Abstract
Rapid non-genomic effects of 17β-estradiol, the principal circulating estrogen, have been observed in a wide variety of cell types. Here we investigate rapid signaling effects of 17β-estradiol in rat hepatocytes. We show that, above a threshold concentration of 1 nm, 17β-estradiol, but not 17α-estradiol, stimulates particulate guanylyl cyclase to elevate cGMP, which through activation and plasma membrane recruitment of protein kinase G isoform Iα, stimulates plasma membrane Ca2+-ATPase-mediated Ca2+ efflux from rat hepatocytes. These effects are extremely rapid in onset and are mimicked by a membrane-impermeant 17β-estradiol-BSA conjugate, suggesting that 17β-estradiol acts at the extracellular face of the plasma membrane. We also show that 17β-estradiol binds specifically to the intact hepatocyte plasma membrane through an interaction that is competed by an excess of atrial natriuretic peptide but also shows many similarities to the pharmacological characteristics of the putative γ-adrenergic receptor. We, therefore, propose that the observed rapid signaling effects of 17β-estradiol are mediated either through the guanylyl cyclase A receptor for atrial natriuretic peptide or through the γ-adrenergic receptor, which is either itself a transmembrane guanylyl cyclase or activates a transmembrane guanylyl cyclase through cross-talk signaling.
Keywords: Calcium ATPase, Calcium Transport, Cyclic GMP (cGMP), Estrogen, Guanylate Cyclase (Guanylyl Cyclase), Hepatocyte, Protein Kinase G (PKG)
Introduction
The steroid 17β-estradiol is the principal and most potent circulating estrogenic hormone. The classical mode of 17β-estradiol signaling involves binding to cytosolic estrogen receptors (ERα or ERβ),3 translocation to the nucleus, and regulation of transcription (1, 2). However, numerous rapid non-genomic actions of 17β-estradiol have been demonstrated in a wide variety of cell types. These include cAMP elevation (3), cGMP elevation (4–9), KATP channel inhibition (5), MAPK activation (10, 11), MAPK inhibition (12), elevation of cytosolic Ca2+ concentration ([Ca2+]c) (5), attenuation of [Ca2+]c (7, 12–14), Ca2+ influx stimulation (15–17), Ca2+ influx inhibition (13, 18, 19), and Ca2+ efflux stimulation (13).
Direct measurements have shown that 17β-estradiol elevates cGMP in PC12 pheochromocytoma cells (4), human umbilical vein endothelial cells (6), pancreatic α (7) and β cells (5), and human coronary smooth muscle cells (9). Moreover, several studies using protein kinase G (PKG) inhibitors have implicated cGMP in rapid effects of 17β-estradiol (5, 7, 14, 20). These include decreased contractility of isolated porcine coronary arterial rings (20), attenuation of acetylcholine-induced Ca2+ transients in hypothalamic GT1–7 neuronal cells (14), inhibition of KATP channel activity in pancreatic β cells (5), increased frequency of [Ca2+]c oscillations in pancreatic β cells (5), and prevention of [Ca2+]c oscillations in pancreatic α cells (7).
Cellular cGMP generation is controlled by two distinct classes of guanylyl cyclases that differ in their cellular location and activation by specific ligands; soluble guanylyl cyclase exists in the cytosol and is activated by nitric oxide (NO) (21), whereas particulate guanylyl cyclases are trans-plasma membrane proteins that are activated by agonist binding to their extracellular receptor portions (22). Seven mammalian particulate guanylyl cyclase isoforms have been identified: GC-A through to GC-G (23). GC-A and GC-B are receptors for atrial natriuretic peptide (ANP) and B-type natriuretic peptide, whereas GC-C mediates the effects of guanylin and uroguanylin (22). GC-D, -E, -F, and -G are orphan receptors; endogenous ligands have yet to be identified (23). GC-A (24) and GC-C (25) have been detected in rat hepatocytes. GC-C is developmentally regulated; it is synthesized by late fetal and early neonatal rat liver but is not expressed by adult rat liver except under conditions of liver regeneration (26).
Differential effects of soluble and particulate GC activation, attributable to cGMP compartmentalization, have been demonstrated in several cell types (27–31). These include our own previous studies in rat hepatocytes; particulate guanylyl cyclase activation by ANP promotes plasma membrane recruitment of PKGIα, Ca2+ efflux stimulation, and [Ca2+]c attenuation, whereas soluble guanylyl cyclase activators are without effect (32–34). Interestingly, both particulate guanylyl cyclase (4–5, 7, 9, 14, 20) and soluble guanylyl cyclase (6, 35–38) have been implicated in the rapid effects of 17β-estradiol in different cell types, which is consistent with its diverse range of effects.
Many of the rapid actions of 17β-estradiol involve regulation of Ca2+ signaling (5,7 12–19). These include a single study that demonstrates 17β-estradiol-mediated stimulation of Ca2+ efflux from porcine coronary arterial smooth muscle cells (13). This is consistent with reports that 17β-estradiol stimulates plasma membrane Ca2+ ATPase (PMCA) of both excitable (rat cortical synaptosomes) (39, 40) and non-excitable (human erythrocytes) cells (40).
The rapidity of the effects of 17β-estradiol is consistent with it acting at the plasma membrane, and indeed, many of the reported rapid effects of 17β-estradiol are mimicked by 17β-estradiol-rendered plasma membrane-impermeant by linkage to a large molecule e.g. BSA (6, 41–44) or horseradish peroxidase (7, 45–46). There is a large body of evidence in favor of the existence of plasma membrane receptors for 17β-estradiol, and numerous such receptors have been proposed. These include classical ERα localized at the plasma membrane (47–49). Variant forms of ERα, including a truncated form, ER46 (50, 51), have been found associated with caveolae in the plasma membrane of some cells. Membrane estrogen receptors that are distinct from the classical estrogen receptors have also been described. These include an orphan G protein-coupled receptor GPR30 (52, 53), ERX, a novel receptor found in post-natal neocortical and uterine plasma membrane (54), and a receptor that has been termed the “γ-adrenergic receptor” (5, 7, 55, 56).
The pharmacology of the putative γ-adrenergic receptor is distinct from any other estrogen receptors; intact cell competition binding studies revealed that the catecholestrogen 2-hydroxyestradiol, noradrenaline, adrenaline, and dopamine blocked the binding of the membrane-impermeant conjugate 17β-estradiol-horseradish peroxidase to the plasma membrane of both pancreatic α (7) and β cells (55). In contrast, selective α-agonists, a β-antagonist, a D2 dopamine receptor antagonist, and the classical ER antagonist ICI 182,780 were without effect. Nadal et al. (5, 7, 55, 56) noted striking similarities to the so-called γ-adrenergic receptor that was first described in vascular cells (57, 58) and then characterized pharmacologically in presynaptic terminals of chick ciliary ganglion (59) and defined as a novel adrenergic receptor that is stimulated by noradrenaline but insensitive to α- and β-adrenergic agonists and antagonists and activates particulate GC to elevate cGMP and activate PKG.
Here we investigate rapid effects of 17β-estradiol on rat hepatocytes. We show that, above a threshold concentration of 1 nm, 17β-estradiol stimulates particulate guanylyl cyclase to elevate cGMP, which through activation and plasma membrane recruitment of PKGIα stimulates PMCA-mediated Ca2+ efflux from rat hepatocytes. These effects are extremely rapid, occurring within seconds, and are mimicked by a membrane-impermeant form of 17β-estradiol, suggesting that 17β-estradiol acts at the plasma membrane. We also show that 17β-estradiol binds specifically to the intact hepatocyte plasma membrane through an interaction that is competed by an excess of ANP but also shows competitive binding characteristics that resemble those of the γ-adrenergic receptor that have been proposed to mediate the effects of 17β-estradiol in pancreatic α and β cells (5, 7, 55, 56).
EXPERIMENTAL PROCEDURES
Materials
Collagenase was from Roche Applied Sciences. Phenol red-free Williams medium E (WME), 17β-estradiol 6-(O-carboxymethyl)oxime:BSA (17β-estradiol-BSA), 17β-estradiol 6-(O-carboxymethyl)oxime:BSA fluorescein isothiocyanate conjugate (17β-estradiol-BSA-FITC), 17β-estradiol encapsulated in 2-hydroxypropyl-β-cyclodextrin, (2-hydroxypropyl)-β-cyclodextrin (cyclodextrin vehicle), 17β-estradiol, 17α-estradiol, sodium nitroprusside, rat ANP 1–28, 3-isobutyl-1-methylxanthine (IBMX), and 8-bromo-cyclic guanosine monophosphate were from Sigma. Guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio)-, Rp-isomer, triethylammonium salt (Rp-8-pCPT-cGMPS), DT-3, NS2028, zaprinast, and 1-(4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)ethanone (G-1) were from CN Biosciences, UK. Fura-2 pentapotassium salt, fura-2 dextran (10 kDa), and Alexa Fluor 595-conjugated donkey anti-goat IgG (H + L; 2 mg/ml) were from Molecular Probes (Invitrogen). Caloxin 1b1 peptide was prepared by custom synthesis by Dalton Pharma Services, Toronto, ON, Canada. Primary anti-PKG antibodies and the corresponding blocking peptides were from Santa Cruz Biotechnology, Inc. (Insight Biotechnology Ltd., Wembley, UK). Citifluor was from Agar Scientific Ltd., Essex, UK. All other chemicals (VWR International Ltd., Lutterworth, UK) were of the purest grade available. For preparation of the efflux medium, ultrapure water was prepared using the Milli-Q RG system (Millipore).
