Abstract
Ganglioside GD3 is widely expressed in human malignant melanoma cell lines and tumors. Previously, we reported that GD3+ cells show stronger tyrosine phosphorylation of focal adhesion kinase (FAK), p130Cas, and paxillin when treated with fetal calf serum than GD3− cells. In this study, we analyzed the changes in the signals mediated by the interaction between integrins and extracellular matrices (ECM) to clarify how GD3 enhances cell signals in the vicinity of the cell membrane. An adhesion assay with a real time cell electronic sensing system revealed that GD3+ cells had stronger adhesion to all extracellular matrices examined. In particular, GD3+ cells attached more strongly to collagen type I and type IV than controls. Correspondingly, they showed stronger tyrosine phosphorylation of FAK and paxillin during adhesion to collagen type I. In the floating pattern of detergent extracts, a high level of integrin β1 was found in glycolipid-enriched microdomain (GEM)/rafts in GD3+ cells before adhesion, whereas a smaller amount of integrin β1 was detected in the GEM/rafts of controls. Some phosphorylated forms of FAK as well as total FAK were found in GEM/rafts during cell adhesion only in GD3+ cells. Another signal consisting of integrin-linked kinase/Akt was also activated during adhesion more strongly in GD3+ cells than in controls. In double stained GD3+ cells, GD3 and integrin β1 co-localized at the focal adhesion with a punctate pattern. All these results suggested that integrins assembled and formed a cluster in GEM/rafts, leading to the enhanced signaling and malignant properties under GD3 expression.
Keywords: Adhesion, Ganglioside, Glycolipids, Integrin, Lipid Raft, Signal Transduction, Tumor, Melanoma, Microdomain
Introduction
Sialic acid-containing glycosphingolipids, known as gangliosides, are expressed at high levels in nerve tissues and various tumor cells (1). Although a number of studies on the roles of gangliosides in the regulation of cell proliferation have been performed (2), the mechanisms of regulation are not well understood. Malignant melanoma is very difficult to cure because of its rapid growth, high tendency of metastasis, rigorous invasion into surrounding tissues, and resistance to therapy (3). Because ganglioside GD3 is widely and specifically expressed in melanomas (4, 5), anti-GD3 monoclonal antibodies (mAbs) were tried for the therapy of melanoma patients first in 1985 (6), and have been expected as one of promising therapeutic trials in melanoma patients (7). Anti-GD3 antibodies have been reported to suppress the growth of cultured melanoma cells (8), although the role of GD3 in malignant melanoma is not precisely understood.
To investigate the roles of GD3, we generated GD3-overexpressing transfectant cells (GD3+) from a GD3− mutant of SK-MEL-28 named N1 (9) by using cloned GD3 synthase cDNA (10), and observed the phenotypic changes and studied the molecular mechanisms of GD3-mediated biosignals. We demonstrated that adaptor molecules such as focal adhesion kinase (FAK),4 p130Cas, and paxillin undergo stronger tyrosine phosphorylation after treatment with fetal calf serum (FCS) in GD3+ cells than in control cells, and that they are actually involved in increased cell proliferation and invasion with GD3 expression (11, 12).
Among extrinsic signals supporting cellular activity, those derived from the interaction between integrin and the extracellular matrix (ECM) should be just as important as growth factor-derived signals. Molecules involved in biosignaling via growth factors/receptors might also be involved in integrin-mediated signaling (13). In fact, integrins play essential roles in causing melanomas to acquire malignant properties based on GD3 expression (14, 15). There are a number of studies indicating that integrin expression is up-regulated during malignant transformation, and integrin-mediated signals consisting of FAK, integrin-linked kinase (ILK), and ERK/MAPK, which determine cell fates such as cell growth and invasion, are activated in melanomas (16). In this study, we examined how GD3 is implicated in increased integrin-mediated adhesion and subsequent signaling, leading to the malignant properties of melanomas.
EXPERIMENTAL PROCEDURES
Cell Cultures
G5, G11, and G12 are GD3+ transfectant cells generated from a GD3− mutant of SK-MEL-28 N1 (9) by using cloned GD3 synthase cDNA (10). V4 and V9 are GD3− control cells transfected with the vector alone. These cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 7.5% FCS at 37 °C in a humidified atmosphere containing 5% CO2.
Antibodies
Anti-GD3 mAb R24 was kindly provided by Dr. L. J. Old at Memorial Sloan-Kettering Cancer Center. FITC-labeled anti-mouse IgG antibody was purchased from ICN/Cappel (Durham, NC). Anti-rabbit IgG antibody conjugated with horseradish peroxidase (HRP) was purchased from Cell Signaling Technology (Beverly, MA). Anti-mouse IgG conjugated with HRP was from Amersham Biosciences. Anti-phosphotyrosine mAb (PY20), rabbit anti-p130Cas, mouse anti-integrin β1, mouse anti-FAK, rabbit anti-phospho-FAK (Tyr-397) antibodies were from BD Transduction Laboratories (San Jose, CA). Rabbit anti-FAK, rabbit anti-phospho-FAK (Tyr-576, Tyr-577, Tyr-861, and Tyr-925), and anti-caveolin-1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse anti-human CD29 (integrin β1)/biotin antibody was from Ancell (Bayport, MN). Anti-CD29 mAb was from BD Transduction. Anti-integrin isoform mAbs were from DAKO (Carpinteria, CA) (α2β1, α4β1, α5β1) and Santa Cruz Biotechnology (α3). Phosphorylation site-specific anti-p130Cas antibodies (Tyr-165, Tyr-249, and Tyr-410) were purchased from Cell Signaling Technology. Anti-phospho-paxillin antibodies, p-Paxillin (Tyr-31 and Tyr-118) were purchased from Santa Cruz Biotechnology, and p-Paxillin (Tyr-118) was from Cell Signaling Technology. Anti-ILK antibodies were purchased from BD Transduction (mouse mAb), and from Cell Signaling Technology (rabbit anti-ILK1). Anti-phospho-Akt (Ser-473), anti-phospho-Akt (Thr-308), anti-Akt, and anti-phosphothreonine antibodies were purchased from Cell Signaling Technology. An anti-phosphoserine mAb was purchased from Sigma (number M65327).
Reagents
Fibronectin (FN) from human plasma, collagen (CL) type I, and CL type IV from human placenta were purchased from Chemicon (Temecula, CA). Laminin (LN) was from human placenta and poly-l-lysine (PLL) was from Sigma. Protein A-FITC was from ICN Immuno-Biologicals (Lisle, IL). Phycoerythrin (PE) -streptavidin was from BD Transduction.
Flow Cytometry
The cell surface expression of integrin β1 was analyzed by flow cytometry (BD Biosciences) as previously described (17).
In Vitro Invasion Assay and BrdU Assay
The in vitro invasion and BrdU assays were performed as described previously (14).
Preparation of Plates Coated with ECM Proteins
ECM in PBS (5 μg/ml) was coated in Petri dishes (Greiner Bio-one, Frickenhausen, Germany) or glass base dishes (Iwaki, Tokyo, Japan) overnight at 4 °C. Then, plates were washed twice with PBS and blocked with serum-free minimal essential medium containing 1% heat-inactivated BSA (10 min at 60 °C). Plates coated with 0.01% PLL (Sigma) in Petri dishes for 5 min at room temperature were washed with PBS and blocked with minimal essential medium/BSA as previously described (18).
