Abstract
Potassium fluxes integrate mitochondria into cellular activities, controlling their volume homeostasis and structural integrity in many pathophysiological mechanisms. The outer mitochondrial membrane (OMM) is thought to play a passive role in this process because K+ is believed to equilibrate freely between the cytosol and mitochondrial intermembrane space. By patch clamping mitochondria isolated from the central nervous systems of adult mitoCFP transgenic mice, we discovered the existence of IOMMKi, a novel voltage-dependent inwardly rectifying K+ conductance located in the OMM. IOMMKi is regulated by osmolarity, potentiated by cAMP, and activated at physiological negative potentials, allowing K+ to enter the mitochondrial intermembrane space in a controlled regulated fashion. The identification of IOMMKi in the OMM supports the notion that a membrane potential could exist across this membrane in vivo and suggests that the OMM possesses regulated pathways for K+ uptake.
Keywords: Bioenergetics, Membrane Biophysics, Mitochondria, Potassium Channels, Potassium Transport
Introduction
Potassium accumulation in mitochondria is known to be regulated by a uniport mechanism that controls mitochondrial volume homeostasis and bioenergetics (1) and works concurrently with a K+-H+ exchanger to prevent mitochondrial swelling (2, 3). In particular, K+ entry into mitochondria has been credited to its passive diffusion through the outer mitochondrial membrane (OMM)5 into the intermembrane compartment and to the K+ uniport mechanism, which comprises distinct channels located in the inner mitochondrial membrane (IMM) that allow K+ to flow into the matrix (4). In contrast to the vast literature on K+ currents through the IMM (4–6), there have been only a few early attempts to study the electrophysiological properties of the OMM in situ (i.e. in intact isolated mitochondria), which fell short of giving a clear picture of K+ across this membrane (7). For instance, the large voltage-dependent anion channel (VDAC) of the outer membrane was thoroughly described in reconstituted membrane lipid bilayers (8), but it has never been observed or its biophysical features described in patch-clamp experiments of intact mitochondria. Rather, a variety of conductances in the range of 10–307 picosiemens (in 150 KCl) have been reported (9, 10), whose function and regulation still await to be assigned (5).
To provide an additional contribution to the existing database on OMM conductances, we applied the patch-clamp technique to intact mitochondria isolated from the central nervous system of adult mice. This is a severe technical challenge mainly because of the small size of the organelle (∼1 μm in diameter) and the difficulty in identifying the single mitochondrion in isolation. We circumvented these problems by using tissue homogenization in combination with a bland trypsin treatment and Percoll gradient purification to isolate single neuronal mitochondria from the spinal cord of a transgenic mouse in which a cyan fluorescent protein (CFP) was fused to a mitochondrial transport sequence derived from cytochrome c and expressed in neurons under the control of the Thy-1 promoter element (11). By patch clamping isolated neuronal mitochondria, we described a novel voltage-dependent K+ conductance in the OMM (IOMMKi) whose identification favors the concept that a membrane potential (EOMM) is maintained in the OMM. We found that IOMMKi is selective for K+, regulated by osmolarity, potentiated by cAMP, and activated at physiological negative potentials, allowing K+ to enter the mitochondrial intermembrane space in a controlled fashion. Traffic of K+ ions firmly integrates mitochondria into cellular activities. Mitochondrial volume homeostasis, which depends on K+ movements across the IMM (K+ uniport), is key to cellular pathophysiology. For instance, it is known that dysregulation of K+ fluxes affects osmotic homeostasis of mitochondria and their structural integrity and function, resulting in opening of the permeability transition pore (3). The existence of a voltage-dependent channel selective for K+ in the OMM could therefore participate in regulating K+ electrophoretic distribution in respiring mitochondria.
EXPERIMENTAL PROCEDURES
Mitochondrial Isolation for Patch-clamp Recording
Mitochondria were prepared from the spinal cord of an adult mitoCFP transgenic mouse, which expresses CFP in neuronal mitochondria (11). Three spinal cords were triturated for 15 min at 37 °C in Ca2+/Mg2+-free phosphate-buffered saline containing 2 mg/ml collagenase A (Roche Applied Science) and 1 mg/ml trypsin (Sigma). After the addition of an equal volume of solution A (70 mm sucrose, 190 mm mannitol, 20 mm Hepes, 2 mm EDTA, 1 mm EGTA, 5 mm sodium pyruvate, and 0.2% bovine serum albumin (pH 7.4 with NaOH)), the cords were homogenized in a Potter homogenizer (Teflon/glass; 4 °C, 600 rpm), and mitochondria were isolated by differential centrifugation and purified on a discontinuous Percoll gradient (modified from Ref. 12). The mitochondrial fraction was resuspended and stored at 4 °C in solution A diluted 1:1 with solution B (150 mm KCl, 20 mm Hepes, 2.5 mm EGTA, and 2 mm EDTA (pH 7.2 with KOH)). Just before the experiments were performed, 1 μl of the mitochondrial suspension was added to 1 ml of solution B and transferred to the recording chamber. Mitochondria were identified by wide-field epifluorescence microscopy as blue vesicles ∼1 μm in diameter and then patched under Nomarski optics (60× objective, water immersion condenser).