Water-soluble 17β-estradiol was supplied as 17β-estradiol encapsulated in 2-hydroxypropyl-β-cyclodextrin (cyclodextrin vehicle), which releases free 17β-estradiol in aqueous solution. This formulation has been used in numerous studies, e.g. Refs. 6, 60, and 61), as it negates the need for organic solvents and thereby more faithfully mimics in vivo conditions. Water-soluble 17β-estradiol was, therefore, used extensively throughout the present study, except for the binding studies, for which it is not recommended. For binding studies, 17β-estradiol was used from a 1000-fold concentrated stock solution dissolved in DMSO. Because 17α-estradiol is not commercially available in cyclodextrin-encapsulated form, it was also used from a 1000-fold concentrated DMSO-dissolved stock solution; in such experiments similarly DMSO-dissolved 17β-estradiol also was used, enabling direct comparison of data.
Isolation of Hepatocytes
Single hepatocytes were isolated from 150–250 g of female Wistar rats by collagenase digestion as described previously (62, 63).
Measurement of cGMP
Isolated rat hepatocytes at a cell density of 1.5 × 106/ml were incubated in suspension in medium of composition 150 mm NaCl, 3.6 mm KCl, 1 mm MgCl2, 10 mm HEPES, 1.8 mm CaCl2, 11 mm glucose, pH 7.4, in a shaking water bath at 37 °C. Agonists were added for the periods indicated. cGMP was measured by using the acetylation method in a cGMP enzyme immunoassay system (Biotrak cellular communication assays; Amersham Biosciences).
Measurement of Ca2+ Efflux from Hepatocytes
Isolated rat hepatocytes were counted using a hemocytometer and then stored in suspension in WME on ice. Before each assay ∼106 cells were washed 3 times in ice-cold efflux medium composed of 150 mm NaCl, 3.6 mm KCl, 1 mm MgCl2, 10 mm HEPES, 11 mm glucose, pH 7.4. Ca2+ efflux from the intact rat hepatocytes was monitored by measuring the extracellular concentration of Ca2+ ([Ca2+]o) in the medium using fura-2 as extracellular Ca2+ reporter. Thus, the cells were resuspended in 1 ml of efflux medium at 37 °C to which the membrane-impermeant pentapotassium salt of fura-2 (7.5 μm) was added. Fluorescence was measured at 37 °C with constant stirring by using a PTI DeltaRam spectrofluorimeter (Photon Technologies International, Inc., Lawrenceville, NJ), exciting alternately at 340 and 380 nm and collecting emission at 510 nm. [Ca2+]o was calculated by calibrating the photometric 340/380 nm ratio signal as described previously (33). ([Ca2+]o) never rose above 1.5 μm, ensuring a true, unidirectional, Ca2+ efflux measurement.
Measurement of [Ca2+]c in Single Hepatocytes
Preparation of Single Cells for Microinjection
After harvesting, the hepatocytes were incubated at 37 °C at low density (∼103 cells/ml) in 1.7% SeaPrep-agarose in WME. Single hepatocytes were transferred to 0.1-mm path length microslides containing 1.2% FMC SeaPlaque-agarose in WME, which was subsequently gelled at 4 °C for 2 min. The cells were then held at 37 °C under a layer of liquid paraffin.
Fura-2 Dextran Preparation
Fura-2 dextran (10 kDa) was dissolved in 150 mm KCl, 1 mm PIPES, pH 7.2, to make a 5 mm stock solution that was stored at −70 °C. For microinjection, a small aliquot of 5 mm fura-2 dextran was held as a droplet under liquid paraffin.
Microinjection and Data Acquisition
Freshly pulled pipettes were filled with fura-2 dextran by dipping the tip for a few seconds in the fura-2 dextran droplet. Individual hepatocytes were injected to ∼0.5% of the cell volume as described previously (64, 65). The fura-2 dextran thus localized in the cytoplasmic compartment reports [Ca2+]c only. Injected cells of healthy appearance were transferred within microslides to a heated (37 °C) stainless steel perfusion chamber on the stage of an Olympus IX71 epifluorescence microscope. Cells were superfused with extracellular buffer to which ATP and 17β-estradiol were added as indicated. Cells were alternately excited at 340- and 380-nm wavelengths by means of a PTI DeltaRAM V high speed random access monchromator (Photon Technologies International Inc., Lawrenceville, NJ). The 510-nm emission was detected by a PTI Q-8 microscope photometer. [Ca2+]c was calculated by calibrating the fura-2 signal in situ as described previously (66).
Immunocytochemistry
Primary Antibodies
The following primary anti-PKG antibodies (Santa Cruz) were used: cGKIα (N-16), goat polyclonal, targeted against a 16-amino acid sequence near the N terminus of PKGIα; cGKIβ (L-16), goat polyclonal, targeted against a 16-amino acid sequence near the N terminal of PKGIβ.
Immunolabeling
Hepatocytes were allowed to attach to glass coverslips for 24 h in WME supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin at 37 °C and then fixed in 4% paraformaldehyde. Nonspecific binding was prevented by blocking for 1 h at 25 °C in phosphate-buffered saline (PBS) plus 0.01% Triton X-100 containing 5% normal rabbit serum. After three 5-min washes with PBS, cells were incubated at 4 °C overnight with the corresponding primary antibody diluted (1:50) in PBS plus 0.01% Triton X-100. After antibody incubation, cells were washed three times with PBS and then incubated with Alexa Fluor 594-conjugated donkey anti-goat IgG (H + L) for 2 h at 25 °C. The secondary antibody was diluted 1:1000 in PBS plus 0.01% Triton X-100. Cells were then washed 3 times for 5 min in PBS, and coverslips were then mounted in Citifluor (glycerol/PBS solution) on glass slides. Cells were examined under oil immersion (×63) using a Leica DMRE upright laser scanning confocal microscope with a TCS SP2 scan head.
Quantification of Fluorescence Localization
Raw confocal images were analyzed using MetaMorph imaging software (Universal Imaging, Marlow, Bucks, UK). A single horizontal transect was made through the widest portion of each cell. Regions of interest (2.5 μm × 2.5 μm) were applied along the transect, and the average fluorescent staining intensity within each region was determined. For each cell, data were normalized by expressing staining intensity per region as a percentage of the average staining intensity across the entire transect. Subsequent analysis determined relative staining intensity at the cell periphery compared with average staining intensity across the entire transect. This was achieved by expressing the average staining intensity of the outer 2 regions of each side of the cell as a percentage of the average staining intensity across the entire transect. Similarly, relative staining intensity of the cell interior compared with average staining intensity across the entire transect was determined by expressing the average staining intensity of all except the outer 2 regions of each side of the cell as a percentage of the average staining intensity across the entire transect.
Assay for 17β-Estradiol-BSA-FITC Binding
Hepatocytes were allowed to attach to glass coverslips for 24 h in WME supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin at 37 °C. The cells were then incubated with 1 μm 17β-estradiol-BSA-FITC for 30 min at 4 °C and then fixed in 4% paraformaldehyde. After three 5-min washes in PBS, the coverslips were mounted on Citifluor on glass slides and then examined under oil immersion (×63) using a Leica DMRE upright laser scanning confocal microscope with a TCS SP2 scan head.
Statistics
Differences between means were compared by Student's t test using a level of significance of p < 0.05. Data are expressed as the mean ± S.E. All experiments are from at least three independent hepatocyte preparations.
RESULTS
17β-Estradiol Elevates Hepatocyte cGMP through Activation of Particulate Guanylyl Cyclase
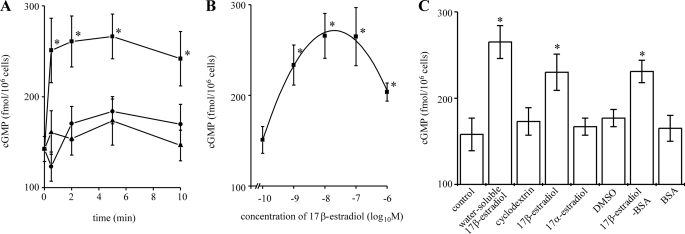
Fig. 1A shows that 10 nm 17β-estradiol rapidly elevated cGMP levels in isolated rat hepatocytes; cGMP was significantly increased above basal levels within 30 s and remained significantly elevated for 10 min. No effect was seen with the cyclodextrin vehicle.
FIGURE 1.
17β-Estradiol elevates cGMP in single rat hepatocytes. Isolated rat hepatocytes were incubated at 37 °C. Total cGMP levels were measured by enzyme immunoassay. All data shown are the mean values of at least six experiments from at least three independent hepatocyte preparations. A, a time course is shown. Cells were incubated with 10 nm water-soluble 17β-estradiol (■), cyclodextrin vehicle (▴) or no treatment (●) for the periods indicated. *, significantly different from cyclodextrin vehicle and no treatment at each corresponding time point. B. concentration dependence is shown. Cells were incubated with water-soluble 17β-estradiol at the concentrations indicated for 5 min. *, significantly different from cyclodextrin vehicle and no treatment. C, effects of 17α-estradiol and 17β-estradiol-BSA are shown. Cells were incubated with no treatment, 10 nm water-soluble 17β-estradiol, 10 nm 17β-estradiol (DMSO-soluble), 17α-estradiol (DMSO-soluble), 10 nm 17β-estradiol-BSA, cyclodextrin vehicle, DMSO vehicle, and BSA alone for 5 min. *, significantly different from no treatment, cyclodextrin vehicle, DMSO vehicle, and BSA alone.
