Abstract
Growth factors modify the structure of the glycosaminoglycan (GAG) chains on biglycan leading to enhanced LDL binding. G-protein receptor-coupled agonists such as thrombin, signal changes the structure of proteoglycans produced by vascular smooth muscle cells (VSMCs). One component of classical G-protein-coupled receptor (GPCR) signaling invokes transactivation of protein tyrosine kinase receptors such as the epidermal growth factor receptor. Serine/threonine receptor growth factors such as transforming growth factor-(TGF)-β are potent activators of proteoglycan synthesis. We have used the model of proteoglycan synthesis to demonstrate that the signaling paradigm of GPCR signaling can be extended to include the transactivation of serine/threonine receptor, specifically the TGF-β type I receptor (TβRI) also known as activin-like kinase (ALK) V. Thrombin stimulated elongation of GAG chains and increased proteoglycan core protein expression and these responses were blocked by the TβRI antagonist, SB431542 and TβRI siRNA knockdown, as well as several protease-activated receptor (PAR)-1 antagonists. The canonical downstream response to TGF-β is increased C-terminal phosphorylation of the transcription factor Smad2 generating phospho-Smad2C (phosphorylation of Smad2 C-terminal region). Thrombin stimulated increased phospho-Smad2C levels, and the response was blocked by SB431542 and JNJ5177094. The proteolytically inactive thrombin mimetic thrombin-receptor activating peptide also stimulated an increase in cytosolic phospho-Smad2C. Signaling pathways for growth factor regulated proteoglycan synthesis represent therapeutic targets for the prevention of atherosclerosis, but the novel finding of a GPCR-mediated transactivation of a serine/threonine growth factor receptor almost certainly has implications well beyond the synthesis of proteoglycans.
Keywords: Atherosclerosis, G-protein-coupled Receptors (GPCR), Glycosaminoglycan, Thrombin, Transforming Growth Factor Beta (TGFbeta)
Introduction
Cardiovascular disease is the largest single cause of mortality, and its major underlying pathology is atherosclerosis (1). The process of atherosclerosis commences with the trapping of lipoproteins in the vessel wall by modified proteoglycans, specifically the glycosaminoglycan (GAG)3 chain elongated and sulfated chondroitin and dermatan sulfate biglycan and decorin (2, 3), and it continues as an inflammatory disease (4). Proteoglycan synthesis and structure is regulated by vasoactive growth factors, and consequently, their receptors and signaling pathways are potential therapeutic targets (5, 6).
G-protein-coupled receptors (GPCRs) are seven transmembrane receptors and are present on vascular smooth muscle cells (VSMCs) where they signal important actions such as vascular contraction, cellular migration and proliferation, and secretion (7, 8). GPCR agonists include key vasoactive molecules associated with physiology and pathophysiology, including thrombin, endothelin-1, and angiotensin II (9). The current paradigm of GPCR signaling covers three major pathways; first, the classic pathway in which ligand engagement causes G-protein binding to the receptor and Gα complexes then regulate the activity of downstream effector molecules (10); second, β-arrestin signaling via ligand-regulated scaffolds (11); and third, as first described by Ullrich in 1996 (12), GPCR agonists through their receptors can transactivate a receptor protein tyrosine kinase (PTK), such as the EGF receptor, platelet-derived growth factor (PDGF) receptor and fibroblast growth factor (FGF) receptor, leading to both Ras-dependent MAPK activation and stimulation of PI3K (13). Thrombin is a serine protease, which causes cellular effects including calcium signaling, proliferation (14, 15), cytoskeletal rearrangement, contraction (16), and regulation of extracellular matrix synthesis (17), including proteoglycans (18). Thrombin has the unique mechanism of receptor activation in that it cleaves the extracellular domain of its GPCR to liberate a tethered ligand that activates the receptor (19).
We have reported recently that thrombin stimulates proteoglycan synthesis by VSMCs (18), and stimulation is associated with an increase in the length of biglycan GAG chains (18). The effects of thrombin on proteoglycan synthesis are completely blocked by the PAR-1 antagonist, JNJ5177094 and are not observed for a catalytically inactive thrombin mimetic, indicating that signaling occurs via the PAR-1 (18, 19). The actions of thrombin on proteoglycan synthesis were also partially (∼40%) mediated via transactivation of the tyrosine kinase EGF receptor (18). We have previously reported on the potent activity of TGF-β to cause GAG elongation in VSMCs via its serine/threonine kinase receptor, TGF-β type I receptor/activin-like kinase V (TβRI/ALK V) and its downstream canonical carboxyl-terminal Smad phosphorylation pathway (20, 21). Being aware of the role of GPCR in transactivation of receptor tyrosine kinases (12) and its involvement in thrombin actions on proteoglycan synthesis in VSMCs (18) and notwithstanding that the current transactivation signaling paradigm is limited to tyrosine kinase receptors (22), we considered and evaluated whether or not the effect of thrombin mediated via PAR-1, might involve transactivation of TβRI/ALK V.
EXPERIMENTAL PROCEDURES
Materials
The following chemicals were purchased from Sigma: thrombin, benzamidine hydrochloride, DEAE-Sephacel, and chondroitin sulfate. Thrombin-receptor activating peptide (TRAP; SFLLRN-NH2) was from Anaspec, Inc.. SB431542, Dulbecco's modified eagle medium (DMEM), and glutamine were from Invitrogen; fetal bovine serum (FBS) and penicillin streptomycin fungzione were obtained from CSL (Parkville, Australia); human recombinant TGF-β was obtained from R&D Systems; carrier-free [35S]SO4 and [35S]-labeled methionine/cysteine were obtained from MP Biomedicals. Rainbow [14C]-methylated protein molecular weight standard was from Amersham Biosciences Pharmacia (Buckinghamshire, England); cetyl pyridinium chloride was from Unilab Chemicals and Pharmaceuticals (India); Whatman 3MM chromatography paper was from Biolab (Mulgrave, Australia); Instagel plus scintillation fluid was from PerkinElmer Life Sciences; poly-Prep columns were from Bio-Rad. The PAR-1 antagonist JNJ5177094 was kindly supplied by Dr. P. Andrade of Johnson and Johnson Pharmaceuticals, Spring House, PA. The PAR-1 inhibitor SCH79797 was from Tocris Biosciences. TβRI/ALK V siRNA (SMARTpool) was purchased from Dharmacon (Lafayette, CO).
