Abstract
Innate immunity is the first line of host defense against invading pathogens, and it is recognized by a variety of pattern recognition molecules, including mannose-binding lectin (MBL). MBL binds to mannose and N-acetylglucosamine residues present on the glycopolymers of microorganisms. Human serum MBL functions as an opsonin and activates the lectin complement pathway. However, which glycopolymer of microorganism is recognized by MBL is still uncertain. Here, we show that wall teichoic acid of Staphylococcus aureus, a bacterial cell surface glycopolymer containing N-acetylglucosamine residue, is a functional ligand of MBL. Whereas serum MBL in adults did not bind to wall teichoic acid because of an inhibitory effect of anti-wall teichoic acid antibodies, MBL in infants who had not yet fully developed their adaptive immunity could bind to S. aureus wall teichoic acid and then induced complement C4 deposition. Our data explain the molecular reasons of why MBL-deficient infants are susceptible to S. aureus infection.
Keywords: Cell Wall, Complement, Innate Immunity, Lectin, Pathogen-associated Molecular Pattern (PAMP), Staphylococcus aureus, Glycopolymer, Ligand, Mannose-binding Lectin, Wall Teichoic Acid
Introduction
Innate immunity is an evolutionarily conserved first-line host defense that senses pathogenic microorganisms through “pattern-recognition” molecules that recognize the conserved molecular patterns on the surface of microbes (1). The complement system is an ancient innate immune defense mediated by soluble pattern recognition molecules, such as mannose-binding lectin (MBL)2 (2). MBL, which consists of a collagen, a neck, and a carbohydrate recognition domain, belongs to the collectin family and is found in many sera of animals including human. It binds to mannose and N-acetylglucosamine (GlcNAc) residues present on the glycopolymers of microorganisms (3). Upon binding, it activates the lectin complement system independently of antibodies via three MBL-associated serine proteases-1, -2, and -3 (MASP-1, -2, and -3) (4–6). MASP-2 cleaves C4 and then C2 to form the C3 convertase, C4bC2a (5) while MASP-1 cleaves factor D of the alternative pathway (7). IgG and IgM immune complexes can also generate the C3 convertase via the activation of C1, leading to the cleavage of C3 to anaphylactic and antimicrobial peptide C3a and opsonic C3b. If activation progresses, the C5 convertase (C4bC2aC3b for the lectin and classical pathways) is generated and cleaves C5 to generate the potent anaphylactic peptide, C5a and C5b. C5b can initiate the assembly of the terminal membrane attack complex on the surface of targets (8).
The deficiency of serum MBL was first identified in human beings in association with a common defect of opsonization in children (9). Subsequent studies have shown that MBL deficiency increases infection susceptibility (10, 11) including acute respiratory tract infections in early childhood (11). In contrast, there is some evidence that MBL deficiency may be protective against some intracellular pathogens (12). Recent studies have demonstrated that MBL- or MBL/MASP complex-mediated opsonophagocytosis is required to inhibit growth of Staphylococcus aureus (13, 16). Although the identity of the receptor remains to be defined, cellular receptors for MBL have been proposed and several studies have shown direct interactions of MBL with phagocytic cells, resulting in enhanced opsonophagocytosis (2).
S. aureus is a common human pathogen in hospital-associated and community-acquired infections, causing wound infections, bacteremia, and sepsis (17). The major cell envelope-associated glycopolymers in S. aureus include wall teichoic acid (WTA) and lipoteichoic acid (LTA) (18). The most common structures of WTAs are composed of a N-acetylmannosamine (ManNAc)-(β1→4)-N-(GlcNAc) disaccharide with one to three glycerol phosphates attached to the C4 hydroxyl of the ManNAc residue (the “linkage unit”) followed by a much longer chain of glycerol- or ribitol phosphate repeats (the “main chain”). The hydroxyls on the glycerol- or ribitol phosphate repeats are tailored with cationic d-alanine esters and monosaccharides, such as glucose or N-GlcNAc. WTA is covalently linked to PG at the C6 position of N-acetylmuramic acid (MurNAc) (Ref. 18 and supplemental Fig. S1). Whereas LTA is anchored to the cell membrane, WTA is covalently linked to peptidoglycan (PG) and functions in the adsorption of S. aureus by certain bacteriophages (19) and in the adherence of S. aureus to nasal epithelial cells (20).
Several reports have demonstrated that MBL bound to S. aureus induces activation of the complement pathway (15, 16, 21), however, which S. aureus glycopolymer is recognized by MBL is still uncertain. We have reported that human MBL binds to S. aureus PG and activates the lectin pathway (21) and two other studies have demonstrated MBL binding to S. aureus LTA (22, 23). S. aureus LTA was also reported to be a ligand of l-ficolin to activate the lectin complement pathway (14). Bacterial glycopolymers may also be important antigens to activate the classical complement pathway and adaptive immunity and can be desirable vaccination targets (18). For example, LTA-specific monoclonal antibodies have yielded promising results as a passive vaccine for severe S. aureus infections (24). Also, anti-capsular polysaccharide 5 and 8 antibodies of S. aureus are on the clinical trial now (25).
In this report, we investigated S. aureus cell wall component mutants including WTA- or LTA-deficient strains, which have recently become available (20, 26) to determine the ligand in the complement system. We demonstrate that S. aureus WTA functions as a natural ligand of human MBL and induces MBL-mediated C4 deposition on the bacterium in vitro. Additionally, MBL-WTA interaction enhances the opsonophagocytosis of S. aureus by human neutrophils. Unexpectedly, serum MBL of adults cannot recognize WTA because of competition by WTA-specific antibodies. In contrast, serum MBL of infants whose adaptive immunity is immature does bind WTA. Infants who have low levels of both MBL and anti-WTA-antibodies have weak ability to activate the complement system. These results indicate that S. aureus WTA is an important pathogen-associated molecular pattern for the complement activation.
EXPERIMENTAL PROCEDURES
Proteins, Sera, and Bacteria
The native human MBL/MASP complex and human l-ficolin were purified from human sera as described (4, 27). Recombinant human MBL was expressed in a CHO cell line and chromatographically purified as described (28). MBL-deficient serum was obtained from a person homozygous for the codon54 mutation of the MBL gene, and C1q-deficient sera were obtained from Calbiochem/EMD Biosciences (San Diego, CA). Infant sera were obtained from the clinical laboratory, and adult sera were obtained from healthy volunteers with informed consent. S. aureus cell surface component mutants were derivatives of RN4220 and are listed in Table 1. All of the bacterial strains were cultured with Luria Bertani medium supplemented with antibiotics wherever required.
TABLE 1.