Investigation of the concentration dependence of the effect of 17β-estradiol (Fig. 1B) showed that a significant increase in cGMP levels was seen above a threshold concentration of 1 nm 17β-estradiol and that the optimum concentration of 17β-estradiol was 10 nm. Interestingly, the magnitude of the increase in cGMP levels was reduced at concentrations of 17β-estradiol above 10 nm. This could be due to a nonspecific effect of 17β-estradiol on the plasma membrane; high concentrations of steroid hormones can interact non-specifically with cell membranes (67). Cytotoxicity arising from high concentrations of 17β-estradiol has been associated with a decrease in membrane fluidity (68). Fig. 1C shows that 10 nm 17β-estradiol-BSA also elevated hepatocyte cGMP to a level similar to that seen with 17β-estradiol, whereas, in contrast, 17α-estradiol, the stereoisomer of 17β-estradiol, did not elevate cGMP.
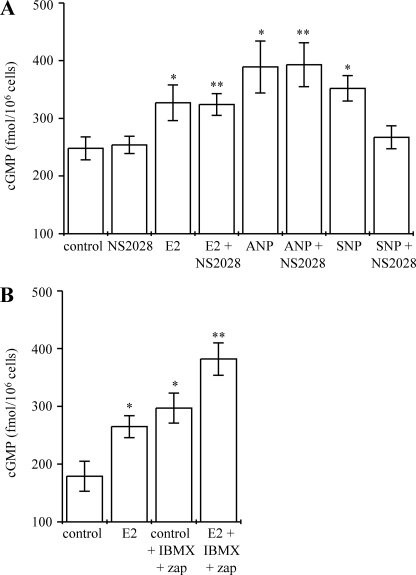
Regarding the mechanism through which 17β-estradiol elevates hepatocyte cGMP, three possibilities exist; that is, soluble guanylyl cyclase activation, particulate guanylyl cyclase activation, or cGMP phosphodiesterase (PDE) inhibition. Because soluble guanylyl cyclase can remain plasma membrane-associated (in caveolae) (69), soluble versus particulate guanylyl cyclase involvement cannot be distinguished using the traditional approach of comparing effects on isolated plasma membrane preparations versus intact cells. Instead, to investigate whether 17β-estradiol elevates cGMP through stimulation of soluble or particulate guanylyl cyclase, NS2028, a potent and specific inhibitor of soluble guanylyl cyclase (70), was used. Agonists were added to hepatocytes in the presence of 10 μm NS2028 hepatocytes (after 5 min of preincubation with NS2028 alone). The specificity and efficacy of this inhibitor is demonstrated in Fig. 2A; NS2028 prevented any significant elevation of cGMP upon the addition of sodium nitroprusside, an NO donor that stimulates soluble guanylyl cyclase (21, 32, 33). In contrast, ANP, a known activator of particulate guanylyl cyclase (22, 24, 32), was still able to elevate cGMP to similar levels in the presence or absence of NS2028. Fig. 2A shows that inhibition of soluble guanylyl cyclase with NS2028 did not prevent 17β-estradiol from elevating cGMP. This suggests that soluble guanylyl cyclase stimulation is not the mechanism responsible for the 17β-estradiol-mediated elevation of cGMP in hepatocytes.
FIGURE 2.
Neither soluble guanylyl cyclase activation nor cyclic nucleotide phosphodiesterase inhibition mediates cGMP elevation by 17β-estradiol. Specific inhibitors were applied to intact rat hepatocytes to investigate the involvement of soluble guanylyl cyclase and cyclic nucleotide phosphodiesterases in the elevation of cGMP by 17β-estradiol. Cells were incubated at 37 °C. Total cGMP levels were measured by enzyme immunoassay. All data shown are the mean values of at least six experiments from at least three independent hepatocyte preparations. A, 17β-estradiol does not stimulate soluble guanylyl cyclase. Cells were incubated with either no treatment (control) or soluble guanylyl cyclase inhibitor NS2028 (10 μm) for 5 min. 10 nm water-soluble 17β-estradiol (E2), 200 nm ANP, or 100 nm sodium nitroprusside (SNP) was then added, and cells were incubated for a further 5 min. *, significantly different from control; **, significantly different from NS2028 alone. B, 17β-estradiol does not elevate cGMP through inhibition of cyclic nucleotide phosphodiesterases. Cells were incubated with either no treatment (control) or 300 μm IBMX plus 300 μm zaprinast (zap) for 10 min. 10 nm water-soluble 17β-estradiol (E2) or no treatment were then added, and cells were incubated for a further 5 min. *, significantly different from control; **, significantly different from control plus IBMX and zaprinast.
Intracellular cGMP can also be elevated through inhibition of cyclic nucleotide PDEs. PDEs 3B, 7B, 9A, and 11A have been identified in hepatocytes (71–73). IBMX is generally used as a universal nonspecific inhibitor of cyclic nucleotide PDEs; however, it has been shown to be ineffective at inhibiting PDE9 (72), a cGMP-specific PDE. PDE9A has the highest affinity for cGMP and has been found in nearly every tissue, including the liver (72, 73). For this reason, a combination of IBMX (300 μm) and zaprinast (300 μm), an inhibitor of PDE9 and PDE5 (72), was used to inhibit hepatocyte PDEs. Agonists were, thus, added in the presence of IBMX and zaprinast (after a 10-min preincubation of hepatocytes with IBMX and zaprinast alone).
Preincubation of cells with IBMX and zaprinast significantly elevated cGMP levels above basal, control levels (Fig. 2B). The addition of 17β-estradiol elevated cGMP to levels significantly greater than those seen in cells preincubated with the PDE inhibitors alone. These data, therefore, suggest that 17β-estradiol elevates cGMP through a mechanism distinct from either stimulation of soluble guanylyl cyclase or inhibition of cyclic nucleotide phosphodiesterases. We, therefore, propose that 17β-estradiol acts by stimulating particulate guanylyl cyclase.
17β-Estradiol Recruits PKGIα to the Hepatocyte Plasma Membrane
PKG is the major mediator of cGMP signaling. Two mammalian isotypes of PKGI have been described; that is, type I, consisting of an α and a β isoform, splice variants of a single gene, and type II (reviewed in Ref. 74). PKGIα and PKGIβ arise from alternative splicing of exons encoding the N-terminal domain of PKGI. These two different PKGI isoforms differ from PKGII and from one another in terms of sensitivity to cGMP, tissue distribution, and substrate specificity, interacting with different proteins through their distinct N termini (74).
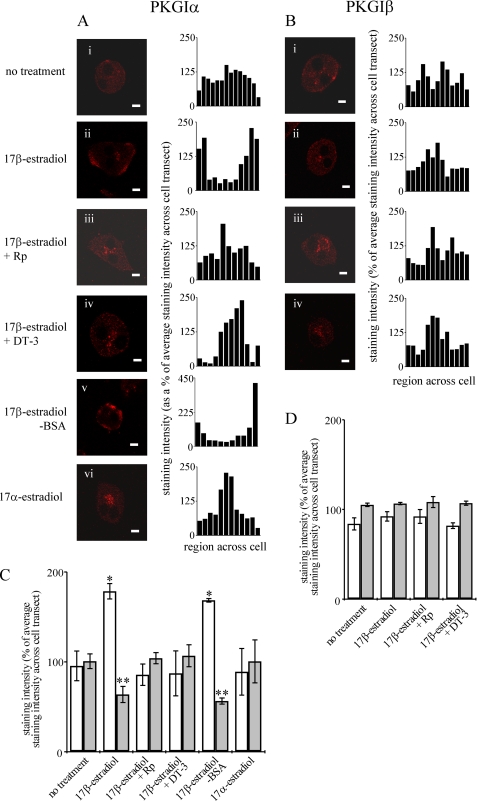
In confirmation of our previous findings (33, 34), immunoreactivity of the commercially available antibodies cGKIα (N-16) and cGKIβ (L-16) (visualized using Alexa 594, red secondary) demonstrated expression of PKGIα and PKGIβ in isolated rat hepatocytes (Fig. 3). No staining was observed using primary antibodies pre-absorbed with corresponding blocking peptides or using the Alexa 594 secondary antibody alone (results not shown). In unstimulated rat hepatocytes, the pattern of staining of both PKGIα (Fig. 3, Ai and C) and PKGIβ (Fig. 3, Bi and D) was punctate and distributed diffusely throughout the cytosol, lacking clear nuclear or plasma membrane localization. Stimulation of hepatocytes with 17β-estradiol (10 nm) for 5 min increased the intensity of plasma membrane-localized immunostaining of PKGIα (Fig. 3, Aii and C), but, in contrast, the pattern of localization of PKGIβ was unaffected by stimulation with 17β-estradiol (Fig. 3, Bii and D). Fluorescence intensity scans of confocal images revealed significantly increased PKGIα immunostaining at the cell periphery and decreased PKGIα immunostaining in the cell interior after treatment of hepatocytes with 17β-estradiol (Fig. 3C). In contrast, the distribution of PKGIβ immunostaining was not significantly altered (Fig. 3D). These observations suggest a selective translocation of PKGIα to the plasma membrane upon stimulation with 17β-estradiol.
