Abstract
It has been known for a long time that the yeast Saccharomyces cerevisiae can assimilate α-methylglucopyranoside and isomaltose. We here report the identification of 5 genes (YGR287c, YIL172c, YJL216c, YJL221c and YOL157c), which, similar to the SUCx, MALx, or HXTx multigene families, are located in the subtelomeric regions of different chromosomes. They share high nucleotide sequence identities between themselves (66–100%) and with the MALx2 genes (63–74%). Comparison of their amino acid sequences underlined a substitution of threonine by valine in region II, one of the four highly conserved regions of the α-glucosidase family. This change was previously shown to be sufficient to discriminate α-1,4- to α-1,6-glucosidase activity in YGR287c (Yamamoto, K., Nakayama, A., Yamamoto, Y., and Tabata, S. (2004) Eur. J. Biochem. 271, 3414–3420). We showed that each of these five genes encodes a protein with α-glucosidase activity on isomaltose, and we therefore renamed these genes IMA1 to IMA5 for IsoMAltase. Our results also illustrated that sequence polymorphisms among this family led to interesting variability of gene expression patterns and of catalytic efficiencies on different substrates, which altogether should account for the absence of functional redundancy for growth on isomaltose. Indeed, deletion studies revealed that IMA1/YGR287c encodes the major isomaltase and that growth on isomaltose required the presence of AGT1, which encodes an α-glucoside transporter. Expressions of IMA1 and IMA5/YJL216c were strongly induced by maltose, isomaltose, and α-methylglucopyranoside, in accordance with their regulation by the Malx3p-transcription system. The physiological relevance of this IMAx multigene family in S. cerevisiae is discussed.
Keywords: Carbohydrate Metabolism, Evolution, Gene Expression, Yeast Genetics, Yeast Metabolism, Isomaltose Metabolism, Multigene Family, Polymorphism, RT-qPCR, Subtelomere
Introduction
Gene duplication, one of the main driving forces in genome evolution, generates paralogous genes that can acquire specificities by sequence divergence. These redundant genes are defined as multigene families, the most often located within subtelomere sequences. Very recently, Brown and co-workers demonstrated that the extraordinary instability of these regions supports rapid adaptation to novel niches. These multigene families, which evolve and expand much faster than families that do not contain subtelomeric genes, indeed reflect the organism's lifestyle (2). The sequenced Saccharomyces cerevisiae BY4741 strain contains about 30% of ORFs that belong to multigene families comprising between 3 and over 20 members (3). Relevant examples can be found in enzymatic systems for sugar uptake and assimilation. The hexose transporters are encoded by a family of 17 genes (HXT1–HXT17) (4, 5), whose expression is dependent on growth conditions, sugar availability, or stress (6, 7). The assimilation of sucrose (α-d-glucopyranosyl-(1→2)-β-d-fructofuranose), maltose (α-d-glucopyranosyl-(1→4)-α-d-glucopyranose), melibiose (α-d-galactopyranosyl-(1→6)-α-d-glucopyranose), and starch is also dependent on the expression of SUCx, MALx, MELx, and STAx multigene families, respectively. These families are not always present in all Saccharomyces species, as for instance in the laboratory strain BY4741, derived from S288c, which does not hydrolyze starch, because STAx genes are absent (8). This strain also harbors only the SUC2 encoding invertase, whereas SUCx genes belong to a multigene family encompassing six unlinked loci (SUC1 through SUC5 and SUC7) (9). The overall organization of the MALx gene family appears to be similar to that of the SUCx gene family. However each of the five MAL loci (MAL1 through MAL4 and MAL6) is a complex locus, which includes three genes that are all required for maltose utilization (10). MALx1 encodes the permease (11), MALx2, the maltase (12), and MALx3, the Malx3p-transactivator required for the expression of the two previous genes (13). The MAL loci are also located in the subtelomeric regions (14).
Yeasts are also able to ferment α-methylglucopyranoside (methyl α-d-glucopyranoside or αMG),2 an analogue of natural substrates like isomaltose (α-d-glucopyranosyl-(1→6)-α-d-glucopyranose). Hawthorne (15) first studied and highlighted the complex genetic situation conferring yeast with the capacity to ferment this substrate. Later on, ten Berge (16) and Naumov (17) identified five complementary MGLx genes (MGLa–e) in other genetic lines of Saccharomyces. This genetic complexity was nicely discussed in a previous study (18), which underlined the difficulty to unravel which of these genes could be associated with transport, cleavage, or regulatory function. From a biochemical view, isomaltase activity was first measured in a commercial yeast maltase preparation (19). Thereafter, an α-methylglucosidase was purified, showing activity on both αMG and isomaltose (20). Khan and Haynes showed that the products of the MGL1 and MGL3 genes have the same phenotypic function, molecular weight, and substrate specificity (isomaltose and αMG), but differ with respect to their specific activity and Michaelis constant (21). More recently, the group of Tabata associated this isomaltase activity with the product of YGR287c and showed that Val-216 in the catalytic site is an important residue to discriminate the α-1,4- and α-1,6-glucosidic linkages of substrates (1).
The in vivo function of YGR287c gene, as well as the function of other homologous ORFs encoding putative glucosidases in the S. cerevisiae genome has not been investigated yet. Our results showed that yeast is endowed with the presence of five genes encoding four distinct isomaltases, all located in the subtelomeric regions of different chromosomes. We report here a complete functional genomic analysis of these five genes, including their respective role in isomaltose and αMG assimilation, their regulation by carbon sources, and their substrate specificities. We have renamed these genes IMAx (IMA1 through IMA5) for IsoMAltase.
EXPERIMENTAL PROCEDURES
Yeast Strains, Media, and Culture Conditions
The prototrophic MAL constitutive CEN.PK113–7D (MATa MAL2-8c SUC2) strain (22) or the mal negative BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) strain (Euroscarf) were used as the wild type, and the host strains were used for all mutant constructions. The CEN.PK derivative strain JF1811 (MATa HIS3 leu2–3,112 ura3–52 TRP1 gph1::URA3 sga1::kanMX4, unpublished) was used for overexpression assays. The transformation was performed according to the lithium acetate method (23). Yeast cells were cultivated in 500-ml shaking flasks at 200 rpm containing 100 ml of yeast nitrogen base (YN) synthetic medium (0.17% yeast nitrogen base without amino acid and without ammonium (Difco), 0.5% ammonium sulfate) buffered at pH 5.0 with sodium succinate/NaOH. Agar was added at 2% for solid media. Glucose, galactose, maltose, isomaltose, or αMG was added at 1% or 2% (w/v) final concentration. YN synthetic medium with glycerol, lactate plus ethanol (3, 2, and 0.5%, respectively) was used for the expression studies. Growth assays for complementation tests were performed in triplicate, after inoculation to an initial A600 of ∼0.05 (Ainitial), and cell growth was measured after 24 h (A24 h). Normalized growth values were expressed as (mean of Δgoi/mean of Δref), with Δgoi and Δref as (A24 h − Ainitial) for the strain transformed by the gene of interest and the reference gene, respectively. Standard error (S.E.) of the normalized ratio was calculated by combining the S.E. of numerator and denominator. Linear approximation was admitted in this combination.
