Abstract
Acetylcholinesterase (AChE) is anchored onto cell membranes by the transmembrane protein PRiMA (proline-rich membrane anchor) as a tetrameric globular form that is prominently expressed in vertebrate brain. In parallel, the PRiMA-linked tetrameric butyrylcholinesterase (BChE) is also found in the brain. A single type of AChE-BChE hybrid tetramer was formed in cell cultures by co-transfection of cDNAs encoding AChET and BChET with proline-rich attachment domain-containing proteins, PRiMA I, PRiMA II, or a fragment of ColQ having a C-terminal GPI addition signal (QN-GPI). Using AChE and BChE mutants, we showed that AChE-BChE hybrids linked with PRiMA or QN-GPI always consist of AChET and BChET homodimers. The dimer formation of AChET and BChET depends on the catalytic domains, and the assembly of tetramers with a proline-rich attachment domain-containing protein requires the presence of C-terminal “t-peptides” in cholinesterase subunits. Our results indicate that PRiMA- or ColQ-linked cholinesterase tetramers are assembled from AChET or BChET homodimers. Moreover, the PRiMA-linked AChE-BChE hybrids occur naturally in chicken brain, and their expression increases during development, suggesting that they might play a role in cholinergic neurotransmission.
Keywords: Acetylcholinesterase, Brain, Multifunctional Enzymes, Neurodevelopment, Protein Assembly, Butyrylcholinesterase, Cholinergic Synapse, Hybrid Tetramer, PRiMA, Protein Assembly
Introduction
In vertebrates, cholinesterases are widespread enzymes present in cholinergic and noncholinergic tissues, as well as in plasma and body fluids (1, 2). Cholinesterases are divided into two classes, acetylcholinesterase (AChE)2 and butyrylcholinesterase (BChE), according to their substrate specificity and susceptibility to inhibitors. The main function of AChE is to rapidly hydrolyze the neurotransmitter acetylcholine at cholinergic synapses, whereas the function of BChE in vertebrates is far less clear, except that it prevents a prolonged neuromuscular blockage after succinylcholine treatment in humans (3). In addition, the survival of AChE knock-out mice suggested that BChE can partially compensate for the absence of AChE in the nervous system (4).
At the molecular level, AChE and BChE are found in a number of forms, depending on alternative splicing and on the association with their interacting partners, ColQ and PRiMA (5). By alternative splicing in the 3′ region of the primary transcripts, AChE exists as different splice variants that possess the same catalytic domain but differ by small C-terminal peptides (6). To date, there are three known variants in mammals: the AChER variant, which produces a soluble monomer that may be up-regulated in the brain during stress (7, 8); the AChEH variant, which produces a glycosylphosphatidylinositol (GPI)-anchored dimer that is mainly expressed in blood cells in mammals (9); and the AChET variant, which is the only type of catalytic subunit expressed in the brain and muscles (10, 11). On the other hand, BChE comes as a single variant: BChET, which possesses a C-terminal t-peptide similar to that of AChET (12, 13).
The t-peptide of AChET, or BChET, also called the tryptophan amphiphilic tetramerization domain, contains seven strictly conserved aromatic residues, including three evenly spaced tryptophans and is organized as an α-helix (14). The major function of the t-peptide is to allow the association of cholinesterase subunits (AChET and BChET) with their structural anchoring proteins: ColQ and PRiMA. Complexes with ColQ represent the collagen-tailed or asymmetric (A) forms in muscle (15, 16), whereas complexes with PRiMA represent membrane-bound tetrameric globular forms (G4), mainly in brain (17–19) and muscle (20). These heteromeric molecules are assembled through the tight coiled-coil association of four tryptophan amphiphilic tetramerization domains (t-peptides) with a proline-rich attachment domain (PRAD) of ColQ or PRiMA (21–23).
The C-terminal peptides of AChET and BChET are very similar; this may explain the existence of asymmetric AChE-BChE hybrid molecules (12, 15); in 1-day-old chicken pectoral muscle, the principal collagen-tailed form of AChE, sedimentating at 20 S, is a mixed oligomer containing both AChET and BChET subunits (12, 15) that is associated with ColQ. The BChET subunits of this 20 S hybrid cholinesterase progressively disappear during muscle development from embryo to adult, ultimately resulting in an homogeneous asymmetric AChE form (24).
Here, we investigated whether PRiMA could organize AChET and BChET subunits into a PRiMA-linked G4 hybrid molecule. In a recombinant system, the co-expression of AChET and BChET subunits never produced hybrid (mixed) dimers. However, when both cholinesterase subunits were co-expressed with PRiMA, we obtained one type of AChE-BChE hybrid tetramer, as well as PRiMA-linked AChE and BChE homotetramers. Several lines of evidence suggest that the hybrids are always composed of two AChET subunits and two BChET subunits, associated with one PRiMA subunit. Because only AChE or BChE homodimers can be formed, they most probably represent an intermediate in the assembly of (AChET)2-(BChET)2-PRiMA hybrids. As expected, the t-peptides of AChET and BChET play a critical role in their tetrameric association with the PRAD-containing protein PRiMA. In addition, PRiMA-linked AChE-BChE hybrid molecules were found in chicken brain. In contrast with the collagen-tailed AChE-BChE hybrids in muscle, the expression level of PRiMA-linked hybrid molecules increased during development in the brain, suggesting a physiological role in the nervous system.
EXPERIMENTAL PROCEDURES
Cell Cultures
A human embryonic kidney fibroblast cell line (HEK293T) was obtained from the American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C in a water-saturated 5% CO2 incubator. All of the reagents for cell cultures were purchased from Invitrogen.
DNA Constructs and Transfection
The cDNAs encoding for human AChET, BChET, AChEBChE-T (AChE catalytic subunit with a BChE t-peptide), BChEAChE-T (BChE catalytic subunit with an AChE t-peptide), AChEΔT, and BChEΔT (catalytic subunits lacking t-peptides) were subcloned in pGS vectors, under a CMV promoter, as described previously (12). The cDNAs encoding full-length rat AChE and the full-length mouse PRiMA I, tagged with an HA epitope (YPYDVPDYA) at the C terminus, were subcloned into pEF-BOS vectors, driven by the EF-1α promoter (18, 25). The cDNA encoding full-length chicken AChET was subcloned in a pcDNA3 vector under a CMV promoter (26). We also used cDNAs encoding mouse PRiMA II (19) and the N-terminal fragment of ColQ, which was linked to a GPI addition signal (QN-GPI) in pEF-BOS vectors (14). HEK293T cells were transfected with cDNA constructs by calcium phosphate precipitation, 1 day after the cells were plated (27). The cell lysates were collected 2 days after the transfection. The media were discarded, because they contained very little or no cholinesterase activity in this cellular system, in contrast with COS cells (2, 28).
Protein Extraction from Cells and Tissues
Two days after the transfection, the cell cultures were collected in low salt lysis buffer (10 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm EGTA, 150 mm NaCl, and 0.5% Triton X-100) with the addition of the following protease inhibitors: 10 μg/ml leupeptin, 10 μg/ml aprotinin, 20 μm pepstatin, and 2.5 mm benzamidine HCl. The homogenization was done by vortexing for 10 min, and the homogenates were clarified by centrifugation for 30 min at 16,000 × g at 4 °C. Frozen tissues from chicken were homogenized in 10 volumes of ice-cold low salt lysis buffer, and the homogenates were clarified by centrifugation for 30 min at 16,000 × g at 4 °C.
Sucrose Density Gradients
Separation of the various molecular forms of AChE and BChE was performed by sucrose density gradient analysis, as described previously (27). In brief, continuous sucrose gradients (5–20%), created in a detergent-containing buffer (10 mm HEPES, pH 7.5, 1 mm EDTA, 1 mm EGTA, 0.2% Brij-97, and 1 m or 150 mm NaCl), were prepared in 12-ml polyallomer ultracentrifugation tubes with a 0.4-ml cushion of 60% sucrose at the bottom. The samples of cell extracts (0.2 ml) containing equal amounts of protein were mixed with the sedimentation markers, alkaline phosphatase (6.1 S) and β-galactosidase (16 S), and loaded onto the gradients to be centrifuged at 38,000 rpm in a Sorvall TH 641 rotor at 4 °C for 16 h. Approximately 45 fractions were collected. AChE and BChE enzymatic activities were determined according to the method of Ellman et al. (29) with minor modifications. For AChE assay, the cell lysates were incubated with 0.1 mm tetraisopropylpyrophosphoramide for 10 min to inhibit chicken BChE activity or 40 μm ethopropazine for 10 min to inhibit mammalian BChE. Samples of ∼5–20 μl were then added to the reaction mixture with final concentrations of 0.625 mm acetylthiocholine iodide (Sigma) and 0.5 mm 5,5′-dithiobis-2-nitrobenzoic acid (Sigma) in 80 mm Na2HPO4 (pH 7.4). The increase in absorbance at 410 nm was recorded, and the specific enzyme activity was expressed as absorbance units/min/μg of protein. BChE activity was assayed in a similar manner, except that the lysates were preincubated with 20 μm BW284c51 (an inhibitor of AChE; Sigma) for 10 min, and the substrate was 0.625 mm butyrylthiocholine iodide (BTCh; Sigma). The assays for both enzymes were highly specific (supplemental Fig. S1A). The amounts of the various AChE or BChE forms were determined by summation of the enzymatic activities corresponding to the peaks of their respective sedimentation profiles. The sedimentation values of the enzymes were calculated from the positions of the markers, alkaline phosphatase and β-galactosidase, as described previously (12).
