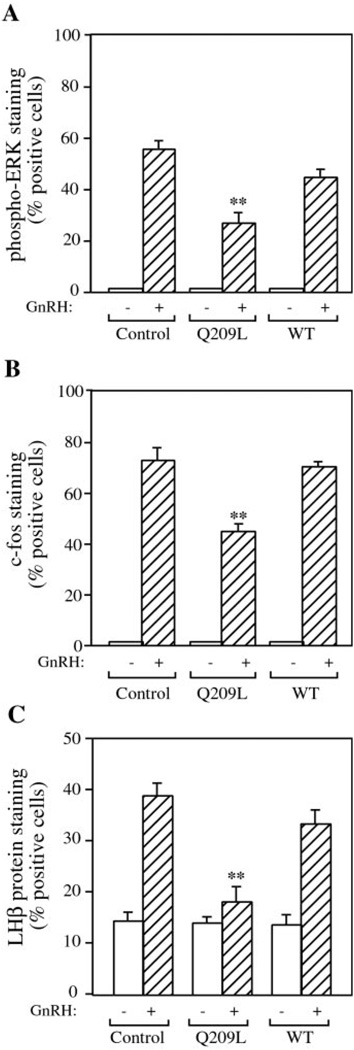
We also examined the effect of chronic expression of a constitutively active Gαq on GnRH signaling. Cells were infected for 16 h and incubated for a further 60 h at 37 °C and then serum-starved and stimulated acutely with GnRH. Infection with control or wild-type Gαq viruses was without effect (Fig. 4). Chronic expression of Gαq (Q209L) had no effect on basal ERK, c-Fos, and LHβ protein expression. However, GnRH stimulation of ERK and c-Fos was reduced 40–50% in Q209L-Gαq-expressing cells. More significantly, GnRH stimulation of LHβ expression was completely abrogated by chronic Gαq signaling. Thus, chronic expression of Gαq (Q209L) induced a state of GnRH resistance and impaired the ability of GnRH to stimulate LHβ gene expression.
FIG. 4. Effect of chronic Gαq expression on GnRH-induced ERK activation, c-Fos and LHβ protein expression in LβT2 cells.
LβT2 cells on coverslips were infected with recombinant adenoviruses expressing WT-Gαq (WT), Q209L-Gαq (Q209L), or lacZ control (CON) at an m.o.i. of 10 for 16 h. Cells were allowed to express the viral protein for a further 60 h, then stimulated with 100 nm GnRH, fixed, and stained. A, cells were stimulated for 5 min and stained with the antibody to phospho-ERK. B, cells were stimulated for 60 min and stained for c-Fos. C, cells were stimulated overnight and stained with the LHβ antibody. Results are the mean ± S.E. of three experiments and are presented as percentage of cells positive for immunofluorescence. Asterisks indicate statistical significance versus GnRH-stimulated control cells (**, p < 0.01).
Generation of Cell-permeable Inhibitory Peptides to Gq/11, Gs, and Gβγ
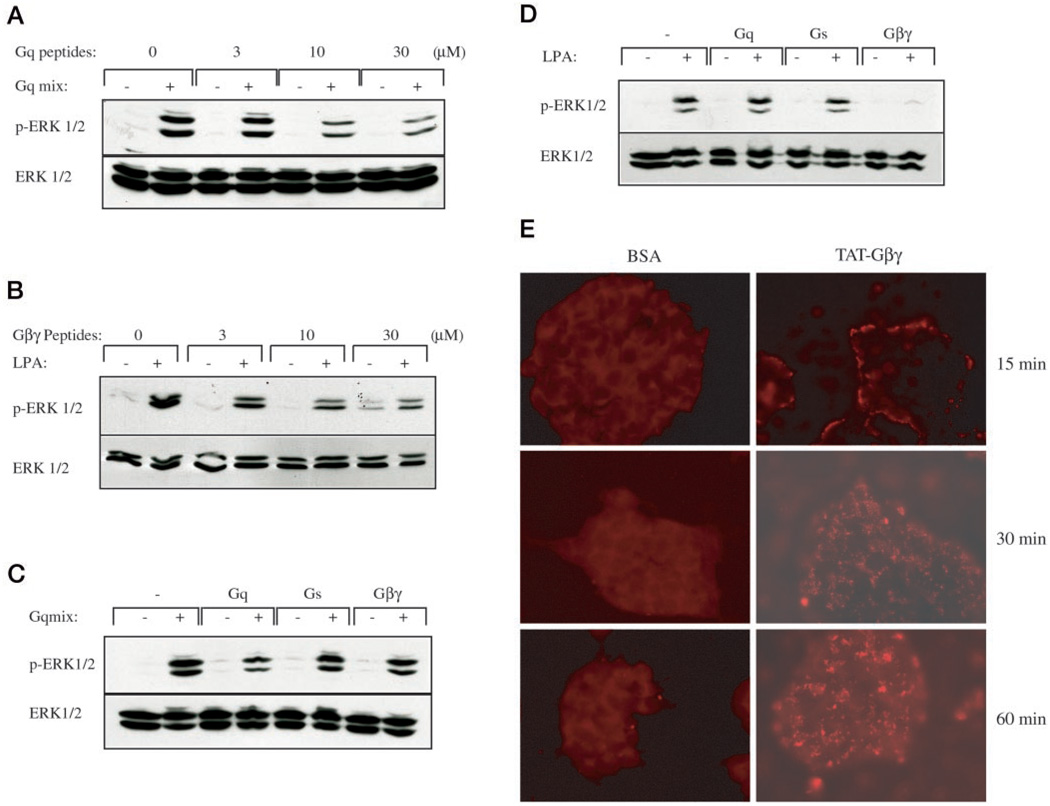
The adenovirus results suggested that the Gαq class of G proteins might be a mediator of GnRH signaling. To confirm the involvement of Gαq, we generated a membrane-permeable peptide to inhibit Gαq signaling. We also generated peptides to inhibit Gαs and Gβγ signaling as controls. These inhibitory peptides are based on published sequences (34). The peptides were expressed as TAT fusion proteins to allow internalization. TAT-GqCT contains amino acids 350–359 of Gq and disrupts GPCR coupling to Gq/11. TAT-GsCT contains amino acids 385–394 of Gs and disrupts GPCR coupling to Gs. TAT-Gβγ contains amino acids 564–583 of PLCβ2 and was designed to sequester free Gβγ-subunits. The peptides were initially tested in the hIRcB fibroblast cell line. Cells were stimulated with the Gq activator mix or LPA to test the Gq and Gβγ peptides, respectively. Pretreatment of cells with increasing doses of TAT-GqCT peptide for 45 min caused a dose-dependent decrease in ERK activation by Gqmix (Fig. 5A). Similarly, pretreatment of cells with increasing doses of TAT-Gβγ caused a dose-dependent decrease in ERK activation by LPA (Fig. 5B). Moreover, neither TAT- GqCT nor TAT-Gβγ alone caused ERK activation. The specificity of the peptides was also verified. Cells were