Abstract
Retinoic acid (RA) and thyroid hormone are critical for differentiation and organogenesis in the embryo. Mct8 (monocarboxylate transporter 8), expressed predominantly in the brain and placenta, mediates thyroid hormone uptake from the circulation and is required for normal neural development. RA induces differentiation of F9 mouse teratocarcinoma cells toward neurons as well as extraembryonal endoderm. We hypothesized that Mct8 is functionally expressed in F9 cells and induced by RA. All-trans-RA (tRA) and other RA receptor (RAR) agonists dramatically (>300-fold) induced Mct8. tRA treatment significantly increased uptake of triiodothyronine and thyroxine (4.1- and 4.3-fold, respectively), which was abolished by a selective Mct8 inhibitor, bromosulfophthalein. Sequence inspection of the Mct8 promoter region and 5′-rapid amplification of cDNA ends PCR analysis in F9 cells identified 11 transcription start sites and a proximal Sp1 site but no TATA box. tRA significantly enhanced Mct8 promoter activity through a consensus RA-responsive element located 6.6 kilobases upstream of the coding region. A chromatin immunoprecipitation assay demonstrated binding of RAR and retinoid X receptor to the RA response element. The promotion of thyroid hormone uptake through the transcriptional up-regulation of Mct8 by RAR is likely to be important for extraembryonic endoderm development and neural differentiation. This finding demonstrates cross-talk between RA signaling and thyroid hormone signaling in early development at the level of the thyroid hormone transporter.
Keywords: Gene Regulation, Neural Stem Cell, Nuclear Receptors, Retinoid, Thyroid, Thyroid Hormone
Introduction
Retinoic acid (RA) and thyroid hormones are essential for vertebrate development (1, 2). The actions of these hormones are mediated by specific nuclear hormone receptors, RA receptor (RAR)3 and thyroid hormone receptor (TR), respectively. RAR signaling plays a critical role in embryonic patterning and in organogenesis (1). TR modulates RA-stimulated neural differentiation as well as expression of some RA-responsive genes in embryonic stem cells (3, 4). The timing of ligand availability and receptor expression is important for normal neural differentiation (4).
RAR and TR form heterodimers with retinoid X (9-cis-RA) receptor (RXR) for regulation of most target genes. The consensus sequence of an RA response element (RARE) contains a direct repeat of the consensus half-sites, 5′-PuG(G/T)(T/A)CA-3′, with spacing of 1, 2, or 5 bases (DR-1, DR-2, or DR-5), whereas that of a retinoid X (or 9-cis-RA) response element contains the same half-sites separated by 1 base (DR-1) (5). Although the difference of half-site spacing provides selectivity for a specific receptor, there are interactions among the various nuclear receptor signaling pathways. Nuclear receptors also share common co-activator(s) and co-repressor(s) for transcriptional regulation (2). A shared requirement for RXR, interaction at related cis-elements, and competition for co-factors are some of the mechanisms underlying cross-talk among nuclear receptor signaling pathways in development (6) and in metabolic regulation (2).
Nuclear receptor ligands are generally lipophilic and have been thought to reach their receptor by passive diffusion through the plasma membrane. Recent studies, however, have demonstrated that uptake of some of these ligands is mediated by selective transporters. Several members of the solute carrier (Slc) family (7–9), including Mct8 (monocarboxylate transporter-8, or Slc16a2) (10), and the solute carrier organic anion transporter family (Slco, or organic anion-transporting polypeptides, Oatp) (9, 11) are known as thyroid hormone transporters. Mct8 loss of function mutations in humans are associated with profound neurological deficits (7), a common manifestation of thyroid hormone insufficiency in embryos and fetuses. Mct8 is expressed in many tissues, including brain, placenta, liver, and kidney (7, 10, 12), which are all important thyroid hormone targets.
F9 teratocarcinoma cells have been widely used as an in vitro model of embryonic stem cell differentiation. A combination treatment of F9 cells with all-trans-RA (tRA), an agonist of RAR, and cAMP induces extraembryonic endoderm (13, 14) or neuron-like cells (15), depending on the composition of culture media. The extraembryonic endoderm supports the developing embryo and facilitates exchange of small molecules between the maternal circulation and the embryo, functioning as an “early placenta” (16). Because mature placenta expresses abundant Mct8 and transports thyroid hormone (12), it may be expressed in F9 cells differentiated into extraembryonic endoderm. Thyroid hormone is critical for brain development, and thus neural differentiation in F9 cells should be accompanied by induction of thyroid hormone transporter(s). To test these hypotheses, we investigated effects of the differentiation inducer, tRA, on expression of Mct8 and other thyroid hormone transporter genes as well as thyroid hormone uptake in F9 cells.
EXPERIMENTAL PROCEDURES
Cell Culture
F9 cells, purchased from ATCC (Manassas, VA), were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) (Invitrogen) in gelatin-coated flasks or Petri dishes as recommended. Cells were subcultivated at a ratio of 1:10 to 1:20 and used at passages 2–6, unless otherwise noted. Neuron-like cells were induced as described previously (15) with minor modifications. Briefly, ∼5 × 105 cells seeded in gelatin-coated Petri dishes were maintained in DMEM/F-12 medium (Invitrogen) with 2% FBS, 1 μm tRA, and 1 mm 8-bromo-cAMP for up to 7 days. The cells were fed every 2 days with fresh medium. The cells were split again at a subcultivation ratio of 1:10 2 days after the first seeding. JEG3 cells, MCF7 cells, and SH-SY5Y cells were purchased from ATCC and maintained as recommended.
Chemicals
Synthetic retinoids were synthesized as described (17, 18), dissolved in dimethyl sulfoxide to 10−2 m, and stored at −20 °C. Restriction enzymes and DNA modification enzymes were purchased from New England Biolabs (Ipswich, MA). Other chemicals were purchased from Sigma unless otherwise noted.
Reverse Transcription (RT)-PCR
Two-step quantitative RT-PCR was carried out by using the DNA Engene Opticon System (MJ Research, Waltham, MA) as described (19) with minor modifications. Briefly, total RNA from culture cells was prepared with the RNeasy minikit (Qiagen, Valencia, CA) with on-column DNase digestion. Three μg of total RNA was reverse-transcribed by using 50 units of Superscript III reverse transcriptase (Invitrogen) in a 20-μl reaction with oligo(dT)12–18 primer or random hexamer. Quantitative PCR of mouse thyroid hormone transporter genes, glyceraldehyde-3-phosphate dehydrogenase gene (Gapdh), 18 S ribosomal RNA, and human MCT8 mRNA was performed with custom DNA primers synthesized by Invitrogen (supplemental Table 1). Quantitative PCR of markers of extraembryonic endoderm and neural differentiation was carried out with the QuantiTect primer assay (Qiagen). Standard curves representing 6-point serial dilution of the corresponding control group were analyzed in each assay and used for calculation of relative expression values. RT-PCR of human GAPDH was performed as previously described (19). The sample quantifications were normalized by the internal control Gapdh or 18 S RNA. Conventional two-step RT-PCR of the 5′-untranslated region of Mct8 was performed with custom primers (supplemental Table 1) by using the Expand High Fidelity PCR system (Roche Applied Science). The cycle number was 35.
Thyroid Hormone Uptake Assay
Uptake of triiodothyronine (T3) and thyroxine (T4) was measured as described previously (20) with minor modifications. Cells, grown in 12-well plates, as well as empty wells for measurement of nonspecific binding of radiolabeled thyroid hormone to the surface of the side wall of the well were rinsed with 1 ml of Dulbecco's PBS, preincubated with 300 μl of Hanks' balanced salt solution with 0.1% bovine serum albumin (BSA) for 15 min at 37 °C, and the medium was replaced with 300 μl of preheated thyroid hormone uptake assay buffer. T3 uptake assay buffer contains Hanks' balanced salt solution, 0.1% BSA, 0.25 μCi/ml 125I-labeled T3 (MP Biomedicals, Solon, OH), and 1.0 nm T3. T4 uptake assay buffer contains Hanks' balanced salt solution, 0.1% BSA, 0.3 μCi/ml 125I-labeled T4 (MP Biomedicals), and 1.0 μm T4. Cells were incubated for 4–30 min at 37 °C in a humidified incubator, rinsed twice with 1 ml of ice-cold Dulbecco's PBS, and lysed with 200 μm passive lysis buffer (Promega). Radioactivity of the whole lysate as well as lysis buffer from the duplicate empty wells was measured in a γ-counter. The background count from the side wall was subtracted from the count of cell lysate. The count was then normalized to the cellular protein content measured in the same cells by using a Bio-Rad protein assay.