Bacterial strains used in this study
| Names | Genotypes and characteristics | Refs. |
|---|---|---|
| S. aureus | ||
| RN4220 | Parent strain | 40 |
| M0702 | RN4220 ΔtagO::pT0702 | 46 |
| T111 | RN4220 ΔtagO::pT0702/pStagO | This work |
| M0674/pM101 | RN4220 ΔltaS::phleo/pM101 | 31 |
| M0793 | RN4220 ΔdltA::pT0793 | 46 |
| T013 | RN4220 Δlgt::pmlgt | 45 |
| CK1001 | RN4220 ΔmprF::pCK20mprF | 47 |
| M0875 | RN4220 ΔypfP::pT0875 | 46 |
| M0107 | RN4220 Δspa::phleo | 31 |
| T258 | M0107 ΔtagO::pT0702 | This work |
| T358 | M0107 ΔtagO::pT0702/pStagO | This work |
| T002 | RN4220 ΔoatA::erm | This work |
Bacterial Strains Constructed in This Study
T002 strain (RN4220 ΔoatA::erm) was constructed via integration of a pMutinT3 plasmid (29) harboring central open reading frame region of the oatA gene. pStagO is a pND50 plasmid (30) harboring the intact S. aureus tagO gene. T258 and T358 strains were constructed via phage transduction using phage80 (31).
WTA-attached and WTA-free PG, and WTA of S. aureus
WTA-attached insoluble PG was prepared from S. aureus strains according to our published method (32) with some modifications. Briefly, WTA-attached insoluble PG was obtained from the S. aureus T002 strain, a PG O-acetyltransferase-deficient oatA mutant, which is sensitive to lysozyme (33). WTA-free PG was obtained from a WTA-deficient S. aureus ΔtagO mutant. To purify WTA, insoluble WTA-attached PG (20 mg) was incubated with lysostaphin (0.17 mg, Sigma-Aldrich) in 1 ml of buffer A (20 mm Tris-HCl, pH 7.0) for 6 h at 37 °C and then with lysozyme (1 mg, Bioshop) for 18 h at 37 °C. The digested materials were boiled for 10 min, passed through a 0.45-μm membrane filter, and 1/10 amounts were then loaded onto a HiTrap-Q column (1 ml) equilibrated with buffer A. The column was washed, followed by elution with a 20-ml gradient from 0 to 1 m NaCl in buffer A. Fractions (1 ml) were collected and assayed for inorganic phosphate (34), and PAGE was performed with silver staining to detect WTA. The pooled WTA fraction (8 mg from 20 mg of PG) was precipitated with acetone and dissolved in phosphate-buffered saline (PBS, pH 7.5) and used for further experiments.
MBL Binding to S. aureus Cells
Fully grown S. aureus cells (2.0 × 109 cells) were fixed with 70% ethanol, washed, incubated with 1 μg of recombinant MBL (rMBL) or l-ficolin in 500 μl of buffer B (20 mm Tris-HCl containing 150 mm NaCl, 20 mm CaCl2, and 0.05% Tween 20, pH 7.4) at 4 °C for 2 h. Bacterial cells were recovered by centrifugation, washed, and treated with 500 μl of 20 mm Tris-HCl, pH 8.0 containing 10 mm EDTA. The supernatant and eluted proteins were precipitated with trichloroacetic acid and analyzed with 12% SDS-PAGE.
Flow Cytometry Analysis of MBL Binding and C4 Deposition on S. aureus Cells
IgG-binding protein A-deficient S. aureus Δspa mutant (M0107) and its derivates were used. Ethanol-fixed S. aureus cells (1.0 × 109 cells) were incubated with MBL/MASP (1 μg) in 500 μl of buffer C (10 mm Tris-HCl, pH 7.4, 150 mm NaCl, 10 mm CaCl2, 1% BSA, and 0.05% Tween 20) for 2 h at 4 °C. After washing with washing buffer (10 mm Tris-HCl, pH 7.4, 140 mm NaCl, 0.05% Tween 20, 1 mm CaCl2), C4 (800 ng, Complement Tech.) was incubated with S. aureus cells in 150 μl of buffer C for 1 h at 37 °C. Alternatively, fixed S. aureus cells were incubated with 2–20% human serum with in buffer C (500 μl) for 1 h at 37 °C. To detect bound C4b, mouse anti-human C4 monoclonal antibody (mAb) (Bioporto, Denmark, diluted 1:500) and goat F(ab′)2 anti-mouse IgG antibodies conjugated with fluorescein 5-isothiocyanate (FITC) (Beckman Coulter, diluted 1:200) were used. To detect bound MBL, mouse anti-human MBL mAb (Dobeel, Korea, diluted 1:1,000) was used. Then, S. aureus cells were sonicated for 30 s to make cells single to perform flow cytometry analysis (Beckman Coulter).
ELISA Assay
MBL binding to purified WTA and resulting C4 deposition were evaluated using enzyme-linked immunosorbent assay (ELISA, 23), with some modifications. Briefly, 5 μg of each ligand in 50 μl of PBS (pH 7.5) were applied to F96 Cert. maxisorp immuno plates (duplicate, Nunc) and absorbed overnight at room temperature. Wells were washed with washing buffer and blocked with 200 μl of a buffer D (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 1% BSA) for 1 h at room temperature. After washing, the wells were incubated with 100 ng of MBL or MBL/MASP in 50 μl of buffer C for 2 h at 4 °C. When the C4 deposition was determined, wells were washed and incubated with C4 (100 ng) in 50 μl of buffer C for 1 h at 37 °C. Alternatively, immobilized ligands were incubated with 6% human serum (3 μl) in 50 μl of buffer C. Primary antibodies for MBL and C4b were used for flow cytometry analyses with a dilution of 1:1,000 and 1:3,000, respectively. Secondary antibodies were goat anti-mouse IgG (H+L) conjugated with horseradish peroxidase (HRP) (Beckman Coulter, 1:10,000 dilution). The resulting plates were developed with the substrate, 3,3′,5,5′-tetramethylbenzidine (Zymed Laboratories Inc.) in the dark and stopped with 2 n H2SO4. Absorbance at 450 nm was recorded using a microplate reader (Thermo Scientific). To monitor the inhibition of MBL binding to WTA by total IgGs, or anti-WTA-IgGs, the tested materials, and rMBL were mixed and used for ELISA. Also, to examine the inhibitory effects of MBL binding to WTA by mannan, mannose, or EDTA, we prepared the mixture of MBL (10 ng) with mannan (0.5 and 5 μg), mannose (final concentration 10 and 100 mm) or EDTA (10 and 100 mm), and then the mixtures were added to the WTA-coated microplate wells. The binding ability of MBL to WTA was estimated as the same method as described above. Data were representative of at least three independent experiments.
Determination of anti-WTA IgG Levels
Levels of anti-WTA-IgGs in human serum were determined by ELISA. WTA (5 μg)-coated microplates were incubated with 0.1 μl of human serum in 50 μl of buffer E (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.05% Tween 20) containing 1% BSA for 2 h at 4 °C, and bound IgGs were detected with mouse monoclonal anti-human-IgG antibodies (Sigma-Aldrich) and Goat F(ab′)2 anti-mouse IgG (H+L) antibodies conjugated with HRP (Human Ads). The concentration was estimated compared with that of control, purified anti-WTA-IgGs.