FIGURE 3.
17β-Estradiol promotes recruitment of PKGIα but not PKGIβ to the plasma membrane. Isolated hepatocytes seeded onto coverslips were incubated for 5 min at 37 °C in the presence of either no treatment (Ai and Bi), 10 nm 17β-estradiol (Aii and Bii), 10 nm 17β-estradiol plus 25 μm Rp-8-pCPT-cGMPS (Rp) (Aiii and Biii) 10 nm 17β-estradiol plus 4 μm DT-3 (Aiv and Biv), 10 nm 17β-estradiol-BSA (Av), or 10 nm 17α-estradiol (Avi). Cells were then immunostained with either anti-PKGIα (cGKIα (N-16)) (Ai–Avi) or anti-PKGIβ (cGKIβ (L-16)) (Bi–Biv). Alexa-594 anti-goat (red) was used to visualize immunoreactivity. The images and their corresponding intensity scans (Ai–vi and Bi–iv) are representative of several experiments. The scale bar represents 10 μm. Mean percentage staining intensities at the cell periphery (white bars) and the cell interior (gray bars) (as a percentage of average staining intensity across the entire cell transect) were calculated from fluorescence intensity scans of several cells from at least three independent hepatocyte preparations immunostained with either anti-PKGIα (C) or anti-PKGIβ (D).
PKGIα is a homodimer, with each subunit containing an N-terminal dimerization domain, a regulatory domain, and a catalytic domain. The regulatory domain contains two tandem cGMP binding sites, occupation of which releases inhibition of the catalytic domain by the autoinhibitory site on the N-terminal domain. The catalytic domain when, thus, exposed phosphorylates serine/threonine residues in target proteins (74). To determine whether the activation of PKGIα is required for its translocation to the plasma membrane, two distinct membrane-permeant inhibitors of PKG were used: Rp-8-pCPT-cGMPS and DT-3. Rp-8-pCPT-cGMPS inhibits all three PKG isoforms (75) by blocking cGMP binding to the regulatory domain, thereby preventing exposure of the catalytic domain. DT-3 acts through a distinct mechanism to that of Rp-8-pCPT-cGMPS; the PKGI-inhibitor peptide portion of DT-3, W45, prevents binding and, hence, phosphorylation of substrates at the PKGI catalytic domain (76). DT-3 inhibits isoforms Iα and the Iβ at low μm concentrations, whereas PKGII is relatively (∼100-fold less) insensitive to this inhibitor (77).
Hepatocytes were incubated with 17β-estradiol in the presence of, and after a 100-s preincubation with Rp-8-pCPT-cGMPs or DT-3. In the presence of Rp-8-pCPT-cGMPs (Fig. 3, Aiii and C) or DT-3 (Fig. 3, Aiv and C), plasma membrane recruitment of PKGIα was prevented. As a control, neither Rp-8-pCPT-cGMPs nor DT-3 altered the pattern of immunostaining of PKGIβ (Fig. 3, Biii, Biv, and D).
Plasma membrane recruitment of PKGIα (Fig. 3, Av and C) was also seen upon treatment of hepatocytes with 17β-estradiol-BSA (10 nm). In contrast, 17α-estradiol did not affect the pattern of localization of PKGIα (Fig. 3, Avi and C).
17β-Estradiol Stimulates PMCA-mediated Ca2+ Efflux from Hepatocytes
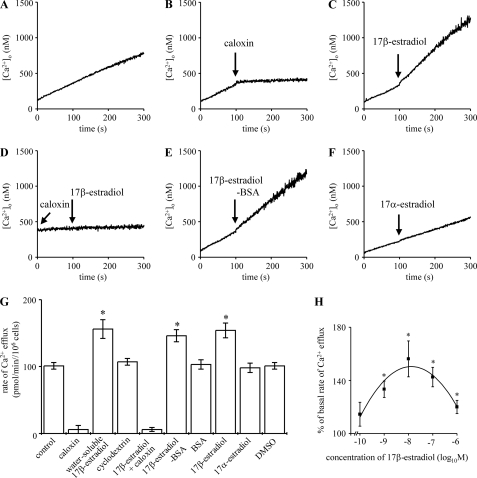
Consistent with our previous findings (32–34, 78), a basal rate of Ca2+ efflux was recorded from intact rat hepatocytes in the absence of agonist (Fig. 4, A and G) and was blocked by caloxin 1b1, a specific PMCA inhibitor (79) (Fig. 4, B and G), confirming that the basal hepatocyte Ca2+ efflux recorded here is predominantly due to PMCA activity (33, 78).
FIGURE 4.
17β-Estradiol stimulates PMCA-mediated Ca2+ efflux from hepatocytes. Ca2+ efflux from populations of intact rat hepatocytes was measured at 37 °C using fura-2 pentapotassium salt in the extracellular medium. The traces shown (A–F) are representative of several experiments. 300 μm caloxin, 10 nm water-soluble estradiol, 10 nm 17β-estradiol-BSA, or 10 nm 17α-estradiol were added where indicated by the arrows. Calculated mean rates of Ca2+ efflux of at least six experiments from at least three independent hepatocyte preparations are shown in G. H shows dependence of Ca2+ efflux stimulation on concentration of 17β-estradiol. *, significantly differently from control.
The addition of 10 nm 17β-estradiol stimulated an approximate 1.6-fold increase in the rate of Ca2+ efflux (Fig. 4, C and G). The increase was extremely rapid, occurring within ∼2–4 s. No effect on the rate of Ca2+ efflux was seen upon the addition of the equivalent concentration of the cyclodextrin vehicle (Fig. 4G). Caloxin 1b1 prevented any stimulation of Ca2+ efflux by 17β-estradiol (Fig. 4, D and G), indicating that the 17β-estradiol-stimulated Ca2+ efflux is PMCA-mediated. Fig. 4, E and G, show that an equivalent concentration of 17β-estradiol-BSA also stimulates Ca2+ efflux from hepatocytes, with an approximate 1.5-fold increase, similar to the increase seen with 17β-estradiol. The stereoisomer 17α-estradiol had no effect on the rate of Ca2+ efflux from hepatocytes (Fig. 4, F and G).
The relationship between hepatocyte Ca2+ efflux stimulation and concentration of 17β-estradiol was investigated (Fig. 4H). The optimum concentration was 10 nm, although a significant increase in the rate of Ca2+ efflux was seen at and above a threshold concentration of 1 nm. Consistent with the effect on cGMP elevation (Fig. 1B), the magnitude of the Ca2+ efflux stimulation was reduced at higher concentrations of 17β-estradiol (Fig. 4H).
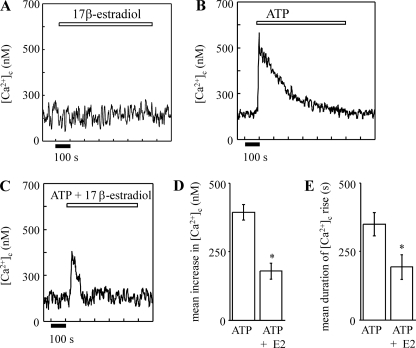
17β-Estradiol Does Not Elevate [Ca2+]c but Attenuates ATP-induced Elevations in [Ca2+]c in Single Hepatocytes
The role of 17β-estradiol in overall hepatocyte [Ca2+]c homeostasis was determined by measuring its effect on (i) resting [Ca2+]c levels and (ii) elevations in [Ca2+]c elicited by extracellular ATP, a physiological agonist for hepatocytes (80).
Fig. 5A shows that 10 nm 17β-estradiol applied alone did not alter resting [Ca2+]c levels in single rat hepatocytes. Single rat hepatocytes responded to 25 μm ATP by the elevation of [Ca2+]c (Fig. 5, B, D, and E). Co-application of 10 nm 17β-estradiol decreased both the amplitude and duration of the ATP-induced [Ca2+]c rise (Fig. 5C, D, and E). It, thus, appears that 17β-estradiol does not elevate hepatocyte [Ca2+]c but, rather, through Ca2+ efflux stimulation attenuates [Ca2+]c elevations evoked by Ca2+-mobilizing hormones.
FIGURE 5.
17β-Estradiol attenuates ATP-induced [Ca2+]c rises in single rat hepatocytes. Single, fura-2 dextran-injected, rat hepatocytes were superfused with 10 nm 17β-estradiol (A), 25 μm ATP (B), and 25 μm ATP plus 10 nm 17β-estradiol (C) for the periods indicated by the addition bars. The traces shown in A-C are representative of several experiments. D and E show, respectively, the calculated mean increase in amplitude (nm) above resting [Ca2+]c and the duration (s) of the [Ca2+]c rise above resting [Ca2+]c for each treatment. *, significantly different from ATP alone.