Construction of the Deletion Mutants
Deletion of the various MALx or IMAx genes in yeast was carried out by homologous recombination with a PCR product that was obtained as follows. A 2677-bp fragment bearing the mal12::kanMX4 allele was amplified by PCR using genomic DNA from the BY mal12::kanMX4 strain (mal12Δ) from the Saccharomyces Genome Deletion Project (purchased from Openbiosystems), and primers were used to clone the wild ORF in pGEM-T easy vector (Table 1). Similarly, a 2660-bp fragment bearing the yjl216c::kanMX4 was amplified by PCR using genomic DNA from the BY yjl216c::kanMX4 strain (yjl216cΔ) and primers used to clone the wild ORF as reported in Table 1. A 2611-bp fragment carrying the ygr287c::kanMX4 allele (ygr287cΔ) was amplified by PCR using genomic DNA from the Euroscarf mutants collection, and the primers were used to clone the wild ORF (Table 1). To obtain the recombinant fragments yil172c::kanMX4 and yjl221c::kanMX4 (yil172cΔ and yjl221cΔ), the Xho1/Hpa1 fragment from the plasmid bearing the wild ORFs was replaced by the Sal1/EcoRV fragment containing the kanMX4 gene from the plasmid pFA-kanMX4 (24). The primers designed to clone the wild ORF YIL172c (id. to YJL221c) were also used to amplify yil172c::kanMX4 or yjl221c::kanMX4 cassettes. The same method was used to obtain the recombinant fragment yol157c::kanMX4 (yol157cΔ) from the plasmid pGEM-T easy bearing the wild ORF YOL157c. The recombinant fragment mal2–8c::kanMX4 (mal2–8cΔ) was obtained from the plasmid pGEM-T easy bearing the MAL2–8c allele, in which the Xma1/EcoRV fragment was replaced by the Xma1/EcoRV kanMX4 fragment from the plasmid pFA-kanMX4. All these PCR fragments were used to transform the CEN.PK113–7D strain, leading to the single deletion mutants. Correct replacement of the gene in the mutant strains were analyzed by PCR.
TABLE 1.
List of plasmids and primers
Forward (F) and reverse (R) primer sequences used to clone the designed ORF are shown. Restriction sites underlined.
| Backbone vector | ORF | Primer sequence |
|---|---|---|
| pGEM-T easy | MAL12 | F: TATGTCCCTCAAGCCCGGTACGTTG |
| R: ACTTGACGTAAACGCATAAGCCGGC | ||
| pGEM-T easy | MAL2–8c | F: GCGGATCCGTCATCCACTACTCTCCCTCCTGAG |
| R: GCACCGGTCTATCAGTATATCTATCTCGTATAAGTGAACGGCG | ||
| pGEM-T easy | YGR287c | F: CGAATATTCATTCAGTCGGACCG |
| R: CGGCCACCATCTCATTCTTTACTAG | ||
| pGEM-T easy | YIL172c (id. to YJL221c) | F: GAAGGTTTTGTGAAGTGTCAGGGAAATGC |
| R: GAAGCCCATTTTCTACCTCCATCACCGTAC | ||
| pGEM-T easy | YJL216c | F: AGAAGCGTTCGGAAATTCCAAAAC |
| R: TTTGTTAAGAACAAGGCCTGCACC | ||
| pGEM-T easy | YOL157c | F: TAATTTTTGTGGGGAACTGAACAAGGTCA |
| R: CGAACCGGTGAATATATTTTGCTGGCTAGAT | ||
| YEplac181-PGK-CYC1 | MAL12 | F: CGGGATCCATGACTATTTCTGATCATCC |
| R: GGCATGCATTTATTTGACGAGGTAGATTCT | ||
| YEplac181-PGK-CYC1 | YGR287c | F: GCCAGATCTATGACTATTTCTTCTGCACATCCAG |
| R: GCCCTGCAGTCATTCGCTGATATATATTCTTCCTTC | ||
| YEplac181-PGK-CYC1 | YIL172c (id. to YJL221c) | F: CGGGATCCATGACTATTTCTTCTGCACATCCAGAAA |
| R: TGCATGCATTCATTCAGATATGTAAATTCTGCCCTCC | ||
| YEplac181-PGK-CYC1 | YJL216c | F: CGGGATCCATGACGATCATCCATAATCCT |
| R: GGCCTGCAGTTACTTCAACAAGTAAAGTCTTC | ||
| YEplac181-PGK-CYC1 | YOL157c | F: GGAAGATCTATGACTATTTCTTCTGCACATCCAGAA |
| R: GCCTGCAGTCATTCAGATATGTAAATTCTGCCCTC |
Construction of Overexpression Strains
The YEplac181-PGK/CYC1 plasmid (unpublished), which was obtained from the cloning of the PGK1/CYC1 cassette from pYPGE2 (25) in the 2μ, LEU2 YEplac181 backbone (26), was used to clone MAL12, YGR287c, YIL172c, YJL216c, and YOL157c coding sequences under the control of the strong PGK1 yeast promoter. Because YIL172c and YJL221c are 100% identical, they could not be differentiated. As a consequence, only one expression plasmid was constructed and used to get the results presented in the Fig. 2. Because of the considerable sequence similarity of these genes, the ORFs with roughly 500-bp upstream and downstream sequences were previously cloned in the pGEM-T easy vector by using the CEN.PK genomic DNA as template, and the obtained plasmids were verified by restriction pattern analysis (Rsa1 enzyme).3 These plasmids were then used as template together with primers indicated in Table 1, to amplify the coding sequences of these five genes. The MAL12, YGR287c, YIL172c (id. to YJL221c), and YOL157c PCR fragments were inserted into the Bgl2/Pst1 or BamH1/Pst1 compatible sites of the YEplac181-PGK/CYC1 plasmid to yield to the final expression vectors. The Yjl216c PCR fragment was inserted first in the pGEM-T easy vector before EcoR1 subcloning in the YEplac181-PGK/CYC1 plasmid. These plasmids were verified by sequencing and used to transform the JF1811 strain or the ygr287cΔ leu2Δ mutant strain (this study). Leu+ transformants were selected on YN agar plates plus 2% glucose, in the absence of leucine.
FIGURE 2.
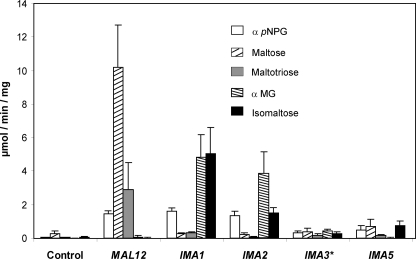
Biochemical activities on α-1,6- and α-1,4-glucosidic substrates. The α-glucosidase activity on different α-glucosidic substrates was assayed on crude extract from JF1811 yeast strain overexpressing MAL12 or ORFs of this gene family. ★, because IMA3 and IMA4 are 100% identical, they could not be differentiated. The cells were grown in YN synthetic medium containing 2% glucose to A600 nm ∼ 1.0, and activities were measured using 20 A600 nm units of cells. Control: JF1811 strain transformed with the empty YEplac181-PGK/CYC1 plasmid. The α-p-nitrophenyl α-d-glucopyranoside (pNPG) was used to measure total α-glucosidase activity. Maltose and maltotriose were used to measure maltase activity. αMG and isomaltose were used to measure isomaltase activity. Activities and standard deviation were calculated from three independent cultures.
Preparation of Extracts and Enzyme Assays
20 ml of exponentially growing yeast cells (A600 nm ∼ 1.0) on YN medium containing 2% glucose were collected by centrifugation (2200 × g, 5 min). The pellet was washed with 1 ml of water and frozen at −20 °C until use. Crude extract was prepared by resuspending the pellets in 700 μl of ice-cold solution containing 0.1 m potassium phosphate buffer, pH 6.8, 1 mm PMSF, and 1 mm DTT, and an equal volume of glass beads (0.4–0.5 mm diameter). The cells were broken open by vortexing the suspension four times each 30 s, keeping the mixture on ice between intervals. The sample was centrifuged for 3 min at 2000 × g, and the supernatant was used for enzymatic assays.
