Abstract
The STAT3 transcription factors are cytoplasmic proteins that induce gene activation in response to growth factor stimulation. Following tyrosine phosphorylation, STAT3 proteins dimerize, translocate to the nucleus, and activate specific target genes involved in cell-cycle progression. Despite its importance in cancer cells, the molecular mechanisms by which this protein is regulated in response to DNA damage remain to be characterized. In this study, we show that STAT3 is activated in response to topoisomerase I inhibition. Following treatment, STAT3 is phosphorylated on its C-terminal serine 727 residue but not on its tyrosine 705 site. We also show that topoisomerase I inhibition induced the up-regulation of the cdk5 kinase, a protein initially described in neuronal stress responses. In co-immunoprecipitations, cdk5 was found to associate with STAT3, and pulldown experiments indicated that it associates with the C-terminal activation domain of STAT3 upon DNA damage. Importantly, the cdk5-STAT3 pathway reduced DNA damage in response to topoisomerase I inhibition through the up-regulation of Eme1, an endonuclease involved in DNA repair. ChIP experiments indicated that STAT3 can be found associated with the Eme1 promoter when phosphorylated only on its serine 727 residue and not on tyrosine 705. We therefore propose that the cdk5-STAT3 oncogenic pathway plays an important role in the expression of DNA repair genes and that these proteins could be used as predictive markers of tumors that will fail to respond to chemotherapy.
Keywords: Cancer Therapy, CDK (Cyclin-dependent Kinase), Drug Resistance, Gene Expression, STAT Transcription Factor
Introduction
Signal transducer and activator of transcription 3 (STAT3)3 proteins are cytoplasmic transcription factors that translocate into the nucleus following growth factor stimulation. In contrast to normal cells where its phosphorylation is only transient, constitutive activation of STAT3 has been reported in several primary cancers and tumor cell lines (1–3). This abnormal activation is due to oncogenic kinases such as epidermal growth factor receptor, Her2/Neu, src, or bcr-abl, which induce STAT3 activation through phosphorylation of its tyrosine 705 residue (4). This phosphorylation allows the nuclear translocation and DNA binding of the STAT3 dimer and the up-regulation of several genes involved in cell-cycle and cell survival such as cyclin D1, Myc, or bclxl. The up-regulation of these cancer genes mediates the oncogenic activity of STAT3 and its ability to transform cells (5).
A second phosphorylation occurs on the serine 727 residue of the C-terminal activation domain. It has been proposed that this phosphorylation is necessary for maximal gene activation, because its mutation prevents STAT3 transcriptional function (6). It is believed that this modification favors the recruitment of transcriptional cofactors such as CBP, NcoA, or P-Tefb that binds to the C-terminal domain of the transcription factor (7–10). However, it remains to be determined if the association of STAT3 with its coactivators is a direct consequence of Ser-727 phosphorylation.
Although it was initially believed that the tyrosine phosphorylation is essential for STAT3 activity, several groups have recently reported that specific forms of the transcription factor, which are only phosphorylated on its Ser-727 residue, can induce gene activation. In prostate cancer, Ser-727 phosphorylation is sufficient to activate STAT3 and drive tumorigenesis in the absence of tyrosine 705 activation (11). Elegant results have shown that tyrosine 705 mutants can associate with NF-κB and induce the expression of genes such as mras or met, which are likely to play an important role in cell transformation by STAT3 (12–14). These results lead to the important conclusion that the influence of STAT3 on cell transformation can be independent of the tyrosine 705 phosphorylation and that this site should not be considered as a unique marker of STAT3 oncogenic activity.
This conclusion also leads to the hypothesis that STAT3 can induce different transcriptional programs, depending on which sites are phosphorylated and certainly on the type of stimulation. Although STAT3 activation is well characterized in response to growth factor stimulation, little is known about its regulation in response to other stimulation such as DNA damage and chemotherapy treatment. Interestingly, several studies have suggested that an abnormal activation of this transcription factor is associated with intrinsic drug resistance (15). STAT3 expression has been associated with resistance to radiation-induced apoptosis (16–18), and it can also confer resistance to Fas or paclitaxel-mediated apoptosis in multiple myeloma and ovarian cancer (19, 20). Most of the time, escape to drug treatment is related to the STAT3-mediated expression of survival proteins such as bcl-xl or survivin (21, 22). In addition, we have recently shown that the epidermal growth factor receptor-src-STAT3 pathway can prevent senescence induction (23) and activate DNA repair genes (24) to confer resistance to chemotherapy treatments.
In this study, we have further characterized the regulation of STAT3 during DNA damage. In colorectal cell lines, we have found that the transcription factor is phosphorylated on its serine 727 residue following topoisomerase I inhibition and that tyrosine 705 phosphorylation is not modified. In addition, we have also observed that this phosphorylation is due to the binding of the cdk5 kinase to the transcription factor. cdk5 is a serine/threonine kinase, which was initially characterized in postmitotic neurons. Once associated with its specific activators p35/p25, this protein plays an important role in neuronal survival, neurite outgrowth, and cytoskeletal functions (25–27). In response to topoisomerase I inhibition, we have observed that cdk5 is activated and that it interacts with STAT3 to induce its serine phosphorylation. Cdk5 appeared to be involved in the STAT3-mediated regulation of the cyclin D1, myc, and Eme1 genes. Importantly, ChIP analysis showed that the transcription factor can be found associated with the Eme1 promoter when phosphorylated only on serine 727. We therefore propose that cdk5 regulates the STAT3-Eme1 pathway and that this is an important step in the response of colorectal tumors to topoisomerase I inhibition.
MATERIALS AND METHODS
Cell Lines
The human colorectal cell lines HT29 (HTB-38) and HCT116 (CCL-247) (ATCC, Manassas, VA) were cultured in RPMI 1640 medium (Lonza, Walkersville, MD). Cell lines were supplemented with 10% fetal bovine serum (PAA Laboratories GmbH, Austria).
Materials
sn38 came from Pfizer (New York, NY). Polyclonal anti-STAT3 (C20), anti-phospho-STAT3-Ser-727 (ser727-R), anti-cdk5 (C8), anti-cdk5 Y15, anti-Erk2 (C14), anti-phospho-Erk1/2 (E4), anti-p35 (C19), anti-lamin A/C (346), anti-β-tubulin (H-235), and hsc70 (B-6) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-H2Ax Alexa fluor was obtained from BD Biosciences, and the anti-phospho-STAT3-Tyr 705 was from Cell Signaling. The cdk5 and STAT3 siRNAs have been obtained from Dharmacon Inc. (Lafayette, CO) and transfected using the Dharmafect 4 (Dharmacon) method. Three different siRNAs were used for each transfection.
Cell Treatment
Cells grown in 3% FBS medium were immediately treated with sn38 (5 ng/ml) for 48 h. Note that this treatment should be done before complete cell adhesion so that every cell can incorporate the drug before entering the next S phase. For siRNA experiment, cells were transfected with the appropriate siRNA using the Dharmafect 4 method and grown up for 48 h in 6-well plates. In 3% FBS medium, cells were then divided into two wells and again immediately treated with sn38 (5 ng/ml) for 48 h.
Immunoprecipitation and Western Blot Analysis
After two washings with cold PBS, cells were lysed in 100 μl (Western blot) or 1 ml (immunoprecipitation) using ice-cold lysis buffer (25 mm HEPES, pH 7.9, 300 mm KCl, 0.2 mm EDTA, 10% glycerol, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5 m NaF, 100 mm Na3VO4). After a 30-min incubation at 4 °C, total extracts were clarified by centrifugation at 12,000 rpm for 10 min.
Immunoprecipitations were performed overnight at 4 °C with whole cell extracts (2–4 mg) in the presence of 0.1 or 1% Nonidet P-40 (CA-630, Sigma). Cell extracts were precleared with 75 μl of protein G-agarose (Sigma-Aldrich, 50% slurry in phosphate-buffered saline) for 2 h at 4 °C, and cleared extracts were immunoprecipitated with 4 μg of the indicated antibodies overnight at 4 °C followed by the addition of 50 μl of protein G-agarose for 1 h at 4 °C. Immunoprecipitated proteins were washed two times in lysis buffer and one time with 10 mm Tris, pH 8, 100 mm EDTA, prior to the addition of sample buffer. Following electrotransfer, membranes (Millipore Corp., Billerica, MA) were blocked for 45 min at room temperature in Tris-buffered saline buffer, 5% bovine serum albumin, 0.05% Tween. Membranes were then incubated overnight with the indicated antibodies diluted in Tris-buffered saline buffer, 1% bovine serum albumin, 0.05% Tween at 4 °C. After three washings, blots were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody for 45 min. Proteins were detected using an enhanced chemiluminescence system (ECL, Bio-Rad).