treated with a single dose of the Gq, Gs, or Gβγ peptide (30 µm) for 45 min and then stimulated with the mixture of Gq agonists or LPA. Activation of ERK was assessed by immunoblotting with the antibody to phosphorylated ERK. Only the Gq peptide caused an appreciable inhibition of ERK phosphorylation in response to Gq agonists (Fig. 5C). Similarly, only the Gβγ peptide inhibited the phosphorylation of ERK in response to LPA (Fig. 5D). The ability of the Gs peptide to inhibit selectively Gαs signaling was verified by measuring increases in cAMP (see below). Next, we labeled the TAT-Gβγ peptide or BSA with rhodamine in order to examine whether these TAT peptides were taken up by LβT2 cells. Incubating cells for 15, 30, or 60 min with rhodamine-TAT-Gβγ showed a time-dependent increase in cellular fluorescence that was maximal by 30–60 min (Fig. 5E). In contrast, rhodamine-BSA did not any label cells at any time demonstrating that uptake required the TAT permeabilization sequence.
FIG. 5. Effect of cell-permeable inhibitory peptides on Gq and LPA-induced ERK activation.
A, effect of TAT-GqCT peptide on Gq-induced ERK activation. hIRcB cells were starved with serum-free medium overnight and then pretreated with TAT-GqCT inhibitory peptide (Gq) at various concentrations for 45 min. Cells were then stimulated with a mixture of Gq agonists (Gqmix: 50 nm bombesin, 50 nm bradykinin, and 10 nm endothelin-1) for 5 min. Whole-cell lysates were separated by SDS-PAGE and immunoblotted with the antibody to phospho-ERK. Blots were stripped and re-blotted for ERK protein to verify equal loading. B, effect of TAT-Gβγ peptide on LPA-induced ERK activation. Serum-starved hIRcB cells were pretreated with TAT-Gβγ peptide (Gβγ) for 45 min and then stimulated with 10 µm LPA for 5 min. Whole-cell lysates were analyzed by immunoblotting as above. Blots are representative of three experiments with similar results. C, effect of TAT peptides on Gq-induced ERK activation. hIRcB cells were starved with serum-free medium overnight and then pretreated with 30 µm TAT-GqCT (Gq), TAT-GsCT (Gs), or TAT-Gβγ (Gβγ) inhibitory peptide for 45 min. Cells were then stimulated with a mixture of Gq agonists (Gqmix: 50 nm bombesin, 50 nm bradykinin, and 10 nm endothelin-1) for 5 min. Whole-cell lysates were analyzed by immunoblotting as above. D, effect of TAT peptides on LPA-induced ERK activation. Serum-starved hIRcB cells were pretreated with 30 µm TAT-GqCT (Gq), TAT-GsCT (Gs), or TAT-Gβγ (Gβγ) inhibitory peptide for 45 min and then stimulated with 10 µm LPA for 5 min. Whole-cell lysates were analyzed by immunoblotting as above. E, rhodamine-labeled TAT-Gβγ loading into LβT2 cells. LβT2 cells were plated on acid-washed coverslips and serum-starved overnight. BSA and TAT-Gβγ labeled with rhodamine (30 µm) were added to the medium for 15, 30, or 60 min. The cells were fixed, and the uptake of labeled peptide was determined by fluorescence microscopy. Representative fields of cells are shown.
