Abstract
The activity of transcription factor FoxO1 is regulated by phosphorylation-dependent nuclear exclusion and deacetylation-dependent nuclear retention. It is unclear whether and how these two post-translational modifications affect each other. To answer this question, we expressed FoxO1 cDNAs with combined mutations of phosphorylation and acetylation sites in HEK-293 cells and analyzed their subcellular localization patterns. We show that mutations mimicking the acetylated state (KQ series) render FoxO1 more sensitive to Akt-mediated phosphorylation and nuclear exclusion and can reverse the constitutively nuclear localization of phosphorylation-defective FoxO1. Conversely, mutations mimicking the deacetylated state (KR series) promote FoxO1 nuclear retention. Oxidative stress and the Sirt1 activator resveratrol are thought to promote FoxO1 deacetylation and nuclear retention, thus increasing its activity. Accordingly, FoxO1 deacetylation was required for the effect of oxidative stress (induced by H2O2) to retain FoxO1 in the nucleus. H2O2 also inhibited FoxO1 phosphorylation on Ser-253 and Thr-24, the key insulin-regulated sites, irrespective of its acetylation. In contrast, the effect of resveratrol was independent of FoxO1 acetylation and its phosphorylation on Ser-253 and Thr-24, suggesting that resveratrol acts on FoxO1 in a Sirt1- and Akt-independent manner. The dissociation of deacetylation from dephosphorylation in H2O2-treated cells indicates that the two modifications can occur independently of each other. It can be envisaged that FoxO1 exists in multiple nuclear forms with distinct activities depending on the balance of acetylation and phosphorylation.
Keywords: Energy Metabolism, Fusion Protein, Insulin, Intracellular Trafficking, Metabolism, Nuclear Translocation, Oxidative Stress, Phosphatase, PP2A
Introduction
FoxO1 and its closely related isoforms FoxO3A and FoxO4 are transcription factors characterized by a conserved winged helix (“forkhead”) DNA binding domain. Genetic epistasis experiments in Caenorhabditis elegans demonstrated a role for these proteins in insulin receptor signaling, spawning studies of their contribution to mammalian metabolism, cellular differentiation, and transformation (1). It is now recognized that FoxOs are critical regulators of hepatic gluconeogenesis (2, 3) and pancreatic β-cell function (4–8), in addition to differentiation of myotubes (9–11) and adipocytes (12). Moreover, the C. elegans FoxO ortholog DAF-16 is required for life extension caused by DAF-2 (insulin receptor) mutations, suggesting that FoxO has a role in longevity (13, 14).
FoxO activity is regulated by post-translational modifications that affect primarily its subcellular localization (15). Insulin and growth factor signaling inhibit FoxO via Akt-dependent phosphorylation and nuclear exclusion (16–18). Several additional serine/threonine kinases, such as Mst1 (19), Jnk (20), and Sgk promote or inhibit FoxO via nuclear translocation (20–22) or nuclear exclusion, respectively (23–25). A second regulatory layer is FoxO acetylation by p300, CBP (cAMP-response element-binding protein-binding protein), and PCAF (p300/CBP-associated factors) in response to oxidative stress or DNA binding (26–28), followed by deacetylation by class I and II histone deacetylases (26, 28–30), including Sirt1, the NAD+-dependent deacetylase encoded by the ortholog of yeast longevity gene Sir2 (31).
The effects of phosphorylation and acetylation on FoxO function have been studied extensively but separately. However, these two modifications are likely to occur concurrently in vivo and to reciprocally affect each other. In this study, we generated an allelic series of FoxO1 mutants containing changes to both acetylation and phosphorylation sites and analyzed their regulation in response to physiologic (insulin) and pathophysiologic cues (oxidative stress, resveratrol) to explore the reciprocal regulation of acetylation and phosphorylation and their combined effects on FoxO1 cellular localization and biological functions.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco's modified Eagle's medium with 4.5 g/liter glucose, fetal bovine serum, calf serum, trypsin/EDTA, and phosphate-buffered saline were purchased from Mediatech (Manassas, VA). Insulin, H2O2, resveratrol, nicotinamide, and cycloheximide were purchased from Sigma, Akti-1/2 from EMD, microcystin-LR from Cayman, and leptomycin B from LC Laboratories. Anti-FLAG (M2) affinity gel was purchased from Sigma. Anti-phospho-S253, phospho-T24 FoxO1, and phospho-Akt antibodies (T308) were purchased from Cell Signaling. Anti-GFP,2 anti-tubulin, and anti-FLAG antibodies were purchased from Santa Cruz Biotechnology.