Quantitative PCR
RNA was extracted using the TRIzol method (Invitrogen), and complementary DNA was synthesized from 2 μg of RNA by random hexamer priming using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). For cDNA quantification, PCR was performed with 4 μl of 20× diluted cDNA, 5 μl of Qiamix (Qiagen), and 1 μl of 5 μm primers. Accumulation of fluorescent products was monitored on the ABI PRISM 7300 real-time PCR system (Applied Biosystems, Foster City, CA). The relative quantification of gene expression was performed using the comparative CT method, with normalization of the target gene to the endogenous housekeeping gene RPLPO. RT-PCR primers were as follows: RPLPO (5′-AACCCAGCTCTGGAGAAACT-3′ and 5′-CCCCTGGAGATTTTAGTGGT-3′), CD1 (5′-CAGTAACGTCACACGGACTAC-3′ and 5′-ACAGGAGCTGGTGTTCCAT-3′), cdk5 (5′-AGCGACAAGAAGCTGACTTT-3′ and 5′-AGAATCCCAGCCCTTTTAGT-3′), and Eme1 (5′-AACGCTTCAGGGCTTTGTAA-3′ and 5′-GCTCCCTGTTTCCCTCTTCT-3′).
ChIP
Attached cells were washed twice with cold PBS, cross-linked with 1% formaldehyde at room temperature for 10 min, and then washed twice with 10 ml of cold PBS. Cells were lysed with 500 μl of lysis buffer (1% SDS, 10 mm EDTA, 150 mm NaCl, 20 mm Tris-HCl, pH 8.1, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5 m NaF, 100 mm Na3VO4), and extracts were sonicated six times for 15 s each. Supernatants were recovered by centrifugation at 12,000 rpm for 10 min at 4 °C, diluted one time in dilution buffer (1% Triton X-100, 20 mm Tris-HCl, pH 8.1, 2 mm EDTA, 150 mm NaCl, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5 m NaF, 100 mm Na3VO4), and subjected to one round of immunoclearing for 2 h at 4 °C using protein-G-agarose coated with salmon sperm DNA (Millipore). Immunoprecipitations were performed overnight with specific antibodies, then 20 μl of protein G-agarose-coated beads with salmon sperm DNA (50%) was added for 1 h at 4 °C. Beads were then washed for 10 min in TSE1 (0.1% SDS, 1% Triton X-100, 2mm EDTA, 20 mm Tris-HCl, pH 8.1, and 150 mmol/NaCl), TSE2 (0.1% SDS, 1% Triton X-100, 2 mm EDTA, 20 mm Tris-HCl, pH 8.1, and 500 mmol/NaCl), and TSE3 (0.25 m LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mm EDTA, and 10 mm Tris-HCl, pH 8.1). Beads were washed once with TE buffer (10 mm Tris, pH 8, 100 mm EDTA) and eluted with 500 μl of elution buffer (1% SDS and 0.1 m NaHCO3) for 1 h. Eluates were heated at 65 °C overnight, and DNA was precipitated using classic procedures. For PCR, 5 μl from a 100-μl DNA preparation was used for 25–30 amplification cycles. The following primers were used: region −34/+89, 5′-CCGGGCTTTGATCTTTGCT-3′ and 5′-GACTCTGCTGCTCGCTGCTA-3′ of the cyclin D1 promoter; region −2760/−2486, 5′-TTGTGCCACTGCTGACTTTGTC-3′ and 5′-AGCCTGAAGAAGGAGGATGTGAGG-3′ of the p21 promoter. Myc and Eme1 primers have been described before (24, 28).
Flow Cytometry
For DNA content analysis, 1.5 × 106 cells were washed twice with PBS and fixed in 70% ethanol. Cells were treated with 100 units/ml RNase A for 20 min at 37 °C, then diluted in PBS/propidium iodide (50 μg/ml), and immediately analyzed by flow cytometry (BD Biosciences). For phospho-H2Ax analysis, 1 × 106 cells were recovered by centrifugation with their supernatant at 1500 rpm for 5 min at room temperature. Cells were fixed with 2% paraformaldehyde at room temperature for 10 min. Cells were then washed twice with PBS and centrifuged at 1500 rpm for 5 min at 4 °C. Cells were incubated with a PBS-2% bovine serum albumin-0.2% Triton solution for 2 min. The primary antibody was diluted at 1/50, and 4′,6-diamidino-2-phenylindole (5 μg/ml) was diluted 500 times. Cells were incubated for 1 h a room temperature and then analyzed by flow cytometry.
Colony Formation Assay
For colony formation assays, 1000 cells were plated per well in 6-well plates, treated with sn38 the next day and allowed to form colonies. After 10–14 days cells were washed twice with PBS and treated with crystal violet for 10 min at room temperature, and then washed five times with water. The percentage of colony-forming cells was calculated as compared with non-treated cells.
Pulldown Assay
Bacteria were grown up in 5 ml of LB medium overnight. 200 ml of ampicillin-LB was inoculated with 2 ml of the overnight culture and grown up until optical density reached 0.6–0.8. Isopropyl 1-thio-β-d-galactopyranoside was then added at 1 mm for 2 h, bacteria were recovered by centrifugation at 4000 rpm for 20 min at 4 °C, resuspended in 8 ml of lysis buffer (50 mm Na2HPO4, pH 8, 300 mm NaCl, 10 mm imidazole, 1 mm phenylmethylsulfonyl fluoride, 1 mg/ml lysozyme), incubated on ice for 30 min and sonicated 6–8 times for 20 s. Triton X-100 was added to the final concentration of 1% and incubated on ice for 15 min. Extracts were recovered by centrifugation at 4000 rpm for 15 min at 4 °C, and supernatants were transferred to a 15-ml conical tube. 250 μl of beads (nickel-nitrilotriacetic acid-agarose, Qiagen) was added for every 200 ml of initial culture, and extracts were incubated for 1 h at 4 °C. Beads were then washed three times with washing buffer (50 mm Na2HPO4, pH 8, 300 mm NaCl, 20 mm imidazole, 1 mm phenylmethylsulfonyl fluoride). Beads (400 ng of fusion protein) were then incubated for 20 min at 4 °C with cell extracts (300 μg) and washed three times with lysis buffer (25 mm HEPES, pH 7.9, 300 mm KCl, 0.2 mm EDTA, 10% glycerol, 1 mm phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin A, 0.5 m NaF, 100 mm Na3VO4) prior to the addition of sample buffer and Western blot analysis.
Kinase Assay
His-Δ1–716 STAT3 proteins were produced as described above and eluted from the beads. In parallel, cdk5 was immunoprecipitated from sn38-treated cells (total extracts, 100 μg), and 15 μl of beads was incubated with 1 μg of His-Δ1–716 STAT3 at room temperature for 10 min with 10 μm cold ATP. The reaction was stopped by the addition of 50 μl of sample buffer. Samples were analyzed by Western blot as described above using a polyclonal antibody directed against the serine-phosphorylated form of STAT3.
RESULTS
STAT3 Is Phosphorylated on Its Serine 727 Residue following Topoisomerase I Inhibition
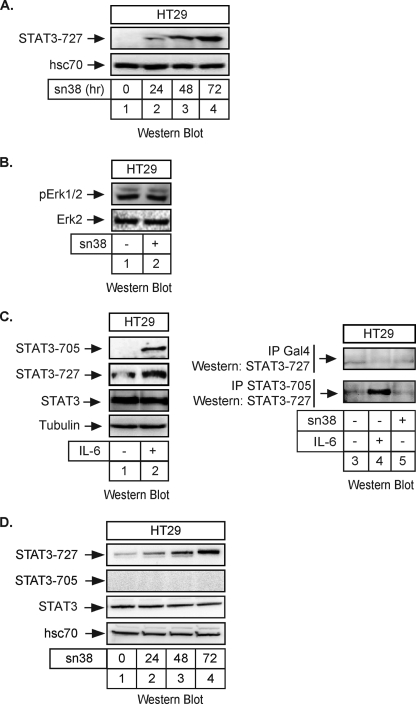
To determine if the STAT3 transcription factor is involved in the response to DNA damage, growing HT29 colorectal cells were treated with sn38, the active metabolite of irinotecan. Using Western blot analysis, we observed that topoisomerase I inhibition induced the phosphorylation of STAT3 on its serine 727 C-terminal residue (Fig. 1A, lanes 3 and 4). As a control, topoisomerase I inhibition did not affect the expression or phosphorylation of the Erk1/2 kinases (Fig. 1B, lanes 1 and 2). Because p53 is mutated in the HT29 cell line, this effect does not appear to rely on the tumor suppressor gene. Under these conditions, we were not able to detect a significant activation of the tyrosine 705 phosphorylation site, whereas this site was normally phosphorylated upon IL-6 stimulation. When HT29 cells were serum-starved for 2 days and then stimulated with this cytokine for 30 min, a significant activation of the two phosphorylation sites was detected as expected (Fig. 1C, lanes 1 and 2). In addition, when the transcription factor was immunoprecipitated using antibodies directed against its tyrosine 705-phosphorylated form, we observed that STAT3 was phosphorylated on its two sites following IL-6 stimulation. However, we were not able to detect any tyrosine phosphorylation following topoisomerase I inhibition (Fig. 1C, compare lanes 4 and 5). Using Western blot analysis, we observed that this phosphorylation remained non-detectable during the 3 days of treatment, whereas the serine 727 phosphorylation was easily detected and declined at 96 h (Fig. 1D, lanes 1–4 and data not shown). Note, however, that we were able to detect a weak constitutive phosphorylation of the tyrosine 705 site when 600 μg of total extract was used. By contrast, 50- 60 μg of proteins were used in this study to detect all protein expression and STAT3 serine 727 phosphorylation. Therefore, in our experimental model, the tyrosine-phosphorylated forms of STAT3 are not highly expressed as compared with the ones presenting a phosphorylation on the serine 727 residue.