Cell Adhesion Assays Using Real Time Cell Electronic Sensing (RT-CES)
ACEA e-plates (ACEA Biosciences, San Diego, CA) were coated with FN, LN, CL type I, CL type IV, or PLL for 1 h at 37 °C. The plates were washed with PBS and coated with 0.5% BSA in PBS for 20 min at 37 °C. The wells were washed with PBS before the addition of culture medium with or without serum, and cells (1 × 104) were added on ACEA e-plates coated with various ECM proteins. The adhesion of cells was monitored continuously using the RT-CES system (Wako Pure Chemical, Osaka, Japan) for 24 h.
Integrin-mediated Adhesion to ECM
Cells were starved for 14–16 h in serum-free DMEM, and harvested with trypsin/EDTA in PBS or 0.02% EDTA in PBS. Trypsin activity was inhibited by adding 100 μg/ml of soybean trypsin inhibitor (Invitrogen) to PBS. To reduce basal phosphorylation of signaling molecules, cells were rotated for 1 h at 37 °C. Cell suspensions (4 × 105) were added to 6-cm dishes precoated with CL type I or CL type IV. Cells were lysed after incubation at 37 °C, and lysates were used for Western immunoblotting.
Preparation of Cell Lysates
Cells were lysed with cell lysis buffer (20 mm Tris-HCl, 150 mm NaCl, 1 mm Na2EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 μg/ml of leupeptin) (Cell Signaling), Protease Inhibitor MixtureTM (Calbiochem, San Diego, CA), and 1 mm PMSF. Insoluble materials were removed by centrifugation at 4 °C at 10,000 × g for 10 min.
Western Immunoblotting
Cell lysates were separated by SDS-PAGE using 10–15% gels. The separated proteins were transferred onto an Immunobilon-PTM membrane (Millipore, Billerica, MA). Blots were blocked with BSA in PBS containing 0.05% Tween 20. The membrane was first probed with primary antibodies. After being washed, the blots were incubated with goat anti-rabbit IgGs or goat anti-mouse IgGs conjugated with HRP (1:1000). Bound conjugates on the membrane were visualized with an Enhanced ChemiluminescenceTM detection system (PerkinElmer Life Sciences).
Preparation of the GEM/Rafts Fractions
Cells (2–2.5 × 107) were lysed with cell lysis buffer, i.e. 1% Lubrol WX (SERVA, Heidelberg, Germany) in MNE buffer (25 mm MES, pH 6.5, 150 mm NaCl, 5 mm EDTA, 1 mm Na3VO4, 1 mm PMSF, 1 μg/μl of aprotinin). After removing insoluble material by centrifugation at 10,000 × g for 10 min, lysates were dounced 10 times with a Digital HomogenizerTM (AS ONE, Osaka, Japan). The lysates were mixed with an equal volume of 80% sucrose in MNE buffer, and a stepwise gradient was prepared by overlaying 30% sucrose in MNE followed by a final layer of 5% sucrose in MNE. The gradient was formed by centrifugation for 16–18 h at 4 °C at 200,000 × g using a Beckman MLS50 rotor (Kent, MI). Fractions of 500 μl were separated from the top of the gradient, and were used for Western immunoblotting.
Immunoprecipitation and Sequential Immunoblotting
Approximately 500 μl of cell lysates were immunoprecipitated with rabbit anti-FAK antibodies (0.2 μg), rabbit anti-ILK antibodies, or normal rabbit IgGs bound to protein A-Sepharose beads at 4 °C for 8 h. The beads were washed three times with a washing buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% Triton X-100, 1 mm Na3VO4) and finally resuspended in 40 μl of 2× SDS sample buffer. The precipitated proteins were separated by SDS-PAGE and then analyzed by immunoblotting with mouse anti-FAK antibodies, an anti-phosphotyrosine mAb (PY20), anti-phosphothreonine antibodies, anti-phosphoserine antibodies, or anti-ILK antibodies.
Knockdown of ILK
Suppression of ILK after 48 h of transfection into GD3+ cell (G5) with 4 kinds of siRNA was examined by immunoblotting using an anti-ILK antibody (BD Biosciences). GL2, siRNA for a firefly luciferase (B-Bridge International, Inc. CA) was used as a negative control. si-ILK3 (5′-AAGUUAAGCUGUUUGAAGUCAAUGC-3′) (Invitrogen) was the most effective (knockdown rate was about 70%).
Immunofluorescence Staining
Cells were fixed in paraformaldehyde (4% in PBS for 10 min) and then incubated with 0.1% Triton X-100 in PBS for 10 min at room temperature. After being washed with PBS, nonspecific binding was blocked with 2.5% BSA in PBS for 60 min at room temperature. Cells were incubated with biotin-conjugated anti-human CD29 (integrin β1) or mAb R24 in PBS containing 0.5% BSA for 60 min at room temperature, then with phycoerythrin-streptavidin or protein A-FITC in PBS containing 0.5% BSA for 30–45 min at room temperature. The resulting staining patterns were imaged using a confocal microscope (Fluoview FV500, Olympus, Tokyo, Japan).
RESULTS
Expression of Integrins on GD3+ Cells and Control Cells
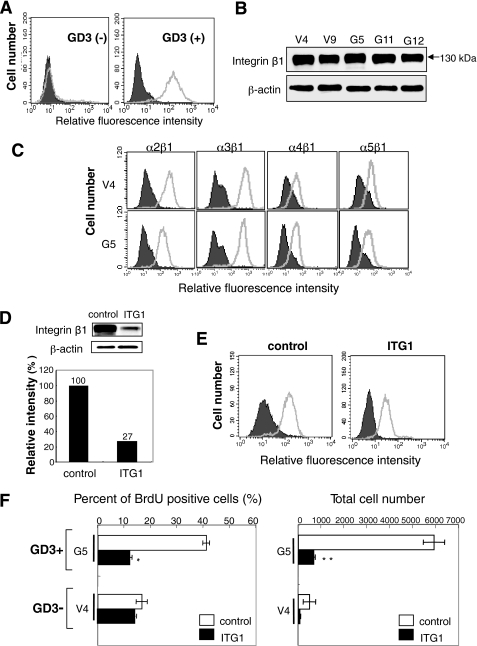
Expression of GD3 on established GD3+ cells (G5) and control cells (V4) was shown in Fig. 1A. Other transfectant cells showed essentially the same pattern as G5. Integrin β1 levels in cell lysates prepared from GD3+ cells and control cells were analyzed by immunoblotting with anti-integrin β1, showing an almost equivalent intensity of bands among all GD3+ cells and control cells (Fig. 1B). Expression of integrins on GD3+ cells (G5, G11) and control cells (V4, V9) was analyzed by flow cytometry using anti-integrin antibodies (α2β1, α3, α4β1, and α5β1) (see Fig. 1C for data on G5 and V4). There were no differences in the expression levels of any integrins between GD3+ cell lines and the controls examined. Expression levels of integrins α2β1 and α3β1 were higher than those of integrins α4β1 and α5β1 in all cell lines.
FIGURE 1.