Plasmids, Adenoviruses, and Cell Culture
cDNAs encoding murine FoxO1 and carrying the following mutations, T24A, T24A-KQ, T24A-KR, and S253A, were subcloned into pEGFP-N1 to generate FoxO1-GFP fusion proteins. Plasmids pEGFP-N1 encoding WT, KQ, KR, ADA, ADA-KR, and ADA-KQ FoxO1 have been described previously (8). We used TransIT transfection reagent from Mirus (Madison, WI) for cell transfection. HEK-293 cells were cultured as described (10). In some experiments, we used adenoviruses encoding FoxO1-KR and -KQ mutants as described previously (8).
Protein Analyses
We harvested cells and prepared protein extracts in buffer containing 20 mm Tris, pH 7.4, 150 mm NaCl, 10% glycerol, 2% Nonidet P-40, 1 mm EDTA, pH 8.0, 0.2% semidehydroascorbate, 0.5% sodium deoxycholate supplemented with protease and phosphatase inhibitors (Boston Bioproducts). We fractionated 40 μg of protein by gel electrophoresis, followed by Western blot. Immunoprecipitation was carried out by standard methods. FLAG immunoprecipitation was carried out according to the manufacturer's instructions, and bound proteins were eluted using a FLAG peptide.
RESULTS AND DISCUSSION
Generation of FoxO1 Mutants
In addition to the previously described constitutively acetylated (FoxO1-KQ, in which lysine at amino acid residues 219, 242, 245, 259, 262, 271, and 291 is replaced with glutamine) and constitutively deacetylated mutants (FoxO1-KR, in which the same lysine residues are replaced with arginine) (Fig. 1A) (8), we generated an allelic series in which the KR and KQ mutations were introduced along with the following mutations of the three main phosphorylation sites: T24A, S253A, or combined T24A/S253D/S316A (ADA mutant) (12). Ser-253 is the main Akt site, whereas Thr-24 is phosphorylated by an insulin-activated kinase(s) distinct from Akt (23, 25). We generated the mutants KR, KQ, S253A, T24A-KQ, T24A-KR, ADA-KQ, and ADA-KR as GFP fusion proteins to facilitate their detection by fluorescence microscopy (30). For simplicity, we omit “FoxO1-GFP” from the nomenclature.
FIGURE 1.
Effect of insulin stimulation and Akt inhibition on FoxO1 acetylation site mutants. A, diagram summarizing the location of phosphorylation (purple circles) and acetylation sites (green triangles) in FoxO1. B, HEK-293 cells transfected with WT FoxO1-GFP or KQ, KR, and S253A mutants. Cells were treated with insulin and Akti for 30 min, and FoxO1 localization was visualized using a fluorescent microscope.
Effects of Acetylation Site Mutations on FoxO1 Subcellular Localization
We studied the effect of mutating acetylation sites on insulin-induced FoxO1 subcellular translocation. To this end, we transfected wild type (WT), KQ, or KR FoxO1-GFP fusion proteins into HEK-293 cells. WT localized to the nucleus in serum-free medium and translocated to the cytoplasm upon insulin stimulation. The Akt inhibitor Akti-1/2 inhibited this process (33) (Fig. 1B). Conversely, the KQ mutant was predominantly cytoplasmic, regardless of whether cells were incubated in serum-free medium or in the presence of insulin and Akt inhibitor. The KR mutant translocated to the cytoplasm after insulin stimulation in a WT-like fashion and was retained in the nucleus after Akti-1/2 treatment, whereas the S253A mutant was constitutively nuclear under all conditions tested (Fig. 1B) (24).