Affinity Purification of Anti-WTA-IgGs from Human IVIG Serum using WTA-coated Nitrocellulose Membrane
Anti-WTA-IgGs were affinity-purified as described (32). Briefly, 1 μg of purified WTA or WTA-free PG in 5 μl of PBS was spotted onto a nitrocellulose membrane (10 × 3 mm, Whatman, pore 0.45 μm), and baked at 100 °C for 1 h. The membranes were washed with buffer E and blocked with buffer D for 1 h at room temperature. Twenty sheets of membrane were prepared for each sample and were incubated with 250 mg of commercially available human intravenous immunoglobulins (IVIG, SK Chemicals, Seoul) in 25 ml of buffer E for 2 h at 4 °C. After washing with buffer E, bound IgGs were eluted with 1 ml of 0.1 m glycine (pH 2.8) and immediately neutralized with 1 n KOH to pH 7.5.
Isolation of Human Neutrophils and Opsonophagocytosis Assay
The peripheral blood neutrophils were isolated from healthy young donors using a method involving dextran sedimentation and differential centrifugation through a Ficoll-Hypaque density gradient as described our previous method (35). The donors were confirmed not to have taken any anti-inflammatory drugs for at least 3 weeks before sampling. Informed consent was obtained from all participants and the local institutional review board at Dong-A University Hospital approved the study. The contaminating monocytes were deficient using anti-CD14 Ab-coated magnetic beads (Milteny Biotec Inc., Auburn, CA). The detection of CD14+ cells in the separated neutrophils was less than 0.1% in flow cytometry analysis using FITC-conjugated anti-CD14 Ab. The neutrophils were shown to be 98% pure by CD66b staining. The neutrophils (1 × 107/1 ml) were labeled by PKH67 Green Fluorescent Cell Linker kit (Sigma-Aldrich) and rMBL- or MBL/MASP-treated S. aureus (1 × 108/1 ml) cells were labeled with PKH26 Red Fluorescent Cell Linker kit (Sigma-Aldrich). The cells were washed three times using PBS. The PKH67-labeled neutrophils (1 × 105/100 μl) were cultured with or without PKH26-labeled wild type or mutant S. aureus (1 × 106/100 μl) cells for 1 h in RPMI 1640 medium supplemented with 1% glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin in the presence or absence of 5% FBS at 37 °C in a humidified atmosphere containing 5% CO2. The percentage of PKH26-staining cells in total neutrophils was analyzed using flow cytometry. Neutrophils were shown to be 99% viable by Trypan Blue exclusion and 98–99% pure by staining with Giemsa.
RESULTS
Human MBL Binding to S. aureus Cells Requires the S. aureus tagO Gene
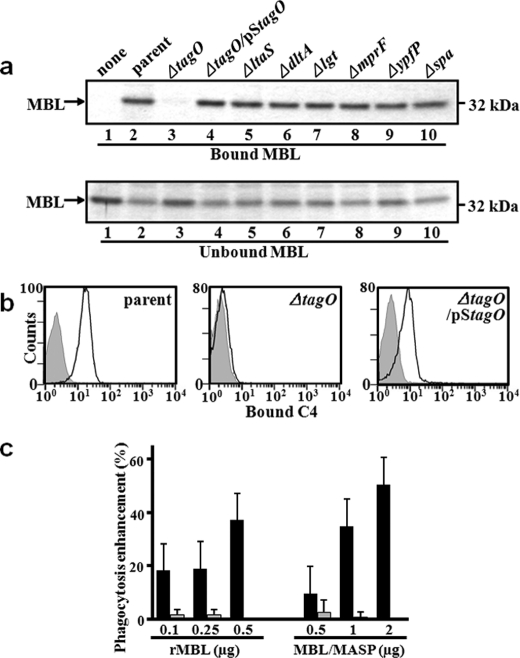
We have previously reported that human MBL binds to S. aureus PG and activates the PG-dependent lectin complement pathway (21). Retrospectively, the commercially available PG in our previous study is suspected to have other bacterial components, including WTA, LTA, and lipoproteins. PG, WTA, and LTA are major glycopolymers containing N-GlcNAc on S. aureus cell surface (see supplemental Fig. S1 for structures of PG and WTA). The recent availability of several S. aureus cell wall component-deficient mutants has prompted us to re-examine which molecule(s) of the S. aureus cell envelope is recognized by human MBL. In this experiment, we used seven S. aureus mutant strains, such as the WTA-deficient ΔtagO mutant (20), the LTA-deficient ΔltaS mutant (25, 31), the d-Ala residue of LTA and the WTA modification-deficient ΔdltA mutant (36), the lipoprotein maturation-deficient Δlgt mutant (37), the lysylphosphatidylglycerol-deficient ΔmprF mutant (38), the glycolipid-deficient ΔypfP mutant (39), the IgG-binding protein A-deficient Δspa mutant (31), and a parental S. aureus RN4220 strain (40). After incubation with human rMBL and S. aureus mutants, bacteria were precipitated, and the MBL bound to bacteria was analyzed by SDS-PAGE. As shown in Fig. 1a, only WTA-deficient ΔtagO mutant cannot bind to MBL (lane 3) and the ΔtagO mutant introduced by a plasmid harboring tagO gene (ΔtagO/pStagO) restored the binding ability to MBL (lane 4). Under the same conditions, human l-ficolin, another serum protein involved in the lectin complement pathway, did not bind any of these S. aureus mutants (data not shown). Requirement of the S. aureus tagO gene for MBL binding to S. aureus cells was also observed in the S. aureus MW2 strain background (41) (data not shown). To further examine whether the tagO-dependent MBL binding to S. aureus cells is functional for complement activation or not, we determined the induction of MBL/MASP complex-mediated C4 deposition on the S. aureus cell surface by flow cytometry analysis. To avoid the nonspecific binding by S. aureus protein-A with primary and secondary IgG antibody, we used S. aureus protein-A-deficient Δspa mutant as a parental strain. As expected, ΔtagO mutant did not show C4 deposition, whereas the parental strain and ΔtagO mutant harboring pStagO clearly induced C4 deposition (Fig. 1b). Taken together, these results demonstrate that a requirement of the S. aureus tagO gene for MBL/MASP activation, suggesting that S. aureus WTA is a ligand for the MBL/MASP complex.
FIGURE 1.