Genomic DNA Sequence Inspection
To determine putative RARE, consensus half-sites, (A/G)G(G/T)(A/T)CA as well as other reported half-sites (5) were searched on both strands of the mouse Mct8 locus (NT_000086) by using MacMolly Tetra Lite (Mologen, Berlin, Germany) as described (21). CpG islands around the transcription start site (TSS) of Mct8 were predicted by the CpG island searcher (hosted on the worldwide web by the laboratory of Dr. P. A. Jones at University of Southern California (USC)) with the following parameters: observed/expected ratio, >0.65; %GC, >50; length, >200 (22). Basic transcription factor binding sites (23, 24) were searched around the TSS of Mct8 by using MacMolly Tetra Lite.
5′-Rapid Amplification of cDNA Ends (RACE)
Total RNA from F9 cells treated with 1 μm tRA for 72 h was isolated with the RNeasy Plus kit (Qiagen). The 5′-end of the Mct8 cDNA was identified by the oligo-capping and RNA ligase-mediated RACE method with the GeneRacer kit (Invitrogen) according to the manufacturer's instructions. Briefly, 5 μg of total RNA was dephosphorylated, decapped, and ligated to GeneRacer RNA oligonucleotides. Reverse transcription was carried out with random primer by using SuperScript III reverse transcriptase (Invitrogen). The 5′-ends of Mct8 were amplified from the cDNA pool as a template with the GeneRacer 5′-primer and +496 primer (supplemental Table 1). Nested PCR was then performed with the GeneRacer 5′-nested primer and the +496 primer. PCR products were cloned into pCR4-TOPO (Invitrogen) and analyzed by automated DNA sequencer (Laguna Scientific Laboratory, Laguna Niguel, CA).
Plasmid Construction
To generate constructs for screening of functional RARE on mouse Mct8, annealed synthetic oligonucleotides, containing the putative RAREs as well as an exogenous BamHI site for confirmation of cloning (supplemental Table 1), were inserted to the SmaI site of the pGL3 promoter vector (Promega, Madison, WI). A DNA fragment of the Mct8 5′-flanking region (−976 to −54; +1 is A in the translation start site) was obtained by genomic PCR from F9 cells with a forward primer (5′-CAGATCTTTCGTGCCTCCCTCCTTTC-3′) and a reverse primer (5′-CAAGCTTGTTTCTGCTGCTACTGCTCCT-3′). To generate pGL3 −976/−54, the PCR products were ligated into the polylinker site of the pGL Basic vector (Promega) with BglII and HindIII. pGL3 −836/−54 and pGL3 −659/−54 were constructed from the pGL3 −976/−54 by deletion of the sequences between ApaI and BglII sites and AgeI and BglII sites, respectively, both of which were treated with T4 DNA polymerase. pGL3 DR5A −976/−54 and pGL3 DR5A −836/−54 were constructed by blunt-end ligation of annealed oligonucleotides of the DR5A (supplemental Table 1) into the SmaI site of the pGL3 −976/−54 and the pGL3 −836/−54, respectively. Mutation of the DR5A element in pGL3 DR5A −836/−54 was generated by using the QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) with custom primers from Invitrogen (supplemental Table 1). Sequences of the constructed vectors were analyzed by an automated DNA sequencer (Laguna Scientific Laboratory).
Transient Expression Analysis
Cells (500–600 cells/well) were seeded in 12-well dishes 24 h before transfection. Unless otherwise noted, 0.2 μg of a Luciferase (Luc) reporter construct and 10 ng of a Renilla Luc reporter vector, pRL-CMV (Promega), were transfected to the cells by using Effectene (Qiagen, Valencia, CA) as recommended. The transfection medium was changed to growth media with or without RA at 24 h, and the Luc assay was performed at 48 h with a dual Luc reporter assay system (Promega). Results of the Luc reporter assay were normalized to Renilla Luc expression.
Chromatin Immunoprecipitation (ChIP) Assay
F9 cells, grown in 100-mm Petri dishes, were treated with tRA (1 μm) for up to 90 min and fixed with 1% formaldehyde. The ChIP assay was carried out with the ChIP-IT express enzymatic kit (Active Motif, Carlsbad, CA) as recommended. Partially digested chromatin was immunoprecipitated with 3 μg of anti-RARα (C-20), anti-RARβ (C-19), or anti-RXRα (D-20) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) overnight at 4 °C. Eluted DNA, as well as the aliquot of sheared chromatin prior to immunoprecipitation (input), was amplified by using the Expand high fidelity PCR system (Roche Applied Science) with primers specific to an RA-responsive region of mouse Mct8 or the mouse Lat1 (L-type amino acid transporter 1) promoter (supplemental Table 1). PCR cycle numbers for Mct8 and Lat1 were 40 and 37, respectively. The amplicons were analyzed by electrophoresis in a 2% agarose gel.
RESULTS
Induction of Mct8 mRNA by RA in F9 Cells
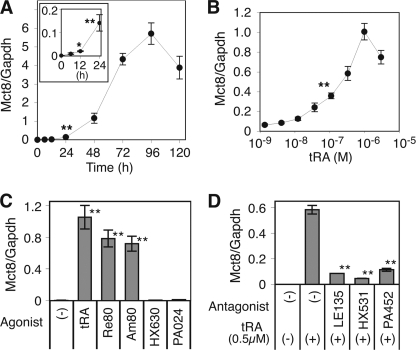
tRA treatment markedly increased Mct8 mRNA expression in F9 cells grown in media with 10% serum. A time course study (Fig. 1A) showed a significant induction of Mct8 mRNA by tRA (1 μm) at 12 h (∼9.5-fold), reaching a maximum at 96 h (∼1120-fold). The effect was dose-dependent with a 50% effective tRA concentration (EC50) of ∼1.47 × 10−7 m and maximum induction at an RA concentration of 10−6 m (Fig. 1B). In the presence of 10% charcoal-stripped FBS, the EC50 for tRA was ∼1.35 × 10−7 m.
FIGURE 1.
Induction of Mct8 mRNA expression by retinoids in F9 cells. Results of quantitative RT-PCR for Mct8 and Gapdh mRNA are shown. A, time course of the induction of Mct8 mRNA by tRA. The inset graph shows induction within the first 24 h. Cells were treated with tRA (1 μm) for the indicated times. B, dose dependence of the induction of Mct8 mRNA by tRA. Cells were treated with the indicated concentration of tRA for 48 h. C, cells were treated with the indicated retinoid receptor agonist (1 μm) for 48 h (Re80 and Am80-RAR agonists, HX630 and PA024-RXR agonists). D, cells were treated with or without tRA (0.5 μm) and the indicated retinoid receptor antagonist (10 μm) for 48 h (LE135-RAR antagonist, HX531 and PA452-RXR antagonists). Treatment with tRA (1 μm) for 48 h was used to generate standard curves to quantify Mct8 and Gapdh. F9 cells were maintained in DMEM with 10% FBS. Values are expressed as means ± S.D. (error bars) (n = 3). *, p < 0.05; **, p < 0.01, when compared with untreated cells (A–C) or cells with only tRA (D).
tRA is a potent agonist of RAR, although some tRA is converted intracellularly to 9-cis-RA, an agonist of RXR. Undifferentiated F9 cells express both RAR and RXR. To determine which receptor is required for Mct8 induction, we utilized synthetic retinoid receptor agonists (17, 18). Treatment with the pan-RAR agonist, Re80 (1 μm), or with an RARα/β-specific agonist, Am80 (1 μm), mimicked the effects of tRA on Mct8 mRNA expression. No significant induction was observed with the pan-RXR agonists, HX630 and PA024 (Fig. 1C). The induction of Mct8 by tRA was significantly inhibited by an RAR antagonist, LE135, as well as RXR antagonists HX531 and PA452 (Fig. 1D). These data indicate that stimulation of RAR is required for the induction of Mct8 in F9 cells. Expression of RXR, but not stimulation by ligand, is also probably required for the up-regulation of Mct8.