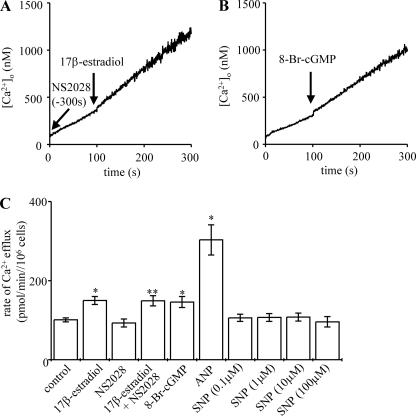
Stimulation of Ca2+ Efflux by 17β-Estradiol Is Not Mediated by Soluble Guanylyl Cyclase
Fig. 6, A and C, shows that soluble guanylate cyclase inhibitor NS2028 did not prevent 17β-estradiol from stimulating hepatocyte Ca2+ efflux. In confirmation of our previous findings, both the membrane-permeant cGMP analogue 8-Br-cGMP (Fig. 6, B and C) and ANP-mediated particulate guanylyl cyclase activation (Fig. 6C) stimulated Ca2+ efflux from rat hepatocytes. However, sodium nitroprusside-mediated soluble guanylyl cyclase activation did not stimulate Ca2+ efflux (Fig. 6C) (32–34).
FIGURE 6.
Stimulation of Ca2+ efflux by 17β-estradiol is not mediated by soluble guanylyl cyclase. Ca2+ efflux from populations of intact rat hepatocytes was measured at 37 °C using fura-2 pentapotassium salt in the extracellular medium. The traces shown (A and B) are representative of several experiments. 10 nm 17β-estradiol (water-soluble) and 100 μm 8-Br-cGMP were added where indicated by the arrows. Cells were preincubated with either 10 μm NS2028 (A) or no treatment for 5 min, washed 3 times in ice-cold efflux medium (containing NS2028/no treatment), then transferred to a cuvette, and Ca2+ efflux was measured at 37 °C, adding agonist where indicated by the arrows. Calculated mean rates of Ca2+ efflux of at least six experiments from at least three independent hepatocyte preparations are shown in C. *, significantly different from control; **, significantly different from NS2028 alone. SNP, sodium nitroprusside.
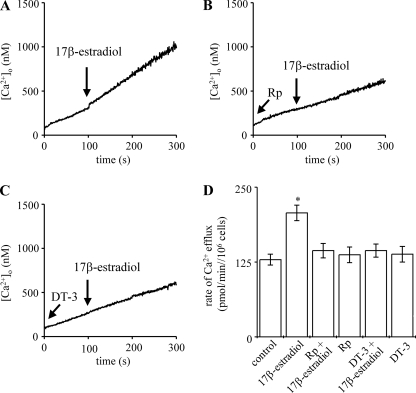
Stimulation of Ca2+ Efflux by 17β-Estradiol Is Mediated by PKG
To investigate a possible link between elevation of cGMP, activation of PKG, and Ca2+ efflux stimulation by 17β-estradiol, the two distinct membrane-permeant PKG inhibitors, Rp-8-pCPT-cGMPS and DT-3, were used.
PKG inhibition by preaddition of either Rp-8-pCPT-cGMPS (Rp) or DT-3 prevented the stimulation of hepatocyte Ca2+ efflux by 17β-estradiol (Fig. 7, B and C, respectively; mean Ca2+ efflux values are shown in Fig. 7D). Neither Rp-8-pCPT-cGMPS nor DT-3 alone affected the rate of Ca2+ efflux (Fig. 7D).
FIGURE 7.
Inhibition of PKG prevents 17β-estradiol-mediated Ca2+ efflux stimulation. Ca2+ efflux from populations of intact rat hepatocytes was measured at 37 °C using fura-2 in the extracellular medium. The traces shown in A–C are representative of several experiments. 10 nm 17β-estradiol (water-soluble), 25 μm Rp-8-pCPT-cGMPS (Rp), and 4 μm DT-3 were added where indicated by the arrows. Calculated rates of Ca2+ efflux are shown in D. *, significantly different from control.
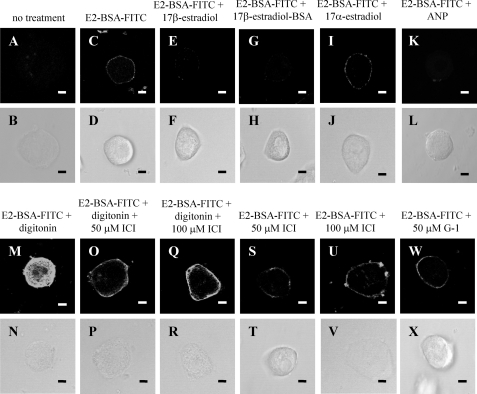
17β-Estradiol Binds Specifically at the Plasma Membrane of Intact Rat Hepatocytes
To investigate whether 17β-estradiol binds at the plasma membrane of intact rat hepatocytes, the membrane-impermeant, fluorescently labeled conjugate 17β-estradiol-BSA-FITC was used. Fig. 8C shows that 17β-estradiol-BSA-FITC binds to the plasma membrane of intact hepatocytes that had been cultured for 24 h. Similar plasma membrane binding of 17β-estradiol-BSA-FITC was also observed in hepatocytes cultured for 6 h (results not shown). An excess of either 17β-estradiol (Fig. 8E) or 17β-estradiol-BSA (Fig. 8G) prevented 17β-estradiol-BSA-FITC binding to the plasma membrane. In contrast, an excess of 17α-estradiol did not compete with the binding of 17β-estradiol-BSA-FITC to the plasma membrane (Fig. 8I). An excess of guanylyl cyclase A receptor agonist ANP was, however, effective in preventing plasma membrane binding of 17β-estradiol-BSA-FITC (Fig. 8K).
FIGURE 8.
Specific binding of 17β-estradiol-BSA-FITC at the hepatocyte plasma membrane. Isolated hepatocytes were seeded onto coverslips and allowed to attach for 24 h. Cells were treated with either no treatment (A and C), 50 μm 17β-estradiol (E), 50 μm 17β-estradiol-BSA (G), 50 μm 17α-estradiol (I), 20 μm ANP (K), 100 μm digitonin (M), 100 μm digitonin plus 50 μm ICI 182,780 (ICI) (O), 100 μm digitonin plus 100 μm ICI (Q), 50 μm ICI 182,780 (S), 100 μm ICI 182,780 (U), or 50 μm G-1 (W) for 20 min at 4 °C. 1 μm 17β-estradiol-BSA-FITC (E2-BSA-FITC) was then added (C–W), and the cells were incubated for a further 20 min at 4 °C. Cells were then fixed and mounted onto coverslips. Corresponding phase images (B–X) demonstrate general cell morphology. The scale bar represents 10 μm, and data are representative of several experiments from at least 3 independent hepatocyte preparations.
Two classical estrogen receptors have been described: ERα and ERβ (2). A previous study has shown that ERα, but not ERβ, is present in rat liver (81). Fig. 8M shows that 17β-estradiol-BSA-FITC stained both the plasma membrane and the cytosol of digitonin-permeabilized hepatocytes. The cytosolic staining is consistent with 17β-estradiol-BSA-FITC binding to classical 17β-estradiol receptors, which have an intracellular location. Indeed, when the cells were preincubated with ICI 182,780, an antagonist of the classical ERs (82), the cytosolic staining was greatly reduced (Fig. 8, O and Q). In marked contrast, however, neither 50 nor 100 μm ICI 182,780 appeared to affect the binding of 17β-estradiol-BSA-FITC at the plasma membrane of either permeabilized (Fig. 8, O and Q) or intact hepatocytes (Fig. 8, S and U). These findings suggest that 17β-estradiol binding to the hepatocyte plasma membrane is not mediated by classical ERα or ERβ. Fig. 8W shows that G-1, a specific agonist (83) for the GPR30 receptor (52, 53), did not compete with 17β-estradiol-BSA-FITC for binding to the intact hepatocyte plasma membrane.
Neither ERα-antagonist ICI 180,270 (ICI) nor GPR30 Agonist G-1 Affects Hepatocyte Ca2+ Efflux
ICI 182,780 at 0.5 and 1 μm had no effect on either basal or 17β-estradiol-stimulated Ca2+ efflux from hepatocytes. Moreover, GPR30 agonist G-1 at 10 and 100 nm did not stimulate hepatocyte Ca2+ efflux (results not shown).
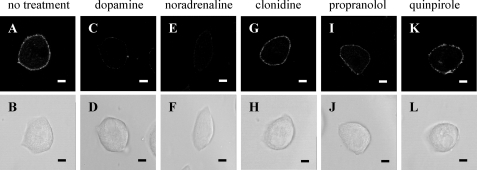
Dopamine and Noradrenaline Compete with 17β-Estradiol-BSA-FITC Binding to the Intact Hepatocyte Plasma Membrane
To investigate whether the 17β-estradiol binding properties of the hepatocyte plasma membrane resemble pharmacologically those of the putative γ-adrenergic receptor (57–59) proposed to mediate the rapid effects of 17β-estradiol on pancreatic α and β cells (5, 7, 55, 56), a competition binding assay was conducted using dopamine, noradrenaline, the α2-adrenergic agonist clonidine, the β-adrenergic antagonist propranolol, and the D2-dopaminergic antagonist quinpirole. Both dopamine (Fig. 9C) and noradrenaline (Fig. 9E) competed with the binding of 17β-estradiol-BSA-FITC to the plasma membrane of rat hepatocytes. In contrast, clonidine (Fig. 9G), propranolol (Fig. 9I), and quinpirole (Fig. 9K) did not compete.
FIGURE 9.