FIGURE 1.
STAT3 is phosphorylated on its serine 727 residue following topoisomerase I inhibition. A, HT29 cells were treated with sn38 (5 ng/ml) or not for the indicated times. Following stimulation, total cell extracts were prepared, and serine 727 phosphorylation was analyzed by Western blot using polyclonal antibodies directed against the phosphorylated form of the protein. The membrane was reprobed with an antibody directed against hsc70 as a loading control (n = 5). Note that, in every experiment, sn38 was added to the cell culture just after cell plating to get an optimal inhibition of cell cycle progression. B, HT29 cells were treated with sn38 (5 ng/ml) for 48 h. Following stimulation, cell extracts were prepared and p-Erk1/2 and Erk2 expression was analyzed by Western blot with polyclonal antibodies directed against these proteins (n = 3). C, HT29 cells were serum-starved for 2 days and stimulated with IL-6 (20 ng/ml) for 30 min. STAT3 activation was analyzed by Western blot using antibodies directed against the different phosphorylated forms of the proteins or against its non-phosphorylated form (n = 2). In parallel, whole cell extracts were immunoprecipitated with polyclonal antibodies directed against the tyrosine-phosphorylated form of STAT3 (−705) or control antibodies (Gal4), and samples were then analyzed by Western blot using polyclonal antibodies directed against STAT3 Ser727 (n = 2). D, HT29 cells were treated with sn38 (5 ng/ml) or not for the indicated times. Following stimulation, total cell extracts were prepared, and the serine 727 and tyrosine 705 phosphorylations were analyzed by Western blot using polyclonal antibodies directed against the phosphorylated forms of the protein. The membrane was reprobed with an antibody directed against STAT3 and then hsc70 as a loading control (n = 3).
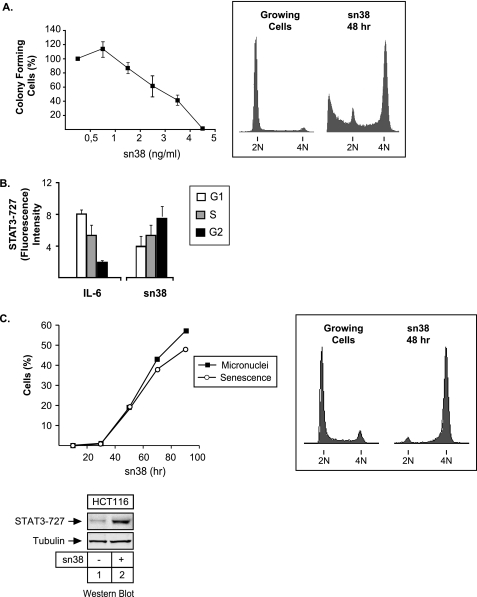
The activation of STAT3 was surprising, because its phosphorylation theoretically occurs in the G1 phase of the cell cycle and in response to growth factor stimulation. Under the conditions used in this study, clonogenic assays indicated that sn38 treatment inhibited cell proliferation, and FACS analysis showed that HT29 cells were arrested in the G2 phase of the cell cycle after 48 h (Fig. 2A). Because STAT3 phosphorylation is maximal at 72 h, this event is probably not driving G2 arrest. To confirm that STAT3 was activated during the G2 phase of the cell cycle upon DNA damage, FACS experiments were performed using propidium iodide staining conjugated with intracellular staining using phospho-serine 727 antibodies. Cells were treated with sn38 or starved and stimulated with IL-6 as a control. As expected, upon cytokine stimulation, the two main STAT3 phosphorylation sites, serine 727 (and tyrosine 705, data not shown) were detected mainly in cells present in the G1 phase of the cell cycle. By contrast, serine phosphorylation following sn38 treatment was detected essentially in the G2 phase of the cell cycle (Fig. 2B).
FIGURE 2.
STAT3 is phosphorylated on serine 727 during G2 arrest. A, HT29 cells were treated or not with different concentration of sn38 for 10–14 days. Colony formation was then counted using an inverted microscope, and the growth of non-treated cells was set up at 100%. Clonogenic survival was then plotted as a fraction relative to these untreated cells (n = 5 ± S.D.). In parallel, growing HT29 cells were treated with sn38 (5 ng/ml) for 48 h, and DNA content and apoptosis were then evaluated by flow cytometry (n = 5). B, HT29 cells were treated with sn38 (5 ng/ml) or IL-6 (20 ng/ml) as indicated, and DNA content and serine phosphorylation were then analyzed by flow cytometry analysis using polyclonal antibodies directed against the serine 727-phosphorylated form of STAT3 (n = 3). C, growing HCT116 cells were treated or not with sn38 for different times as indicated. The percentage of senescent cells was evaluated as the number of cells expressing SA-β-gal activity and micronuclei (left part, n = 3). In parallel, DNA content was evaluated by flow cytometry after 48 h (right part), and the phosphorylation of STAT3 on its serine residue was analyzed by Western blot as described above (n = 3, bottom).
We noticed that a fraction of HT29 cells died by apoptosis following sn38 treatment as shown by the reproducible presence of a subG1 propidium iodide staining (Fig. 2A, right panel). This escape to G2 arrest has been previously reported and is due to the inactivation of the p53-p21waf1 pathway in this cell line (29). To determine if STAT3 phosphorylation was due to the induction of apoptosis, we used HCT116 cells, because topoisomerase I inhibition induces senescence in this cell line due to intact p53 signaling. Results presented in Fig. 2C confirm that sn38 induced G2 arrest and the appearance of cells with multiple micronuclei and an increase in the number of β-galactosidase-positive cells, two hallmarks of senescence induction and mitotic catastrophe. Under this condition, Western blot analysis showed that STAT3 was phosphorylated on its serine 727 residue to the same extent as compared with HT29 cells (Fig. 2C, lanes 1 and 2). This result suggests that the phosphorylation of the transcription factor is not due to apoptosis. Importantly, it also indicates that this effect is not cell line-specific. Altogether, these results indicate that the STAT3 transcription factor is phosphorylated on its serine 727 residue in response to topoisomerase I inhibition.
The cdk5 Kinase Is Up-regulated upon Topoisomerase I Inhibition
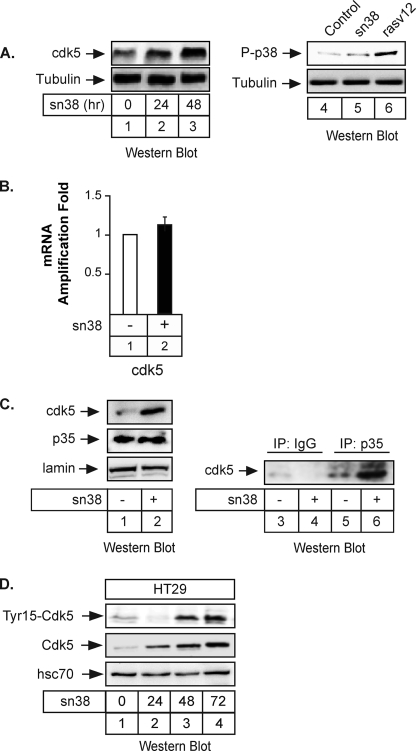
We then wanted to determine which kinase is involved in STAT3 phosphorylation upon genotoxic treatment. During the course of these experiments, we have noticed that the expression of the cdk5 kinase was increased in response to topoisomerase I inhibition. Although cdk5 has been essentially characterized in neurons (25), it has been recently shown that this protein is also involved in the response to DNA damage and in the induction of senescence programs (30–33). Interestingly, this kinase has also been shown to regulate STAT3 in neuronal cells (34). Using total cell extracts, Western blot experiments showed that cdk5 expression was significantly enhanced in response to sn38 (Fig. 3A, lanes 2 and 3). As a control, we did not detect any significant activation of p38, although it was phosphorylated as expected in response to the rasV12 oncogene (Fig. 3A, lanes 4–6). To determine if the increased expression of the kinase was regulated at the transcriptional level, cells were treated with sn38, and the level of the cdk5 mRNA was analyzed by quantitative RT-PCR experiments. Results presented Fig. 3B showed that topoisomerase I inhibition had no effect on this mRNA, suggesting that the expression of cdk5 was mainly regulated at the translational level. In neuronal cells, it has been shown that cdk5 is activated following its association with its p35/p25 coactivator (25). Western blot experiments showed that p35 was constitutively expressed in HT29 cells and that sn38 did not affect its expression level (Fig. 3C, lanes 1 and 2). We were not able to detect p25. We then asked whether cdk5 interacts with p35 upon drug treatment. To this end, HT29 cells were treated with sn38, total cell extracts were recovered, and co-immunoprecipitations were performed with polyclonal antibodies directed against p35 or nonspecific antibodies (Fig. 3C, lanes 3–6). Proteins present in the immunoprecipitates were revealed by immunoblotting with anti-cdk5 antibodies. Under these conditions, cdk5 was found to co-immunoprecipitate with p35 proteins (Fig. 3C, compare lane 6 and 4). Note that these co-immunoprecipitations were carried out using non-transfected cells, so that the association does not require the proteins to be overexpressed. It has been recently proposed that the binding to p35 is not sufficient to initiate Cdk5 kinase activation, which is also dependent on the phosphorylation of the kinase on its tyrosine 15 residue (35). We thus determined using total cell extracts whether sn38 also modulates Cdk5 phosphorylation. As shown in Fig. 3D, lanes 1–4, we found that phosphorylation of Cdk5 at tyrosine 15 was also increased in response to topoisomerase I inhibition. Note that, using total cell extracts, we were not able to detect a significant activation of the kinase before 48 h of treatment (see below). Therefore, we concluded from these results that the cdk5 kinase is activated following topoisomerase I inhibition.