Western Blot Analysis of Mitochondrial Preparation
Isolated mitochondria were tested by Western blotting for contamination of synaptic membranes using synapsin (anti-synapsin monoclonal antibody (1:500), Stressgen) and cytochrome c oxidase complex IV (anti-cytochrome c oxidase polyclonal antibody (1:1000), Cell Signaling) as markers of synaptic membranes and mitochondria, respectively.
Mitochondrion-attached Recordings
Gigohm seals were obtained with high resistance electrodes (30-80 megohms in solution B. Currents were recorded in the mitochondrion-attached configuration, and membrane voltages were described relative to the resting potential, which under the conditions used was assumed to be 0 mV; inward currents deflected inwardly. Whole mitochondrial, perforated, or excised patch configurations were unachievable, always resulting in loss of the tight seal. In the mitochondrion-attached configuration, the patch and bulk (outside the patch) membranes were in series. The properties of the resulting voltage divider depend on the patch and bulk membrane areas (13, 14). A spherical mitochondrion 1 μm in diameter would have a surface area of 3.14 μm2. With a patch area of ∼0.13 μm2 (∼0.4-μm internal diameter of the pipette tip), the voltage drop across the bulk membrane would be ∼4% of the applied voltage, assuming equal specific resistance for both membrane areas (15). No correction has been made for the voltage divider error. The bath and pipette solutions contained solution B unless specified otherwise. The bath K+ concentration was varied from 150 to 5 mm by diluting solution A with solution B, thus maintaining iso-osmolarity. 150 mm Na+ (K+) gluconate, NaCl, and CsCl solutions were prepared by substituting KCl in solution B with the corresponding salt. The osmolarity of the solutions was 320 mosm. Voltage steps and ramps were applied as specified in the figures.
Data Analysis
Signals were recorded using an Axopatch 200B patch-clamp amplifier (Axon Instruments), filtered at 2-5 kHz, and sampled at either 10 or 20 kHz. In some figures, traces were further filtered at 350 Hz. The Boltzmann equation was used to fit Fig. 2A. Time courses of activation and deactivation could be best fitted by a single-exponential function. Data in Fig. 3C were fitted by linear regression. Statistical data were calculated as means ± S.D. except where indicated otherwise.
FIGURE 2.
Voltage dependence and kinetics of the inward current. A, preconditioning voltage steps to potentials between +60 and −100 mV were applied for durations ranging between 6 and 10 s before the membrane potential was stepped to +60 mV to elicit tail currents. Normalized tail currents were fitted with a Boltzmann function with values of V½ and slope factor k of −45.6 ± 2.3 mV and 21.6 ± 2.1 mV, respectively (n = 7). Vh = 0 mV, and ΔV = 20 mV. The first and second blue arrows represent the areas of recording that have been magnified to better visualize tail currents. B, activation curve obtained by a tail current protocol. C and D, τact and τdeact, respectively, of Iinward assessed by voltage step protocols. For τact, the membrane was stepped to hyperpolarized potentials from −20 to −100 mV for 20 s to allow steady-state activation of the inward current; for τdeact, the same protocol as described for B was followed. Vh = 0 mV, and ΔV = 20 mV. Both activation and deactivation kinetics of the inward current followed a single-exponential time course (fitted by I = I0exp(−t/τ). Symmetrical [K+]pipette/[K+]bath was 150/150 mm.
FIGURE 3.
