Abstract
Dopamine is a catecholamine neurotransmitter, which plays an important role in the regulation of T cell functions. In activated T cells from normal volunteers, stimulation of D1 and D2 dopamine receptors inhibit cell proliferation and cytokine secretion. However, there is no report yet regarding the regulatory role of D1 and D2 dopamine receptors in abnormally proliferating T cells. The present study investigates the expression and effect of activation of these dopamine receptors in Jurkat cells, a leukemic T cell line showing uncontrolled proliferation. Like normal human T cells, in Jurkat cells, D1 and D2 dopamine receptors are also expressed; however, unlike activated normal T cells, stimulation of these dopamine receptors in Jurkat cells fails to inhibit their T cell receptor-induced proliferation. This alteration is due to failure of D1 dopamine receptor-mediated activation of cyclic AMP signaling and a missense mutation at the third cytoplasmic loop of D2 dopamine receptors affecting inhibition of phosphorylation of ZAP-70, an important downstream protein transducing signal from the T cell receptor. These results help to understand the biology of abnormal proliferation of T cells in pathophysiological conditions where dopamine plays an important role.
Keywords: Cyclic AMP (cAMP), Immunology, Lymphocyte, Neurotransmitter Receptors, Signal Transduction, Dopamine, Jurkat Cells, Neuroimmunology, ZAP-70, Proliferation
Introduction
Dopamine (DA)3 is one of the major neurotransmitters in the brain. In the central nervous system, it regulates various important functions like emotions, motivations, feelings of pleasure, addiction, and movement (1, 2). In the periphery, it regulates blood pressure, heart rate, gut motility, endocrine, and kidney functions (3–5). Recent reports indicate that DA influences different functions of the immune effector cells, most importantly normal T lymphocytes (6–11). T lymphocytes can synthesize, transport, and reuptake DA (12, 13). DA, in turn, modulates the functions of these immune effector cells by acting through its specific classes of receptors expressed in these cells.
The DA receptors are further subdivided into two categories, the D1 class consisting of D1 and D5 subtypes, which, when activated, increases intracellular cyclic AMP (cAMP) and the D2 class consisting of D2, D3, and D4 subtypes that inhibit intracellular cAMP on stimulation (1). Human T cells express both the D1 and D2 class of DA receptors (14–16). Changes in the expression of DA receptors and their signaling pathways in T cells are associated with altered immune functions in disorders like schizophrenia (17), Parkinson disease (18), Alzheimer disease (19), attention-deficit hyperactivity disorder (20), migranes (21), multiple sclerosis (22), and Tourette syndrome (23).
Our previous reports indicate that in activated normal human T cells, stimulation of D1 DA receptors inhibit cell proliferation by elevating intracellular cAMP (24). Similarly, stimulation of D2 receptors in T cells has been shown to inhibit activated T cell receptor (TCR)-induced cell proliferation and secretion of IL-2, IFN-γ, and IL-4 (25). Also, DA D4 receptors have a pivotal role in maintaining T cell quiescence (26). All of these reports indicate that other than being an important regulator of immunity (10, 11), DA also plays a key role in the regulation of normal human T cell functions (27, 28).
Therefore, it is interesting to find out the expression profile of DA receptors and their regulatory role, if any, in pathologically abnormal T cells with respect to their uncontrolled proliferation. Accordingly, to examine the role of dopaminergic regulation in abnormally proliferating T cells, acute T lymphoblastic leukemic cells (Jurkat) were selected (29).
EXPERIMENTAL PROCEDURES
Cell Line
The acute lymphoblastic leukemia T cell line (Jurkat cells) was obtained from the American Type Culture Collection. The cells were grown in RPMI 1640 medium with 1.5 mm l-glutamine, Earle's salt, and sodium bicarbonate and supplemented with 10% fetal bovine serum (29).
Isolation and Culture of Normal Human Lymphocytes
Blood was collected from normal volunteers according to the norms of Institutional Review Board. Resting T cells were isolated by Dynal T cell negative isolation kit II (Dynal Biotech, Invitrogen) following manufacturer's protocol (26).
DA Receptor Expression in Activated Normal and Leukemic T Cells by Semiquantitative RT-PCR
Total RNA was extracted from activated normal T cells and Jurkat cells by RNA isolation kit following manufacturer's protocol (Ambion, Inc.). Reverse transcription followed by PCR was carried out in a DNA thermal cycler (GeneAmp-9700; Applied Biosystems) with D1 and D2 DA receptor primers. The sequence of primers for DA receptors were as follows: DA D1 receptor, 5′-CAGTCCACGCCAAGAATTGCC-3′ and 5′-ATTGCACTCCTTGGAGATGGAGCC-3′; and DA D2 receptor, 5′-GCAGCCGAGCTTTCAGGGCC-3′ and 5′-GCAGCCGAGCTTTCAGGGCC-3′ (30).
Stimulation of D1 or D2 DA Receptors with TCR Activation
Resting T cells and Jurkat cells were stimulated with plate-bound anti-CD3 (Sigma-Aldrich) followed by the addition of soluble anti-CD28 (eBioscience) (4 μg/ml each) in a 96-well plate together with D1 (SKF 82526, Sigma-Aldrich) or D2 DA receptor agonist (quinpirole hydrochloride, Sigma-Aldrich) in different concentrations. From dose-response experiments, the dose showing the highest proliferation inhibition was selected for further experiments. In experiments where phosphodiesterase inhibitor was used, CD3/CD28 activated Jurkat cells were pretreated with 1 mm theophylline for 30 min followed by D1 DA receptor agonist stimulation. The D1 DA receptor specific antagonist, SCH 23390 (100 μm; Sigma-Aldrich) when used, added 10 min before theophylline, and followed thereafter by the D1 DA receptor agonist stimulation.