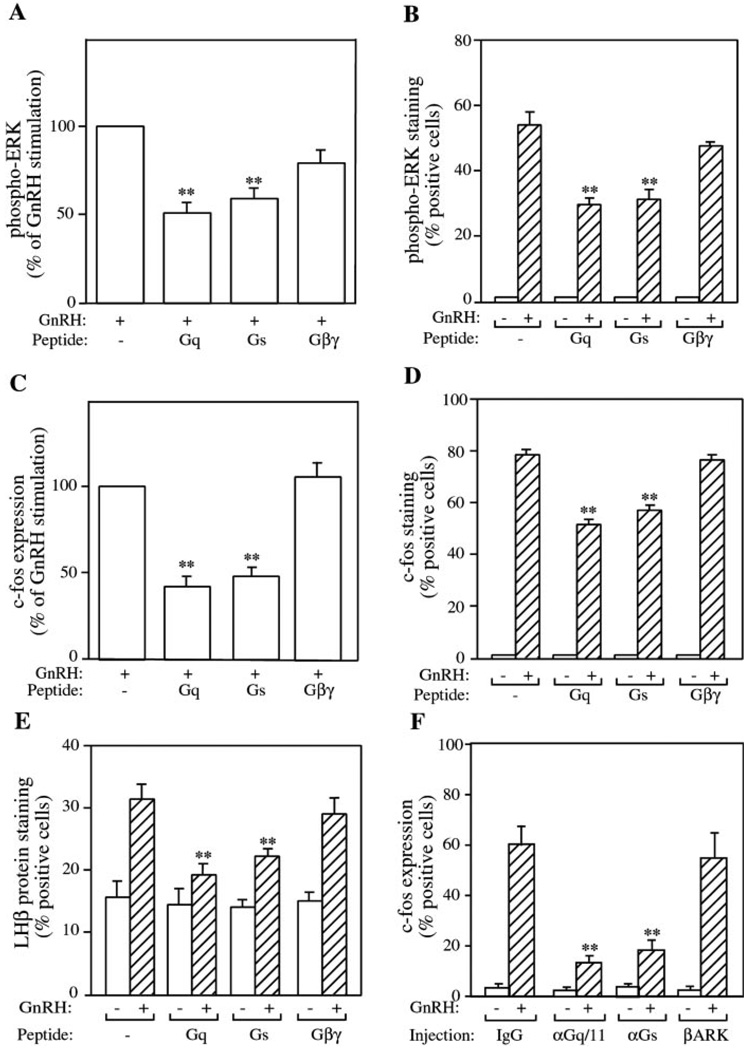
Effect of Inhibitory Peptides on GnRH-induced ERK Activation and c-Fos and LHβ Expression in LβT2 Cells
Next, we used these inhibitory peptides to investigate GnRH signaling. LβT2 cells were pretreated with 30 µm TAT- GqCT, TAT-GsCT, and TAT-Gβγ for 45 min and then were stimulated with 100 nm GnRH for 5 min. Whole-cell lysates were immunoblotted for phospho-ERK and quantified by densitometry (Fig. 6A). Both Gq and Gs peptides inhibited GnRH-induced ERK activation, but Gβγ peptides had no effect. Activation of ERK was also measured by immunofluorescence. The Gq and Gs peptides inhibited the appearance of phospho-ERK in the nucleus following stimulation with GnRH (Fig. 6B). As for the immunoblotting earlier, the Gβγ peptide had no effect. These results confirmed that GnRH signals via Gq to activate ERK and suggested that signaling via Gs may also contribute.
FIG. 6. Effect of inhibitory peptides on GnRH receptor signaling in LβT2 cells.
LβT2 cells plated on coverslips or 24-well plates were starved with serum-free DMEM overnight and then pretreated with various peptides for 45 min before stimulation with 100 nm GnRH at 37 °C. A and B, cells were stimulated for 5 min. ERK activation was monitored by immunoblotting of whole-cell lysates followed by densitometry (A) or by immunofluorescent staining (B) as before. C and D, cells were stimulated for 60 min. Induction of c-Fos was monitored by immunoblotting of whole-cell lysates followed by densitometry (C) or by immunofluorescent staining (D) as before. E, cells were stimulated overnight. Induction of LHβ was monitored by immunofluorescent staining as before. Results are the mean ± S.E. of three experiments. Staining results are presented as percentage of cells positive for immunofluorescence. Immunoblotting results are presented as the percentage of the GnRH-stimulated value. Asterisks indicate statistical significance versus GnRH-stimulated cells (**, p < 0.01). F, effect of microinjection of inhibitory Gq/11 and Gs antibodies on GnRH-induced c-Fos expression. Serum-starved LβT2 cells on coverslips were microinjected with an anti-Gq/11 antibody (αGq/11), an anti-Gs antibody (αGs), a GST-βARK fusion protein (βARK), or preimmune IgG at 5 mg/ml. Sheep IgG was co-injected in all cases as an injection marker. Cell were incubated with or without 100 nm GnRH for 1 h and then fixed and processed for c-Fos immunofluorescence. Data are presented as the mean ± S.E. from three separate experiments. Asterisks indicate statistical significance versus GnRH-stimulated IgG-injected cells (**, p < 0.01).
The induction of c-Fos expression was investigated, and cells were pretreated with 30 µm TAT-GqCT, TAT-GsCT, or TAT-Gβγ for 45 min and then stimulated with 100 nm GnRH for 60 min. Immunoblotting of whole-cell lysates showed that both the Gq and Gs peptides partially inhibited c-Fos induction, but the Gβγ peptide again had no effect (Fig. 6C). These results were also confirmed by immunostaining. As before, both the Gq and Gs peptides blocked GnRH-stimulated c-Fos expression, but the Gβγ peptide had no effect (Fig. 6D). These data indicated that both Gq and Gs may be involved in GnRH-stimulated c-Fos expression. The induction of LHβ expression was also assessed, and LβT2 cells were pretreated with TAT-GqCT, TAT-GsCT, or TAT-Gβγ for 45 min and then stimulated with 100 nm GnRH for 16 h. LHβ expression was quantified by immunofluorescent staining. Both the Gq and Gs peptides inhibited GnRH-stimulated LHβ protein expression, but the Gβγ peptide did not. None of the peptides altered LHβ expression in the absence of GnRH (Fig. 6E). This result suggests that Gq and Gs may also mediate GnRH-stimulated LHβ protein expression. The lack of an effect with the TAT-Gβγ inhibitory peptide on all three end points shows that these peptides are not toxic to the cells and excludes GnRH signaling through the Gβγ-subunits derived from either Gq or Gs.