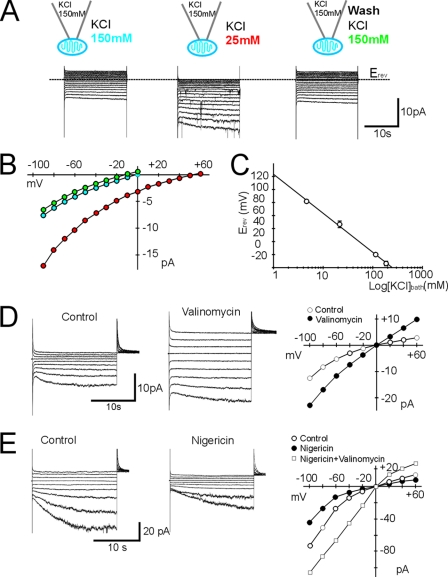
K+ selectivity and effect of ionophores on the inward current. A and B, representative traces with corresponding I/V plots in response to voltage steps from +60 to −90 mV (Vh = 0 mV, and ΔV = 10 mV) during bath perfusion with 150 and 25 mm KCl. Erev shifted from 0 mV in symmetrical [K+]pipette/[K+]bath = 150/150 mm to +48 mV in [K+]pipette/[K+]bath = 150/25 mm. The effect was reversible upon returning to [K+]bath = 150 mm KCl. C, semilogarithmic plot of Erev against activity of [K+]bath calculated according to the activity coefficient of the ions in solution. Erev was 81.8 ± 2.3 mV (n = 5), 46.5 ± 5.08 mV (n = 6), 0 ± 0 mV (n = 20), and −13.75 ± 3.5 mV (n = 4) in 5, 25, 150, and 250 mm K+ nominal bath solutions. The activity coefficients for K+ and Cl− in solution were calculated using the Debye-Hückel equation for 5 and 25 mm KCl and the Davies equation for 150 and 250 mm KCl in binary aqueous solution at 25 °C and were 0.925 (5 mm KCl), 0.852 (25 mm KCl), 0.764 (150 mm KCl), and 0.743 (250 mm KCl). [K+]pipette was 150 mm KCl. The ionic activity was calculate by a = activity coefficient × concentration. Data (means ± S.D.) were fitted by linear regression (r2 = 0.9970). D, representative traces and corresponding I/V plots of the control and 5 μm valinomycin application right before loss of the seal. Bath application of valinomycin (in 0.1% Me2SO) reduced the membrane seal resistance by ∼6 times (n = 3) and caused swelling of the mitochondrion and loss of the patch seal. Note the linearity of the trace after valinomycin application. Voltage steps (ΔV = 20 mV) from Vh = 0 mV to potentials between +60 and −100 mV were applied for 10 or 20 s before the membrane potential was stepped to +60 mV. E, representative traces and corresponding I/V plots of control bath application of 1 μm nigericin in 0.1% Me2SO and co-application of 1 μm nigericin + 5 μm valinomycin in 0.2% Me2SO. Nigericin alone increased the apparent seal resistance by ∼3 times and reduced the inward current (n = 4) by ∼2 times, as expected because the addition of nigericin to mitochondria is known to prevent K+ accumulation, whereas co-application of nigericin and valinomycin prevented loss of the membrane seal. The voltage step protocol was as described for D.
Electron Microscopy
Mitochondria were fixed overnight at 4 °C in 2% glutaraldehyde with 1% tannic acid buffered in phosphate buffer (pH 7.4), rinsed in the same buffer, and then exposed to 2% osmium tetroxide. Following a rinse with double-distilled water, samples were incubated in 0.5% uranyl acetate and then dehydrated in graded steps of acetone, filtrated with Spurr resin, and polymerized in a 65 °C convection oven. The blocks were thin-sectioned (70 nm) with a DiATOME diamond knife on a Leica UCT ultramicrotome; sections were analyzed under an FEI Tecnai 12T transmission electron microscope; and images were digitally recorded with an AMT XR111 camera.
RESULTS
Slowly Developing Inward Current Activates at Hyperpolarizing Potentials in the OMM
To study K+ currents through the OMM, we patch-clamped purified isolated central nervous system mitochondria possessing intact outer and inner membranes (Fig. 1A). Mitochondria from the spinal cords of mitoCFP transgenic mice, which express CFP in neuronal mitochondria, were purified on a Percoll gradient (11) and identified for recording by fluorescence microscopy (Fig. 1, B and C). In the mitochondrion-attached configuration and symmetrical pipette and bath solutions containing 150 mm KCl, 2.5 mm EGTA, and 2 mm EDTA, application of voltage steps from +20 to −10 mV elicited an instantaneous current that displayed a linear I/V relationship followed, at hyperpolarizing voltages (−20 to −80 mV), by a slowly developing inward current that did not inactivate during voltage steps of up to 1 min (Fig. 1D). This current was detected in ∼90% of the patch-clamped mitochondria.
