Abstract
Interleukin-6 (IL-6) is a multifunctional cytokine, which may block apoptosis during inflammation to protect cells under very toxic conditions. However, IL-6 also activates STAT3 in many types of human cancer. Recent studies demonstrate that high levels of IL-6 are associated with hepatocellular carcinoma, the most common type of liver cancer. Here we reported that IL-6 promoted survival of human liver cancer cells through activating STAT3 in response to doxorubicin treatment. Endogenous IL-6 levels in SNU-449 cells were higher than in Hep3B cells. Meanwhile, SNU-449 cells were more resistant to doxorubicin than Hep3B cells. Addition of IL-6 induced STAT3 activation in Hep3B cells and led to protection against doxorubicin. In contrast, neutralizing IL-6 with anti-IL-6 antibody decreased survival of SNU-449 cells in response to doxorubicin. To elucidate the mechanism of the anti-apoptotic function of IL-6, we investigated if STAT3 mediated this drug resistance. Targeting STAT3 with STAT3 siRNA reduced the protection of IL-6 against doxorubicin-induced apoptosis, indicating that STAT3 signaling contributed to the anti-apoptotic effect of IL-6. Moreover, we further explored if a STAT3 small molecule inhibitor could abolish this anti-apoptotic effect. LLL12, a STAT3 small molecule inhibitor, blocked IL-6-induced STAT3 phosphorylation, resulting in attenuation of the anti-apoptotic activity of IL-6. Finally, neutralization of endogenous IL-6 with anti-IL-6 antibody or blockade of STAT3 with LLL12 lowered the recovery in SNU-449 cells after doxorubicin treatment. Therefore, our results demonstrated that targeting STAT3 signaling could interrupt the anti-apoptotic function of IL-6 in human liver cancer cells.
Keywords: Apoptosis, Drug Resistance, Interleukin, Liver, STAT Transcription Factor
Introduction
Interleukin-6 is a multifunctional cytokine that was originally characterized acting in immune and inflammatory responses, and inhibits apoptosis in toxic environments during inflammation (1). Growing evidence has indicated that cancers are associated with chronic inflammation. Although serum IL-6 levels were known elevated in colon cancer patients, only recent studies by the Karin group (2) document the role of IL-6 in promoting colitis-associated cancer (CAC) tumorigenesis, enhancing tumor initiation cell proliferation, and preventing apoptosis of premalignant intestinal epithelial cells. High levels of IL-6 have been detected in many types of human epithelial cancers (3), and correlate with proliferation or survival of multiple myeloma, breast cancer stem cells, lung adenocarcinomas, prostate cancer, cervical cancer, gastric cancer, and esophageal carcinoma (4–14). IL-6 antibody alone can induce PC-3 xenograft regression (15). Thus, blocking IL-6-mediated signaling cascades has a potential for treatment of these human cancers (4, 16, 17).
IL-6 signals through IL-6 receptor (IL-6-R), GP130, and Janus kinases (JAKs). IL-6-induced JAK family members activate three major pathways, STAT3, MAPK, and PI3K (1, 18). The signal transducer and activator of transcription 3 (STAT3) is considered as an oncogene and found constitutively activated in many types of human malignancies (19–22). STAT3 signaling is a major pathway for cancer inflammation. It induces a lot of genes crucial for inflammation. Environmental factors, including UV radiation, chemical carcinogen, infection, stress, and smoke, can activate STAT3 via cytokine receptor, toll-like receptor, adrenergic receptor, or nicotinic receptor (22, 23). STAT3 is activated when tyrosine 705 (Tyr-705) is phosphorylated. Phosphorylated STAT3 molecules dimerize and translocate into the nucleus, where they bind to specific DNA response elements and induce the transcription of proliferation and anti-apoptosis associated genes (19, 24), such as BCL-2, BCL-XL, IL-17, IL-23, MCL1, and Survivin (20, 21, 25–28). The activation of STAT3 also promotes tumor angiogenesis via HIF-1 and VEGF (11, 29–31). Therefore, STAT3 is a potential target for cancer therapy. Evidence shows that inhibiting STAT3 using dominant-negative STAT3, antisense oligonucleotides and RNA interference induces tumor cell death (22, 32).
A growing numbers of evidence demonstrate that tumorigenesis caused by STAT3 is mediated by IL-6 signaling (1, 33). Therefore, targeting IL-6/STAT3 signaling pathway should be considered for the treatment of patients with elevated levels of IL-6/STAT3 signaling. IL-6 levels in liver cancer patients are 25-fold higher than healthy adults (34). The high levels of IL-6 are associated with hepatocellular carcinoma (HCC), the most common liver cancer (35).
In this study, we explored the biological role of IL-6 in human liver cancer cells and found that IL-6 stimulated STAT3 phosphorylation and promoted cell survival upon doxorubicin treatment. Interestingly, targeting STAT3 by STAT3 siRNA, anti-IL-6 antibody or a small molecule STAT3 inhibitor, attenuated IL-6-induced drug resistance, suggesting that activated STAT3 contributed to the IL-6-induced cell survival.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and RNA Interference
Human hepatoma cell lines, Hep3B, SNU-387, SNU-398, SNU-449 were obtained from American Type Culture Collection (ATCC, Manassas, VA). Hep3B cells were cultured in Minimum Essential Medium, Eagle (MEM) (ATCC) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. SNU-398 and SNU-449 cells were cultured in RPMI 1640 medium (ATCC) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Human hepatocytes were purchased from ScienCell Research Laboratories (Carlsbad, CA). Human hepatocytes were cultured in Hepatocyte Medium (HM, ScienCell Research Laboratories) including 5% FBS, 1% hepatocyte growth supplement, and 1% penicillin/streptomycin. Interleukin-6 (IL-6) and interferon-γ (IFN-γ) were purchased from Cell Sciences (Canton, MA). Antibody to human IL-6, which can neutralize IL-6 bioactivity, was from R&D Systems (Minneapolis, MN). The human IL-6 ELISA kit was from Pepro Tech Inc (Rocky Hill, NJ).
siRNA was transfected into Hep3B cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. STAT3 small interfering RNA (siRNA) kit and scrambled control siRNA were purchased from Cell Signaling Technology (Beverly, MA).