MBL binding to S. aureus cells requires the S. aureus tagO gene. a, screening for a MBL ligand using S. aureus cell wall mutants. Ethanol-fixed S. aureus mutant cells (2.0 × 109 cells) were incubated with rMBL (1 μg), recovered by centrifugation, washed, and the bound MBL was eluted with EDTA-containing buffer. MBL in the eluate (bound) and supernatant (unbound) was detected on 12% SDS-PAGE with CBB staining. S. aureus mutants are described in Table 1 and in the text. Among them, the ΔtagO mutant loses WTA. b, tagO-dependent MBL binding induces C4 deposition on S. aureus cells. S. aureus M0107, T258 (ΔtagO), and T358 (ΔtagO/pStagO) cells (1.0 × 109 cells) were incubated with the MBL/MASP complex (1 μg) at 4 °C for 2 h, washed, and further incubated with C4 (800 ng) at 37 °C for 1 h. Bound C4b on S. aureus cells was detected by flow cytometry (Beckman Coulter) with mouse anti-human C4 mAb and goat F(ab′)2 anti-mouse antibody conjugated with FITC. Gray area represents data without serum. c, tagO-dependent opsonin activity of MBL on S. aureus cells for neutrophils. Ethanol-fixed S. aureus parental (M0107) or ΔtagO mutant (T258) cells labeled with PKH26 (1.0 × 106 cells) were incubated with rMBL (250 ng) or MBL/MASP complex (1 μg) at 4 °C for 2 h. Bacterial cells were washed, and further incubated with human neutrophils labeled with PKH67 (1.0 × 105 cells) at an MOI of 10:1 at 37 °C for 1 h. The phagocytosis of S. aureus cells by neutrophils was analyzed by flow cytometry. The opsonin activity of rMBL or MBL/MASP is shown as relative increase in phagocytosis of S. aureus cells by neutrophilis.
Requirement of S. aureus tagO on MBL-mediated Opsonophagocytosis by Neutrophils
To investigate whether the tagO-dependent MBL binding to S. aureus cells functions as an opsonin, parental or WTA-deficient ΔtagO mutant cells were incubated with rMBL or MBL/MASP complex, and were examined for neutrophilic phagocytosis (Fig. 1c). When neutrophilic phagocytosis was determined after 1 h of incubation, rMBL (500 ng) and MBL/MASP complex (2 μg) increased the phagocytosis of S. aureus parental cells by 37.3 and 50.5%, respectively, while there was no or only little increase for ΔtagO mutants (0% by MBL and 7.5% by MBL/MASP complex, respectively) (flow cytometry data are shown in supplemental Fig. S2). These results suggest that the binding of the rMBL or MBL/MASP complex to S. aureus WTA enhances neutrophilic opsonophagocytosis.
S. aureus WTA Is a Ligand of Human MBL
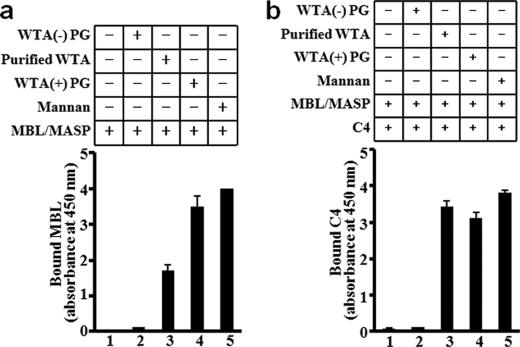
The logical next experiment was to purify WTA from S. aureus parental cells to confirm that WTA is a MBL ligand. S. aureus WTA is known to be covalently bound to the N- MurNAc residue of the PG layer. Therefore, we took the following purification scheme: First we purified insoluble PG from S. aureus and then treated it with two well-known PG-degrading enzymes: lysozyme and lysostaphin. This purification method yielded soluble WTA harboring N-GlcNAc-N-MurNAc disaccharide with l-Ala-d-isoGln-l-Lys-d-Ala tetrapeptide (named purified WTA) as shown in supplemental Fig. S1. As expected, WTA was obtained in a single peak by anion ion-exchange column chromatography, and the presence of WTA was confirmed both by phosphorus assay and by the silver staining of PAGE (supplemental Fig. S3). The purified S. aureus WTA was immobilized on 96-well plates and used to estimate the binding of the native MBL/MASP complex to it and following C4 deposition by ELISA (Fig. 2, a and b). As controls, we used WTA-free soluble polymeric PG (WTA(−)-PG), WTA-containing insoluble polymeric PG (WTA(+)-PG) prepared by our previous method (32) and commercially available mannan as a ligand of MBL. The MBL/MASP complex showed a binding ability to the purified WTA and WTA(+)-PG and to mannan (Fig. 2a), and these three ligands mediated comparable C4 deposition (Fig. 2b). Under the same conditions, WTA(−)-PG did not allow MBL/MASP complex binding and C4 deposition (Fig. 2b). Furthermore, because MBL binding to microbial polysaccharides is through a carbohydrate recognition domain in a Ca2+-dependent manner (42), we examined the effects of mannan, mannose, and EDTA on MBL binding to WTA (supplemental Fig. S4). Mannan, mannose, and EDTA inhibited the MBL binding to WTA, suggesting that the binding is through the carbohydrate recognition domain of MBL. Taken together, these results suggest that WTA of S. aureus functions as a ligand of the MBL and MBL/MASP complex in vitro and that MBL binds to WTA but not other glycopolymers on the S. aureus cell surface.
FIGURE 2.
S. aureus WTA is a functional ligand of human MBL. MBL/MASP complex (0.1 μg) were incubated at 4 °C for 2 h with immobilized ligands (5 μg) including WTA-free-PG [WTA(−)-PG], purified WTA, WTA-containing PG [WTA(+)-PG], or mannan. After washing of the unbound MBL/MASP complex, C4 (0.1 μg) was added and incubated at 37 °C for 1 h. Bound MBL (a) or C4 (b) was detected using mouse anti-human MBL mAb or mouse anti-human C4 mAb, respectively, with goat anti-mouse IgG (H+L) antibody conjugated with HRP and a substrate, 3,3′,5,5′-tetramethylbenzidine (450 nm). The results are representative of three independent experiments.