Culture of Human VSMCs
Human VSMCs were obtained by the explant method (23) from otherwise discarded sections of saphenous veins from patients undergoing coronary artery bypass grafting, at the Alfred Hospital, with approval by the Alfred Ethics committee. VSMC were grown in DMEM with 5 mm glucose, 10% FBS and 1% penicillin-streptomycin-fungzione solution. For experimentation, VSMC were seeded into 24-well plates, grown until confluency, and then rendered quiescent by serum starvation for 48 h.
Quantitation of Proteoglycan Synthesis
Quiescent cells were changed to fresh medium containing 50 μCi/ml of [35S]sulfate in the presence or absence of thrombin for 24 h. Medium from the cell cultures was harvested with added protease inhibitors (5 mm benzamidine in 0.1 m 6-aminocaproic acid). Incorporation of the radiolabel into proteoglycans was measured by cetyl pyridinium chloride precipitation assay, as described previously (24).
SDS-PAGE Analysis of Proteoglycan Size
Proteoglycans labeled with [35S]sulfate were prepared for SDS-PAGE by isolation through DEAE-Sephacel anionic exchange mini columns. Samples were added to pre-equilibrated columns and then washed extensively with low salt buffer (8 m urea, 0.25 m NaCl, 2 mm disodium EDTA, 0.5% Triton X-100). Proteoglycans were eluted with high salt buffer (8 m urea, 3 m NaCl, 2 mm disodium EDTA, 0.5% Triton X-100) and fractions containing the highest number of 35S cpm were pooled. Aliquots (25,000 cpm) were precipitated (1.3% potassium acetate, 95% ethanol) and chondroitin sulfate was added as a “cold carrier.” Samples were resuspended in buffer (8 m urea, 2 mm disodium EDTA, pH 7.5), to which an equal volume of sample buffer was added. Radiolabeled proteoglycans were separated on 4–13% acrylamide gels with a 3% stacking gel at 50 V overnight. A radiolabeled ([14C]) protein molecular weight marker was run simultaneously. Processed and dried gels were exposed to a phosphorimaging screen (Fuji Photo Film Co) for ∼3 days and then scanned on a Bio-imaging analyser BAS-1000 MacBas (Fuji Photo Film Co, Japan).
Western Blotting
Total cell lysates were resolved on 10% SDS-PAGE and transferred onto PVDF. Membranes were blocked with 5% skim milk powder, incubated with anti-phosphorylated Smad2 (phospho-Smad2) rabbit monoclonal antibody, and followed by HRP anti-rabbit IgG and ECL detection.
Measurement of IP3 Production
VSMCs were grown to ∼80% confluency in DMEM with 5 mm glucose, 10% FBS, and 1% penicillin-streptomycin-fungzione solution. Cells were then washed and incubated in inositol phosphate-free DMEM (DMEM/−IP) containing 6.25μCi of myo-[3H]inositol for 4–6 h, followed by an additional volume of DMEM/−IP, and cells were incubated for a further 18–20 h. Cells were then washed with DMEM/−IP, and DLB solution (DMEM/−IP, 10 mm LiCl, 0.1% BSA) was added, followed by incubation at 37% for 10 min without antagonist or 30 min with antagonist and then stimulated with agonists. Media was removed and cold TEP (5% TCA, 2.5 mm EDTA, 5 mm phytic acid) was added to terminate inositol triphosphate production. Lysates were then collected, and a 1:1 solution of tri-n-octylamine:1,1,2-tricholorethane was added. Samples were vortexed, and a volume of top phase was collected and added to 10 ml distilled H2O. Inositol 1,4,5-trisphosphate (IP3) was isolated through Dowex-1-chloride mini columns. After loading, samples were washed with distilled H2O and then 60 mm ammonium formate. IP3 were eluted with buffer (0.1 m formic acid, 1 m ammonium formate). Samples were then analyzed on a liquid scintillation counter for 3H.
siRNA Knockdown of TβRI/ALKV
VSMCs were maintained in DsMEM (5 mm glucose, 10% FBS, and 1% penicillin-streptomycin-fungzione) and grown to 90% confluence before transfection with siRNA using DharmaFECTTM according to the manufacturer's instructions. Culture was continued for 48 h and then treated as required.
RESULTS
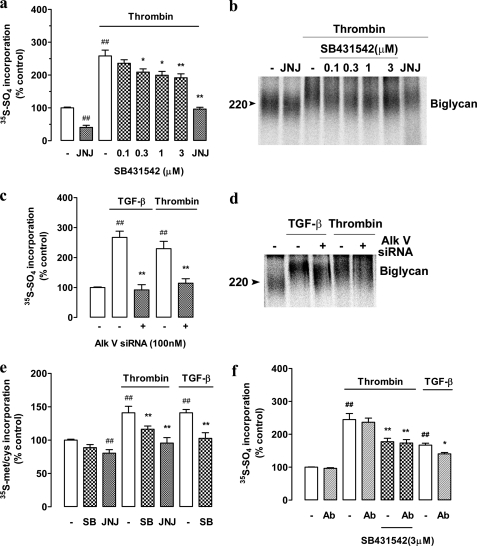
The small molecule inhibitor, SB431542, is a well characterized antagonist of serine/threonine kinase activity of the TβRI (25, 26). SB431542 inhibits the action of TGF-β on VSMC proteoglycan synthesis (21), and we used it as a tool to investigate the role of TβRI in mediating the effects of thrombin on proteoglycan synthesis in human VSMCs. Radiosulfate incorporation into secreted proteoglycans is used as a measure of proteoglycan synthesis, and it represents the sum of increased proteoglycan core protein synthesis (and hence more GAG initiation sites), elongation of the GAG chains and increased sulfation of the GAG chains (5). Thrombin treatment of confluent serum-deprived VSMCs caused a 2.5-fold increase in [35S]sulfate incorporation, and this was blocked (partially) in a concentration-dependent manner by SB431542 (0.1–3 μm) (Fig. 1a). Inhibition was apparent at very low concentrations (0.1 μm) of SB431542 consistent with the specificity of action being limited to the proposed target. SB431542 blocked almost 50% of the response to thrombin with an IC50 of ∼250 nm. siRNA knockdown of the TβRI/ALKV (100 nm) also totally inhibited TGF-β, but importantly, thrombin mediated radiosulfate incorporation (Fig. 1c). We have demonstrated previously that the size of the GAG chains from thrombin-treated cells is increased when analyzed by SDS-PAGE and confirmed by the gold standard technique of size exclusion chromatography of the free GAG chains chemically released from the core proteins (18). Furthermore, the action of thrombin extends to the synthesis of short GAG chains on exogenous xyloside and is therefore occurring at the level of the synthesis of the GAG chains in the Golgi apparatus (18). We analyzed the size of the proteoglycans secreted by the cells as shown in Fig. 1a by SDS-PAGE (Fig. 1b). Thrombin treatment increased the size of the major secreted proteoglycan, the dermatan sulfate proteoglycan, biglycan, and this was inhibited in a concentration-dependent manner by SB431542 (Fig. 1b). The increase in size of biglycan by thrombin also was inhibited partially by siRNA knockdown of the TβRI/ALKV (Fig. 4d). The size of the core protein does not change, and we have previously demonstrated for multiple agonists that the change in size of the proteoglycan is due to a change in size of the GAG chains (18, 20).