Microinjection of Antibodies to the C Terminus of Gαq/11 or Gαs Inhibits c-Fos Induction by GnRH
The cell-permeable inhibitory peptide results suggested that Gαq/11 signaling contributed to ERK, c-Fos, and LHβ induction. To confirm this finding we utilized the approach of single cell microinjection of an inhibitory antibody to block Gαq/11 or Gαs signaling. The antibodies were rabbit polyclonals raised against the C terminus of Gαq, which recognizes both Gαq and Gα11, or the C terminus of Gαs. The antibodies were inhibitory as the epitopes corresponded to the sites of interaction of the G proteins and the activated GPCR. As a control, cells were injected with preimmune IgG or a recombinant GST fusion protein containing the Gβγ binding domain of βARK. Sheep IgG was used as an injection marker in all cases. Cells were allowed to recover from the injection, stimulated with 100 nm GnRH for 1 h, fixed, and then stained for c-Fos using a goat anti-c-Fos antibody. We were unable to stain for phospho-ERK or LHβ because these antibodies were also rabbit polyclonals and could not be distinguished from the injected antibodies. Injected cells were identified by staining for the co-injected sheep IgG. Cells that were positive for injection of sheep IgG were scored for the presence of c-Fos fluorescence. Injection of the preimmune IgG or the GST-βARK did not alter the ability of GnRH to induce c-Fos expression, but injection of the inhibitory Gαq/11 or Gαs antibody reduced c-Fos induction by GnRH (Fig. 6F). This confirmed that GnRH signals via Gαq and Gαs to induce the c-fos gene.
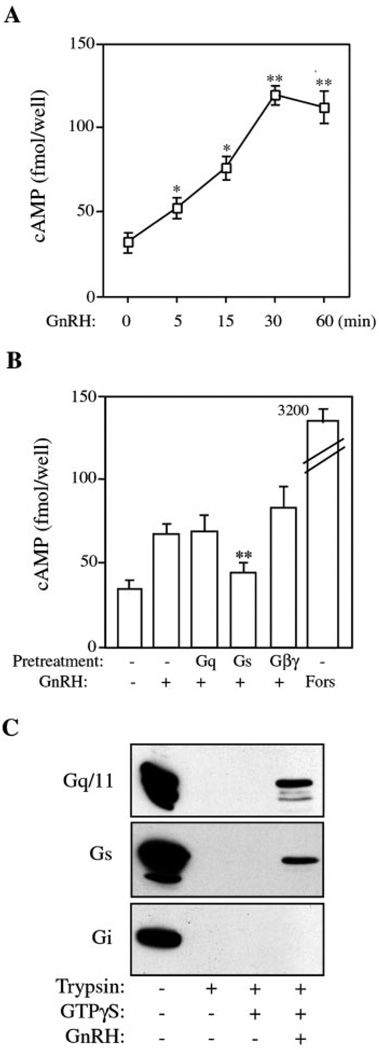
GnRH Elevates Intracellular cAMP
The above data demonstrated that GnRH signals via Gq to induce c-Fos and LHβ expression. This is consistent with both the results from the adenoviral expression of an active mutant of Gαq and our previous data (23) showing a requirement for calcium signaling. However, the finding that GnRH signals through Gs was not expected. So we verified that GnRH activates Gs in LβT2 cells by measuring cAMP levels following GnRH stimulation. The cells were treated with 100 nm GnRH for increasing times, and cAMP levels were measured by enzyme-linked immunosorbent assay. GnRH increased cAMP as early as 5 min, reaching a peak at 30 min (Fig. 7A). Cells were pretreated with TAT-GqCT, TAT-GsCT, or TAT-Gβγ peptides for 45 min and then stimulated with 100 nm GnRH for 30 min. Both the Gq and Gβγ had no effect on cAMP, but the Gs peptide completely blocked that GnRH-stimulated increase in cAMP production (Fig. 7B). This result showed that GnRH signals through Gs to elevate cAMP and confirmed the specificity of these peptides, as only the Gs peptide blocked the increase in cAMP. Activation of G protein complexes caused loading of GTP onto the α-subunit. GTP-bound Gα can be detected by a trypsin sensitivity assay. This assay is based on the observation that binding of GTP to the α-subunit protects it from cleavage by trypsin. We used this assay to demonstrate that GnRH activated both Gαq and Gαs but not Gαi. Membranes from LβT2 cells were stimulated with GnRH in the presence of GTPγS and then subjected to rapid digestion with TPCK-treated trypsin. The digestion products were separated by SDS-PAGE and immunoblotted with antibodies to Gαq, Gαs, and Gαi. Trypsin digestion caused the rapid disappearance of the band corresponding to the α-subunit (Fig. 7C). Addition of GTPγS had no effect, but simultaneous incubation with GnRH partially protected the Gαq and Gαs proteins from digestion but had no ability to protect Gαi (Fig. 7C). This is evidence that both the Gq/11 and Gs complexes are activated by the GnRH receptor in LβT2 cells. The Gi complex did not appear to be activated although the Gαi subunit was detected in these cells.
FIG. 7. GnRH activates Gs and stimulates cAMP production in LβT2 cells.
A, time course of GnRH stimulation of cAMP production. LβT2 cells in 96-well plates were starved with serum-free DMEM overnight and then stimulated with 100 nm GnRH for the indicated times. cAMP levels in cell extracts were measured using a competitive enzyme-linked immunosorbent assay. Results are expressed as fmol/well and show the mean ± S.E. from three similar experiments performed in triplicate. Asterisks indicate statistical significance versus the cAMP value at time 0 (*, p < 0.05; **, p < 0.01). B, effect of inhibitory peptides on cAMP production. LβT2 cells in 96-well plates were starved with serum-free medium overnight and then pretreated with various peptides for 45 min before stimulation with 100 nm GnRH or 10 µm forskolin (Fors) for 30 min. cAMP measurements were performed as above. Results are expressed as fmol/well and show the mean ± S.E. from three similar experiments performed in triplicate. Asterisks indicate statistical significance versus GnRH-stimulated cells (**, p < 0.01). C, activation of G proteins by trypsin sensitivity. Membranes from LβT2 cells were incubated with GTPγS in the absence or presence of 100 nm GnRH for 5 min. Samples were then rapidly digested with TPCK-treated trypsin, separated by SDS-PAGE, and immunoblotted with antibodies against Gq/11, Gs, or Gi. Blot is representative of five experiments.