FIGURE 3.
The cdk5 kinase is activated following topoisomerase I inhibition. A, growing HT29 cells were treated or not with sn38 (5 ng/ml) for 24 or 48 h. Following stimulation, total cell extracts were prepared, and cdk5 expression was analyzed by Western blot using polyclonal antibodies directed against the kinase (lanes 1–3, n = 5). Under the same conditions, the phosphorylation of the p38 kinase was investigated. As a control, cells were transfected with the rasv12 oncogene to induce p38 activation (lanes 4–6, n = 3). The membranes were reprobed with an antibody directed against tubulin as a loading control. B, growing HT29 cells were incubated with sn38 (5 ng/ml) for 48 h, and the expression of the cdk5 mRNA was analyzed by quantitative RT-PCR experiments (n = 3). C, growing HT29 cells were treated as described above, and after 48 h, whole cell extracts were prepared and Western blot analysis was performed with polyclonal antibodies directed against cdk5, p35, or lamin as a loading control (lanes 1 and 2). In parallel, extracts were immunoprecipitated with polyclonal antibodies directed against p35 (lanes 5 and 6) or a control serum (lanes 3 and 4). Samples were then analyzed by Western blot using polyclonal antibodies directed against cdk5 (n = 3). D, HT29 cells were treated with sn38 as described previously, and the expression and phosphorylation of cdk5 on its tyrosine 15 residue were analyzed by Western blot (n = 3).
Cdk5 Interacts with STAT3 and Induces Its Serine 727 Phosphorylation upon Topoisomerase I Inhibition
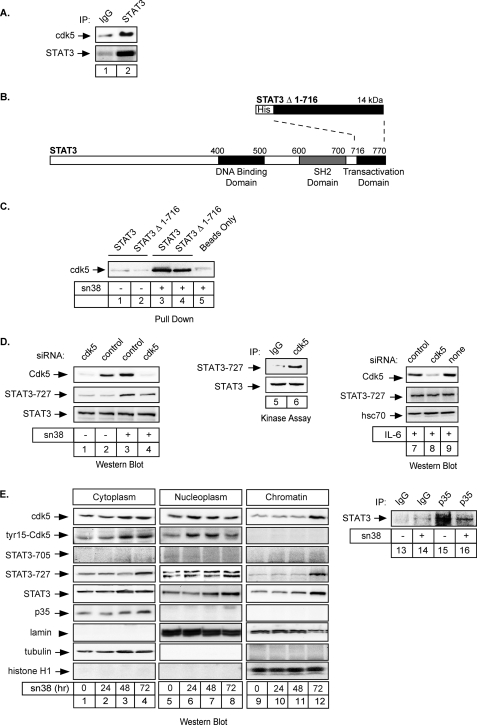
To determine if cdk5 is involved in STAT3 serine phosphorylation following sn38 treatment, we first asked if the kinase could bind to the transcription factor. To this end, HT29 cells were treated with sn38, total cell extracts were recovered, and immunoprecipitations were performed with polyclonal antibodies directed either against STAT3 or nonspecific antibodies (Fig. 4A, lanes 1 and 2). Proteins present in the immunoprecipitates were revealed by immunoblotting with the reciprocal cdk5 or STAT3 antibodies. Under these conditions, STAT3 was found to co-immunoprecipitate with cdk5. These interactions were specific, because almost no interaction was observed using a control IgG antibody (Fig. 4A, compare lanes 1 and 2). Importantly, these co-immunoprecipitations were performed with extracts from non-transfected cells; therefore, the association between STAT3 and cdk5 did not rely on the overexpression of the two proteins.
FIGURE 4.
Cdk5 interacts with STAT3 to induce its phosphorylation on Serine 727. A, HT29 cells were treated with sn38 for 48 h, and whole cell extracts were then immunoprecipitated with polyclonal antibodies directed against STAT3 proteins (lane 2) or a control serum (lane 1), separated by SDS-PAGE, transferred to a nitrocellulose filter, and probed with polyclonal antibodies directed against STAT3 or cdk5 proteins as indicated. B, representation of the fusion proteins used in the pulldown experiments. C, total cell extracts (300 μg) were incubated with histidine, with His-tagged STAT3Cter (STAT3Δ1–716), or with the full-length STAT3 (his-STAT3) immobilized on nickel-agarose beads (400 ng). Samples were then separated on polyacrylamide gels, and cdk5 binding was detected by Western blot using anti-cdk5 polyclonal antibodies (lanes 1–4). D, growing HT29 cells were either transfected with cdk5-specific siRNA oligonucleotides or control oligonucleotides as indicated. Cdk5 expression and phosphorylation of STAT3 on its serine residue were monitored after treatment with sn38 for 48 h (lanes 1–4, n = 4). In parallel, cdk5 was immunoprecipitated from sn38-treated cells and incubated with STAT3Δ1–716 for 10 min at RT in the presence of cold ATP. The phosphorylation of STAT3 on its serine 727 residue was analyzed by Western blot as described above (lanes 5 and 6). In parallel, growing HT29 cells were either transfected with cdk5-specific siRNA or control siRNA as indicated, serum-starved, and then stimulated with IL-6 for 30 min. Cdk5 expression and STAT3 phosphorylation were monitored as above (n = 2). E, HT29 cells were treated with sn38 for the indicated times and cytoplasmic, nuclear, or chromatin extracts were prepared and analyzed by Western blot analysis using antibodies directed against the indicated proteins (n = 2, lanes 1–12). Lamin, tubulin, and histone expression were used as loading controls for each compartment. In parallel, HT29 cells were treated or not with sn38 for 48 h, and whole cell extracts were then immunoprecipitated with polyclonal antibodies directed against p35 (lanes 15 and 16) or a control serum (lanes 13 and 14), separated by SDS-PAGE, transferred to a nitrocellulose filter, and probed with polyclonal antibodies directed against STAT3 proteins as indicated.
To confirm this observation, in vitro pulldown experiments were performed using bacterially produced 6× histidine-tagged STAT3 containing either the full-length protein or the 716–770 amino acids corresponding to the activation domain of STAT3 (Fig. 4B, STAT3 or STAT3Δ1–716). His-tagged STAT3 proteins were immobilized on beads and incubated with total cell extracts prepared from cells treated or not with sn38. As previously shown using extracts from neuronal cells (34), we found that the endogenous cdk5 kinase was retained by the full-length his-STAT3 protein as well as by the STAT3Δ1–716 fusion protein immobilized on beads. By contrast, the kinase was not retained by the beads alone (Fig. 4C, compare lanes 3–5). We also noticed that this interaction was dependent on sn38, because almost no signal was observed using extracts from non-treated cells (Fig. 4C, compare lanes 1–2 with 3–4).