FIGURE 1.
Slowly developing hyperpolarization-activated inward current through the OMM. A, electron microscopy of mitochondria isolated from the spinal cords of mitoCFP mice by differential centrifugation and purified on a discontinuous Percoll gradient showing integral outer and inner membranes. The lower panel shows loss of the synaptic marker synapsin (∼80 kDa) and enrichment of the mitochondrial marker cytochrome c oxidase complex IV (Cox IV; ∼17 kDa) in the Percoll-purified fraction compared with the “crude” mitochondrial fraction. B, mitochondria were incubated with 300 nm MitoTracker Red (MitoRed; Molecular Probes) for 3–5 min at room temperature. Panels show MitoTracker Red-stained mitochondria merged with CFP-expressing neuronal mitochondria. In the Merge panel, four of eight red positive mitochondria are neuronally derived (arrows). C, transmitted and fluorescent image of gigohm seal patch between the recording pipette and one mitochondrion. D, representative recording (n = 75) elicited in response to a voltage step protocol applied to a mitochondrion patched in the mitochondrion-attached configuration. Vh = 0 mV, and ΔV = 10 mV. Inward currents flow from the cytoplasmic (pipette) side of the outer membrane to the inside (bath) of the mitochondrion. Symmetrical (pipette and bath) 150 KCl solutions contained solution B.
Biophysical Characterization of the Inward Current
The inward current was voltage-dependent (Fig. 2, A and B), with a time constant of activation (τact) that decreased with hyperpolarization (Fig. 2C), and was unique to the OMM because we failed to detect it in mitoplasts, which instead displayed the well studied inner membrane centum picosiemen anion channel (supplemental Fig. 1, B–D) (16). Mitochondria were easily distinguished from mitoplasts on morphological grounds, as the latter are typically bigger (4–10 μm in diameter), with the characteristic “ghost” membrane and the well recognizable “cap” region (supplemental Figs. 1A and 2, A and B).
We assessed the voltage dependence from Boltzmann fits to tail currents (Fig. 2, A and B). After an initial delay, the inward current followed a sigmoidal time course of activation and exhibited strong voltage dependence (Fig. 2, A and B). At −20 mV, τact was 1.8 ± 0.1 s, increasing to 4.8 ± 0.2 s at −100 mV (n = 7) (Fig. 2C). The termination of hyperpolarizing steps evoked outward relaxations, indicating deactivation of the conductance (Fig. 2, B and D). The kinetics of deactivation (τdeact) of the outward tail currents at +60 mV did not show voltage dependence on the preceding hyperpolarization (τdeact = 800 ms, n = 8) (Fig. 2D).
Inward Current is K+-selective
The OMM conductance responsible for the inward current was K+-selective. Reduction of [K+]bath by dilution with an iso-osmotic solution resulted in increased current amplitudes at voltages negative to −20 mV and a positive shift in the reversal potential (Erev) as expected for the increased driving force due to equilibration of [K+]bath with the intermembrane space (Fig. 3, A–C). The rationale for the effectiveness of the mitochondrion-attached configuration to study the ionic selectivity of the conductance (Fig. 3, A–C) was based on the assumption that the average small diameter of the mitochondria (∼1 μm) allowed fast equilibration of the internal mitochondrial space with the ionic composition of the bath solution. Under our experimental conditions, the I/V relationships showed the features expected for ionic equilibration between the intramitochondrial space and the bath solution. When the K+ concentration was reduced on the bath side of the membrane (which corresponded to [K+]in), at voltages expected to elicit the slowly activating inwardly rectifying current, the conductance to K+ inward current was increased as expected for a channel whose permeability varies greatly with the force acting on the K+ ions (Fig. 3B). In addition, under symmetrical conditions (150 mm KCl on both sides of the membrane, i.e. pipette [K+]out and bath [K+]in solutions), the I/V relationships passed through the pipette zero potential (Fig. 3B), and changes in the shape of the I/V relationships could be reversed and were reproducible from one patch to the next (n = 35) (Fig. 3, A–C). The changes in I/V relationships occurred within 1 min of application. With [K+]pipette (cytoplasmic face of the membrane) at 150 mm and [K+]bath ranging from 5 to 250 mm (chemical concentrations), Erev approached the theoretical equilibrium potential for each of the imposed K+ gradients (EK). The shift was 50 mV per 10-fold change in [K+]bath, indicating a high selectivity for K+ (Erev versus KCl activity) (Fig. 3C). A similar effect was obtained by application of 150 mm bath CsCl, which shifted Erev to more positive