Expression and roles of integrins in GD3+ melanoma cells. A, expression of GD3 on transfectant cells (G5)(right) and control cells (V4)(left) was analyzed by flow cytometry using mAb R24 and FITC-labeled anti-mouse IgG antibody. Closed histograms and open histograms indicate controls and samples, respectively (A, C, and E). B, cell lysates were prepared from GD3+ cells (G5, G11, and G12) and control cells (V4 and V9) and used for immunoblotting with an anti-integrin β1 antibody. SDS-PAGE was done under reducing conditions, showing bands at 130 kDa. C, expression of integrins on GD3+ cells (G5) and control cells (V4) was analyzed by flow cytometry using anti-integrin α2β1, -integrin α3, -integrin α4β1, or -integrin α5β1 antibodies. Controls were prepared with the secondary antibody alone. D, knockdown of integrin β1 with siRNA ITG 1. The sequence of ITG 1 was 5′-AUAAUGUUCCUACUGCUGACUUAGG-3′. Control siRNA was GL-2 (anti-firefly luciferase siRNA), and the sequence described above. After 2 days of transfection of an anti-integrin β1 siRNA into G5, immunoblotting was performed using cell lysates. This was representative data among experiments repeated at least 3 times with similar results. E, reduction of the integrin expression as analyzed with flow cytometry. F, suppression of cell growth (left) and invasion (right) activity with knockdown of integrin β1. Cell growth was measured by uptake of BrdU at 2 days after siRNA transfection. Cell invasion was analyzed by a Boyden chamber invasion assay by counting the cell number on the reverse side of the filter. Bars indicate mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
Roles of Integrins in the Proliferation and Invasion of Melanoma Cells
As shown in Fig. 1, D and E, knockdown of integrin β1 with siRNA ITG1 resulted in reduced protein levels (Fig. 1D) and reduced surface expression (Fig. 1E). Then, it was shown that knockdown of integrin β1 resulted in suppressed cell growth and invasion activity, especially in the GD3+ transfectant cells (Fig. 1F). These results were essentially the same as those reported in a previous study (14).
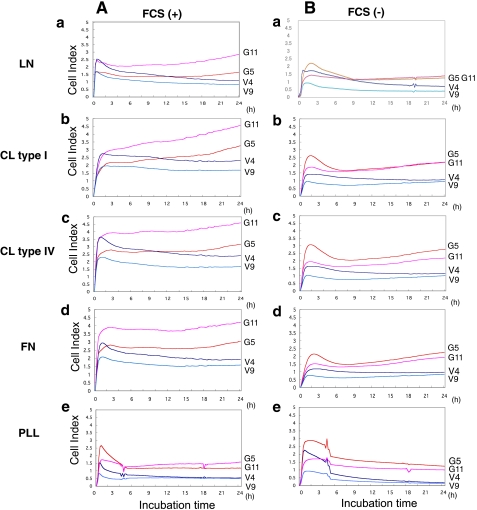
Cell Adhesion Analyzed by the RT-CES System
The intensity of adhesion and spreading of GD3+ cells and control cells was analyzed by the RT-CES system (Fig. 2). This system allowed us to observe cell adhesion activity in real time. ACEA e-plates were coated with LN, CL type I, CL type IV, FN, or PLL as a control. GD3+ cells (G5 and G11) adhered to LN, CL type I, CL type IV, or FN more strongly than control cells (V4 and V9) in the presence of FCS (left). Similarly, GD3+ cells showed more intense adhesion to LN, CL type I, CL type IV, or FN than control cells under FCS-free conditions (right), although the adhesion activity of these cells was generally lower under FCS-free conditions than in the presence of FCS. For PLL, the adhesion intensity was very low and did not increase in the latter phase in either GD3+ or control cells.
FIGURE 2.
Dynamic monitoring of cell adhesion to LN, CL type I, CL type IV, FN, or PLL-coated surfaces. A, GD3+ cells (G5 and G11) and control cells (V4 and V9) were seeded in the wells of 96-well e-plates at 104 cells/well with FCS, and cell attachment and spreading were monitored by the RT-CES system. The e-plates were pre-coated with LN (a), CL type I (b), CL type IV (c), FN (d), or PLL (e) as described under “Experimental Procedures.” B, similar experiments were performed without FCS in wells.
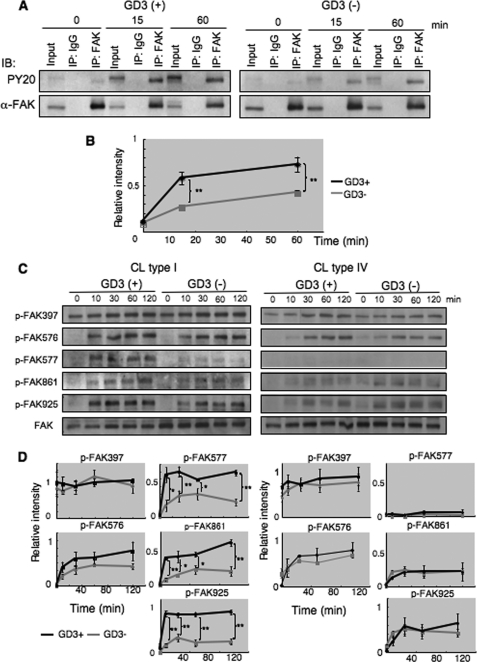
Stronger Phosphorylation of FAK in GD3+ Cells during Adhesion to CL Type I
To investigate integrin signaling triggered by cell adhesion to ECM, we analyzed tyrosine phosphorylation of FAK in GD3+ and control cells because FAK has been implicated in integrin-mediated signaling. After serum starvation and rotation, cells were plated on dishes pre-coated with CL type I or CL type IV under serum-free conditions, and incubated at 37 °C for 0, 15, or 60 min (Fig. 3A). After incubation, cells were lysed and the lysates were used for immunoprecipitation using an anti-FAK antibody and subsequent immunoblotting using PY20 or an anti-FAK antibody. As shown in Fig. 3, A and B, the phosphorylation levels of FAK in GD3+ cells were higher than those of control cells during adhesion to CL type I for 15 or 60 min incubation. Immunoblotting with phosphorylation site-specific antibodies revealed that phosphorylation levels were generally higher in GD3+ cells than in control cells. In particular, Tyr-577, Tyr-861, and Tyr-925 showed stronger phosphorylation in GD3+ cells than in control cells (Fig. 3, C and D, left). When the same experiment was performed for CL type IV, the phosphorylation levels of Tyr-577 and Tyr-861 were much lower with no definite differences between either type of cell (Fig. 3, C and D, right). These results suggested that integrin-mediated signaling is enhanced with GD3 expression during adhesion to CL type I.
FIGURE 3.