FIGURE 1.
Thrombin-mediated proteoglycan synthesis is inhibited by TGF-β receptor ALK V inhibition, which is not due to the release and autocrine/paracrine action of TGF-β. VSMCs were treated with SB431542 (SB; 0.1–3 μm) in the presence of thrombin (10 units/ml) and [35S]SO4 (50 μCi/ml) for 24 h. JNJ5177094 (JNJ; 30 μm) was used as a positive control. a, harvested medium containing secreted proteoglycans was spotted onto Whatman paper and run through cetyl pyridinium chloride precipitation, as outlined under “Experimental Procedures,” to assess radiolabel incorporation. b, secreted proteoglycans were isolated using DEAE-loaded ion exchange chromatography followed by concentration using ethanol/potassium acetate precipitation. Electrophoretic mobility relating to the overall size of complete proteoglycans was assessed by SDS-PAGE over a 4–13% acrylamide gradient gel. The gel reveals biglycan as the proteoglycan of interest and is representative of three identical experiments. c and d, VSMCs were transfected with ALK V siRNA for 48 h followed by treatment with TGF-β (2 ng/ml) or thrombin (10 units/ml) for 24 h. Radiolabel incorporation and electrophoretic mobility were assessed as described above. e, VSMCs were treated with SB431542 (3 μm) or JNJ5177094 (30 μm) in the presence of thrombin (10 units/ml) and [35S]-Met/Cys (50 μCi/ml) for 24 h to asses proteoglycan core protein synthesis. TGF-β alone and with SB431542 (3 μm) was used as a positive control. Radiolabeled incorporation was assessed as described above. f, VSMCs were treated with a pan-TGF-β neutralizing antibody (Ab) with or without SB431542 (3 μm) in the presence of thrombin (10 units/ml) or TGF-β (2 ng/ml) and [35S]SO4 (50 μCi/ml) for 24 h. Radiolabeled incorporation was assessed as described above. Results are the mean ± S.E. of data normalized to control from three separate experiments in triplicate. **, p < 0.01 and *, p < 0.05 versus thrombin or TGF-β alone and ##, p < 0.01 versus control, using a one-way ANOVA.
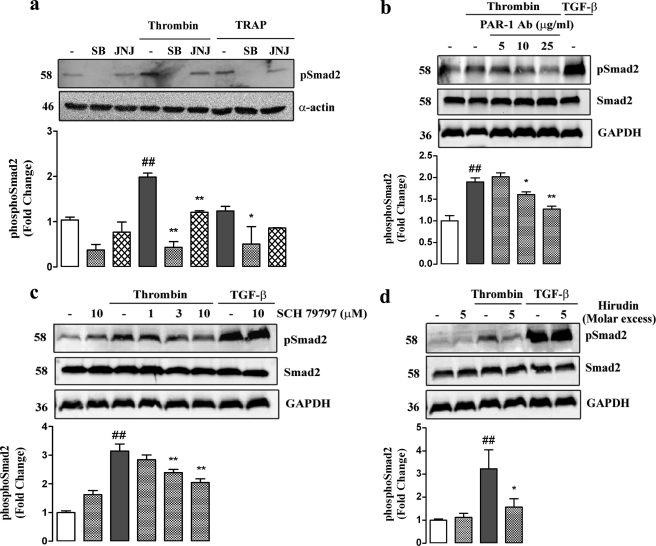
FIGURE 4.
Blockade of PAR-1 and ALK V inhibits thrombin stimulated phosphorylation of Smad2. a, VSMCs were treated with SB431542 (SB; 3 μm) or JNJ5177094 (JNJ; 30 μm for thrombin and 10 μm for TRAP) in the presence of thrombin (10 units/ml) or TRAP (500 μm) and cellular lysates collected at 4 h. Lysate proteins (50 ng/ml) were resolved over 10% acrylamide SDS-PAGE and transferred to a PVDF membrane. The membrane was probed with anti-Smad2 (Ser-465/467) monoclonal antibody (1:1000) followed by peroxidise-labeled anti-rabbit IgG secondary antibody (Ab). Reprobing with anti-smooth muscle α actin (1:1000) followed by peroxidase-labeled anti-mouse IgG secondary antibody indicated equal loading of proteins. The gel is a representation of three separate experiments. b, VSMCs were preincubated for 30 min with monoclonal anti-PAR-1 antibody (5–25 μg/ml) before addition of thrombin (10 units/ml). TGF-β stimulation for 4 h was used as a positive control. Cellular lysates were collected at 4 h and separated (50 ng/ml) by SDS-PAGE on a 10% acrylamide gel. Proteins were transferred and probed as described in Fig. 3a. The gel is a representation of three separate experiments. c, VSMCs were treated with SCH79797 (SCH; 1–10 μm) in the presence of thrombin (10 units/ml). SCH79797 (10 μm) in the presence of TGF-β (2 ng/ml) was used as a positive control. Cellular lysates were collected at 4 h and separated (50 ng/ml) by SDS-PAGE on a 10% acrylamide gel. Proteins were transferred and probed as described in Fig. 3a. The gel is a representation of three separate experiments. d, VSMCs were preincubated for 15 min with 5× molar excess hirudin before addition of thrombin (10 units/ml). TGF-β alone and in the presence of hirudin (5× molar excess) was used as a positive control. Cellular lysates were collected at 4 h and separated (50 ng/ml) by SDS-PAGE on a 10% acrylamide gel. Proteins were transferred and probed as described in Fig. 3a. The gel is a representation of three separate experiments. Histograms represent band density expressed as fold over basal from at least three separate experiments. ##, p < 0.01 versus untreated control, *, p < 0.05 versus thrombin or TRAP alone, and **, p < 0.01 versus thrombin or TRAP alone using a one-way ANOVA. b, c, and d do not show quantitation of TGF-β bands as they appear off the scale.
