Abstract
Patients with diabetes suffer disproportionately from impaired lipid metabolism and cardiovascular disease, but the relevant roles of insulin resistance and hyperglycemia in these processes are unclear. Transcription factor FoxO1 is regulated dually by insulin and nutrients. In this study, we addressed the hypothesis that, in addition to its established role to regulate hepatic glucose production, FoxO1 controls aspects of lipid metabolism in the diabetic liver. Mice with a liver-specific deletion of FoxO1 (l-FoxO1) and their control littermates were rendered hyperglycemic by streptozotocin administration. Subsequently, we monitored serum lipids, liver VLDL secretion, and hepatic expression of genes related to lipid metabolism. Hepatic FoxO1 ablation resulted in increased VLDL secretion, increased cholesterol, and increased plasma free fatty acids, three hallmarks of the diabetic state. l-FoxO1 mice expressed increased levels of SREBP-2 and FGF21 without affecting lipogenic genes. We propose that FoxO1 fine tunes lipolysis through its actions on FGF21 and that hepatic FoxO1 ablation increases availability of substrates for hepatic triglyceride and cholesterol synthesis and VLDL secretion. The implications of these findings are that FoxO1 protects against excessive hepatic lipid production during hyperglycemia and that its inhibition by intensive insulin treatment may exacerbate paradoxically the lipid abnormalities of diabetes.
Keywords: Fatty Acid Metabolism, Glucose Metabolism, Insulin Resistance, Lipoprotein, Transcription Factors
Introduction
Alterations of lipid metabolism are an integral part of the metabolic syndrome and diabetes and can lead to the development of cardiovascular diseases (1). Individuals with type 2 diabetes have a 2–4-fold increase in their lifetime risk of developing cardiovascular diseases, and heart disease is the leading cause of death among diabetics (2). The primary abnormalities of lipid metabolism in diabetes are increased free fatty acid (FFA) levels, and increased plasma triglyceride (TG)2 and cholesterol in atherogenic lipoprotein fractions, due in part to excessive secretion of very low density lipoproteins (VLDL) by the liver (3). However, it is unclear how insulin resistance and diabetes affect the development of these defects. Two mutually nonexclusive causes have been proposed: insulin resistance and hyperglycemia. Delineating the relative roles of these two states has been difficult because they typically coexist, although increased TG and VLDL can be observed in insulin-resistant humans long before the development of hyperglycemia (4). Furthermore, most prospective studies indicate that tight glucose control has little benefit, or possible untoward effects, on macrovascular complications of diabetes (5). Thus, insulin resistance appears to have an independent effect on the development of the lipid abnormalities that lead to cardiovascular disease.
An early step in the development of atherosclerosis is increased production of hepatic TG and low density lipoproteins (namely, VLDL) (6). But the mechanism by which insulin resistance affects hepatic lipid metabolism remains elusive. In recent years, a consensus has emerged, indicating that insulin regulates hepatic lipid synthesis through transcription factor SREBP-1c, and glucose production through transcription factor FoxO1 (7, 8). The forkhead protein FoxO1 is regulated by both insulin (through phosphorylation-dependent inactivation) and high glucose (through deacetylation-dependent activation) to promote or repress gene expression, providing a metabolic sensor linking the nutritional status of the cell to regulation of gene expression (9). Accordingly, liver-specific FoxO1 knock-out mice (l-FoxO1) show increased glucose disposal and reduced hepatic glucose production during hyperinsulinemic/euglycemic clamps and hypoglycemia following a prolonged fast (10).
The role of FoxO1 in lipid metabolism has been less clear. Gain-of-function experiments have yielded conflicting results (11–13) that may be accounted for partly by differences in experimental design and indirect effects of the FoxO1 manipulations on systemic insulin and glucose levels. Hepatic loss-of-function experiments have been notable for the lack of effects on lipid metabolism genes (10, 14). We have shown previously that hyperglycemia-induced oxidative stress leads to FoxO1 activation by targeting it to nuclear PML bodies by way of an acetylation-dependent mechanism (15). Thus, we reasoned that the effect of FoxO1 in lipid metabolism might only become apparent in hyperglycemic conditions. To test this hypothesis, we used streptozotocin (STZ) to induce diabetes in liver-specific FoxO1 knock-out (l-FoxO1) and control mice, generating a model of diabetes where both glucose and insulin levels were similar between the two genotypes, minimizing these as potential confounders. Unexpectedly, we found that hyperglycemic l-FoxO1 animals have high serum TG, cholesterol, and FFA and increased VLDL secretion from the liver. We provide a mechanism suggesting that the effect of FoxO1 ablation is due to increased FGF21 secretion from the liver, acting in turn to promote elevation of FFAs in vivo (16).
EXPERIMENTAL PROCEDURES
Mice
l-FoxO1 mice were described previously (10) and were fed a standard chow diet. Male mice, with an average age of 14–20 weeks, were injected intraperitoneally with streptozotocin (Sigma-Aldrich) at a dose of 225 mg/kg or a similar volume of 0.9% saline. The Columbia University Institutional Animal Care and Use Committee approved all experiments.
Serum and Liver Lipid Analysis
Colorimetric assay kits included TG (Thermo Scientific, Waltham, MA), β-hydroxybutyrate (Stanbio, Boerne, TX), cholesterol E, and nonesterified fatty acids (NEFAs; Wako, Richmond, VA). The OneTouch glucose monitor and strips were from LifeScan (Milpitas, CA). “HI” glucose measurements were read in 7 of 38 mice ad libitum fed and 1 of 32 overnight fasted STZ-treated control mice as well as 2 of 57 ad libitum fed and 5 of 10 refed STZ-treated l-FoxO1 mice; these were treated as 600 mg/dl. Serum glucagon was measured at the St. Luke's Hormone and Metabolite Core by radioimmunoassay (Millipore, Billerica, MA). For fasting serum lipids, measurements from untreated and saline-treated mice were combined, as there were no differences due to saline. Liver lipid content (n = 5–7) was measured by Folch extraction (17) and confirmed by both Oil Red O staining of snap-frozen liver sections and saponification in ethanolic KOH as described previously (18). Serum FGF21 was measured by ELISA (Millipore).