Phosphorylation of FAK during adhesion. A, tyrosine phosphorylation of FAK during adhesion was examined with immunoprecipitation and immunoblotting. GD3+ (G5) or control cells (V4) were detached with 0.02% EDTA in PBS after culturing in serum-free conditions at 37 °C for 14–16 h, and were rotated for 1 h at 37 °C to cancel adhesion signals. Cell suspensions (4 × 105 cells) were added to pre-coated plates with CL type I, and incubated for 0, 15, and 60 min. After incubation, cells were lysed and lysates were used for immunoprecipitation as described under “Experimental Procedures.” Immunoprecipitates were served for immunoblotting using antibodies reactive to FAK or anti-phosphotyrosine antibody PY20. B, bands in A were scanned and the results presented after correction with total FAK bands. Relative phosphorylation levels of FAK in GD3+ cells are shown as black lines, and those of control cells are shown as gray lines. C and D, phosphorylation levels of FAK at individual phosphorylation sites during adhesion to ECM. C, GD3+ (G5) or control cells (V4) were detached after culturing in serum-free conditions at 37 °C for 14–16 h, and rotated for 1 h at 37 °C to cancel adhering signals. Cell suspensions (4 × 105 cells) were added to pre-coated plates with CL type I or CL type IV, and incubated for 0, 10, 30, 60, or 120 min. After incubation, cells were lysed and used (6 μg total proteins) for immunoblotting using antibodies specifically reactive to the individual phosphorylation sites of FAK. Bands in autofluorograms (C) were quantified by a scanner, and the relative intensities of the bands were plotted after correction with total FAK bands (D). Bars indicate mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
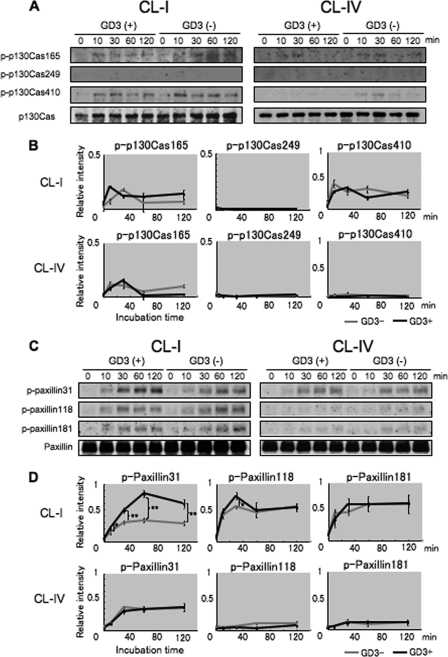
Increased Phosphorylation of Paxillin with GD3 Expression during Adhesion to CL Type I
We analyzed phosphorylation of p130Cas and paxillin during adhesion to CL type I or CL type IV (Fig. 4). The phosphorylation of p130Cas and paxillin was detected by immunoblotting with phosphorylation site-specific antibodies of p130Cas (p-p130Cas 165, 249, and 410) or paxillin (p-paxillin 31, 118, and 181) as well as anti-p130Cas and anti-paxillin antibodies. GD3+ cells (G5) showed stronger phosphorylation of paxillin than the control cells (V4) during adhesion to CL type I as shown for FAK (Fig. 4, C and D). Notably, the strongest levels of phosphorylation of paxillin (p-paxillin 31 and 118) were observed in GD3+ cells at 30 min incubation. On the other hand, no differences in the phosphorylation of p130Cas were found between GD3+ cells and GD3− cells in any sites of p130Cas (Fig. 4, A and B). These results suggested that signaling via integrin and its downstream was differentially enhanced for paxillin, not for p130Cas with GD3 expression in melanomas. As for adhesion to CL type IV, phosphorylation levels of these molecules were much lower at all time points in both types of cells (Fig. 4, A and C, right, and B and D, lower). These results suggested that phosphorylation of Tyr-577 and Tyr-861 in FAK is important for subsequent activation of paxillin, leading to malignant phenotypes under GD3 expression.
FIGURE 4.
Phosphorylation levels of p130Cas and paxillin in GD3+ cells or control cells during adhesion to ECM. A, to analyze tyrosine phosphorylation of p130Cas, similar cell preparations as described in the legend to Fig. 3 were performed using pre-coated plates with CL type I or CL type IV. Phosphorylation of p130Cas (p-p130Cas) was detected by antibodies specifically reactive with individual phosphorylation sites (p-p130Cas 165, 249, and 410). Total p130Cas was also detected with an anti-p130Cas antibody. B, bands in A were quantified by a scanner, and the relative intensities were plotted after correction with those of p130Cas. C, the same experiments were performed to examine tyrosine phosphorylation of paxillin. Immunoblotting was performed by antibodies specifically reactive with individual phosphorylation sites (p-paxillin 31, 118, and 181) as well as an anti-paxillin antibody. D, bands in C were quantified by a scanner, and the relative intensities were plotted after correction with those of paxillin. Bars indicate mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
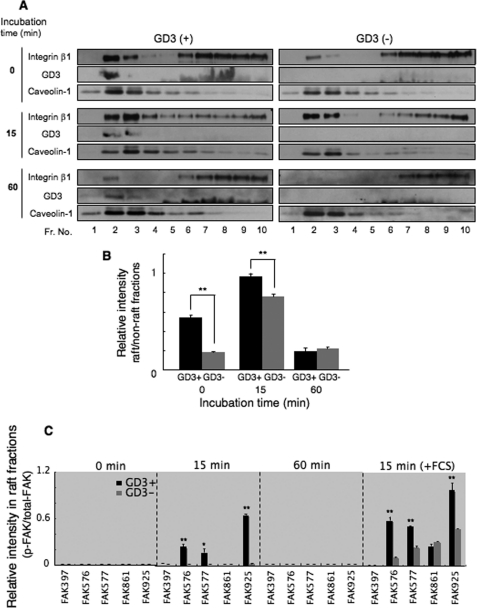
A Shift of Integrins to the GEM/Raft Fraction in GD3+ Cells
We examined the mechanisms with which integrin-mediated signaling is enhanced by GD3 expression. The assembly of integrins in GEM/rafts under GD3 expression might be a possible mechanism. We therefore separated fractions from GD3+ (G5) or control cells (V4) during adhesion to CL type I using sucrose density gradient ultracentrifugation, and immunoblotting with anti-integrin β1 antibodies was performed using the fractions (Fig. 5A). Caveolin-1 was used as a GEM/raft marker. In these experiments, the majority of GD3 existed in GEM/raft fractions at all time points in GD3+ cells. Fundamental data to confirm GD3 bands in immunoblotting were shown under supplemental Fig. S1. It is noteworthy that a significant amount of integrin β1 was already present in GEM/raft fractions in GD3+ cells at approximately three times or more of that of control cells before incubation (time 0). The majority of integrins moved to the GEM/raft fractions in both GD3+ cells and control cells upon a 15-min incubation with CL type I. This result indicated that integrins in GEM/rafts are involved in cell adhesion, and that integrin-mediated signaling is generated there. After incubation for 60 min, only low levels of integrins remained in the GEM/rafts in both GD3+ and control cells, which were almost equivalent to the level of control cells at time 0 (Fig. 5B).
FIGURE 5.
Integrin β1 is found in GEM/rafts before and during adhesion to CL type I in GD3+ cells. A, cells were treated as described in the legend to Fig. 3, and added to plates coated with CL type I. GD3+ or control cells were lysed with 1% Lubrol WX in MNE buffer during adhesion to pre-coated plates with CL type I after incubation for 0, 15, or 60 min at 37 °C. The lysates were separated by sucrose density gradient ultracentrifugation and used for immunoblotting using anti-integrin β1, mAb R24, or anti-caveolin-1 antibodies. Fractions 1–4 contained low density fractions and fractions 6–10 corresponded to high density fractions. B, bands in A were quantified by a scanner, and the relative intensities in the GEM/raft fractions (fractions 2 and 3) against those in the non-GEM/raft fraction (fractions 7–10) were plotted. Black boxes represent GD3+ cells and gray boxes are control cells. Results are presented as mean ± S.D. (n = 3). **, p < 0.01. C, cells were treated as described in the legend to Fig. 3. The fractions were analyzed with an anti-FAK antibody or antibodies specifically reactive to the individual tyrosine-phosphorylation sites of FAK as described under supplemental Fig. S2. Cells were resuspended in medium without FCS (or with FCS exceptionally), and were added to pre-coated plates with CL type I. Results in supplemental Fig. S2 are summarized by calculating band intensities as ratios between p-FAK and total FAK in the GEM/rafts. Bars indicate mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
FAK Localized and Was Activated in GEM/Rafts during Adhesion
We analyzed floating patterns of FAK, p130Cas, and paxillin, and their phosphorylated forms during cell adhesion in GD3+ transfectant cells (G5) and control cells (V4). The amounts of total FAK detected in the GEM/raft fractions were fairly high in both types of cells before and after 15 min of adhesion reaction (supplemental Fig. S2). Phosphorylated FAK bands were detected in the GEM/raft fractions at 15 min incubation only in GD3+ cells. A strong tyrosine phosphorylation band at Tyr-925 of FAK, and weak bands at Tyr-576 and Tyr-577, were found in the GEM/rafts at 15 min in GD3+ cells. These phosphorylation bands were not found in the GEM/raft fractions at 60 min incubation in either type of cells. The results in supplemental Fig. S2 were summarized in the graph of Fig. 5C. In addition, we examined the floating patterns of FAK during adhesion in the presence of FCS. As shown in supplemental Fig. S2B, bands of total FAK and phosphorylated FAK in GEM/rafts were more definite and more intense than those in the mere adhesion experiments (supplemental Fig. S2, A and B). These results were also summarized in Fig. 5C.