Cell Proliferation Assay
[3H]thymidine incorporation assay was undertaken to measure cell proliferation. [3H]thymidine was added 18 h before the termination of 72 h of culture of cells (25).
Intracellular cAMP Assay
Intracellular cAMP was measured by a parameter ELISA kit (R&D Systems) following the manufacturer's protocol from CD3/CD28-stimulated and D1 agonist (SKF 82526, 1 μm)-treated Jurkat cells pretreated with or without theophylline (1 mm) in separate experiments. To further determine the specificity of D1 DA receptor action on the elevation of cAMP, the D1 DA receptor-specific antagonist SCH 23390 (100 μm) was used, and then intracellular cAMP was measured. In addition, intracellular cAMP also was measured in CD3/CD28-stimulated Jurkat cells treated with or without the D2 DA receptor-specific agonist quinpirole (2 μm) (31).
Western Blot Analysis of DA Receptors and Phosphorylated ZAP-70 in Activated Normal T Cells and Jurkat Cells
For Western blot analysis of DA receptors, equal amounts of proteins from lysed activated T cells and Jurkat cells were immunoblotted with primary antibodies against DA receptors (D1and D2) (Santa Cruz Biotechnology). For phosphorylation study, proteins from CD3/CD28-costimulated and D2 agonist (quinpirole, 2 μm)-treated normal T cells and Jurkat cells were immunoblotted against antiphospho-ZAP-70 (Upstate). Membrane was reprobed against anti-ZAP-70 (Upstate) for total protein expression (24, 25).
Genotyping of D1 and D2 DA Receptors in Jurkat Cells
Total DNA was isolated from Jurkat cells by QIAamp DNA mini kit (Qiagen) according to the manufacturer's protocol. The human D1 DA receptor gene (GenBankTM accession no. NC_000005.9) is composed of two exons separated by a small intron (32), whereas the DA D2 receptor gene (GenBankTM accession no. NC_000011.9) consists of eight exons separated by seven introns (33). PCR reactions were established with suitable primers to produce overlapping products for D1 (3488 bp) and D2 (65,575 bp) DA receptor genes. Bidirectional sequencing reactions were performed for D1 and D2 DA receptors in an automated sequencing platform (ABI 3730xl, Applied Biosystems) using an energy transfer terminator. Sequences were analyzed to identify single nucleotide polymorphisms (SNPs) by Polyphred and validated manually as described previously (34, 35).
Statistical Analysis
All data are expressed as the mean ± S.E. Statistical comparisons were performed using Student's t test. p < 0.05 was considered significant (25).
RESULTS
Expression of DA Receptors in T Cells from Normal Volunteers and Jurkat Cells
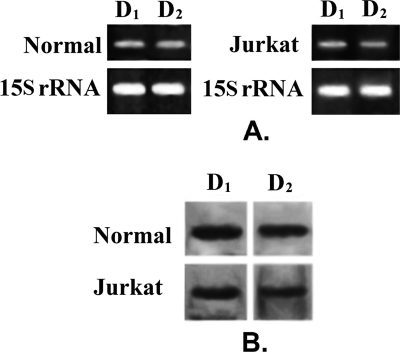
From RT-PCR and Western blot analysis, it was evident that T lymphocytes from normal volunteers expressed both D1 and D2 DA receptors (Fig. 1, A and B). Similarly, considerable expressions of both D1 and D2 DA receptors also were shown in Jurkat leukemic T cells (Fig. 1, A and B). However, unlike normal T cells, very low expressions of D3, D4, and D5 DA receptors were observed in Jurkat T cells (data not shown). Thus, D1 and D2 DA receptors were the predominant DA receptor subtypes present in these cells.
FIGURE 1.
Expression of D1 and D2 dopamine receptors in Normal and Jurkat cells. A, semiquantitative reverse transcription-polymerase chain reaction analysis of mRNA shows presence of D1 and D2 dopamine receptor expression in normal activated T cells and Jurkat cells. 15 S rRNA expression is the positive control. B, Western blot with specific antibodies against receptors also revealed similar expression pattern of D1 and D2 dopamine receptor proteins in normal and Jurkat T lymphocytes. Results are representative of six separate experiments.