potentials and created a K+ gradient across the OMM, increasing the inward current at negative potentials, as expected for a K+-selective conductance (supplemental Fig. 3). The slowly activated inward current was blocked by 150 mm pipette CsCl (n = 10). Conversely, tetraethylammonium (5–20 mm) and 4-aminopyridine (1 mm), selective blockers of plasma membrane voltage-activated K+ channels, delivered in the bath and/or in pipette solution did not affect the inward K+ current. Erev approached EK also when the bath solution contained 150 mm potassium gluconate without disturbing the slowly developing inward current at potentials negative to −20 mV, indicating little, if any, contribution of Cl− to either the inward current or the Erev of the OMM. Physiological cytosolic [Na+] (5–10 mm) in both the pipette and bath solutions affected neither Erev nor the amplitude of the inward current, whereas in symmetrical 150 mm NaCl or sodium gluconate, only low resistance patches were obtained (∼300 megohms), displaying linear current response at all potentials (−140 to +120 mV) with slightly outward rectification upon leak subtraction (n = 3) (supplemental Fig. 4). This could represent either the activation of an alternative conductance for Na+ or the disruption of the membrane seal due to supraphysiological Na+ concentrations. In view of its biophysical properties, we refer to the OMM voltage-dependent inwardly rectifying K+ conductance as IOMMKi.
IOMMKi Is Regulated by K+ Homeostasis and Changes in Osmolarity
A plethora of information on mitochondrial transport of monovalent cations, specifically K+, was obtained from kinetic measurements of volume changes with optical methods using ionophores such as valinomycin and nigericin and by elucidating their transport properties in both artificial and natural membranes (1, 17). Here, we tested the effect of these compounds on mitochondrial membranes for the first time with the patch-clamp technique to determine whether changes in K+ fluxes across the OMM affect IOMMKi conductance.
It is well established that respiring mitochondria in KCl media swell beyond the point of outer membrane rupture upon the addition of valinomycin, a ionophore that allows rapid and selective K+ uptake into mitochondria (1, 17). Bath perfusion of valinomycin (5 μm) in symmetrical 150 mm KCl led (within seconds from the application) to an irreversible increase in the K+ inward conductance (Fig. 3D) and to an overall increased permeability of the OMM to K+, resulting in a linear (ohmic) current added to IOMMKi (Fig. 3D). Over time (within 10 min from the bath application of valinomycin), the seal resistance broke up, with complete loss of the gigohm seal as monitored by the disappearance of the capacitative transients and swelling of the organelle observed in real time under the microscope (Nomarski optics, 60× water immersion condenser; see “Experimental Procedures”). The ohmic current response added to IOMMKi was likely due to the formation of additional K+-selective pores in the OMM, as it is obvious that valinomycin itself should increase K+ conductance in symmetrical high K+ solutions. Nevertheless, it is clear from the outset of these experiments that alteration of mitochondrial K+ fluxes had also an effect on IOMMKi, further confirming the permeability of the channel to K+. Biochemical studies have shown that the addition of nigericin (which promotes K+ exit in exchange for H+) to mitochondria causes loss of endogenous K+ ions and shrinkage of the mitochondrial matrix (1) and that simultaneous application of excess nigericin with valinomycin prevents swelling, causing permanent uncoupling (1). In our hands, bath application of nigericin (1 μm) decreased IOMMKi without altering its voltage dependence, yet increased the apparent seal resistance (n = 3) (Fig. 3E). As expected, co-application of nigericin (1 μm) with valinomycin (5 μm) prevented loss of the patch seal, as seen with valinomycin alone, despite increasing the overall K+ permeability of the OMM (Fig. 3E, I/V plot; compare with Fig. 3D). It is likely that under our control conditions (no nigericin and valinomycin added), the endogenous K+-H+ exchanger was functional, preventing the swelling of mitochondria in symmetrical 150 mm KCl, whereas the addition of valinomycin in the bath solution induced an excessive K+ influx, exceeding the K+ extrusion capacity of the endogenous K+-H+ exchanger. This unbalanced condition could only be counteracted by nigericin.
The experiments with ionophores known to affect the overall K+ homeostasis in mitochondria further confirmed the nature of IOMMKi as a K+-selective/regulated channel. Hence, we cannot rule out the possibility of IOMMKi being involved, at least in part, in mitochondrial volume homeostasis.