Total α-glucosidase activity was assayed using α-p-nitrophenyl α-d-glucopyranoside as substrate as outlined by Dubin et al. (12). The reaction mixture containing 50 μl of crude extract and 400 μl of 5 mm α-p-nitrophenyl α-d-glucopyranoside prepared in 0.1 m potassium phosphate buffer, pH 6.8, was incubated at 30 °C. The reaction was stopped by adding 1 ml of 1 m Na2CO3. Released p-nitrophenyl was followed at 405 nm, with a calculated extinction coefficient of 25 690 liter.mol−1.cm−1. Specific activity was expressed as micromoles of p-nitrophenyl released per milligram of protein/min. Activity of α-glucosidase on maltose (maltase), αMG (α-methyl glucosidase), and isomaltose (isomaltase) was determined by adding 30 μl of crude extract in a final volume of 300 μl of 50 mm potassium phosphate buffer, pH 6.8, containing either 0.1 m maltose, 0.1 m maltotriose, 0.1 m αMG, or 25 mm isomaltose. After 5 min of incubation at 30 °C the reaction was stopped by placing the tube in a water bath set at 80 °C for 5 min. The tubes were then centrifuged, and the glucose released was measured in the supernatant by the GLOX method (Sigma). The activity was expressed as micromoles of glucose released per milligram of protein/min. Assays were carried out in triplicate using three independent cultures. Protein concentrations were determined by the Bradford method using the Bio-Rad protein assay dye reagent.
RNA Sampling and Total RNA Extraction
The cells were grown in YN medium containing 3% glycerol (w/v), 2% lactate (w/v) plus 0.5% ethanol (v/v) to A600 nm ∼ 1.0. At this time, the sugar (maltose, isomaltose, αMG, glucose, or galactose) was added at 2% (w/v) final. Samples (∼108 cells) were collected (2200 × g, 4 °C, 3 min) before (T0 control) and 1 h after the addition of the sugar. The pellets were immediately transferred in 2-ml microcentrifuge tubes, frozen in liquid nitrogen, and stored at −80 °C until RNA extraction. Frozen cells were mechanically disrupted for 3 min using the TissueLyserII apparatus from Qiagen, with one stainless steel bead per tube. Total RNA was extracted using the SV Total RNA purification kit from Promega. The extracted RNA was analyzed and quantified using the Bioanalyzer 2100 and the RNA 6000 Nano LabChip kit (Agilent Technologies), and the ND-1000 UV-visible light spectrophotometer (NanoDrop Technologies).
Quantitative RT-PCR
The oligonucleotides for real-time PCR (Table 2) were designed using Beacon Designer 2.0 software (PREMIER Biosoft International). An analysis of primers on the Mfold server avoided positioning on risky secondary structures, while a BLAST analysis against S. cerevisiae genome sequence was included for specificity confidence. However, as the sequences of the studied genes were highly homologous, most of the primer pairs from this multigene family were manually designed and positioned on non-conserved nucleotides. Specificity of these primer sets was therefore validated by using the plasmids bearing the individual ORFs on the pGEM-T easy vector (Table 1). One microgram of total RNA was reverse-transcribed into cDNA in a 20-μl reaction mixture using the iScript cDNA synthesis kit (Bio-Rad). The cDNA levels were then analyzed using the MyIQ real-time PCR system from Bio-Rad. Each sample was tested in duplicate in a 96-well plate (Bio-Rad, CA). The reaction mix (25 μl of final volume) consisted of 12.5 μl of iQ SYBR Green Supermix (Bio-Rad), 2.5 μl of each primer (200 nm final concentration), 2.5 μl of H2O, and 5 μl of a 1/10 dilution of the cDNA preparation. The absence of genomic DNA in RNA samples was checked by real-time PCR before cDNA synthesis (minus RT control). A blank (No Template Control) was also incorporated in each assay. The thermocycling program for MALx2, YGR287c, and YIL172c (id. to YJL221c) amplifications consisted of one hold at 95 °C for 4 min, followed by 40 cycles of 10 s at 95 °C and 45 s at 54 °C. For YJL216c and YOL157c amplifications, the hybridizing-elongation step was performed at 55.7 °C. After completion of these cycles, melting-curve data were then collected to verify PCR specificity, contamination, and the absence of primer dimers.
TABLE 2.
List of primers used for qPCR analysis
Forward (F) and reverse (R) primer sequences and PCR amplification efficiency (Eff) are shown.
| ORF | Primer sequence | Eff. |
|---|---|---|
| % | ||
| MAL12 (id. to MAL32) | F: GGCGTTGATGCTATTTGGGTTTGT | 99 |
| R: GAGGTCTCCAGAAGAACCAGTCAC | ||
| YGR287c/IMA1 | F: CGATGCCATTTGGATCTCACCATTC | 88 |
| R: ACCAGTCACGCTTTGGATTAGTCTTC | ||
| YOL157c/IMA2 | F: GACTTAGTCATCAACCATTGCTCG | 85 |
| R: ACAGTCCTCATTCTCCCAATTCAA | ||
| YIL172c/IMA3 (id. to YJL221c/IMA4) | F: GACTTAGTCATCAACCATTGCTCC | 92 |
| R: GCAGTCTTCGTTCTCCCAGTTTAG | ||
| YJL216c/IMA5 | F: ACAATGACGGATGGGGTGATTTAGC | 90 |
| R: CAACAATAACCTTGATACCTCTCTTATGAGC |
The PCR efficiency of each primer pair (Eff, Table 2) was evaluated by the dilution series method using a mix of sample cDNAs as the template. Normalization was carried out using ALG9, TFC1 and UBC6 genes after their validation in this new experimental setup, as described in (27).
RESULTS
Identification of Five Genes Encoding Putative α-1,6-Glucosidases
While searching for uncharacterized genes encoding α-glucosidases in the S. cerevisiae genome, we blasted the whole yeast genome with the MAL12/YGR292w sequence. We retrieved, besides MAL32/YBR299w, five additional genes: YGR287c, YIL172c, YJL216c, YJL221c, and YOL157c (Table 3). Interestingly, these five genes are all located in the subtelomeric regions of different chromosomes, like other multigene families involved in sugar metabolism (see the Saccharomyces Genome Database (SGD) ORF map, available on-line). YIL172c, YJL216c, YJL221c, and YOL157c are in the vicinity of HXT12, HXT8, HXT9, and HXT11, respectively, which all belong to the HXTx multigene family encoding hexose transporters (28), whereas YGR287c is located near the MAL1 locus. From these five genes, YJL221c was previously known as FSP2, which stands for Flocculent Specific Protein, but no function has yet been reported for this gene (29). On the opposite, YGR287c has not been given a gene name despite the demonstration that it encodes an enzyme with α-1,6-glucosidase activity (1). These five genes are roughly 70% identical to MALx genes at the nucleotide sequence level (Table 3). However, when compared among them, it was found that YIL172c and YJL221c, both located on different chromosomes, are 100% identical (Table 3). YOL157c on Chr XV is 98% identical to YIL172c (id. to YJL221c), and YGR287c, possesses 89 and 88% identities with YOL157c and YIL172c or YJL221c, respectively. YJL216c shares the least sequence identity, because it only exhibits an overall 67% with the other four genes. We also extended the sequence analysis up to 1000 bp upstream of the start codon (Table 3). Similarity varies between the flanking sequences: from no significant (∼46%) to 99.8% between YIL172c and YJL221c upstream regions, which are different from each other by only a single-nucleotide deletion at position −309. This could be explained by systematic sequencing errors as reported for other genes in SGD. The gene order in the neighborhood of YIL172c and YJL221c is PAU14-VTH1-YIL172c-HXT12 and PAU1-VTH2-YJL221c-HXT9, respectively. It is therefore obvious that the gene pair YIL172c and YJL221c arose from a segmental duplication that spans at least 19,559 kb, between the left end of chromosome IX and the left end of chromosome X.
TABLE 3.