Stimulation of D1 and D2 DA Receptors in Jurkat Cells Did Not Inhibit the Proliferation of These Cells
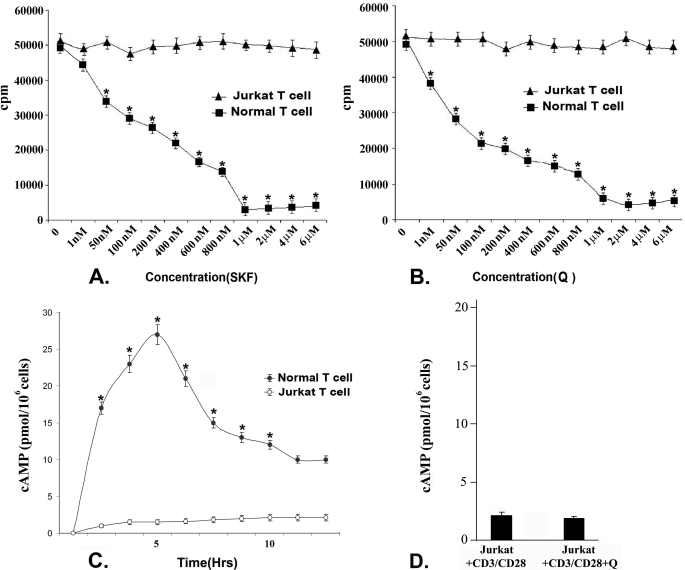
As stimulation of D1 and D2 DA receptors in activated T cells from normal volunteers has been shown to negatively regulate their proliferation (24, 25) and because our present results indicate that D1 and D2 are the predominant DA receptors expressed in Jurkat cells, it was therefore interesting to determine the role of DA-mediated regulation of cell proliferation through these receptors in Jurkat cells. Following stimulation of D1 DA receptors with different concentrations (1 nm–6 μm) of D1 receptor-specific agonist SKF 82526, together with anti CD3/CD28, no significant change in the proliferation of Jurkat cells was observed, whereas CD3/CD28 costimulated and D1 DA agonist-treated (1 nm–6 μm) T cells from normal volunteers showed significant dose-dependent cell proliferation inhibition with the maximum inhibition observed at a 1 μm concentration (Fig. 2A). Similarly in CD3/CD28-costimulated Jurkat cells, no significant inhibition of proliferation was observed following treatment with a specific D2 DA receptor agonist, quinpirole hydrochloride, in different concentrations (1 nm–6 μm). In contrast, CD3/CD28 co-stimulation-induced proliferation of T cells from normal volunteers was inhibited significantly in a dose-dependent manner with maximum inhibition observed when treated with 2 μm quinpirole (D2 DA receptor agonist) (Fig. 2B).
FIGURE 2.
Effect of stimulation of D1 and D2 dopamine receptors on proliferation of activated normal T cells and Jurkat cells by [3H]thymidine incorporation assay. Treated cells were cultured for 72 h, and radiolabeled thymidine was added 18 h before termination of experiments. Incorporated radioactivity was measured as representative of cellular proliferation. Intracellular cAMP concentration also was measured from these treatment groups. A, stimulation of D1 dopamine receptors by its specific agonist (SKF, SKF 82526) in CD3/CD28-stimulated normal T cells showed significant dose-dependent inhibition of proliferation with maximum inhibition found at a 1 μm concentration. In contrast, no significant inhibition of proliferation was observed in CD3/CD28-stimulated Jurkat cells following D1 agonist treatment. B, CD3/CD28-stimulated normal T cells showed significant dose-dependent inhibition of proliferation following stimulation of D2 DA receptors by a specific D2 agonist (Q, quinpirole hydrochloride), and maximum inhibition was found at 2 μm concentration, but no significant inhibition of proliferation was observed in Jurkat cells following D2 DA receptor agonist treatment. Results are mean ± S.E. of six separate experiments (*, p < 0.05). C, intracellular cAMP measured at different time points after D1 agonist stimulation (1 μm) showed a significantly elevated level in normal activated T cells where the peak was reached at 5 h and then declined. But similar treatment failed to elevate intracellular cAMP pool of Jurkat cells. D, stimulation of D2 dopamine receptors could not inhibit the cAMP concentration in CD3/CD28-activated Jurkat cells. Results are mean ± S.E. of six separate experiments (*, p < 0.05). Culture and treatment protocols are as described under “Experimental Procedures.”
Evaluation of the Functions of D1 and D2 DA Receptors in Jurkat Cells
Activation of D1 and D2 DA receptors has shown to inhibit normal activated T cell proliferation by elevating intracellular cAMP and down-regulating expression of important signaling intermediates of TCR activation pathway, respectively (24, 25). Our present results showed that unlike normal T cells, D1 and D2 DA receptor stimulation did not show any inhibitory effect in Jurkat cell proliferation. Therefore, we evaluated whether in these abnormally proliferating cells, stimulation of D1 and D2 DA receptors were able to generate respective proliferation inhibitory signaling events like that of activated normal T cells. When D1 DA receptors, which are Gs protein-coupled receptors, were stimulated with a specific D1 receptor agonist, SKF 82526 (1 μm, the inhibitory concentration selected from a dose-response curve; Fig. 2A) in activated normal T cells, cAMP started to increase within 5 min and reached to peak in 5 h (Fig. 2C) and thereafter, started to decline (Fig. 2C). On the contrary, no significant alteration in intracellular cAMP pool was observed in Jurkat cells following similar D1 DA receptor stimulation, suggesting cAMP, the second messenger, could not effectively accumulate in these proliferating T cells (Fig. 2C).
As stimulation of D2 DA receptors in normal activated T cells inhibited intracellular cAMP (1), the function of D2 DA receptors in Jurkat cells was determined in respect to their ability to inhibit intracellular cAMP accumulation. Unlike normal activated T cells, stimulation of D2 DA receptors in Jurkat cells with a specific agonist, quinpirole (2 μm, the inhibitory concentration selected from a dose-response curve), did not show any effect on intracellular cAMP level (Fig. 2D).