MBL in Adult Serum Does Not Bind to WTA
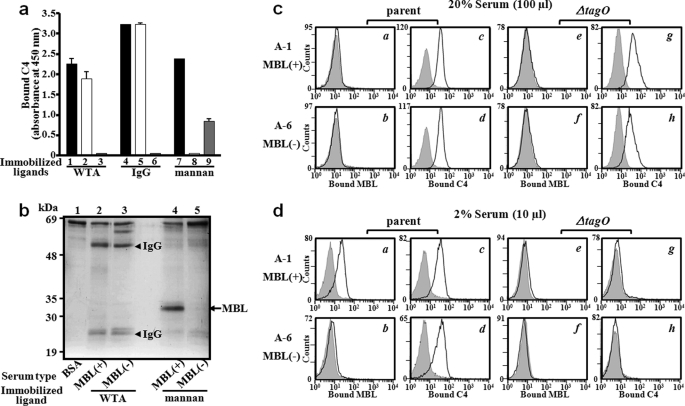
To determine whether WTA functions as a ligand of MBL in human serum, WTA-mediated C4 deposition was measured using MBL-sufficient and MBL-deficient adult sera. Whereas MBL-sufficient serum can induce WTA-dependent C4 deposition (column 1 in Fig. 3a), MBL-deficient serum still induced WTA-mediated C4 deposition (column 2). Unexpectedly, WTA-mediated C4 deposition was not induced in the C1q-deficient serum (column 3). As a control, immunoglobulin G (IgG)-mediated dependent C4 deposition (columns 4 and 5) was not induced in the in C1q-deficient serum (column 6). Mannan-dependent C4 deposition was induced in an MBL-dependent manner (columns 7 and 8) and in a C1q-independent manner (column 9). These results suggest that WTA-mediated C4 deposition in adult serum is not induced by the MBL/MASP complex but rather by C1q. To provide the biochemical evidences as to why WTA-mediated C4 deposition took place in a C1q- dependent but not MBL-dependent manner, we determined serum proteins that were recruited onto WTA or mannan (Fig. 3b). Mannan recruited serum MBL (lane 4). However, WTA recruited human heavy and light chain IgGs, but not MBL, from adult serum regardless of MBL (lanes 2 and 3). To further confirm this observation on S. aureus whole cells, we performed flow cytometry analysis. For this purpose, we collected the sera from six adults with different MBL serum levels (Table 2) and performed MBL binding and C4 deposition on S. aureus parent and ΔtagO mutant cells (supplemental Fig. S5). Among them, representative results from two adults are shown in Fig. 3, c and d, respectively. Consistent with Fig. 3a, serum MBL did not bind to S. aureus parental cells in a final 20% MBL-sufficient adult serum (panel a in Fig. 3c). In contrast, we found that 2% serum allowed MBL binding to S. aureus cells that was dependent on the S. aureus tagO gene (panels a and e in Fig. 3d), which was not observed in MBL-deficient adult serum (panel b in Fig. 3d). The data suggest that serum MBL binding to WTA in adults is inhibited by some serum factors. In addition, being consistent with Fig. 3a again, and C4 deposition was activated regardless of MBL in both 20 and 2% adult sera (panels c and d in Fig. 3, c and d). Interestingly, the C4 depositions in 2% adult sera were dependent on the S. aureus tagO gene (panels c, d, g, and h in Fig. 3d), suggesting that WTA on S. aureus cell surface serves as a strong ligand to induce C1q-dependent C4 deposition in adult serum.
FIGURE 3.
Serum MBL in adults does not bind to S. aureus WTA. a, C1q-dependent C4 deposition on purified WTA by MBL- and C1q-deficient serums. Purified WTA, IgG, or mannan (5 μg) was immobilized and incubated with adult serum (6%) at 37 °C for 1 h, and bound C4 was detected as described in the legend to Fig. 2. Adult serum used was MBL-sufficient (black bar columns of 1, 4, and 7), MBL-deficient (white bar columns of 2, 5, and 8), or C1q-deficient (gray columns of 3, 6, and 9). The data are shown as the mean ± S.D. using at least three independent experiments. b, MBL in adult serum could not bind to S. aureus WTA. WTA or mannan-coated 96-well plates were incubated at 4 °C for 2 h with 25 μl of MBL-sufficient [(MBL(+)] or MBL-deficient [MBL(−)] adult serum. Bound proteins were eluted by SDS and analyzed by 12% SDS-PAGE with CBB (Coomassie Brilliant Blue) staining. c and d, WTA is a strong inducer for the classical complement pathway in adult serum. S. aureus M0107 (parent) or T258 (ΔtagO) cells were incubated with MBL-sufficient (A-1) or MBL-deficient (A-6) adult serum. Serum concentration used was 20% (c) or 2% (d). Bound MBL or C4 on S. aureus cells were detected by flow cytometry as described in the legend of Fig. 1b. The gray area represents data without serum.
TABLE 2.
Estimation of serum IgGs, MBL, anti-WTA-IgGs, MBL binding, and C4 deposition of adult and infant sera on S. aureus parent and ΔtagO mutant cells
| SerumAdult(A) Infant(I) | Age, year(y) month(m)/gender, male(M) female(F) | IgG levels | MBL levels | Anti-WTA-IgGs A450x dilution factor | 20% Serum |
2% Serum |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent |
ΔtagO |
Parent |
ΔtagO |
|||||||||
| Bound MBL | Bound C4 | Bound MBL | Bound C4 | Bound MBL | Bound C4 | Bound MBL | Bound C4 | |||||
| mg/ml | μg/ml | % | % | % | % | % | % | % | % | |||
| A-1 | 17y/F | 15.60 | 5.38 | 808.25 | 7.41 | 98.21 | 8.74 | 98.46 | 65.95 | 93.82 | 6.24 | 4.77 |
| A-2 | 82y/M | 16.10 | 4.70 | 645.25 | 4.37 | 99.25 | 7.66 | 98.91 | 96.24 | 77.05 | 9.37 | 3.27 |
| A-3 | 25y/F | 14.90 | 4.61 | 955.75 | 4.34 | 97.92 | 6.50 | 96.50 | 82.22 | 85.77 | 5.55 | 4.34 |
| A-4 | 26y/F | 13.00 | <0.01 | 1225.80 | 4.01 | 94.03 | 3.24 | 97.72 | 5.76 | 82.37 | 7.09 | 2.47 |
| A-5 | 50y/F | 9.38 | <0.01 | 1185.00 | 11.10 | 93.37 | 5.44 | 98.37 | 12.58 | 61.78 | 6.61 | 3.07 |
| A-6 | 49y/F | 12.20 | <0.01 | 1448.00 | 2.67 | 98.30 | 8.75 | 92.51 | 7.53 | 88.91 | 5.49 | 2.51 |
| I-1 | 6m/M | 4.62 | 6.49 | 49.75 | 99.46 | 99.04 | 8.90 | 61.44 | 92.83 | 86.47 | 3.83 | 2.33 |
| I-2 | 5m/M | 3.54 | 6.24 | 72.00 | 99.65 | 99.16 | 10.45 | 95.11 | 90.35 | 87.09 | 8.74 | 2.70 |
| I-3 | 9m/F | 5.28 | 4.70 | 64.00 | 99.82 | 96.49 | 6.01 | 29.05 | 62.18 | 93.09 | 3.57 | 2.54 |
| I-4 | 4m/M | 3.99 | <0.01 | 93.75 | 5.79 | 99.78 | 6.12 | 60.70 | 3.10 | 8.01 | 11.45 | 0.97 |
| I-5 | 5m/F | 6.36 | <0.01 | 69.50 | 3.36 | 99.31 | 6.85 | 43.30 | 4.03 | 3.52 | 5.39 | 2.42 |
| I-6 | 4m/F | 3.50 | <0.01 | 98.25 | 2.37 | 99.16 | 8.39 | 53.55 | 3.01 | 5.99 | 6.01 | 2.02 |
Affinity-purified Anti-WTA Antibodies Specifically Inhibit MBL Binding to WTA
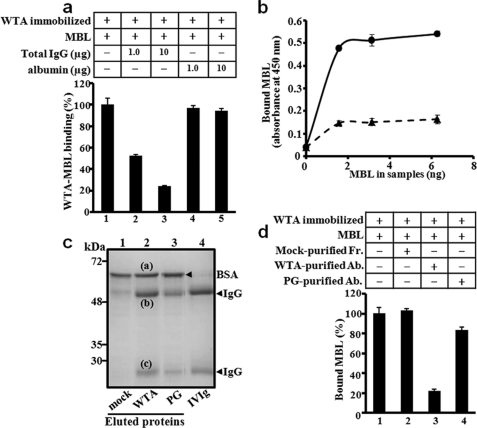