Both RAR and RXR are expressed in many types of cancer cells, including MCF-7 breast cancer cells (25) and SH-SY5Y neuroblastoma cells. Our quantitative RT-PCR study indicated abundant Mct8 expression in untreated SH-SY5Y cells (∼36% of the level in tRA-treated F9 cells) (Table 1). tRA, however, did not significantly increase the MCT8 mRNA expression in SH-SY5Y or MCF7 cells (Table 1). JEG3 cells express abundant RXR but not RAR, and treatment with tRA did not induce MCT8 (Table 1).
TABLE 1.
Expression of MCT8 mRNA in various cancer cell lines
Cells were treated with or without tRA (1 μm) for 48 h, and quantitative RT-PCR of Mct8 and Gapdh was performed. For comparison of relative Mct8 expression among the cell lines, Mct8 expression was quantified with standard curves of Gapdh in each cell line and condition, and the average of F9 cells treated without tRA was set as 1. Values are means ± S.D. (n = 3).
| −tRA | +tRA | +tRA/−tRA | |
|---|---|---|---|
| F9 | 1.00 ± 0.08 | 77.4 ± 4.46a | 76.9 ± 8.32 |
| JEG3 | 2.03 × 10−4 ± 3.27 × 10−5 | 1.41 × 10−4 ± 8.50 × 10−5 | 0.74 ± 0.05 |
| MCF7 | 0.30 ± 0.01 | 0.51 ± 0.04 | 1.71 ± 0.19 |
| SH-SY5Y | 27.8 ± 3.34 | 20.5 ± 3.54 | 0.73 ± 0.04 |
a p < 0.01, when compared with untreated cells.
Uptake of Thyroid Hormones in tRA-treated F9 Cells
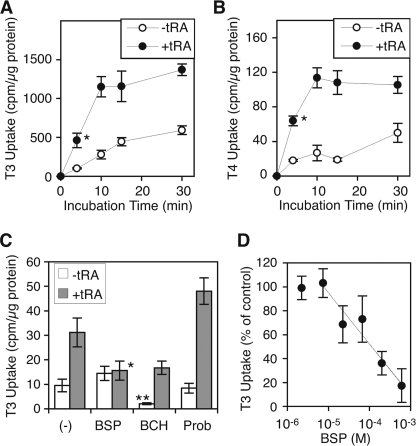
Transfection of Mct8 into mammalian cells induces uptake of both T3 and T4 (20). To investigate if the induction of endogenous Mct8 by tRA increases functional Mct8 expression, we measured the accumulation of T3 as well as T4 in F9 cells treated with or without tRA (1 μm) for 6 days. Cells were incubated with 125I-labeled T3 or T4 for 4–30 min in the presence of 0.1% BSA as a carrier. T3 uptake in tRA-treated cells was significantly increased in the first 4 min and partially saturated at 10 min (Fig. 2A). T3 uptake in tRA-treated cells at 10 min was significantly higher (∼4.1-fold) than that in untreated cells (Fig. 2A). T4 uptake was also significantly increased in the first 4 min and saturated at 10 min (Fig. 2B). T4 uptake in tRA-treated cells at 10 min was significantly higher (∼4.3-fold) than that in untreated cells (Fig. 2B). These results are consistent with previous data of thyroid hormone uptake in mammalian cells transfected with vectors expressing Mct8 (20).
FIGURE 2.
Uptake of thyroid hormones by F9 cells. Cells were treated with or without tRA (1 μm) in the presence of 10% FBS for 6 days before the assay. A and B, time courses of T3 accumulation (A) and T4 accumulation (B). Cells were incubated with the 125I-labeled T3 uptake buffer (A) or 125I-labeled T4 uptake buffer (B) for the indicated times. Values are expressed as means ± S.D. (n = 4). *, p < 0.01, when compared with untreated cells. C, pharmacological inhibition of T3 uptake. Cells were incubated with the 125I-labeled T3 uptake buffer for 10 min in the presence of BSP (Mct8 inhibitor), BCH (Lat inhibitor), or probenecid (Prob, pan-Oatp inhibitor) (1 mm each). Values are expressed as means ± S.D. (n = 3). *, p < 0.05, when compared with +tRA/−inhibitor. **, p < 0.02, when compared with −tRA/−inhibitor or +tRA/+BCH. D, dose dependence of BSP inhibition of tRA-induced T3 uptake. Cells were incubated with 125I-labeled T3 uptake buffer in the presence of BSP at the indicated concentrations. T3 uptake without BSP was simultaneously measured in tRA-treated and -untreated cells. Uptake, normalized to protein content, without BSP or tRA was subtracted from uptake at each concentration of BSP in tRA-treated cells, and uptake without BSP in tRA-treated cells was set at 100%. Values are expressed as means ± S.D. (error bars) (n = 3). The best fit line (R2 = 0.93) is shown.
Although Mct8 is one of the most efficient transporters of both T3 and T4, several other transporters have also been reported to mediate thyroid hormone uptake, including other SLC family members, Mct10 (or SLC16A10) (8), sodium/taurocholate-cotransporting polypeptide-1 (NTCP1, or SLC10A1), L-type amino acid transporter-1 (LAT1, or SLC7A5) and LAT2 (or SLC7A8) (9), and eight of the 15 members of the Oatp/Slco family (9, 11). To determine if the tRA-induced thyroid hormone uptake in F9 cells is mediated by Mct8, we utilized pharmacological inhibitors of thyroid hormone transporters (26). An inhibitor of T3 uptake by Mct8, bromosulfophthalein (BSP), but not a broad spectrum inhibitor of Oatp, probenecid, significantly reduced the tRA-induced T3 uptake (Fig. 2C) with an IC50 of 112 μm (Fig. 2D). Modest T3 uptake was observed in the cells without tRA treatment, although it was not inhibited by BSP or probenecid (Fig. 2A). A Lat inhibitor, 2-amino-2-norbornane carboxylic acid (BCH), significantly reduced the T3 uptake in both tRA-treated and -untreated F9 cells, whereas the induction by tRA was not abolished (Fig. 2C). These data suggest that the basal modest T3 uptake is mediated by Lat, whereas tRA-induced T3 uptake is dependent on the BSP-sensitive thyroid hormone transporter, Mct8.
Expression of Other Thyroid Hormone Transporter Genes in F9 Cells
To determine if the tRA-induced thyroid hormone uptake (Fig. 2) was due to induction of Mct8, we measured the expression of mouse orthologs of other reported thyroid hormone transporter genes in response to tRA treatment in F9 cells (9, 11). The expression level of each gene was quantified by RT-PCR with a Gapdh standard for comparison of the relative expression among each gene.
tRA treatment for 96 h increased expression of Mct10 and Lat2 (Table 2), although the magnitudes of induction (∼2.1- and ∼4.6-fold, respectively) were much less than that of Mct8 (∼678-fold). Interestingly, abundant expression of Lat1 (∼7 × 10−2-fold compared with Gapdh), as well as modest expression of Oatp4a1 (∼10−3-fold compared with Gapdh), was observed in both tRA-treated and untreated F9 cells, although tRA did not significantly influence the expression of these transporters (Table 2). Expression levels of Ntcp1 and the other seven Oatp genes were relatively small (less than 5 × 10−5-fold compared with Gapdh) and were not significantly increased by the tRA treatment (Table 2). These data indicate that, among the thyroid hormone transporter genes tested in F9 cells, only Mct8 was markedly induced by tRA.