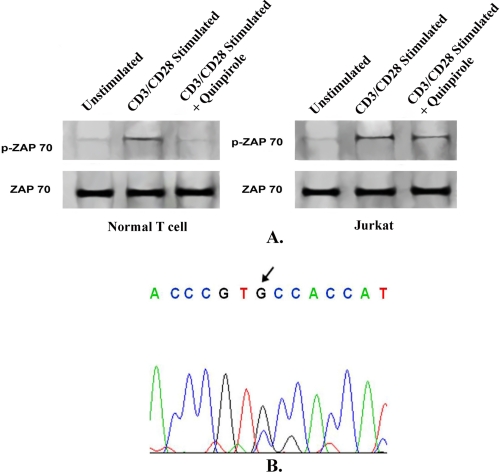
Furthermore, as stimulation of D2 DA receptors inhibits the TCR stimulation pathway (25), to examine D2 DA receptor function in Jurkat cells, we chose to study the phosphorylation status of ZAP-70, an important signaling intermediate of the TCR-activated mitogenic pathway leading to cytokine release and subsequent cell proliferation (36). Stimulation of D2 DA receptors by its specific agonist, quinpirole (2 μm, Fig. 2B), significantly inhibited phosphorylation of ZAP-70 in activated normal T cells (Fig. 3A), but stimulation of D2 DA receptors failed to show any significant inhibitory effect on ZAP-70 phosphorylation in CD3/CD28-stimulated Jurkat cells (Fig. 3A). Total ZAP-70 expression remained unchanged (Fig. 3A).
FIGURE 3.
Effect of stimulation of D2 dopamine receptors on ZAP-70 phosphorylation in activated normal T lymphocytes and Jurkat cells. A, phosphorylation of ZAP-70, an important signaling protein in T cell activation was abrogated following stimulation of D2 dopamine receptors by specific agonist (Q, quinpirole hydrochloride; 2 μm) in CD3/CD28-activated normal T cells. However, similar D2 agonist treatment failed to inhibit ZAP-70 phosphorylation in activated Jurkat cells. Protein loading was verified by reblotting the membrane by anti-ZAP-70, which showed that total ZAP-70 expression remained unchanged. Results are representative of six separate experiments. B, chromatogram of DNA from PCR product amplified from D2 dopamine receptor of Jurkat cells shows a nonsynonymous mutation (C → G transversion). Base change to guanine at the indicated position of codon 311 results in substitution of amino acid cysteine and functional alteration of D2 receptor activity in Jurkat cells.
Mutation Screening of D1 and D2 DA Receptor Genes in Jurkat Cells Showed Synonymous and Nonsynonymous SNPs
The present results reveal that unlike normal T cells, stimulation of D1 and D2 DA receptors in Jurkat cells failed to show any inhibitory effect on Jurkat cell proliferation. Therefore, it is rational to screen these two DA receptor genes in Jurkat cells for any possible mutation altering their functions. Whole gene sequence analysis of D1 and D2 DA receptors revealed several SNPs both at exon and intron regions (Tables 1 and 2), which were reported previously in the SNP database (www.ncbi.nlm.nih.gov/projects/SNP). Polymorphisms at exon of D1 DA receptor were found to be synonymous in nature. This mutation, including the intronic mutations of D1 and D2 DA receptors, however, were not sufficient to significantly alter the function of these receptors.
TABLE 1.
D1 DA receptor allele of Jurkat cells with detected SNP positions in chromosome
| Allele type | Position on chromosome | Adjacent sequence | Accession no. | Type | Mutation type and amino acid change |
|---|---|---|---|---|---|
| A/G | 174803508 | GGAAGTGGGC(C/T)GCCGCCGCCT | rs265981 | Intron | |
| C/G | 174803379 | CTCAACGTTT(C/G)GGAGCCGCTG | Novel | Intron | |
| C/T | 174802756 | TTCCCTGCTT(G/A)GGAACTTGAG | rs4532 | Intron | |
| C/T | 174801446 | CAGCCCTATC(A/G)GTCATATTGG | rs155417 | Exon | Synonymous (Ser → Ser) |
| A/G | 174801306 | TGCTCTGGGG(C/T)TTGCTATTAA | rs686 | Intron | |
| A/G | 174800505 | AAAAATTTAA(A/G)AAAGTATAGC | rs4867798 | Intron |
TABLE 2.
D2 DA receptor allele of Jurkat cells with detected SNP positions in chromosome
| Allele type | Position on chromosome | Adjacent sequence | Accession no. | Type | Mutation type and amino acid change |
|---|---|---|---|---|---|
| G/C | 112788694 | CCCGACCCGT(G/C)CCACCATGGT | rs1801028 | Exon | Nonsynonymous (Ser → Cys) |
| T/A | 112800208 | GCTTTTACCA(T/A)TGAGTTTCCT | rs12808482 | Intron |
In addition to these mutations, interestingly, one nonsynonymous SNP change also was detected at the exon region of only D2 DA receptors in Jurkat cells (Table 2). Further analysis revealed that this mutation (C → G nucleotide transversion) is present in the heterozygote pattern in D2 DA receptors of Jurkat cells (Fig. 3B). This substitution is reported at the NCBI Single Nucleotide Polymorphism database (http://www.ncbi.nlm.nih.gov/projects/SNP) as S311C (NCBI SNP ID rs1801028) (37), resulting in an important change at 311th amino acid position (serine → cysteine) in D2 DA receptor variants present in Jurkat cells. In addition, analysis with bioinformatics tools revealed that this substituted amino acid cysteine is at the third cytoplasmic loop of the human D2 DA receptor, which is responsible for effective signal transduction by these receptors.