We then determined if cdk5 was involved in the serine phosphorylation of STAT3 in response to genotoxic treatment. To this end, cells were transfected with a pool of three siRNA directed against cdk5 or the corresponding control siRNA, cells were treated or not, and STAT3 phosphorylation was then investigated by Western blot. Following siRNA transfection, we observed as expected that the expression of cdk5 was down-regulated (Fig. 4D, lanes 1 and 4, top panel). Interestingly, we also noticed that STAT3 serine phosphorylation was reduced upon genotoxic treatment in the absence of the kinase (Fig. 4D, compare lanes 3 and 4, middle panel). Note, however, that we were not able to completely down-regulate STAT3 phosphorylation, suggesting either that the S727-phosphorylated form of STAT3 has an increased half-life or that other kinases are also phosphorylating STAT3. To further confirm that cdk5 was able to phosphorylate STAT3, the kinase was immunoprecipitated from sn38-treated cells and incubated in vitro in the presence of cold ATP and a purified preparation of STAT3Δ1–716. Western blot analysis confirmed that the transcription factor was effectively phosphorylated on its serine 727 residue under these conditions (Fig. 4D, lanes 5 and 6). In addition, we also determined if cdk5 was involved in STAT3 phosphorylation in other experimental conditions. To this end, cells were transfected with siRNA as described above, serum-starved, and then stimulated with IL-6 for 30 min. Results indicates that cdk5 was not involved in the activation of STAT3 by this cytokine (Fig. 4D, lanes 7–9).
It is well known that STAT3 is present in the cytoplasm and in the nucleus. In addition, it has also been reported that cdk5 is localized in the cytoplasm to regulate the neuronal architecture. To determine the localization of these proteins following topoisomerase I inhibition, fractionation experiments have been performed (Fig. 4E, lanes 1–12). Results showed that both STAT3 and cdk5 were present in the nucleus and on the chromatin following sn38 treatment (Fig. 4E, lanes 6, 7, and 12). As observed in Fig. 1, STAT3 phosphorylation was maximal at 72 h. Both proteins were also activated in the cytoplasm; however, cdk5 activation occurred first in the nucleus, suggesting that the initial activation event might occur in this compartment between 24 and 48 h. Interestingly, we were also able to detect cdk5 on chromatin but only its non-phosphorylated form. However, ChIP experiments indicated that cdk5 was not associated with STAT3 on its target genes (see below). These fractionation experiments also indicated that p35 was not present in the nucleus. Because p35 and cdk5 interact, we then asked whether p35 binds to STAT3 in response to sn38. As described above, total cell extracts were recovered, and immunoprecipitations were performed with polyclonal antibodies directed either against p35 or nonspecific antibodies (Fig. 4E, lanes 13–16). Proteins present in the immunoprecipitates were revealed by immunoblotting with the reciprocal STAT3 antibodies. Under these conditions, STAT3 was found to co-immunoprecipitate with p35. Interestingly, this association was inhibited following sn38 treatment (Fig. 4E, compare lanes 15 and 16). This was expected if these proteins localized in two different compartments following topoisomerase I inhibition. Taken together, these results suggest that cdk5 binds to STAT3 upon topoisomerase I inhibition to induce its phosphorylation on serine 727.
The cdk5 Kinase Regulates the Activation of STAT3 Target Genes following Topoisomerase I Inhibition
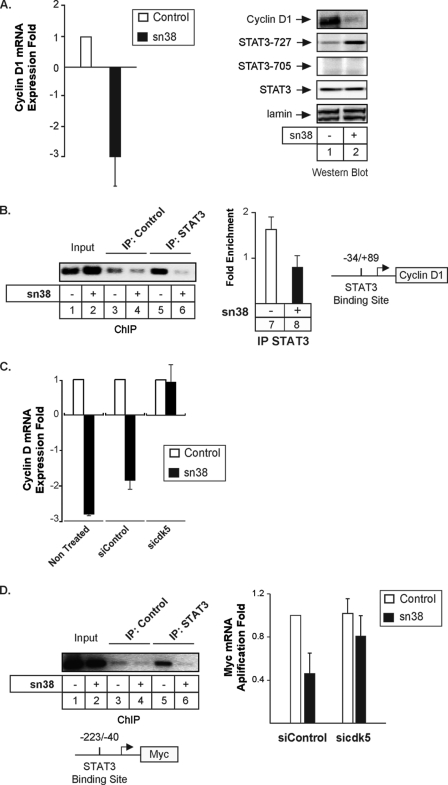
We then wanted to determine if cdk5 is involved in the regulation of STAT3 target genes following sn38 treatment. It is well known that STAT3 regulates the expression of cyclin D1 in growing cells to induce cell cycle progression (36). Because sn38 treatment induced growth inhibition (see Fig. 2), we then determined if topoisomerase I inhibition prevented cyclin D1 expression and if this was linked to cdk5 activation. To this end, cells were treated or not with sn38, and the expression of the cyclin was evaluated by quantitative RT-PCR and Western blot experiments. As expected, results indicated that genotoxic treatment down-regulated the expression of the cyclin D1 mRNA and protein (Fig. 5A). STAT3 was phosphorylated as expected on the serine 727 under these conditions. To determine if STAT3 is associated with the cyclin D1 promoter and if this binding is regulated in response to SN38, ChIP experiments were then performed using STAT3 antibodies and primers encompassing the proximal promoter where a binding site for the transcription factor has been recently described (37). Antibodies directed against the Ras protein were used as negative controls. As previously shown, ChIP experiments confirmed that STAT3 was present on the proximal cyclin D1 promoter in growing cells. Importantly, the association of the transcription factor with DNA was significantly inhibited following sn38 treatment (Fig. 5B, lanes 5 and 6). As a control, PCR analysis did not detect any occupancy of the −2760/−2486 region of the p21waf1 gene by STAT3 (data not shown). The ChIP result was obtained by semi-quantitative PCR (Fig. 5B, compare lanes 5 and 6) and quantified by quantitative-PCR (Fig. 5B, lanes 7 and 8). We then determined if cdk5 was involved in the inhibition of the cyclin D1 gene upon sn38 treatment. As described above, cells were transfected with a pool of three siRNA directed against cdk5 or the corresponding control siRNA, and the expression of cyclin D1 was then investigated following sn38 treatment by quantitative RT-PCR analysis. As expected, genotoxic treatment reduced the expression of the cyclin D1 mRNA, and the same effect was observed when cells were transfected with control siRNA (Fig. 4C). Interestingly, the sn38-mediated inhibition of cyclin D1 was not observed anymore in the absence of cdk5.
FIGURE 5.
Cdk5 is involved in the down-regulation of cyclin D1 and myc following topoisomerase I inhibition. A, growing HT29 cells were incubated with sn38 (5 ng/ml) for 48 h, and the expression of the cyclin D1 mRNA was analyzed by quantitative RT-PCR experiments (n = 3). In parallel, Western blot experiments were also performed to confirm the down-regulation of the cyclin D1 protein and the phosphorylation of STAT3 on its serine residue (lanes 1 and 2). B, HT29 growing cells were treated as described above, and soluble chromatin was prepared from the indicated cells and immunoprecipitated with antibodies directed against STAT3 or control antibodies. DNA was amplified using one pair of primers that covers the STAT3 proximal binding site of the cyclin D1 promoter. ChIP assays were analyzed on agarose gel (left part) or quantified by real-time PCR (n = 3, right part of the figure). C, growing HT29 cells were left untreated or transfected with cdk5-specific or control siRNA oligonucleotides as indicated. Cyclin D1 mRNA expression was analyzed by quantitative RT-PCR experiments following sn38 treatment (n = 3). D, growing HT29 cells were treated as described above, and the association of STAT3 with the myc proximal promoter was analyzed by ChIP (lanes 1–6). In parallel, myc expression was evaluated by quantitative RT-PCR in the presence or absence of cdk5 (right part, n = 4 ± S.D.).
Besides cyclin D1, we and others have also shown that the Myc-cdc25a pathway is also an important target of the STAT3 oncogene (38–41). To extend our results, we therefore determined if cdk5 was involved in the regulation of the myc gene upon genotoxic treatment. As expected, ChIP experiments indicated that STAT3 was associated with the proximal promoter of the myc gene in growing HT29 cells (Fig. 5D, compare lanes 3 and 5). As described above for the cyclin D1 gene, the association of the transcription factor with the myc promoter was significantly inhibited following sn38 treatment (Fig. 5D, lanes 5 and 6). The expression of the Myc mRNA was then investigated following sn38 treatment and transfection with a pool of three siRNA directed against cdk5 or with the corresponding controls. As previously shown (23, 28), Myc expression was down-regulated following topoisomerase I inhibition. Interestingly, results showed that this inhibition was reduced in the absence of cdk5 (Fig. 5D, right panel). Although this does not rule out the participation of others regulators, these results suggest that cdk5 also regulates the STAT3-mediated activation of myc following DNA damage.