Western Blot
Whole cell extracts were prepared by lysing the cells in RIPA buffer containing mixture proteasome inhibitor. Lysates were then centrifuged at 13,300 rpm for 10 min at 4 °C, and the supernatant was collected. Protein samples were separated by SDS-PAGE, transferred onto PVDF membrane, and immunoblotted with the appropriate antibody. Antibodies to p-STAT3 (Y705), STAT3, p-STAT1 (Y701), STAT1, p-ERK1/2 (T202/Y204), p-JAK2, JAK2, p-JAK1, JAK1, GP130, GAPDH, and HRP-conjugated secondary antibodies were from Cell Signaling Technology. The target protein was examined by chemiluminescence (Cell Signaling Technology).
Immunofluorescence
Cells were seeded on a glass slide and fixed with cold methanol for 15 min. After a washing in phosphate-buffered saline (PBS), the slide was blocked with 5% normal goat serum and 0.3% Triton X-100 in PBS for at least 1 h. Then the slide was incubated with primary antibody. After overnight incubation, the slide was washed with PBST and then incubated with anti-rabbit FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). The cells were mounted in Vectashield HardSet mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Pictures were captured by Leica Microsystems (Bannockburn, IL).
Apoptosis Assay
Apoptosis was measured with caspase3/7 assay kit (Promega, Madison, WI) according to the manufacturer's protocol. Briefly, 20,000 cells were seeded into 96-well plates. After the treatment, 100 μl of Apo-One caspase3/7 reagent was added to each well and was incubated at 37 °C for 30 min. The fluorescence was measured at an excitation wavelength range of 485 nm and an emission wavelength range of 530 nm.
Viability Assay
Viability was performed with CyQUANT NF Assay (Invitrogen) according to the manufacturer's protocol. Briefly, 2,000 cells were seeded into a 96-well plate. After the 48-h treatment, medium was removed from the plate, and 50 μl of dye binding solution were added to each well. The plate was covered and incubated at 37 °C for 30 min. The fluorescence was measured at an excitation wavelength range of 485 nm and an emission wavelength range of 530 nm.
Clonogenic Assay
Cells were washed with PBS twice and fixed with cold methanol at −20 °C for 10 min. After 10 min, cells were stained with 1% crystal violet (25% methanol) at room temperature for 10 min. After the staining, the plates were washed with distill water and dried.
Statistical Analysis
The statistical significance was calculated using Student's t test. p values of <0.05 were considered significant.
RESULTS
IL-6 Induces STAT3 Phosphorylation
To examine the endogenous IL-6 levels in different liver cancer cells, four human liver cancer cell lines (Hep3B, SNU-387, SNU-398, and SNU449) and human hepatocytes were cultured in the same medium, hepatocyte medium, for 24 h. After the incubation, the IL-6 levels in the culture medium were evaluated using IL-6 ELISA assay. As illustrated in Fig. 1A, the IL-6 levels were remarkably elevated in SNU-387 and SNU-449 cell lines compared with human hepatocytes, Hep3B and SNU-398. To explore if IL-6 would induce STAT3 phosphorylation in cells with lower endogenous IL-6, Hep3B, and SNU-398 cells were cultured in serum-free medium for 24 h and then were treated with different concentrations of IL-6 (0–50 ng/ml) for 30 min. As illustrated in Fig. 1B, IL-6 induced STAT3 phosphorylation in a dose-dependent manner, but did not alter the expression of total STAT3. To confirm if IL-6-induced phosphorylated STAT3 would translocate to the nucleus, the localization of phosphorylated STAT3 was determined by staining with anti-phosphorylated STAT3 primary antibody and FITC-conjugated secondary antibody. The nucleus was stained with DAPI. Fig. 1C demonstrated that phosphorylated STAT3 translocated to the nucleus following IL-6 treatment.
FIGURE 1.
IL-6 induces STAT3 phosphorylation. A, human hepatocyte, Hep3B, SNU-387, SNU-398, and SNU-449 cells were cultured in Hepatocyte Medium for 24 h. Endogenous IL-6 was accessed by IL-6 ELISA assay. B, Hep3B and SNU-398 cells were cultured in serum-free medium. After 24 h of serum deprivation, cells were treated with various concentrations of IL-6 for 30 min. STAT3 phosphorylation and total STAT3 were detected by Western blot. C, Hep3B and SNU-398 cells were cultured under the same conditions and then were treated with 50 ng/ml of IL-6 for 30 min. The distribution of phosphorylated STAT3 was analyzed by immunofluorescence.
SNU-449 Cells Are More Doxorubicin-resistant than Hep3B Cells
To determine if liver cancer cells with higher endogenous IL-6 would be more resistant to drug treatment than those with lower endogenous IL-6, we treated SNU-449 and Hep3B cells with different concentrations of doxorubicin. After 24 h of treatment, the characteristic morphology of cell death was observed in Hep3B cells but not in SNU-449 cells (Fig. 2A). After another 24 h, cell viability assay was performed. Cell viability did not show significant change in SNU-449 treated by 2.5 or 5 μm doxorubicin whereas 40% decrease was observed when treated by 10 μm doxorubicin (Fig. 2B). In contrast, cell viability decreased around 60, 80, and 80% when Hep3B cells were treated by 2.5, 5, and 10 μm doxorubicin, respectively (Fig. 2B). These results demonstrated that SNU-449 cells were more resistant to doxorubicin than Hep3B cells. To examine if liver cancer cells with higher IL-6 would also show enhanced endogenous STAT3, we analyzed phosphorylated STAT3 in SNU-449 and Hep3B cells. The Western blot results showed that phosphorylated STAT3 was elevated in SNU-449 cells compared with Hep3B cells, whereas total STAT3 level did not change (Fig. 2C).