Increases in radiosulfate incorporation (Fig. 1a) arise from increased expression of proteoglycan core proteins and elongation of GAG chains (5). The synthesis of proteoglycan core proteins can be quantitated by providing the cells with a radiolabeled amino acid (35S-Met/Cys) and determining secreted levels of radioactivity by the cetyl pyridinium chloride precipitation method, which is specific for the identification of proteoglycans (27). Treatment of VSMCs with thrombin and TGF-β each resulted in an ∼30% increase in secreted proteoglycan core proteins over 24 h (Fig. 1e). The stimulation by thrombin was completely blocked by the PAR-1 antagonist JNJ5177094 and partially (∼50%) blocked by the TβRI/ALK V antagonist SB431542. The response to TGF-β was completely blocked by SB431542 (Fig. 1e). These data indicate that the stimulation of proteoglycan core protein synthesis and secretion by thrombin involves PAR-1 and TβRI/ALK V.
We reported previously (21) that the effect of PDGF on radiosulfate incorporation into secreted proteoglycans and GAG elongation in VSMCs was blocked by SB431542 (21). However, in that case, we utilized a pan-TGF-β antibody to immunoneutralize any released TGF-β and demonstrated that the effect of PDGF was due to the release and subsequent auto/paracrine action of TGF-β (21). Thrombin can release TGF-β from the pericellular matrix (28). To determine whether a similar mechanism applied to the action of thrombin on biglycan synthesis and secretion by human VSMCs, we utilized the pan-TGF-β antibody immunoneutralization strategy in VSMCs treated with thrombin (Fig. 1f). The pan TGF-β-neutralizing antibody greatly attenuated the effect of exogenously added TGF-β, but it did not inhibit the action of thrombin to stimulate radiosulfate incorporation into these cells (Fig. 1f). Furthermore, when TGF-β actions were inhibited by SB431542, there was no further inhibition apparent in the presence of high concentrations of the anti TGF-β antibody (Fig. 1f). We further examined the effect of thrombin on the size of biglycan molecules as assessed by SDS-PAGE, and again, the effect of thrombin was not attenuated in the presence of an immunoblocking concentration of a pan-TGF-β antibody (data not shown). Thus, either VSMCs are not releasing TGF-β or if they are synthesizing and releasing TGF-β or TGF-β is being released from storage sites in the matrix, then the amount that is released is insufficient to have an auto-paracrine action on the cells to activate TβRI/ALK V receptors and affect proteoglycan synthesis. It is noteworthy that although TGF-β is released from the pericellular matrix the majority is in the inactive latent, rather than biologically active patent form (28).
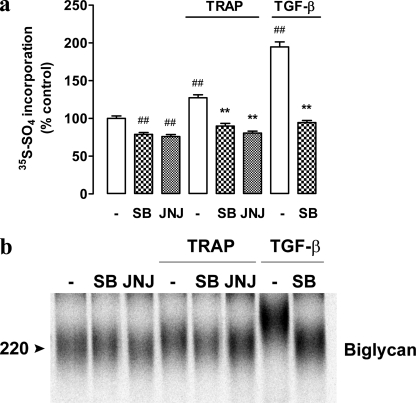
Thrombin is a trypsin-like serine protease, which extremely rapidly activates PAR-1 by binding to and cleaving the amino-terminal exodomain of the PAR-1 at the Arg-41/Ser-42 peptide bond to unmask a new receptor amino terminus and unveils a tethered peptide ligand, containing the hexapeptide recognition sequence, SFLLRN, which activates the receptor (19). Peptide mimetics have been developed, which can activate the PAR-1 receptor but do not possess protease activity (29), giving the opportunity to study the effect of PAR-1 activation in the absence of the potentially confounding protease activity of thrombin. TRAP, the hexapeptide amine SFLLRN, treatment of VSMCs for 24 h lead to increased [35S]sulfate into secreted proteoglycans (Fig. 2a). The effect of TRAP was blocked by the PAR-1 antagonist JNJ5177094, and it was also blocked by SB431542 (Fig. 2a). Further analysis demonstrated that treatment of cells with TRAP leads to the synthesis of biglycan molecules that are of increased molecular size (Fig. 2b, lane 4 versus 1). Thus, the [35S]sulfate incorporation (Fig. 2a) and GAG elongation effect (Fig. 2b) of TRAP is blocked by SB431542. SB431542 and JNJ5177094 had a small effect on basal sulfate incorporation (Fig. 2a) but a minimal effect on biglycan size (Fig. 2b). These data strongly suggest that the effect of thrombin on proteoglycan synthesis, secretion, and GAG hyperelongation was mediated via PAR-1 and was not due to the proteolytic activity of thrombin releasing or activating TGF-β from the extracellular matrix.
FIGURE 2.
