Abstract
The purpose of this study was to determine the effects of contamination with smoker’s and non-smoker’s saliva on the bond strength of resin composite to superficial dentin using different adhesive systems. The interfacial structure between the resin and dentin was evaluated for each treatment using environmental scanning electron microscopy (ESEM). Freshly extracted human molars were ground with 600-grit SiC paper to expose the superficial dentin. Adhesives [One-Up-Bond-F-Plus (OUFP) and Adper-Prompt-L-Pop (APLP)] and resin composite (TPH-Spectrum) were bonded to the dentin (n = 8/group, 180 total specimens) under five surface conditions: control (adhesive applied following manufacturers’ instructions); saliva, then 5-s air dry, then adhesive; adhesive, saliva, 5-s air dry; adhesive, saliva, 5-s water rinse, 5-s air dry (ASW group); and adhesive, saliva, 5-s water rinse, 5-s air dry, reapply adhesive (ASWA group). After storage in water at 37°C for 24 h, the specimens were debonded under tension at a speed of 0.5 mm/min. ESEM photomicrographs of the dentin/adhesive interfaces were taken. Mean bond strength ranged from 8.1 to 24.1 MPa. Fisher’s protected least significant difference (P = 0.05) intervals for critical adhesive, saliva, and surface condition differences were 1.3, 1.3, and 2.1 MPa, respectively. There were no significant differences in bond strength to dentin between contamination by smoker’s and non-smoker’s saliva, but bond strengths were significantly different between adhesive systems, with OUFP twice as strong as APLP under almost all conditions. After adhesive application and contamination with either smoker’s or nonsmoker’s saliva followed by washing and reapplication of the adhesive (ASWA group), the bond strength of both adhesive systems was the same as that of the control group.
Keywords: Bond strength, Saliva, Smokers, SEM
Introduction
Several kinds of contamination can affect the structural and chemical properties of dental restorative materials. Oral fluids such as saliva, blood, and crevicular fluid can cause chemical incompatibility with dental materials.1
Saliva is composed of 99.4% water and 0.6% solids, including macromolecules such as proteins (26% mucins), glycoproteins, sugar, and amylase; inorganic particles such as calcium, sodium, and chloride; and organic particles such as urea, amino acids, fatty acids, and free glucose.2,3 Saliva is an essential fluid in the mouth that serves as a barrier against desiccation and environmental injury. Furthermore, the different components of saliva interact to modulate pH and provide buffering, cleanse the oral tissues in general, prevent demineralization of the teeth, and serve as an antibacterial agent.4,5
Several studies have reported the effects of different contaminants found or used commonly in the oral cavity on the bond strength of dentin and enamel. For example, the effects of water, saliva, astringents, plasma, handpiece lubricant, zinc oxide–eugenol cement, and non-eugenol–zinc oxide cement on bond strength have been studied.6–11
Several studies have also reported that saliva contamination of enamel and dentin did not affect the bond strength of single-bottle (total-etch) adhesive systems.12 However, studies have shown decreases in bond strength with contaminants such as astringents, blood, and saliva with different self-etching adhesive systems and have suggested ways to correct and improve the bond strength in cases of contamination.7–9 Some self-etching adhesive systems seem to be more compatible with the oral environment for reasons such as low moisture sensitivity and resistance to saliva contamination.12–15
This low sensitivity may be caused by the use of a water-based solvent in the primer. In the oral cavity, it is difficult to obtain dry conditions because as soon as the patient breathes there is moisture in the environment. It is important to study contamination factors such as saliva because some studies have reported that the proteins present in saliva are the main factors responsible for the improper formation of the dentin–adhesive interface.3,15
One billion men and a quarter of a billion of women worldwide consume some kind of tobacco.16 A cigarette contains at least 5000 chemicals that produce many changes in the oral environment, such as teeth pigmentation and inflammatory and degenerative illnesses. Furthermore, smoking causes cancer and is the main cause of death worldwide.17–19 Tobacco may also affect the chemical interaction between oral biomaterials and the teeth. Thus, smoker’s saliva may affect restorative procedures and properties of restorations such as bond strength and interfacial microstructure. In this study, we worked with smoker’s saliva but we did not specifically work with smoker’s teeth because we wanted to focus specifically on the effects of saliva. Future studies should be done with teeth of documented smokers, because we think that frequent use of tobacco probably affects the oral tissues and teeth substrates as well.