TABLE 2.
Expression of thyroid hormone transporter genes in F9 cells
Cells were treated with or without tRA (1 μm) for 96 h, and quantitative RT-PCR of the indicated genes was performed. For comparison among the genes tested, expression of each gene was quantified with standard curves of Gapdh from cDNA mixture of +tRA and −tRA. Values are means ± S.D. (n = 3). NA, not applicable.
| −tRA | +tRA | +tRA/−tRA | |
|---|---|---|---|
| Mct8 (Slc16a2) | 1.92 × 10−5 ± 1.29 × 10−6 | 1.29 × 10−2 ± 1.25 × 10−3a | 678.1 ± 78.6 |
| Mct10 (Slc16a10) | 1.51 × 10−4 ± 3.73 × 10−5 | 3.11 × 10−4 ± 2.78 × 10−5a | 2.05 ± 0.53 |
| Ntcp1 (Slc10a1) | <10−6 | <10−6 | NA |
| Lat1 (Slc7a5) | 7.26 × 10−2 ± 2.11 × 10−3 | 6.68 × 10−2 ± 2.08 × 10−3 | 0.96 ± 0.18 |
| Lat2 (Slc7a8) | 8.83 × 10−5 ± 5.51 × 10−6 | 4.08 × 10−4 ± 5.49 × 10-6a | 4.63 ± 0.21 |
| Oatp (Slco) 1a1 | 4.66 × 10−5 ± 5.23 × 10−7 | 3.37 × 10−5 ± 3.21 × 10−6 | 0.72 ± 0.05 |
| Oatp (Slco) 1a4 | <10−6 | <10−6 | NA |
| Oatp (Slco) 1a5 | <10−6 | <10−6 | NA |
| Oatp (Slco) 1b2 | 1.10 × 10−5 ± 6.45 × 10−7 | 5.20 × 10−6 ± 4.26 × 10−7 | 0.47 ± 0.05 |
| Oatp (Slco) 1c1 | <10−6 | <10−6 | NA |
| Oatp (Slco) 4a1 | 1.45 × 10−3 ± 9.48 × 10−5 | 2.35 × 10−4 ± 2.15 × 10−5 | 0.16 ± 0.002 |
| Oatp (Slco) 6b1 | <10−6 | <10−6 | NA |
| Oatp (Slco) 6c1 | <10−6 | <10−6 | NA |
| Gapdh | 1.29 ± 0.02 | 0.93 ± 0.14 | 0.72 ± 0.07 |
a p < 0.01, when compared with −tRA.
Differentiation Status and Mct8 Gene Expression in F9 Cells
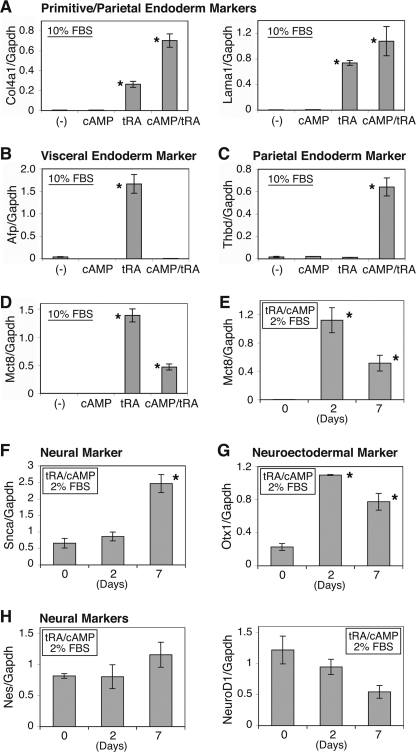
Treatment of F9 cells with tRA and/or cAMP under normal (10%) serum conditions induces extraembryonic endoderm, primitive, parietal, and visceral (13, 14). Combination treatment with tRA and cAMP, under low serum conditions, induces morphologically neuron-like cells with neuron-specific acetylcholinesterase activity (15). tRA treatment induced primitive/visceral endoderm differentiation markers, Col4a1, Lama1, and Afp, in F9 cells, whereas the combination with tRA and 8-bromo-cAMP induced parietal endoderm differentiation markers, Col4a1, Lama1, and Thbd (Fig. 3, A–C), consistent with previous reports (13, 14). The expression of Mct8 mRNA was markedly increased with any extraembryonic endoderm differentiation induced, although the addition of 8-bromo-cAMP modestly reduced the magnitude of induction (Fig. 3D). The combination treatment of tRA and 8-bromo-cAMP in low serum (2%) conditions significantly induced Mct8 (∼354-fold; Fig. 3E) as well as a neural differentiation marker, Snca (Fig. 3F), and a neuroectodermal marker, Otx1 (Fig. 3G), but not other neural markers, Nes or NeuroD1 (Fig. 3H).
FIGURE 3.
Expression of differentiation markers and Mct8 mRNA in F9 cells. A–D, induction of markers of endoderm differentiation in F9 cells. Cells were grown in DMEM supplemented with 10% FBS and treated with or without tRA (1 μm) and/or 8-bromo-cAMP (1 mm) for 96 h, and quantitative RT-PCR of the indicated genes was performed. Quantification of those genes as well as the internal control Gapdh was obtained from standard curves of serial dilution series of cDNA mixture of tRA-treated cells and tRA/cAMP-treated cells. The sample quantification was then normalized to Gapdh. E–H, induction of neural differentiation markers in F9 cells. Cells were treated with or without tRA (1 μm) and 8-bromo-cAMP (1 mm) in DMEM/F-12 (50:50) with 2% FBS for 2 days or 7 days, and quantitative RT-PCR of the indicated gene was performed. Quantification was obtained from standard curves of serial dilution series of cDNA at day 2 and normalized to Gapdh. Values are expressed as means ± S.D. (n = 3). *, p < 0.01, when compared with untreated cells (day 0). Col4a1, collagen type IV α-1; Afp, α-fetoprotein, Lama1, laminin α1; Thbd, thromobomodulin; Mct8, monocarboxylate transporter 8; Snca, synuclein α; Otx1, orthodenticle homeobox 1; Nes, nestin; NeuroD1, neurogenic differentiation 1.
Transcriptional Regulation of Mct8 Expression in F9 Cells
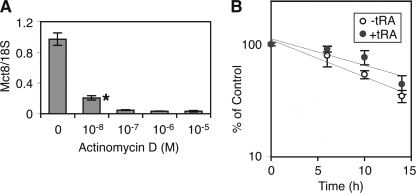
To determine if the effects of tRA on Mct8 expression are at the transcriptional level, we utilized a transcription inhibitor, actinomycin D. Treatment with actinomycin D for 14 h significantly reduced the tRA-induced Mct8 expression (Fig. 4A). A time course study demonstrated that degradation of Mct8 mRNA in tRA-treated F9 cells was slightly slower than that in untreated cells, although the difference was not significant (p = 0.062; Fig. 4B). The half-life of Mct8 mRNA in tRA-treated F9 cells and untreated cells was 15.0 ± 2.53 and 10.3 ± 1.15 h, respectively. Treatment with a protein synthesis inhibitor, cycloheximide, did not significantly decrease the Mct8 mRNA expression in F9 cells treated with tRA (1 μm) for 24 h (data not shown). These data indicate that tRA stimulates Mct8 expression predominantly at the transcriptional level, without a requirement for newly synthesized protein induced by tRA.
FIGURE 4.
Effects of a transcription inhibitor, actinomycin D, on Mct8 mRNA expression in F9 cells. A, cells were treated with tRA (1 μm) and the indicated concentration of actinomycin D for 21 h, and quantitative RT-PCR of Mct8 was performed. Results were normalized to 18 S ribosomal RNA. Values are expressed as means ± S.D. (n = 3). *, p < 0.01, when compared with control (0 m). B, effects of tRA on degradation of Mct8 mRNA in F9 cells. To induce abundant Mct8, cells were pretreated with tRA (1 μm) for 24 h. Cells were rinsed twice with Dulbecco's PBS, cultured in growth media for 48 h, treated with actinomycin D (10 μm) for 1 h, and then treated with or without tRA (1 μm) in the presence of actinomycin D for the indicated time. Results of quantitative RT-PCR of Mct8 were normalized to 18 S ribosomal RNA. Data at 0 h were set at 100%. Values are expressed as means ± S.D. (n = 3).