FIGURE 2.
SNU-449 cells are more doxorubicin-resistant than Hep3B cells. A, SNU-449 cells and Hep3B cells were treated with various concentrations of doxorubicin. After 24 h, morphological examination was performed. B, SNU-449 cells and Hep3B cells were treated under the same conditions. After 48 h, cell viability assay was performed. The data showed the percent decrease in cell viability compared with untreated cells and represented three independent results. C, endogenous phosphorylated STAT3 and total STAT3 in Hep3B and SNU-449 cells were analyzed by Western blot.
IL-6 Confers Resistance to Apoptosis Induced by Doxorubicin
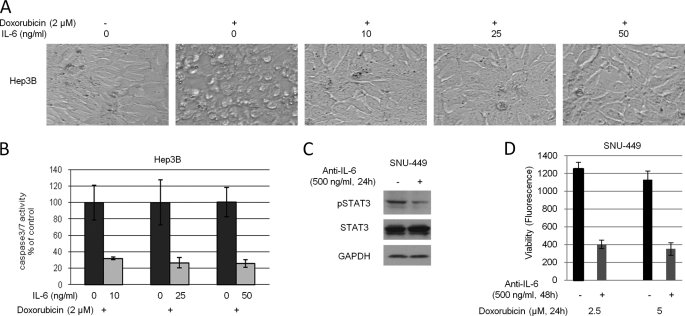
To explore if IL-6 would protect cells upon drug treatment, Hep3B cells were pretreated with 2 μm doxorubicin for 2 h followed by different doses of IL-6 (0–50 ng/ml). After 6 h of incubation, the characteristic morphology of cell death was observed in cells treated with doxorubicin whereas the addition of IL-6 rescued doxorubicin-caused cell death (Fig. 3A). 10 ng/ml of IL-6 effectively displayed anti-apoptotic effect. To further confirm our results, Hep3B cells were treated under the similar conditions and after 24 h, apoptotic levels were measured using caspase3/7 assay. Fig. 3B showed that 10 ng/ml of IL-6 treatment exhibited 70% decrease in doxorubicin-caused apoptosis. Similar results were observed in cells treated with 25 and 50 ng/ml of IL-6.
FIGURE 3.
IL-6 protects cells against doxorubicin-induced apoptosis. A, Hep3B cells were pretreated with 2 μm doxorubicin for 2 h followed by various concentrations of IL-6 for 6 h. Morphological examination was performed after treatment. B, Hep3B cells were treated under the same conditions. After 24 h, caspase3/7 activity was analyzed. The data showed the percent decrease in csapase3/7 activity compared with control cells and represented three independent results. C, SNU-449 cells were treated with 500 ng/ml of anti-IL-6 antibody or control IgG for 24 h. After the incubation, whole cell lysates were extracted. Phosphorylated STAT3 and GAPDH were detected by Western blot. D, SNU-449 cells were pretreated with 500 ng/ml of anti-IL-6 antibody or control IgG for 24 h. After the pretreatment, cells were further treated with doxorubicin for 24 h. After the 24 h treatment, cell viability was measured. The data represented eight independent results.
To investigate if disruption of IL-6 autocrine would down-regulate STAT3 phosphorylation and would interrupt the anti-apoptotic effect of IL-6, SNU-449 cells were treated with anti-IL-6 antibody and phosphorylated STAT3 was analyzed by Western blot. Our results demonstrated that the treatment with anti-IL-6 antibody reduced phosphorylated STAT3 levels (Fig. 3C). Then we pretreated SNU-449 cells with anti-IL-6 antibody overnight to neutralize the endogenous IL-6. After the pretreatment, cells were treated with doxorubicin for 24 h followed by cell viability assay. Fig. 3D showed that cells treated with doxorubicin and anti-IL-6 antibody exhibited 70% decrease in viability compared with cells treated with doxorubicin alone. The data in Fig. 3 suggested that IL-6 promoted cell survival in response to drug treatment.
IL-6 Causes Anti-apoptosis through Activating STAT3
To explore if STAT3 signaling would contribute to the anti-apoptotic activity of IL-6, we targeted STAT3 by STAT3 siRNA. siRNA was transfected into Hep3B cells with less than 50% transfection efficiency (data not shown). The transfected cells were cultured in serum-free medium overnight and then were treated with 50 ng/ml of IL-6 for 30 min. Western blot results demonstrated a decrease in phosphorylated STAT3 compared with control scrambled siRNA-transfected cells (Fig. 4A). Phosphorylated AKT and GAPDH were used as controls, which were not affected by STAT3 siRNA.
FIGURE 4.
Inhibition of phosphorylated STAT3 blocks IL-6-induced cell survival. A, two siRNAs targeting STAT3 were transfected into Hep3B cells. After overnight incubation, the cells were cultured in serum-free medium for 24 h followed by IL-6 for 30 min. Phosphorylated STAT3, phosphorylated AKT, and GAPDH were detected by Western blot. B, STAT3 siRNA-transfected Hep3B cells were treated with 2 μm doxorubicin for 2 h followed by 50 ng/ml of IL-6 for 6 h. Morphological examination was performed after treatment. C, STAT3 siRNA-transfected Hep3B cells were pretreated with doxorubicin for 2 h followed by 50 ng/ml of IL-6 for 24 h. Caspase3/7 activity was assayed. The data showed the percent increase in caspase3/7 compared with scrambled siRNA-transfected control groups and represented three independent experiments. *, p < 0.02.