Pairwise nucleotide and amino acid sequence identities
The non-underlined numbers in the table give the nucleotide sequence identities (%) between the indicated ORFs (start to stop codon). Italic numbers in parentheses give the amino acid sequences identities (%). The underlined numbers give the identity (%) between promoter sequences (1000 bp upstream of the start codon). Nucleotide and amino acid sequence were retrieved from the SGD (available on-line). This comparative analysis was done using default settings from the Pairwise Alignment Tool (ClustalW) from Vector NTI Advance 10 software.
| MAL12 | MAL32 | YJL216c | YGR287c | YIL172c | YJL221c | YOL157c | |
|---|---|---|---|---|---|---|---|
| MAL12 | 100 | 71 | 44 | 47 | 44 | 44 | 45 |
| MAL32 | 99.5 (100) | 100 | 45 | 45 | 47 | 47 | 47 |
| YJL216c | 67 (61) | 63 (60) | 100 | 46 | 44 | 44 | 45 |
| YGR287c | 74 (72) | 73 (72) | 66 (65) | 100 | 46 | 47 | 46 |
| YIL172c | 73 (72) | 73 (72) | 67 (66) | 88 (93) | 100 | 99.8 | 91 |
| YJL221c | 73 (72) | 73 (72) | 67 (66) | 88 (93) | 100 | 100 | 91 |
| YOL157c | 73 (72) | 73 (72) | 67 (66) | 89 (93) | 98 (99) | 98 (99) | 100 |
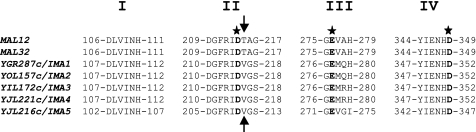
Alignment of the protein sequences (see Table 3 for pairwise sequence identities) revealed the presence of the four regions that are conserved among the α-amylase superfamily (30). This family corresponds to the glycoside hydrolase 13 family (GH13) as defined in the Carbohydrate-Active enZYmes database (CAZY, available on-line) (Fig. 1). Although regions I, II, and IV are strictly identical, the consensus III sequence shows some variability between these five proteins. In particular, Ima1p and Ima2p exhibit the same GEMQH consensus, whereas the Ima3p and Ima4p consensus is GEMRH, and the Ima5p consensus diverges from the others with the GEVGI sequence. The Asp residue in region II has been described as the catalytic nucleophile of α-glucosidases, whereas Glu (region III) and Asp (region IV) are likely involved in acid/base catalysis (31). The only pertinent difference between the five putative α-glucosidases encoded by YGR287c, YIL172c (id. to YJL221c), YJL216c, and YOL157c, and the maltases encoded by MAL12 and MAL32 is at the level of the consensus region II. The two latter genes bear a threonine residue, whereas the five newly identified genes possess a valine residue. This Val-216 in the protein encoded by YGR287c has been ascribed as an important amino acid residue that discriminates α-1,6- from α-1,4-linkages of glucosidic substrates in S. cerevisiae (1). Taken all together, these in silico analysis suggested that these five genes encode proteins with α-1,6-glucosidase activity, which will be biochemically validated in vitro and in vivo (see below). We therefore decided to rename these genes IMAx for IsoMAltase, with YGR287c as IMA1, YOL157c as IMA2, YIL172c as IMA3, YJL221c as IMA4, and YJL216c as IMA5 (see Addendum).
FIGURE 1.
Four conserved regions of the GH13 family. ClustalW amino acid sequence alignment of MAL12, MAL32, and five unlinked genes encoding putative α-glucosidase, which have been renamed IMA1 through IMA5. The three most important catalytic residues are written in boldface and marked with a star. The arrows indicate the position of the amino acid residue that discriminates α-1,6- (Val) from α-1,4- (Thr) linkages of glucosidic substrates.
Biochemical Evidence for Isomaltase Activity
To measure α-glucosidase activity in crude extracts, we used the JF1811 control strain that is deleted for SGA1 encoding the α-1,4/α-1,6-amyloglucosidase. Total α-glucosidase activity was measured by hydrolysis of α-p-nitrophenyl α-d-glucopyranoside (12). The α-1,4-glucosidase activity was obtained using maltose or maltotriose as substrates, whereas the α-1,6-glucosidase activity was measured on isomaltose or αMG. Because no detectable activity on αMG or isomaltose was measured in this control strain (Fig. 2), overexpression of the five genes was performed in this strain by cloning the corresponding ORFs downstream to the strong PGK1 promoter and upstream to the CYC1 terminator in the 2μ plasmid YEplac181 (Table 1). As is shown in Fig. 2, overexpression of these genes led to measurable α-glucosidase activity, although to different levels according to the protein and substrate used for the assay. The overexpression of MAL12 confirmed that this gene encodes an α-1,4-glucosidase acting on maltose and maltotriose, but not on αMG and isomaltose. On the other hand, remarkable α-glucosidase activity was measured on αMG and isomaltose in crude extracts prepared from cells overexpressing IMA1 and IMA2, indicating that these two genes encode proteins cleaving preferentially α-1,6-linkages. In contrast, extracts from IMA5-overexpressing cells was able to act on isomaltose (16 times more than in the control strain), not on αMG, but also on maltose (2.5 times more than in the control). Finally, crude extract from cells overexpressing IMA3, which could not be differentiated from IMA4 due to 100% identity between the two ORFs, was weakly active on the different sugars tested, but also showed a broad substrate specificity with both α-1,4- and α-1,6-glucosidic activity. None of these five proteins showed activity on polysaccharides such as amylose, limit dextrin, and glycogen (not shown). In conclusion, we proved biochemically that these five unlinked genes encode proteins bearing isomaltase activity.
Ima1p Isomaltase and Agt1p Transporter Are Essential for Isomaltose Assimilation
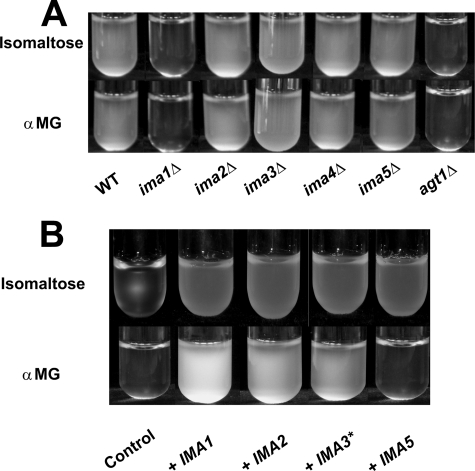
It can be seen from the Fig. 3A that the CEN.PK113–7D strain was able to grow on synthetic medium containing 1% isomaltose or αMG. Metabolic activity was also visualized by gas production after 24 h of incubation at 30 °C in Durham tubes containing 1% isomaltose or αMG in synthetic minimal medium (not shown). Therefore, and based on the finding that all the IMAx genes encoded a protein with α-1,6-glucosidase, these substrates were used as carbon sources to identify which of these genes are involved in their assimilation. As is shown in Fig. 3A, the deletion of IMA1 totally eliminated the capacity of yeast cells to grow on isomaltose and αMG, whereas the deletion of any of the other four genes didn't lead to growth defect on these two substrates in a IMA1 strain background, indicating that the bulk of α-1,6-glucosidase was made up of IMA1 gene product. Nonetheless, to verify the functionality of IMA2, IMA3 (id. to IMA4), and IMA5 on these substrates, we overexpressed these genes in the ima1Δ mutant. Cultures examined after 72 h showed that the growth of this mutant on isomaltose was restored with a plasmid carrying any one of the genes, even if quantitative analysis of A600 nm after 24 h underlined a slower growth for the strain transformed by the plasmids carrying IMA3 and IMA5 as compared with the strain overexpressing IMA1 or IMA2 (Fig. 3B). The growth of ima1Δ mutant was also restored on αMG with any of these genes with the exception of IMA5, although IMA3 overexpression led to a much lower growth rate than IMA1 and IMA2, which perfectly agreed with in vitro activity data (see Fig. 2).
FIGURE 3.
Qualitative growth assay of deletion mutants and overexpression strains on isomaltose and αMG. The yeast strains were grown on YN liquid medium containing 1% sugar (isomaltose or αMG). A, growth of the wild-type strain CEN.PK113–7D, its five isogenic single deletion mutants from this new gene family, and its agt1Δ derivative (MATa MAL2–8c SUC2 agt1::lacZ loxP-kanMX4-loxP (66)). The cultivation tubes were incubated at 30 °C for 24 h prior to being photographed. B, the ygr287cΔ leu2Δ mutant strain was transformed by plasmids overexpressing the different ORFs of this gene family. Control: empty YEplac181-PGK/CYC1 plasmid. The tubes were photographed when cells reached the stationary phase after 72 h of incubation at 30 °C. Normalized values of A600 nm (±2 S.E., see “Experimental Procedures”), after 24 h of cultivation on isomaltose and αMG, were respectively: 1.0 ± 0.14 and 1.0 ± 0.27 (IMA1); 0.95 ± 0.11 and 0.88 ± 0.20 (IMA2); 0.69 ± 0.13 and 0.25 ± 0.06 (IMA3*); 0.76 ± 0.08 and 0.008 ± 0.002 (IMA5); and 0.09 ± 0.03 and 0.02 ± 0.01 (empty plasmid). ★, IMA3 or IMA4.