The purpose of this study was to measure the bond strength of resin composite to saliva-contaminated dentin and to analyze the interfacial microstructure between smoker’s and nonsmoker’s saliva by using environmental scanning electron microscopy (ESEM). Contaminated teeth were bonded with one of two self-etching adhesive systems and we also evaluated two methods of correcting the saliva contamination. The null hypothesis was that there were no differences in the bond strength or the microstructure of the dentin adhesive interface between contamination with smoker’s and that with nonsmoker’s saliva.
Material and methods
Names, manufacturers, compositions, and lot numbers of the products used in this study are shown in Table 1. Eighty human molars with no restorations or decay were used in this study. Saliva was collected from three smoker and three nonsmoker participants. These saliva samples were applied to the specimens the same day that they were collected, about 30 min after collection. The teeth, which had been extracted within 3 months of the initiation of this study, were stored in 0.25% sodium azide/saline solution at 4°C prior to specimen preparation. The teeth were sectioned mesiodistally with a slow-speed diamond saw (Isomet, Buehler, Lake Forest, IL, USA), and embedded in acrylic potting resin to make a total of 160 buccal or lingual specimens. The specimens were ground to expose the superficial dentin using 600-grit SiC paper (Buehler). The specimens were then randomly divided into four groups with different contamination conditions and a control group (n = 8) for each bonding system.
Table 1.
Products, manufacturers, and batch numbers of products
| Product | Manufacturer | Chemical composition | Component | Lot. No. |
|---|---|---|---|---|
| One-Up Bond F Plus | Tokuyama Dental Corporation | MAC-10, photoinitiator, methacryloylalkyl acid phosphate, multifunctional methacrylic monomer | Bonding A | MS-13 |
| MMA, HEMA, water, F-deliverable microfiller (fluoroaluminosilicate glass), photoinitiator | Bonding B | MS-13 | ||
| Adper Prompt L-Pop | 3M ESPE | Methacrylated phosphoric esters, bis-GMA, initiators based on camphoroquinone, stabilizer | Liquid A | 176296 (BOX 178281) |
| Water, HEMA, polyalkenoic acid, stabilizer | Liquid B | |||
| TPH Spectrum | Dentsply Caulk | Bis-GMA-adduct (adduct of 2,2-bis[4-2-hydroxy-3-methacryloyloxpropoxy)-phenyl] propane with hexamethylene diisocyanate), ethoxylated bisphenol-A-dimethacrylate (bis-EMA, 2,2-bis[4-(2-methacryloyloxyethoxy)-phenyl]propane,) and triethylene glycol dimethacrylate. Barium aluminoboro silicate glass filler, colloidal silica |
A2 | 0306102 |
MMA, methyl methacrylate; MAC-10, 11-methacryloxy-1,1-un-decanedicarboxylic acid; HEMA, 2-hydeoxyethyl methacrylate; bis-EMA, bisphenol-A-dimethacrylate; bis-GMA, bisphenol A glycidyl methacrylate
The treatment groups were contaminated with either smoker’s or non-smoker’s saliva, and the adhesives were applied according to manufacturers’ instructions. The bonding protocols used in the four treatment groups and the control group were as follows:
Control group: adhesive was applied to dentin following the manufacturers’ instructions
SA group: 10 μl of saliva was dispensed and rubbed on the dentin surface with an applicator for 10 s, followed by air drying for 5 s with a hard stream from a distance of 5 mm, followed by adhesive
AS group: adhesive was applied, and then 10 μl of saliva was dispensed and rubbed on the dentin surface with an applicator for 10 s, followed by air drying for 5 s with a hard stream from a distance of 5 mm
ASW group: adhesive was applied, and then 10 μl of saliva was dispensed and rubbed on the dentin surface with an applicator for 10 s. This was followed by a 5 s water rinse and a 5 s air dry with a hard stream from a distance of 5 mm
ASWA group: adhesive was applied, and then 10 μl of saliva was dispensed and rubbed on the dentin surface with an applicator for 10 s, followed by a 5 s water rinse with a hard stream from a distance of 5 mm and a 5 s air dry. Then adhesive was reapplied
Bonding agents and resin composite [One-Up Bond F Plus (OUFP), Tokuyama Dental Corporation, Tsukuba, Japan; Adper Prompt L-Pop (APLP), 3M ESPE, St. Paul, MN, USA], and resin composite [TPH Spectrum (TPH), Denstply/Caulk, Milford, DE, USA] were bonded to the dentin surface by using a polytetrafluoroethylene jig in the shape of an inverted truncated cone with a diameter of 3 mm at the bonded surface and 5 mm at the top.20 The composite was dispensed in 2-mm increments and cured as recommended by the manufacturers (20 s per layer). Photopolymerization was accomplished with an Elipar Highlight (Kerr Demetron, Danbury, CT, USA) curing light. The light output was verified as greater than 450 mW cm−2 throughout the study with a curing radiometer (Kerr Demetron, Danbury, CT, USA).