Volume homeostasis depends on regulated K+ transport across the mitochondrial membranes (2, 3, 18, 19) as well as changes in osmolarity (20, 21). This prompted us to examine the effect of osmolarity on IOMMKi. A hyperosmotic solution applied to either the bath or pipette completely suppressed IOMMKi (Fig. 4A), whereas IOMMKi was augmented by hyposmosis (Fig. 4B), suggesting that IOMMKi was involved in the swelling-contraction cycle of the mitochondrion. Application of the hyposmotic solution also caused activation of a large conductance at very negative potentials (Fig. 4B), which eventually caused loss of the patch seal. To identify IOMMKi pharmacologically, we initially tested ruthenium red (0.2–1 μm), a well known blocker of K+/Na+-non-selective leak currents (1), which had no effect on IOMMKi. Neither diazoxide (50, 100, and 300 μm) nor 5-hydroxydecanoate (1 mm), activator and inhibitor of mitochondrial KATP, respectively (17), delivered in the pipette and/or bath solution affected IOMMKi (n = 3). Unitary openings of IOMMKi were non-resolvable, indicating that the conductance was small. Our attempts to determine the channel conductance through non-stationary noise analysis were unsuccessful due to activation, over long periods of repeated hyperpolarization, of a clear-cut macropore (0.5–2-nanosiemen conductance), which surmounted IOMMKi (supplemental Fig. 5). Nevertheless, the appearance of this large conductance, described previously in the outer membrane of presynaptic and ischemic mitochondria (22, 23), confirmed that our recordings were made from the OMM.
FIGURE 4.
Osmolarity affects IOMMKi. A, IOMMKi was blocked by bath application of a hyperosmotic solution (135 mm sucrose in 150 mm KCl; ∼455 mmol/kg). Shown are representative traces and an I/V plot of n = five recordings. The effect of the hyperosmotic solution was reversible upon returning to 150 mm KCl (bath). The voltage step protocol was as described for Fig. 2B. Pipette (cytoplasmic face) application of the hyperosmotic solution had the same effect (n = 7/7). B, IOMMKi was increased by bath application of a hyposmotic solution (5 mm sucrose, 5 mm Hepes, and 1 mm EGTA (pH 7.2 with KOH); ∼13 mmol/kg). Of note is the appearance of a large voltage-dependent conductance active at potentials negative to −100 mV, which surmounted IOMMKi. Shown are representative traces and an I/V plot of n = three recordings. The voltage step protocol was as described for Fig. 3A, except that that the initial and final voltage steps were done at +50 and −140 mV, respectively.
IOMMKi Is Regulated by cAMP Acting on the Inside of the Mitochondrion
The biophysical properties of IOMMKi resembles those of Ih, a hyperpolarization-activated cation current (24). We wanted to determine whether cAMP, shown to regulate Ih, could also influence IOMMKi. Bath application of the membrane-permeable cAMP analog 8-bromo-cAMP (1 μm, Kd for Ih) increased IOMMKi peak conductance by >100% between −20 and −60 mV (Fig. 5, A and B) and markedly decreased both τact and τdeact (2–3-fold) (Fig. 5C). The activation curve of IOMMKi was unfeasible to study in the presence of 8-bromo-cAMP due to excessive activation of the current at voltages negative to −60 mV, which made the patch unstable. Pipette and bath application of the membrane-impermeable cAMP had no effect on IOMMKi, ruling out that 8-bromo-cAMP could act from the cytosolic side. The responsiveness to cAMP suggests that IOMMKi could be a potential target for regulation of the recently described CO2/HCO3−-activated soluble adenylate cyclase contained within mitochondria, which was shown to serve as a metabolic sensor in response to nutrient availability (25).
FIGURE 5.
IOMMKi is regulated by cAMP. A and B, bath application of 1 μm 8-bromo-cAMP increased IOMMKi by ≥100% within 6 min of application (n = 3). Vh = 0 mV, and ΔV = 20 mV. Symmetrical (pipette and bath) 150 KCl solutions contained solution B. C, 8-bromo-cAMP decreased both τact (∼2-fold) at all voltages between −20 and −60 mV (at −60 mV: control, 2.9 ± 0.25 ms; and in 8-bromo-cAMP, 1.3 ± 0.075 ms (mean ± S.D., n = 3)) and τdeact (∼3-fold) (at +60 mV after −60-mV preconditioning step: control, 795 ± 12.6; and in 8-bromo-cAMP, 266 ± 29.8 ms (mean ± S.D., n = 3)). The voltage protocol and solutions were as described for Fig. 4A.