Role of Phosphodiesterase in cAMP Metabolism in Jurkat Cells
Present results reveal that stimulation of Jurkat cell D1 DA receptors, which are Gs protein-coupled receptors, did not generate intracellular cAMP. Increased cAMP production was shown to correlate strongly with the inhibition of activated normal T cell proliferation (24). Furthermore, mutation screening of the D1 DA receptor gene in Jurkat cells demonstrated changes in the intron regions and a synonymous change at an exon, which are of little significance in terms of alteration of receptor function (Table 1). Therefore, it was pertinent to look into other important factors regulating metabolism of cAMP pathway in Jurkat cells like activity of phosphodiesterase (PDE) responsible for cAMP breakdown.
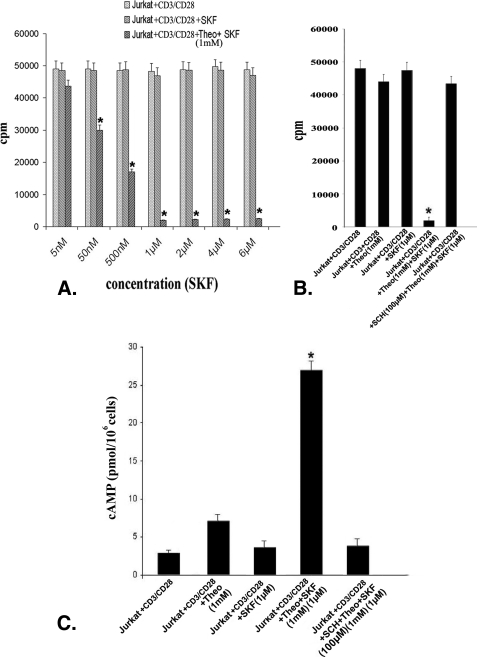
Therefore, in the present investigation, it is rational to study whether higher PDE activity is related to failure in intracellular cAMP elevation in Jurkat cells even after Gs protein-coupled D1 DA receptor stimulation. CD3/CD28-stimulated Jurkat cells were pretreated with 1 mm of the PDE inhibitor theophylline and stimulated with different concentrations of the D1 DA receptor agonist SKF 82526 (5 nm–6 μm). Treatment resulted in significant dose-dependent inhibition of cell proliferation, as evident from [3H]thymidine incorporation in Jurkat cells (Fig. 4A). Among different concentrations of SKF 82526, a 1 μm concentration showed maximum proliferation inhibition (Fig. 4A) and was associated with a significant rise in intracellular cAMP (Fig. 4C). However, in experiments where CD3/CD28 co-stimulated Jurkat cells were treated with theophylline (1 mm) alone, proliferation (Fig. 4B) as well as intracellular cAMP (Fig. 4C) did not change significantly. However, pretreatment with D1 DA antagonist (SCH 23390, 100 μm) abrogated this theophylline and D1 DA agonist-mediated cAMP accumulation (Fig. 4C) and inhibition of proliferation (Fig. 4B). Therefore, the failure of D1 DA receptor stimulation to elevate intracellular cAMP was not due to the functional defect of these receptors, but due to high PDE activity in these cells, which in turn inhibited intracellular cAMP.
FIGURE 4.
Inhibition of phosphodiesterase activity, together with stimulation of D1 dopamine receptors in Jurkat cells, is associated with significant elevation in intracellular cAMP level and inhibition of cell proliferation. A, the D1 DA receptor agonist (SKF, SKF 82526) induced dose-dependent inhibition of proliferation with 1 μm concentration, showing the maximum inhibition observed in CD3/CD28-stimulated Jurkat T cells when pretreated with phosphodiesterase inhibitor (Theo, theophylline; 1 mm), but not in CD3/CD28-stimulated Jurkat cells similarly treated with D1 agonist alone. Results are based on [3H]thymidine incorporation assay. Results are mean ± S.E. of six separate experiments (*, p < 0.05). B, inhibition of phosphodiesterase activity by theophylline (1 mm) or stimulation with D1 DA receptor-specific agonist (1 μm) separately was not sufficient to inhibit the CD3/CD28-induced Jurkat T cell proliferation, but stimulation of D1 DA receptors with specific agonist (1 μm) together with inhibition of phosphodiesterase activity (theophylline; 1 mm) significantly inhibited proliferation of these cells as evident from [3H]thymidine incorporation assay. Inhibition of D1 DA receptor activity by specific D1 DA receptor antagonist (SCH, SCH 23390; 100 μm) abrogated this above-mentioned proliferation inhibition in Jurkat, suggesting the functional competence of D1 DA receptors, and both inhibition of phosphodiesterase activity and D1 receptor stimulation are required for effective inhibition of proliferation in Jurkat cells. C, significant increase in intracellular cAMP observed in CD3/CD28-stimulated Jurkat cells treated with phosphodiesterase inhibitor (theophylline; 1 mm) and D1 DA receptor agonist (SKF 82526; 1 μm), when compared with only theophylline-treated (1 mm) or D1 DA receptor agonist (SKF 82526; 1 μm)-treated groups which showed little or no increase of cAMP, respectively. Inhibition of D1 DA receptor activity by D1 DA receptor-specific antagonist (SCH 23390; 100 μm) and then treatment with theophylline (1 mm) and D1 DA receptor agonist (SKF 82526; 1 μm) also showed only negligible increase in intracellular cAMP in Jurkat cells. Results are mean ± S.E. of six separate experiments (*, p < 0.05). Culture and treatment protocols are as described under “Experimental Procedures.”