TSSs of the Mct8 Gene in F9 Cells
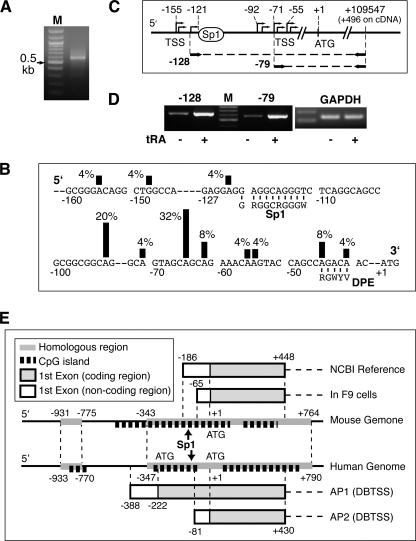
To elucidate the mechanism of transcriptional regulation by RA, the proximal promoter region of Mct8 was characterized. We determined the TSS of Mct8 in F9 cells by oligo-capping and RNA ligase-mediated 5′-RACE, in which cDNA from full-length 5′-capped mRNA, but not incomplete mRNA, is selectively amplified (27). The identified 5′-end of cDNA, therefore, represents the TSS. PCR amplification of the RACE-ready cDNA from tRA-treated F9 cells with a 5′ adaptor primer and a gene-specific primer (+496, relative to the translation start site of Mct8) produced DNA fragments of around 600 bp (Fig. 5A). Sequence analysis of 25 clones, following ligation of the PCR products into a pCR4 vector, identified 11 TSSs between −155 and −41 (Fig. 5B). A highly utilized TSS has a consensus dinucleotide sequence, pyrimidine-purine, especially CG, TG, or CA (23). Eight of 11 determined TSSs, including the two most used TSSs at −65 and −92, conserve the dinucleotide consensus (Fig. 5B). Although the TSS of the reference sequence of Mct8 mRNA in the National Center for Biotechnology Information data base (MN_009197) is at −186, no clone contained the sequence around −186.
FIGURE 5.
TSS of Mct8 in F9 cells. A, results of 5′-RACE from tRA-treated F9 cells. Shown is agarose gel electrophoresis of the second round PCR with GeneRacer 5′ nested primer and 3′ +496 primer. Marker (M) was a 100-bp ladder. B, distribution of the TSS of Mct8 in F9 cells. The sequence of the 5′-flanking region of Mct8 is shown. The positions of the identified TSSs are marked with vertical lines, the height of which indicates the frequency of RACE clones found to initiate at each site. Putative core promoter elements are shown. DPE, downstream core promoter element. C, diagram of the promoter region and amplification of upstream transcripts, including the Sp1 site (−128) and downstream transcripts without the Sp1 site (−79). D, determination of the influence of tRA treatment on transcripts. RT-PCR of the 5′ region of Mct8 in F9 cells treated with or without tRA (1 μm) for 48 h is shown. The internal control, Gapdh, is shown on the right. E, comparison of TSS positions in the mouse and human MCT8 genes. Alternative first exons, CpG islands, canonical Sp1 sites (arrow), and homologous regions between the two species are shown. TSSs of mouse Mct8 as well as that of the short variation of human MCT8 flanked by AP2 (alternative promoter 2) (DBTSS) are located in CpG islands in high homologous regions (79%) between the two species. The long variation of human MCT8, flanked by AP1 (DBTSS), is transcribed from a human-specific region outside of the CpG island.
Two isoforms of human MCT8 have been reported: an isoform with high homology to mouse Mct8 and a longer isoform with an additional 74-amino acid-long human specific sequence at the N-terminal portion (7). A comprehensive study of TSS in the human genome (28, 29) has shown at least two putative alternative promoters corresponding to the Mct8 mRNA variants. The 5′-flanking region of mouse Mct8 from −343 to +1, containing every TSS detected, had a strong homology (83.2%) with one of the human alternative promoters, AP2, controlling the shorter variant of human MCT8 (Fig. 5E).
Sequence inspection of the core promoter elements indicated that the 5′-flanking region of Mct8 contained a canonical Sp1 site, between −120 and −110 (GRGGCRGGGW), but no TATA box (Table 3). An epigenetically regulatable CpG island was found between −404 and +124, included in all TSSs determined in our experiments (Fig. 5E). These results are compatible with a recent consensus that most of the TATA-less (GC-rich) core promoters initiate transcription at multiple sites and are located in a CpG island (23).
TABLE 3.
Inspection for core promoter elements in mouse Mct8 and its human ortholog
| Core promoter elementsa | Consensus sequence | Mouse Mct8 | Human MCT8 |
|---|---|---|---|
| D-BRE | RTDKKKK | NPb | NP |
| U-BRE | SSRCGCC | NP | NP |
| Dinucleotide (−1/+1) | YR (Py-Pu) | 8 of 11 TSS | At −81c |
| DPEd | RGWYVT | Between −45 and −41 | Between −15 and −11c |
| Inr | YYANWYY (YYRRWYY) | NP | NP |
| MTE | CSARCSSAAC | NP | NP |
| Sp1 | GRGGCRGGGW | Between −121 and −112 | Between −116 and −107c |
| TATA box | TATAWAAR | NP | NP |
| XCPE1 | DSGYGGRASM | NP | NP |
a U-BRE, upstream-TFIIB (transcription factor for RNA polymerase IIB) recognition element; D-BRE, downstream-BRE; DPE, downstream core promoter element; Inr, initiator; MTE, motif ten element; XCPE1, X core promoter element-1.
b NP, sequence not present.
c A in the translation start site of the short variation of MCT8 mRNA is +1 (see Fig. 5E).
d A role in transcription and the consensus sequence of DPE are still controversial (23).
A majority (88%) of determined TSSs in the Mct8 promoter were mapped to a region downstream of the proximal Sp1 site (Fig. 5B), suggesting a role of Sp1 in the Mct8 promoter activation. Full induction of several genes by RA requires Sp1 (30–32). To assess whether the up-regulation of Mct8 by RA requires the Sp1 site, we performed RT-PCR of the 5′-untranslated region of Mct8 in F9 cells treated with or without tRA (Fig. 5C). The expression of transcripts containing the Sp1 site was increased by tRA treatment (Fig. 5D), indicating that transcription from TSSs upstream of the Sp1 site is also up-regulated by RA. The Sp1 site, therefore, is not required for tRA induction.
Functionality of the Mct8 Promoter in F9 Cells
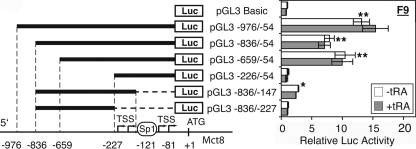
The 5′-flanking region of mouse Mct8 was evaluated for promoter activity in firefly Luc reporter vectors containing progressive deletion mutants of the 5′-flanking region from −976 to −226. Significant promoter activity (7.9–13.1-fold compared with the background) was observed in the constructs containing the fragment between −659 and −54 (pGL3 −976/−54, pGL3 −836/−54, and pGL3 −659/−54; Fig. 6), whereas the deletion of the sequence between −659 and −227 completely abolished the promoter activity (pGL3 −226/−54; Fig. 6). Deletion of the 3′ portion of the 5′-flanking region (from −146 to −54), containing the Sp1 site, significantly decreased the promoter activity, although a modest activity (∼2.8-fold) was still observed (pGL3 −836/−147; Fig. 6). Further deletion of the 3′ side from −226 to −147 abolished the promoter activity (pGL3 −836/−227; Fig. 6). These results demonstrate that the sequence from −659 to −54, including the proximal Sp1 site, is necessary for full promoter activity. This is consistent with the results of TSS mapping, showing that the majority of TSSs are located downstream of the Sp1 site (Fig. 5B).