Because serum IgGs were recruited onto WTA, we hypothesized that anti-WTA antibodies in serum might compete with MBL for WTA binding and also induce C4 deposition in a C1q-dependent manner. If this hypothesis is correct, MBL binding to WTA should be inhibited by addition of human IgGs. As expected, purified human IgGs inhibited MBL binding to purified WTA in a dose-dependent manner (Fig. 4a). In addition, depletion of IgGs from the MBL-sufficient adult serum recovered MBL binding to purified WTA (Fig. 4b) and also to S. aureus parental cells, but not to the ΔtagO mutant (supplemental Fig. S6). These results demonstrate that serum IgGs in adults inhibit MBL binding to S. aureus WTA. Next, we examined whether the anti-WTA antibody was responsible for this inhibition. For this purpose, we attempted to purify anti-WTA antibodies from IVIG using purified WTA-blotted nitrocellulose membrane as an affinity purification media. The membranes blotted with soluble WTA-free PG or mock treatment were prepared as controls. Three proteins bands were eluted from the purified WTA-blotted and WTA-free PG-blotted membranes (Fig. 4c). N-terminal sequences by Edman degradation identified that the top protein band (a), which was also eluted from the mock-treated membrane (lane 1), was determined as BSA, a component of the blocking solution. The second (b) and third (c) bands, which appeared specifically in lanes 2 and 3 and showed the same gel mobility with IVIG, were determined to be human heavy and light chain IgGs, respectively. Additionally, the IgGs were re-confirmed using human anti-IgG-specific polyclonal antibodies (data not shown). Then we performed an inhibition assay using the affinity-purified antibodies (Fig. 4d). 1 μg of the purified anti-WTA antibodies inhibited 80% of MBL binding to WTA (column 3). Under the same conditions, the eluted proteins from mock and WTA-free PG-blotted membranes showed 0 and 17% inhibition, respectively (columns 2 and 4). The inhibitory effect of purified anti-WTA antibodies was further examined on S. aureus whole cells using ELISA. In the ELISA assay, MBL bound to S. aureus parental cells but not ΔtagO mutant cells, (supplemental Fig. S7). 1 μg of the purified anti-WTA antibodies inhibited 90% of MBL binding to S. aureus parental cells (column 6 in supplemental Fig. S7). Under the same conditions, the equivalent fractions eluted from mock and WTA-free PG-blotted membranes showed 23 and 25% inhibition, respectively (columns 4 and 8 in supplemental Fig. S7). Taken together, these results demonstrate that anti-WTA antibodies specifically inhibit the MBL binding to both purified WTA and S. aureus whole cells.
FIGURE 4.
Anti-WTA-antibodies inhibit MBL binding to WTA. a, inhibitory effects of serum IgGs on MBL binding to WTA. Effect of total IgGs or BSA on MBL (10 ng) binding to WTA (5 μg) was determined by ELISA. Total IgGs were prepared from adult serum using a protein G column. Data were representative of at least three independent experiments. b, IgG-depletion recovers MBL binding to WTA in adult serum. Serum MBL binding to purified WTA was determined for IgG-depleted (line) or parental adult serum (dot-line) by ELISA. Data were plotted on MBL amounts in each serum. IgGs were depleted using a protein-G column. c, affinity purification of anti-WTA antibodies. Nitrocellulose membranes spotted by mock (PBS, lane 1), purified WTA (lane 2), or WTA-free PG (lane 3) were incubated with commercially available human IVIG for 2 h at 4 °C. Eluted proteins from the membranes were analyzed by 12% SDS-PAGE with CBB staining. BSA from the blocking reagent (band a), IgG heavy chain (b), and IgG light chain (c) were determined by N-terminal sequencing. d, anti-WTA antibodies inhibit MBL binding to WTA. The effects of affinity-purified IgGs (1 μg) prepared in Fig. 4c were examined by ELISA. MBL binding to WTA was determined in ELISA as described in the legend to Fig. 4a. Data were representative of at least three independent experiments.
Serum MBL of Infants with Immature Adaptive Immunity Senses WTA of S. aureus
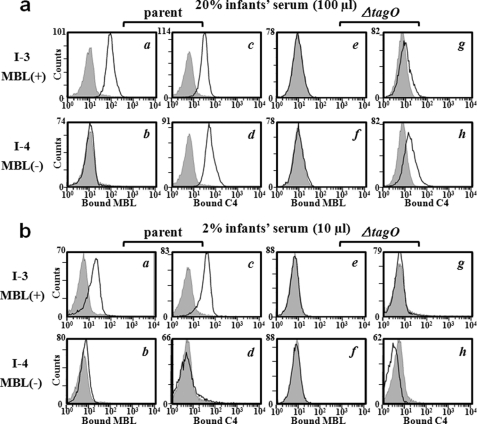
During pregnancy, maternal IgG antibodies are transported across the placenta into the fetal circulation, providing the newborn infant with antibody levels comparable to those of their mothers (43). From 6 to 12 months after birth, as the maternally derived IgG of the infant is catabolized, the antibody level gradually decreases while the infant immune system begins to produce its own antibody at about six months of age. In particular, MBL-deficient infants who have not yet fully developed adaptive immune system are known to be susceptible to S. aureus infection (9–11) However, the detailed molecular mechanisms of this susceptibility have not yet been clearly elucidated. We hypothesized that serum MBL in infants could bind to WTA and S. aureus whole cells due to the low concentrations of anti-WTA antibodies. To test this hypothesis, we collected serum from six adults and six infants with different MBL serum levels (Table 2). First, when the total serum IgGs and anti-WTA-IgG amounts of all six adults and infants were determined, the amounts of IgG and anti-WTA-IgG in the adults averaged 3 and 14 times higher than those of the six infants, respectively, indicating that adaptive immunity is immature in all infants. Finding of higher titers of serum anti-WTA-antibodies in MBL-deficient adults than MBL-sufficient adults supports previous reports that deficiency in MBL enhances antigen-specific IgG production following immunization with bacterial polysaccharide (9). Using these sera, we assayed MBL binding and C4 deposition on S. aureus parent and ΔtagO mutant cells (supplemental Fig. S8). Among them, representative results from two infants are shown in Fig. 5. As expected, in contrast to the adult serum (Fig. 3c), MBL-sufficient infant serum at 20% showed MBL binding on S. aureus parental cells in an S. aureus tagO gene-dependent manner (panels a and e in Fig. 5a). MBL was not bound on S. aureus cells when the serum was deficient for MBL (panel b in Fig. 5a). Under the same conditions, C4 deposition was induced on S. aureus parental cells regardless of the MBL concentrations (panels c and d in Fig. 5a) and that on ΔtagO cells it was low regardless of MBL (panels g and h in Fig. 5a), which is distinct from adult sera (Fig. 3c). In addition, whereas MBL-sufficient serum-activated C4 deposition on S. aureus parental cells (panel c in Fig. 5b), MBL-deficient infant serum at 2% with low serum levels of anti-WTA-IgGs did not activate it (panel d in Fig. 5b). Therefore, MBL-sufficient infant serum, which has immature adaptive immunity, can recognize WTA on the S. aureus cell surface. In addition, MBL-deficient infant sera have a weak C4 deposition ability to activate the complement pathway.