Serum Lipoprotein Analysis and TG Production
For FPLC analysis, sera from three mice per genotype were pooled after a 5-h fast and separated as described (19). TG production was measured by intravenous injection of Triton WR 1339 (Tyloxapol, Sigma) at 500 mg/kg followed by serum collection at 1 and 2 h post-injection. Post-Triton serum samples were separated by density ultracentrifugation as described (19). Fractions were separated by SDS-PAGE and stained with Coomassie Blue for apoB visualization. Bands were quantitated using Image J software.
RNA and Gene Expression
Total RNA from liver was isolated using RNeasy (Qiagen, Germantown, MD) and RNA from perigonadal white adipose tissue was isolated using TRIzol (Invitrogen). cDNA was synthesized using Superscript III (Invitrogen), and quantitative PCR was performed in triplicate with 5–9 mice per group using SyBr Green (New England Biolabs) in a Bio-Rad Peltier thermal cycler (96 well). All genes are normalized to 18 S. qPCR primer sequences are available in supplemental Table S2, with the exception of liver pyruvate kinase, which was purchased from SABiosciences (Frederick, MD). Rabbit anti-LDL receptor (LDLR) was a gift from Jay Horton.
Statistical Analysis
All data are presented as mean ± S.E. Data were analyzed by either two-way analysis of variance followed by post hoc Tukey tests, or two-tailed Student's t tests, where appropriate.
RESULTS
Hyperglycemic l-FoxO1 Mice Are Hyperlipidemic
To identify the effects of FoxO1 knock-out on lipid metabolism, we first analyzed serum lipid levels (Fig. 1, A–C) after an overnight fast and a subsequent 4-h refeeding period in untreated mice (n ≥ 19 for fasting and n ≥ 6 for refeeding). In control mice, refeeding caused an increase in serum TG, a decline in cholesterol, and a large reduction in NEFAs. We obtained similar results in l-FoxO1 mice, indicating that FoxO1-ablated animals can respond normally to fasting-feeding regulation of lipid metabolism, similar to what has been observed previously (10). To determine whether hyperglycemia affected this response, we injected WT control and l-FoxO1 littermates with a single high dose of STZ.
FIGURE 1.
Metabolic characteristics of fasting and refeeding in untreated and STZ-treated mice. A–C, serum TG, cholesterol, and NEFA in untreated or STZ-treated mice, fasted overnight (n = 16–44) or fasted overnight then refed for 4 h (n = 5–10). D, blood glucose levels 1 week after STZ injection (n = 5–57 per group). E–F, hepatic TG and cholesterol in untreated or STZ-treated mice, fasted overnight or fasted overnight then refed for 4 h (n = 5–7). For blood glucose, *, p < 0.05 by two-tailed Student's t test. For all other analyses, *, p < 0.05 and **, p < 0.01 by two-way analysis of variance.
One week after STZ injection, both control and l-FoxO1 mice exhibited insulin levels below the limit of detection and few, if any, insulin-positive islet cells (data not shown). There were no differences in fasting serum glucagon levels between control and l-FoxO1 animals (75.5 ± 9.7 pg/ml versus 74.9 ± 10.3 pg/ml, respectively). As expected, STZ-treated animals from both genotypes were found to be hyperglycemic in fed and fasted states (Fig. 1D). Interestingly, l-FoxO1 mice showed lower glucose levels after a 5-h fast, consistent with the established role of FoxO1 in promoting glucose production (10).
STZ-treated control and l-FoxO1 mice were fasted overnight and refed for 4 h, and serum lipids were analyzed (Fig. 1, A–C) (n ≥ 16 for fasting and n ≥ 6 for refeeding). In control mice, STZ treatment had little effect on fasted or refed serum TG, but, in l-FoxO1 mice, STZ treatment caused TG to rise to a level 60% higher than control animals during the fasted state (p < 0.01 and p < 0.05 for the interaction of l-FoxO1 and STZ). TG in l-FoxO1 remained 60% higher than control after refeeding. Fasting cholesterol levels rose similarly in both genotypes in response to STZ (p < 0.05). However, after refeeding, serum cholesterol declined in control animals, similar to what we observed in untreated mice, but failed to do so in l-FoxO1 mice, such that levels were 40% higher in l-FoxO1 (p < 0.01). Fasting serum NEFA were increased only marginally due to STZ in control mice but were significantly higher, by 60%, in STZ l-FoxO1 mice compared with controls (p < 0.01 and p < 0.01 for the interaction of l-FoxO1 and STZ). Refed NEFAs were significantly elevated in STZ-treated mice of both genotypes (p < 0.01) but were still significantly higher in l-FoxO1 compared with control (p < 0.01). The increases in serum lipids were not associated with lipid accumulation in the liver, as STZ treatment greatly reduced liver TG content in both genotypes, as reported previously (20) and had only a modest effect on liver cholesterol, which also was similar between the two genotypes (Fig. 1, E–F).
Overall, STZ-treated mice of both genotypes were able to initiate lipid metabolic responses to refeeding, with the exception of the serum cholesterol in l-FoxO1 mice, which did not decline as it did in the other three groups. These responses should be regulated by both glucose and the trace amounts of insulin remaining after STZ. During fasting, the differences between control and l-FoxO1 mice are magnified, consistent with the expectation that in the fasted state hyperglycemic, hypoinsulinemic control mice have active nuclear FoxO1 in hepatocytes, whereas l-FoxO1 mice do not.
Increased VLDL Secretion in STZ-treated l-FoxO1 Mice
To investigate the difference in fasting TG between hyperglycemic control and l-FoxO1 mice, we performed a less prolonged 5-h fast on STZ-treated mice and subjected pooled serum samples to fast protein liquid chromatography (FPLC) to analyze lipoprotein distribution. As shown in Fig. 2A, the VLDL fraction in l-FoxO1 animals contained a nearly 2-fold increase of TG compared with control animals. Thus, the difference in total serum TG can be primarily accounted for by the VLDL fraction. Cholesterol levels in the 5-h fasted state were similar between knock-out and control mice (Fig. 2B). Serum FFAs in the 5-h fasted state were increased in l-FoxO1 compared with control (0.89±.04 meq/l versus 0.73±.04 meq/l, respectively; p < 0.05).
FIGURE 2.