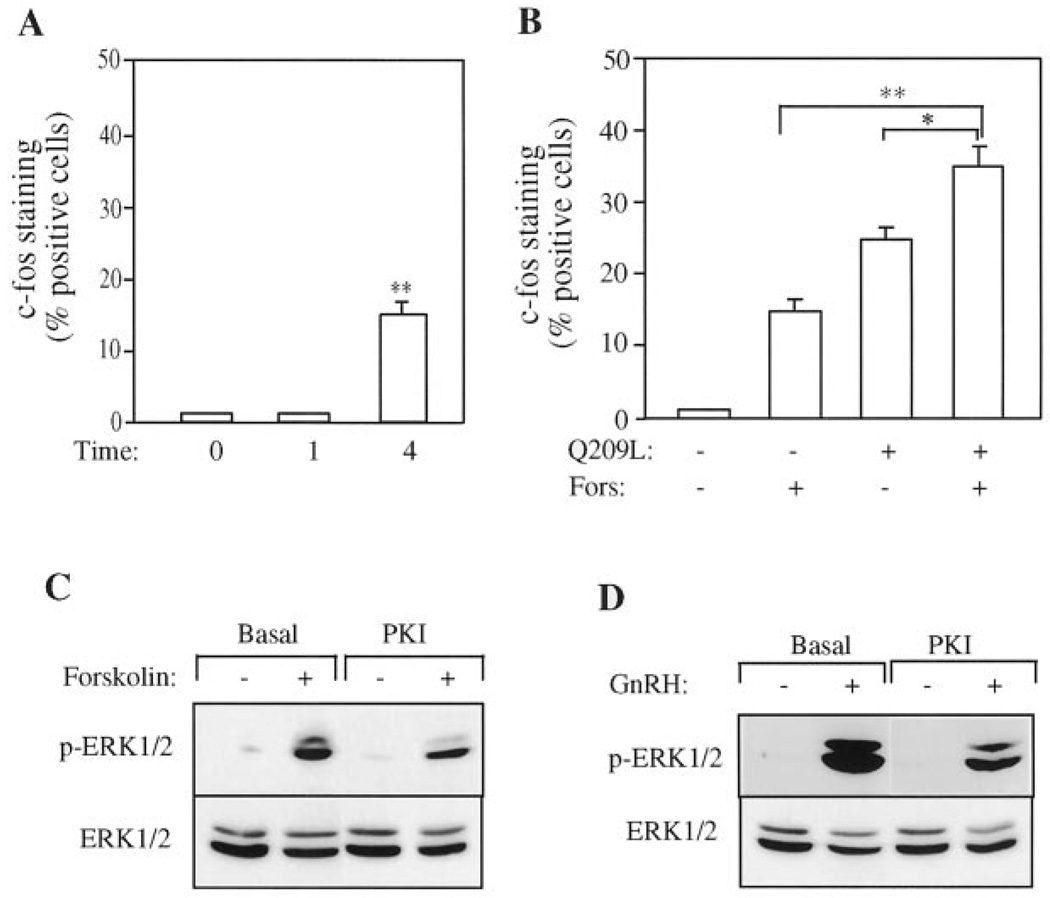
To investigate whether increases in cAMP could contribute to GnRH action, we used forskolin as an artificial stimulator of adenylate cyclase. Treatment of LβT2 cells with forskolin caused a very strong elevation of cAMP (Fig. 7B). Forskolin treatment for 1 h did not induce c-Fos expression, but treatment for 4 h led to an increase in the number of cells positive for c-Fos (Fig. 8A). The induction of c-Fos by forskolin was slower than with GnRH or PMA, which gave maximal c-Fos levels after 1 h. To test whether the effects of the Gs and Gq pathways were additive, cells were infected with the Gαq (Q209L) adenovirus and then treated with forskolin for 4 h. Both Gαq (Q209L) and forskolin induced c-Fos on their own, and the effect of the two was additive (Fig. 8B). We have shown previously that GnRH induces c-Fos and LHβ expression via the MEK-ERK cascade (23). Because signaling via Gs was involved in induction of c-Fos, we investigated whether cAMP signaling led to activation of ERK in LβT2 cells, and whether GnRH-evoked increases in cAMP activated ERK via the PKA. Cells were pretreated with the cell-permeable PKI peptide that inhibits PKA and then stimulated with either forskolin to elevate cAMP artificially or GnRH. Whole-cell lysates were immunoblotted with the phospho-ERK antibody. Elevation of cAMP alone was able to activate ERK in these cells, and this activation was blocked by the PKI peptide (Fig. 8C). This PKI peptide also reduced ERK activation to a similar extent following stimulation with GnRH showing that cAMP signaling contributed to activation of ERK (Fig. 8D). The inhibition was not complete with either forskolin or GnRH suggesting that other cAMP-dependent pathways, not involving PKA, might be involved (35). These results confirm that GnRH signals through both Gq and Gs to activate ERK and induce the c-Fos and LHβ proteins. This is consistent with the partial inhibition of signaling seen earlier with the Gq or Gs inhibitory peptides.
FIG. 8. Effect of forskolin on ERK activation and c-Fos expression in LβT2 cells.