A thrombin-mimetic, TRAP-mediated proteoglycan synthesis is blocked by inhibition of ALK V. VSMCs were treated with SB431542 (3 μm) or JNJ5177094 (10 μm) in the presence of TRAP (500 μm) and [35S]SO4 (50 μCi/ml) for 24 h. a, radiolabeled incorporation was assessed as described in Fig. 1A. Results are the mean ± S.E. of data normalized to control from three separate experiments in triplicate. **, p < 0.01 versus TRAP alone and ##, p < 0.01 versus control using a one-way ANOVA. b, complete proteoglycans were isolated and separated over SDS-PAGE (4–13% acrylamide gradient) as described in Fig. 1b. The gel is a representative of three independent experiments.
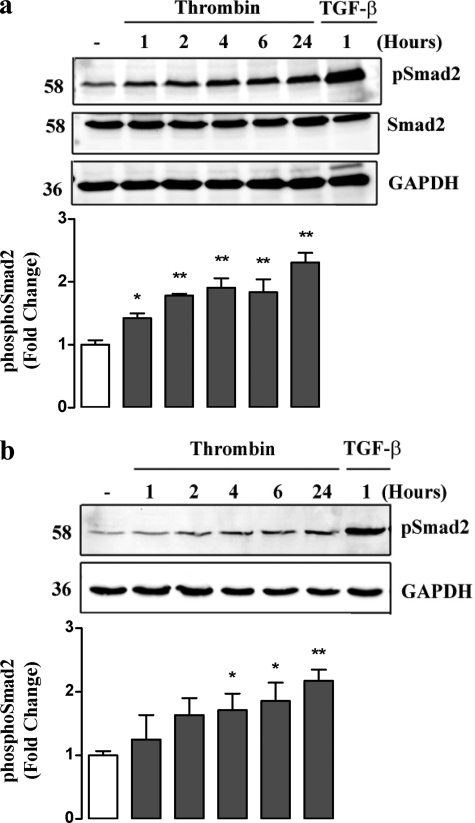
The initial downstream signaling consequences of the activation of the TβRI/ALK V are the phosphorylation of the Smad transcription factors, specifically Smad2 and -3 in the carboxyl-terminal generating, phospho-Smad2C and phospho-Smad3C, respectively (30). The exact consequences depend upon the cell type and context (30). We have demonstrated previously and reported that TGF-β activates proteoglycan synthesis in VSMCs via a mechanism involving TβRI and phosphorylation of Smad2 (21). Here, we evaluated the temporal effects of thrombin treatment of VSMCs on the level of phospho-Smad2C (Fig. 3a). Assessed over a 24-h period, we observed an increase in the level of phospho-Smad2C at 1–2 h and a slowly increasing level of phospho-Smad2C out to 24 h. (Fig. 3a). This response is slower than the effect of TGF-β itself on activation of phospho-Smad2C but is consistent with a direct action of the PAR-1 on the TβRI.
FIGURE 3.
Thrombin stimulates Smad2 phosphorylation over 24 h. Thrombin causes nuclear translocation of phospho-Smad2. a, VSMCs were treated with thrombin (10 units/ml) for up to 24 h. VSMCs stimulated with TGF-β (2 ng/ml) for 1 h (TGF-β) were used as a positive control. Cell lysates were collected and proteins (50 μg) were resolved on SDS-PAGE 10% acrylamide gel and then transferred to a PVDF membrane. The membrane was then incubated with anti-Smad2(Ser-465/467) monoclonal antibody (1:1000) followed by peroxidise-labeled anti-rabbit IgG secondary antibody. The membrane was then reprobed with unphosphorylated-Smad2 and anti-GAPDH monoclonal antibody's (1:1000) followed by peroxidise labeled anti-rabbit IgG secondary antibody to determine equal loading. b, VSMCs were treated with thrombin (10 units/ml) for up to 24 h. TGF-β (2 ng/ml) stimulation at 1 h was used as a positive control. Nuclear fractions were collected using cellular disruption and centrifugation and separated (50 ng/ml) by SDS-PAGE on a 10% acrylamide gel. Proteins were transferred and probed as described in a. The gel is a representation of three separate experiments. Histograms represent band density expressed as fold over basal from at least three separate experiments. **, p < 0.01 versus untreated control using a one-way ANOVA. a and b do not show quantitation of TGF-β bands as they appear off of the scale.
The question arises whether thrombin generated phospho-Smad2C is a physiologically relevant response such that it can lead to translocation of the phospho-Smad2C to the cell nucleus, where it has the capacity to act in a complex as a transcription factor (31). To answer this question, we treated serum-deprived human VSMCs with thrombin and TGF-β, isolated a nuclear fraction by cellular disruption and centrifugation, and assessed the levels of phospho-Smad2C by Western blotting. Thrombin elicited an early increase in nuclear levels of phospho-Smad2C (1 h) and subsequently a rise to higher levels at 24 h (Fig. 3b). As a positive control, TGF-β also caused a marked increase of phospho-Smad2C in the nuclear fraction (Fig. 3b). These data indicate that the action of thrombin to trans-activate TβRI receptors generates functionally active phospho-Smad2C, which translocates to the nucleus as if it were generated classically from TGF-β activation of TβRI/ALK V.
The effect of thrombin to increase phospho-Smad2C levels was blocked by inhibition of PAR-1 using multiple approaches, including blockade with the small molecule inhibitors JNJ5177094 (Fig. 4a), and dose-dependently with SCH79797 (Fig. 4b). In addition, preincubation with a PAR-1-specific antibody, which competitively binds to the tethered ligand region, completely blocked the stimulation of phospho-Smad2C (Fig. 4c). A similar effect is seen when interfering with the thrombin active site using 5× molar excess hirudin (Fig. 4d). Confirming the role of ALK V, SB431542 also completely inhibited the ability of thrombin to stimulate phospho-Smad2C (Fig. 4a). We also evaluated the effect of the thrombin mimetic, TRAP, to activate carboxyl-terminal phosphorylation of Smad2. Treatment of VSMCs with TRAP lead to an increase in cytosolic phospho-Smad2C, and this response was attenuated by SB431542 and JNJ5177094 (Fig. 4a). Thus, we have demonstrated that activation of PAR-1 with either its natural ligand thrombin or a peptide mimetic TRAP leads to increased cytoplasmic levels of the classic TβRI/ALK V receptor product, phospho-Smad2C.