FIGURE 6.
Activity of the Mct8 proximal promoter in F9 cells. Cells were transfected with the indicated Luc reporter vector and pRL-CMV, treated with or without tRA (1 μm) for 24 h, and harvested for the luciferase assay. Data of pGL3 Basic in untreated F9 cells were set at 1. Values are means ± S.D. (n = 3). *, p < 0.05; **, p < 0.01, when compared with pGL3 Basic without tRA.
tRA induced Mct8 expression (Figs. 4 and 5D), whereas tRA did not significantly increase proximal promoter activity (Fig. 6). RAREs have been reported both within the proximal promoter (5, 33, 34) and outside of the proximal promoter (35, 36).
RARE in the Locus of the Mouse Mct8 Gene
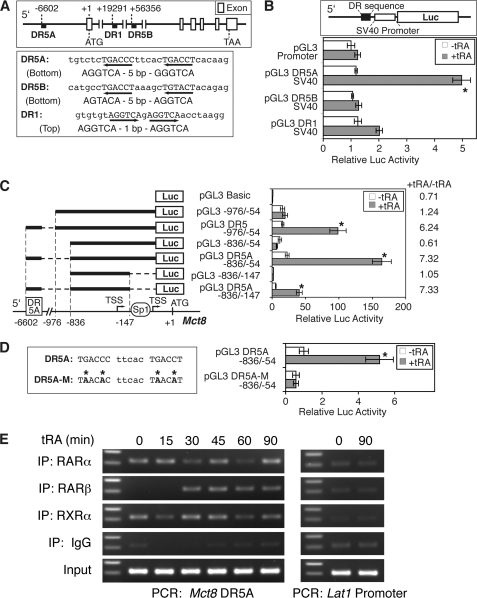
To determine an RA-responsive enhancer in the Mct8 locus, we inspected the mouse genomic sequence from −10,724 to +134,953, including exon and introns, and more than 10,000 bp of flanking regions (Fig. 7A) for consensus sequences of RARE, DR-1, DR-2, and DR-5 (5). Based on the half-site sequences and configuration (21), we identified three candidate elements: two DR-5 elements, DR5A at −6602 (in the 5′-flanking region) and DR5B at +56,356 (in the first intron), and one DR-1 element at +19,291 (in the first intron) (Fig. 7A).
FIGURE 7.
Characterization of mouse Mct8 RARE in F9 cells. A, location and sequence of putative RAREs in the mouse Mct8 locus. B, functional analysis of putative RAREs in F9 cells. Three putative RAREs were fused to a heterologous SV40 promoter in Luc reporter vector constructs (upper panel) and transfected into F9 cells with pRL-CMV. Cells were treated with or without tRA (1 μm) for 24 h, and the luciferase assay was performed. Normalized data of the pGL promoter (−tRA) were set at 1. C, enhancer activity of the DR5A RARE with the Mct8 proximal promoter. Cells were transfected with the indicated Luc reporter constructs as well as pRL-CMV, treated with or without tRA (1 μm) for 24 h, and harvested for the luciferase assay. Normalized data of pGL3 Basic (−tRA) were set at 1. D, mutation analysis of the DR5A RARE. In the left panel, mutated bases in the DR5A RARE are indicated by an asterisk. Cells were transfected with the mutated vector (pGL3 DR5A-M −836/−54) or the original vector with wild DR5A (pGL3 DR5A −836/−54) and treated with or without tRA (1 μm) for 24 h. Normalized data of pGL3 DR5A −836/−54 (−tRA) were set at 1. Values are means ± S.D. (n = 3). *, p < 0.01, when compared with −tRA in each vector. E, binding of endogenous retinoid receptors to the region of DR5A RARE. F9 cells were treated with tRA (1 μm) for the indicated time, and a ChIP assay was performed with the indicated antibodies or preimmune IgG. The DR5A region, as well as the tRA-unresponsive Lat1 promoter, was amplified by PCR from the immunoprecipitated (IP) chromatin and analyzed by agarose gel electrophoresis. Expected amplicon sizes were 217 and 203 bp, respectively. The DNA size marker indicates 300 and 200 bp.
The three putative RAREs were inserted into a Luc reporter vector with the heterologous SV40 promoter driving the reporter gene expression (Fig. 7B) and evaluated in F9 cells for tRA induction. Our transient transfection study indicated a significant tRA response to the DR5A at −6602 (∼4.2-fold) but not the DR5B or DR1 (Fig. 7B).
To determine if the identified RARE enhances the mouse Mct8 promoter, we fused the DR5A element upstream of the Mct8 5′-flanking sequence (−976 to −54 or −836 to −54) in Luc reporter vectors and transfected it into F9 cells. tRA treatment (1 μm) significantly increased the Mct8 promoter activity (6.2–7.3-fold) with DR5A (pGL3 DR5A −976/−54 and pGL3 DR5A −836/−54; Fig. 7C) but not without DR5A (pGL3 −976/−54 and pGL3 −836/−54; Fig. 7C). Mutations of the DR5A element completely abolished induction by tRA (Fig. 7D), indicating the critical role for the tRA response. The function of RARE was observed even in the Sp1 element-deleted construct (pGL3 DR5A −836/−147; Fig. 7C), consistent with the tRA induction of Mct8 transcripts from the TSSs upstream of the Sp1 site (Fig. 5D).
tRA treatment did not increase endogenous Mct8 mRNA expression in JEG3 cells (Table 1). Significant activity of the Mct8 proximal promoter was observed in JEG3 cells; however, tRA did not enhance the DR5A RARE (0.8 ± 0.1-fold), even with exogenous RARα expression. This suggests that RA induction of Mct8 is cell type-specific and may require specific factors to be present or be susceptible to inhibitors.
To determine if retinoid receptors bound directly to the DR5A element, we performed a ChIP assay in F9 cells treated with tRA at various time points (0–90 min). The binding of RARβ to the DR5A region was induced by tRA in 30 min, whereas both RARα and RXRα bound consistently, even in the absence of tRA treatment (Fig. 7E). In contrast, no significant binding of RARα, RARβ, or RXRα was observed in the promoter region of Lat1 (Fig. 7E), with no putative RARE or induction of endogenous mRNA by tRA (Table 2). These data demonstrate direct binding of RAR and RXR to the DR5A region in F9 cells.
DISCUSSION
We have shown that T3 and T4 uptake is significantly increased in F9 teratocarcinoma cells differentiated by tRA. A significant reduction of the tRA-induced T3 uptake by a selective Mct8 inhibitor suggests a dependence of the thyroid hormone uptake on Mct8. Transcription of Mct8 is markedly induced, at least partially by RAR stimulation of a DR5 RARE identified 6.6 kilobases upstream of the coding region. The induction was dependent on RA treatment, but not limited by differentiation status, in F9 cells.
Five members of the Slc family, including Mct8, and eight members of the Oatp/Slco family have been reported as thyroid hormone transporters (11). We analyzed the expression levels of the known thyroid hormone transporter genes in F9 cells and found that only Mct8 was significantly induced by tRA. Modest inductions of Mct10 and Lat2, however, were also observed in the tRA-treated cells. Our T3 uptake study demonstrated that a Lat inhibitor, BCH, did not abolish the tRA-induced T3 uptake, indicating a lesser contribution of the modest Lat2 induction to the increase of uptake. Mct8 transports both T3 and T4, whereas Mct10 transports predominantly T3 in mammalian cells (8). Because the tRA treatment induced uptake of both T3 and T4, the robust induction of Mct8, rather than the modest induction of Mct10, probably plays a predominant role in the induction of thyroid hormone uptake in tRA-treated F9 cells.