To investigate if knocking down STAT3 would decrease the anti-apoptotic effect of IL-6, STAT3 siRNA-transfected cells were treated under the similar conditions employed in Fig. 4A. As seen in Fig. 4B, STAT3 siRNA-transfected cells exhibited more characteristic morphology of apoptosis induced by doxorubicin compared with scrambled control siRNA transfected cells. Caspase3/7 assay further showed that knocking down STAT3 increased doxorubicin-induced apoptosis when treated with 1 or 2 μm doxorubicin (Fig. 4C). Therefore, the data in Fig. 4 suggested that IL-6 promoted cell survival via the enhanced STAT3 signaling.
STAT3 Small Molecule Inhibitor Blocks IL-6/STAT3 Signaling
To investigate if a STAT3 inhibitor could block IL-6-induced STAT3 phosphorylation, Hep3B and SNU-398 cells were cultured in serum-free medium for 24 h and then were pretreated with 5 or 10 μm LLL12 for 2 h followed by 50 ng/ml of IL-6 for 30 min. Fig. 5A showed that IL-6 induced STAT3 phosphorylation but had no effect on LLL12-pretreated cells. IL-6 or LLL12 treatment did not alter the expression levels of total STAT3.
FIGURE 5.
A STAT3 small molecule inhibitor LLL12 blocks IL-6-induced STAT3 phosphorylation. A, Hep3B and SNU-398 cells were cultured in serum-free medium for 24 h and then were pretreated with DMSO or LLL12 for 2 h followed by IL-6 for 30 min. Phosphorylated STAT3 and total STAT3 were detected by Western blot. B, Hep3B and SNU-398 cells were cultured in serum-free medium for 24 h and then were pretreated with LLL12 for 2 h followed by IFN-γ for 30 min. The expression levels of phosphorylated STAT1, and total STAT1 were analyzed by Western blot. C and D, Hep3B cells were treated under the same conditions, and the distribution of phosphorylated STAT3 and total STAT3 was analyzed by immunofluorescence.
To explore if this STAT3 small molecule inhibitor would affect STAT1 activity, we pretreated Hep3B and SNU-398 cells for 2 h followed by IFN-γ for 30 min. Fig. 5B showed that IFN-γ induced STAT1 phosphorylation, whereas LLL12 pretreatment did not block IFN-γ-induced STAT1 phosphorylation.
We have shown in Fig. 1C that phosphorylated STAT3 translocated to the nucleus after IL-6 treatment. To investigate if LLL12 would block this translocation, we pretreated cells with 5 μm LLL12 for 2 h and then treated the cells with 50 ng/ml of IL-6 for 30 min. The cells were fixed and stained by anti-phosphorylated STAT3 primary antibody and FITC conjugated secondary antibody. The nucleus was stained with DAPI. As illustrated in Fig. 5C, LLL12 pretreated cells did not show phosphorylated STAT3 in the nucleus. Nonactivated STAT3 is in the cytoplasm. STAT3 translocates to the nucleus when it is activated (36). To confirm if the phosphorylation induced by IL-6 would translocate STAT3 to the nucleus and if LLL12 pretreatment could reverse this process, we cultured Hep3B cells in serum-free medium for 24 h. Then, the starved cells were pretreated with 5 μm LLL12 for 2 h followed by 50 ng/ml of IL-6 for 30 min. The cells were stained by anti-STAT3 primary antibody and secondary antibody. Fig. 5D demonstrated that STAT3 was in the cytoplasm when cultured in serum-free medium. IL-6 treatment translocated STAT3 to the nucleus whereas LLL12 pretreatment blocked IL-6-induced STAT3 nuclear translocation.
LLL12 Inhibits IL-6-induced STAT3 Phosphorylation in a Dose- and Time-dependent Manner
We further examined if the inhibitory effect of LLL12 would be dose dependent. We pretreated cells with different concentrations of LLL12 (0–5 μm) for 2 h and then treated the cells with 50 ng/ml of IL-6 for 30 min. Fig. 6A demonstrated a LLL12 dose dependence. Exposure of cells to 0.5 μm LLL12 was able to suppress STAT3 phosphorylation.
FIGURE 6.
The STAT3 small molecule inhibitor LLL12 blocks STAT3 phosphorylation in a dose- and time-dependent manner. A, Hep3B and SNU-398 cells were cultured in serum-free medium for 24 h and then were pretreated with various concentrations of LLL12 (0.5–5 μm) for 2 h followed by IL-6 for 30 min. Phosphorylated STAT3 and total STAT3 were detected by Western blot. B, Hep3B and SNU-398 cells were cultured under the same conditions and then were treated with 5 μm LLL12 for different time points (0–2 h). After pretreatment, IL-6 was added to the cultured cells. Phosphorylated STAT3 and total STAT3 were detected by Western blot. C, Hep3B cells were cultured in serum-free medium for 24 h and then were pretreated with 5 μm LLL12 for 2 h. After the 2 h pretreatment, medium was discarded, and fresh medium without LLL12 was added. After the indicated incubation (0–24 h), the cells were treated with IL-6 for 30 min. Phosphorylated STAT3 and total STAT3 were detected by Western blot. D, Hep3B cells were cultured in serum-free medium for 24 h and then were pretreated with 5 μm LLL12 for 2 h. After 2 h of pretreatment, the medium was discarded, and fresh medium without LLL12 was added. Cycloheximide was also added into the medium to block protein synthesis. After 12 h, the cells were treated with IL-6 for 30 min. Phosphorylated STAT3 and total STAT3 were detected by Western blot.
To study if the inhibitory effect of LLL12 would also be time dependent, we pretreated cells with LLL12 for different time points (0–2 h) followed by 50 ng/ml of IL-6 for 30 min. As illustrated in Fig. 6B, IL-6-induced STAT3 phosphorylation was suppressed by LLL12 in a time-dependent manner.