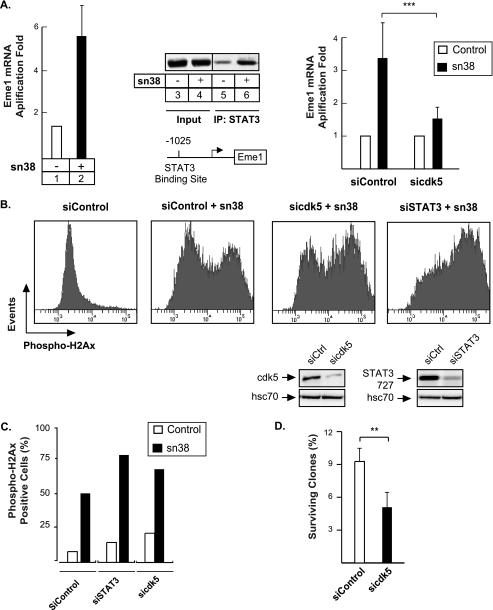
To further extend this observation, we then determined if cdk5 was only involved in the regulation of proliferative genes such as cyclin D1 or myc, or if its effects could also be observed on other genes regulated by STAT3. We have recently shown that STAT3 can bind to the promoter of the Eme1 gene to induce its expression (24). Eme1 is an endonuclease that is implicated in the rescue of broken replication forks in response to topoisomerase I inhibition (42–44). Using quantitative PCR analysis, we confirmed in HT29 cells that Eme1 expression was increased in response to sn38 (Fig. 6A, lanes 1 and 2). In addition, ChIP experiments also indicated that STAT3 effectively bound to the Eme1 promoter following DNA damage (Fig. 6A, lanes 5 and 6). To determine if cdk5 was involved in the activation of the endonuclease, its expression was then investigated in the presence or absence of siRNA directed against the kinase. As expected, Eme1 expression was increased in the presence of control siRNA in response to sn38 (Fig. 6A, right part). However, when cells were transfected with a pool of three siRNAs directed against cdk5, the sn38-mediated expression of the endonuclease was significantly reduced (Fig. 6A, right part). The expression of Eme1 has been correlated with DNA damage, chromosomal aberrations, and genetic stability. Based on these observations, we made the hypothesis that the down-regulation of cdk5 or STAT3 might potentiate the effect of sn38 on DNA damage through a reduced expression of the endonuclease. To this end, cells were transfected with pools of three siRNA directed against STAT3 or cdk5, treated or not with sn38, and DNA damage was investigated by FACS analysis using an antibody directed against the Ser-139-phosphorylated form of histone H2Ax. Results presented Fig. 6B show as expected that topoisomerase I inhibition induced a significant increase in H2Ax phosphorylation (compare the first and second panel). Interestingly, we also observed that DNA damage was enhanced in the absence of cdk5 or STAT3 (compare the second panel with panels 3 and 4). FACS quantification (Fig. 6C) confirmed that the percentage of cells with increased DNA damage is higher in the absence of STAT3 or cdk5.
FIGURE 6.
The cdk5-STAT3 pathway regulates the expression of Eme1 and reduces DNA damage. A, Eme1 mRNA expression was analyzed by quantitative RT-PCR (lanes 1 and 2), and STAT3 association with the Eme1 promoter was characterized by ChIP (lanes 3–6) following sn38 treatment. In parallel, cells were transfected with control or cdk5 siRNA and then treated with sn38 (5 ng/ml) for 48 h. The expression of the Eme1 mRNA was analyzed by RT-QPCR experiments (n = 3 ± S.D., p < 0.001). B and C, growing HT29 cells were transfected with specific or control siRNA and treated or not with sn38 (5 ng/ml). The generation of DNA double strand breaks was quantified by FACS analysis using polyclonal antibodies directed against the ser139 phosphorylated form of histone H2Ax (one experiment representative of three). D, HT29 cells were transfected with pools of siRNAs directed against cdk5 or control siRNAs for 48 h. Cells were then split and treated with sn38 for 10–14 days. The percentage of colony-forming cells was evaluated as compared with non-treated cells (n = 3 ± S.D., p < 0.01).
To further extend this result, we then determined if cdk5 down-regulation enhanced cell death following topoisomerase I inhibition. This would be expected as a consequence of increased DNA damage. To this end, cells were transfected with control siRNA or a pool of siRNAs directed against cdk5 for 2 days, and cells were then split and treated for 10–14 days with sn38. Results from clonogenic assays presented in Fig. 6D showed that cdk5 down-regulation resulted in a significant decrease of cell viability as compared with control cells. Altogether, these results suggest that cdk5 interacts with STAT3 to regulate the expression of Eme1 and that this allows DNA repair in response to topoisomerase I inhibition.
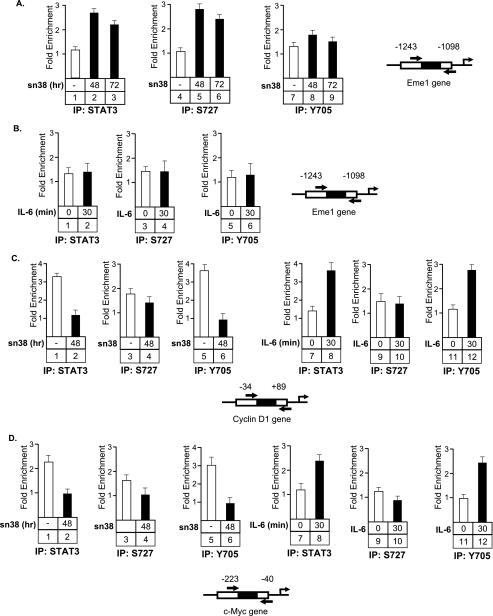
STAT3 Is Associated with the Eme1 Promoter, Phosphorylated on Serine 727, but Not on Tyrosine 705
These results suggest that STAT3 can function as a transcriptional regulator following serine 727 phosphorylation, in the absence of tyrosine 705 phosphorylation. To test this hypothesis, ChIP experiments were performed in HT29 cells using antibodies directed against either the tyrosine (Tyr-705), the serine (Ser-727) phosphorylated forms of the transcription factor or one polyclonal antibody directed against all forms of STAT3 (Fig. 7). Two conditions have been used, growing cells treated or not with sn38 for the indicated times, or cells that have been serum-starved and stimulated with IL-6 for 30 min. DNA binding has been characterized on the Eme1, cyclin D1, and Myc promoters. We observed as expected that STAT3 was recruited to the Eme1 gene following sn38 treatment (Fig. 7A, lanes 1-3). Interestingly, the same recruitment was noticed using the Ser-727 antibody, but the Tyr-705 antibody did not detect any STAT3 binding (Fig. 7, lanes 4–9). This observation further suggests that a serine-phosphorylated form of STAT3 can be found associated with a target gene in the absence of tyrosine phosphorylation. When cells were stimulated with IL-6 (Fig. 7B), STAT3 was not recruited to the Eme1 gene, indicating that this promoter is not a target of the transcription following cytokine stimulation. On the cyclin D1 and Myc promoters (Fig. 7, C and D), results showed that STAT3 was present on both promoters in growing cells and that its binding was inhibited following sn38 treatment. This was observed using either a “total” antibody or an antibody directed against the tyrosine-phosphorylated form of the transcription factor (Fig. 7, C and D, lanes 1–2 and 5–6). In serum-starved cells, STAT3 was not present on these promoters, but the transcription factor was recruited following IL-6 stimulation. As expected, this promoter-associated form was phosphorylated on tyrosine 705 (Fig. 7, C and D, lanes 7–8 and 11–12). This observation corresponds to results that have been published previously by our group and others, showing that STAT3 can activate the myc and cyclin D1 genes following JAK-mediated phosphorylation of tyrosine 705 and the recruitment of transcriptional activators such as CBP, SRC, or P/Tefb. Interestingly, we were not able to detect a significant recruitment of STAT3 phosphorylated on its serine residue on these two promoters. Note, however, that this site may not be accessible when the dimer is bound to DNA, whereas this would be the case following DNA damaged if STAT3 has a different conformation or different partners. Taken together, these results indicate that STAT3 can be found associated with the Eme1 promoter in response to DNA damage when phosphorylated only on its serine 727 residue.
FIGURE 7.
STAT3 is recruited to the Eme1 promoter when phosphorylated only on its serine 727 residue. Growing HT29 cells were treated or not with sn38 as indicated above and soluble chromatin was prepared and immunoprecipitated with antibodies directed against STAT3 (IP:STAT3) or its serine or tyrosine phosphorylated forms (IP:S727 or IP:Y705). In parallel, cells were serum-starved and stimulated or not with IL-6 (10 ng/ml) for 30 min, and the chromatin was immunoprecipitated under the same conditions. DNA was amplified using pair of primers that covers the STAT3 proximal binding sites of the cyclin D1 (panel C), Myc (panel D), and Eme1, (panel A and B) promoters as indicated. ChIP assays were then quantified by real-time PCR as compared with the signals obtained on each genes with a control IgG (n = 3). Note that sn38 (−) in the legend means growing cells, whereas IL6 (0) means serum-starved cells.