We next examined whether acetylation trumps phosphorylation as a signal for FoxO1 retention in the nucleus. To this end, we compared the phosphorylation-defective mutant ADA (12) with combined phosphorylation/acetylation site mutants, ADA-KQ or ADA-KR. ADA was constitutively nuclear regardless of the culture conditions (Fig. 2A). Surprisingly, ADA-KQ had predominantly cytoplasmic localization, whereas ADA-KR was retained in the nucleus (Fig. 2A). These data indicate that acetylation trumps phosphorylation as a signal regulating FoxO1 cellular localization. Inhibition of nuclear export by leptomycin B resulted in nuclear accumulation of both KQ and ADA-KQ, indicating that acetylation doesn't prevent nuclear targeting of FoxO1 but likely accelerates its export to the cytoplasm or retards its nuclear import (Fig. 2B).
FIGURE 2.
Subcellular localization of FoxO1 acetylation site mutants in response to insulin. A, HEK-293 cells transfected with GFP-tagged WT or ADA-KQ and ADA-KR FoxO1 and incubated in the absence and presence of insulin prior to visualizing FoxO1 localization by fluorescence microscopy. B, HEK-293 cells transfected with GFP-tagged KQ and ADA-KQ and incubated in the absence and presence of serum or leptomycin B (LMB) prior to visualizing FoxO1 localization by fluorescence microscopy. C, FoxO1 acetylation was determined by immunoblotting with anti-acetyllysine antiserum following immunoprecipitation with anti-FLAG (M2) antibody of protein extracts from HEK-293 cells transfected with FLAG-tagged FoxO1 mutants S253A (SA) or T24A (TA), the latter treated with and without insulin.
To rule out that mutation of the phosphorylation sites affected FoxO1 acetylation, we measured acetyl-FoxO1 levels in the phosphorylation site mutants, S253A and T24A. As the former is unaffected by insulin treatment, we measured only basal acetylation in the absence of insulin; in the latter, we compared acetylation levels in the absence and presence of insulin. However in neither case did we observe changes to FoxO1 acetylation (Fig. 2C).
Effects of Acetylation Site Mutations on Insulin-induced FoxO1 Phosphorylation
To understand why acetylation promotes FoxO1 nuclear exclusion, we examined whether it affects phosphorylation of Ser-253, the site required for insulin-dependent nuclear translocation (24). To avoid the potential confounding effects of Thr-24 phosphorylation on subcellular localization (24), we measured Ser-253 phosphorylation in T24A-KQ and T24A-KR mutants following exposure of cells to different doses of insulin. Insulin-induced Ser-253 phosphorylation of the T24A mutant paralleled Akt phosphorylation in a dose-dependent manner, with an ED50 ∼ 0.3 nm (Fig. 3). In contrast, the ED50 for Ser-253 phosphorylation decreased to <0.15 nm in the T24A-KQ mutant and rose to >1.5 nm in the T24A-KR mutant, resulting in a 10-fold difference between the two mutants. Interestingly, levels of the T24A-KQ mutant decreased in insulin-treated cells. Based on prior studies, this is likely to reflect increased protein degradation through the proteasome (8). This process was reversed by Akt inhibition, as was Ser-253 phosphorylation (Fig. 3). These data indicate that acetylation increases FoxO1 sensitivity to Akt phosphorylation and degradation, suggesting that FoxO1 nuclear exclusion and protein turnover are integrated through acetylation-based mechanisms.
FIGURE 3.
Phosphorylation of FoxO1 acetylation site mutants. HEK-293 cells were transfected with FLAG-tagged T24A, T24A-KQ, or T24A-KR FoxO1 and treated with different concentrations of insulin. Phosphorylation of FoxO1 and Akt was determined by Western blotting with anti-phospho-S253 FoxO1 (p-S253), phospho-T24 FoxO1 (p-T24), and phospho-Akt (T308) antibodies. Tubulin was used as the gel loading control.
Uncoupling of Acetylation from Phosphorylation following H2O2-induced Oxidative Stress
Oxidative stress and the polyphenol resveratrol promote FoxO1 nuclear retention (30). Their effects have been ascribed to FoxO1 deacetylation (27, 28, 34). However, the data in Fig. 3 raise the possibility that they also inhibit FoxO1 phosphorylation or promote its dephosphorylation. To answer this question, we used acetylation site FoxO1 mutants to examine FoxO1 localization and phosphorylation following incubation of cells with insulin and H2O2, a chemical agent used to mimic oxidative stress (35), or insulin and resveratrol, a Sirt1 and AMP-activated protein kinase agonist (29, 30). Addition of H2O2 to insulin-treated cells prevented FoxO1 nuclear export. This effect was reversed by the constitutively acetylated KQ mutant but not by the deacetylated KR mutant, indicating that H2O2 promotes FoxO1 deacetylation or requires that FoxO1 be deacetylated to keep it in the nucleus (Fig. 4A). Resveratrol also prevented FoxO1 nuclear exclusion in response to insulin but, unlike H2O2, failed to prevent nuclear exclusion of either KQ or KR mutants (Fig. 4A), indicating that its effects are independent of FoxO1 acetylation.