As for maltose, the assimilation of α-glucoside should require a coupling between sugar transport across the membrane and its cleavage in the cytoplasm. It is known that αMG and isomaltose can be actively taken up in the yeast cells by a transporter encoded by AGT1 (18). We demonstrated in this work that Agt1p is necessary for growth on isomaltose and αMG (Fig. 3A). This result indicated that none of the other MAL-dependent permeases loci, i.e. MAL21 and MAL31, nor the MPH2 and MPH3 genes characterized as α-glucoside transporters and present in the CEN.PK strain (32, 33) can substitute for AGT1 to allow significant αMG or isomaltose influx and subsequent growth on these sugars.
Growth on Isomaltose Is Dependent on the Mal23p Transcriptional Activator
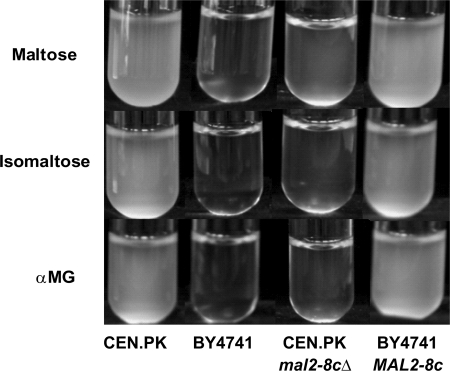
While using a set of laboratory strains known for their ability to ferment maltose, it was observed that the only strain able to grow on maltose, isomaltose, and αMG was the CEN.PK strain (Fig. 4). This strain possesses the constitutive MAL activator gene MAL2–8c (34), whereas the others, including the BY4741 strain, are known to bear a non-functional MAL-activator MALx3 (35). As expected, loss of MAL2–8c function abolished growth of the CEN.PK strain on isomaltose and αMG, whereas the introduction of this MAL23 allele in the mal− BY4741 strain recovered growth of this strain on the three carbon sources (Fig. 4).
FIGURE 4.
Qualitative growth assay of yeast strains harboring different Mal phenotypes on maltose, isomaltose, and αMG. The wild-type CEN.PK113–7D strain (Mal+) and its MAL2–8c deletion mutant, the wild-type BY4741 strain (Mal−), and the BY4741 strain transformed with a CEN plasmid bearing the constitutively expressed MAL2–8c allele of the MAL23 gene, were grown on YN liquid medium containing 1% sugar (maltose, isomaltose, or αMG). The cultivation tubes were incubated at 30 °C for 24 h prior to being photographed.
The Expression of the IMAx Genes Is Regulated by Carbon Sources
Because growth on isomaltose relied on a MAL23 activator (see above), we investigated whether the expression of this multigene family is under the control of the MAL-regulatory system and is regulated by the nature of the carbon source, as is the case for MALx genes (36). To this end, we studied the expression levels of IMAx genes by RT-qPCR. Technical features of the real-time PCR are indeed especially relevant to the study of multigene families, as was illustrated in plant research due to plant genomes complexity (37). This technology offers a high sensitivity and does guarantee a specific detection, which is particularly well adapted to relatively low expressed genes sharing a high percentage of sequence identity within the family. Despite these advantages, IMA3 and IMA4 transcripts could not be analyzed individually because they present 100% identity. The MALx2 primers were chosen to detect both MAL12/YGR292w as well as MAL32/YBR299w transcripts. Because all RT-PCR reactions were performed with equal quantity of total RNA, we could assume that equal cycle thresholds (Ct) corresponded to equal transcripts number. Using RNA extracted from the wild-type CEN.PK113–7D strain cultivated in YN glycerol-lactate-ethanol medium as a permissive, non-repressible culture condition, the average Ct values spanned from 26 for IMA1 to 28.5 for the remaining IMAx, as compared with 22 for MALx2. This result indicated 10- to 100-fold lower expression levels of the IMAx as compared with MALx2 in this control growth condition.
The effects of various carbon sources on transcripts levels of each of the IMAx genes were then analyzed 1 h after the addition of sugar to exponentially growing cells on YN glycerol-lactate-ethanol medium. As is shown in Table 4, the IMA1 gene showed a remarkable induction in response to the addition of maltose, isomaltose, and αMG. IMA5 also responded to the addition of these three different sugars, but the extent of the induction was weaker than for IMA1. It is also worth noting that IMA5 was induced by αMG, although the product of this gene was not active on this substrate (see Fig. 2). In contrast, IMA2, and the pool of IMA3 and IMA4 transcripts were almost insensitive to the addition of these sugars. Interestingly, the MALx2 transcripts, used as positive controls, accumulated to a lesser extent than IMA1 transcripts after maltose addition and were also weakly responsive to the addition of isomaltose and αMG. As it could be expected, the deletion of the MAL2–8c gene in the CEN.PK strain completely abolished the induction of IMA1 and IMA5 genes by these sugars (Table 4).
TABLE 4.
IMAx gene expression profiles in response to various carbon sources by RT-qPCR
Yeast strains were grown in YN medium containing 3% glycerol, 2% lactate, and 0.5% ethanol. Sugar (2% final concentration of maltose, αMG, isomaltose, galactose, or glucose) was then added to the culture medium when yeast cells reached the exponential phase (A600 nm ∼ 1.0), and samples were collected after 1 h in the presence of the sugar. RNA levels were quantified by RT-qPCR as described under “Experimental Procedures.” Cultures were duplicated, and each of the RNA samples were then analyzed with two independent reverse transcription reactions. Four qPCR technical duplicates were therefore used for the calculation of expression data and their standard deviation. For each gene, the exponential phase sample before the addition of sugar (T0 control) was used as a calibrator sample (normalized fold expression set to 1). The MALx2 transcripts (both MAL12 and MAL32) were used as positive control for sugar induction or repression experiments. The transcripts of FBP1, which encodes fructose-1,6-bisphosphase, were used as an another positive control gene for glucose repression. Upper part: wild type CEN.PK113–7D strain; Lower part: mal2–8c null mutant.
| MALx2 | IMA1 | IMA2 | IMA3*a | IMA5 | FBP1 | |
|---|---|---|---|---|---|---|
| Wild type | ||||||
| Control | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Maltose | 19.3 ± 4.6 | 55.0 ± 4.4 | 0.5 ± 0.2 | 1.4 ± 0.3 | 12.2 ± 3.2 | NDb |
| αMG | 5.7 ± 3.1 | 32.3 ± 4.1 | 1.2 ± 0.4 | 1.8 ± 0.2 | 29.7 ± 6.2 | ND |
| Isomaltose | 3.7 ± 1.9 | 36.8 ± 3.2 | 1.0 ± 0.3 | 1.4 ± 0.3 | 19.5 ± 1.0 | ND |
| Galactose | 0.4 ± 0.2 | 1.1 ± 0.3 | 0.9 ± 0.4 | 1.3 ± 0.5 | 1.1 ± 0.3 | 0.21 |
| Glucose | 0.03 ± 0 | 0.66 ± 0.1 | 0.31 ± 0.1 | 0.47 ± 0.3 | 1.26 ± 0.3 | 0.09 |
| mal2–8c null mutant | ||||||
| Maltose | 1.0 | 1.4 | 0.5 | 1.1 | 1.5 | ND |
| αMG | 1.1 | 1.2 | 0.7 | 0.9 | 1.6 | ND |
| Isomaltose | 1.5 | 0.9 | 0.6 | 0.6 | 1.2 | ND |
a The star (★) represents the pool of IMA3 and IMA4 transcripts.
b ND, not determined.