Immunoprecipitation of AChE and BChE
Five hundred μl of G2 or G4 fractions, obtained from the sucrose density gradients, were incubated with anti-mammalian AChE antibody E-19 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA), anti-mammalian BChE antibody N-15 (1:200; Santa Cruz Biotechnology), or anti-chicken AChE monoclonal antibody (3D10, 1:200, purified at 2 μg/ml) (15) at 4 °C overnight. These anti-AChE and anti-BChE antibodies are highly specific and did not cross-react with BChE and AChE, respectively. Each sample was added with 30 μl of protein G-agarose (Roche Applied Science) and incubated for 4 h at 4 °C. The protein G beads were centrifuged in a microcentrifuge tube and washed three times with ice-cold extraction buffer. The supernatant was discarded by careful aspiration, and the protein G beads were then subjected to enzymatic assays for either AChE or BChE.
Nondenaturing Gel Electrophoresis, Denaturing Nonreducing SDS-PAGE, and Denaturing Reducing SDS-PAGE
Fractions containing dimers (G2) and tetramers (G4) obtained from the sucrose density gradients were subjected to horizontal nondenaturing gel electrophoresis (30). The gel contained 7.5% polyacrylamide, 0.5 m glycine-Tris-HCl (pH 8.9), 0.25% Triton X-100, and 0.05% sodium deoxycholate and was run at 400 V for 5 h. The enzymes were visualized by the histochemical method of Karnovsky and Roots (31). The staining solution contained 150 mm sodium acetate (pH 6.5), 5 mm sodium citrate, 3 mm CuSO4, and 0.5 mm K3Fe(CN)6. The activity of AChE was visualized by adding 40 μm ethopropazine (BChE inhibitor) and 1 mg/ml acetylthiocholine iodide; BChE activity was visualized by adding 20 μm BW284c51 (AChE inhibitor) and 1 mg/ml BTCh iodide.
For electrophoresis in denaturing but nonreducing conditions, the G2 or G4 fractions obtained from the sucrose density gradients were denatured at 100 °C for 5 min in a buffer containing 2% SDS and separated by electrophoresis in 8% SDS-PAGE. For standard SDS-PAGE, the protein lysate was denatured in the presence of 2% SDS and 100 mm β-mercaptoethanol. Anti-mammalian AChE antibody E-19 (1:2,000), anti-mammalian BChE antibody N-15 (1:2,000), anti-chicken AChE antibody (1:5,000), and anti-HA antibody (1:5,000) were used for Western blot analyses. The immune complexes were visualized using the ECL method (Amersham Biosciences).
To verify the presence of HA-labeled PRiMA in cholinesterase tetramers, 0.2 ml of G4 fractions from AChET, BChET, and PRiMA I-HA triple transfected cell lysate were incubated for 4 h at room temperature at different doses of anti-HA antibody (1:100, 1:500, 1:2,500, and 1:12,500 dilutions; Sigma). Then 30 μl of washed protein-G agarose was added and incubated for 4 h at 4 °C. After centrifugation, the supernatants were loaded onto nondenaturing gel electrophoresis, and different G4 enzymes were visualized by enzymatic activity staining as described above. Another method to prove that these tetramers are PRiMA-linked was by Western blotting using anti-HA antibody. The three bands corresponding to G4 AChET, G4 BChET, and G4 hybrid molecules in the G4 fractions of AChET, BChET, and PRiMA I-HA triple transfected cell lysate were isolated from nondenaturing gel. The gels were chopped into small pieces and boiled at 100 °C for 10 min in a buffer containing 2% SDS and 100 mm β-mercaptoethanol. The supernatant containing the protein extracts was used for denaturing reducing SDS-PAGE and was plotted with anti-HA antibody (1:5000).
Deglycosylation
Ninety μl of G2 and G4 fractions of AChE or BChE obtained from the sucrose density gradients were mixed with 10 μl of 10× incubation buffer (200 mm sodium phosphate, pH 6, 100 mm EDTA, 1% SDS, and 5% Triton). 1 mm PMSF was added to inhibit protease activity. The reaction was incubated with 0.5 μl of peptide N-glycosidase F (Roche Applied Science) for 16 h at 37 °C. After the addition of nonreducing SDS gel loading buffer, the samples were boiled for 5 min and applied to nonreducing electrophoresis.
ELISA
The anti-chicken AChE antibody was diluted to 10 μg/ml in coating buffer (15 mm Na2CO3, 35 mm NaHCO3, pH 9.5), and 50-μl aliquots were dispensed into each well of 96-well ELISA plate (Nunc Maxisorp Immunoplate, Roskilde, Denmark). The plates were preincubated overnight at 4 °C with 3% BSA in PBS for 2 h at room temperature to reduce nonspecific binding. The wells were washed three times with PBS containing 0.1% Tween 20. Samples of tissue lysates with equal amounts of AChE activity from chicken cerebrum at different developmental stages were loaded on anti-AChE antibody-precoated ELISA plates. After 4 h, the plates were washed three times. The amount of AChE and BChE activities immobilized on the well surface was assayed directly in the wells with the Ellman reagents, described above.
Other Assays
Protein concentrations were measured by the Bradford method (32) with a kit from Bio-Rad.
RESULTS
Formation of PRiMA-linked AChE-BChE G4 Hybrids in Transfected Cells
To investigate whether AChET and BChET could form a hybrid oligomer in vitro, cultured HEK293T fibroblast cells, possessing very low levels of endogenous AChE and BChE activities, were employed as an expression system. The cDNAs encoding human AChET and human BChET were single or double expressed with or without mouse PRiMA in cultured HEK293T cells. In the absence of PRiMA, expression of AChET or BChET produced mainly G2 dimers, together with a minor proportion of G1 monomers, forming an overlapping peak in sedimentation profiles, but no G4 tetramers (Fig. 1A). The profiles obtained for each enzyme when they were co-expressed in the cultures were identical to those obtained when they were expressed separately (Fig. 1A), indicating that they did not form AChET-BChET hybrid dimers. The absence of AChET-BChET dimer was confirmed by immunoprecipitation experiments of G2-enriched fractions collected from sucrose density gradients using anti-AChE (E-19) and anti-BChE (N-15) specific antibodies. Both of these antibodies are highly specific for each enzyme (supplemental Fig. S1, B and C). No BChE activity was co-precipitated by the anti-AChE antibody, or vice versa, in G2 fractions from double transfected cells (Fig. 1B). The G2 fractions from AChET and BChET singly transfected cells served as controls.
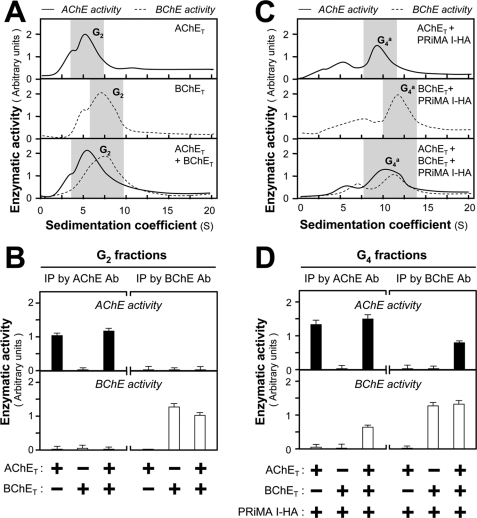
FIGURE 1.
Formation of PRiMA-linked AChE-BChE G4 hybrid molecules in transfected HEK293T cells. A, HEK293T cells were transfected with equal amounts of cDNAs encoding AChET and BChET for 2 days. Equal amounts of protein from cell lysates were subjected to sucrose density gradient analysis. The enzymatic activities were plotted as a function of the S value, estimated from the position of the sedimentation markers. B, G2 fractions shaded in A were collected for immunoprecipitation by either anti-AChE antibody or anti-BChE antibody. The enzymatic activities of the immobilized AChE and BChE on the beads were determined. The lack of co-immunoprecipitation indicates that only homodimers were produced. C, HEK293T cells were transfected with equal amounts of cDNAs encoding AChET and BChET together with cDNA encoding PRiMA I-HA for 2 days. The amount of PRiMA I-HA cDNA was doubled in the triple transfection to ensure that the ratio of PRiMA to AChE-BChE was the same as in the double transfection. Equal amounts of protein from cell lysates were subjected to sucrose density gradient analysis to analyze the molecular forms, as in A. D, G4 fractions shaded in C were collected for immunoprecipitation as in B. In contrast with B, the co-immunoprecipitation of AChE and BChE indicates the existence of G4 hybrid (mixed) tetramers. The enzymatic activities are expressed in arbitrary units. The values are the means ± S.E., each with triplicate samples (n = 3). Representative gradient profiles and gels are shown (n = 4). Ab, antibody; IP, immunoprecipitation.