To examine if the inhibitory effect of LLL12 would be reversible, we pretreated cells with 5 μm LLL12 for 2 h. Then LLL12-supplemented medium was removed and fresh medium without LLL12 was added to the cells. After different incubation time points, the cells were treated with 50 ng/ml of IL-6 for 30 min. As seen in Fig. 6C, STAT3 phosphorylation recovered in Hep3B cells 12 h after LLL12 treatment. The results suggested that the inhibitory effect of LLL12 on STAT3 phosphorylation was reversible. We further blocked protein synthesis using cycloheximide and repeated the experiment in Fig. 6C to examine if IL-6 would induce STAT3 phosphorylation 12 h after LLL12 treatment. As illustrated in Fig. 6D, STAT3 phosphorylation recovered in cycloheximide-treated cells, indicating that the recovered STAT3 phosphorylation was not due to the new synthesized STAT3 protein.
LLL12 Reduces the Anti-apoptotic Activity of IL-6
To investigate if a small molecule STAT3 inhibitor would decrease anti-apoptotic activity of IL-6, we chose a low dose (0.5 μm) of LLL12 to pretreat Hep3B cells for only 2 h. After pretreatment, LLL12 was removed, and cells were treated with doxorubicin and IL-6 overnight. Fig. 7A clearly demonstrated that LLL12-pretreated cells showed more cell death compared with the cells without LLL12 pretreatment. This result was quantified by cell viability assay (Fig. 7B). LLL12 pretreatment completely blocked IL-6-induced doxorubicin resistance. Interestingly, the 2 h pretreatment with LLL12 did not cause cell death (Fig. 7, A and B). These data suggested that low dose of STAT3 inhibitor alone might not lead to cell death but was able to attenuate IL-6-induced cell survival upon drug treatment.
FIGURE 7.
LLL12 decreases IL-6-induced cell survival. A, Hep3B cells were pretreated with 0.5 μm LLL12 for 2 h. After the pretreatment, LLL12 was removed, and fresh medium was added. The cells were treated as indicated. After overnight treatment, morphological examination was performed. B, results from A were quantified by cell viability assay. C and D, Hep3B and SNU-398 cells were pretreated with 0.5 μm LLL12 for 2 h. After the pretreatment, cells were treated with IL-6 for 30 min. The levels of JAK1, JAK2, GP130, p-AKT, and p-ERK1/2 were analyzed by Western blot.
To explore if STAT3 inhibitor LLL12 would affect other components of IL-6/STAT3 signaling or other IL-6 signaling, we pretreated Hep3B and SNU-398 cells with 0.5 μm LLL12 for 2 h, followed by IL-6 for 30 min. JAK1, JAK2, GP130, phosphorylated AKT, and phosphorylated ERK were analyzed by Western blot. Fig. 7, C and D showed that 0.5 μm LLL12 did not affect other proteins of IL-6 signaling pathways. The data in Fig. 7 suggested that the low dose of STAT3 small molecule inhibitor LLL12 attenuated IL-6-induced cell survival through specifically inhibiting STAT3 phosphorylation.
Blockade of IL-6 or STAT3 Reduces Cell Recovery from Doxorubicin
To investigate if blocking IL-6 or STAT3 would reduce cell recovery from doxorubicin, SNU-449 cells were treated with 5 μm doxorubicin alone, doxorubicin plus anti-IL-6 antibody, or doxorubicin plus LLL12, respectively. After overnight treatment, live cells were counted, and 5,000 cells were re-plated. The cells were cultured in doxorubicin-free medium for 7 days. Anti-IL-6 antibody or LLL12 was supplemented in medium. After 7 days, cells were fixed and stained with 1% crystal violet. Fig. 8 clearly showed that many cells recovered from control cells, whereas fewer cells recovered from anti-IL-6 antibody or LLL12-treated cells. The data in Fig. 8 suggested that blockade of IL-6 or STAT3 reduced cell recovery from drug treatment.
FIGURE 8.
Blockade of IL-6 or STAT3 reduces cell recovery from doxorubicin. SNU-449 cells were treated with 2 μm doxorubicin, anti-IL-6 antibody, or LLL12 as indicated. After overnight treatment, live cells were counted and 5,000 cells were re-plated. Cells were treated with control IgG, anti-IL-6 antibody, or LLL12, respectively. After 7 days, cells were fixed and stained with 1% crystal violet. A, pictures were captured under microscopy at different magnification. B, original plates were scanned. Three independent experiments were performed.
Blockade of IL-6/STAT3 Does Not Affect Cell Viability in Human Primary Hepatocytes Treated with Doxorubicin
To investigate if blocking IL-6 or STAT3 would reduce viability in normal liver cells, human primary hepatocytes were treated with 500 ng/ml of anti-IL-6 antibody or 0.5 μm LLL12 for 2 or 24 h, respectively. After 24 h, cell viability was measured. As shown in Fig. 9A, anti-IL-6 antibody or LLL12 did not reduce cell viability compared with untreated cells.
FIGURE 9.
Blockade of IL-6 or STAT3 does not affect cell viability in human primary hepatocytes treated with doxorubicin. A, human primary hepatocytes were treated with 500 ng/ml of anti-IL-6 antibody or 0.5 μm LLL12 for 2 h and 24 h, respectively. After 24 h, cell viability was measured. Non-treated cells (NT) were used as control. The data represented three independent results. B, human primary hepatocytes were pretreated with 500 ng/ml of anti-IL-6 antibody or 0.5 μm LLL12 for 2 h. After the pretreatment, LLL12 was discarded and fresh medium with different concentrations of doxorubicin was added. After 24 h, cell viability was measured. The data represented three independent results.