DISCUSSION
The present investigation demonstrates that among the DA receptors, D1 and D2 DA receptors were predominantly expressed in Jurkat cells, and the expression of other subtypes of DA receptors (D3, D4, and D5) were very low in comparison to normal T cells. Thus, it was prudent to investigate the functional role of these D1 and D2 DA receptors, which, when stimulated in activated normal T cells, have been reported to show proliferation inhibition (24, 25). However, activation of these D1 and D2 DA receptors by their specific agonists failed to inhibit proliferation of Jurkat cells. Therefore, the DA-mediated proliferation regulation through D1 and D2 DA receptors as observed in activated normal T cells, was lost in Jurkat cells. As intracellular cAMP accumulation following Gs protein-coupled receptor D1 DA stimulation resulted in proliferation inhibition in normal activated T cells, nonresponsiveness to D1 DA receptor-mediated dopaminergic regulation in Jurkat cells was evaluated in relation to intracellular second messenger cAMP accumulation following D1 DA receptor stimulation. Interestingly, no significant increase in intracellular cAMP was observed in Jurkat cells following D1 DA receptor stimulation. Therefore, to find out whether the failure of D1 DA receptor mediated increase of its second messenger cAMP in Jurkat cells is due to structural alteration in these DA receptors, the full-length gene of D1 DA was sequenced and analyzed for structural changes. Mutation analysis of the D1 DA receptor gene sequence of Jurkat T cells revealed synonymous polymorphisms at the exon region or nonfunctional intron regions, which suggest no functional significance of these alterations to failure of D1 DA receptors to generate its second messenger, cAMP.
In addition, as we had shown previously that D1 DA receptor stimulation inhibited activated normal T cells through cAMP production (24), it is rational to conclude from the present experiment that this absence of D1 DA receptor activity in Jurkat cells might be due to the alteration in cAMP metabolism in these cells (38–40) because our present results indicate that pharmacological inhibition of PDE activity with theophylline along with D1 DA receptor stimulation resulted in robust cAMP accumulation with concomitant inhibition of proliferation in Jurkat T cells. It is thus logical to interpret from our data that failure of D1 DA receptor-mediated inhibition of proliferation of Jurkat cells was due to high catabolic activity of the PDE enzyme resulting in hydrolysis of intracellular cAMP in these leukemic T cells. This observation corroborates well with other findings where high PDE activity was observed in Jurkat cells (38, 39), and thereby, cAMP hydrolysis was found to be significantly higher in these abnormally proliferating cells than normal T cells (40).
Because inhibition of PDE activity followed by stimulation of D1 DA receptors in Jurkat cells resulted in increased level of intracellular cAMP, which in turn inhibited their proliferation, the failure of D1 DA receptor-mediated inhibition of Jurkat cell proliferation in our study was not as the result of defect in coupling of this receptor with Gs protein and its downstream signaling but was due to high PDE activity in these cells. However, inhibition of PDE activity in these cells alone was not sufficient to elevate cAMP in these cells to inhibit their proliferation. Our results indicate that not only inhibition of higher PDE activity but D1 DA receptor-mediated elevation of intracellular cAMP level also is important for the inhibition of proliferation of these leukemic T cells.
D2 DA receptors are Gi protein-coupled receptors, and stimulation of these receptors inhibit intracellular cAMP accumulation (1). In our study, we observed that unlike normal activated T cells, stimulation of D2 DA receptors failed to inhibit Jurkat cell proliferation. Also, no significant change in intracellular cAMP level was observed in these cells treated with the specific D2 DA receptor agonist quinpirole. These data therefore indicate a defect in the downstream signaling pathway of these receptor subtypes in these leukemic T cells. Furthermore, although stimulation of D2 DA receptors in activated normal T cells inhibited their proliferation by down-regulating phosphorylation of ZAP-70, an important downstream signaling molecule that follows TCR activation in normal T cells (36), in contrast, no such effect was shown in Jurkat cells, thereby suggesting a defect in the signal transduction mechanism of D2 DA receptors in these cells.
Therefore, the D2 DA receptor gene was sequenced to reveal any structural change responsible for this functional abnormality. Results revealed that other than mutation at intron, one nonsynonymous SNP change was present at coding region of the D2 DA receptor allele of Jurkat cells, which resulted in substitution of cysteine in place of serine at 311th amino acid position. This S311C mutation (NCBI SNP ID rs1801028) (37) is present at third cytoplasmic loop of the human D2 DA receptor, which is responsible for effective signal transduction of these receptors (41, 42). It is further reported that D2 DA receptor S311C allelic variants do not severely affect ligand binding of the receptor and its coupling with the G protein; rather, the receptor variant becomes significantly less effective than the wild type in relation to lower efficiency in activating the α subunit of the G protein heterotrimer to transduce downstream signals (41, 42).This mutation in the human D2 DA receptor also has been reported to be associated with wide range of cellular dysfunctions (43–47). These data thus explain the failure of the D2 DA receptors to down-regulate ZAP-70 phosphorylation and inhibition of TCR-activated proliferation in Jurkat cells.
Finally, Jurkat T cells are well characterized abnormal T cells with uncontrolled proliferation (48). Among several regulators of proliferation, neurotransmitter DA is an important modulator of normal activated T cell proliferation, mainly acting through its D1 and D2 receptors present on T cells (24, 25). However, our present investigation reveals that in pathologically abnormal T cells with uncontrolled proliferation, stimulation of these D1 and D2 DA receptors have no significant effect on proliferation inhibition. While investigating the failure of DA mediated proliferation regulation in Jurkat cells, we observed that altered cAMP metabolism in these cells affected D1 DA receptor-mediated inhibition of cell proliferation, and a missense mutation in D2 DA receptor abrogated its effect on inhibition of ZAP-70 phosphorylation, a critical down stream signaling pathway necessary for T cell receptor-induced cell proliferation and cytokine release (36).