Serum lipoprotein analysis. A–B, shown are TG and cholesterol in FPLC-separated lipoprotein fractions from 5-h fasted STZ-treated control and l-FoxO1 mice. Sera were pooled from three mice per genotype. C, shown is the serum TG accumulation after Triton WR 1339 injection (n = 6–9 per group). Shown are TG (D) and cholesterol (E) from the VLDL fraction 2 h after Triton injection. F, VLDL and LDL ApoB proteins were analyzed by SDS-PAGE followed by Coomassie blue staining (n = 6 per group). G, shown is the quantitation of ApoB48 and ApoB100 (n = 6 per group). *, p < 0.05 and **, p < 0.01 by two-tailed Student's t test.
We wanted to determine whether the increase in TG levels was due to increased production or decreased uptake by the liver. To this end, we used tail-vein injection of Triton WR 1339 to block lipoprotein clearance from the circulation, and measured accumulation of serum TG and apolipoprotein B (apoB), the apoprotein required for hepatic VLDL assembly. Using similar methods, several previous studies have found that STZ treatment alone causes minimal changes in TG and apoB production (21–23). We found that hyperglycemic l-FoxO1 mice secreted significantly more TG than hyperglycemic control animals (Fig. 2C). We separated individual serum samples from the final (2-h) time point after Triton WR 1339 injection using density ultracentrifugation into VLDL, LDL, and HDL fractions. We found higher TG, cholesterol, apoB100 and apoB48 levels in post-Triton serum VLDL fractions from l-FoxO1 mice, compared with controls (Fig. 2, D–G). These data indicate that hypertriglyceridemia in l-FoxO1 mice is due to increased production of apoB-containing VLDL by the liver.
SREBP-1c, LXR, and ChREBP Pathways Are Not Up-regulated
FoxO1 is capable of exerting both positive and negative transcriptional regulation (24–26). Because the mutation in these hyperlipidemic mice was liver-specific, we hypothesized that the primary cause of the defect in hyperglycemic l-FoxO1 was aberrant expression of lipogenic genes in liver. Thus, we measured mRNA levels of lipogenic genes in hyperglycemic control and l-FoxO1 mice.
The SREBP (sterol regulatory element binding protein)-1c transcription factor regulates lipogenesis by promoting transcription of genes in the TG biosynthetic pathway (27). Although Srebp-1c expression is known to decline dramatically due to STZ treatment (28, 29), we found that Srebp-1c mRNA was 5-fold higher in fasted, hyperglycemic l-FoxO1 mice compared with similarly treated controls (Table 1) but not under basal fasting conditions (supplemental Table S1 and (10)). Increased Srebp-1c mRNA is not necessarily predictive of increased target gene transcription, as SREBP proteins must be activated post-translationally (30); thus, we measured expression of several SREBP-1c target genes (Table 1). Although fatty acid synthase showed a 75% increase in l-FoxO1 mice (p < 0.05), overall SREBP-1c target genes failed to show large or statistically significant increases in expression. Furthermore, transgenic expression of SREBP-1c in liver that increases expression of Acc, fatty acid synthase, and Scd1 more robustly than this model (2–4-fold) fails to increase, and indeed decreases, plasma TG, while markedly increasing hepatic TG accumulation (31). Thus, it did not appear that the level of Srebp-1c we observed could explain the elevation in TG secretion. The apparent discrepancy between elevated Srebp-1c expression and activity was examined by measuring the expression of Insig-1, -2a, and -2b, inhibitors of the SREBP-1c chaperone Scap (SREBP cleavage-activating protein) that is required for transport of SREBP proteins out of the endoplasmic reticulum and into the Golgi for cleavage and activation. Insig proteins normally bind Scap under conditions of high cholesterol, although high Insig expression can lower the threshold for association (32). Three Insig transcripts have been identified: Insig-1 levels are high in fed animals, and its transcription is promoted by SREBPs; Insig-2a levels are high during fasting, and its transcription is repressed by insulin; and Insig-2b transcription is not known to be regulated by fasting and feeding (29). We measured Insig expression in STZ-treated mice and found that in the fasted state all three Insig transcripts were significantly up-regulated in l-FoxO1 mice (Table 1). The large increase in Insig levels is consistent with Scap inhibition and subsequent SREBP-1c endoplasmic reticulum retention, despite elevated mRNA expression. There were no significant differences in Insig-2a expression in untreated l-FoxO1 compared with controls (supplemental Table S1).
TABLE 1.
Expression of SREBP-1c, LXR, and ChREBP pathways in liver
FAS, fatty acid synthase; l-pk, liver pyruvate kinase.
| STZ-treated, fasted |
STZ-treated, refed |
|||
|---|---|---|---|---|
| Control | l-FoxO1 | Control | l-FoxO1 | |
| Srebp-1c | 1 ± 0.14 | 5.16 ± 0.58a | 7.88 ± 2.21 | 12.54 ± 1.23 |
| Acc1 | 1 ± 0.08 | 0.89 ± 0.03 | 0.94 ± 0.04 | 0.98 ± 0.07 |
| Acc2 | 1 ± 0.37 | 0.51 ± 0.06b | 0.95 ± 0.19 | 0.54 ± .06b |
| FAS | 1 ± 0.20 | 1.72 ± 0.16a | 4.21 ± 0.58 | 5.15 ± 0.79 |
| Scd1 | 1 ± 0.39 | 2.90 ± 0.55 | 3.05 ± 1.24 | 2.30 ± 0.71 |
| Acss2 | 1 ± 0.09 | 1.1 ± 0.10 | 1.21 ± 0.13 | 1.46 ± 0.15 |
| Gpam | 1 ± 0.10 | 1.52 ± 0.14 | 2.06 ± 0.31 | 1.84 ± 0.21 |
| Insig-1 | 1 ± 0.26 | 2.82 ± 0.47a | 4.99 ± 1.98 | 7.20 ± 1.05 |
| Insig-2a | 1 ± 0.29 | 3.38 ± 0.53a | 1.53 ± 0.32 | 0.68 ± 0.15b |
| Insig-2b | 1 ± 0.18 | 1.66 ± 0.08a | 1.95 ± 0.31 | 1.72 ± 0.12 |
| LXRα | 1 ± 0.02 | 1.07 ± 0.05 | 1.40 ± 0.17 | 1.61 ± 0.06 |
| Abcg5 | 1 ± 0.22 | 1.36 ± 0.24 | 0.88 ± 0.13 | 0.51 ± 0.02a |
| Abcg8 | 1 ± 0.32 | 1.35 ± 0.26 | 0.77 ± 0.19 | 0.34 ± 0.04b |
| ChREBP | 1 ± 0.15 | 0.98 ± 0.08 | 0.87 ± 0.17 | 0.81 ± 0.12 |
| l-pk | 1 ± 0.15 | 1.47 ± 0.18 | 1.84 ± 0.37 | 1.94 ± 0.16 |
a p < 0.01.
b p < 0.05.