As for p130Cas and paxillin, paxillin underwent stronger phosphorylation in GD3+ cells during cell adhesion as shown in Fig. 4, whereas the majority of those proteins were persistently found in non-GEM/raft fractions (supplemental Fig. S3). Phosphorylation bands of p130Cas and paxillin were also absent in the GEM/raft fractions at all stages in both types of cells (G5 and V4).
Integrin-ILK-Akt Signal Was Also Enhanced in GD3+ Cells
In addition to FAK and paxillin, the ILK-Akt signaling pathway was also enhanced during cell adhesion to CL type I in GD3+ cells. Both pAkt (Ser-473) and pAkt (Thr-308) were more strongly activated in GD3+ cells at 10 min of incubation with CL type I (Fig. 6, A and B). To examine the involvement of ILK in Akt activation, siRNA:si-ILK3 was used for its highest efficiency (∼80%) in knockdown of ILK (Fig. 6C). In GD3+ cells treated with si-ILK3, stronger suppression in band intensities of p-Akt (Ser-473) and p-Akt (Thr-308) was observed at 20 min after cell adhesion to CL type I than in GD3− cells (Fig. 6D).
FIGURE 6.
Integrin-ILK-Akt signaling was also enhanced in GD3+ cells. A, phosphorylation of Akt during adhesion to CL type I in GD3+ cells (G5) and GD3− cells (V4) was examined. Cells were prepared as described in the legend to Fig. 3, and immunoblotting was performed using anti-phospho-Akt (Ser-473), anti-phospho-Akt (Thr-308), and anti-total Akt antibodies. B, band intensities were plotted after correction with those of total Akt. C, knockdown of ILK. To knockdown ILK, 4 kinds of anti-ILK siRNAs were analyzed. siRNA:si-ILK3 suppressed ILK levels most efficiently (70–80%), and was used hereafter. GL-2 (anti-firefly luciferase siRNA) was used as a control. D, ILK is involved in Akt phosphorylation. Effects of ILK knockdown on phosphorylation levels of Akt in GD3+ (a) and GD3− cells (b) during adhesion to CL type I were examined. After 48 h of transfection, cells were plated on pre-coated CL type I, and lysed for immunoblotting at incubation time 0 and 20 min. Definite reduction in Akt phosphorylation was detected in GD3+ cells. Bars indicate mean ± S.D. (n = 3). *, p < 0.05; **, p < 0.01. These were representative results among experiments repeated at least 3 times with similar results (C and D).
ILK Localization and Phosphorylation during Adhesion
Using fractions by sucrose density gradient fractionation, floating patterns of ILK were examined (supplemental Fig. S4). It was detected only in non-GEM/rafts. Phosphorylation at Ser/Thr of ILK was also examined, showing low levels of phosphorylation regardless of incubation intervals.
ERK Phosphorylation during Adhesion
To examine activation of ERK during cell adhesion, immunoblotting of ERKs was performed. Consequently, no definite increase in phosphorylation levels of ERKs was found in cells after vigorous washing of FCS. Furthermore, no bands of p-ERK were found in GEM/rafts (supplemental Fig. S5, A–C). Interestingly, stronger phosphorylation reaction of ERKs could be found in GD3+ cells when adhesion was performed in the presence of the remaining FCS (supplemental Fig. S5D).
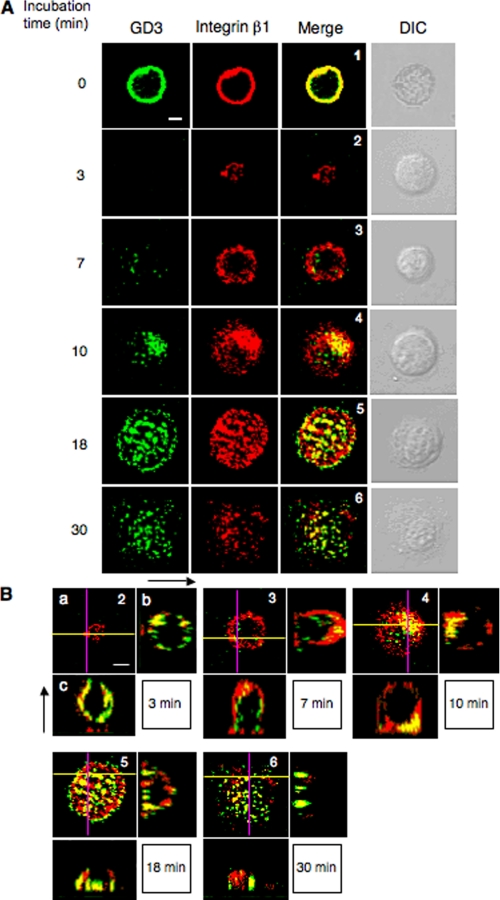
Integrin β1 Colocalized with GD3 at the Focal Adhesion during Cell Adhesion
We analyzed the intracellular localization of integrin β1 and GD3 by double immunofluorescence staining. Cells (G5) were plated on pre-coated plates with CL type I, and fixed after incubation for 0, 3, 7, 10, 18, and 30 min. The intracellular localization of integrin β1 and GD3 at various stages of adhesion is shown in Fig. 7A. Evidence to confirm definite the remaining levels of GD3 in Triton X-100-treated cells were shown under supplemental Fig. S6. Although integrin β1 and GD3 were uniformly merged on the cell membrane at time 0 (14), integrin β1 was detected at the peripheral regions of cells at the early stage of adhesion (7 min). Then, GD3 appeared at the central area of the cells and colocalized with integrin β1 (10 min). They co-localized with a punctate pattern at cell adhesion spots until around 30 min incubation. Furthermore, scanned images in Fig. 7B show integrins located at the cell attachment sites on the matrix first, and GD3 coming down to the focal adhesion sites following the integrins, leading to colocalization until about 30 min.
FIGURE 7.
Spatio-temporal relationships between integrins and GD3 during adhesion. A, cells (G5) were plated on pre-coated plates with CL type I after being treated and rotated under serum-free conditions as described in the legend to Fig. 3. After incubation for 0, 3, 7, 10, 18, and 30 min, cells were fixed with 4% paraformaldehyde and treated with 0.1% Triton X-100 in PBS. Then cells were stained for integrin β1 (red) and GD3 (green), and their images were observed using a confocal microscope. It was confirmed that there is no cross-reaction between the authentic antigens and non-relevant second reagents. DIC indicates images of differential interference contrast microscope. B, a, similar images as shown in A. The numbers in the images correspond to the numbers in A. b, images of the y-z axis in a (purple line). The left side in the image is the adhesion site. c, images of the x-z axis in a (yellow line). The bottom side in the image is the adhesion site. Scale bars indicate 10 μm.
DISCUSSION
GD3 is highly and specifically expressed in melanomas, and has therefore been used as a target for immune therapy (6, 7). There have been a number of reports indicating that GD3 is associated with malignant properties such as cell growth (9), invasion (19), and cell adhesion to the extracellular matrix (20). To investigate the roles of GD3, we have established a set of melanoma cell lines with or without GD3 expression using a GD3-deficient mutant, N1, which was generated by treatment of SK-MEL-28 with an anti-GD3 mAb and complement (9). Re-expression of GD3 with GD3 synthase cDNA resulted in the establishment of cell lines that enabled us to identify the phenotypic changes due to GD3 expression, and to investigate the molecular mechanisms of the enhanced malignant properties under GD3 expression (11). Effects of 9-O-acetyl-GD3 might be negligible due to lack of its expression in these cells.