DISCUSSION
In this study, we have found that the STAT3 transcription factor is phosphorylated on its serine C-terminal residue but not on tyrosine 705 upon topoisomerase I inhibition. Our results indicate that this is due to the activation of the cdk5 kinase, which binds to the C-terminal of domain of the transcription factor to induce its phosphorylation. Importantly, cdk5 is involved in the down-regulation of early G1 genes such as myc and cyclin D1 and in the STAT3-mediated up-regulation of the Eme1 gene, an endonuclease involved in the processing of damaged replication forks. In light of these results, we propose that the cdk5-STAT3-Eme1 pathway plays an important role in the response to topoisomerase I inhibition and chemotherapy treatments.
It is well known that STAT3 is activated at the G0-G1 transition following cytokine or growth factor stimulation. In this condition, the transcription factor binds to the promoter of several cell cycle genes such as myc, cyclin D1, fos, or cdc25A to induce their expression and activate progression toward S phase. Gene activation by STAT3 during the G0-G1 transition is due to the phosphorylation of STAT3 on its tyrosine residue, followed by nuclear translocation and DNA binding. The second phosphorylation of STAT3 on its serine residue allows the contact of the tyrosine-phosphorylated dimer with transcriptional cofactors such as CBP, NcoA, or Ptefb. However, this pathway is probably not the only mechanism by which STAT proteins are activated, because several results have shown that these transcription factors induce transcription in the absence of tyrosine phosphorylation. This was originally described with STAT1 when it was shown that this transcription factor can drive the expression of several genes in the absence of tyrosine phosphorylation (45). Using non-phosphorylated forms of STAT3 on its tyrosine residue, Yang et al. have shown that these mutants can induce the expression of genes such as met and mras, which certainly play an important role in the oncogenic activity of STAT3. Under these conditions, gene activation is a consequence of the formation of a STAT3-NF-κB enhanceosome that plays a key role in transformed cells (12, 13). Most importantly, the genes regulated by STAT3 in these conditions are normally not activated when the transcription factor is phosphorylated on its tyrosine residue. This leads to the important conclusion that the STAT3 transcriptional targets depends on its post-translational modifications.
Importantly, using ChIP analysis, we have been able to detect STAT3 on the Eme1 promoter when phosphorylated only on its Ser-727 residue. We therefore propose that STAT3 is activated by DNA damage during the G2 phase of the cell cycle and that its serine phosphorylation allows the specific up-regulation of DNA repair genes such as the Eme1 endonuclease. Surprisingly, the role of STAT3 in the response to genotoxic treatment has not been well characterized. By contrast, it is known that both STAT1 and STAT5 are regulated following DNA damage. STAT1 is involved in the S and G2/M checkpoints and can associate with repair signaling proteins such as Chk2 and Mdc1 in response to γ-irradiation (46, 47). In addition, this transcription factor is also phosphorylated in response to topoisomerase inhibitors (47). STAT5 has been shown to regulate the expression of rad51 and, importantly, this has been linked to the ability of several oncogenic kinases such as bcr-abl or tel-jak2 to induce drug resistance (48, 49). Interestingly, recent results also suggest that STAT3 plays an important role in the regulation of genome stability. The inactivation of the T-cell protein tyrosine phosphatase induces a constitutive activation of STAT3 probably as a consequence of replication fork stalling, and this leads to aberrant mitoses with lagging chromosomes (50). Unfortunately, the link between STAT3 and DNA repair has not been characterized in this study, because this effect has been linked to a sustained expression of cyclin D1 during S phase. Further suggesting a link between STAT3 and DNA stability, it is well known that a direct target of STAT3, myc, can induce DNA damage and dysregulate genomic stability and DNA repair pathways (51). In this study, we further extend these observations, showing that this transcription factor is activated by Cdk5 in response to topoisomerase I inhibitors. We speculate that this kinase allows the formation of a new STAT3 enhanceosome that would specifically regulate the expression of DNA repair genes upon genotoxic treatment. In light of recent results showing an essential role of NF-κB in the response to DNA damage (52), one interesting hypothesis is that genes involved in the response to sn38 are controlled by a specific STAT3-NF-κB complex that would be activated by cdk5. It will be interesting to determine if this enhanceosome preferentially binds DNA repair genes as opposed to more conventional STAT3 targets such as myc or cdc25A.
As a consequence of DNA repair genes regulation, our results indicate that the cdk5-STAT3 pathway reduces DNA damage in response to topoisomerase I inhibition. This suggests that these proteins might play an essential role in the resistance of cancer cells to chemotherapy. Further confirming the importance of this oncogenic cascade, recent results have shown that the cdk5-STAT3 pathway plays an essential role in thyroid carcinomas (53). In addition, we and others have recently shown that STAT3 prevents the induction of senescence through p53-p21 inactivation (18, 40, 54, 55). Interestingly, cdk5 is also involved in senescence programs, because this kinase regulates cell morphology through ezrin and rac1 modulation (32, 33). It will be interesting to determine if cdk5 is also involved in the inactivation of the p53-p21 pathway by the STAT3 oncogene during senescence induction.
In light of this study and other results (53), we therefore propose that cdk5 plays an important role in cell transformation by the STAT3 oncogene. Because it has been proposed that cell transformation induces an intrinsic resistance program to chemotherapy (56), we speculate that cdk5-STAT3 provides cancer cells with intrinsic resistance capacities due to enhanced Eme1 expression and that this a corollary of cell transformation. We propose that the early detection on tumor biopsies of the cdk5-STAT3 oncogenic pathway, both of its phosphorylation status and of its target genes, will provide oncologists with a resistance profile indicative of tumors that will fail to respond to chemotherapy (15, 57). In addition, we also propose that STAT3 inhibitors, which are emerging as new targeted cancer therapies (2, 57, 58) should be tested in clinical trials in combination with irinotecan to reduce DNA repair and enhance the efficiency of genotoxic treatments.
This work was supported by fellowships (to S. C. and A. V.), by a grant (Equipe Labelisée) from the Ligue Contre le Cancer and Institut du Cancer, and a fellowship from Inserm-Pays de Loire (to S. C. T.), and by the Ministère de la Recherche (to H. S.).
- STAT3
- signal transducers and activators of transcription 3
- ChIP
- chromatin immunoprecipitation
- CBP
- CREB-binding protein
- cdk
- cyclin-dependent kinase
- siRNA
- small interference RNA
- PBS
- phosphate-buffered saline
- RT
- reverse transcription
- IL-6
- interleukin-6
- FACS
- fluorescent-activated cell sorting.