We further examined if blockade of IL-6 or STAT3 signaling in human primary normal liver cells would reduce cell viability when treated with doxorubicin. Human primary hepatocytes were pretreated with 500 ng/ml of anti-IL-6 antibody or 0.5 μm LLL12 for 2 h. After the pretreatment, LLL12 was discarded, and fresh medium with different concentrations of doxorubicin was added. After 24 h, cell viability was measured. Our results showed that when doxorubicin concentration increased from 0.5 to 1 μm, cell viability decreased significantly. However, the treatment by anti-IL-6 antibody or LLL12 with doxorubicin did not markedly reduce viability in human primary hepatocytes compared with the cells treated by doxorubicin alone (Fig. 9B), indicating that unlike liver cancer cells, liver normal cells did not rely on IL-6/STAT3 signaling.
DISCUSSION
Growing evidence has shown that inflammatory signals from the surrounding microenvironment promote tumor growth and progression. They are also associated with survival pathways and immunosuppression (37–39). It has been well documented that inflammatory cytokine IL-6/STAT3 signaling is involved in many types of human cancer (2, 6, 7, 9, 40–42). IL-6 can promote cell proliferation in multiple myelomas, ovarian carcinoma and prostate cancer cells in vitro (41, 43, 44) and tumor growth in vivo in prostate, lung, and breast (37, 44). It is also related to cell migration and adhesion (45).
Liver cancer is a major health problem because of the poor prognosis. IL-6 plays a critical role in liver cancer (34). Hepatocellular carcinoma (HCC) is the most common liver tumor with a 7% five-year survival rate. It mainly occurs in males due to differences in IL-6 production between males and females (35). Moreover, obesity may increase the risk of liver and other cancer due to the chronic inflammatory response caused by enhanced levels of IL-6/STAT3 and TNF/STAT3 signaling (46).
IL-6 is a key event in tumorigenesis, but its biological function in liver cancers is not clear. Previous studies have demonstrated that IL-6/STAT3 provides hepatoprotection against liver damage (47). Based on these results, we hypothesized that IL-6 would promote survival of liver cancer cells via STAT3 upon drug treatment. We found that the endogenous levels of IL-6 in SNU-387 and SNU-449 liver cancer cell lines were much higher than those in human hepatocytes. Hep3B and SNU-398 cells showed similar levels of IL-6 to human hepatocytes. Because IL-6 levels in liver cancer patients are 25-fold higher than healthy adults (34), we wondered how Hep3B and SNU-398 would respond upon IL-6. We found that IL-6 activated STAT3 in these two cell lines. To study the phenotype of IL-6 in liver cancer cells, we induced apoptosis in Hep3B cells using doxorubicin. Interestingly, exogenous addition of IL-6 protected cells toward apoptosis induced by doxorubicin. To explore if IL-6-promoted cell survival upon doxorubicin would be caused by activated STAT3, we targeted STAT3 by siRNA and observed that STAT3 knocked down cells showed higher level of apoptosis. When SNU-449 cells with enhanced endogenous IL-6 were treated with IL-6 antibody, phosphorylated STAT3 was down-regulated. As a result, IL-6 antibody treated SNU-449 cells exhibited lowered cell viability, suggesting that IL-6 induced cell survival via STAT3. In human cervical cancer, IL-6 causes anti-apoptosis induced by doxorubicin via PI3K/AKT instead of STAT3 (10). Therefore, IL-6 exerts its activity through different mechanisms depending on cell types. In prostate cancer, IL-6 also causes anti-apoptosis via Mcl-1 (48). In pancreatic cancer, PANC-1 cells, however, we found that IL-6 induced STAT3 in a dose-dependent manner, but it did not cause anti-apoptosis (data not shown), suggesting that the anti-apoptotic effect of IL-6 is cell type-specific.
Treatment with anti-IL-6 monoclonal antibody or with lentivirus expressing shSTAT3 has been shown to induce apoptosis in prostate cancer and glioblastoma multiforme in vitro or in vivo (15, 49, 50). To study if STAT3 small molecule inhibitor would show similar effect to STAT3 siRNA and IL-6 antibody on IL-6/STAT3 signaling in liver cancer cells, we used a STAT3 small molecule inhibitor, which was reported by our laboratory (51). Our previous results show that high concentration of this STAT3 small molecule inhibitor may cause apoptosis in different types of human cancer with elevated STAT3. In this study, we demonstrated that this STAT3 small molecule inhibitor blocked IL-6-induced STAT3 phosphorylation in a dose-dependent manner. Low concentration of LLL12 did not cause cell death whereas it attenuated IL-6-induced cell survival upon doxorubicin treatment via down-regulation of phosphorylated STAT3. Our data indicate that blockade of STAT3 signaling with STAT3 small molecule inhibitor may reduce IL-6-induced anti-apoptosis.
Although LLL12 is a STAT3 structure-based molecule, we wondered if LLL12 could affect other components of IL-6/STAT3 signaling pathway. We examined JAK1, JAK2, and GP130. JAK1 and JAK2 phosphorylate STAT3 (52, 53). GP130 provides docking site for STAT3 (52). LLL12 did not change JAK1, JAK2, or GP130. IL-6 can also activate PI3K and ERK signaling pathways (9), but LLL12 did not alter them either. In conclusion, IL-6 could protect human liver cancer cells toward apoptosis via STAT3 signaling. Targeting STAT3 with STAT3 siRNA, anti-IL-6 antibody, or a STAT3 small molecule inhibitor may decrease or block IL-6-induced liver cancer cell survival upon drug treatment.
Short term and long term IL-6 exposure show paradoxical effects on liver injury and repair (54). Short term production of IL-6 is a natural response of the body during the liver injury to protect cells (35). In contrast, long term exposure to IL-6 can impair liver regeneration and even increase liver injury (54). Our results also demonstrated that ablation of IL-6/STAT3 in human primary hepatocytes did not affect their viability under normal conditions and did not induce more cell death when treated with chemotherapeutic agents.