Because stronger phosphorylation of FAK, p130Cas, and paxillin in GD3+ cells after treatment with FCS has been observed (12), we questioned whether these molecules are also involved in integrin-mediated signaling. In this study, we tried to elucidate whether GD3 is implicated in integrin-mediated cell adhesion using the transfectant cells of GD3 synthase cDNA.
Integrins are cell surface receptors consisting of two subunits, α and β. Immunoblotting and flow cytometry revealed that the expression levels of integrins examined were almost equivalent between GD3+ cells and negative controls (Fig. 1, B and C), whereas they were very low or undetectable in the GD3-positive parent SK-MEL-28 cells (9). This should mean that introduction of GD3 synthase cDNA into N1 cells did not necessarily suppress the expression levels of these integrins, and that N1 cells might be deficient in not only GD3, but also other factors regulating integrin expression. They interact with ECMs and mediate various intracellular signaling (21, 22). There are some reports indicating that integrin β1 is important in melanoma cell migration and migration-associated matrix reorganization (23), and that GD3 might be involved in increased proliferation and invasion via enhancement of integrin signaling. The roles of glycosphingolipids including GD3 in the regulation of cell adhesion have been recognized for a long time (24), but the mechanisms for this regulation remain unclear. Several studies on the modulation of integrins by gangliosides have been reported. For example, membrane cholesterol and sphingolipids have been shown to modify the function of adhesion molecules present on the membrane (25, 26). Gangliosides extracted from neuroblastoma cells or atherosclerotic plaques enhance platelet adhesion via integrin binding to collagen (27–29). Furthermore, highly sialylated gangliosides appear to regulate integrin α5β1-mediated adhesion of epithelial cells to fibronectin through carbohydrate-carbohydrate interactions (30).
In this study, using an RT-CES system, a high cell index value (resistance) was observed when GD3+ cells were plated on pre-coated plates with ECMs compared with control cells, particularly when GD3+ cells adhered to CL type I and CL type IV under FCS-free conditions (Fig. 2). The cell index value reflects the numbers of adhering cells and the morphological changes of cells after adhesion. In particular, definite differences in the early phase of adhesion to ECMs could be detected between GD3+ and GD3− cells under the FCS-free condition (Fig. 2B, b, c, and e). These results suggested that strong cell adhesion was induced by GD3 expression based on increased interaction between integrins and CL type I or CL type IV as previously described (31). In fact, it has been reported that integrins α1β1, α2β1, α3β1, and αvβ1 interact with CL type I and/or type IV (16, 32).
We detected stronger tyrosine phosphorylation of FAK and paxillin in GD3+ cells during adhesion to CL type I than in control cells (Figs. 3 and 4). Above all, FAK, a tyrosine-phosphorylated protein kinase, interacts with integrins and plays a critical role in intracellular processes of cell adhesion, motility, survival, and cell cycle progression as a signaling platform (33, 34). FAK has several autophosphorylation sites in the intracellular region. Among them, Tyr-576 and Tyr-577 are important phosphorylation sites for gaining kinase activity (35). High phosphorylation levels of Tyr-576 and Tyr-577 observed when GD3+ cells adhered to CL type I suggested that GD3 expression enhances FAK-mediated signaling via these phosphorylation sites, leading to the malignant features of melanomas. Phosphorylated Tyr-861 and Tyr-925 are recognized by p130Cas and paxillin, respectively, leading to their binding to FAK, and to their involvement as downstream signaling molecules of FAK (36). Moreover, it was reported that efficient EGF-stimulated cell migration requires FAK to be targeted to sites of integrin clustering by its carboxyl-terminal domain, indicating the importance of FAK in a receptor-proximal link between growth factor receptor and integrin signaling pathways (37).
p130Cas is now considered to be a significant adaptor molecule in a variety of biological processes, including cell adhesion (38), migration (39), growth factor stimulation (40), and cytokine receptor engagement (41). Paxillin is a multidomain protein that primarily localizes to cell adhesions forming a linkage structure between the ECM and the actin cytoskeleton, and is also an important site for signal transduction (42), particularly in melanomas (43). Strong phosphorylation of FAK and paxillin, but not of p130Cas during the adhesion of GD3+ cells to CL type I suggests that GD3 expression in melanoma cells enhances malignant properties, such as cell growth or invasion activity, based on increased integrin functions. The fact that tyrosine phosphorylation of p130Cas was scarcely observed even in GD3+ cells suggested that it largely depends on the presence of FCS.
During the adhesion to CL type IV, no distinct differences in phosphorylation levels of FAK, p130Cas, or paxillin were observed between GD3+ and control cells, although the cell index values to CL type I or CL type IV in the RT-CES system were very similar in both GD3+ cells and control cells. Moreover, the phosphorylation levels of FAK (Tyr-577 and Tyr-861) and paxillin (Tyr-31 and Tyr-118) were much lower during adhesion to CL type IV than to CL type I. This might be due to differences in the structures of CL type I and CL type IV. CL type I is fibrillar, whereas CL type IV is nonfibrillar, so that CL type I might be better recognized by integrins to facilitate the transmission of downstream signaling under GD3 expression (44).
Involvement of another signaling pathway downstream of integrin receptors in GD3-derived enhancing effects was examined, i.e. ILK/Akt pathway (16). ILK is a ubiquitously expressed protein kinase that can bind to the cytoplasmic domains of integrin β1 and β3 (45). The kinase activity is stimulated upon integrin engagement in a phosphatidylinositol 3-kinase-dependent manner. Among intracellular substrates of ILK, Akt, glycogen synthase kinase-3, and myosin light chain are most notable, playing roles in oncogenic transformation and cancer phenotypes (46). Actually, phosphorylation of Akt during adhesion could be observed more strongly in GD3+ cells (Fig. 6, A and B), and knockdown of ILK resulted in the reduction of Akt phosphorylation levels (Fig. 6D). ILK was detected in non-GEM/raft, and showed no changes in the phosphorylation levels during adhesion (supplemental Fig. S4). This fact suggests that ILK plays a role as an adaptor molecule (46), causing Akt activation.
Then, phosphorylation of ERKs during adhesion was analyzed, because integrin-mediated signalings are considered to lead to the activation of ERKs (16). Intriguingly, no clear increase in activation levels of ERKs was found, and their localization in non-GEM/raft did not change during adhesion (supplemental Fig. S5). However, in the presence of the remaining FCS, GD3+ cells showed definite activation of ERKs (supplemental Fig. S5). This fact together with our previous data (14) might indicate that efficient growth signals can be transduced only when both the growth factor receptor-mediated pathway and integrin-mediated pathway are simultaneously switched on.
The mechanism for how integrin-mediated signaling is enhanced with GD3 expression is a key issue in the functional analysis of GD3. Gangliosides are known to exist in clusters and to be constituents of microdomains consisting of cholesterol, glycosylphosphatidylinositol-anchored proteins, and sphingomyelin on the surface of the plasma membrane (47). These microdomains are referred to as lipid rafts or GEM/rafts, and have been considered to be involved in signal transduction, because a variety of signaling molecules, such as Src-family kinases and trimeric G proteins, are also physically associated with the microdomains (48). Moreover, a number of studies have been reported concerning the association of integrins and GEM/rafts (26). For example, the glycosylphosphatidylinositol-anchored protein CD24 as a marker for poor prognosis of carcinomas (ovary, breast, and pancreas), induced localization of integrin β1 to GEM/rafts with its transfection into A125 and MDA-MB-435S cell lines (49). The association of integrin α3β1 and α6β1 with the transmembrane glycoprotein CD36 leads to the sequestration of integrins into the GEM/rafts and promotes cell migration in human melanoma cells (50). Based on these findings, we thought that GD3 might recruit integrins to GEM/rafts, leading to the formation of an integrin cluster so that enhanced integrin-mediated signaling was transduced in GD3+ cells.