The expression levels of IMAx genes were also measured after glucose and galactose addition. The MALx2 and FBP1 genes (fructose-1,6-bisphosphatase (38)) were used as positive controls for sugar repression (Table 4). Although these two genes were repressed ∼2.5- and 5-fold by galactose, respectively, the IMAx genes were almost insensitive to the addition of this sugar. Glucose exerted a potent repression on MALx2 and FBP1 (∼30- and 10-fold, respectively), but it moderately repressed IMA2 and IMA3 or IMA4 (3- and 2-fold, respectively) and was inactive on IMA1 and IMA5.
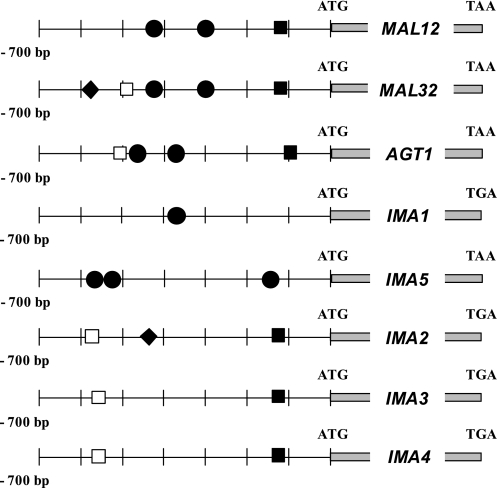
These transcriptional patterns prompted us to have a look at the promoter sequences of these genes. These sequences were limited to 700 bp upstream of the ATG and were searched for putative binding sites of transcriptional regulators using the YEASTRACT database (available on-line) (39). Two of the genes, namely IMA1 and IMA5, exhibit, respectively, one and three MALx3 binding motifs (Fig. 5), which is in agreement with their MAL-dependent transcriptional induction in response to maltose and isomaltose addition (see Table 4). Only IMA2 harbors a consensus motif for the transcriptional repressor Mig1p (40), which should explain the moderate glucose repression that was observed for this gene. No such consensus could be identified in the promoter sequence of IMA3 and IMA4, which makes possible that not-identified consensus variants of Mig1p binding site allow glucose repression. Alternatively, a MIG1-independent glucose regulation may apply to these genes and explain their modest glucose repression, as was already described for the GAL and MAL systems (41, 42). Finally, three of the genes (IMA2, IMA3, and IMA4) harbor an STRE motif for the binding of Msn2/Msn4 proteins (43) together with a TATA box at −125 before the start codon, whose physiological relevance is discussed below.
FIGURE 5.
Putative binding sites of transcriptional regulators. The 700-bp sequences upstream of the start codon of MAL12, MAL32, and AGT1 and the five IMAx genes were analyzed for consensus binding motifs using the YEASTRACT database (available on-line) and using the fuzznuc program for TATA box patterns searching (Mobyle database, available on-line). TATA Box: TATAA/TAA/TA/G (■) (60); Msn2/4p binding site (STRE consensus): CCCCT (□) (43); Mal63p binding site: MGCN{9}MGS (●) (67); Mig1p binding site: ATTTTTGTGGGG (among 12 published consensus) (◆) (68).
DISCUSSION
After the release of the complete S. cerevisiae genome in 1996 (44), there were still in 2007 over 1000 uncharacterized yeast genes (45). For years, the fermentation of α-methylglucopyranoside or isomaltose has been genetically attributed to several unlinked MGLx genes (15–17). Here, we provide evidences that these MGLx genes correspond to a new multigene family that is composed of five members, YGR287c, YIL172c, YJL216c, YJL221c/FSP2, and YOL157c. They are all located in the subtelomeric regions of different chromosomes, as is the case for the SUCx, MALx, and MELx gene families involved in the assimilation of alternative carbon sources (46). As was particularly well illustrated for the large PAUx (47), DUPx (48), or HXTx (5) multigene families, this chromosomal localization clearly provides an adaptive advantage to the cells in terms of easier functional diversification through faster gene duplications and specialization (2, 49–51). Relative to these five genes from S. cerevisiae, only one ORF, YGR287c, was previously reported to encode for a protein with α-1,6-glucosidase activity (1). The other four were only assigned to putative α-glucosidases in the SGD. Although these five genes present very high pairwise identity scores within the family, they share less than 75% sequence identity with MALx2 genes. This divergence was further verified in this work at the functional level: biochemical data indeed showed that these genes code for α-glucosidases, which preferentially act on isomaltose. We therefore renamed these genes IMA1–IMA5 for IsoMAltase genes.
The first clues toward understanding the structure-function relationship of α-glucosidases were recently provided by the group of Tabata (1). While studying YGR287c, these authors demonstrated that Val-216 from the consensus region II, which is one of the four regions conserved in GH13 family proteins, is an important residue discriminating the α-1,4- and 1,6-glucosidic linkages of substrates. This result suggested that this new protein family from S. cerevisiae exhibits an isomaltase activity. This hypothesis was validated in this work through constitutive expression of each of the five IMAx open reading frames, but the significant differences of activity between the five proteins pointed out large efficacy disparities in hydrolyzing the α-1,6-glucoside substrates. Under the standard conditions of the in vitro assay, we nevertheless observed a cross-reactivity on α-1,4-linkages for some of these proteins. This functional diversity may therefore rely on some of the few amino acid residues that differentiate these highly similar proteins. The consensus III sequence is the only one that shows some variability between the five Imax proteins. Whether this consensus brings differences in hydrolysis efficiencies and substrate specificities of the proteins awaits further work.
The fact that overexpression of any one of the five IMAx genes restored growth on isomaltose in the ima1Δ mutant, further provided functional evidence that these five genes encode isomaltase. Our results nevertheless allowed understanding of the absence of functional redundancy within this new gene family for growth on isomaltose, which was illustrated by the fact that the single deletion of IMA1 totally abolished growth on this carbon source, as well as on αMG. Because these α-glucosidase enzymes are likely to be intracellular like maltase, in contrast to other extracellular systems such as the invertase (52) or the Ath1p trehalase (53), transport systems are required for α-glucoside uptake. Han and co-workers reported that the AGT1 gene encodes an α-glucoside permease with broad substrate specificity, including isomaltose and αMG (18). We confirmed in this work that Agt1p is essential for growth on isomaltose. This implied that the two other α-glucoside transporters also present in the CEN.PK strain, MPH2 and MPH3 (33), are probably not expressed. This expression defect may be due to the impossibility to bind the Mal23p transcriptional activator to their promoters (see below), even if these genes have been shown to allow functional complementation when expressed in the W303 strain transformed with a plasmid expressing the Mal63p transcriptional activator (32). Altogether, our results showed that the isomaltose assimilation pathway is dependent on the coupling between the Agt1p active transporter and the major α-1,6-glucosidase protein encoded by IMA1.