Dopamine and noradrenaline compete with 17β-estradiol-BSA-FITC for binding to the hepatocyte plasma membrane. Isolated hepatocytes were seeded onto coverslips and allowed to attach for 24 h. Cells were treated with either no treatment (A), 50 μm dopamine (C), 50 μm noradrenaline (E), 50 μm clonidine (G), 50 μm propranolol (I), or 50 μm quinpirole (K) for 20 min at 4 °C. 1 μm 17β-estradiol-BSA-FITC was then added, and the cells incubated for a further 20 min at 4 °C. Cells were then fixed and mounted onto coverslips. Corresponding phase images (B–L) demonstrate general cell morphology. The scale bar represents 10 μm, and data are representative of several experiments from at least 3 independent hepatocyte preparations.
DISCUSSION
We have shown here that 17β-estradiol stimulates particulate guanylyl cyclase to rapidly elevate rat hepatocyte cGMP and promote plasma membrane localization of PKGIα. We also show that 17β-estradiol, above a similar threshold concentration of 1 nm, stimulates PMCA-dependent Ca2+ efflux from rat hepatocytes through a PKGI-mediated mechanism. Our study is the first to observe particulate guanylyl cyclase activation and Ca2+ efflux stimulation by 17β-estradiol in one cell type and to demonstrate that they are linked by the activation and translocation of PKGIα to the plasma membrane.
Our model proposes that a spatially and functionally distinct pool of cGMP close to the plasma membrane (30, 84), generated by activation of particulate guanylyl cyclase but not soluble guanylyl cyclase, recruits activated PKGIα into its vicinity. PKGIα thus localized at the plasma membrane activates the PMCA (85, 86) thereby stimulating plasma membrane Ca2+ efflux (33, 34). PKGIα, but not PKGIβ, stimulates PMCA partially purified from aortic smooth muscle cells (86), which like rat hepatocytes (87), predominantly express PMCA isotype I.
How PKGIα is recruited to the hepatocyte plasma membrane is not known. However, we propose the involvement of an interaction between PKGIα and PKG-anchoring proteins. In addition to holding the homodimers together and controlling activation/inhibition of the catalytic domain, the N-terminal domain of PKGIα has a further important function in that it mediates binding to PKG-anchoring proteins, which target PKGIα to different intracellular locations (74). Such PKG-anchoring proteins include regulator of G protein signaling 2 (RGS-2) and the myosin-binding subunit of phosphatase I. Indeed, studies in both primary mouse vascular smooth muscle cells (88) and isolated mouse cardiomyocytes (89) have shown that activated PKGIα phosphorylates and binds to RGS-2, resulting in translocation of the PKGIα-RGS-2 complex to the plasma membrane. In both cell types, consistent with our data, PKG inhibition prevents PKGIα plasma membrane recruitment by preventing PKGIα-mediated phosphorylation of the N terminus of RGS2 (88, 89).
We have previously presented a novel mechanism through which ANP protects hepatocytes by particulate guanylyl cyclase-mediated Ca2+ efflux stimulation, thereby attenuating harmful elevations in [Ca2+]c (33). 17β-estradiol also protects liver and isolated hepatocytes under pathophysiological conditions associated with a rise in [Ca2+]c (90–93) through an unknown mechanism that appears to be unrelated to classical ER activation (91, 92). Our finding that 17β-estradiol can also, like ANP, stimulate Ca2+ efflux through particulate guanylyl cyclase activation and attenuate [Ca2+]c elevations suggests that such a cytoprotective mechanism may underlie 17β-estradiol-mediated hepatoprotection and may, thus, prove to be of more widespread physiological significance.
Our data show that the plasma membrane-impermeant conjugate 17β-estradiol-BSA closely mimicked all of the effects of free 17β-estradiol, elevating cGMP, promoting PKGIα plasma membrane localization, and stimulating Ca2+ efflux. We, therefore, propose that the rapid effects of 17β-estradiol on hepatocyte cGMP, PKGIα plasma membrane recruitment, and Ca2+ efflux observed here are mediated by 17β-estradiol acting at the extracellular face of the plasma membrane.
The nature of the plasma membrane interaction was investigated by both competition binding and functional (Ca2+ efflux) studies using agonists and antagonists of characterized plasma membrane receptors for 17β-estradiol. Our observations that the classical ER antagonist ICI 182,780 (82) prevented intracellular 17β-estradiol-BSA-FITC binding in permeabilized hepatocytes but not 17β-estradiol-BSA-FITC binding at the plasma membrane of either intact or permeabilized hepatocytes and did not prevent 17β-estradiol-stimulated Ca2+ efflux indicate that neither classical receptor, ERα or ERβ, mediates either hepatocyte plasma membrane 17β-estradiol binding or the rapid signaling effects observed here. Likewise, because the GPR30 agonist G–1 (83) did not compete with 17β-estradiol-BSA-FITC for binding to intact hepatocytes nor mimic the ability of 17β-estradiol to stimulate Ca2+ efflux, we have discounted the receptor GPR30 (52, 53). This conclusion is further supported by the absence of effect of ICI 182,780 on Ca2+ efflux; ICI 182,780 is, in addition to its action as a classical ER antagonist, a potent GPR30 agonist (52). Furthermore, our observations that 17α-estradiol is without effect on plasma membrane binding (consistent with the findings of an early study (94)) of 17β-estradiol-BSA-FITC, cGMP, PKGIα translocation or Ca2+ efflux rules out receptor ERX, which is preferentially activated by 17α-estradiol (61).
Our finding that an excess of ANP competes with 17β-estradiol-BSA-FITC for hepatocyte plasma membrane binding raises the possibility that 17β-estradiol binds directly to the extracellular receptor portion of the GC-A receptor for ANP, thus stimulating the intrinsic particulate guanylyl cyclase activity of this receptor and thereby promoting plasma membrane recruitment of PKGIα and PMCA stimulation (33, 34). However, there are other feasible explanations of this observation. First, an excess of ANP may simply block binding of 17β-estradiol to its distinct plasma membrane receptor. Second, the GC-A receptor and the plasma membrane 17β-estradiol receptor may reside in close proximity such that binding of ANP to the GC-A receptor blocks binding of 17β-estradiol to its distinct receptor. Therefore, it remains possible that 17β-estradiol acts through a distinct plasma membrane receptor.
Our observation that dopamine and noradrenaline compete with 17β-estradiol-BSA-FITC binding to the plasma membrane of intact rat hepatocytes, whereas a specific α2-adrenergic agonist, a β-adrenergic antagonist and D2 dopamine receptor antagonist are without effect, is consistent with the properties of the putative γ-adrenergic receptor characterized pharmacologically in chick ciliary ganglion (59). The γ-adrenergic receptor was proposed by Nadal et al. (5, 7, 55, 56) to mediate the rapid signaling effects of 17β-estradiol in α and β pancreatic cells, which resemble, in terms of both particulate guanylyl cyclase stimulation and insensitivity to ICI 182,780, the plasma membrane-initiated signaling events we have observed in hepatocytes.
Despite compelling pharmacological evidence for its existence (57–59), the molecular identity of the γ-adrenergic receptor remains to be elucidated. Perhaps the γ-adrenergic receptor is one of the orphan particulate guanylyl cyclase isoforms (22–23) for which 17β-estradiol may be revealed to be the endogenous ligand. However, sensitivity of 17β-estradiol signaling to pertussis toxin in pancreatic α cells suggests that this putative receptor is a G protein-coupled receptor (46). Pharmacological similarities have been noted to a recently identified cloned and characterized Drosophila melanogaster G protein-coupled receptor, DmDopEcR (95), that mediates the effects of ecdysteroids and catecholamines. It is an intriguing possibility that the γ-adrenergic receptor may be a G protein-coupled receptor that activates a transmembrane guanylyl cyclase, perhaps the GC-A receptor, by cross-talk signaling mediated by G protein activation. There are, indeed, several reports of coupling of G protein-coupled receptors to transmembrane guanylyl cyclases (96–97).
In summary, we have shown here that 17β-estradiol, acting at the extracellular face of the plasma membrane, stimulates particulate guanylyl cyclase, rapidly elevating cGMP, which through activation and plasma membrane recruitment of PKGIα, stimulates PMCA-mediated Ca2+ efflux from hepatocytes. We have also shown that 17β-estradiol binds specifically at the hepatocyte plasma membrane through an interaction that is competed by an excess of ANP but that also shows many similarities to the pharmacological characteristics of the putative γ-adrenergic receptor. We, therefore, propose that either the observed rapid signaling effects of 17β-estradiol are mediated through the GC-A receptor or they are mediated by the γ-adrenergic receptor, which is either itself a transmembrane guanylyl cyclase or activates a transmembrane guanylyl cyclase perhaps the GC-A receptor through cross-talk signaling.
This work was supported by The University of Warwick Research Development Fund and a Biotechnology and Biological Sciences Research Council-funded Ph.D. studentship (to R. C. S.).