In parallel experiments, cultured HEK293T cells were co-transfected with cDNA encoding PRiMA I-HA. In the presence of PRiMA I-HA, G4 AChE and G4 BChE (sedimenting at 9.6 and 11 S, respectively) were produced (Fig. 1C). In triple transfected cells expressing both AChET and BChET with PRiMA I-HA, the G4 peaks corresponding to AChE and BChE activities were markedly broader than those observed for AChET or BChET alone and appeared to include a component of intermediate sedimentation coefficient, suggesting the presence of mixed PRiMA-linked tetramers. Immunoprecipitation of the G4 fractions of triple transfected cells showed that AChE activity could be precipitated by the anti-BChE antibody, and BChE activity could be precipitated by the anti-AChE antibody (Fig. 1D). The G4 fractions from cells expressing only AChET or BChET with PRiMA I-HA served as negative controls. These results suggest that AChET and BChET produce only homodimers in the G2 fractions but can associate with PRiMA I into PRiMA I-linked AChET-BChET G4 hybrid molecules.
To understand the molecular assembly of the G4 hybrid molecules, the subunit composition was studied by nondenaturing gel electrophoresis. The G2-enriched fractions, collected from cells expressing AChET, BChET, or both enzymes in the absence of PRiMA, contained only monomers and homodimers. AChET and BChET oligomers were clearly separated; in particular, BChET dimers migrated much slower than AChET dimers (Fig. 2A). In the double transfected cells, we observed only homodimers of both AChET and BChET subunits, but no hybrid dimers that would present an intermediate electrophoretic migration.
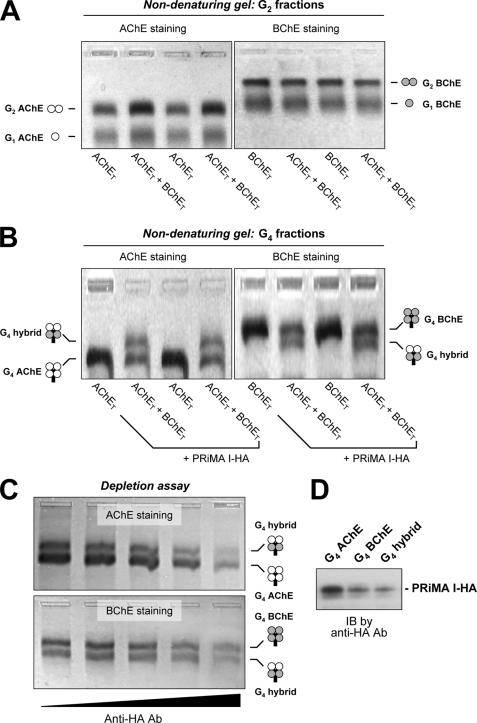
FIGURE 2.
Analysis of AChE and BChE oligomers by nondenaturing gel electrophoresis: existence of a single species of PRiMA I-linked AChE-BChE G4 hybrid tetramer. A, G2-enriched fractions from cells expressing AChET, BChET, or AChET + BChET. B, G4-enriched fractions from cells expressing AChET, BChET, or AChET + BChET, together with PRiMA I-HA. The enzymatic activities of AChE and BChE were revealed by Karnovsky staining using acetylthiocholine iodide with ethopropazine (BChE inhibitor) and BTCh with BW284c51 (AChE inhibitor), respectively. BChE oligomers migrate markedly more slowly than the corresponding AChE oligomers; a single intermediate species, possessing both AChE and BChE activities, was observed in G4 fractions from cells expressing both AChET and BChET with PRiMA. C, G4-enriched fractions from cells expressing AChET + BChET + PRiMA I-HA were depleted by anti-HA antibody (from left to right, negative control, 1:12,500, 1:2,500, 1:500, and 1:100 dilutions). Different G4 enzymes in the supernatant were visualized by Karnovsky staining after nondenaturing gel electrophoresis. The decrease of G4 AChE, G4 BChE, and G4 hybrid after the depletion with anti-HA antibody indicates that they are PRiMA-linked. D, gel extracts from G4 AChE, G4 BChE, and G4 hybrid bands in the AChET + BChET + PRiMA I-HA overexpressed cells were analyzed by Western blotting and probed with an anti-HA antibody. Representative gels are shown (n = 3). Ab, antibody; IB, immunoblot.
We analyzed the G4 fractions obtained from cells expressing AChET and/or BChET together with PRiMA I-HA in the same manner. Extracts from double transfected cells showed that PRiMA-linked BChET tetramer migrated more slowly than PRiMA-linked AChET tetramers (Fig. 2B). In the case of triple transfected cells expressing both AChET and BChET with PRiMA I, we observed an additional molecular species, with an intermediate migration between G4 AChE and G4 BChE, which possessed both AChE and BChE activities (Fig. 2B). In addition, the G4 AChE, G4 BChE, and G4 hybrid molecules, detected on the native gel, were depleted by the anti-HA antibody in a dose-dependent manner, suggesting that they were PRiMA I-linked (Fig. 2C). The presence of PRiMA I-HA in G4 AChE, G4 BChE, and G4 hybrid enzymes produced by triple transfected cells expressing AChET, BChET, and PRiMA I-HA was further confirmed by Western blotting with the anti-HA antibody (Fig. 2D).
The single type of hybrid tetramer that we observed might represent one of the following combinations: (AChET)1-(BChET)3-PRiMA, (AChET)2-(BChET)2-PRiMA, and (AChET)3-(BChET)1-PRiMA. Because AChET and BChET do not form hybrid dimers, the molecular composition of the hybrid tetramer most likely corresponds to one AChET dimer, one BChET dimer, and one PRiMA, i.e. (AChET)2-(BChET)2-PRiMA, excluding the other two combinations.
In mammals, PRiMA has two splicing variants, PRiMA I and PRiMA II; PRiMA II differs from PRiMA I by its conspicuously shorter C-terminal cytoplasmic domain. The two splice variants appear equivalent in their capacity to anchor tetramers of AChET at the cell surface (19). In our study, we expressed AChET, BChET, and PRiMA II in cultured HEK293T cells. We found that the co-expression of AChET or BChET with PRiMA II produced amphiphilic tetramers of AChE and BChE, in the same way as with PRiMA I (Fig. 3A). Immunoprecipitation with anti-AChE or anti-BChE antibodies indicated the formation of AChE and BChE G4 hybrid tetramers when they were co-expressed with PRiMA II (Fig. 3B). In nondenaturing gel electrophoresis, a single molecular species possessing both enzymatic activities migrated between PRiMA II-linked AChET and BChET tetramers (Fig. 3C). Thus, PRiMA II, like PRiMA I, is able to direct the assembly of a hybrid tetramer, i.e. (AChET)2-(BChET)2-PRiMA II, indicating that the intracellular cytoplasmic tail of PRiMA I is not required for this oligomerization process.
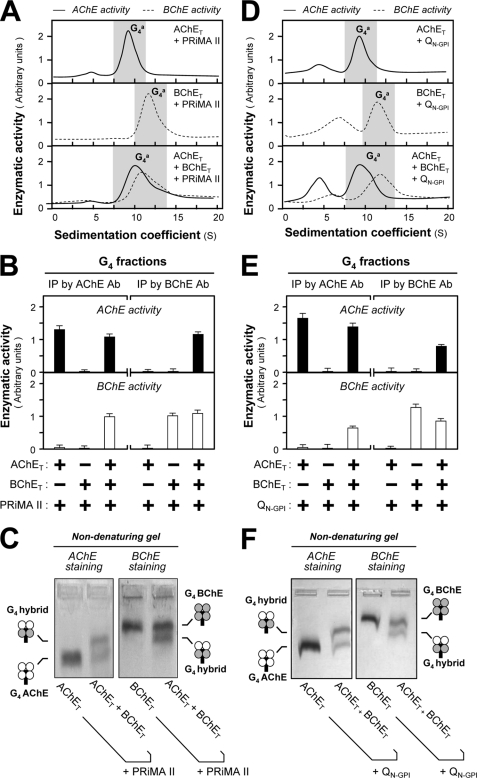
FIGURE 3.
Formation of AChE-BChE G4 hybrid tetramer in the presence of PRiMA II and QN-GPI. HEK293T cells were transfected with cDNAs encoding AChET and/or BChET with PRiMA (A) or QN-GPI (D) (14) for 2 days. Sucrose density gradient analysis was performed as in Fig. 1A. B and E, G4-enriched fractions shaded in A and D were collected for immunoprecipitation by either anti-AChE or anti-BChE antibody as in Fig. 1B. The co-immunoprecipitation of AChE and BChE indicates the existence of G4 hybrid tetramers. The enzymatic activities are expressed in arbitrary units. The values are the means ± S.E. (n = 3), each with triplicate samples. C and F, G4-enriched fractions shaded in A and D were analyzed by nondenaturing electrophoresis. The enzymatic activities of AChE and BChE were visualized by Karnovsky staining. A new oligomer migrating between G4 AChE and G4 BChE was found in the triple transfection, suggesting that PRiMA II and QN-GPI, like PRiMA I, induce the formation of the AChE-BChE hybrid tetramer. Ab, antibody; IP, immunoprecipitation.