DISCUSSION
We have described the existence of a novel voltage-activated inwardly rectifying K+-selective conductance located in the OMM (IOMMKi). The existence of IOMMKi, which is activated at negative voltages, warrants some level of intermembrane [K+] regulation. A K+ channel sensitive to voltage fluctuations in the OMM also favors the idea that this membrane maintains a membrane potential (EOMM), which could drive K+ accumulation. Energized mitochondria maintain a low passive permeability to H+ and therefore maintain a transmembrane electrical potential difference (Δψm), which is the main driving force for K+ accumulation. The existence of EOMM was first proposed by Colombini (26), who ascribed it to the presence of VDAC in the OMM. Indeed, the presence of different charged macromolecules and proteins in the cytosol and intermembrane space requires that a Donnan potential be established across the OMM. More recently, Porcelli et al. (27) measured a pH gradient across the OMM. This H+ gradient is most likely at equilibrium with a potential that would have been established across the outer membrane. Lemeshko (28) also proposed that the IMM potential (Δψm) could be translated to the OMM either by capacitive coupling through contact sites or by a voltage division mechanism. The presence of IOMMKi reinforces the concept that the voltage sensitivity of the OMM might be conserved in vivo. Potassium ion movements within mitochondria integrate these organelles into cellular activities. Mitochondrial volume homeostasis, which depends on K+ fluxes across the IMM (K+ uniport), is key to cellular pathophysiology. Alterations in these fluxes affect mitochondrial osmotic homeostasis, leading to opening of the permeability transition pore (3). In this context, it is possible that a regulated pathway for K+ uptake located in the OMM (IOMMKi) could affect K+ fluxes across this membrane and impact the K+ uniport mechanism(s) of the IMM.
Our recordings also showed that VDAC, the main conductive pathway for small solutes of the OMM (8), could exist in a fully closed state under our experimental conditions. Indeed, we never observed a conductance resembling VDAC biophysical properties as reported for VDAC purified and reconstituted in artificial lipid bilayers (29), suggesting that VDAC could be differently regulated in vivo (30). However, at this stage of our investigation, because VDAC is the most abundant protein in the outer membrane (31, 32), we also cannot rule out the possibility that IOMMKi could be a further, in situ regulated, subconductive state of VDAC.
Finally, IOMMKi modulation by osmolarity and cAMP makes it a perfect candidate to link mitochondrial volume changes to oxidative phosphorylation and metabolism. The high negative value of Δψm required for oxidative phosphorylation is not only a powerful driving force for K+ uptake but may also assure that K+ fluxes into mitochondria are sensitive to physiological fluctuations of Δψm. Thus, dynamic regulation of mitochondrial K+ flux in vivo through IOMMKi in the OMM, which is voltage-gated, would be an important factor for maintaining the structural and functional integrity necessary for oxidative phosphorylation (33).
Supplementary Material
Acknowledgments
We are grateful to Drs. R. Horn and A. Plested for experimental advice and Drs. J. Hoek and P. Bernardi for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1-NS064488 and RO1-NS044942 (to D. T.) and RO1-NS051488 (to P. P.). This work was also supported by the ALS Association (to P. P.) and the Muscular Dystrophy Association (to D. T.). The generation of the mitoCFP mice was supported by grants from the Christopher and Dana Reeve Foundation, the Dana Foundation, and the Deutsche Forschungsgemeinschaft. The Weinberg Unit for ALS Research is sponsored by the Farber Family Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- OMM
- outer mitochondrial membrane
- IMM
- inner mitochondrial membrane
- VDAC
- voltage-dependent anion channel
- CFP
- cyan fluorescent protein.