Having excluded a major contribution of the SREBP-1c pathway to hypertriglyceridemia in this model, we examined other pathways. The liver X receptor (LXR) is a sterol-sensing nuclear receptor that can promote lipogenic gene expression by heterodimerization with retinoid X receptor and binding to LXR response elements. Although the induction of lipogenesis in response to LXR is executed largely by SREBP-1c, mice lacking SREBP-1c still can respond to LXR stimulation, leaving room for alternative effectors. To test the contribution of LXR to the observed phenotype, we measured expression of LXRα and its SREBP-independent targets. Neither LXRα nor its liver target genes, the cholesterol transporters Abcg5 and -8, were up-regulated (Table 1), indicating that LXR is not responsible for the lipid phenotype in these mice. Notably, both Abcg5 and -8 were significantly down-regulated in refed l-FoxO1 mice, consistent with their identification as direct FoxO1 targets (33).
A third chief regulator of lipid synthesis is the carbohydrate response element binding protein (ChREBP), which is activated by glucose and also is a target of LXR. However, ChREBP mRNA was not different between l-FoxO1 and control animals (Table 1), and the small increase in liver pyruvate kinase, a ChREBP-specific target gene, did not reach significance. Thus, ChREBP is unlikely to be the cause of the excess lipids in this model.
Dgat1, Dgat2, and apoB are involved in TG synthesis but are not regulated at the gene expression level by the three transcriptional pathways mentioned above (supplemental Table S1). However, overexpression of Dgat1 or Dgat2 in liver, even at high levels, does not increase TG or apoB production (34, 35), and apoB itself is thought to be regulated by post-translational degradative pathways, not apoB mRNA expression (36). The absence of any large changes in SREBP-1c, LXR, or ChREBP pathways suggested the possibility that a different metabolic network was influencing lipid production in hyperglycemic l-FoxO1 mice. Although we expect that these pathways are still functional to mediate the refeeding response in this STZ model, where the FA and TG synthesis genes are up-regulated (Table 1) and serum TG rise (Fig. 1A), we find that differences in expression of these genes fails to explain the effect of FoxO1 ablation on fasting serum TGs or TG production.
SREBP-2 Cholesterol Biosynthetic Pathway Is Up-regulated
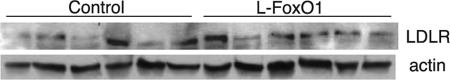
SREBP-2 promotes expression of cholesterol synthetic genes (27) and is potentially able to substitute for SREBP-1c in the absence of the latter (37). We observed a 60% increase in Srebp-2 mRNA in l-FoxO1 in the refed state (p < 0.05) and a similar trend in the fasted state. Consistent with this finding, all tested SREBP-2 target genes were up-regulated in l-FoxO1 mice compared with control mice (Table 2), particularly in the refed state. SREBP-2 is also post-translationally regulated by Scap and Insigs; thus, the considerable decline of Insig-2a in refed l-FoxO1 mice (see Table 1) may contribute to the ability of SREBP-2 to promote expression of its target genes in that condition. Furthermore, the stability of HMG-CoA reductase, the rate-limiting enzyme in cholesterol synthesis, is regulated directly by Insigs (32), such that a down-regulation of Insig-2a in the refed state might lead directly to increased cholesterol production. An important transcriptional target of SREBP-2 is the LDLR. We found no defect in LDLR mRNA expression; if anything, LDLR mRNA was increased slightly (p = 0.065 and 0.054 in fasted and refed states, respectively), similar to other SREBP-2 target genes (Table 2), nor did we find any decrease in LDLR protein levels (Fig. 3). This suggests that defective lipoprotein clearance via LDLR is unlikely to be the cause of serum lipoprotein accumulation. Overall, the up-regulation of cholesterol biosynthetic genes is consistent with the elevated serum cholesterol but is not sufficient to explain the increased TG synthesis.
TABLE 2.
Expression of cholesterol biosynthesis genes in liver
| STZ-treated, fasted |
STZ-treated, refed |
|||
|---|---|---|---|---|
| Control | l-FoxO1 | Control | l-FoxO1 | |
| Srebp-2 | 1 ± 0.30 | 1.78 ± 0.23 | 1.54 ± 0.32 | 2.42 ± 0.13b |
| Acly | 1 ± 0.06 | 1.39 ± 0.13 | 2.48 ± 0.36 | 5.21 ± 0.68b |
| HMG-CoA synthase | 1 ± 0.21 | 4.39 ± 0.89a | 4.69 ± 1.83 | 10.72 ± 1.02b |
| HMG-CoA reductase | 1 ± 0.33 | 2.33 ± 0.39a | 4.04 ± 1.44 | 6.19 ± 0.69 |
| Fdps | 1 ± 0.15 | 1.98 ± 0.32 | 4.41 ± 1.62 | 8.38 ± 1.10 |
| LDLR | 1 ± 0.30 | 2.94 ± 0.48 | 5.38 ± 2.15 | 9.34 ± 0.73 |
a p < 0.01.
b p < 0.05.
FIGURE 3.
LDL receptor protein visualized by Western blot in STZ-treated control and l-FoxO1 mice that were fasted overnight and subsequently refed for 4 h.
Increased FFA Production in Adipose Cells of Hyperglycemic l-FoxO1 Mice
Having ruled out the role of TG synthesis genes, we hypothesized that the increase in TG release was due to increased supply of FFAs from white adipose tissue, as suggested by the elevated serum FFAs (Fig. 1C). Hormone-sensitive lipase and adipose TG lipase, the latter a FoxO1 target in fat cells (38), promote TG hydrolysis for release as FFAs. In perigonadal fat, we found a 2.9-fold increase of hormone-sensitive lipase in overnight-fasted, STZ-treated l-FoxO1 mice (p < 0.05). We detected no differences in adipose TG lipase expression. Although adipose TG lipase is thought to be rate-limiting under fasting conditions, its role may be overshadowed by other factors in the hyperglycemic conditions, compounded with the prolonged fast, in which we examined its expression. The surprising alteration in white adipose tissue gene expression in mice with a liver-specific mutation suggested to us that hepatic FoxO1 regulates a secreted factor that affects this process.