The first AChE-BChE hybrid molecule was discovered in muscles of a 1-day-old chicken as a ColQ-associated asymmetric form (15, 24). We did not use the full-length ColQ subunit in the following study, because it forms a highly complex oligomer containing 12 catalytic subunits and three collagenous subunits and because its aggregation properties do not allow analysis by nondenaturing gel electrophoresis. An N-terminal fragment of ColQ (QN) has been shown to organize cholinesterase tetramers (21, 22, 33). Here, we used this fragment, linked with a C-terminal GPI addition signal (QN-GPI). As expected, the expression of QN-GPI with AChET or BChET in HEK293T cells produced QN-linked, cell surface GPI-anchored tetramers (Fig. 3D). Immunoprecipitation and nondenaturing electrophoresis showed that the triple expression of both AChET and BChET with QN-GPI produced a hybrid tetramer, composed of two AChE subunits and two BChE subunits, i.e. (AChET)2-(BChET)2-QN-GPI (Fig. 3, E and F). Therefore, these findings suggest that ColQ-linked A12 hybrid molecules contain three ColQ-linked AChE-BChE tetramers, with equal numbers of AChET and BChET subunits (15).
The Assembly of AChE or BChE Homodimers
Both AChET and BChET possess a cysteine residue near the C-terminal extremity of their t-peptide; this residue has been found to participate in the formation of disulfide-linked dimers in asymmetric AChE (28, 34) and in the human BChE G4 form (13). In addition, mouse PRiMA I contains four cysteines upstream of the PRAD (Cys6, Cys13, Cys17, and Cys19 in the mature protein) (25), which may form disulfide linkages with associated catalytic subunits. Using nonreducing electrophoresis and Western blotting, we analyzed the intercatenary disulfide bonds in G2 dimers and PRiMA-linked G4 complexes, formed by co-expressing human AChET or human BChET with mouse PRiMA I-HA, in cultured HEK293T cells. In Western blotting, both AChET and BChET were detected by their specific antibodies, whereas PRiMA I-HA was detected by an anti-HA antibody. The G2 fraction obtained from cells expressing AChET and PRiMA I-HA contained AChE dimers, recognized by the anti-AChE antibody but not by the HA antibody (Fig. 4A), indicating that they represent homodimers that possess an intercatenary disulfide bond between the two AChET subunits and are not associated with PRiMA. The G4 fractions produced several bands in nonreducing Western blots. The faster migrating band near 140 kDa was labeled by the anti-AChE antibody but not by the HA antibody, suggesting that it consists of two disulfide-linked AChET subunits (“light dimer”); it appeared to migrate more slowly than the dimer band obtained in the G2 fractions, perhaps because recruitment by PRiMA into tetramers is accompanied by a modification in glycosylation. A slower migrating band (∼160 kDa) was labeled by both anti-AChE and anti-HA antibodies, suggesting that it corresponds to two AChET subunits linked with a PRiMA subunit (“heavy dimer”) (Fig. 4A). In addition, we observed a heavier band that was also labeled by both antibodies, suggesting that it consisted of four AChET subunits disulfide-linked with PRiMA (Fig. 4A). We obtained similar results for PRiMA-linked G4 BChET (Fig. 4C). The presence of a clear band ∼180 kDa, in addition to the light dimer at ∼165 kDa that appears in the G2 fraction, might be also due to modifications in glycosylation correlated with the assembly of tetramers, and this band would thus represent the light dimer derived from PRiMA-linked G4 molecules; the difference between its mass and that of the heavy dimer band at ∼200 kDa is compatible with the presence of a PRiMA subunit.
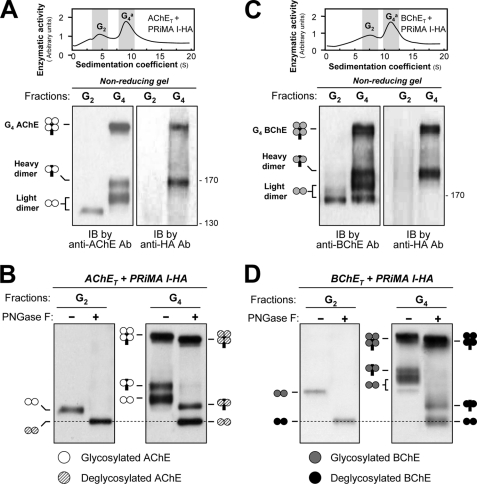
FIGURE 4.
Analysis of disulfide bonds between subunits of G4 AChE and G4 BChE by nonreducing electrophoresis and Western blotting. A, AChET subunits and PRiMA I-HA were co-expressed in HEK293T cells. G2 and G4 fractions (shaded) from cell lysates were collected following sedimentation in sucrose gradients. Disulfide linkages between AChET subunits and between AChET subunits and PRiMA were analyzed by nonreducing electrophoresis and Western blotting with anti-AChE and anti-HA antibodies, as indicated. The G2 fractions produced a single dimeric band, whereas the G4 fractions produced a dimeric band that did not include PRiMA (light dimer), as well as a dimeric band associated with PRiMA (heavy dimer) and a heavier component, probably representing an AChE tetramer in which all four subunits are disulfide-linked with PRiMA. B, G2 and G4 fractions of AChE were treated with or without peptide N-glycosidase F and analyzed by nonreducing electrophoresis and Western blotting with anti-AChE antibody. AChEs in all different oligomers were N-glycosylated, because of the apparent mass decrease after deglycosylation. The mass of the light dimers in the G4 AChE fraction appeared the same as that in the G2 fractions after the deglycosylation, as indicated by the dotted lines. C, BChET subunits and PRiMA I-HA were co-expressed in HEK293T cells. Disulfide linkages between BChET subunits and between BChET subunits and PRiMA in the G2 and G4 fractions were analyzed by nonreducing electrophoresis and Western blotting with anti-BChE and anti-HA antibodies (lower panel). D, G2 and G4 fractions of BChE were treated with or without peptide N-glycosidase F and analyzed by nonreducing electrophoresis and Western blotting with anti-BChE antibody. The details of the analyses were as for B. Representative gradient profiles and gels are shown, n = 4. Ab, antibody; IB, immunoblot; PNGase F, peptide N-glycosidase F.
To eliminate the influence of glycosylation on AChE and BChE G2 forms and PRiMA-linked G4 enzymes in nonreducing gel electrophoresis, we used N-glycosidase F, which removed all N-linked carbohydrates. After the digestion, the light dimers in the PRiMA-linked AChE and BChE G4 enzymes became identical to their G2 fractions at ∼125 kDa, and the heavy dimers were shifted to ∼145 kDa (Fig. 4, B and D). In addition, the heavier bands corresponding to PRiMA-linked AChET and BChET tetramers also migrated more quickly.
Therefore, both PRiMA-linked AChE and BChE complexes appear to be organized in two possible manners: (i) one pair of catalytic subunits are linked to each other by disulfide bonds, forming a light dimer, whereas the other two catalytic subunits are disulfide-linked with two cysteines of PRiMA, forming a heavy dimer, and (ii) all four catalytic subunits are disulfide-linked with four cysteines of PRiMA, generating the heavy band. The fact that only AChET and BChET homodimers exist in the absence of PRiMA and also in PRiMA-linked G4 tetramers suggests that they may represent intermediates in the assembly of these complexes. In addition, the glycosylation in AChET and BChET in the PRiMA-linked G4 enzymes appeared more extensive than in the G2 dimers.
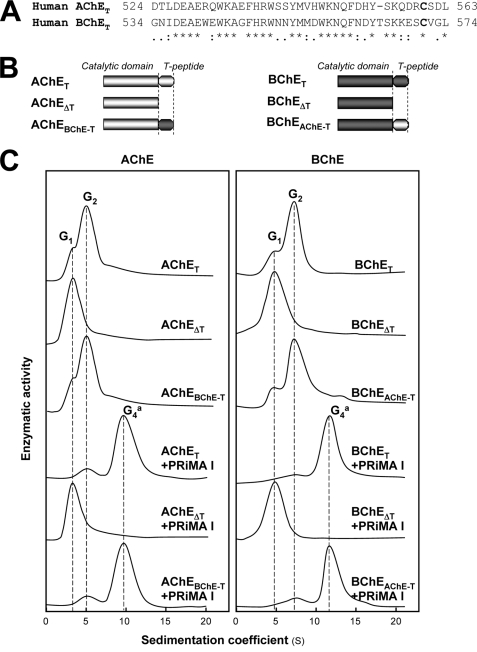
Role of the C-terminal t-peptides in Cholinesterase Oligomerization
AChET and BChET possess C-terminal t-peptides of 40 and 41 residues, respectively, which are known to be required for their oligomerization. The t-peptides of human AChET and BChET are highly homologous, with 60% residue identity (Fig. 5A). The fact that co-expression of the AChET and BChET subunits did not produce AChET-BChET heterodimers led us to determine the role of t-peptides. We constructed mutants AChEΔT and BChEΔT, in which the t-peptides of AChET and BChET were deleted. As expected (35), these t-peptide-depleted mutants produced only monomers, when they were co-expressed with or without PRiMA, indicating the importance of t-peptides for both dimerization and tetramerization (Fig. 5C).
FIGURE 5.