To address these issues, we fractionated extracts from GD3+ cells and control cells before or during adhesion to CL type I (Fig. 5). To our surprise, high amounts of integrins were already present in GEM/rafts in GD3+ cells (approximately three times or more than the control cells) at time 0 (before cell adhesion). In turn, integins in GEM/rafts were almost equivalent between GD3+ cells and control cells after incubation for 15 or 60 min, i.e. integrins were enriched in GEM/rafts at 15 min and released to non-GEM/rafts at 60 min in both types of cells. These data suggested that GD3 might recruit integrins to and/or hold integrins in the GEM/rafts. This finding corresponds with a previous report that integrin β1 was enriched in GEM/rafts in murine neuroepithelial cells in which GD3 was highly expressed (51). High amounts of integrins in GEM/rafts in GD3+ cells should induce stronger adhesion and integrin-mediated signaling than in control cells. Higher phosphorylation levels of FAK in GEM/rafts were detected in GD3+ cells at 15 min of incubation (Fig. 5C), suggesting that more efficient transduction of adhesion signals occurred in GD3+ cells than in the controls based on differences in integrin localization as shown in Fig. 5, A and B. In fact, in immunofluorescence staining, GD3 and integrins co-localized at the focal adhesion during cell adhesion with a punctate pattern (Fig. 7). The possibility that positive reaction with mAb R24 after detergent treatment was due to cross-reactivity of the antibody with protein-bound antigens seemed low because anti-glycolipid antibodies including mAb R24 seem to require the lipid part for reactivity. Although they co-localized uniformly on the cell membrane before cell adhesion, higher amounts of integrins might be in GEM/rafts under GD3 expression. Moreover, integrins formed clusters at the focal adhesion with a different time course of movement toward the focal adhesion from that of GD3 (Fig. 7). These results suggested that attachment of integrins to ECM at an early stage was exploited for mere adhesion, and that co-localization of integrins with GD3 might cause higher levels of cell adhesion and transduce integrin-mediated signaling based on cluster formation.
In conclusion, GD3 may assemble integrins in the GEM/rafts and cause cluster formation, so that the intensities of adhesion and integrin-mediated signaling might be enhanced immediately after attachment to ECMs. Furthermore, the fact that p130Cas and paxillin were not phosphorylated after stimulation with FCS in non-adherent GD3+ cells and control cells (14) might indicate that FCS stimulation via the growth factor receptor could be effective only when cells were receiving adhesion signals via the integrin-ECM interaction (14), and that these signals should be essential for cells to prepare to efficiently accept growth signals via receptors on the cell surface. As shown in this study, only adhesion signals are also unable to fully transduce growth signals such as ERK phosphorylation in the absence of FCS. In addition, there are differences in the cell adhesion activity and adhesion signaling among ECMs. CL type I should be the best one to induce malignant properties in melanoma cells. It might be advantageous for melanoma cells, because a large quantity of CL type I is in the dermis where they invade.
GD3 synthase-transfected melanoma cells expressed high levels of GD3, but also showed decreased levels of GM3 and low levels of GD2. The possibility that the decrease of GM3 and/or the increase of GD2 might enhance malignant properties cannot be completely ruled out. However, the facts that 1) inhibition of the synthesis of glucosylceramide resulted in the lowered level of GM3 and decreased cell growth (52), or 2) addition of GM3 to fibroblasts promoted growth factor-induced proliferation (53) suggest that decreased levels of GM3 should not enhance cell growth and activity. The comparison of double knock-out mice of GM2/GD2 synthase/GD3 synthase (54) and those of GM2/GD2 synthase/GM3 synthase (55) also indicate that the presence of GM3 apparently enhances cell survival and activity, suggesting that decreased levels of GM3 should not enhance cell growth and activity. As for the increase of GD2, the expression levels of GM2/GD2 synthase or GD2 are much less than that of GD3, suggesting that the increased level of GD3 in the transfected cells should be responsible for the enhanced malignant properties.
In this study, we demonstrated mechanisms for how melanomas acquire enhanced adhesion signals and resulting malignant properties under the expression of GD3. These observations could lead to novel strategies to regulate the metastatic nature of melanomas and develop novel therapeutics for melanoma patients.
Supplementary Material
Acknowledgments
We thank Dr. J. Nakano and Dr. K. O. Lloyd for providing N1 cells, and T. Mizuno, Y. Nakayasu, and T. Yamamori for technical assistance.
This work was supported by a Grant-in-aid from Japan Science and Technology Agency (JST), a Grant-in-aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT), and a grant from the COE Project for Private Universities from MEXT.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- FAK
- focal adhesion kinase
- GEM
- glycolipid-enriched microdomain
- ECM
- extracellular matrix
- ILK
- integrin-linked kinase
- RT-CES
- real time cell electronic sensing
- FN
- fibronectin
- CL
- collagen
- LN
- laminin
- PLL
- poly-L-lysine.
REFERENCES
- 1.Fredman P., Hedberg K., Brezicka T. (2003) BioDrugs 17, 155–167 [DOI] [PubMed] [Google Scholar]
- 2.Hakomori S., Igarashi Y. (1993) Adv. Lipid Res. 25, 147–162 [PubMed] [Google Scholar]
- 3.Furukawa K., Lloyd K. O. (1990) in Human Melanoma: From Basic Research to Clinical Application (Ferrone S. ed) pp. 15–30, Springer, Heidelberg [Google Scholar]
- 4.Portoukalian J., Zwingelstein G., Doré J. F. (1979) Eur. J. Biochem. 94, 19–23 [DOI] [PubMed] [Google Scholar]
- 5.Carubia J. M., Yu R. K., Macala L. J., Kirkwood J. M., Varga J. M. (1984) Biochem. Biophys. Res. Commun. 120, 500–504 [DOI] [PubMed] [Google Scholar]
- 6.Houghton A. N., Mintzer D., Cordon-Cardo C., Welt S., Fliegel B., Vadhan S., Carswell E., Melamed M. R., Oettgen H. F., Old L. J. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 1242–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forero A., Shah J., Carlisle R., Triozzi P. L., LoBuglio A. F., Wang W. Q., Fujimori M., Conry R. M. (2006) Cancer Biother. Radiopharm. 21, 561–568 [DOI] [PubMed] [Google Scholar]