We report here for the first time that abnormal T lymphocytes, which lack proliferation regulatory mechanisms, also are associated with disrupted DA receptor signaling, thereby suggesting that this neurotransmitter has an important role in T cell homeostasis (10, 11). The absence of inhibitory dopaminergic effects on T cells by these two major DA receptors in Jurkat cells may thus indicate the role of this neurotransmitter in abnormal T cell functions reported in many pathological conditions (17–23).
Acknowledgment
We thank Nidhan K. Biswas (Human Genetics Unit, Indian Statistical Institute, Kolkata, India) for comments regarding the SNP experiments.
This work was supported, in whole or in part, by National Institutes of Health Grants CA118265 (to S. B.) and CA124763 (to S. B.). This work was also supported by Council of Scientific and Industrial Research Government of India Fellowships 9/30(43)/2005-EMR-1 (to B. B.) and 9/30(54)/2009-EMR-1 (to S. S.) and Department of Defense Grant W81XWH-07-1-0051 (to S. B.).
- DA
- dopamine
- PDE
- phosphodiesterase
- TCR
- T cell receptor.
REFERENCES
- 1.Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G. (1998) Physiol. Rev. 78, 189–225 [DOI] [PubMed] [Google Scholar]
- 2.Riederer P., Sofic E., Konradi C. (1985) in The Role of Brain Dopamine (Flückiger E., Müller E. E., Thorner M. O. eds.) pp. 1–17, Springer Verlag, Berlin [Google Scholar]
- 3.Clark B. J. (1985) The Dopaminergic System (Flückiger E., Müller E. E., Thorner M. O. eds.) pp. 27–39, Springer Verlag, Berlin [Google Scholar]
- 4.Flemström G., Säfsten B., Jedstedt G. (1993) Gastroenterology 104, 825–833 [DOI] [PubMed] [Google Scholar]
- 5.Haskel Y., Hanani M. (1994) Dig. Dis. Sci. 39, 2364–2367 [DOI] [PubMed] [Google Scholar]
- 6.Levite M. (2008) Curr. Opin. Pharmacol. 8, 460–471 [DOI] [PubMed] [Google Scholar]
- 7.Bergquist J., Josefsson E., Tarkowski A., Ekman R., Ewing A. (1997) Electrophoresis 18, 1760–1766 [DOI] [PubMed] [Google Scholar]
- 8.Carr L., Tucker A., Fernandez-Botran R. (2003) J. Neuroimmunol. 137, 87–93 [DOI] [PubMed] [Google Scholar]
- 9.Cioca D. P., Watanabe N., Isobe M. (2000) Jap. Heart J. 41, 385–398 [DOI] [PubMed] [Google Scholar]
- 10.Basu S., Dasgupta P. S. (2000) J. Neuroimmunol. 102, 113–124 [DOI] [PubMed] [Google Scholar]
- 11.Sarkar C., Basu B., Chakroborty D., Dasgupta P. S., Basu S. (2010) Brain Behav. Immun. 24, 525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cosentino M., Fietta A. M., Ferrari M., Rasini E., Bombelli R., Carcano E., Saporiti F., Meloni F., Marino F., Lecchini S. (2007) Blood 109, 632–642 [DOI] [PubMed] [Google Scholar]
- 13.Bergquist J., Tarkowski A., Ekman R., Ewing A. (1994) Proc. Natl. Acad. Sci. 91, 12912–12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna F., McLaughlin P. J., Lewis B. J., Sibbring G. C., Cummerson J. A., Bowen-Jones D., Moots R. J. (2002) J. Neuroimmunol. 132, 34–40 [DOI] [PubMed] [Google Scholar]
- 15.Kirillova G. P., Hrutkay R. J., Shurin M. R., Shurin G. V., Tourkova I. L., Vanyukov M. M. (2008) J. Neurosci. Methods 174, 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari M., Cosentino M., Marino F., Bombelli R., Rasini E., Lecchini S., Frigo G. (2004) Biochem. Pharmacol. 67, 865–873 [DOI] [PubMed] [Google Scholar]
- 17.Ilani T., Ben-Shachar D., Strous R. D., Mazor M., Sheinkman A., Kotler M., Fuchs S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 625–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai Y., Ueno S., Saeki Y., Soga F., Hirano M., Yanagihara T. (1996) Neurology 46, 791–795 [DOI] [PubMed] [Google Scholar]
- 19.Barbanti P., Fabbrini G., Ricci A., Bruno G., Cerbo R., Bronzetti E., Amenta F., Luigi Lenzi G. (2000) Mech. Ageing Dev. 120, 65–75 [DOI] [PubMed] [Google Scholar]
- 20.DiMaio S., Grizenko N., Joober R. (2003) J. Psychiatry Neurosci. 28, 27–38 [PMC free article] [PubMed] [Google Scholar]
- 21.Barbanti P., Bronzetti E., Ricci A., Cerbo R., Fabbrini G., Buzzi M. G., Amenta F., Lenzi G. L. (1996) Neurosci. Lett. 207, 73–76 [DOI] [PubMed] [Google Scholar]