FGF21 Is Up-regulated, Independent of Hyperglycemia-induced Ketosis
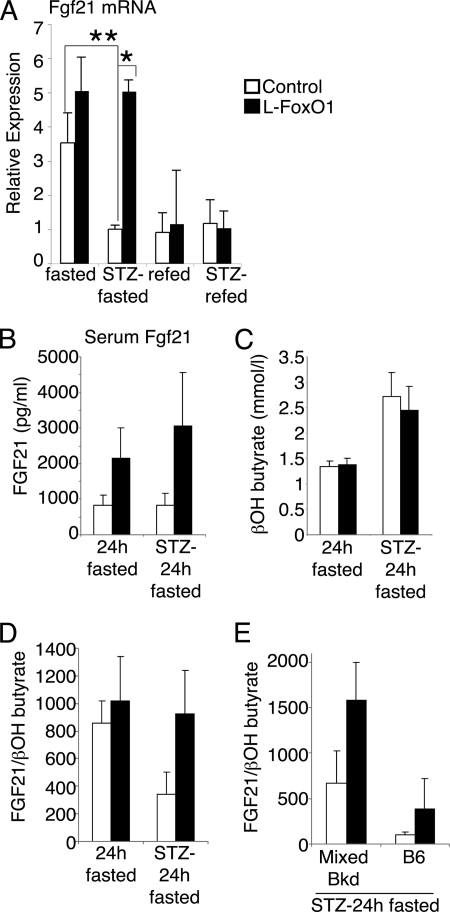
One liver-secreted factor that has been implicated to cause elevated adipose tissue lipolysis in vivo is fibroblast growth factor (FGF)-21, an insulin-sensitizing peptide that is secreted from the liver in response to fasting and mediates FA oxidation and ketosis (16, 39). Increased liver expression of FGF21 has been reported to enhance lipolysis in white adipose tissue by up-regulation of hormone-sensitive lipase and adipose TG lipase, leading to FFA release (16). To identify changes in the expression of FGF21, we measured liver mRNA levels. Compellingly, we found a 5-fold up-regulation of FGF21 in overnight fasted l-FoxO1 compared with control mice during hyperglycemia (p < 0.05) (Fig. 4A). Previous studies have found that robust induction of FGF21 occurs after prolonged fasting (≥24 h) (16, 39). Thus, we fasted untreated and STZ-treated mice for 24 h and measured serum FGF21 levels. In both conditions, l-FoxO1 mice showed increases in serum FGF21 versus control: 2.6-fold in the untreated state and 3.7-fold after STZ treatment (p = 0.055 by two-way analysis of variance) (Fig. 4B). To confirm that increased FGF21 mRNA and protein levels corresponded to increased FGF21 action, we measured expression of several transcriptional targets of FGF21 in the liver, namely pancreatic lipases (16). We found trends of increased liver mRNA expression of Cel (p = 0.05), Clps (p = 0.06), and Pnlip (p = 0.15) in STZ-treated l-FoxO1 mice (Table 3).
FIGURE 4.
FGF21 expression. A, FGF21 mRNA from livers of untreated or STZ-treated mice, fasted overnight or fasted overnight then refed for 4 h (n = 5–9 per group). For comparison, quantitative PCR analyses were combined from three separate 96-well plates: untreated fasted versus untreated refed; untreated fasted versus STZ-treated fasted; and STZ-treated fasted versus STZ-treated refed. Each sample was measured in triplicate. B, serum FGF21 levels after a 24 h fast in untreated or STZ treated mice (n = 6–12). C, serum β-hydroxybutyrate (n = 9–13). D, the ratio of serum FGF21 to β-hydroxybutyrate was calculated for individual mice; the mean of these ratios is presented (n = 6–12). E, ratio of serum FGF21 to β-hydroxybutyrate in STZ-treated mice, separated by strain background. Mixed background line is ∼80% B6 and 15% 129, with minor contributions from FVB and DBA. The B6 line is ∼97% B6. Ratios were calculated for individual mice; the mean of these ratios is presented (n = 4–7). *, p < 0.05 and **, p < 0.01 by two-way analysis of variance.
TABLE 3.
Expression of FGF21 target genes, Pparα, and fatty acid oxidation genes in liver
| STZ-treated, fasted |
STZ-treated, refed |
Saline-treated, fasted |
||||
|---|---|---|---|---|---|---|
| Control | l-FoxO1 | Control | l-FoxO1 | Control | l-FoxO1 | |
| Cel | 1 ± 0.25 | 2.39 ± 0.55 | ||||
| Clps | 1 ± 0.23 | 2.18 ± 0.47 | ||||
| Pnlip | 1 ± 0.23 | 2.24 ± 0.74 | ||||
| PPARα | 1 ± 0.13 | 1.77 ± 0.22a | 0.98 ± 0.18 | 1.43 ± 0.12 | 1.09 ± 0.18 | 1.33 ± 0.12 |
| Acox1 | 1 ± 0.30 | 3.18 ± 0.53b | 1.18 ± 0.08 | 1.47 ± 0.14 | ||
| Cpt1a | 1 ± 0.21 | 1.86 ± 0.19b | 0.69 ± 0.02 | 1.06 ± 0.09b | ||
| Acadm | 1 ± 0.24 | 1.58 ± 0.30 | 0.62 ± 0.10 | 1.38 ± 0.26a | ||
a p < 0.05.
b p < 0.01.