FIGURE 4.
Subcellular localization and phosphorylation of FoxO1 acetylation site mutants in response to treatment with H2O2 or resveratrol. A, representative images of fluorescence microscopy of HEK-293 cells transfected with GFP-tagged WT, KQ, and KR FoxO1 and incubated in the conditions indicated. B, immunoblot analysis using anti-phospho-S253 FoxO1 (p-S253), phospho-T24 FoxO1 (p-T24), and phospho-T308 Akt (T308) antibodies and tubulin. Extracts were prepared from HEK-293 cells transfected as in B. C, Western blot analysis of HEK-293 cells transfected with GFP-tagged WT FoxO1 and incubated with insulin (Ins) and cycloheximide (CHX) prior to the addition of H2O2 or resveratrol (Resv).
Next we compared the effects of H2O2 and resveratrol on insulin-dependent phosphorylation of Ser-253 and Thr-24. Insulin promoted FoxO1 phosphorylation on both sites. Addition of H2O2 to insulin decreased phosphorylation of both sites. The effect of H2O2 was preserved in the KQ and KR mutants, indicating that it is independent of FoxO1 acetylation (Fig. 4B). Addition of resveratrol to insulin also decreased insulin-dependent Ser-253 and Thr-24 phosphorylation in WT FoxO1, but not in the KQ and KR mutants (Fig. 4B). Neither H2O2 nor resveratrol affected insulin-induced Akt phosphorylation to a significant extent (Fig. 4B), and their effect on Ser-253 phosphorylation was independent of changes in FoxO1 protein levels, as indicated by the fact that they retained their ability to decrease Ser(P)-253 in the presence of the protein synthesis inhibitor, cycloheximide (Fig. 4C).
From these experiments, we concluded that the effects of resveratrol are mediated neither by changes in FoxO1 acetylation nor by dephosphorylation of Ser-253 and Thr-24. In contrast, H2O2 promotes FoxO1 nuclear retention through a dual mechanism: deacetylation and reduced insulin-dependent phosphorylation of Ser-253 and Thr-24. Interestingly, the two effects can occur independently. Thus, despite their apparent similarities, resveratrol and H2O2 affect FoxO1 activity in mechanistically distinct fashions.
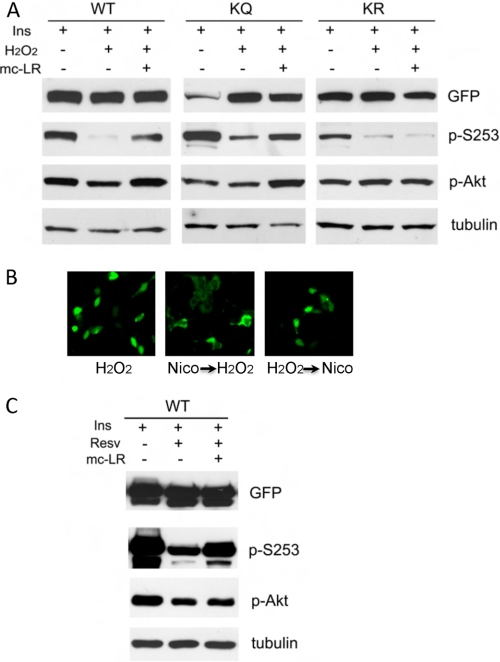
H2O2 might promote FoxO1 dephosphorylation either by preventing access of the relevant kinases to these sites or by easing access by the relevant phosphatases. To address this question, we examined whether the ability of H2O2 to prevent FoxO1 phosphorylation on Ser-253 was reversed by inhibition of PP2A, a FoxO1 Ser-253 phosphatase (36). H2O2 decreased Ser-253 phosphorylation, and its effect was partly reversed by the PP2A inhibitor microcystin-LR (Fig. 5A). The ability of microcystin-LR to offset H2O2 inhibition of Ser-253 phosphorylation was preserved in the KQ, but not in the KR, mutant (Fig. 5A). Given that the KR mutant is predominantly nuclear, these data are consistent with the interpretation that PP2A-dependent FoxO1 dephosphorylation occurs outside the nucleus. The fact that the effect of microcystin-LR is partial indicates that H2O2 also regulates other phosphatases or prevents access of Akt to FoxO1.