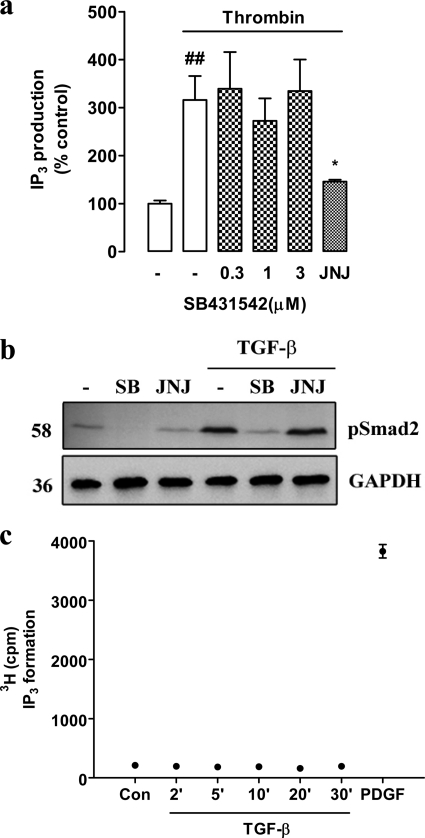
Although the data show that thrombin can activate Smad2C phosphorylation and its nuclear translocation is an outcome known only to occur as a downstream response to activation of TβRI/ALK V (30), further aspects of the data rely on the specificity of the inhibitors of each of the receptors involved in the transactivation pathway, namely PAR-1 and TβRI/ALK V, so we explored this issue. A classic GPCR response that we have characterized previously in these VSMCs is agonist-mediated activation of phospholipase C leading to the cleavage of phosphatidylinositol bisphosphate yielding diacylglycerol and IP3 (32). We evaluated the effect of SB431542 (0–3 μm) and JNJ5177094, respectively, on thrombin-stimulated IP3 accumulation in human VSMCs (Fig. 5a). Thrombin caused a 3-fold increase in the level of IP3, which was not blocked by the highest concentration of SB431542 but was antagonized completely by JNJ5177094 (Fig. 5a). The fact that the response was sensitive to JNJ5177094 confirmed that it is mediated via PAR-1. Thus, there is no evidence that SB431542 has any direct inhibitory activity toward the thrombin receptor PAR-1. We evaluated the potential inhibitory activity of the thrombin receptor antagonist toward the TβRI by stimulating cells with TGF-β in the presence and absence of SB431542 and JNJ517094 and assessing phospho-Smad2C levels by Western blotting (Fig. 5b). The effect of TGF-β to increase phospho-Smad2C levels was blocked by SB431542 but was totally unaffected by the PAR-1 antagonist JNJ5177094 (Fig. 5b). SCH79797 also exhibited no inhibitory action on the ability of TGF-β to stimulate phospho-Smad2C (Fig. 4b, lanes 7 and 8). A further interesting question relates to the directionality and specificity of the signaling pathways downstream of PAR-1 and TβRI/ALK V. To address these questions, we examined the ability of TGF-β to activate GPCRs, including PAR-1, which are coupled to phospholipase C and thus generate IP3. Human VSMCs were treated with TGF-β for up to 30 min, and the response was compared with PDGF used as a positive control. TGF-β did not increase IP3 levels over 30 min, a time over which PDGF caused a 10-fold increase in IP3 levels (Fig. 5c). Thus, PAR-1 activates TβRI/ALK V, but TβRI/ALK V does not activate PAR-1; furthermore, there is no cross-talk from the TβRI signaling pathway to activation of phospholipase C associated with PAR-1 or most likely any GPCR.
FIGURE 5.
The respective inhibitors of ALK V and PAR-1 do not cross react with each others receptors. TGF-β does not activate IP3 production. a, VSMCs were incubated with myo-[3H]inositol (6.25 μCi/ml) for 24 h in IP-free medium. Cells were then washed and pretreated with SB431542 (SB; 0.3–3 μm) for 30 min before stimulation with thrombin (10 units/ml) for 5 min. JNJ5177094 (JNJ; 30 mm) in the presence of thrombin (10 units/ml) was used as a positive control. Whole cell lysates were collected, and IP3 was isolated and measured using Dowex 1 loaded ion-exchange chromatography. Results are the mean ± S.E. of data normalized to control from three separate experiments in triplicate. *, p < 0.05 versus thrombin alone using a one-way ANOVA. b, VSMCs were treated with SB431542 (3 μm) or JNJ5177094 (30 μm) in the presence of TGF-β (2 ng/ml). Whole cell lysates were collected at 4 h, and proteins (50 ng/ml) were resolved by SDS-PAGE using a 10% acrylamide gel. Proteins were transferred and probed as described in Fig. 3a. c, VSMCs were incubated with myo-[3H]inositol (6.25 μCi/ml) for 24 h in IP-free medium. Cells were then washed and treated with TGF-β (2 ng/ml) for up to 30 min. A 30-min stimulation with PDGF (50 ng/ml) was used as a positive control. Whole cell lysates were collected, and IP3 accumulation was assessed as described in the legend to Fig. 4. Results are the mean ± S.E. of data normalized to control from one experiment in duplicate.