Because FGF21 is known to mediate FA oxidation and ketosis, we investigated the induction of these processes. We measured mRNA levels of peroxisome proliferator-activated receptor α (PPARα), a master transcriptional regulator of FA oxidation, and its target genes. We found that hyperglycemic l-FoxO1 mice expressed higher levels of PPARα, Acox1, Cpt1a, and Acadm (Table 3). As these increases may reflect increased rates of FA oxidation, we measured serum β-hydroxybutyrate levels but failed to detect differences between control and l-FoxO1 mice (Fig. 4C), suggesting that FA oxidation is similar between the two genotypes. Given the large individual variation in FGF21 and serum or urinary ketone levels, we calculated ratios of serum FGF21 to β-hydroxybutyrate in individual mice. We found that in the untreated state, control and l-FoxO1 mice had similar FGF21/ketone ratios; however, after STZ treatment, l-FoxO1 animals had a nearly 3-fold higher ratio than control mice (Fig. 4D), indicating that FGF21 rose disproportionately to β-oxidation. Interestingly, the ratio was highly dependent on a strain background, although it was consistently higher in l-FoxO1 compared with matched controls: 2.4- and 3.9-fold higher on a mixed 129×BL6 and inbred BL6 background, respectively (Fig. 4E).
Taken together, these results indicate that hepatic FoxO1 ablation causes inappropriately high expression of FGF21, an inducer of adipose TG lipolysis, during hyperglycemia. Despite the rise in PPARα and its target genes, ketone levels are similar in l-FoxO1 and control mice, suggesting that the liver is unable to oxidize the excessive supply of FFAs, which are instead channeled into esterification, resulting in elevated VLDL secretion.
DISCUSSION
The pathogenesis of disordered lipid metabolism in diabetes remains unclear, especially with regard to the relevant contributions of insulin resistance and hyperglycemia to the process. Given the material costs and human suffering associated with the long term complications of dyslipidemia, this condition represents a significant unmet need in public health. Having demonstrated a critical role of transcription factor FoxO1 in various metabolic functions (9), in this study, we sought to address its potential role in lipid metabolism in diabetes.
To this end, we used a model system of hepatic FoxO1 ablation in the setting of hyperglycemia and hypoinsulinemia to show that the absence of FoxO1 in the liver increases VLDL production, circulating FFAs, and fed cholesterol levels. Gene expression data and plasma peptide levels point to a potential role of increased FGF21 in this process. Although the ability of FGF21 to regulate adipose tissue lipolysis in a cell-nonautonomous manner remains controversial (40), in vivo evidence suggests that increased liver FGF21 expression increases release of FFAs from adipose tissue (16). The deleterious effect of FoxO1 ablation on lipid metabolism following STZ indicates that FoxO1 plays a protective role during hyperglycemia by curtailing FGF21 expression and indirectly limiting FFA release from adipose tissue. In addition, FoxO1 appears to decrease expression of Srebp-2 and its target genes, resulting in increased fed cholesterol in l-FoxO1 compared with control mice, similar to the situation in diabetic patients (41). While seemingly paradoxical, this “protective” effect of FoxO1 during hyperglycemia is wholly consistent with the demonstration that FoxO1 activation in response to hyperglycemia-induced oxidative stress protects pancreatic β-cells against apoptosis and promotes expression of insulin gene transcription factors (15).
FoxO1 ablation may increase FGF21 expression in hyperglycemia by multiple mechanisms. One possibility is that FoxO1 represses FGF21. This may occur by FoxO1 binding to the FGF21 promoter through a co-repressor, requiring no direct binding of FoxO1 to DNA, or by binding directly with subsequent co-repressor recruitment, as seen previously (42). There are at least two putative FoxO1 binding sites in the proximal upstream region of FGF21 (data not shown). Alternatively, FoxO1 may exert a repressive effect on a pathway regulating FGF21 transcription, such as PPARα (16, 39). It is possible that FoxO1 deletion relieves a repressive influence on the PPARα pathway, consistent with the up-regulation of the latter in STZ-treated l-FoxO1 mice (Table 3) and with its repression in acute FoxO1 gain-of-function experiments (12). Other pathways involved in FA metabolism also may be involved. The FoxO1 coactivator peroxisome proliferator-activated receptor γ coactivator protein-1α (PGC-1α) (43) has been implicated in regulation of fatty acid oxidation (44) and can be regulated indirectly by FGF21. However, we found no differences in expression of Pgc-1α or its target gene cytochrome c (supplemental Table S1). Overall, although we cannot exclude the possibility that changes in expression of FGF21 and PPARα pathway genes occur secondarily to other FoxO1 effects, the fundamental conclusion remains that the absence of FoxO1 specifically in the liver increases FFA levels, leading to increased VLDL secretion. Note that the normal effect of refeeding to increase fatty acid and TG synthetic genes and thereby increase TG production, even while circulating FFAs are reduced compared with fasted levels, still exists in this model.
Similarly the effect of FoxO1 on Srebp-2 expression may be direct or indirect, and putative FoxO1 binding sites exist within the first intron of this gene (data not shown). Modest increases in expression and activation of SREBP-2 can be expected to cause not only elevated cholesterol synthesis but also induction of Srebp-1c mRNA expression (45). Although we did not observe more accumulation of cholesterol in liver tissue in l-FoxO1 compared with controls, this may be because the VLDL particles being secreted at a higher rate in this model include cholesterol esters (Fig. 2).
The rise of FGF21 in l-FoxO1 mice reveals a paracrine liver-adipose circuit regulated by FoxO1 that may underlie the pathogenesis of diabetic dyslipidemia. The elevated FGF21/β-hydroxybutyrate ratios and increased FFAs in l-FoxO1 mice suggest that lipolysis exceeds β-oxidation, by virtue of either FGF21 resistance, or inefficient β-oxidation, or both. Thus, excess FFAs become substrates for TG synthesis and secretion, resulting in higher plasma TG.
The association of elevated TGs and increased FGF21 in our study is seemingly at odds with the reported elevation of TGs in FGF21 knockdown mice fed a ketogenic diet (39). However, substantive differences between the two models render a direct comparison moot. Badman and colleagues used a very high (78.9%) fat diet that, in combination with the FGF21 knockdown, reduced expression of many fatty acid oxidation genes, including Cpt1a and Acox1, and led to very high liver TG accumulation (>4 fold higher than chow-fed controls). The elevation in plasma TGs was attributed to reduced TG clearance due to reduced expression of Angptl4, an inhibitor of lipoprotein lipase. In contrast, the same experiments in chow-fed mice showed neither differences in liver TGs, plasma TGs, nor in Angptl4, although Acox1 was reduced. Hyperglycemic l-FoxO1 mice do show the converse increase in Acox1 (Table 3), but have no differences in Angptl4 expression (supplemental Table S1). Furthermore, STZ treatment resulted in considerable liver TG reduction in both control and l-FoxO1 mice (Fig. 1), demonstrating that hepatic lipid metabolism is markedly different during hyperglycemia as compared with a ketogenic diet.