Oligomerization of human AChE, BChE, and mutants lacking the C-terminal t-peptide or containing exchanged t-peptides. A, comparison of the amino acid sequences of C-terminal t-peptides from human AChE and BChE. Identical residues are indicated by asterisks. Highly and moderately similar residues are indicated by double dots and single dots, respectively. B, schematic representations of AChET, BChET, and their mutants. AChET and BChET are wild-type human AChE and BChE, respectively. AChEΔT and BChEΔT are mutants in which the t-peptides were deleted. AChEBChE-T and BChEAChE-T are chimeras in which the t-peptides of AChE and BChE were exchanged. C, HEK293T cells were transfected with cDNAs encoding AChET, AChEΔT, AChEBChE-T, BChET, BChEΔT, or BChEAChE-T with and without cDNA encoding PRiMA for 2 days. Cell extracts containing equal amounts of enzymatic activity were subjected to sucrose density gradient analysis as in Fig. 1A. The positions of the peaks corresponding to the G1, G2, and G4a forms of AChE (left panel) and BChE (right panel) are shown by vertical dashed lines. Enzymatic activities are expressed in arbitrary units, and one representative result is shown (n = 4).
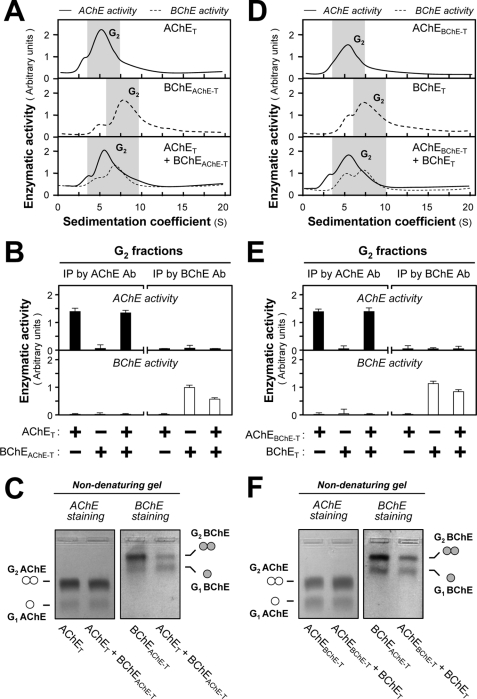
Effect of Exchanging the C-terminal t-peptides between AChET and BChET on Dimerization
The difference between their t-peptides might explain why hybrid AChET-BChET dimers are not produced. To test this hypothesis, we constructed mutants AChEBChE-T and BChEAChE-T, in which the t-peptides of AChET and BChET were exchanged with each other (Fig. 5B) and expressed them in various combinations with wild-type AChET and BChET in HEK293T cells. We found that the formation of dimers was very similar for AChEBChE-T and AChET as well as for BChEAChE-T and BChET (Fig. 5C), indicating that the t-peptides of AChET and BChET conferred essentially the same expression level and the same ability to dimerize, in good agreement with their high degree of sequence identity. Sucrose density gradient analysis showed that the co-expression of AChET and BChEAChE-T produced G2 forms of AChE and BChE (Fig. 6A), and we obtained a similar profile by co-expression of BChET and AChEBChE-T (Fig. 6D). Immunoprecipitation experiments with G2-enriched fractions from single and double transfected cells did not show any evidence of co-immunoprecipitation of AChET with BChEAChE-T (Fig. 6B) or of BChET with AChEBChE-T, when these subunits were co-expressed (Fig. 6E). This result was further confirmed by nondenaturing gel electrophoresis, which showed the presence only of homodimers and not of hybrid dimers AChET-BChEAChE-T (Fig. 6C) and BChET-AChEBChE-T (Fig. 6F). Therefore, the exclusive formation of homodimers is not determined by the nature of the t-peptide, suggesting that the catalytic domains of AChET and BChET must play a critical role in dimerization. This is consistent with the fact that the catalytic domains of cholinesterases interact through a dimeric contact zone called the “four-helix bundle” in T variants possessing a t-peptide and also in H variants that do not possess this peptide, forming dimers that are stabilized by a C-terminal disulfide bond (36, 37).
FIGURE 6.
AChE and BChE subunits do not form hybrid dimers, even when they contain the same t-peptide. A and D, HEK293T cells were single or double transfected with cDNAs encoding AChE and BChE catalytic subunits, which contained the same t-peptides (AChET and BChEAChE-T or AChEBChE-T and BChET). Sucrose density gradient analysis was performed as in Fig. 1A. B and E, G2 fractions from single and double transfected cells (shaded in A and D) were immunoprecipitated by either anti-AChE or anti-BChE antibodies and absorbed on protein G beads. The enzymatic activities of AChE and BChE immobilized on the beads were determined. C and F, G2 fractions from single and double transfected cell lysates (shaded in A and D) were analyzed by nondenaturing electrophoresis coupled with Karnovsky staining as in Fig. 2. The absence of hybrid dimer in the double transfection indicates that AChE and BChE subunits do not associate, even when containing the same t-peptide. The enzymatic activities are expressed in arbitrary units. The values are the means ± S.E., each with triplicate samples (n = 3). Representative gradient profiles and gels are shown (n = 4). Ab, antibody; IP, immunoprecipitation.
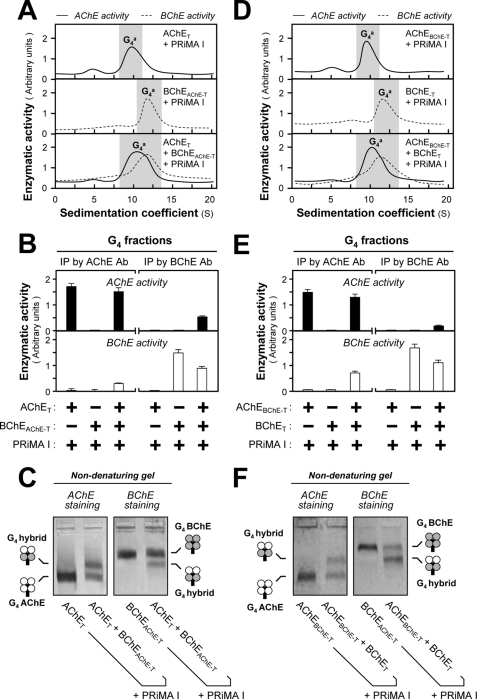
PRiMA Recruits Homodimers of AChET and/or BChET to Assemble PRiMA-linked Cholinesterase Tetramers
Similar co-transfection experiments were performed by using AChET, BChET, AChEBChE-T, and BChEAChE-T in the presence of PRiMA. The co-expression of BChEAChE-T and PRiMA produced a G4 enzyme that did not differ in its sedimentation from that produced with BChET and PRiMA (Fig. 7, A and D). Similarly, the G4 complex formed by co-expressing AChET and PRiMA did not differ from that formed by AChEBChE-T and PRiMA (Fig. 7, A and D). The triple expression of AChET, BChEAChE-T, and PRiMA produced a PRiMA-linked AChE-BChE hybrid, i.e. (AChET)2-(BChEAChE-T)2-PRiMA. The formation of this hybrid was confirmed by (i) co-immunoprecipitation of AChE activity by an anti-BChE antibody and vice versa (Fig. 7B) and (ii) formation of a single intermediate migrating species in nondenaturing electrophoresis, showing the presence of complexes composed of AChET and BChET homodimers (2/2), excluding AChET and BChET in 1/3 or 3/1 combinations (Fig. 7C). Similarly, the triple expression of BChET, AChEBChE-T, and PRiMA formed a (BChET)2-(AChEBChE-T)2-PRiMA G4 hybrid (Fig. 7, D–F). These results also confirm that the complexes consist of two cholinesterase homodimers linked with a PRiMA subunit. The selectivity of dimerization depends on the catalytic domain but not on the t-peptide, even though the presence of its C-terminal cysteine is necessary for the stabilization of dimmers (14, 38, 39).
FIGURE 7.
Formation of G4 hybrids by AChE and BChE subunits containing the same t-peptide. A and D, HEK293T cells were single or double transfected with cDNAs encoding AChE and BChE catalytic subunits, which contained the same t-peptides (AChET and BChEAChE-T or AChEBChE-T and BChET) together with cDNA encoding PRiMA I. The amount of PRiMA I cDNA was doubled in the triple transfection. Sucrose density gradient analysis was performed as in Fig. 1A. B and E, G4 fractions from single and double transfected cells (shaded in A and D) were immunoprecipitated by either anti-AChE or anti-BChE antibody and absorbed on protein G beads; the retained AChE and BChE activities were determined by Ellman assays. C and F, analysis of G4 fractions from double and triple transfected cells by nondenaturing electrophoresis coupled with Karnovsky staining as in Fig. 2. The presence of a new oligomer migrating between G4 AChE and G4 BChE indicates that AChE and BChE, possessing the same t-peptides, can form a PRiMA-linked G4 hybrid. The enzymatic activities are expressed in arbitrary units. The values are the means ± S.E., each with triplicate samples (n = 3). Representative gradient profiles and gels are shown (n = 4). Ab, antibody; IP, immunoprecipitation.