REFERENCES
- 1.Bernardi P. (1999) Physiol. Rev. 79, 1127–1155 [DOI] [PubMed] [Google Scholar]
- 2.Kaasik A., Safiulina D., Zharkovsky A., Veksler V. (2007) Am. J. Physiol. Cell Physiol. 292, C157–C163 [DOI] [PubMed] [Google Scholar]
- 3.Nowikovsky K., Schweyen R. J., Bernardi P. (2009) Biochim. Biophys. Acta 1787, 345–350 [DOI] [PubMed] [Google Scholar]
- 4.Bednarczyk P. (2009) Acta Biochim. Pol. 56, 385–392 [PubMed] [Google Scholar]
- 5.O'Rourke B. (2007) Annu. Rev. Physiol. 69, 19–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szewczyk A., Jarmuszkiewicz W., Kunz W. S. (2009) IUBMB Life 61, 134–143 [DOI] [PubMed] [Google Scholar]
- 7.Sorgato M. C., Moran O. (1993) Crit. Rev. Biochem. Mol. Biol. 28, 127–171 [DOI] [PubMed] [Google Scholar]
- 8.Colombini M. (2004) Mol. Cell. Biochem. 256–257, 107–115 [DOI] [PubMed] [Google Scholar]
- 9.Moran O., Sciancalepore M., Sandri G., Panfili E., Bassi R., Ballarin C., Sorgato M. C. (1992) Eur. Biophys. J. 20, 311–319 [DOI] [PubMed] [Google Scholar]
- 10.Moran O., Sorgato M. C. (1992) J. Bioenerg. Biomembr. 24, 91–98 [DOI] [PubMed] [Google Scholar]
- 11.Misgeld T., Kerschensteiner M., Bareyre F. M., Burgess R. W., Lichtman J. W. (2007) Nat. Methods 4, 559–561 [DOI] [PubMed] [Google Scholar]
- 12.Brustovetsky N., Brustovetsky T., Jemmerson R., Dubinsky J. M. (2002) J. Neurochem. 80, 207–218 [DOI] [PubMed] [Google Scholar]
- 13.Horn R., Brodwick M. S. (1980) J. Gen. Physiol. 75, 297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn R., Patlak J. (1980) Proc. Natl. Acad. Sci. U.S.A. 77, 6930–6934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S. M., Bergsman J. B., Harata N. C., Scheller R. H., Tsien R. W. (2004) Neuron 41, 243–256 [DOI] [PubMed] [Google Scholar]
- 16.Sorgato M. C., Keller B. U., Stühmer W. (1987) Nature 330, 498–500 [DOI] [PubMed] [Google Scholar]
- 17.Bednarczyk P., Barker G. D., Halestrap A. P. (2008) Biochim. Biophys. Acta 1777, 540–548 [DOI] [PubMed] [Google Scholar]
- 18.Garlid K. D., Dos Santos P., Xie Z. J., Costa A. D., Paucek P. (2003) Biochim. Biophys. Acta 1606, 1–21 [DOI] [PubMed] [Google Scholar]
- 19.Korge P., Honda H. M., Weiss J. N. (2005) Am. J. Physiol. Heart Circ. Physiol. 289, H66–H77 [DOI] [PubMed] [Google Scholar]
- 20.Devin A., Guérin B., Rigoulet M. (1997) J. Bioenerg. Biomembr. 29, 579–590 [DOI] [PubMed] [Google Scholar]
- 21.Hansson M. J., Morota S., Teilum M., Mattiasson G., Uchino H., Elmér E. (2010) J. Biol. Chem. 285, 741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonas E. A., Buchanan J., Kaczmarek L. K. (1999) Science 286, 1347–1350 [DOI] [PubMed] [Google Scholar]
- 23.Bonanni L., Chachar M., Jover-Mengual T., Li H., Jones A., Yokota H., Ofengeim D., Flannery R. J., Miyawaki T., Cho C. H., Polster B. M., Pypaert M., Hardwick J. M., Sensi S. L., Zukin R. S., Jonas E. A. (2006) J. Neurosci. 26, 6851–6862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pape H. C. (1996) Annu. Rev. Physiol. 58, 299–327 [DOI] [PubMed] [Google Scholar]
- 25.Acin-Perez R., Salazar E., Kamenetsky M., Buck J., Levin L. R., Manfredi G. (2009) Cell Metab. 9, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombini M. (1979) Nature 279, 643–645 [DOI] [PubMed] [Google Scholar]
- 27.Porcelli A. M., Ghelli A., Zanna C., Pinton P., Rizzuto R., Rugolo M. (2005) Biochem. Biophys. Res. Commun. 326, 799–804 [DOI] [PubMed] [Google Scholar]
- 28.Lemeshko V. V. (2002) Biophys. J. 82, 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zizi M., Forte M., Blachly-Dyson E., Colombini M. (1994) J. Biol. Chem. 269, 1614–1616 [PubMed] [Google Scholar]
- 30.Mannella C. A., Kinnally K. W. (2008) J. Bioenerg. Biomembr. 40, 149–155 [DOI] [PubMed] [Google Scholar]
- 31.Mannella C. A. (1982) J. Cell Biol. 94, 680–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wunder U. R., Colombini M. (1991) J. Membr. Biol. 123, 83–91 [DOI] [PubMed] [Google Scholar]
- 33.Halestrap A. P. (1989) Biochim. Biophys. Acta 973, 355–382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.