- ER
- estrogen receptor
- PKGIα
- protein kinase GIα
- PMCA
- plasma membrane Ca2+ ATPase
- [Ca2+]c
- cytosolic concentration of free Ca2+
- PKG
- protein kinase G
- ANP
- atrial natriuretic peptide
- 17β-estradiol-BSA
- 17β-estradiol 6-(O-carboxymethyl)oxime:BSA
- 17β-estradiol-BSA-FITC
- 17β-estradiol 6-(O-carboxymethyl)oxime:BSA-FITC
- cyclodextrin vehicle
- 2-hydroxypropyl-β-cyclodextrin
- IBMX
- 3-isobutyl-1-methylxanthine
- Rp-8-pCPT-cGMPS
- guanosine 3′,5′-cyclic monophosphorothioate, 8-(4-chlorophenylthio)-, Rp-isomer, triethylammonium salt
- G-1
- 1-(4-(6-bromobenzo[1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)ethanone
- PDE
- phosphodiesterase
- WME
- Williams medium E
- RGS-2
- regulator of G protein signaling 2
- GC
- guanylyl cyclase.
REFERENCES
- 1.Murdoch F. E., Gorski J. (1991) Mol. Cell. Endocrinol. 78, C103–C108 [DOI] [PubMed] [Google Scholar]
- 2.Gruber C. J., Tschugguel W., Schneeberger C., Huber J. C. (2002) N. Engl. J. Med. 346, 340–352 [DOI] [PubMed] [Google Scholar]
- 3.Filardo E. J., Quinn J. A., Frackleton A. R., Jr., Bland K. I. (2002) Mol. Endocrinol. 16, 70–84 [DOI] [PubMed] [Google Scholar]
- 4.Chen Z. J., Yu L., Chang C. H. (1998) Biochem. Biophys. Res. Commun. 252, 639–642 [DOI] [PubMed] [Google Scholar]
- 5.Ropero A. B., Fuentes E., Rovira J. M., Ripoll C., Soria B., Nadal A. (1999) J. Physiol. 521, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell K. S., Haynes M. P., Sinha D., Clerisme E., Bender J. R. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 5930–5935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ropero A. B., Soria B., Nadal A. (2002) Mol. Endocrinol. 16, 497–505 [DOI] [PubMed] [Google Scholar]
- 8.Abou-Mohamed G., Elmarakby A., Carrier G. O., Catravas J. D., Caldwell R. W., White R. E. (2003) Pharmacology 69, 20–26 [DOI] [PubMed] [Google Scholar]
- 9.El-Mowafy A. M., Alkhalaf M., Jaffal S. M. (2007) Nutr. Metab. Cardiovasc. Dis. 17, 508–516 [DOI] [PubMed] [Google Scholar]
- 10.Migliaccio A., Di Domenico M., Castoria G., de Falco A., Bontempo P., Nola E., Auricchio F. (1996) EMBO J. 15, 1292–1300 [PMC free article] [PubMed] [Google Scholar]
- 11.Song R. X., McPherson R. A., Adam L., Bao Y., Shupnik M., Kumar R., Santen R. J. (2002) Mol. Endocrinol. 16, 116–127 [DOI] [PubMed] [Google Scholar]
- 12.Numakawa Y., Matsumoto T., Yokomaku D., Taguchi T., Niki E., Hatanaka H., Kunugi H., Numakawa T. (2007) Endocrinology 148, 627–637 [DOI] [PubMed] [Google Scholar]
- 13.Prakash Y. S., Togaibayeva A. A., Kannan M. S., Miller V. M., Fitzpatrick L. A., Sieck G. C. (1999) Am. J. Physiol. 276, H926–H934 [DOI] [PubMed] [Google Scholar]
- 14.Morales A., Díaz M., Guelmes P., Marín R., Alonso R. (2005) Eur. J. Neurosci. 22, 2207–2215 [DOI] [PubMed] [Google Scholar]
- 15.Zaitsu M., Narita S., Lambert K. C., Grady J. J., Estes D. M., Curran E. M., Brooks E. G., Watson C. S., Goldblum R. M., Midoro-Horiuti T. (2007) Mol. Immunol. 44, 1977–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samadi A., Carlson C. G., Gueorguiev A., Cenedella R. J. (2002) Pflugers Arch. 444, 700–709 [DOI] [PubMed] [Google Scholar]
- 17.Sarkar S. N., Huang R. Q., Logan S. M., Yi K. D., Dillon G. H., Simpkins J. W. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15148–15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi A. M., Picotto G., de Boland A. R., Boland R. L. (2002) J. Cell. Biochem. 87, 324–333 [DOI] [PubMed] [Google Scholar]
- 19.Wang J., Green P. S., Simpkins J. W. (2001) J. Neurochem. 77, 804–811 [DOI] [PubMed] [Google Scholar]
- 20.Keung W., Vanhoutte P. M., Man R. Y. K. (2005) Br. J. Pharmacol. 144, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyriochou A., Papapetropoulos A. (2005) Cell. Signal. 17, 407–413 [DOI] [PubMed] [Google Scholar]
- 22.Potter L. R., Abbey-Hosch S., Dickey D. M. (2006) Endocr. Rev. 27, 47–72 [DOI] [PubMed] [Google Scholar]
- 23.Biswas K. H., Shenoy A. R., Dutta A., Visweswariah S. S. (2009) J. Mol. Evol. 68, 587–602 [DOI] [PubMed] [Google Scholar]
- 24.Bilzer M., Jaeschke H., Vollmar A. M., Paumgartner G., Gerbes A. L. (1999) Am. J. Physiol. 276, G1137–G1144 [DOI] [PubMed] [Google Scholar]
- 25.Rambotti M. G., Giambanco I., Spreca A. (2000) Histochem. J. 32, 231–238 [DOI] [PubMed] [Google Scholar]
- 26.Scheving L. A., Russell W. E. (1996) Cancer Res. 56, 5186–5191 [PubMed] [Google Scholar]
- 27.Castro L. R., Verde I., Cooper D. M., Fischmeister R. (2006) Circulation 113, 2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zolle O., Lawrie A. M., Simpson A. W. M. (2000) J. Biol. Chem. 275, 25892–25899 [DOI] [PubMed] [Google Scholar]
- 29.Piggott L. A., Hassell K. A., Berkova Z., Morris A. P., Silberbach M., Rich T. C. (2006) J. Gen. Physiol. 128, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su J., Scholz P. M., Weiss H. R. (2005) Exp. Biol. Med. 230, 242–250 [DOI] [PubMed] [Google Scholar]
- 31.Rho E. H., Perkins W. J., Lorenz R. R., Warner D. O., Jones K. A. (2002) J. Appl. Physiol. 92, 257–263 [DOI] [PubMed] [Google Scholar]
- 32.Green A. K., Zolle O., Simpson A. W. M. (2002) Gastroenterology 123, 1291–1303 [DOI] [PubMed] [Google Scholar]
- 33.Green A. K., Stratton R. C., Squires P. E., Simpson A. W. M. (2007) J. Biol. Chem. 282, 34542–34554 [DOI] [PubMed] [Google Scholar]
- 34.Stratton R. C., Squires P. E., Green A. K. (2008) Biochem. Biophys. Res. Commun. 368, 965–970 [DOI] [PubMed] [Google Scholar]
- 35.Bracamonte M. P., Jayachandran M., Rud K. S., Miller V. M. (2002) Am. J. Physiol. Heart Circ. Physiol. 283, H2389–H2396 [DOI] [PubMed] [Google Scholar]
- 36.Prevot V., Croix D., Rialas C. M., Poulain P., Fricchione G. L., Stefano G. B., Beauvillain J. C. (1999) Endocrinology 140, 652–659 [DOI] [PubMed] [Google Scholar]
- 37.Kan W. H., Hsu J. T., Schwacha M. G., Choudhry M. A., Bland K. I., Chaudry I. H. (2008) Ann. Surg. 248, 294–302 [DOI] [PubMed] [Google Scholar]
- 38.Kim K. H., Bender J. R. (2009) Mol. Cell. Endocrinol. 308, 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zylińska L., Gromadzińska E., Lachowicz L. (1999) Biochim. Biophys. Acta 1437, 257–264 [DOI] [PubMed] [Google Scholar]
- 40.Szemraj J., Kawecka I., Lachowicz L., Zylińska L. (2003) Pol. J. Pharmacol. 55, 887–893 [PubMed] [Google Scholar]
- 41.Watters J. J., Campbell J. S., Cunningham M. J., Krebs E. G., Dorsa D. M. (1997) Endocrinology 138, 4030–4033 [DOI] [PubMed] [Google Scholar]
- 42.Walf A. A., Frye C. A. (2008) Steroids 73, 997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taguchi Y., Koslowski M., Bodenner D. L. (2004) Nuclear Receptor 2, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morales A., Díaz M., Ropero A. B., Nadal A., Alonso R. (2003) Eur. J. Neurosci. 18, 2505–2514 [DOI] [PubMed] [Google Scholar]
- 45.Nadal A., Rovira J. M., Laribi O., Leon-quinto T., Andreu E., Ripoll C., Soria B. (1998) FASEB J. 12, 1341–1348 [DOI] [PubMed] [Google Scholar]
- 46.Alonso-Magdalena P., Laribi O., Ropero A. B., Fuentes E., Ripoll C., Soria B., Nadal A. (2005) Environ. Health Perspect. 113, 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razandi M., Pedram A., Greene G. L., Levin E. R. (1999) Mol. Endocrinol. 13, 307–319 [DOI] [PubMed] [Google Scholar]
- 48.Razandi M., Pedram A., Merchenthaler I., Greene G. L., Levin E. R. (2004) Mol. Endocrinol. 18, 2854–2865 [DOI] [PubMed] [Google Scholar]