In conclusion, IL-6/STAT3 signaling may be not crucial in human primary hepatocytes. However, this signaling can promote hepatocellular carcinoma survival upon drug treatment. Blockade of IL-6/STAT3 may be able to render hepatocellular carcinoma with elevated endogenous IL-6/STAT3 more sensitive to chemotherapeutic agent-induced apoptosis.
Footnotes
This work was supported, in whole or in part, by National Institutes of Health R21 Grant R21CA133652-01 (to J. L.). This work was also supported by a Pancreatic Cancer Action Network-AACR, National Foundation for Cancer Research grant.
REFERENCES
- 1.Hodge D. R., Hurt E. M., Farrar W. L. (2005) Eur. J. Cancer 41, 2502–2512 [DOI] [PubMed] [Google Scholar]
- 2.Grivennikov S., Karin E., Terzic J., Mucida D., Yu G. Y., Vallabhapurapu S., Scheller J., Rose-John S., Cheroutre H., Eckmann L., Karin M. (2009) Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer Z. T., Brugge J. S. (2007) J. Clin. Invest. 117, 3660–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharti A. C., Donato N., Aggarwal B. B. (2003) J. Immunol. 171, 3863–3871 [DOI] [PubMed] [Google Scholar]
- 5.Sansone P., Storci G., Tavolari S., Guarnieri T., Giovannini C., Taffurelli M., Ceccarelli C., Santini D., Paterini P., Marcu K. B., Chieco P., Bonafè M. (2007) J. Clin. Invest. 117, 3988–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao S. P., Mark K. G., Leslie K., Pao W., Motoi N., Gerald W. L., Travis W. D., Bornmann W., Veach D., Clarkson B., Bromberg J. F. (2007) J. Clin. Invest. 117, 3846–3856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou W., Ni Z., Dyer K., Tweardy D. J., Gao A. C. (2000) Prostate 42, 239–242 [DOI] [PubMed] [Google Scholar]
- 8.Spiotto M. T., Chung T. D. (2000) Prostate 42, 88–98 [DOI] [PubMed] [Google Scholar]
- 9.Culig Z., Steiner H., Bartsch G., Hobisch A. (2005) J. Cell. Biochem. 95, 497–505 [DOI] [PubMed] [Google Scholar]
- 10.Wei L. H., Kuo M. L., Chen C. A., Chou C. H., Cheng W. F., Chang M. C., Su J. L., Hsieh C. Y. (2001) Oncogene 20, 5799–5809 [DOI] [PubMed] [Google Scholar]
- 11.Wei L. H., Kuo M. L., Chen C. A., Chou C. H., Lai K. B., Lee C. N., Hsieh C. Y. (2003) Oncogene 22, 1517–1527 [DOI] [PubMed] [Google Scholar]
- 12.Lin M. T., Juan C. Y., Chang K. J., Chen W. J., Kuo M. L. (2001) Carcinogenesis 22, 1947–1953 [DOI] [PubMed] [Google Scholar]
- 13.Leu C. M., Wong F. H., Chang C., Huang S. F., Hu C. P. (2003) Oncogene 22, 7809–7818 [DOI] [PubMed] [Google Scholar]
- 14.Meng F., Yamagiwa Y., Ueno Y., Patel T. (2006) J. Hepatol 44, 1055–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith P. C., Keller E. T. (2001) Prostate 48, 47–53 [DOI] [PubMed] [Google Scholar]
- 16.Liu S., Ishikawa H., Li F. J., Ma Z., Otsuyama K., Asaoku H., Abroun S., Zheng X., Tsuyama N., Obata M., Kawano M. M. (2005) Cancer Res. 65, 2269–2276 [DOI] [PubMed] [Google Scholar]
- 17.Liu S., Ma Z., Cai H., Li Q., Rong W., Kawano M. (2010) Eur. J. Haematol. 84, 137–144 [DOI] [PubMed] [Google Scholar]
- 18.Yu C. Y., Wang L., Khaletskiy A., Farrar W. L., Larner A., Colburn N. H., Li J. J. (2002) Oncogene 21, 3949–3960 [DOI] [PubMed] [Google Scholar]
- 19.Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. (1999) Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 20.Yu H., Kortylewski M., Pardoll D. (2007) Nat. Rev. Immunol. 7, 41–51 [DOI] [PubMed] [Google Scholar]
- 21.Kortylewski M., Xin H., Kujawski M., Lee H., Liu Y., Harris T., Drake C., Pardoll D., Yu H. (2009) Cancer Cell 15, 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H., Pardoll D., Jove R. (2009) Nat. Rev. Cancer 9, 798–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhari S. R., Khan M. A., Harris G., Picker D., Jacob G. S., Block T., Shailubhai K. (2007) Mol. Cancer Ther. 6, 112–121 [DOI] [PubMed] [Google Scholar]
- 24.Real P. J., Sierra A., De Juan A., Segovia J. C., Lopez-Vega J. M., Fernandez-Luna J. L. (2002) Oncogene 21, 7611–7618 [DOI] [PubMed] [Google Scholar]
- 25.Epling-Burnette P. K., Liu J. H., Catlett-Falcone R., Turkson J., Oshiro M., Kothapalli R., Li Y., Wang J. M., Yang-Yen H. F., Karras J., Jove R., Loughran T. P., Jr. (2001) J. Clin. Invest. 107, 351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoki Y., Feldman G. M., Tosato G. (2003) Blood 101, 1535–1542 [DOI] [PubMed] [Google Scholar]