- 8.Dippold W. G., Knuth A., Meyer zum Büschenfelde K. H. (1984) Cancer Res. 44, 806–810 [PubMed] [Google Scholar]
- 9.Nakano J., Raj B. K., Asagami C., Lloyd K. O. (1996) J. Invest. Dermatol. 107, 543–548 [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi M., Yamashiro S., Yamamoto A., Furukawa K., Takamiya K., Lloyd K. O., Shiku H., Furukawa K. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 10455–10459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamamura K., Furukawa K., Hayashi T., Hattori T., Nakano J., Nakashima H., Okuda T., Mizutani H., Hattori H., Ueda M., Urano T., Lloyd K. O., Furukawa K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 11041–11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamamura K., Tsuji M., Ohkawa Y., Nakashima H., Miyazaki S., Urano T., Yamamoto N., Ueda M., Furukawa K., Furukawa K. (2008) Biochim. Biophys. Acta 1780, 513–519 [DOI] [PubMed] [Google Scholar]
- 13.Ogita H., Takai Y. (2008) Int. Rev. Cytol. 265, 1–54 [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa Y., Miyazaki S., Miyata M., Hamamura K., Furukawa K., Furukawa K. (2008) Biochem. Biophys. Res. Commun. 373, 14–19 [DOI] [PubMed] [Google Scholar]
- 15.Wong R. P., Ng P., Dedhar S., Li G. (2007) Mol. Cancer Ther. 6, 1692–1700 [DOI] [PubMed] [Google Scholar]
- 16.Kuphal S., Bauer R., Bosserhoff A. K. (2005) Cancer Metastasis Rev. 24, 195–222 [DOI] [PubMed] [Google Scholar]
- 17.Yoshida S., Fukumoto S., Kawaguchi H., Sato S., Ueda R., Furukawa K. (2001) Cancer Res. 61, 4244–4252 [PubMed] [Google Scholar]
- 18.Toledo M. S., Suzuki E., Handa K., Hakomori S. (2005) J. Biol. Chem. 280, 16227–16234 [DOI] [PubMed] [Google Scholar]
- 19.Iliopoulos D., Ernst C., Steplewski Z., Jambrosic J. A., Rodeck U., Herlyn M., Clark W. H., Jr., Koprowski H., Herlyn D. (1989) J. Natl. Cancer Inst. 81, 440–444 [DOI] [PubMed] [Google Scholar]
- 20.Cheresh D. A., Pierschbacher M. D., Herzig M. A., Mujoo K. (1986) J. Cell Biol. 102, 688–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calderwood D. A., Shattil S. J., Ginsberg M. H. (2000) J. Biol. Chem. 275, 22607–22610 [DOI] [PubMed] [Google Scholar]
- 22.Giancotti F. G., Ruoslahti E. (1999) Science 285, 1028–1032 [DOI] [PubMed] [Google Scholar]
- 23.Friedl P., Zänker K. S., Bröcker E. B. (1998) Microsc. Res. Tech. 43, 369–378 [DOI] [PubMed] [Google Scholar]
- 24.Griffiths S. L., Perkins R. M., Streuli C. H., Critchley D. R. (1986) J. Cell Biol. 102, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pande G. (2000) Curr. Opin. Cell Biol. 12, 569–574 [DOI] [PubMed] [Google Scholar]
- 26.Chen H. H., Fukumoto S., Furukawa K., Nakao A., Akiyama S., Urano T., Furukawa K. (2003) Int. J. Cancer. 103, 169–176 [DOI] [PubMed] [Google Scholar]
- 27.Fang L. H., Lucero M., Kazarian T., Wei Q., Luo F. Y., Valentino L. A. (1997) Clin. Exp. Metastasis 15, 33–40 [DOI] [PubMed] [Google Scholar]
- 28.Wen F. Q., Jabbar A. A., Patel D. A., Kazarian T., Valentino L. A. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 519–524 [DOI] [PubMed] [Google Scholar]
- 29.Valentino L. A., Ladisch S. (1996) Biochim. Biophys. Acta 1316, 19–28 [DOI] [PubMed] [Google Scholar]
- 30.Wang X., Sun P., Al-Qamari A., Tai T., Kawashima I., Paller A. S. (2001) J. Biol. Chem. 276, 8436–8444 [DOI] [PubMed] [Google Scholar]
- 31.Nakano J., Yasui H., Lloyd K. O., Muto M. (1999) J. Investig. Dermatol. Symp. Proc. 4, 173–176 [DOI] [PubMed] [Google Scholar]
- 32.Charalabopoulos K., Mittari E., Karakosta A., Golias C., Batistatou A. (2005) Exp. Oncol. 27, 86–90 [PubMed] [Google Scholar]
- 33.Tilghman R. W., Parsons J. T. (2008) Semin. Cancer Biol. 18, 45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra S. K., Schlaepfer D. D. (2006) Curr. Opin. Cell Biol. 18, 516–523 [DOI] [PubMed] [Google Scholar]
- 35.Hanks S. K., Polte T. R. (1997) BioEssays 19, 137–145 [DOI] [PubMed] [Google Scholar]
- 36.Schaller M. D., Parsons J. T. (1994) Curr. Opin. Cell Biol. 6, 705–710 [DOI] [PubMed] [Google Scholar]
- 37.Sieg D. J., Hauck C. R., Ilic D., Klingbeil C. K., Schaefer E., Damsky C. H., Schlaepfer D. D. (2000) Nat. Cell Biol. 2, 249–256 [DOI] [PubMed] [Google Scholar]
- 38.Saito M., Narayana A. S. (1999) J. Bone Miner Res. 14, 65–72 [DOI] [PubMed] [Google Scholar]
- 39.Nojima Y., Morino N., Mimura T., Hamasaki K., Furuya H., Sakai R., Sato T., Tachibana K., Morimoto C., Yazaki Y. (1995) J. Biol. Chem. 270, 15398–15402 [DOI] [PubMed] [Google Scholar]
- 40.Cary L. A., Han D. C., Polte T. R., Hanks S. K., Guan J. L. (1998) J. Cell Biol. 140, 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribon V., Saltiel A. R. (1996) J. Biol. Chem. 271, 7375–7380 [DOI] [PubMed] [Google Scholar]
- 42.Turner C. E. (2000) J. Cell Sci. 113, 4139–4140 [DOI] [PubMed] [Google Scholar]
- 43.Schraw W., Richmond A. (1995) Biochemistry 34, 13760–13767 [DOI] [PubMed] [Google Scholar]
- 44.Prockop D. J., Kivirikko K. I. (1995) Annu. Rev. Biochem. 64, 403–434 [DOI] [PubMed] [Google Scholar]
- 45.Persad S., Dedhar S. (2003) Cancer Metastasis Rev. 22, 375–384 [DOI] [PubMed] [Google Scholar]
- 46.Zervas C. G., Gregory S. L., Brown N. H. (2001) J. Cell Biol. 152, 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simons K., Toomre D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 48.Hur E. M., Park Y. S., Lee B. D., Jang I. H., Kim H. S., Kim T. D., Suh P. G., Ryu S. H., Kim K. T. (2004) J. Biol. Chem. 279, 5852–5860 [DOI] [PubMed] [Google Scholar]
- 49.Runz S., Mierk C. T., Joumaa S., Behrens J., Fabry B., Altevogt P. (2008) Biochem. Biophys. Res. Commun. 365, 35–41 [DOI] [PubMed] [Google Scholar]
- 50.Thorne R. F., Marshall J. F., Shafren D. R., Gibson P. G., Hart I. R., Burns G. F. (2000) J. Biol. Chem. 275, 35264–35275 [DOI] [PubMed] [Google Scholar]
- 51.Yanagisawa M., Nakamura K., Taga T. (2004) Genes Cells 9, 801–809 [DOI] [PubMed] [Google Scholar]
- 52.Weiss M., Hettmer S., Smith P., Ladisch S. (2003) Cancer Res. 63, 3654–3658 [PubMed] [Google Scholar]
- 53.Li R., Manela J., Kong Y., Ladisch S. (2000) J. Biol. Chem. 275, 34213–34223 [DOI] [PubMed] [Google Scholar]
- 54.Inoue M., Fujii Y., Furukawa K., Okada M., Okumura K., Hayakawa T., Furukawa K., Sugiura Y. (2002) J. Biol. Chem. 277, 29881–29888 [DOI] [PubMed] [Google Scholar]
- 55.Yamashita T., Yamashita T., Wu Y. P., Sandhoff R., Werth N., Mizukami H., Ellis J. M., Dupree J. L., Geyer R., Sandhoff K., Proia R. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2725–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.