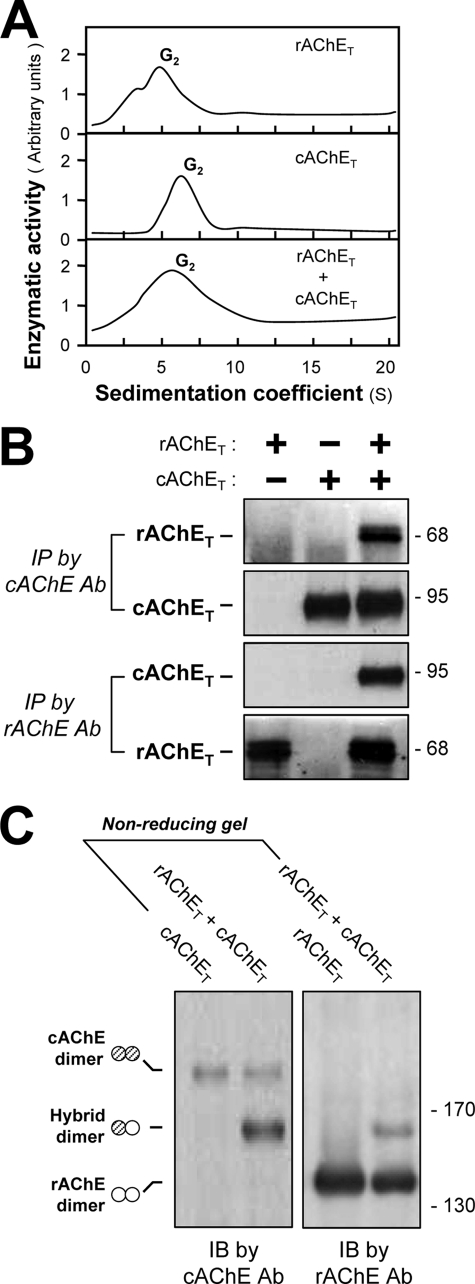
Role of the Catalytic Domain in the Selectivity of Dimerization
The selectivity of cholinesterase dimerization was further analyzed by using AChET from different species. Both rat AChET (rAChET) and chicken AChET (cAChET) form dimers when expressed in HEK293T cells, separately or together (Fig. 8A). To determine whether their co-expression produced a hybrid dimer, the cell extracts were immunoprecipitated with anti-chicken AChE or anti-rat AChE antibodies, which were highly specific for their own enzyme (24). The immunoprecipitated complex was analyzed by SDS-PAGE and labeled in Western blots with the same antibodies. rAChET and cAChET produced distinct protein bands at ∼68 and ∼95 kDa, respectively. Immunoprecipitation of cell extracts expressing both enzymes (rAChET and cAChET) showed that each one was partly pulled down together with the other (Fig. 8B), indicating the formation of hybrid rAChET-cAChET dimers. Extracts from cells separately expressing rAChET or cAChET served as controls.
FIGURE 8.
Formation of chicken-rat AChE hybrid dimers. A, HEK293T cells were single or double transfected with cDNAs encoding rAChET and cAChET. Sucrose density gradient analysis was performed as in Fig. 1A. Enzymatic activities are expressed in arbitrary units, and one representative result is shown (n = 3). B, the cell lysates were immunoprecipitated by anti-cAChE or anti-rAChE antibodies and analyzed by SDS-PAGE and Western blotting with anti-cAChE or anti-rAChE antibodies. C, extracts from cells expressing rAChET and cAChET separately or together were analyzed by nonreducing SDS-PAGE and labeled with anti-cAChE and anti-rAChE antibodies. The presence of an intermediate band between cAChE and rAChE dimers, recognized by both antibodies, demonstrates the formation of cAChET-rAChET hybrid dimer. Representative gels are shown (n = 3). Ab, antibody; IP, immunoprecipitation; IB, immunoblot.
We also performed gel electrophoresis under nonreducing conditions (Fig. 8C). We observed cAChET dimers (∼190 kDa), rAChET dimers (∼140 kDa), and intermediate heterodimers (∼165 kDa), which were labeled by both anti-chicken and anti-rat AChE antibodies (Fig. 8C), indicating that they correspond to disulfide-linked rAChET-cAChET hybrid dimers. Therefore, these mammalian and avian AChEs have conserved the capacity to co-dimerize, despite their evolutionary distance.
Expression of PRiMA-linked AChE-BChE Hybrid Tetramers in Chicken Brain
We used avian brain to explore the possible existence of PRiMA-linked AChE-BChE G4 hybrid molecules in native tissues for two reasons. First, asymmetric AChE-BChE hybrid molecules were initially discovered in chicken muscle (15). Second, we possess monoclonal antibodies specifically recognizing chicken AChE (3D10) and BChE (7D11), which allow an accurate detection of the hybrid molecules in chicken tissues. Fig. 9A illustrates the presence of both dimeric G2 and tetrameric G4 molecular forms of AChE and BChE in adult chicken brain. The chicken G4 AChE was characterized as a PRiMA-linked membrane-bound enzyme (40–42). BChE activity could be co-immunoprecipitated with AChE, using the 3D10 antibody (against AChE), in the G4 fractions but not in the G2 fractions (Fig. 9B). Conversely, some AChE activity from the G4 fractions could also be co-precipitated with BChE activity by using the anti-BChE antibody (7D11) (Fig. 9B). The existence of AChE-BChE hybrid tetramers was further confirmed by the fact that the anti-AChE antibody 3D10 was able to partially deplete the G4 form of BChE in a chicken brain extract, without any effect on the G2 form; the amount of this hybrid was ∼15% of total G4 BChE activity (Fig. 9C). Thus, AChE-BChE hybrid G4 tetramers exist in chicken brain, in agreement with the results obtained with the transfected cells.
FIGURE 9.
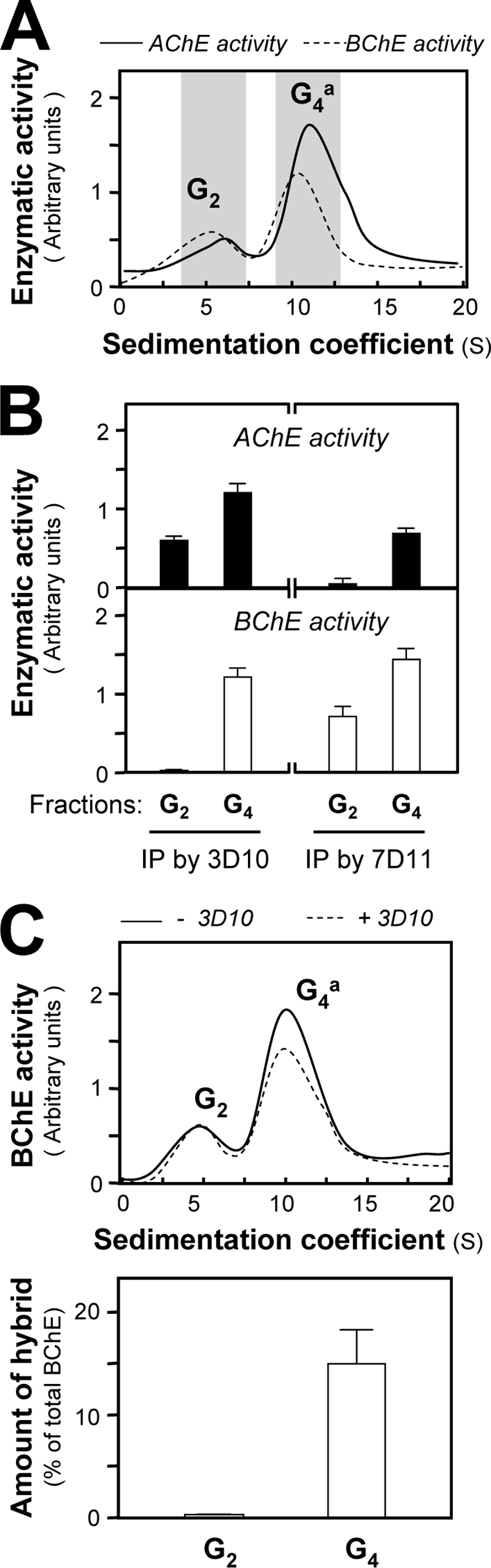
Existence of PRiMA-linked G4 AChE-BChE hybrid tetramers in chicken brain. A, sedimentation profiles of AChE and BChE molecular forms in extracts from adult chicken brain, as analyzed in Fig. 1A. B, G2 fractions and G4 fractions shaded in A were immunoprecipitated with anti-chicken AChE antibody (3D10) or anti-chicken BChE antibody (7D11) and absorbed onto protein G beads. AChE and BChE activities were determined by Ellman assays. C, immunodepletion of AChE-BChE hybrid molecules with 3D10 antibody. Samples of chicken brain extracts were incubated with or without 3D10 antibody and absorbed onto protein G-agarose beads, and the supernatant was analyzed by sedimentation in sucrose density gradients, as in Fig. 1A. The observed decrease of BChE activity after immunoprecipitation by 3D10 antibody showed that in BChE tetramers, ∼15% of BChE subunits were included in AChE-BChE hybrid tetramers (lower panel). The enzymatic activities are expressed in arbitrary units. The values are the means ± S.E., each with triplicate samples (n = 3). Representative gradient profiles are shown (n = 3). IP, immunoprecipitation.