- 22.Giorelli M., Livrea P., Trojano M. (2005) J. Interferon. Cytokine Res. 25, 395–406 [DOI] [PubMed] [Google Scholar]
- 23.Ferrari M., Termine C., Franciotta D., Castiglioni E., Pagani A., Lanzi G., Marino F., Lecchini S., Cosentino M., Balottin U. (2008) J. Psych. Res. 43, 24–29 [DOI] [PubMed] [Google Scholar]
- 24.Saha B., Mondal A. C., Majumder J., Basu S., Dasgupta P. S. (2001) Neuroimmunomodulation 9, 23–33 [DOI] [PubMed] [Google Scholar]
- 25.Ghosh M. C., Mondal A. C., Basu S., Banerjee S., Majumder J., Bhattacharya D., Dasgupta P. S. (2003) Int. Immunopharmacol. 3, 1019–1026 [DOI] [PubMed] [Google Scholar]
- 26.Sarkar C., Das S., Chakroborty D., Chowdhury U. R., Basu B., Dasgupta P. S., Basu S. (2006) J. Immunol. 177, 7525–7529 [DOI] [PubMed] [Google Scholar]
- 27.Jameson S. C. (2002) Nat. Rev. Immunol. 2, 547–556 [DOI] [PubMed] [Google Scholar]
- 28.Stockinger B., Barthlott T., Kassiotis G. (2004) Immunology 111, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss A., Wiskocil R. L., Stobo J. D. (1984) J. Immunol. 133, 123–128 [PubMed] [Google Scholar]
- 30.Lemmer K., Ahnert-Hilger G., Höpfner M., Hoegerle S., Faiss S., Grabowski P., Jockers-Scherübl M., Riecken E. O., Zeitz M., Scherübl H. (2002) Life Sci. 71, 667–678 [DOI] [PubMed] [Google Scholar]
- 31.Cianfarani F., Baldini E., Cavalli A., Marchioni E., Lembo L., Teson M., Persechino S., Zambruno G., Ulisse S., Odorisio T., D'Armiento M. (2010) J. Invest. Dermatol. 130, 93–101 [DOI] [PubMed] [Google Scholar]
- 32.Grandy D. K., Zhou Q. Y., Allen L., Litt R., Magenis R. E., Civelli O., Litt M. (1990) Am. J. Hum. Genet. 47, 828–834 [PMC free article] [PubMed] [Google Scholar]
- 33.Grandy D. K., Litt M., Allen L., Bunzow J. R., Marchionni M., Makam H., Reed L., Magenis R. E., Civelli O. (1989) Am. J. Hum. Genet. 45, 778–785 [PMC free article] [PubMed] [Google Scholar]
- 34.Ramensky V., Bork P., Sunyaev S. (2002) Nucleic Acids Res. 30, 3894–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunyaev S., Ramensky V., Koch I., Lathe W., 3rd., Kondrashov A. S., Bork P. (2001) Hum. Mol. Genet. 10, 591–597 [DOI] [PubMed] [Google Scholar]
- 36.Mustelin T., Taskén K. (2003) Biochem. J. 371, 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itokawa M., Arinami T., Futamura N., Hamaguchi H., Toru M. (1993) Biochem. Biophys. Res. Comm. 196, 1369–1375 [DOI] [PubMed] [Google Scholar]
- 38.Epstein P. M., Mills J. S., Ross C. P., Strada S. J., Hersh E. M., Thompson W. J. (1977) Cancer Res. 37, 4016–4023 [PubMed] [Google Scholar]
- 39.Ekholm D., Mulloy J. C., Gao G., Degerman E., Franchini G., Manganiello V. C. (1999) Biochem. Pharmacol. 58, 935–950 [DOI] [PubMed] [Google Scholar]
- 40.Seybold J., Newton R., Wright L., Finney P. A., Suttorp N., Barnes P. J., Adcock I. M., Giembycz M. A. (1998) J. Biol. Chem. 273, 20575–20588 [DOI] [PubMed] [Google Scholar]
- 41.Cravchik A., Sibley D. R., Gejman P. V. (1996) J. Biol. Chem. 271, 26013–26017 [DOI] [PubMed] [Google Scholar]
- 42.Kaiser R., Tremblay P. B., Klufmöller F., Roots I., Brockmöller J. (2002) Mol. Psychiatry 7, 695–705 [DOI] [PubMed] [Google Scholar]
- 43.Arinami T., Itokawa M., Enguchi H., Tagaya H., Yano S., Shimizu H., Hamaguchi H., Toru M. (1994) Lancet 343, 703–704 [DOI] [PubMed] [Google Scholar]
- 44.Higuchi S., Muramatsu T., Murayama M., Hayashida M. (1994) Biochem. Biophys. Res. Commun. 204, 1199–1205 [DOI] [PubMed] [Google Scholar]
- 45.Noble E. P. (2003) Am. J. Med. Genet. B. Neuropsychiatr. Genet. 116B, 103–125 [DOI] [PubMed] [Google Scholar]
- 46.Campa D., Zienolddiny S., Lind H., Ryberg D., Skaug V., Canzian F., Haugen A. (2007) Lung Cancer 56, 17–23 [DOI] [PubMed] [Google Scholar]
- 47.Gemignani F., Landi S., Moreno V., Gioia-Patricola L., Chabrier A., Guino E., Navarro M., Cambray M., Capellà G., Canzian F. (2005) Cancer Epidemiol. Biomarkers Prev. 14, 1633–1638 [DOI] [PubMed] [Google Scholar]
- 48.Abraham R. T., Weiss A. (2004) Nat. Rev. Immunol. 4, 301–308 [DOI] [PubMed] [Google Scholar]