FIGURE 5.
Effect of PP2A inhibition on the phosphorylation of FoxO1 acetylation site mutants. A, HEK-293 cells transduced with GFP-tagged WT, KQ, and KR adenovirus and treated with H2O2 (1 mm) and microcystin-LR (mc-LR) for 30 min. B, HEK-293 cells transfected with GFP-tagged WT FoxO1 and incubated sequentially with H2O2 and nicotinamide or vice versa. C, HEK-293 cells transduced with GFP-tagged WT FoxO1 and exposed to resveratrol (Resv) (50 μm) with and without microcystin-LR.
The ability of H2O2 to promote FoxO1 nuclear retention was preempted, but not reversed, by the deacetylase inhibitor nicotinamide (8). Thus, cell pretreatment with nicotinamide blocked H2O2-induced nuclear translocation, but addition of nicotinamide after H2O2 treatment was unable to reverse this effect (Fig. 5B), indicating that FoxO1 acetylation can prevent its nuclear entry but cannot promote its nuclear exclusion.
The effect of resveratrol, like that of H2O2, appears to entail reduced FoxO1 phosphorylation on Ser-253 and Thr-24. Decreased Ser-253 phosphorylation in resveratrol-treated cells was partly reversed by microcystin-LR (Fig. 5C), indicating that part of the effect of resveratrol is PP2A-dependent.
Because 14-3-3 participates in nucleocytoplasmic shuttling of FoxO by binding phosphorylated Thr-24 and Ser-253 (37, 38) and given the tight relationship between the phosphorylation of these sites and acetylation, we asked whether acetylation affected binding of 14-3-3 to the Ser-253 site. Using immunoprecipitation of FLAG-tagged T24A, T24A-KQ, and T24A-KR mutants, followed by immunoblotting with anti-14-3-3 antibody, we observed that insulin-induced phosphorylation of Ser-253 was associated with increased binding of T24A to 14-3-3 (Fig. 6). Constitutively deacetylated T24A-KR bound 14-3-3 more efficiently than T24A (Fig. 6, lanes WT and KR under +Insulin), even as its phosphorylation on Ser-253 was reduced. The constitutively acetylated T24A-KQ mutant also showed increased 14-3-3 binding, but, unlike the KR mutant, it was associated with increased Ser-253 phosphorylation (Fig. 6, compare lanes WT and KQ under −Insulin with the same lanes under +Insulin). These data indicate that FoxO1 binding to 14-3-3 is also modulated by its acetylation state, lending further support to the idea that acetylation affects FoxO1 nucleocytoplasmic shuttling.
FIGURE 6.

Binding of FoxO1 acetylation site mutants to 14-3-3. HEK-293 cells were transfected with FLAG-tagged T24A, T24A-KQ, or T24A-KR FoxO1, treated with insulin, and immunoprecipitated with anti-FLAG antibody (M2) prior to Western blot with the indicated antibodies.
Conclusions
The goal of this study was to examine the reciprocal regulation of two primary posttranslational modifications of FoxO1, acetylation and phosphorylation, and their combined effects on FoxO1 function. Using constitutively deacetylated (KR) and acetylated (KQ) mutants, we show that acetylation causes a leftward shift in the dose-response curve for insulin-induced FoxO1 phosphorylation, whereas deacetylation causes a rightward shift. As a result, the two mutants differ by ∼10-fold in their insulin sensitivity, suggesting that acetylation is a major determinant of FoxO1 activity in vivo.