We investigated the developmental profile of PRiMA-linked tetramers in chicken brain, from embryonic day 9 (E9) to the adult stage. Tissue extracts containing equal amounts of protein were assayed to determine AChE and BChE activities (Fig. 10A). The AChE activity increased steadily (∼5-fold), whereas BChE activity increased only slightly (less than 2-fold) until the early postnatal stage (postnatal day 12) and then reached over 3-fold of its E9 value at the adult stage (Fig. 10A). We used semi-quantitative RT-PCR to determine the expression profile of PRiMA I and II (40) during the development of chicken brain (supplemental Fig. S2A). PRiMA I mRNA remained predominant and did not change conspicuously during the brain development, whereas the level of PRiMA II mRNA gradually increased from the E9 stage, reaching a maximum at the postnatal day 12 stage (supplemental Fig. S2B). During this period, the levels and proportions of G4 tetramers increased for both AChE and BChE in the chicken cerebrum (data not shown) (40, 43). The relative amounts of PRiMA-linked AChE-BChE hybrid tetramers in chicken brain were determined by ELISA at different stages of development. Chicken brain extracts containing equal AChE activity were incubated with immobilized anti-chicken AChE antibody (3D10). The amount of AChE being captured on the plates occurred in a dose-dependent manner and was identical for all developmental stages (Fig. 10B). In parallel, the BChE activity, retained by anti-AChE 3D10 antibody, was taken to reflect the proportion of the G4 hybrid molecule in the chicken brain; it increased during development, most markedly between postnatal day 12 and reaching adulthood (2–3-fold) (Fig. 10C). This strong increase suggests that the AChE-BChE G4 hybrid molecules may play a physiological role in the chicken brain.
FIGURE 10.
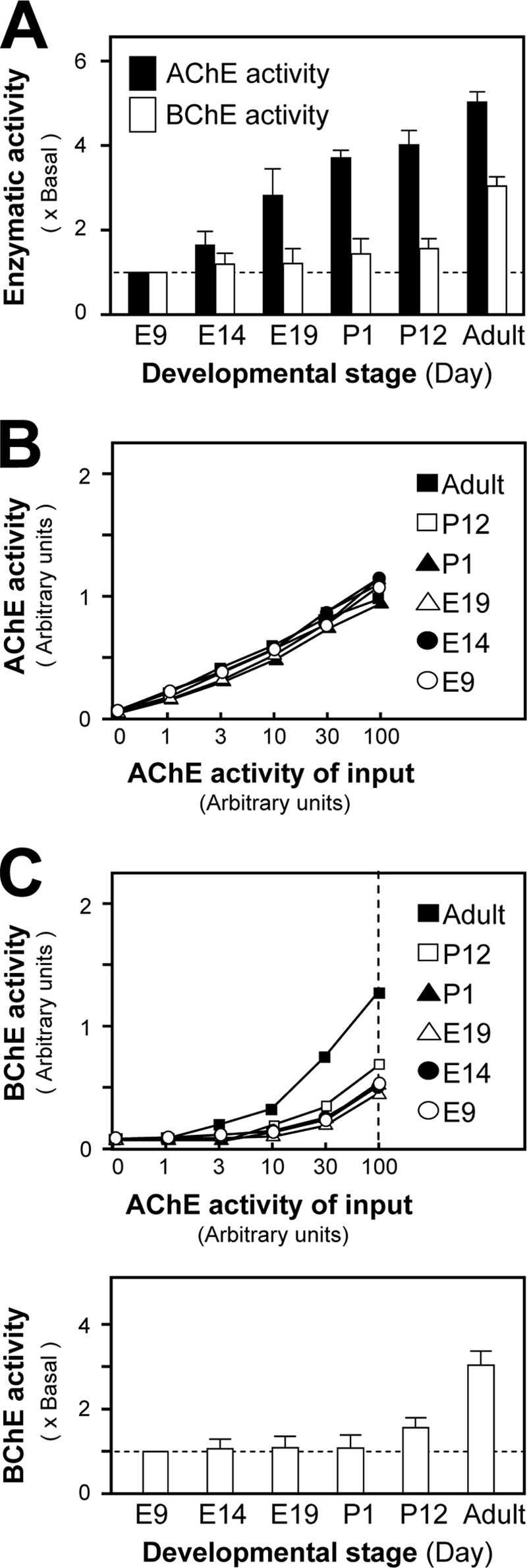
Developmental profile of PRiMA-linked G4 AChE-BChE hybrid in chicken brain. A, AChE and BChE activities were determined in protein extracts from chicken brain of embryonic (E), postnatal (P), and adult stages, all containing equal amounts of protein. The data were normalized to the basal level of E9. B and C, the level of PRiMA-linked AChE-BChE G4 hybrid molecule was quantified by ELISA. Extracts with equal amounts of AChE activity from chicken brain at different developmental stages were loaded onto ELISA plates precoated with a saturating amount of anti-chicken AChE antibody and incubated for 4 h. B and C, the immobilized AChE activity (B) and BChE activity (C) were determined directly in the ELISA plate after washing. The enzymatic activity was expressed in arbitrary units. The lower panel in C showed the quantitative data of the BChE activity on the hybrid molecules obtained when the AChE activity of input was 100 arbitrary units. The data were normalized by the basal level of E9. All of the values are the means ± S.E., each with triplicate samples (n = 3).
DISCUSSION
The asymmetric form (A12) of AChE, purified by immunoaffinity chromatography from 1-day-old chicken pectoral muscles, was shown to contain AChE and BChE catalytic subunits in a 1:1 ratio (15). The existence of this type of hybrid molecule was supported by several lines of evidence: (i) using anti-BChE antibody 7D11 in immunoprecipitation could precipitate AChE activity (15) and (ii) in the purified asymmetric AChE, three subunits corresponding to AChE, BChE, and ColQ were detected on the silver-stained SDS-PAGE gel (24). Here, we extend this study by showing the formation of PRiMA-linked AChE-BChE tetrameric hybrid molecules, both in transfected cells in culture and in intact chicken brain.
We expressed AChET and BChET subunits in HEK293T cells, with or without a PRAD-containing protein (PRiMA I, PRiMA II, or QN-GPI). In the absence of a PRAD-containing protein, (i) cholinesterase subunits formed only monomers (G1) and dimers (G2); (ii) we obtained homodimers of AChET and of BChET, but co-expression of both subunits never produced heterodimers (i.e. AChET-BChET); and (iii) interspecies dimers could be formed between rat and chicken AChET subunits (rAChET-cAChET). As previously shown (33), cholinesterase subunits lacking their t-peptides remained monomeric, indicating that the t-peptide is necessary for the formation of dimers; however, mixed AChET-BChET dimers were not formed even when the C-terminal t-peptides were exchanged. Subunits of type H also form dimers, as well as subunits lacking either a t-peptide or a h-peptide but possessing a cysteine downstream of the catalytic domain (37). Thus, the presence of a cysteine stabilizes cholinesterase dimers, but dimerization depends on the formation of a four-helix bundle, in which two α-helices of each catalytic domain are closely apposed to the corresponding helices of the other one (36, 37). Because we observed the formation of interspecies dimers between rat and chicken AChETs but not between mammalian AChET and BChET, the selectivity of dimerization seems to be based on features that are largely shared between vertebrate AChEs (including mammalian and avian enzymes) but distinguish them from vertebrate BChEs. A comparison between the sequences of helices forming the four-helix bundle is shown in the supplemental Fig. S3.
The PRAD-containing proteins induced the formation of tetramers, consisting of two dimers, which could be either identical or of different types, with two AChET subunits and two BChET subunits. Thus, the assembly of cholinesterase tetramers by a PRAD-containing protein appears to proceed through a stepwise recruitment of two homodimers. The core of this heteromeric association is the formation of a coiled-coil cylinder of four α-helical t-peptides around the PRAD, organized as a polyproline II helix (22). Our results show that t-peptides derived from vertebrate AChET and BChET subunits are compatible to form mixed coiled-coils associated with a PRAD, despite conserved differences between their sequences (12).
Thus, the formation of cholinesterase dimers of either H or T variants depends on a compatible contact zone formed between two catalytic domains as a four-helix bundle and on the presence of a C-terminal cysteine. In contrast, the formation of tetramers depends on the presence of a t-peptide, either for AChET subunits (12, 37) or for BChET subunits (13). Although subunits of type T can probably form nonamphiphilic homotetramers by themselves, the assembly of tetramers is efficiently induced by a PRAD-containing protein, ColQ or PRiMA (21, 25), or by a polyproline peptide in the case of soluble BChE tetramers (44).
The PRAD-linked AChET-BChET tetrameric hybrids do not represent an artifact of the recombinant system in transfected cells, because they also exist in chicken muscle (in association with ColQ) (15) and brain (in association with PRiMA) (Figs. 9 and 10). Although we do not have sufficient evidence to show the existence of this hybrid enzyme in mammalian brain, a soluble hybrid tetramer having AChE and BChE activity was also found in cyst fluids derived from a human astrocytoma (45). Previous studies have shown that collagen-tailed asymmetric hybrid forms are relatively abundant in young chicken muscle but tend to disappear at the adult stage (24). In contrast, the present study showed that the amount of the PRiMA-linked AChE-BChE hybrid molecules in chicken brain was strongly increased from the embryonic stages to adulthood (Fig. 10). At the adult stage, AChE-BChE hybrids contain ∼15% of the total BChE subunits in the tetrameric fractions. Based on the findings in the transfected cellular expression system, we assume that the molecular organization of the AChE-BChE hybrid tetramer in chicken brain is (AChET)2-(BChET)2-PRiMA. The observed increase of these hybrid molecules during brain development suggests that they may exert a specific functional role, which remains to be established. At this stage, we have no idea of this possible function. Important issues need to be addressed regarding AChE-BChE hybrid tetramers: (i) their localization in the brain and whether they are lipid raft-associated as in the case of G4 PRiMA-linked AChE (46); (ii) possible control mechanisms directing the formation of either homotetramers or heterotetramers; and (iii) the role of BChE in brain, because our present lack of understanding of the precise role of BChE makes it difficult to evaluate the possible benefit of associating AChE and BChE activities in the same hybrid oligomer.