Modest thyroid hormone uptake in undifferentiated F9 cells was sensitive to the Lat inhibitor BCH but not a Mct8 inhibitor, BSP, or a broad spectrum Oatp inhibitor, probenecid, suggesting a central role of a Lat in the thyroid hormone uptake before the tRA treatment. Our RT-PCR in the undifferentiated F9 cells indicated abundant expression of Lat1 and much less Lat2 (∼1,000-fold less than Lat1). Those results indicate that Lat1 probably mediates the modest thyroid hormone uptake in undifferentiated F9 cells. Although Lat1 was originally reported as an amino acid transporter, the apparent Km for T3 is the lowest of any reported Lat1 substrate (7, 37). LAT1 is also important for cell growth and survival (38). Overexpression of LAT1 has been reported in some cancer tissues as well as many cell lines, including PA1 teratocarcinoma cells (39).
Some nuclear hormone receptors, such as those for glucocorticoid and androgen, reside predominantly in the cytoplasm as unliganded receptor bound to heat shock protein. The addition of ligand disrupts binding to heat shock protein and rapidly induces translocation of these receptors from the cytoplasm into the nuclei to induce target genes (40). Although ligand-induced translocation of RAR has been reported (41, 42), unliganded RAR is predominantly localized in the nucleus and binds to RAREs as a heterodimer with RXR, in association with co-repressors (34). Binding of ligand changes the conformation of the RAR/RXR heterodimer, resulting in release of co-repressors and subsequent recruitment of co-activators (34). tRA strongly induces RARβ through an RARE in its promoter in association with RARα (33), whereas RAR binding to the RARE is not dependent on tRA (43, 44). Our ChIP assay in F9 cells showed binding of both RARα and RXRα to the RARE in Mct8, in the presence or absence of tRA. Induction of RARβ binding to the DR5A element by tRA, shown in our ChIP assay, may be correlated with the induction of RARβ expression by tRA. Our study with a protein synthesis inhibitor, cycloheximide, however, showed that de novo protein synthesis is not required for tRA induction of Mct8.
Recent genome-wide studies of mammalian RNA polymerase II core promoters have identified two classes of promoters, sharp type promoters and broad type promoters. About 20% of mammalian promoters are the sharp type promoter, containing a TATA box and a single TSS, whereas the others are broad type promoters, containing CpG island(s) with multiple TSSs but without a TATA box (23). Our analysis of the Mct8 promoter demonstrated that the promoter was TATA-less, containing a CpG island with more than 10 TSS, consistent with the broad type promoter.
Several consensus sequences have been identified in the eukaryotic core promoter region, although no universal element has yet been discovered (23). We found a canonical Sp1 site in the mouse Mct8 promoter, located 56 bases upstream of the most frequently used TSS at −65. The position of the putative Sp1 site is in the consensus region for its efficient functioning, 40–80 bases upstream of the TSS (45). Indeed, our analysis of the Mct8 promoter demonstrated the requirement of Sp1 site for full promoter activity, although the Sp1 site is not necessary for function of the Mct8 upstream RARE, DR5A.
Results of a genome-wide study of TSS in several species, based on the oligo-capping method with RNA from multiple organs (28, 29), are available on-line as the data base of transcriptional start sites (DBTSS; available on the World Wide Web). Our in silico search for TSS of mouse Mct8 with the DBTSS identified 12 TSSs between −211 and −81. The results, however, did not match any TSSs identified in our study. The discrepancy may be due to the difference in RNA origin; RNA used in the DBTSS is derived from brain, kidney, spleen, and joint but not testis, the origin of F9 cells. The results of a DBTSS search have shown distinct TSS of Mct8 among those tissues.
The structure of the mouse Mct8 proximal promoter was similar to that of the short variant of human Mct8. The functional RARE in the mouse is located around 6.6 kilobases upstream of the coding region. A homologous sequence to the mouse RARE was not found in the MCT8 locus in human; however, there is a similar RARE sequence in an intron of human MCT8. The difference in gene structure may reflect the differential regulation of Mct8 in various species and tissues. There is likely to be cell type specificity because we found RA induction of Mct8 only in F9 cells.
RA stimulates differentiation of F9 cells into extraembryonic tissues, parietal and visceral endoderm (13). These endoderm-derived tissues support the embryo and transport maternal nutrients as an “early placenta” between 5 and 10 days of gestation (16). Maternal retinol is transported to embryo/fetus through the visceral endoderm (46). TRα is expressed in embryo during the embryogenesis, even in the fertilized egg in Xenopus laevis (47), whereas overexposure of thyroid hormone to the embryo causes malformation (48). Visceral endoderm, therefore, might adjust the influx of thyroid hormone into embryonic layers and proamniotic cavity via Mct8, although the detailed pattern of in vivo expression of Mct8 in development has not been described.
F9 cells have been reported to differentiate into neuron-like cells with acetylcholinesterase activity (15). The marked Mct8 induction was observed in the neuron-like F9 cells. SH-SY5Y neuroblastoma cells expressed abundant Mct8 even without RA treatment. The simultaneous expression of Mct8 and neural differentiation markers is consistent with enhancement of neural differentiation by overexpression of Mct8 in embryonic stem cells (49).
The activity of many nuclear receptors is regulated by the intracellular concentration of its ligand(s), which is modulated by the ligand-selective transporter(s) expressed in the cell membrane. Regulation of such transporters by nuclear receptor signaling has been studied in cholesterol metabolism and inflammation pathways (50). We have demonstrated that RA signaling enhances the TR signaling pathway by up-regulating a thyroid hormone transporter, Mct8. The concept of developmental competence for the thyroid hormone signal in Xenopus metamorphosis, linked to TR and RXR expression, is well described (6, 51). This cooperative cross-talk of RA induction of thyroid hormone transport probably plays an important role in signaling pathways of extraembryonic endoderm and neural development.
Supplementary Material
Acknowledgments
We thank Drs. Vasanthi Narayan and Sun Wook Kim for assistance with the experiments and Dr. Jerome Hershman for helpful discussions.
This work was supported, in whole or in part, by Veterans Affairs Merit Review Funds. This work was also supported by National Institutes of Health Grant RO1 CA089364 (to G. A. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
- RAR
- retinoic acid receptor
- BCH
- 2-amino-2-norbornane carboxylic acid
- BSP
- bromosulfophthalein
- DBTSS
- database of transcriptional start sites
- DR
- direct repeat
- Lat
- L-type amino acid transporter
- Luc
- luciferase
- RACE
- rapid amplification of cDNA ends
- RARE
- retinoic acid response element
- RXR
- retinoid X receptor
- T3
- triiodothyronine
- T4
- thyroxine
- TR
- thyroid hormone receptor
- tRA
- all-trans-retinoic acid
- TSS
- transcription start site.