A new finding of the present study is that the two modifications, acetylation and phosphorylation, can be uncoupled from each other. Using H2O2 to mimic oxidative stress and induce FoxO1 deacetylation, we show that H2O2 can antagonize insulin signaling by promoting either FoxO1 deacetylation or dephosphorylation of Ser-253 and Thr-24, the former in part through the serine/threonine phosphatase PP2A. In either case, the expectation is that FoxO1 will be retained in the nucleus.
In contrast, and somewhat surprisingly, resveratrol promotes FoxO1 nuclear localization independent of acetylation, as well as of Ser-253 and Thr-24 phosphorylation. These data support the recent observation that resveratrol acts by deacetylation-independent mechanisms (e.g. AMP-activated protein kinase activation) (39). We cannot exclude the possibility that resveratrol is unable to induce dephosphorylation of the KQ and KR mutants, because of structural alterations caused by the replacement of acetyllysine with arginine or glutamine.
The uncoupling of FoxO1 phosphorylation from acetylation in H2O2-treated cells has important ramifications for FoxO1 nuclear function. In fact, it has been shown that acetylation affects FoxO1 affinity to bind its DNA targets (8, 26) and may thus favor DNA binding-independent modalities of FoxO1 function, e.g. cell differentiation versus replication and metabolism (10). Likewise, it has previously been demonstrated that phosphorylation affects transactivation properties of FoxO1 (32), making it theoretically possible that FoxO1 be nuclear and inactive.
In conclusion, the findings that phosphorylated FoxO1 can be retained in the nucleus by decreasing its acetylation and, conversely, that dephosphorylated FoxO1 can be targeted to the cytoplasm through increased acetylation underscore that phosphorylation and acetylation are regulated through partly overlapping, and partly independent, mechanisms and suggest new research directions to develop agents that modulate pleiotropic functions of FoxOs.
Acknowledgment
We thank members of the Accili laboratory for helpful discussions and advice.
This work was supported by National Institutes of Health Grants DK57539 and DK63608 from the Columbia University Diabetes and Endocrinology Research Center.
- GFP
- green fluorescent protein
- ADA
- T24A/S253D/S316A
- WT
- wild type.
REFERENCES
- 1.Accili D., Arden K. C. (2004) Cell 117, 421–426 [DOI] [PubMed] [Google Scholar]
- 2.Nakae J., Biggs W. H., 3rd, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. (2002) Nat. Genet. 32, 245–253 [DOI] [PubMed] [Google Scholar]
- 3.Nakae J., Kitamura T., Silver D. L., Accili D. (2001) J. Clin. Invest. 108, 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buteau J., Accili D. (2007) Diabetes. Obes. Metab. 9, Suppl. 2, 140–146 [DOI] [PubMed] [Google Scholar]
- 5.Buteau J., Shlien A., Foisy S., Accili D. (2007) J. Biol. Chem. 282, 287–293 [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T., Kitamura Y. I., Kobayashi M., Kikuchi O., Sasaki T., Depinho R. A., Accili D. (2009) Mol. Cell. Biol. 29, 4417–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., 3rd, Wright C. V., White M. F., Arden K. C., Accili D. (2002) J. Clin. Invest. 110, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitamura Y. I., Kitamura T., Kruse J. P., Raum J. C., Stein R., Gu W., Accili D. (2005) Cell Metab. 2, 153–163 [DOI] [PubMed] [Google Scholar]
- 9.Hribal M. L., Nakae J., Kitamura T., Shutter J. R., Accili D. (2003) J. Cell Biol. 162, 535–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura T., Kitamura Y. I., Funahashi Y., Shawber C. J., Castrillon D. H., Kollipara R., DePinho R. A., Kitajewski J., Accili D. (2007) J. Clin. Invest. 117, 2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker S. H., Goldberg A. L. (2004) Cell 117, 399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakae J., Kitamura T., Kitamura Y., Biggs W. H., 3rd, Arden K. C., Accili D. (2003) Dev. Cell 4, 119–129 [DOI] [PubMed] [Google Scholar]