A, forskolin induces c-Fos expression. LβT2 cells on coverslips were starved with serum-free medium overnight and then stimulated with 10 µm forskolin (Fors) for 0, 1, or 4 h. Cells were fixed and stained for c-Fos expression as before. Cells with nuclear c-Fos immunofluorescence were counted as positive. Data are presented as the percentage of cells positive for c-Fos immunofluorescence and show the mean ± S.E. from three separate experiments. Asterisks indicate statistical significance versus cells at time 0 (**, p < 0.01). B, effect of Gαq activation on forskolin-induced c-Fos expression. LβT2 cell on coverslips were infected with the recombinant adenovirus expressing Q209L-Gαq (Q209L) at an m.o.i. of 10. After 16 h of infection, cells were stimulated with 10 µm forskolin (Fors) for 4 h and then fixed and processed for immunofluorescence as above. Results are the mean ± S.E. of three experiments and are presented as the percentage of cells positive for c-Fos staining. Asterisks indicate statistical significance (*, p < 0.05; **, p < 0.01). C and D, inhibition of protein kinase A reduces GnRH- or forskolin-induced ERK activation. LβT2 cells were starved overnight and pretreated with the cell-permeable PKI for 30 min. Cells were then stimulated with 100 nm GnRH or 10 µm forskolin for 5 min. Whole-cell lysates were subjected to SDS-PAGE and immunoblotted with the phospho-ERK antibody as before. Blots were stripped and re-blotted for ERK protein to verify equal loading. Blots are representative of two experiments.
DISCUSSION
Previously, we showed that GnRH activates ERK and induces c-Fos and LHβ protein expression in LβT2 cells. In this study, we examined which G proteins are involved in these GnRH effects. We show that GnRH induction of ERK, c-Fos, and LHβ is not inhibited by pertussis toxin or a peptide that sequesters Gβγ, effectively ruling out signaling via Gi. This agrees with the results from Naor and co-workers (9) showing that Gi is not involved in the GnRH response in αT3-1 cells (9). Many studies have shown that binding of GnRH to the receptor leads to the activation of phospholipase C and the formation of inositol 1,4,5-triphosphate and diacylglycerol, which leads directly to the elevation of intracellular Ca2+ and the activation of protein kinase C. This is mediated via the coupling of the receptor to the Gq/11 family of G proteins. Expression of a mutant Q209L-Gαq, which lacks GTPase activity, enhances phospholipase C stimulation and transformation in NIH-3T3 cells (36). Here we demonstrate that expression of this same Gq mutant (Q209L) by adenoviral infection partially mimics the induction of c-Fos and LHβ by GnRH. However, the extent of induction is only 50% that seen with GnRH suggesting the presence of additional signals. We were unable to detect the activation of ERK, most likely due to its transient nature. We also show that chronic activation of Gαq signaling via Gαq (Q209L) results in a state of GnRH resistance. The mechanisms of GnRH resistance are not known, but it may be related to either the down-regulation of diacylglycerol-dependent PKC isoforms or the rapid down-regulation of Gq/11 proteins that have been observed with chronic PMA and GnRH treatment.
Signaling cascades often require an activated protein to contact its immediate downstream mediator. Disrupting this protein-protein interaction, by introducing one of the binding domains into cell, can thus block specific pathways. Previous studies (34, 37, 38) have shown that the GPCR/G protein interaction can be disrupted in vitro by peptides derived from the C terminus of the G protein. This approach to analyze signaling has been limited, however, as most peptides do not readily penetrate the cell membrane. Some success has been achieved by lipid or chemical attachment to a membrane permeabilization sequence. In this study, we rendered the blocking peptides cell-permeable by expressing them as fusion proteins with the TAT protein transduction domain (31). We generated TAT fusion peptides containing decapeptides from the carboxyl termini of Gαq (GqCT) and Gαs (GsCT) and a 20-amino acid peptide from phospholipase Cβ2. The α-subunit peptides block the interaction of GPCRs with their respective G proteins, and the PLCβ2 peptide binds to free Gβγ-subunits. We used these peptides here to show that both Gq and Gs proteins participate in GnRH receptor signaling leading to ERK activation and c-Fos and LHβ protein expression in LβT2 cells.
It is now well documented that Gβγ-subunits, as well as the Gα-subunits, have the ability to signal to downstream effectors. Effector activation by Gβγ released from both Gs and Gi heterotrimers has been reported (39–41). It is possible that some of the activation of GnRH signaling is caused by Gβγ released from either Gq or Gs. In particular, the β2 isoform of phospholipase C is activated by Gβγ, as is adenylate cyclase 2. Thus, Gβγ signaling could potentially contribute to both Gq and Gs pathways. However, the lack of an effect with the Gβγ blocking peptide and the injection of the GST-βARK protein indicates that GnRH signaling is mediated primarily by α-subunits.
Although it is thought that most of the biological actions of GnRH are mediated by Gq-coupled pathways, studies have suggested a physiological role for cAMP as a mediator of GnRH actions in the pituitary gland (42). The third intracellular loop of the rat GnRH-R couples to both Gs and Gq/11-mediated signaling pathways in G-GH3 cells, and cAMP signaling is dependent on specific residues in the loop that are not essential for activation of the phosphoinositide signaling pathway (43, 44). Both GnRH and cAMP activate the mouse GnRH-R gene promoter via the cAMP-response element in G-GH3 cells (45, 46). In contrast, there was no evidence for activation of Gs in βT3-1 cells (47). A recent study in tilapia pituitary cells demonstrated that GnRH induction of both α and FSHβ subunit genes was sensitive to inhibition of PKA, suggesting activation of cAMP signaling (48). Induction of LHβ on the other hand was relatively resistant to inhibition of PKA but sensitive to PKC and MEK signaling. Our data suggest that both Gq and Gs are involved in GnRH receptor signaling in LβT2 cells similar to the tilapia study. We showed that artificial elevation of cAMP with forskolin can induce c-Fos protein expression on its own and can enhance c-Fos induction due to Gαq (Q209L), suggesting that the two pathways are independent and additive. Unfortunately, we are unable to detect changes in α-subunit protein in the LβT2 cells, and the FSHβ protein is expressed at an extremely low level. Despite this limitation, it is interesting to speculate that Gq and Gs pathways may be used differentially to regulate gonadotropin gene expression.