FIGURE 5.
MBL binding, IgGs binding in infant sera. S. aureus M0107 (parent) or T258 (ΔtagO) cells were incubated with MBL-sufficient (I-3) or MBL-deficient (I-4) infant serum, and bound MBL or C4 on S. aureus cells was detected by flow cytometry as described in the legend to Fig. 3c. The serum concentration used was 20% in the upper 8 columns (panels a–h) or 2% in the lower 8 columns (panels a–h). The gray area represents data without serum.
DISCUSSION
The availability of S. aureus cell wall mutant strains led us to screen MBL ligand. Among the mutants, the WTA-deficient S. aureus ΔtagO mutant was impaired in its binding capacity to MBL, and introduction of a plasmid-encoded tagO gene restored the binding ability, enabling us to demonstrate the evidence of direct binding between the MBL/MASP complex and purified S. aureus WTA. Finding of the inhibition of the MBL-WTA interaction by human adult serum allowed us to understand the inhibitory effect of anti-WTA antibodies on the MBL-WTA interaction and, thus, allowed us to find that MBL in sera from infant, who have not yet fully developed the adaptive immune system, could bind to WTA. The latter observation is consistent with the idea that the MBL-mediated lectin complement pathway has been suggested to be a primitive immune response compared with the antigen-antibody complex-mediated classical complement pathway (44). In addition, while it is known that MBL-deficient infants are susceptible to S. aureus infection (9–11), our biochemical and immunological evidence highlights the biological importance of MBL in children. We also found that WTA on the S. aureus cell surface is a strong antigen to activate C1-dependent classical complement pathway in adult serum. To our knowledge, this is the first biochemical and immunological study demonstrating the importance of S. aureus WTA as a ligand for MBL. We propose that S. aureus WTA is a ligand of serum MBL in infants and also serves as a strong antigen in developing anti-WTA IgGs, which trigger efficient opsonization and complement activation against S. aureus infection. The identification and characterization of neutrophil receptors recognizing S. aureus cells opsonized with MBL or MBL/MASP complex will shed further insight into the molecular mechanism of MBL-mediated opsonophagocytosis.
We recently demonstrated that S. aureus SitC lipoprotein functions as a native ligand of human Toll-like receptor 2 (TLR2) (45). This SitC lipoprotein was also purified from S. aureus insoluble PG, indicating that SitC is also exposed on the cell wall surface of S. aureus. Our current and our previous studies suggest that S. aureus WTA is recognized by MBL, a serum soluble pattern recognition protein, which activates the complement system and functions as an opsonin to facilitate neutrophilic phagocytosis, while S. aureus lipoproteins are recognized by TLR2, a membrane-associated pattern recognition receptor that induces inflammatory cytokine production. These observations imply that the host has dual innate immunity for sensing S. aureus infections. Additionally, MBL binds to mannose and N-GlcNAc residues in sugar chains presented on microorganisms and a high mannose-type glycans with high affinity (3, 42) and functions in a host defense system against invading pathogens and cancer cells. Because MBL binding to mannan was not abolished in adult serum (Fig. 3a), the inhibition of MBL-WTA interactions by anti-WTA antibodies can keep MBL concentrations high in adult serum, which may be helpful in combating other important targets, such as cancer cells.
The molecular structures of bacterial cell wall glycopolymers are highly diverse and species- or strain-specific (18). Despite recent developments in analytical techniques in the glycobiology field, our knowledge of bacterial cell wall compositions and structures are still limited. In our study, we used S. aureus mutant strains for screening MBL ligands. Definition of the biological and immunological significances of other cell wall glycopolymers, which may regulate host defense responses or be involved in linking innate and adaptive immunity, and their application to the medical field is a challenge. More comprehensive and functional studies in this field are anticipated in the near future.
Supplementary Material
Acknowledgments
We thank Drs. J. S. Yum and H. M. Moon of the Dobeel Corp. for providing the recombinant human MBL.
This work was supported by Programs M10400000028-04J0000-02, 2009-032-2000, 2008-8057-6001, and BK21 of the National Research Foundation of Korea.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
- MBL
- mannose-binding lectin
- GlcNAc
- acetylglucosamine
- rMBL
- recombinant MBL
- MASP
- MBL-associated serine protease
- WTA
- wall teichoic acid
- LTA
- lipoteichoic acid
- PG
- peptidoglycan
- ManNAc
- acetylmannosamine
- MurNAc
- acetylmuramic acid
- IVIG
- human intravenous immunoglobulins
- TLR
- Toll-like receptor.