The exacerbation of the lipogenic profile by hyperglycemia likely explains differences between our observations and previous findings in FoxO1 transgenic mice (11–13). One report used transgenic mice overexpressing a constitutively nuclear FoxO1, finding increased TG secretion from the liver compared with wild-type controls, an effect attributed to expression of the VLDL assembly protein microsomal TG transfer protein (11), which is unaltered in our model (supplemental Table S1). However, unlike l-FoxO1 mice, these transgenic mice are insulin-resistant (46), and therefore, the effect on lipoprotein metabolism has probably multiple components. Another group used low-level, liver-specific transgenic overexpression of constitutively nuclear human FoxO1 cDNA and observed an opposite effect; plasma TG levels and hepatic lipogenesis were markedly lower than controls, and lipogenic gene expression was decreased (13). Similar to the other transgenic model, however, these mice were insulin-resistant. Thus, it is possible that in these models, the apparent effect of FoxO1 on lipid metabolism is secondary to systemic insulin resistance. In the model presented here, we have eliminated major differences in glucose and insulin levels.
A second reason for the differences between these models lies in the effect of hyperglycemia on FoxO1 activity. FoxO1 is regulated not only by the canonical insulin receptor-PI3K-Akt pathway, but also by ubiquitination (15), O-glycosylation (47), and by oxidative stressors, including high glucose, which cause FoxO1 deacetylation by Sirt1 and nuclear retention (48). Although the expression of several known FoxO1 target genes is similarly regulated by FoxO1 mutants that mimic constitutive deacetylation or by mutants that prevent phosphorylation (15), it is possible that alterations in post-translational modifications differentially influence a subset of FoxO1 targets (49). In other words, all forms of nuclear FoxO1 may not be equal. This intriguing possibility suggests that specifically during hyperglycemia FoxO1 plays a protective role against diabetic dyslipidemia.
In summary, our data provide evidence for a role of FoxO1 in lipid metabolism and VLDL secretion. With regard to the clinical implications of these observations, we propose the following model: in florid hyperglycemia, FoxO1 is activated through oxidative stress and dampens the dyslipidemic response by curtailing FGF21 and SREBP-2 expression. When poorly controlled diabetic patients are treated intensively with insulin, FoxO1 is excluded from the nucleus, relieving its repression of FGF21, with the end result that dyslipidemia becomes exacerbated, thus paving the way for deteriorating cardiovascular outcomes. Consistent with this model, recent evidence reveals that increased hepatic insulin sensitivity causes both increased TG secretion and increased atherosclerosis (19). Conversely, loss of insulin sensitivity by liver-specific deletion of the insulin receptor decreases TG secretion, although this model is also prone to atherosclerosis due to severe defects in cholesterol metabolism (50). These data argue for a reappraisal of intensive insulin treatment in hyperglycemic patients and provide a working model to understand the pathophysiology of lipid metabolism in diabetes.
Supplementary Material
Acknowledgments
We thank Drs. Alan Tall and Ira Tabas for useful discussions and critical reading of the manuscript.
This work was supported by National Institutes of Health Grants P01HL87123, T32DK07328, and P30DK63608 (Columbia Diabetes & Endocrinology Research Center).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2.
- TG
- triglyceride
- NEFA
- nonesterified fatty acid
- ChREBP
- carbohydrate response element binding protein
- LXR
- liver X receptor
- PPARα
- peroxisome proliferator-activated receptor α.
REFERENCES
- 1.Nathan D. M., Cleary P. A., Backlund J. Y., Genuth S. M., Lachin J. M., Orchard T. J., Raskin P., Zinman B. (2005) N. Engl. J. Med. 353, 2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobel B. E. (2007) Am. J. Med. 120, S3–11 [DOI] [PubMed] [Google Scholar]
- 3.Savage D. B., Petersen K. F., Shulman G. I. (2007) Physiol. Rev. 87, 507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis G. F., Steiner G. (1996) Diabetes Care 19, 390–393 [DOI] [PubMed] [Google Scholar]
- 5.Gerstein H. C., Miller M. E., Byington R. P., Goff D. C., Jr., Bigger J. T., Buse J. B., Cushman W. C., Genuth S., Ismail-Beigi F., Grimm R. H., Jr., Probstfield J. L., Simons-Morton D. G., Friedewald W. T. (2008) N. Engl. J. Med. 358, 2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsberg H. N., Zhang Y. L., Hernandez-Ono A. (2005) Arch. Med. Res. 36, 232–240 [DOI] [PubMed] [Google Scholar]
- 7.Brown M. S., Goldstein J. L. (2008) Cell Metab. 7, 95–96 [DOI] [PubMed] [Google Scholar]
- 8.Haeusler R. A., Accili D. (2008) Cell Metab. 8, 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Accili D., Arden K. C. (2004) Cell 117, 421–426 [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M., Pocai A., Rossetti L., Depinho R. A., Accili D. (2007) Cell Metab. 6, 208–216 [DOI] [PubMed] [Google Scholar]
- 11.Kamagate A., Qu S., Perdomo G., Su D., Kim D. H., Slusher S., Meseck M., Dong H. H. (2008) J. Clin. Invest. 118, 2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto M., Han S., Kitamura T., Accili D. (2006) J. Clin. Invest. 116, 2464–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W., Patil S., Chauhan B., Guo S., Powell D. R., Le J., Klotsas A., Matika R., Xiao X., Franks R., Heidenreich K. A., Sajan M. P., Farese R. V., Stolz D. B., Tso P., Koo S. H., Montminy M., Unterman T. G. (2006) J. Biol. Chem. 281, 10105–10117 [DOI] [PubMed] [Google Scholar]