In summary, we have provided evidence for the participation of both Gq and Gs signaling in the GnRH activation of ERK and induction of c-Fos and LHβ protein in LβT2 cells. These results are consistent with studies in primary pituitary cell cultures and confirm that the LβT2 cells are a good model system for in vitro studies of GnRH action. In addition, we demonstrated that a state of GnRH resistance can be induced by chronic Gq signaling. Further studies are planned to investigate whether this in vitro model of GnRH resistance is comparable with GnRH resistance seen in vivo.
Acknowledgments
We thank Dr. A. F. Parlow (NIDDK) for the gift of LHβ antibody and Dr. Joan Brown (University of California, San Diego) for providing the control recombinant adenovirus containing the lacZ gene and the recombinant adenoviruses expressing wild-type Gαq and Q209L mutant Gαq. We also thank Dr. Steve Dowdy (University of California, San Diego) for the pTAT-HA plasmid and Dr. Robert Lefkowitz (Duke University) for the GST-βARK expression plasmid. We also thank Dr. Takeshi Imamura for technical assistance with adenovirus infection experiments.
Footnotes
This work was supported by a U54 Center Grant HD-12303 from the National Institutes of Health.
The abbreviations used are: GnRH, gonadotropin-releasing hormone; GPCR, G protein-coupled receptor; PMA, phorbol 12-myristate 13-acetate; LPA, lysophosphatidic acid; PTX, pertussis toxin; PKA, cAMP-dependent protein kinase; PKC, protein kinase C; PLC, phospholipase C; MAPK, mitogen-activated protein kinase; LH, luteinizing hormone; TRITC, tetramethylrhodamine isocyanate; m.o.i., multiplicity of infection; IP, inositol phosphate; ERK, extracellularly regulated kinase; MEK, MAPK and ERK kinase; lacZ, β-galactosidase; FBS, fetal bovine serum; DMEM, Dulbecco’s modified Eagle’s medium; BSA, bovine serum albumin; PBS, phosphate-buffered saline; TPCK, N-tosyl-l-phenylamine chloromethyl ketone; GST, glutathione S-transferase; GTPγS, guanosine 5′-3-O-(thio)triphosphate; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; PKI, protein kinase A inhibitor.
REFERENCES
- 1.Gudermann T, Kalkbrenner F, Schultz G. Annu. Rev. Pharmacol. Toxicol. 1996;36:429–459. doi: 10.1146/annurev.pa.36.040196.002241. [DOI] [PubMed] [Google Scholar]
- 2.Gudermann T, Schoneberg T, Schultz G. Annu. Rev. Neurosci. 1997;20:399–427. doi: 10.1146/annurev.neuro.20.1.399. [DOI] [PubMed] [Google Scholar]
- 3.Hur E, Kim K. Cell. Signal. 2002;14:397–405. doi: 10.1016/s0898-6568(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 4.Albert PR, Robillard L. Cell. Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- 5.Neer EJ. Protein Sci. 1994;3:3–14. doi: 10.1002/pro.5560030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wess J. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 7.Hamm HE. J. Biol. Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 8.Gutkind JS. J. Biol. Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 9.Shacham S, Cheifetz MN, Lewy H, Ashkenazi IE, Becker OM, Seger R, Naor Z. Ann. Endocrinol. (Paris) 1999;60:79–88. [PubMed] [Google Scholar]
- 10.Shacham S, Harris D, Ben-Shlomo H, Cohen I, Bonfil D, Przedecki F, Lewy H, Ashkenazi IE, Seger R, Naor Z. Vitam. Horm. 2001;63:63–90. doi: 10.1016/s0083-6729(01)63003-6. [DOI] [PubMed] [Google Scholar]
- 11.Ando H, Hew CL, Urano A. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;129:525–532. doi: 10.1016/s1096-4959(01)00356-6. [DOI] [PubMed] [Google Scholar]
- 12.Rebers FE, Bosma PT, van Dijk W, Goos HJ, Schulz RW. Am. J. Physiol. 2000;278:R1572–R1578. doi: 10.1152/ajpregu.2000.278.6.R1572. [DOI] [PubMed] [Google Scholar]
- 13.Kaiser UB, Conn PM, Chin WW. Endocr. Rev. 1997;18:46–70. doi: 10.1210/edrv.18.1.0289. [DOI] [PubMed] [Google Scholar]
- 14.Hawes BE, Barnes S, Conn PM. Endocrinology. 1993;132:2124–2130. doi: 10.1210/endo.132.5.8386608. [DOI] [PubMed] [Google Scholar]
- 15.Hawes BE, Marzen JE, Waters SB, Conn PM. Endocrinology. 1992;130:2465–2475. doi: 10.1210/endo.130.5.1315244. [DOI] [PubMed] [Google Scholar]
- 16.Imai A, Takagi H, Horibe S, Fuseya T, Tamaya T. J. Clin. Endocrinol. Metab. 1996;81:3249–3253. doi: 10.1210/jcem.81.9.8784077. [DOI] [PubMed] [Google Scholar]