REFERENCES
- 1.Mark M., Ghyselinck N. B., Chambon P. (2009) Nucl. Recept. Signal. 7, e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen P. M. (2001) Physiol. Rev. 81, 1097–1142 [DOI] [PubMed] [Google Scholar]
- 3.Lee L. R., Mortensen R. M., Larson C. A., Brent G. A. (1994) Mol. Endocrinol. 8, 746–756 [DOI] [PubMed] [Google Scholar]
- 4.Liu Y. Y., Tachiki K. H., Brent G. A. (2002) Endocrinology 143, 2664–2672 [DOI] [PubMed] [Google Scholar]
- 5.Giguère V. (1994) Endocr. Rev. 15, 61–79 [DOI] [PubMed] [Google Scholar]
- 6.Furlow J. D., Neff E. S. (2006) Trends Endocrinol. Metab. 17, 40–47 [DOI] [PubMed] [Google Scholar]
- 7.Jansen J., Friesema E. C., Milici C., Visser T. J. (2005) Thyroid 15, 757–768 [DOI] [PubMed] [Google Scholar]
- 8.Friesema E. C., Jansen J., Jachtenberg J. W., Visser W. E., Kester M. H., Visser T. J. (2008) Mol. Endocrinol. 22, 1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Deure W. M., Peeters R. P., Visser T. J. (2007) Best Pract. Res. Clin. Endocrinol. Metab. 21, 339–350 [DOI] [PubMed] [Google Scholar]
- 10.Friesema E. C., Ganguly S., Abdalla A., Manning Fox J. E., Halestrap A. P., Visser T. J. (2003) J. Biol. Chem. 278, 40128–40135 [DOI] [PubMed] [Google Scholar]
- 11.Hagenbuch B. (2007) Best Pract. Res. Clin. Endocrinol. Metab. 21, 209–221 [DOI] [PubMed] [Google Scholar]
- 12.Chan S. Y., Franklyn J. A., Pemberton H. N., Bulmer J. N., Visser T. J., McCabe C. J., Kilby M. D. (2006) J. Endocrinol. 189, 465–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soprano D. R., Teets B. W., Soprano K. J. (2007) Vitam. Horm. 75, 69–95 [DOI] [PubMed] [Google Scholar]
- 14.Komiya S., Shimizu M., Ikenouchi J., Yonemura S., Matsui T., Fukunaga Y., Liu H., Endo F., Tsukita S., Nagafuchi A. (2005) Genes Cells 10, 1065–1080 [DOI] [PubMed] [Google Scholar]
- 15.Kuff E. L., Fewell J. W. (1980) Dev. Biol. 77, 103–115 [DOI] [PubMed] [Google Scholar]
- 16.Cross J. C., Werb Z., Fisher S. J. (1994) Science 266, 1508–1518 [DOI] [PubMed] [Google Scholar]
- 17.Kagechika H., Kawachi E., Hashimoto Y., Himi T., Shudo K. (1988) J. Med. Chem. 31, 2182–2192 [DOI] [PubMed] [Google Scholar]
- 18.Kagechika H., Kawachi E., Hashimoto Y., Shudo K. (1989) J. Med. Chem. 32, 834–840 [DOI] [PubMed] [Google Scholar]
- 19.Kogai T., Kanamoto Y., Li A. I., Che L. H., Ohashi E., Taki K., Chandraratna R. A., Saito T., Brent G. A. (2005) Endocrinology 146, 3059–3069 [DOI] [PubMed] [Google Scholar]
- 20.Friesema E. C., Kuiper G. G., Jansen J., Visser T. J., Kester M. H. (2006) Mol. Endocrinol. 20, 2761–2772 [DOI] [PubMed] [Google Scholar]
- 21.Kogai T., Ohashi E., Jacobs M. S., Sajid-Crockett S., Fisher M. L., Kanamoto Y., Brent G. A. (2008) J. Clin. Endocrinol. Metab. 93, 1884–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardiner-Garden M., Frommer M. (1987) J. Mol. Biol. 196, 261–282 [DOI] [PubMed] [Google Scholar]
- 23.Sandelin A., Carninci P., Lenhard B., Ponjavic J., Hayashizaki Y., Hume D. A. (2007) Nat. Rev. Genet 8, 424–436 [DOI] [PubMed] [Google Scholar]
- 24.Frith M. C., Valen E., Krogh A., Hayashizaki Y., Carninci P., Sandelin A. (2008) Genome Res. 18, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kogai T., Kanamoto Y., Che L. H., Taki K., Moatamed F., Schultz J. J., Brent G. A. (2004) Cancer Res. 64, 415–422 [DOI] [PubMed] [Google Scholar]
- 26.Wirth E. K., Roth S., Blechschmidt C., Hölter S. M., Becker L., Racz I., Zimmer A., Klopstock T., Gailus-Durner V., Fuchs H., Wurst W., Naumann T., Bräuer A., de Angelis M. H., Köhrle J., Grüters A., Schweizer U. (2009) J. Neurosci. 29, 9439–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama K., Sugano S. (1994) Gene 138, 171–174 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki Y., Yamashita R., Nakai K., Sugano S. (2002) Nucleic Acids Res. 30, 328–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakaguri H., Yamashita R., Suzuki Y., Sugano S., Nakai K. (2008) Nucleic Acids Res. 36, D97–D101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann J. M., Zhang X. K., Pfahl M. (1992) Mol. Cell. Biol. 12, 2976–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merchiers P., Bulens F., De Vriese A., Collen D., Belayew A. (1999) FEBS Lett. 456, 149–154 [DOI] [PubMed] [Google Scholar]
- 32.Horie S., Ishii H., Matsumoto F., Kusano M., Kizaki K., Matsuda J., Kazama M. (2001) J. Biol. Chem. 276, 2440–2450 [DOI] [PubMed] [Google Scholar]
- 33.de Thé H., Vivanco-Ruiz M. M., Tiollais P., Stunnenberg H., Dejean A. (1990) Nature 343, 177–180 [DOI] [PubMed] [Google Scholar]
- 34.Bastien J., Rochette-Egly C. (2004) Gene 328, 1–16 [DOI] [PubMed] [Google Scholar]
- 35.Bulens F., Merchiers P., Ibañez-Tallon I., De Vriese A., Nelles L., Claessens F., Belayew A., Collen D. (1997) J. Biol. Chem. 272, 663–671 [DOI] [PubMed] [Google Scholar]
- 36.Liu Y., Chen H., Chiu J. F. (1994) Mol. Cell. Endocrinol. 103, 149–156 [DOI] [PubMed] [Google Scholar]
- 37.Friesema E. C., Docter R., Moerings E. P., Verrey F., Krenning E. P., Hennemann G., Visser T. J. (2001) Endocrinology 142, 4339–4348 [DOI] [PubMed] [Google Scholar]
- 38.Fuchs B. C., Bode B. P. (2005) Semin. Cancer Biol. 15, 254–266 [DOI] [PubMed] [Google Scholar]
- 39.Yanagida O., Kanai Y., Chairoungdua A., Kim D. K., Segawa H., Nii T., Cha S. H., Matsuo H., Fukushima J., Fukasawa Y., Tani Y., Taketani Y., Uchino H., Kim J. Y., Inatomi J., Okayasu I., Miyamoto K., Takeda E., Goya T., Endou H. (2001) Biochim. Biophys. Acta 1514, 291–302 [DOI] [PubMed] [Google Scholar]
- 40.Hager G. L., Lim C. S., Elbi C., Baumann C. T. (2000) J. Steroid Biochem. Mol. Biol. 74, 249–254 [DOI] [PubMed] [Google Scholar]
- 41.Han Y. H., Zhou H., Kim J. H., Yan T. D., Lee K. H., Wu H., Lin F., Lu N., Liu J., Zeng J. Z., Zhang X. K. (2009) J. Biol. Chem. 284, 18503–18514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santos N. C., Kim K. H. (2010) Endocrinology 151, 2361–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flajollet S., Lefebvre B., Rachez C., Lefebvre P. (2006) J. Biol. Chem. 281, 20338–20348 [DOI] [PubMed] [Google Scholar]
- 44.Gillespie R. F., Gudas L. J. (2007) J. Mol. Biol. 372, 298–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smale S. T., Kadonaga J. T. (2003) Annu. Rev. Biochem. 72, 449–479 [DOI] [PubMed] [Google Scholar]
- 46.Johansson S., Gustafson A. L., Donovan M., Romert A., Eriksson U., Dencker L. (1997) Anat. Embryol. 195, 483–490 [DOI] [PubMed] [Google Scholar]
- 47.Banker D. E., Bigler J., Eisenman R. N. (1991) Mol. Cell. Biol. 11, 5079–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraft J. C., Willhite C. C., Juchau M. R. (1994) J. Craniofac. Genet. Dev. Biol. 14, 75–86 [PubMed] [Google Scholar]
- 49.Sugiura M., Nagaoka M., Yabuuchi H., Akaike T. (2007) Biochem. Biophys. Res. Commun. 360, 741–745 [DOI] [PubMed] [Google Scholar]
- 50.Teng S., Piquette-Miller M. (2008) Mol. Pharm. 5, 67–76 [DOI] [PubMed] [Google Scholar]
- 51.Brown D. D., Cai L. (2007) Dev. Biol. 306, 20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.