DISCUSSION
GPCR transactivation of PTK receptors was first described by Ulrich in 1996 (12). Subsequent work has described two major pathways of receptor transactivation. In the first instance, GPCR stimulation induces activation of matrix metalloproteinase, membrane-bound ADAM family proteases, that cause ectodomain shedding of an agonist such as heparin-binding EGF, which acts as a ligand at its cognate receptor (33). EGF activation leads to growth stimulation attributable to the GPCR activation (33). GPCR involved in this pathway include the receptors for thrombin, angiotensin, endothelin, acetylcholine, and lysophosphatidic acid. The second mode of GPCR to PTK receptor transactivation is independent of the cognate ligand for the PTK receptor. PTK activation occurs via a multitude of intracellular signaling pathways downstream of the GPCR that lead to PTK receptor activation. Such intracellular signaling involves intracellular calcium, reactive oxygen species, and PTKs such as Src. We have no information on the mechanism through which PAR-1 trans-activates TβRI. Thrombin has protease activity. We have excluded the possibility that the response is due to the release of TGF-β either from the matrix or the cells, so the mechanism of cognate ligand release that applies to GPCR activation of PTK receptor does not apply. Furthermore, we have found that the response of GPCR transactivation of TβRI/ALK V also occurs for endothelin-1 acting through an endothelin receptor (data not shown). Endothelin does not possess proteolytic activity further arguing against a role of the proteolytic activity of thrombin other than its action to activate PAR-1. Our data shows that the pharmacological antagonists are more efficacious in inhibiting TRAP than thrombin stimulated transactivation of TβRI in both the immediate response of an increase in phospho-Smad2 and the functional readout of the stimulation of biglycan synthesis and GAG elongation. This is most certainly a property of the thrombin receptor, PAR-1, in that being a protease-activated receptor the highly thermodynamically favorably interaction of a tethered ligand (generated by the proteolytic action of thrombin) with its receptor is much more difficult to block than is the interaction of a free peptide ligand, TRAP, in its interaction with PAR-1.
We have established that the transactivation of TβR is unidirectional in the direction of GPCR → TβR because treatment of the VSMCs with TGF-β does not increase the accumulation of IP3, which is the classic response that is strongly evoked by the GPCRs, thrombin, and endothelin (data not shown). The absence of an effect of TGF-β on IP3 accumulation in these cells clearly indicates that there is no backwards signaling to any GPCR that can activate phospholipase C.
We also show that the thrombin induced increase in cytosolic phospho-Smad2C has functional consequences whereby the phospho-Smad2C actively translocates to the nucleus albeit at different levels upon thrombin and TGF-β stimulation. The difference in the maximum level of phospho-Smad2C in the nucleus as a consequence of thrombin or TGF-β stimulation correlates with and is almost certainly a direct consequence of the increase levels of phospho-Smad2 induced by TGF-β compared with thrombin in the cytosol of these cells. The differing levels of nuclear phospho-Smad2C may have consequences with regard to transcriptional activity and gene expression. The reason and the potential outcomes of the different levels of nuclear phospho-Smad2 induced by TGF-β and thrombin is part of ongoing studies in our laboratory.
Studies have shown that in endothelial cells, TGF-β can inhibit thrombin signaling (34) and, importantly, that thrombin, via PAR-1 down-regulates TGF-β signaling due to endocytosis of the TβRII, using the type III receptor endoglin (35). This is in contrast to our data but may highlight the complex interplay between context and cell types, specifically endothelial cells such as those used in the above mentioned studies and the vascular smooth muscle cells used in this study.
The existing paradigm of GPCR signaling via transactivation invokes only PTK receptors although there seems no a priori reason why transactivation of other receptors should not be possible. As the experiments described in this report were evolving there was a report of the GPCR activation of a serine/threonine kinase receptor, which was offered to be the first such demonstration of this novel transactivation pathway (36). In that report, serotonin receptors on pulmonary artery smooth muscle cells transactivate bone morphogenic protein receptors to generate phospho-Smad1/-5/-8 (36). Our data thus represent the first report of the GPCR transactivation of the important and ubiquitous TβRI/ALK V receptor and the generation of phospho-Smad2C. Taken with the earlier report (36), this provides support for this novel extension of the current GPCR signaling paradigm to include the activation of serine/threonine kinase receptors of the TGF-β receptor superfamily.
There are several questions of major importance to be answered including how wide spread is the phenomenon in terms of GPCR ligands, what is the mechanism through which GPCRs lead to activation of serine/threonine kinase receptors and is downstream signaling from the serine/threonine kinase receptor different when it is initiated by cognate ligand interaction or GPCR transactivation? In the final context the phosphorylation cascade in the situation where a serine/threonine kinase receptor, or a PTK for that matter, is activated by a GPCR, the downstream signaling may be different from classical signaling to the extent that novel therapeutic targets may emerge.
In conclusion, the current paradigm of GPCR signaling involves the classical G protein coupled pathway, β-arrestin scaffold signaling and transactivation of PTK receptors. The paradigm relates to up to six GPCR ligands and three PTK receptors although it has not been demonstrated for all possible pairs of receptors. Our data extends the paradigm to include the serine/threonine kinase receptor, for TGF-β, TβR and is based on activation via the GPCRs for thrombin being PAR-1. The pathway is described in the context of proteoglycan synthesis in VSMCs, but its applicability most certainly is much broader. Due to the very extensive expression of both GPCR and TβRs in nature and their involvement in major and widespread pathologies, the implications of this interaction potentially are immense. One example would be the area of cancer metastasis in which PTKs have been targeted but with only modest clinical success. PAR-1 has been targeted for diseases such as thrombosis, atherosclerosis, inflammation, and cancer (37). Targeting GPCRs now needs to take into consideration that some of the effects may be mediated via transactivation of the TβRI and consequently the actions of Smad transcription factors.
Acknowledgments
We thank all of the students and post-docs in the laboratory over the last decade who have undertaken the experiments and added to the development of the concepts, which have formed the foundation of the finding presented in this paper.
This work was supported by National Health and Medical Research Council of Australia Project Grants 268928 and 472611, Development Grant 418934 (to P. J. L.) and Fellowship (to P. J. L.); project grants from the Diabetes Australia Research Trust (to P. J. L. and N. O.); and a grant-in-aid from the National Heart Foundation of Australia (to P. J. L.).
- GAG
- glycosaminoglycan
- VSMC
- vascular smooth muscle cell
- IP3
- inositol 1,4,5-trisphosphate
- GPCR
- G-protein-coupled receptor
- DMEM/−IP
- inositol phosphate-free DMEM
- ALK
- activin-like kinase
- PTK
- protein tyrosine kinase
- TβRI
- TGF-β type I receptor
- IP
- inositol phosphate
- ANOVA
- analysis of variance.