- 27.Amin H. M., McDonnell T. J., Ma Y., Lin Q., Fujio Y., Kunisada K., Leventaki V., Das P., Rassidakis G. Z., Cutler C., Medeiros L. J., Lai R. (2004) Oncogene 23, 5426–5434 [DOI] [PubMed] [Google Scholar]
- 28.Gritsko T., Williams A., Turkson J., Kaneko S., Bowman T., Huang M., Nam S., Eweis I., Diaz N., Sullivan D., Yoder S., Enkemann S., Eschrich S., Lee J. H., Beam C. A., Cheng J., Minton S., Muro-Cacho C. A., Jove R. (2006) Clin. Cancer Res. 12, 11–19 [DOI] [PubMed] [Google Scholar]
- 29.Niu G., Wright K. L., Huang M., Song L., Haura E., Turkson J., Zhang S., Wang T., Sinibaldi D., Coppola D., Heller R., Ellis L. M., Karras J., Bromberg J., Pardoll D., Jove R., Yu H. (2002) Oncogene 21, 2000–2008 [DOI] [PubMed] [Google Scholar]
- 30.Wei D., Le X., Zheng L., Wang L., Frey J. A., Gao A. C., Peng Z., Huang S., Xiong H. Q., Abbruzzese J. L., Xie K. (2003) Oncogene 22, 319–329 [DOI] [PubMed] [Google Scholar]
- 31.Xu Q., Briggs J., Park S., Niu G., Kortylewski M., Zhang S., Gritsko T., Turkson J., Kay H., Semenza G. L., Cheng J. Q., Jove R., Yu H. (2005) Oncogene 24, 5552–5560 [DOI] [PubMed] [Google Scholar]
- 32.Gao L., Zhang L., Hu J., Li F., Shao Y., Zhao D., Kalvakolanu D. V., Kopecko D. J., Zhao X., Xu D. Q. (2005) Clin. Cancer Res. 11, 6333–6341 [DOI] [PubMed] [Google Scholar]
- 33.Hodge D. R., Li D., Qi S. M., Farrar W. L. (2002) Biochem. Biophys. Res. Commun. 292, 287–291 [DOI] [PubMed] [Google Scholar]
- 34.Porta C., De Amici M., Quaglini S., Paglino C., Tagliani F., Boncimino A., Moratti R., Corazza G. R. (2008) Ann. Oncol. 19, 353–358 [DOI] [PubMed] [Google Scholar]
- 35.Naugler W. E., Sakurai T., Kim S., Maeda S., Kim K., Elsharkawy A. M., Karin M. (2007) Science 317, 121–124 [DOI] [PubMed] [Google Scholar]
- 36.Darnell J. E., Jr. (1997) Science 277, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 37.Bromberg J., Wang T. C. (2009) Cancer Cell 15, 79–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balkwill F., Mantovani A. (2001) Lancet 357, 539–545 [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov S., Karin M. (2008) Cancer Cell 13, 7–9 [DOI] [PubMed] [Google Scholar]
- 40.Spiotto M. T., Chung T. D. (2000) Prostate 42, 186–195 [DOI] [PubMed] [Google Scholar]
- 41.Rabinovich A., Medina L., Piura B., Segal S., Huleihel M. (2007) Anticancer Res. 27, 267–272 [PubMed] [Google Scholar]
- 42.Bollrath J., Phesse T. J., von Burstin V. A., Putoczki T., Bennecke M., Bateman T., Nebelsiek T., Lundgren-May T., Canli O., Schwitalla S., Matthews V., Schmid R. M., Kirchner T., Arkan M. C., Ernst M., Greten F. R. (2009) Cancer Cell 15, 91–102 [DOI] [PubMed] [Google Scholar]
- 43.Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. (1988) Nature 332, 83–85 [DOI] [PubMed] [Google Scholar]
- 44.Malinowska K., Neuwirt H., Cavarretta I. T., Bektic J., Steiner H., Dietrich H., Moser P. L., Fuchs D., Hobisch A., Culig Z. (2009) Endocr Relat Cancer 16, 155–169 [DOI] [PubMed] [Google Scholar]
- 45.Santer F. R., Malinowska K., Culig Z., Cavarretta I. T. (2010) Endocr. Relat. Cancer 17, 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park E. J., Lee J. H., Yu G. Y., He G., Ali S. R., Holzer R. G., Osterreicher C. H., Takahashi H., Karin M. (2010) Cell 140, 197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taub R. (2003) J. Clin. Invest. 112, 978–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavarretta I. T., Neuwirt H., Untergasser G., Moser P. L., Zaki M. H., Steiner H., Rumpold H., Fuchs D., Hobisch A., Nemeth J. A., Culig Z. (2007) Oncogene 26, 2822–2832 [DOI] [PubMed] [Google Scholar]
- 49.Kudo M., Jono H., Shinriki S., Yano S., Nakamura H., Makino K., Hide T., Muta D., Ueda M., Ota K., Ando Y., Kuratsu J. (2009) J. Neurosurg 111, 219–225 [DOI] [PubMed] [Google Scholar]
- 50.Li G. H., Wei H., Chen Z. T., Lv S. Q., Yin C. L., Wang D. L. (2009) J. Neurooncol. 91, 165–174 [DOI] [PubMed] [Google Scholar]
- 51.Lin L., Hutzen B., Li P. K., Ball S., Zuo M., DeAngelis S., Foust E., Sobo M., Friedman L., Bhasin D., Cen L., Li C., Lin J. (2010) Neoplasia 12, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heinrich P. C., Behrmann I., Müller-Newen G., Schaper F., Graeve L. (1998) Biochem. J. 334, 297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G., Schaper F. (2003) Biochem. J. 374, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin X., Zimmers T. A., Perez E. A., Pierce R. H., Zhang Z., Koniaris L. G. (2006) Hepatology 43, 474–484 [DOI] [PubMed] [Google Scholar]