Supplementary Material
This work was supported by Research Grants Council of Hong Kong Grants N_HKUST629/07, 662407, 662608, 660409, and F-HK21/06T and Croucher Foundation Grant CAS-CF07/08.SC03 (to K. W. K. T.) and grants from Centre National de la Recherche Scientifique, Ecole Normale Supérieure, Association Française contre les Myopathies, and French Ministry of Foreign Affairs (to J. M.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- AChE
- acetylcholinesterase
- BChE
- butyrylcholinesterase
- GPI
- glycosylphosphatidylinositol
- ColQ
- collagen Q
- PRAD
- proline-rich attachment domain
- r
- rat
- c
- chicken
- En
- embryonic day n.
REFERENCES
- 1.Massoulié J. (2002) Neurosignals 11, 130–143 [DOI] [PubMed] [Google Scholar]
- 2.Falasca C., Perrier N., Massoulié J., Bon S. (2005) J. Biol. Chem. 280, 878–886 [DOI] [PubMed] [Google Scholar]
- 3.Lockridge O. (1990) Pharmacol. Ther. 47, 35–60 [DOI] [PubMed] [Google Scholar]
- 4.Xie W., Stribley J. A., Chatonnet A., Wilder P. J., Rizzino A., McComb R. D., Taylor P., Hinrichs S. H., Lockridge O. (2000) J. Pharmacol. Exp. Ther. 293, 896–902 [PubMed] [Google Scholar]
- 5.Massoulié J., Pezzementi L., Bon S., Krejci E., Vallette F. M. (1993) Prog. Neurobiol. 41, 31–91 [DOI] [PubMed] [Google Scholar]
- 6.Massoulié J., Anselmet A., Bon S., Krejci E., Legay C., Morel N., Simon S. (1998) J. Physiol. Paris 92, 183–190 [DOI] [PubMed] [Google Scholar]
- 7.Kaufer D., Friedman A., Seidman S., Soreq H. (1998) Nature 393, 373–377 [DOI] [PubMed] [Google Scholar]
- 8.Perrier N. A., Salani M., Falasca C., Bon S., Augusti-Tocco G., Massoulié J. (2005) J. Neurochem. 94, 629–638 [DOI] [PubMed] [Google Scholar]
- 9.Li Y., Camp S., Rachinsky T. L., Getman D., Taylor P. (1991) J. Biol. Chem. 266, 23083–23090 [PubMed] [Google Scholar]
- 10.Legay C., Huchet M., Massoulié J., Changeux J. (1995) Eur. J. Neurosci. 7, 1803–1809 [DOI] [PubMed] [Google Scholar]
- 11.Massoulié J., Bon S., Perrier N., Falasca C. (2005) Chem. Biol. Interact. 157–158, 3–14 [DOI] [PubMed] [Google Scholar]
- 12.Liang D., Blouet J. P., Borrega F., Bon S., Massoulié J. (2009) FEBS J. 276, 94–108 [DOI] [PubMed] [Google Scholar]
- 13.Blong R. M., Bedows E., Lockridge O. (1997) Biochem. J. 327, 747–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bon S., Dufourcq J., Leroy J., Cornut I., Massoulié J. (2004) Eur. J. Biochem. 271, 33–47 [DOI] [PubMed] [Google Scholar]
- 15.Tsim K. W., Randall W. R., Barnard E. A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 1262–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krejci E., Thomine S., Boschetti N., Legay C., Sketelj J., Massoulié J. (1997) J. Biol. Chem. 272, 22840–22847 [DOI] [PubMed] [Google Scholar]
- 17.Xie H. Q., Choi R. C., Leung K. W., Chen V. P., Chu G. K., Tsim K. W. (2009) Brain Res. 1265, 13–23 [DOI] [PubMed] [Google Scholar]
- 18.Perrier A. L., Massoulié J., Krejci E. (2002) Neuron 33, 275–285 [DOI] [PubMed] [Google Scholar]
- 19.Perrier N. A., Khérif S., Perrier A. L., Dumas S., Mallet J., Massoulié J. (2003) Eur. J. Neurosci. 18, 1837–1847 [DOI] [PubMed] [Google Scholar]
- 20.Xie H. Q., Choi R. C., Leung K. W., Siow N. L., Kong L. W., Lau F. T., Peng H. B., Tsim K. W. (2007) J. Biol. Chem. 282, 11765–11775 [DOI] [PubMed] [Google Scholar]
- 21.Bon S., Coussen F., Massoulié J. (1997) J. Biol. Chem. 272, 3016–3021 [DOI] [PubMed] [Google Scholar]
- 22.Dvir H., Harel M., Bon S., Liu W. Q., Vidal M., Garbay C., Sussman J. L., Massoulié J., Silman I. (2004) EMBO J. 23, 4394–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noureddine H., Schmitt C., Liu W., Garbay C., Massoulié J., Bon S. (2007) J. Biol. Chem. 282, 3487–3497 [DOI] [PubMed] [Google Scholar]
- 24.Tsim K. W., Randall W. R., Barnard E. A. (1988) EMBO J. 7, 2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noureddine H., Carvalho S., Schmitt C., Massoulié J., Bon S. (2008) J. Biol. Chem. 283, 20722–20732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gough N. R., Randall W. R. (1995) J. Neurochem. 65, 2734–2741 [DOI] [PubMed] [Google Scholar]
- 27.Choi R. C., Leung P. W., Dong T. T., Wan D. C., Tsim K. W. (1996) Neurosci. Lett. 217, 165–168 [PubMed] [Google Scholar]
- 28.Bon S., Massoulié J. (1997) J. Biol. Chem. 272, 3007–3015 [DOI] [PubMed] [Google Scholar]
- 29.Ellman G. L., Courtney K. D., Andres V., Jr., Feather-Stone R. M. (1961) Biochem. Pharmacol. 7, 88–95 [DOI] [PubMed] [Google Scholar]
- 30.Bon S., Toutant J. P., Méflah K., Massoulié J. (1988) J. Neurochem. 51, 786–794 [DOI] [PubMed] [Google Scholar]
- 31.Karnovsky M. J., Roots L. (1964) J. Histochem. Cytochem. 12, 219–221 [DOI] [PubMed] [Google Scholar]
- 32.Bradford M. M. (1976) Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 33.Duval N., Krejci E., Grassi J., Coussen F., Massoulié J., Bon S. (1992) EMBO J. 11, 3255–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S. L., Heinemann S., Taylor P. (1982) J. Biol. Chem. 257, 12282–12291 [PubMed] [Google Scholar]
- 35.Duval N., Massoulié J., Bon S. (1992) J. Cell Biol. 118, 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. (1991) Science 253, 872–879 [DOI] [PubMed] [Google Scholar]
- 37.Morel N., Leroy J., Ayon A., Massoulié J., Bon S. (2001) J. Biol. Chem. 276, 37379–37389 [DOI] [PubMed] [Google Scholar]
- 38.Belbeoc'h S., Falasca C., Leroy J., Ayon A., Massoulié J., Bon S. (2004) Eur. J. Biochem. 271, 1476–1487 [DOI] [PubMed] [Google Scholar]
- 39.Belbeoc'h S., Massoulié J., Bon S. (2003) EMBO J. 22, 3536–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mok M. K., Leung K. W., Xie H. Q., Guo A. J., Chen V. P., Zhu J. T., Choi R. C., Tsim K. W. (2009) Neurosci. Lett. 461, 202–206 [DOI] [PubMed] [Google Scholar]
- 41.Rotundo R. L. (1984) J. Biol. Chem. 259, 13186–13194 [PubMed] [Google Scholar]
- 42.Randall W. R., Tsim K. W., Lai J., Barnard E. A. (1987) Eur. J. Biochem. 164, 95–102 [DOI] [PubMed] [Google Scholar]
- 43.Anselmet A., Fauquet M., Chatel J. M., Maulet Y., Massoulié J., Vallette F. M. (1994) J. Neurochem. 62, 2158–2165 [DOI] [PubMed] [Google Scholar]
- 44.Li H., Schopfer L. M., Masson P., Lockridge O. (2008) Biochem. J. 411, 425–432 [DOI] [PubMed] [Google Scholar]
- 45.García-Ayllón M. S., Sáez-Valero J., Muñoz-Delgado E., Vidal C. J. (2001) Neuroscience 107, 199–208 [DOI] [PubMed] [Google Scholar]
- 46.Xie H. Q., Liang D., Leung K. W., Chen V. P., Zhu K. Y., Chan W. K., Choi R. C., Massoulié J., Tsim K. W. (2010) J. Biol. Chem. 285, 11537–11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.