- 17.Janovick JA, Conn PM. Endocrinology. 1994;135:2214–2219. doi: 10.1210/endo.135.5.7956944. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh KP, Martin TFJ. Mol. Endocrinol. 1992;6:1673–1681. doi: 10.1210/mend.6.10.1333052. [DOI] [PubMed] [Google Scholar]
- 19.Grosse R, Schmid A, Schoneberg T, Herrlich A, Muhn P, Schultz G, Gudermann T. J. Biol. Chem. 2000;275:9193–9200. doi: 10.1074/jbc.275.13.9193. [DOI] [PubMed] [Google Scholar]
- 20.Han XB, Conn PM. Endocrinology. 1999;140:2241–2251. doi: 10.1210/endo.140.5.6707. [DOI] [PubMed] [Google Scholar]
- 21.Lin X, Conn PM. Endocrinology. 1998;139:3896–3902. doi: 10.1210/endo.139.9.6214. [DOI] [PubMed] [Google Scholar]
- 22.Stanislaus D, Ponder S, Ji TH, Conn PM. Biol. Reprod. 1998;59:579–586. doi: 10.1095/biolreprod59.3.579. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Mol. Endocrinol. 2002;16:419–434. doi: 10.1210/mend.16.3.0791. [DOI] [PubMed] [Google Scholar]
- 24.Alarid ET, Windle JJ, Whyte DB, Mellon PL. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- 25.Turgeon JL, Kimura Y, Waring DW, Mellon PL. Mol. Endocrinol. 1996;10:439–450. doi: 10.1210/mend.10.4.8721988. [DOI] [PubMed] [Google Scholar]
- 26.Drissi H, Lasmoles F, Le Mellay V, Marie PJ, Lieberherr M. J. Biol. Chem. 1998;273:20168–20174. doi: 10.1074/jbc.273.32.20168. [DOI] [PubMed] [Google Scholar]
- 27.Murthy KS, Makhlouf GM. J. Biol. Chem. 1998;273:4695–4704. doi: 10.1074/jbc.273.8.4695. [DOI] [PubMed] [Google Scholar]
- 28.Imamura T, Vollenweider P, Egawa K, Clodi M, Ishibashi K, Nakashima N, Ugi S, Adams JW, Brown JH, Olefsky JM. Mol. Cell. Biol. 1999;19:6765–6774. doi: 10.1128/mcb.19.10.6765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClain DA, Maegawa H, Lee J, Dull TJ, Ulrich A, Olefsky JM. J. Biol. Chem. 1987;262:14663–14671. [PubMed] [Google Scholar]
- 30.Luttrell LM, Van Biesen T, Hawes BE, Koch WJ, Touhara K, Lefkowitz RJ. J. Biol. Chem. 1995;270:16495–16498. doi: 10.1074/jbc.270.28.16495. [DOI] [PubMed] [Google Scholar]
- 31.Becker-Hapak M, McAllister SS, Dowdy SF. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 32.Nishida M, Schey KL, Takagahara S, Kontani K, Katada T, Urano Y, Nagano T, Nagao T, Kurose H. J. Biol. Chem. 2002;277:9036–9042. doi: 10.1074/jbc.M107392200. [DOI] [PubMed] [Google Scholar]
- 33.Romoser VA, Graves TK, Wu D, Jiang H, Hinkle PM. Mol. Endocrinol. 2001;15:125–135. doi: 10.1210/mend.15.1.0588. [DOI] [PubMed] [Google Scholar]
- 34.Chang M, Zhang L, Tam JP, Sanders-Bush E. J. Biol. Chem. 2000;275:7021–7029. doi: 10.1074/jbc.275.10.7021. [DOI] [PubMed] [Google Scholar]
- 35.Richards JS. Mol. Endocrinol. 2001;15:209–218. doi: 10.1210/mend.15.2.0606. [DOI] [PubMed] [Google Scholar]
- 36.De Vivo M, Chen J, Codina J, Iyengar R. J. Biol. Chem. 1992;267:18263–18266. [PubMed] [Google Scholar]
- 37.Hamm HE, Rarick HM. Methods Enzymol. 1994;237:423–436. doi: 10.1016/s0076-6879(94)37079-6. [DOI] [PubMed] [Google Scholar]
- 38.Taylor JM, Neubig RR. Cell. Signal. 1994;6:841–849. doi: 10.1016/0898-6568(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 39.Dickenson JM, Hill SJ. Eur. J. Pharmacol. 1998;355:85–93. doi: 10.1016/s0014-2999(98)00468-3. [DOI] [PubMed] [Google Scholar]
- 40.Murthy KS, Coy DH, Makhlouf GM. J. Biol. Chem. 1996;271:23458–23463. doi: 10.1074/jbc.271.38.23458. [DOI] [PubMed] [Google Scholar]
- 41.de la Pena P, del Camino D, Pardo LA, Dominguez P, Barros F. J. Biol. Chem. 1995;270:3554–3559. doi: 10.1074/jbc.270.8.3554. [DOI] [PubMed] [Google Scholar]
- 42.Bourne GA, Baldwin DM. Am. J. Physiol. 1987;253:E296–E299. doi: 10.1152/ajpendo.1987.253.3.E296. [DOI] [PubMed] [Google Scholar]
- 43.Arora KK, Krsmanovic LZ, Mores N, O’Farrell H, Catt KJ. J. Biol. Chem. 1998;273:25581–25586. doi: 10.1074/jbc.273.40.25581. [DOI] [PubMed] [Google Scholar]
- 44.Ulloa-Aguirre A, Stanislaus D, Arora V, Vaananen J, Brothers S, Janovick JA, Conn PM. Endocrinology. 1998;139:2472–2478. doi: 10.1210/endo.139.5.6022. [DOI] [PubMed] [Google Scholar]
- 45.Maya-Nunez G, Conn PM. Biol. Reprod. 2001;65:561–567. doi: 10.1095/biolreprod65.2.561. [DOI] [PubMed] [Google Scholar]
- 46.Maya-Nunez G, Conn PM. Endocrinology. 1999;140:3452–3458. doi: 10.1210/endo.140.8.6945. [DOI] [PubMed] [Google Scholar]
- 47.Shah BH, Milligan G. Mol. Pharmacol. 1994;46:1–7. [PubMed] [Google Scholar]
- 48.Gur G, Bonfil D, Safarian H, Naor Z, Yaron Z. Mol. Cell. Endocrinol. 2002;189:125–134. doi: 10.1016/s0303-7207(01)00724-9. [DOI] [PubMed] [Google Scholar]