- 13.Lin K., Dorman J. B., Rodan A., Kenyon C. (1997) Science 278, 1319–1322 [DOI] [PubMed] [Google Scholar]
- 14.Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. (1997) Nature 389, 994–999 [DOI] [PubMed] [Google Scholar]
- 15.Vogt P. K., Jiang H., Aoki M. (2005) Cell Cycle 4, 908–913 [DOI] [PubMed] [Google Scholar]
- 16.Biggs W. H., 3rd, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. (1999) Proc. Natl. Acad. Sci. U. S. A. 96, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunet A., Bonni A., Zigmond M. J., Lin M. Z., Juo P., Hu L. S., Anderson M. J., Arden K. C., Blenis J., Greenberg M. E. (1999) Cell 96, 857–868 [DOI] [PubMed] [Google Scholar]
- 18.Nakae J., Park B. C., Accili D. (1999) J. Biol. Chem. 274, 15982–15985 [DOI] [PubMed] [Google Scholar]
- 19.Lehtinen M. K., Yuan Z., Boag P. R., Yang Y., Villén J., Becker E. B., DiBacco S., de la Iglesia N., Gygi S., Blackwell T. K., Bonni A. (2006) Cell 125, 987–1001 [DOI] [PubMed] [Google Scholar]
- 20.Essers M. A., Weijzen S., de Vries-Smits A. M., Saarloos I., de Ruiter N. D., Bos J. L., Burgering B. M. (2004) EMBO J. 23, 4802–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asada S., Daitoku H., Matsuzaki H., Saito T., Sudo T., Mukai H., Iwashita S., Kako K., Kishi T., Kasuya Y., Fukamizu A. (2007) Cell Signal. 19, 519–527 [DOI] [PubMed] [Google Scholar]
- 22.Kawamori D., Kaneto H., Nakatani Y., Matsuoka T. A., Matsuhisa M., Hori M., Yamasaki Y. (2006) J. Biol. Chem. 281, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 23.Brunet A., Park J., Tran H., Hu L. S., Hemmings B. A., Greenberg M. E. (2001) Mol. Cell. Biol. 21, 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakae J., Barr V., Accili D. (2000) EMBO J. 19, 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakae J., Kitamura T., Ogawa W., Kasuga M., Accili D. (2001) Biochemistry 40, 11768–11776 [DOI] [PubMed] [Google Scholar]
- 26.Daitoku H., Hatta M., Matsuzaki H., Aratani S., Ohshima T., Miyagishi M., Nakajima T., Fukamizu A. (2004) Proc. Natl. Acad. Sci. U. S. A. 101, 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuoka M., Daitoku H., Hatta M., Matsuzaki H., Umemura S., Fukamizu A. (2003) Int. J. Mol. Med. 12, 503–508 [PubMed] [Google Scholar]
- 28.van der Horst A., Tertoolen L. G., de Vries-Smits L. M., Frye R. A., Medema R. H., Burgering B. M. (2004) J. Biol. Chem. 279, 28873–28879 [DOI] [PubMed] [Google Scholar]
- 29.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., Hu L. S., Cheng H. L., Jedrychowski M. P., Gygi S. P., Sinclair D. A., Alt F. W., Greenberg M. E. (2004) Science 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 30.Frescas D., Valenti L., Accili D. (2005) J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]
- 31.Imai S., Armstrong C. M., Kaeberlein M., Guarente L. (2000) Nature 403, 795–800 [DOI] [PubMed] [Google Scholar]
- 32.Tomizawa M., Kumar A., Perrot V., Nakae J., Accili D., Rechler M. M., Kumaro A. (2000) J. Biol. Chem. 275, 7289–7295 [DOI] [PubMed] [Google Scholar]
- 33.Logie L., Ruiz-Alcaraz A. J., Keane M., Woods Y. L., Bain J., Marquez R., Alessi D. R., Sutherland C. (2007) Diabetes 56, 2218–2227 [DOI] [PubMed] [Google Scholar]
- 34.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 35.Nemoto S., Finkel T. (2002) Science 295, 2450–2452 [DOI] [PubMed] [Google Scholar]
- 36.Yan L., Lavin V. A., Moser L. R., Cui Q., Kanies C., Yang E. (2008) J. Biol. Chem. 283, 7411–7420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Obsil T., Ghirlando R., Anderson D. E., Hickman A. B., Dyda F. (2003) Biochemistry 42, 15264–15272 [DOI] [PubMed] [Google Scholar]
- 38.Rena G., Prescott A. R., Guo S., Cohen P., Unterman T. G. (2001) Biochem. J. 354, 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. (2009) Nature 458, 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]