- 49.Acconcia F., Ascenzi P., Bocedi A., Spisni E., Tomasi V., Trantalance A., Visca P., Marino M. (2005) Mol. Biol. Cell 16, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denger S., Reid G., Kos M., Flouriot G., Parsch D., Brand H., Korach K. S., Sonntag-Buck V., Gannon F. (2001) Mol. Endocrinol. 15, 2064–2077 [DOI] [PubMed] [Google Scholar]
- 51.Li L., Haynes M. P., Bender J. R. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4807–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas P., Pang Y., Filardo E. J., Dong J. (2005) Endocrinology 146, 624–632 [DOI] [PubMed] [Google Scholar]
- 53.Funakoshi T., Yanai A., Shinoda K., Kawano M. M., Mizukami Y. (2006) Biochem. Biophys. Res. Commun. 346, 904–910 [DOI] [PubMed] [Google Scholar]
- 54.Toran-Allerand C. D., Guan X., MacLusky N. J., Horvath T. L., Diano S., Singh M., Connolly E. S., Jr., Nethrapalli I. S., Tinnikov A. A. (2002) J. Neurosci. 22, 8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nadal A., Ropero A. B., Laribi O., Maillet M., Fuentes E., Soria B. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11603–11608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadal A., Ropero A. B., Fuentes E., Soria B., Ripoll C. (2004) Steroids 69, 531–536 [DOI] [PubMed] [Google Scholar]
- 57.Hirst G. D., Neild T. O. (1980) Nature 283, 767–768 [DOI] [PubMed] [Google Scholar]
- 58.Benham C. D., Tsien R. W. (1988) J. Physiol. 404, 767–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yawo H. (1999) J. Neurosci. 19, 5293–5300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagner A. H., Schroeter M. R., Hecker M. (2001) FASEB J. 15, 2121–2130 [DOI] [PubMed] [Google Scholar]
- 61.Nethrapalli I. S., Tinnikov A. A., Krishnan V., Lei C. D., Toran-Allerand C. D. (2005) Endocrinology 146, 56–63 [DOI] [PubMed] [Google Scholar]
- 62.Kummer U., Krajnc B., Pahle J., Green A. K., Dixon C. J., Marhl M. (2005) Biophys. J. 89, 1603–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perc M., Green A. K., Dixon C. J., Marhl M. (2008) Biophys. Chem. 132, 33–38 [DOI] [PubMed] [Google Scholar]
- 64.Marhl M., Gosak M., Perc M., Jane Dixon C., Green A. K. (2008) J. Theor. Biol. 252, 419–426 [DOI] [PubMed] [Google Scholar]
- 65.Pahle J., Green A. K., Dixon C. J., Kummer U. (2008) BMC Bioinformatics 9, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Bueno A., Greenwood M. R., Varela-Nieto I., Marrero I., Gil B., Mato J. M., Cobbold P. H. (1997) Cell Calcium 21, 125–133 [DOI] [PubMed] [Google Scholar]
- 67.Golden G. A., Mason P. E., Rubin R. T., Mason R. P. (1998) Clin. Neuropharmacol. 21, 181–189 [PubMed] [Google Scholar]
- 68.Clarke R., Leonessa F., Welch J. N., Skaar T. C. (2001) Pharmacol. Rev. 53, 25–71 [PubMed] [Google Scholar]
- 69.Zabel U., Kleinschnitz C., Oh P., Nedvetsky P., Smolenski A., Müller H., Kronich P., Kugler P., Walter U., Schnitzer J. E., Schmidt H. H. H. W. (2002) Nat. Cell Biol. 4, 307–311 [DOI] [PubMed] [Google Scholar]
- 70.Olesen S. P., Drejer J., Axelsson O., Moldt P., Bang L., Nielsen-Kudsk J. E., Busse R., Mülsch A. (1998) Br. J. Pharmacol. 123, 299–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bender A. T., Beavo J. A. (2006) Pharmacol. Rev. 58, 488–520 [DOI] [PubMed] [Google Scholar]
- 72.Fisher D. A., Smith J. F., Pillar J. S., St Denis S. H., Cheng J. B. (1998) J. Biol. Chem. 273, 15559–15564 [DOI] [PubMed] [Google Scholar]
- 73.Soderling S. H., Bayuga S. J., Beavo J. A. (1998) J. Biol. Chem. 273, 15553–15558 [DOI] [PubMed] [Google Scholar]
- 74.Hofmann F. (2005) J. Biol. Chem. 280, 1–4 [DOI] [PubMed] [Google Scholar]
- 75.Vaandrager A. B., Edixhoven M., Bot A. G., Kroos M. A., Jarchau T., Lohmann S., Genieser H. G., de Jonge H. R. (1997) J. Biol. Chem. 272, 11816–11823 [DOI] [PubMed] [Google Scholar]
- 76.Pinkse M. W., Rijkers D. T., Dostmann W. R., Heck A. J. (2009) J. Biol. Chem. 284, 16354–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dostmann W. R., Tegge W., Frank R., Nickl C. K., Taylor M. S., Brayden J. E. (2002) Pharmacol. Ther. 93, 203–215 [DOI] [PubMed] [Google Scholar]
- 78.Green A. K., Cobbold P. H., Dixon C. J. (1997) Cell Calcium 22, 99–109 [DOI] [PubMed] [Google Scholar]
- 79.Pande J., Mallhi K. K., Sawh A., Szewczyk M. M., Simpson F., Grover A. K. (2006) Am. J. Physiol. Cell Physiol. 290, C1341–C1349 [DOI] [PubMed] [Google Scholar]
- 80.Green A. K., Cobbold P. H., Dixon C. J. (1999) Cell Calcium 25, 173–178 [DOI] [PubMed] [Google Scholar]
- 81.Kuiper G. G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J. A. (1997) Endocrinology 138, 863–870 [DOI] [PubMed] [Google Scholar]
- 82.Howell A., Osborne C. K., Morris C., Wakeling A. E. (2000) Cancer 89, 817–825 [DOI] [PubMed] [Google Scholar]
- 83.Bologa C. G., Revankar C. M., Young S. M., Edwards B. S., Arterburn J. B., Kiselyov A. S., Parker M. A., Tkachenko S. E., Savchuck N. P., Sklar L. A., Oprea T. I., Prossnitz E. R. (2006) Nat. Chem. Biol. 2, 207–212 [DOI] [PubMed] [Google Scholar]
- 84.Dodge-Kafka K. L., Langeberg L., Scott J. D. (2006) Circ. Res. 98, 993–1001 [DOI] [PubMed] [Google Scholar]
- 85.Rashatwar S. S., Cornwell T. L., Lincoln T. M. (1987) Proc. Natl. Acad. Sci. U.S.A. 84, 5685–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshida Y., Toyosato A., Islam M. O., Koga T., Fujita S., Imai S. (1999) Mol. Cell. Biochem. 190, 157–167 [PubMed] [Google Scholar]
- 87.Howard A., Barley N. F., Legon S., Walters J. R. F. (1994) Biochem. J. 303, 275–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang K. M., Wang G. R., Lu P., Karas R. H., Aronovitz M., Heximer S. P., Kaltenbronn K. M., Blumer K. J., Siderovski D. P., Zhu Y., Mendelsohn M. E., Tang M., Wang G. (2003) Nat. Med. 9, 1506–1512 [DOI] [PubMed] [Google Scholar]
- 89.Takimoto E., Koitabashi N., Hsu S., Ketner E. A., Zhang M., Nagayama T., Bedja D., Gabrielson K. L., Blanton R., Siderovski D. P., Mendelsohn M. E., Kass D. A. (2009) J. Clin. Invest. 119, 408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vilatoba M., Eckstein C., Bilbao G., Frennete L., Eckhoff D. E., Contreras J. L. (2005) Transplant. Proc. 37, 399–403 [DOI] [PubMed] [Google Scholar]
- 91.de Vries A. H., Ponds F. A. M., Padbury R. T. A., Porte R. J., Nieuwenhuijs V. B., Barritt G. J. (2009) J. Hepatol. 50, suppl. 1, S66–S66 [Google Scholar]
- 92.Leal A. M., Begoña Ruiz-Larrea M., Martínez R., Lacort M. (1998) Biochem. Pharmacol. 56, 1463–1469 [DOI] [PubMed] [Google Scholar]
- 93.Ricchi M., Bertolotti M., Anzivino C., Carulli L., Canedi I., Bormioli M. L., Tiozzo R., Croce M. A., Lonardo A., Carulli N., Loria P. (2006) J. Gastroenterol. Hepatol. 21, 894–901 [DOI] [PubMed] [Google Scholar]
- 94.Pietras R. J., Szego C. M. (1980) Biochem. J. 191, 743–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Srivastava D. P., Yu E. J., Kennedy K., Chatwin H., Reale V., Hamon M., Smith T., Evans P. D. (2005) J. Neurosci. 25, 6145–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yunoki M., Nakahara T., Mitani A., Sakamoto K., Ishii K. (2003) Naunyn-Schmiedebergs Arch. Pharmacol. 367, 76–79 [DOI] [PubMed] [Google Scholar]
- 97.Bruges G., Borges A., Sánchez de Villarroel S., Lippo de Bécemberg I., Francis de Toba G., Pláceres F., González de Alfonzo R., Alfonzo M. J. (2007) J. Recept. Signal Transduct. Res. 27, 189–216 [DOI] [PubMed] [Google Scholar]