REFERENCES
- 1.Hoffmann J. A., Kafatos F. C., Janeway C. A., Ezekowitz R. A. (1999) Science 284, 1313–1318 [DOI] [PubMed] [Google Scholar]
- 2.Ip W. K., Takahashi K., Ezekowitz R. A., Stuart L. M. (2009) Immunol. Rev. 230, 9–21 [DOI] [PubMed] [Google Scholar]
- 3.Weis W. I., Drickamer K. (1996) Annu. Rev. Biochem. 65, 441–473 [DOI] [PubMed] [Google Scholar]
- 4.Matsushita M., Fujita T. (1992) J. Exp. Med. 176, 1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel S., Vorup-Jensen T., Stover C. M., Schwaeble W., Laursen S. B., Poulsen K., Willis A. C., Eggleton P., Hansen S., Holmskov U., Reid K. B., Jensenius J. C. (1997) Nature 386, 506–510 [DOI] [PubMed] [Google Scholar]
- 6.Dahl M. R., Thiel S., Matsushita M., Fujita T., Willis A. C., Christensen T., Vorup-Jensen T., Jensenius J. C. (2001) Immunity 15, 127–135 [DOI] [PubMed] [Google Scholar]
- 7.Takahashi M., Ishida Y., Iwaki D., Kanno K., Suzuki T., Endo Y., Homma Y., Fujita T. (2010) J. Exp. Med. 207, 29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walport M. J. (2001) N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 9.Super M., Thiel S., Lu J., Levinsky R. J., Turner M. W. (1989) Lancet 2, 1236–1239 [DOI] [PubMed] [Google Scholar]
- 10.Summerfield J. A., Sumiya M., Levin M., Turner M. W. (1997) BMJ 314, 1229–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch A., Melbye M., Sørensen P., Homøe P., Madsen H. O., Mølbak K., Hansen C. H., Andersen L. H., Hahn G. W., Garred P. (2001) JAMA 285, 1316–1321 [DOI] [PubMed] [Google Scholar]
- 12.Santos I. K., Costa C. H., Krieger H., Feitosa M. F., Zurakowski D., Fardin B., Gomes R. B., Weiner D. L., Harn D. A., Ezekowitz R. A., Epstein J. E. (2001) Infect. Immun. 69, 5212–5215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neth O., Jack D. L., Johnson M., Klein N. J., Turner M. W. (2002) J. Immunol. 169, 4430–4436 [DOI] [PubMed] [Google Scholar]
- 14.Lynch N. J., Roscher S., Hartung T., Morath S., Matsushita M., Maennel D. N., Kuraya M., Fujita T., Schwaeble W. J. (2004) J. Immunol. 172, 1198–1202 [DOI] [PubMed] [Google Scholar]
- 15.Neth O., Jack D. L., Dodds A. W., Holzel H., Klein N. J., Turner M. W. (2000) Infect. Immun. 68, 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L., Takahashi K., Dundee J., Shahroor-Karni S., Thiel S., Jensenius J. C., Gad F., Hamblin M. R., Sastry K. N., Ezekowitz R. A. (2004) J. Exp. Med. 199, 1379–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Archer G. L., Climo M. W. (2001) N. Engl. J. Med. 344, 55–56 [DOI] [PubMed] [Google Scholar]
- 18.Weidenmaier C., Peschel A. (2008) Nat. Rev. Microbiol. 6, 276–287 [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee A. N. (1969) J. Bacteriol. 98, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weidenmaier C., Kokai-Kun J. F., Kristian S. A., Chanturiya T., Kalbacher H., Gross M., Nicholson G., Neumeister B., Mond J. J., Peschel A. (2004) Nat. Med. 10, 243–245 [DOI] [PubMed] [Google Scholar]
- 21.Ma Y. G., Cho M. Y., Zhao M., Park J. W., Matsushita M., Fujita T., Lee B. L. (2004) J. Biol. Chem. 279, 25307–25312 [DOI] [PubMed] [Google Scholar]
- 22.Polotsky V. Y., Fischer W., Ezekowitz R. A., Joiner K. A. (1996) Infect. Immun. 64, 380–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ip W. K., Takahashi K., Moore K. J., Stuart L. M., Ezekowitz R. A. (2008) J. Exp. Med. 205, 169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisman L. E. (2007) Arch. Pediatr. 14, Suppl. 1, S31–S34 [DOI] [PubMed] [Google Scholar]
- 25.Fattom A. I., Horwith G., Fuller S., Propst M., Naso R. (2004) Vaccine 22, 880–887 [DOI] [PubMed] [Google Scholar]
- 26.Gründling A., Schneewind O. (2007) J. Bacteriol. 189, 2521–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita M., Endo Y., Taira S., Sato Y., Fujita T., Ichikawa N., Nakata M., Mizuochi T. (1996) J. Biol. Chem. 271, 2448–2454 [DOI] [PubMed] [Google Scholar]
- 28.Kang H. J., Lee S. M., Lee H. H., Kim J. Y., Lee B. C., Yum J. S., Moon H. M., Lee B. L. (2007) Immunology 122, 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vagner V., Dervyn E., Ehrlich S. D. (1998) Microbiology 144, 3097–3104 [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi J., Kojima T., Oyamada Y., Fujimoto K., Hattori H., Nakamura S., Inoue M. (1996) Antimicrob. Agents. Chemother. 40, 1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oku Y., Kurokawa K., Matsuo M., Yamada S., Lee B. L., Sekimizu K. (2009) J. Bacteriol. 191, 141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J. W., Kim C. H., Kim J. H., Je B. R., Roh K. B., Kim S. J., Lee H. H., Ryu J. H., Lim J. H., Oh B. H., Lee W. J., Ha N. C., Lee B. L. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 6602–6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bera A., Herbert S., Jakob A., Vollmer W., Götz F. (2005) Mol. Microbiol. 55, 778–787 [DOI] [PubMed] [Google Scholar]
- 34.Bartlett G. R. (1959) J. Biol. Chem. 234, 466–468 [PubMed] [Google Scholar]
- 35.Jo E. J., Lee H. Y., Lee Y. N., Kim J. I., Kang H. K., Park D. W., Baek S. H., Kwak J. Y., Bae Y. S. (2004) J. Immunol. 173, 6433–6439 [DOI] [PubMed] [Google Scholar]
- 36.Neuhaus F. C., Baddiley J. (2003) Microbiol. Mol. Biol. Rev. 67, 686–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmaler M., Jann N. J., Fierracin E., Landolt L. Z., Biswas L., Götz E., Landmann R. (2009) J. Immunol. 182, 7110–7118 [DOI] [PubMed] [Google Scholar]
- 38.Peschel A., Jack R. W., Otto M., Collins L. V., Staubitz P., Nicholson G., Kalbacher H., Nieuwenhuizen W. F., Jung G., Tarkowski A., van Kessel K. P., van Strijp J. A. (2001) J. Exp. Med. 193, 1067–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiriukhin M. Y., Debabov D. V., Shinabarger D. L., Neuhaus F. C. (2001) J. Bacteriol. 183, 3506–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novick R. P., Ross H. F., Projan S. J., Kornblum J., Kreiswirth B., Moghazeh S. (1993) EMBO J. 12, 3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baba T., Takeuchi F., Kuroda M., Yuzawa H., Aoki K., Oguchi A., Nagai Y., Iwama N., Asano K., Naimi T., Kuroda H., Cui L., Yamamoto K., Hiramatsu K. (2002) Lancet 359, 1819–1827 [DOI] [PubMed] [Google Scholar]
- 42.Weis W. I., Drickamer K., Hendrickson W. A. (1992) Nature 360, 127–134 [DOI] [PubMed] [Google Scholar]
- 43.Ghetie V., Ward E. S. (2000) Annu. Rev. Immunol. 18, 739–766 [DOI] [PubMed] [Google Scholar]
- 44.Ezekowitz R. A. (1991) Curr. Biol. 1, 60–62 [DOI] [PubMed] [Google Scholar]
- 45.Kurokawa K., Lee H., Roh K. B., Asanuma M., Kim Y. S., Nakayama H., Shiratsuchi A., Choi Y., Takeuchi O., Kang H. J., Dohmae N., Nakanishi Y., Akira S., Sekimizu K., Lee B. L. (2009) J. Biol. Chem. 284, 8406–8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaito C., Sekimizu K. (2007) J. Bacteriol. 189, 2553–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ichihashi N., Kurokawa K., Matsuo M., Kaito C., Sekimizu K. (2003) J. Biol. Chem. 278, 28778–28786 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.