As for the maltose assimilation pathway, we found that isomaltose assimilation is under the control of the Malx3p transcriptional activator, which is constitutively expressed from the MAL2–8c allele of MAL23 in the CEN.PK strain. This regulatory protein was indeed necessary to induce the transcription of the two essential genes of the pathway, AGT1 (32) and IMA1 (this study). We nevertheless observed a wide heterogeneity in the transcriptional regulatory patterns of the different members of this new gene family, thus highlighting another level of polymorphism complexity in multigene families. With glucose repression, the only marked, albeit moderate response as compared with MALx genes, was observed for IMA2, which is the only gene harboring a Mig1p-binding site in its promoter. Glucose also acts at the post-translational level by decreasing the activity and stability of enzymes and transporters, including maltose permease, leading to their inactivation and/or degradation (54–58). However, preliminary results indicated that, like for maltase (56), yeast isomaltase is not the target of catabolite inactivation, because no significant drop of activity was observed after the addition of glucose to yeast cultivated on αMG (not shown). Similarly to glucose repression, transcriptional activation in response to the addition isomaltose (or αMG) could not be generalized to all the members of this gene family. Three of these IMAx genes (IMA2, IMA3, and IMA4) were insensitive to maltose, probably because of the lack of the Malx3p-binding motif in their promoter. Curiously, however, these three genes contain one copy of the Msn2/4p binding site (CCCCT, or STRE motif) together with a well identified TATA box, which were not found in the two maltose-responsive genes IMA1 and IMA5. Only 20% of the yeast genes contain a TATA box, and these TATA box-containing genes are more prevalent in the subtelomeric regions than elsewhere in the genome (59, 60), are highly regulated, and are generally associated with responses to stress. Wapinski and co-workers showed that duplicated genes diversify most frequently at the level of regulation and rarely diverge with respect to biochemical function (61). Altogether, our results supported this view in showing that divergence of nucleotide sequences in the IMAx gene family, both in coding and 5′-uncoding regions, led to interesting variability of expression even if all proteins exhibited isomaltase activity.
However, the function of isomaltase still remains enigmatic, especially when expressed under stress conditions, but it might be important under conditions that are not common in laboratory assays. Starch is the major carbohydrate in dough and its partial hydrolysis during malting generates mainly maltose but also a significant amount of isomaltose and a mixture of dextrin, large fragments made up of glucose molecules joined by α-1,4- and α-1,6- linkages (62, 63). Therefore, this α-1,6-glucosidase activity could be an asset in brewing fermentation for S. cerevisiae strains in agreement with the fact that some yeast strains can assimilate isomaltose from the wort. From a phylogeny viewpoint, we also searched for IMAx orthologs in the genomes of closely related Saccharomyces and in several yeast species, which diverged from Saccharomyces prior to the whole genome duplication. We could not find any orthologs by using the yeast gene order browser (64, 65), probably because the dynamic turnover of these subtelomeric regions breaks down the synteny. However, by using the fungal sequence alignment in SGD, homologous sequences were found in Saccharomyces paradoxus, S. mikatae, S. bayanus, and S. kudriavzevii. No homologous sequence of the IMAx genes was found in S. castellii, the more distantly related species existing after the whole genome duplication. While searching for homologous genes in the genomes of ancient yeast species that existed before the whole genome duplication (Génolevures-3 consortium, available on-line), we identified 21 genes constituting the GL3C0220 family that includes the 2 MALx and 5 IMAx from S. cerevisiae. This family also contains 5 genes from Saccharomyces kluyveri, 4 from Kluyveromyces thermotolerans, 3 from Kluyveromyces lactis, and 2 from Debaryomyces hansenii. A sound phylogenetic analysis of three gene families linked with maltose metabolism was also very recently reported by Brown and co-workers (2). These authors concluded that the evolutionary rate at which these changes have taken place is exceptional, with wide differences in copy number within different species and even strains. Very interestingly, we found that the Val residue in consensus II, attributed to isomaltase activity, was also present in KLTH0B00308p from K. thermotolerans, and in SAKL0A00154p and SAKL0C00176p from S. kluyveri, raising the possibility of isomaltase expression in these ancient yeasts. Their presence in all these species and polymorphism indicate that these genes may have evolved to give yeast cells advantages in adapting to novel niches (2, 51). Growth screening on isomaltose to S. cerevisiae and S. paradoxus yeast collections (Saccharomyces Genome Resequencing Project), will probably be relevant to explore the natural gene diversity and obtain an accurate view of the overall function of this gene family.
Acknowledgments
We thank Manon Duquenne for technical assistance in cloning and Philippe V. Baret for fruitful discussions on gene families analysis in hemiascomycetous yeast species. We particularly acknowledge Christine Rettew (Drexel University) for critical reading and copyediting of the manuscript.
Addendum
While this work was under the revision process and submitted to SGD for Gene Name Reservation, the SGD curators warned us about an in-press publication from another group in which this set of genes was also named IMA. To avoid a very confusing situation, we agreed to change the numbering system in this report to correspond to the nomenclature found in Naumoff's work (69).
This work was supported by ANR Blanc 05-2-42128 and Genopole Toulouse (to J. M. F.).
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-BankTM/EBI Data Bank with accession number(s) HM74861–HM74865.
DNA sequences of the IMAx genes, which were amplified from the CEN.PK113-7D strain, can be retrieved from the GenBank database with accession numbers HM748761 for IMA1, HM748762 for IMA2, HM748764 for IMA4 and HM748765 for IMA5. The CEN.PK MAL12 gene can be retrieved from accession number HM748763.
- αMG
- α-methylglucopyranoside or methyl α-d-glucopyranoside
- SGD
- Saccharomyces Genome Database
- GH13
- glycoside hydrolase 13 family
- id. to
- identical to.
REFERENCES
- 1.Yamamoto K., Nakayama A., Yamamoto Y., Tabata S. (2004) Eur. J. Biochem. 271, 3414–3420 [DOI] [PubMed] [Google Scholar]
- 2.Brown C. A., Murray A. W., Verstrepen K. J. (2010) Curr. Biol. 20, 895–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blandin G., Durrens P., Tekaia F., Aigle M., Bolotin-Fukuhara M., Bon E., Casarégola S., de Montigny J., Gaillardin C., Lépingle A., Llorente B., Malpertuy A., Neuvéglise C., Ozier-Kalogeropoulos O., Perrin A., Potier S., Souciet J., Talla E., Toffano-Nioche C., Wésolowski-Louvel M., Marck C., Dujon B. (2000) FEBS Lett. 487, 31–36 [DOI] [PubMed] [Google Scholar]
- 4.Leandro M. J., Fonseca C., Gonçalves P. (2009) FEMS Yeast Res. 9, 511–525 [DOI] [PubMed] [Google Scholar]
- 5.Ozcan S., Johnston M. (1999) Microbiol. Mol. Biol. Rev. 63, 554–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greatrix B. W., van Vuuren H. J. (2006) Curr. Genet. 49, 205–217 [DOI] [PubMed] [Google Scholar]