REFERENCES
- 1.Bromberg J. (2002) J. Clin. Invest. 109, 1139–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu H., Jove R. (2004) Nat. Rev. Cancer 4, 97–105 [DOI] [PubMed] [Google Scholar]
- 3.Levy D. E., Lee C. K. (2002) J. Clin. Invest. 109, 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bromberg J. F., Horvath C. M., Besser D., Lathem W. W., Darnell J. E., Jr. (1998) Mol. Cell. Biol. 18, 2553–2558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. (1999) Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 6.Wen Z., Zhong Z., Darnell J. E., Jr. (1995) Cell 82, 241–250 [DOI] [PubMed] [Google Scholar]
- 7.Giraud S., Bienvenu F., Avril S., Gascan H., Heery D. M., Coqueret O. (2002) J. Biol. Chem. 277, 8004–8011 [DOI] [PubMed] [Google Scholar]
- 8.Giraud S., Hurlstone A., Avril S., Coqueret O. (2004) Oncogene 23, 7391–7398 [DOI] [PubMed] [Google Scholar]
- 9.Paulson M., Pisharody S., Pan L., Guadagno S., Mui A. L., Levy D. E. (1999) J. Biol. Chem. 274, 25343–25349 [DOI] [PubMed] [Google Scholar]
- 10.Nakashima K., Yanagisawa M., Arakawa H., Kimura N., Hisatsune T., Kawabata M., Miyazono K., Taga T. (1999) Science 284, 479–482 [DOI] [PubMed] [Google Scholar]
- 11.Qin H. R., Kim H. J., Kim J. Y., Hurt E. M., Klarmann G. J., Kawasaki B. T., Duhagon Serrat M. A., Farrar W. L. (2008) Cancer Res. 68, 7736–7741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Chatterjee-Kishore M., Staugaitis S. M., Nguyen H., Schlessinger K., Levy D. E., Stark G. R. (2005) Cancer Res. 65, 939–947 [PubMed] [Google Scholar]
- 13.Yang J., Liao X., Agarwal M. K., Barnes L., Auron P. E., Stark G. R. (2007) Genes Dev. 21, 1396–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee H., Herrmann A., Deng J. H., Kujawski M., Niu G., Li Z., Forman S., Jove R., Pardoll D. M., Yu H. (2009) Cancer Cell 15, 283–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barré B., Vigneron A., Perkins N., Roninson I. B., Gamelin E., Coqueret O. (2007) Trends Mol. Med. 13, 4–11 [DOI] [PubMed] [Google Scholar]
- 16.Sano S., Chan K. S., Kira M., Kataoka K., Takagi S., Tarutani M., Itami S., Kiguchi K., Yokoi M., Sugasawa K., Mori T., Hanaoka F., Takeda J., DiGiovanni J. (2005) Cancer Res. 65, 5720–5729 [DOI] [PubMed] [Google Scholar]
- 17.Shen Y., Devgan G., Darnell J. E., Jr., Bromberg J. F. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1543–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu G., Wright K. L., Ma Y., Wright G. M., Huang M., Irby R., Briggs J., Karras J., Cress W. D., Pardoll D., Jove R., Chen J., Yu H. (2005) Mol. Cell. Biol. 25, 7432–7440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catlett-Falcone R., Landowski T. H., Oshiro M. M., Turkson J., Levitzki A., Savino R., Ciliberto G., Moscinski L., Fernández-Luna J. L., Nuñez G., Dalton W. S., Jove R. (1999) Immunity 10, 105–115 [DOI] [PubMed] [Google Scholar]
- 20.Duan Z., Foster R., Bell D. A., Mahoney J., Wolak K., Vaidya A., Hampel C., Lee H., Seiden M. V. (2006) Clin. Cancer Res. 12, 5055–5063 [DOI] [PubMed] [Google Scholar]
- 21.Diaz N., Minton S., Cox C., Bowman T., Gritsko T., Garcia R., Eweis I., Wloch M., Livingston S., Seijo E., Cantor A., Lee J. H., Beam C. A., Sullivan D., Jove R., Muro-Cacho C. A. (2006) Clin. Cancer Res. 12, 20–28 [DOI] [PubMed] [Google Scholar]
- 22.Gritsko T., Williams A., Turkson J., Kaneko S., Bowman T., Huang M., Nam S., Eweis I., Diaz N., Sullivan D., Yoder S., Enkemann S., Eschrich S., Lee J. H., Beam C. A., Cheng J., Minton S., Muro-Cacho C. A., Jove R. (2006) Clin. Cancer Res. 12, 11–19 [DOI] [PubMed] [Google Scholar]
- 23.Vigneron A., Roninson I. B., Gamelin E., Coqueret O. (2005) Cancer Res. 65, 8927–8935 [DOI] [PubMed] [Google Scholar]
- 24.Vigneron A., Gamelin E., Coqueret O. (2008) Cancer Res. 68, 815–825 [DOI] [PubMed] [Google Scholar]
- 25.Dhavan R., Tsai L. H. (2001) Nat. Rev. Mol. Cell Biol. 2, 749–759 [DOI] [PubMed] [Google Scholar]
- 26.Gong X., Tang X., Wiedmann M., Wang X., Peng J., Zheng D., Blair L. A., Marshall J., Mao Z. (2003) Neuron 38, 33–46 [DOI] [PubMed] [Google Scholar]
- 27.Wang C. X., Song J. H., Song D. K., Yong V. W., Shuaib A., Hao C. (2006) Cell Death Differ. 13, 1203–1212 [DOI] [PubMed] [Google Scholar]
- 28.Vigneron A., Cherier J., Barré B., Gamelin E., Coqueret O. (2006) J. Biol. Chem. 281, 34742–34750 [DOI] [PubMed] [Google Scholar]
- 29.Le H. V., Minn A. J., Massagué J. (2005) J. Biol. Chem. 280, 32018–32025 [DOI] [PubMed] [Google Scholar]
- 30.Turner N. C., Lord C. J., Iorns E., Brough R., Swift S., Elliott R., Rayter S., Tutt A. N., Ashworth A. (2008) EMBO J. 27, 1368–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian B., Yang Q., Mao Z. (2009) Nat. Cell Biol. 11, 211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander K., Yang H. S., Hinds P. W. (2004) Mol. Cell. Biol. 24, 2808–2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H. S., Hinds P. W. (2003) Mol. Cell 11, 1163–1176 [DOI] [PubMed] [Google Scholar]
- 34.Fu A. K., Fu W. Y., Ng A. K., Chien W. W., Ng Y. P., Wang J. H., Ip N. Y. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6728–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J. H., Jeong M. W., Kim W., Choi Y. H., Kim K. T. (2008) J. Biol. Chem. 283, 19826–19835 [DOI] [PubMed] [Google Scholar]
- 36.Leslie K., Lang C., Devgan G., Azare J., Berishaj M., Gerald W., Kim Y. B., Paz K., Darnell J. E., Albanese C., Sakamaki T., Pestell R., Bromberg J. (2006) Cancer Res. 66, 2544–2552 [DOI] [PubMed] [Google Scholar]
- 37.Lo H. W., Hsu S. C., Ali-Seyed M., Gunduz M., Xia W., Wei Y., Bartholomeusz G., Shih J. Y., Hung M. C. (2005) Cancer Cell 7, 575–589 [DOI] [PubMed] [Google Scholar]
- 38.Kiuchi N., Nakajima K., Ichiba M., Fukada T., Narimatsu M., Mizuno K., Hibi M., Hirano T. (1999) J. Exp. Med. 189, 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowman T., Broome M. A., Sinibaldi D., Wharton W., Pledger W. J., Sedivy J. M., Irby R., Yeatman T., Courtneidge S. A., Jove R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 7319–7324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barré B., Avril S., Coqueret O. (2003) J. Biol. Chem. 278, 2990–2996 [DOI] [PubMed] [Google Scholar]
- 41.Barré B., Vigneron A., Coqueret O. (2005) J. Biol. Chem. 280, 15673–15681 [DOI] [PubMed] [Google Scholar]
- 42.Dendouga N., Gao H., Moechars D., Janicot M., Vialard J., McGowan C. H. (2005) Mol. Cell. Biol. 25, 7569–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osman F., Whitby M. C. (2007) DNA Repair 6, 1004–1017 [DOI] [PubMed] [Google Scholar]
- 44.Pommier Y., Redon C., Rao V. A., Seiler J. A., Sordet O., Takemura H., Antony S., Meng L., Liao Z., Kohlhagen G., Zhang H., Kohn K. W. (2003) Mutat. Res. 532, 173–203 [DOI] [PubMed] [Google Scholar]
- 45.Chatterjee-Kishore M., Wright K. L., Ting J. P., Stark G. R. (2000) EMBO J. 19, 4111–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Townsend P. A., Cragg M. S., Davidson S. M., McCormick J., Barry S., Lawrence K. M., Knight R. A., Hubank M., Chen P. L., Latchman D. S., Stephanou A. (2005) J. Cell Sci. 118, 1629–1639 [DOI] [PubMed] [Google Scholar]
- 47.Thomas M., Finnegan C. E., Rogers K. M., Purcell J. W., Trimble A., Johnston P. G., Boland M. P. (2004) Cancer Res. 64, 8357–8364 [DOI] [PubMed] [Google Scholar]
- 48.Slupianek A., Schmutte C., Tombline G., Nieborowska-Skorska M., Hoser G., Nowicki M. O., Pierce A. J., Fishel R., Skorski T. (2001) Mol. Cell 8, 795–806 [DOI] [PubMed] [Google Scholar]
- 49.Slupianek A., Hoser G., Majsterek I., Bronisz A., Malecki M., Blasiak J., Fishel R., Skorski T. (2002) Mol. Cell. Biol. 22, 4189–4201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shields B. J., Hauser C., Bukczynska P. E., Court N. W., Tiganis T. (2008) Cancer Cell 14, 166–179 [DOI] [PubMed] [Google Scholar]
- 51.Vafa O., Wade M., Kern S., Beeche M., Pandita T. K., Hampton G. M., Wahl G. M. (2002) Mol. Cell 9, 1031–1044 [DOI] [PubMed] [Google Scholar]
- 52.Campbell K. J., Witty J. M., Rocha S., Perkins N. D. (2006) Cancer Res. 66, 929–935 [DOI] [PubMed] [Google Scholar]
- 53.Lin H., Chen M. C., Chiu C. Y., Song Y. M., Lin S. Y. (2007) J. Biol. Chem. 282, 2776–2784 [DOI] [PubMed] [Google Scholar]
- 54.Flørenes V. A., Lu C., Bhattacharya N., Rak J., Sheehan C., Slingerland J. M., Kerbel R. S. (1999) Oncogene 18, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 55.Bienvenu F., Barre B., Giraud S., Avril S., Coqueret O. (2005) Mol. Biol. Cell 16, 1850–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnstone R. W., Ruefli A. A., Lowe S. W. (2002) Cell 108, 153–164 [DOI] [PubMed] [Google Scholar]
- 57.Henderson B. W., Daroqui C., Tracy E., Vaughan L. A., Loewen G. M., Cooper M. T., Baumann H. (2007) Clin. Cancer Res. 13, 3156–3163 [DOI] [PubMed] [Google Scholar]
- 58.Benekli M., Baumann H., Wetzler M. (2009) J. Clin. Oncol. 27, 4422–4432 [DOI] [PMC free article] [PubMed] [Google Scholar]