REFERENCES
- 1.Hozawa A., Folsom A. R., Sharrett A. R., Chambless L. E. (2007) Arch. Intern Med. 167, 573–579 [DOI] [PubMed] [Google Scholar]
- 2.Nakashima Y., Fujii H., Sumiyoshi S., Wight T. N., Sueishi K. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1159–1165 [DOI] [PubMed] [Google Scholar]
- 3.Little P. J., Osman N., O'Brien K. D. (2008) Curr. Opin. Lipidol. 19, 448–454 [DOI] [PubMed] [Google Scholar]
- 4.Ross R. (1999) N. Engl. J. Med. 340, 115–126 [DOI] [PubMed] [Google Scholar]
- 5.Ballinger M. L., Nigro J., Frontanilla K. V., Dart A. M., Little P. J. (2004) Cell Mol. Life Sci. 61, 1296–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Little P. J., Ballinger M. L., Osman N. (2007) Vasc. Health Risk Manag. 3, 1–8 [PMC free article] [PubMed] [Google Scholar]
- 7.Smith N. J., Luttrell L. M. (2006) Hypertension 48, 173–179 [DOI] [PubMed] [Google Scholar]
- 8.Lefkowitz R. J. (2007) Biochim. Biophys. Acta 1768, 748–755 [DOI] [PubMed] [Google Scholar]
- 9.Lefkowitz R. J. (2007) Acta Physiol. 190, 9–19 [DOI] [PubMed] [Google Scholar]
- 10.McCudden C. R., Hains M. D., Kimple R. J., Siderovski D. P., Willard F. S. (2005) Cell Mol. Life Sci. 62, 551–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefkowitz R. J., Shenoy S. K. (2005) Science 308, 512–517 [DOI] [PubMed] [Google Scholar]
- 12.Daub H., Weiss F. U., Wallasch C., Ullrich A. (1996) Nature 379, 557–560 [DOI] [PubMed] [Google Scholar]
- 13.Gavi S., Shumay E., Wang H. Y., Malbon C. C. (2006) Trends Endocrinol Metab. 17, 48–54 [DOI] [PubMed] [Google Scholar]
- 14.Andrade-Gordon P., Maryanoff B. E., Derian C. K., Zhang H. C., Addo M. F., Darrow A. L., Eckardt A. J., Hoekstra W. J., McComsey D. F., Oksenberg D., Reynolds E. E., Santulli R. J., Scarborough R. M., Smith C. E., White K. B. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 12257–12262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damiano B. P., Derian C. K., Maryanoff B. E., Zhang H. C., Gordon P. A. (2003) Cardiovasc Drug Rev. 21, 313–326 [DOI] [PubMed] [Google Scholar]
- 16.Janssens W. J., Verhaeghe R. H. (1982) Blood Vessels 19, 126–134 [PubMed] [Google Scholar]
- 17.Dabbagh K., Laurent G. J., McAnulty R. J., Chambers R. C. (1998) Thromb. Haemost 79, 405–409 [PubMed] [Google Scholar]
- 18.Ivey M. E., Little P. J. (2008) Thromb. Res. 123, 288–297 [DOI] [PubMed] [Google Scholar]
- 19.Coughlin S. R. (2000) Nature 407, 258–264 [DOI] [PubMed] [Google Scholar]
- 20.Little P. J., Tannock L., Olin K. L., Chait A., Wight T. N. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 55–60 [DOI] [PubMed] [Google Scholar]
- 21.Dadlani H., Ballinger M. L., Osman N., Getachew R., Little P. J. (2008) J. Biol. Chem. 283, 7844–7852 [DOI] [PubMed] [Google Scholar]
- 22.Saito Y., Berk B. C. (2001) J. Mol. Cell Cardiol. 33, 3–7 [DOI] [PubMed] [Google Scholar]
- 23.Neylon C. B., Little P. J., Cragoe E. J., Jr., Bobik A. (1990) Circ. Res. 67, 814–825 [DOI] [PubMed] [Google Scholar]
- 24.Nigro J., Dilley R. J., Little P. J. (2002) Atherosclerosis 162, 119–129 [DOI] [PubMed] [Google Scholar]
- 25.Mori Y., Ishida W., Bhattacharyya S., Li Y., Platanias L. C., Varga J. (2004) Arthritis Rheum 50, 4008–4021 [DOI] [PubMed] [Google Scholar]
- 26.Ungefroren H., Groth S., Ruhnke M., Kalthoff H., Fändrich F. (2005) J. Biol. Chem. 280, 2644–2652 [DOI] [PubMed] [Google Scholar]
- 27.Wasteson A., Uthne K., Westermark B. (1973) Biochem. J. 136, 1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taipale J., Koli K., Keski-Oja J. (1992) J. Biol. Chem. 267, 25378–25384 [PubMed] [Google Scholar]
- 29.Coughlin S. R. (1999) Thromb. Haemost. 82, 353–356 [PubMed] [Google Scholar]
- 30.Derynck R., Zhang Y. E. (2003) Nature 425, 577–584 [DOI] [PubMed] [Google Scholar]
- 31.Massagué J., Seoane J., Wotton D. (2005) Genes Dev. 19, 2783–2810 [DOI] [PubMed] [Google Scholar]
- 32.Neylon C. B., Nickashin A., Little P. J., Tkachuk V. A., Bobik A. (1992) J. Biol. Chem. 267, 7295–7302 [PubMed] [Google Scholar]
- 33.Konishi A., Berk B. C. (2003) J. Biol. Chem. 278, 35049–35056 [DOI] [PubMed] [Google Scholar]
- 34.Harris H., Kirschenlohr H., Szabados N., Metcalfe J. (2004) Cytokine 25, 85–93 [DOI] [PubMed] [Google Scholar]
- 35.Tang H., Low B., Rutherford S. A., Hao Q. (2005) Blood 105, 1977–1985 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Ren W., Warburton R., Toksoz D., Fanburg B. L. (2009) FASEB J. 23, 2299–2306 [DOI] [PubMed] [Google Scholar]
- 37.Maryanoff B. E., Zhang H. C., Andrade-Gordon P., Derian C. K. (2003) Curr. Med. Chem. Cardiovasc Hematol Agents 1, 13–36 [DOI] [PubMed] [Google Scholar]