- 14.Dong X. C., Copps K. D., Guo S., Li Y., Kollipara R., DePinho R. A., White M. F. (2008) Cell Metab. 8, 65–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. (2005) Cell Metab. 2, 153–163 [DOI] [PubMed] [Google Scholar]
- 16.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Cell Metab. 5, 415–425 [DOI] [PubMed] [Google Scholar]
- 17.Folch J., Lees M., Sloane Stanley G. H. (1957) J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 18.Norris A. W., Chen L., Fisher S. J., Szanto I., Ristow M., Jozsi A. C., Hirshman M. F., Rosen E. D., Goodyear L. J., Gonzalez F. J., Spiegelman B. M., Kahn C. R. (2003) J. Clin. Invest. 112, 608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S., Liang C. P., Westerterp M., Senokuchi T., Welch C. L., Wang Q., Matsumoto M., Accili D., Tall A. R. (2009) J. Clin. Invest. 119, 1029–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuzaka T., Shimano H., Yahagi N., Amemiya-Kudo M., Okazaki H., Tamura Y., Iizuka Y., Ohashi K., Tomita S., Sekiya M., Hasty A., Nakagawa Y., Sone H., Toyoshima H., Ishibashi S., Osuga J., Yamada N. (2004) Diabetes 53, 560–569 [DOI] [PubMed] [Google Scholar]
- 21.Reaven E. P., Reaven G. M. (1974) J. Clin. Invest. 54, 1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi T., Hirano T., Okada K., Adachi M. (2003) Metabolism 52, 1354–1359 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg I. J., Hu Y., Noh H. L., Wei J., Huggins L. A., Rackmill M. G., Hamai H., Reid B. N., Blaner W. S., Huang L. S. (2008) Diabetes 57, 1674–1682 [DOI] [PubMed] [Google Scholar]
- 24.Buteau J., Shlien A., Foisy S., Accili D. (2007) J. Biol. Chem. 282, 287–293 [DOI] [PubMed] [Google Scholar]
- 25.Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., 3rd, Wright C. V., White M. F., Arden K. C., Accili D. (2002) J. Clin. Invest. 110, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramaswamy S., Nakamura N., Sansal I., Bergeron L., Sellers W. R. (2002) Cancer Cell 2, 81–91 [DOI] [PubMed] [Google Scholar]
- 27.Horton J. D., Goldstein J. L., Brown M. S. (2002) J. Clin. Invest. 109, 1125–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimomura I., Bashmakov Y., Ikemoto S., Horton J. D., Brown M. S., Goldstein J. L. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yabe D., Komuro R., Liang G., Goldstein J. L., Brown M. S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3155–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McPherson R., Gauthier A. (2004) Biochem. Cell Biol. 82, 201–211 [DOI] [PubMed] [Google Scholar]
- 31.Shimano H., Horton J. D., Shimomura I., Hammer R. E., Brown M. S., Goldstein J. L. (1997) J. Clin. Invest. 99, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldstein J. L., DeBose-Boyd R. A., Brown M. S. (2006) Cell 124, 35–46 [DOI] [PubMed] [Google Scholar]
- 33.Biddinger S. B., Haas J. T., Yu B. B., Bezy O., Jing E., Zhang W., Unterman T. G., Carey M. C., Kahn C. R. (2008) Nat. Med. 14, 778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., Hevener A. L., Sr., Farese R. V., Jr. (2007) Cell Metab. 6, 69–78 [DOI] [PubMed] [Google Scholar]
- 35.Millar J. S., Stone S. J., Tietge U. J., Tow B., Billheimer J. T., Wong J. S., Hamilton R. L., Farese R. V., Jr., Rader D. J. (2006) J. Lipid Res. 47, 2297–2305 [DOI] [PubMed] [Google Scholar]
- 36.Fisher E. A., Ginsberg H. N. (2002) J. Biol. Chem. 277, 17377–17380 [DOI] [PubMed] [Google Scholar]
- 37.Liang G., Yang J., Horton J. D., Hammer R. E., Goldstein J. L., Brown M. S. (2002) J. Biol. Chem. 277, 9520–9528 [DOI] [PubMed] [Google Scholar]
- 38.Chakrabarti P., Kandror K. V. (2009) J. Biol. Chem. 284, 13296–13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. (2007) Cell Metab. 5, 426–437 [DOI] [PubMed] [Google Scholar]
- 40.Arner P., Pettersson A., Mitchell P. J., Dunbar J. D., Kharitonenkov A., Rydén M. (2008) FEBS Lett. 582, 1725–1730 [DOI] [PubMed] [Google Scholar]
- 41.Annuzzi G., De Natale C., Iovine C., Patti L., Di Marino L., Coppola S., Del Prato S., Riccardi G., Rivellese A. A. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 2397–2402 [DOI] [PubMed] [Google Scholar]
- 42.Kitamura T., Feng Y., Kitamura Y. I., Chua S. C., Jr., Xu A. W., Barsh G. S., Rossetti L., Accili D. (2006) Nat. Med. 12, 534–540 [DOI] [PubMed] [Google Scholar]
- 43.Puigserver P., Rhee J., Donovan J., Walkey C. J., Yoon J. C., Oriente F., Kitamura Y., Altomonte J., Dong H., Accili D., Spiegelman B. M. (2003) Nature 423, 550–555 [DOI] [PubMed] [Google Scholar]
- 44.Burgess S. C., Leone T. C., Wende A. R., Croce M. A., Chen Z., Sherry A. D., Malloy C. R., Finck B. N. (2006) J. Biol. Chem. 281, 19000–19008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horton J. D., Shimomura I., Brown M. S., Hammer R. E., Goldstein J. L., Shimano H. (1998) J. Clin. Invest. 101, 2331–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakae J., Biggs W. H., 3rd, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. (2002) Nat. Genet. 32, 245–253 [DOI] [PubMed] [Google Scholar]
- 47.Housley M. P., Rodgers J. T., Udeshi N. D., Kelly T. J., Shabanowitz J., Hunt D. F., Puigserver P., Hart G. W. (2008) J. Biol. Chem. 283, 16283–16292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frescas D., Valenti L., Accili D. (2005) J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]
- 49.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 50.Biddinger S. B., Hernandez-Ono A., Rask-Madsen C., Haas J. T., Alemán J. O., Suzuki R., Scapa E. F., Agarwal C., Carey M. C., Stephanopoulos G., Cohen D. E., King G. L., Ginsberg H. N., Kahn C. R. (2008) Cell Metab. 7, 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.