- 7.Rintala E., Wiebe M. G., Tamminen A., Ruohonen L., Penttilä M. (2008) BMC Microbiol. 8, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamaki H. (1978) Mol. Gen. Genet. 164, 205–209 [Google Scholar]
- 9.Carlson M., Botstein D. (1983) Mol. Cell Biol. 3, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Needleman R. (1991) Mol. Microbiol. 5, 2079–2084 [DOI] [PubMed] [Google Scholar]
- 11.Cheng Q., Michels C. A. (1991) J. Bacteriol. 173, 1817–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin R. A., Needleman R. B., Gossett D., Michels C. A. (1985) J. Bacteriol. 164, 605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y. S., Dubin R. A., Perkins E., Forrest D., Michels C. A., Needleman R. B. (1988) Curr. Genet. 14, 201–209 [DOI] [PubMed] [Google Scholar]
- 14.Charron M. J., Read E., Haut S. R., Michels C. A. (1989) Genetics 122, 307–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawthorne D. C. (1958) Heredity 12, 273–283 [Google Scholar]
- 16.ten Berge A. M. (1972) Mol. Gen. Genet. 115, 80–88 [DOI] [PubMed] [Google Scholar]
- 17.Naumov G. I., Bashkrova E. V. (1985) Dokl. Akad. Nauk SSSR 279, 1496–1499 [Google Scholar]
- 18.Han E. K., Cotty F., Sottas C., Jiang H., Michels C. A. (1995) Mol. Microbiol. 17, 1093–1107 [DOI] [PubMed] [Google Scholar]
- 19.Hutson D. H., Manners D. J. (1965) Biochem. J. 94, 783–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan N. A., Eaton N. R. (1967) Biochim. Biophys. Acta 146, 173–180 [DOI] [PubMed] [Google Scholar]
- 21.Khan N. A., Haynes R. H. (1972) Mol. Gen. Genet. 118, 279–285 [DOI] [PubMed] [Google Scholar]
- 22.van Dijken J. P., Bauer J., Brambilla L., Duboc P., Francois J. M., Gancedo C., Giuseppin M. L., Heijnen J. J., Hoare M., Lange H. C., Madden E. A., Niederberger P., Nielsen J., Parrou J. L., Petit T., Porro D., Reuss M., van Riel N., Rizzi M., Steensma H. Y., Verrips C. T., Vindeløv J., Pronk J. T. (2000) Enzyme Microb. Technol. 26, 706–714 [DOI] [PubMed] [Google Scholar]
- 23.Gietz R. D., Schiestl R. H. (2007) Nat. Protoc. 2, 31–34 [DOI] [PubMed] [Google Scholar]
- 24.Wach A., Brachat A., Pöhlmann R., Philippsen P. (1994) Yeast 10, 1793–1808 [DOI] [PubMed] [Google Scholar]
- 25.Brunelli J. P., Pall M. L. (1993) Yeast 9, 1299–1308 [DOI] [PubMed] [Google Scholar]
- 26.Gietz R. D., Sugino A. (1988) Gene 74, 527–534 [DOI] [PubMed] [Google Scholar]
- 27.Teste M. A., Duquenne M., François J. M., Parrou J. L. (2009) BMC Mol. Biol. 10, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boles E., Hollenberg C. P. (1997) FEMS Microbiol. Rev. 21, 85–111 [DOI] [PubMed] [Google Scholar]
- 29.Backhus L. E., DeRisi J., Bisson L. F. (2001) FEMS Yeast Res. 1, 111–125 [DOI] [PubMed] [Google Scholar]
- 30.Svensson B. (1994) Plant Mol. Biol. 25, 141–157 [DOI] [PubMed] [Google Scholar]
- 31.McCarter J. D., Withers S. G. (1996) J. Biol. Chem. 271, 6889–6894 [DOI] [PubMed] [Google Scholar]
- 32.Day R. E., Higgins V. J., Rogers P. J., Dawes I. W. (2002) Yeast 19, 1015–1027 [DOI] [PubMed] [Google Scholar]
- 33.Vidgren V., Ruohonen L., Londesborough J. (2005) Appl. Environ. Microbiol. 71, 7846–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson A. W., Wojciechowicz L. A., Danzi S. E., Zhang B., Kim J. H., Hu Z., Michels C. A. (1997) Genetics 146, 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu Z., Gibson A. W., Kim J. H., Wojciechowicz L. A., Zhang B., Michels C. A. (1999) Curr. Genet. 36, 1–12 [DOI] [PubMed] [Google Scholar]
- 36.Hu Z., Yue Y., Jiang H., Zhang B., Sherwood P. W., Michels C. A. (2000) Genetics 154, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gachon C., Mingam A., Charrier B. (2004) J. Exp. Bot. 55, 1445–1454 [DOI] [PubMed] [Google Scholar]
- 38.Zaragoza O., Vincent O., Gancedo J. M. (2001) Biochem. J. 359, 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teixeira M. C., Monteiro P., Jain P., Tenreiro S., Fernandes A. R., Mira N. P., Alenquer M., Freitas A. T., Oliveira A. L., Sa-Correia I. (2006) Nucleic Acids Res. 34, D446–D451, database issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nehlin J. O., Ronne H. (1990) EMBO J. 9, 2891–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alberti A., Lodi T., Ferrero I., Donnini C. (2003) Yeast 20, 1085–1096 [DOI] [PubMed] [Google Scholar]
- 42.Hu Z., Nehlin J. O., Ronne H., Michels C. A. (1995) Curr. Genet. 28, 258–266 [DOI] [PubMed] [Google Scholar]
- 43.Martínez-Pastor M. T., Marchler G., Schüller C., Marchler-Bauer A., Ruis H., Estruch F. (1996) EMBO J. 15, 2227–2235 [PMC free article] [PubMed] [Google Scholar]
- 44.Goffeau A., Barrell B. G., Bussey H., Davis R. W., Dujon B., Feldmann H., Galibert F., Hoheisel J. D., Jacq C., Johnston M., Louis E. J., Mewes H. W., Murakami Y., Philippsen P., Tettelin H., Oliver S. G. (1996) Science 274, 563–567 [DOI] [PubMed] [Google Scholar]
- 45.Peña-Castillo L., Hughes T. R. (2007) Genetics 176, 7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pryde F. E., Gorham H. C., Louis E. J. (1997) Curr. Opin. Genet. Dev. 7, 822–828 [DOI] [PubMed] [Google Scholar]
- 47.Luo Z., van Vuuren H. J. (2009) Microbiology 155, 4036–4049 [DOI] [PubMed] [Google Scholar]
- 48.Despons L., Wirth B., Louis V. L., Potier S., Souciet J. L. (2006) Trends Genet. 22, 10–15 [DOI] [PubMed] [Google Scholar]
- 49.Brown C. J., Todd K. M., Rosenzweig R. F. (1998) Mol. Biol. Evol. 15, 931–942 [DOI] [PubMed] [Google Scholar]
- 50.Codón A. C., Benítez T., Korhola M. (1998) Appl. Microbiol. Biotechnol. 49, 154–163 [DOI] [PubMed] [Google Scholar]
- 51.Fabre E., Muller H., Therizols P., Lafontaine I., Dujon B., Fairhead C. (2005) Mol. Biol. Evol. 22, 856–873 [DOI] [PubMed] [Google Scholar]
- 52.Carlson M., Botstein D. (1982) Cell 28, 145–154 [DOI] [PubMed] [Google Scholar]
- 53.He S., Bystricky K., Leon S., François J. M., Parrou J. L. (2009) FEBS J. 276, 5432–5446 [DOI] [PubMed] [Google Scholar]
- 54.Holzer H. (1989) Revis. Biol. Cell. 21, 305–319 [PubMed] [Google Scholar]
- 55.Medintz I., Jiang H., Han E. K., Cui W., Michels C. A. (1996) J. Bacteriol. 178, 2245–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brondijk T. H., van der Rest M. E., Pluim D., de Vries Y., Stingl K., Poolman B., Konings W. N. (1998) J. Biol. Chem. 273, 15352–15357 [DOI] [PubMed] [Google Scholar]
- 57.Jiang H., Medintz I., Zhang B., Michels C. A. (2000) J. Bacteriol. 182, 647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucero P., Moreno E., Lagunas R. (2002) FEMS Yeast Res. 1, 307–314 [DOI] [PubMed] [Google Scholar]
- 59.Kellis M., Patterson N., Endrizzi M., Birren B., Lander E. S. (2003) Nature 423, 241–254 [DOI] [PubMed] [Google Scholar]
- 60.Basehoar A. D., Zanton S. J., Pugh B. F. (2004) Cell 116, 699–709 [DOI] [PubMed] [Google Scholar]
- 61.Wapinski I., Pfeffer A., Friedman N., Regev A. (2007) Nature 449, 54–61 [DOI] [PubMed] [Google Scholar]
- 62.Dale C. J. (1991) J. Inst. Brew. 97, 187–195 [Google Scholar]
- 63.Allosio-Ouarnier N., Quemener B., Bertrand D., Boivin P. (2000) J. Inst. Brew. 106, 45–52 [Google Scholar]
- 64.Byrne K. P., Wolfe K. H. (2006) Nucleic Acids Res. 34, D452–D455, database issue [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gordon J. L., Byrne K. P., Wolfe K. H. (2009) PLoS Genet. 5, e1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jules M., Guillou V., François J., Parrou J. L. (2004) Appl. Environ. Microbiol. 70, 2771–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sirenko O. I., Ni B., Needleman R. B. (1995) Curr. Genet. 27, 509–516 [DOI] [PubMed] [Google Scholar]
- 68.Lundin M., Nehlin J. O., Ronne H. (1994) Mol. Cell Biol. 14, 1979–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naumoff D. G., Naumov G. I. (2010) Doklady Biochem. Biophys. 432, 114–116 [DOI] [PubMed] [Google Scholar]