The adhesion protocol followed for the APLP self-etching adhesive system was as follows:
After preparation, lightly dry to remove excess water.
Apply adhesive with a rubbing motion for 15 s.
Gently but thoroughly air-dry to remove the aqueous solvent.
Apply a second coat (no waiting time for the second layer).
Gently but thoroughly air-dry to remove the aqueous solvent.
Light cure for 10 s.
The adhesion protocol followed for the OUFP self-etching adhesive system was:
Rinse the prepared cavity. If no cavity preparation has been made, clean the tooth surface with a rubber cup and a fluoride-free cleaning paste; rinse thoroughly with water.
Remove water by blowing air to keep the cavity in a dry, or in a moist condition but without visible puddles or a shiny appearance.
Using the provided dispenser, place one drop of bonding agent A and one drop of bonding agent B into the mixing well.
Mix thoroughly and evenly until the mixed bonding agent turns completely and evenly pink.
Apply the mixed bonding agent to the cavity, both the enamel and dentin, with the disposable applicator tip.
After applying the mixed bonding agent, keep rubbing the mixed bonding agent on the surface for at least 10 s.
OUFP does not require air blowing because it contains no volatile solvent such as ethanol or acetone.
Light irradiate the mixed bonding agent for at least 10 s. keeping the curing light tip within about 2 mm from the cavity surface.
After the specimens were bonded, they were stored in water in an incubator at 37°C for 24 h. The specimens were then debonded under tension using a universal testing machine (Model 4465, Instron, Canton, MA, USA) at a crosshead speed of 0.5 mm/min. Bond strength means were calculated and compared using three-way analysis of variance (ANOVA; StatView, SAS Institute, Cary, NC, USA). Fisher’s protected least significant difference (PLSD) post hoc test (StatView, SAS Institute) was used to determine significant differences at the 0.05 level. Failure modes were observed under 10 × magnification and recorded.
For the SEM analysis, 20 additional extracted human molars with the same characteristics as those used for the bonding were ground with 600-grit SiC paper (Buehler) on the occlusal surface to expose the dentin.
These specimens were randomly divided into ten groups of two specimens per group and then treated and bonded as described above. The specimens were sectioned perpendicular to the adhesive interface with a slow-speed diamond saw (Isomet, Buehler) to produce two slices, each 2–3 mm thick. One slice was acid-etched with 5 N HCl for 30 s, followed by 5% NaOCl for 30 min, and then rinsed thoroughly with distillated water. The other slice was fractured perpendicularly to the interface. Each slice was then dehydrated with 33% ethanol for 30 min, 67% ethanol for 30 min, 85% ethanol for 30 min, and 100% ethanol for 1 h.
The specimens were left overnight to dry and then mounted on 12-mm aluminum stubs and sputter-coated with approximately 20 nm of gold-palladium alloy. The specimens were viewed at three levels of magnification (1000×, 2500×, and 5000×) and tilt angles in a Field Emission Philips XL30 SEM at high vacuum and 10 kV. Analyses of the dentin–adhesive interface characteristics were based on at least 20 images taken along the length of the interface.
Results
Bond strength
Means (SD) of bond strength (MPa) are shown in Table 2. Results of the three-way ANOVA of the data are shown in Table 3. Fisher’s PLSD intervals (P < 0.05) for critical adhesive, saliva, and surface condition differences were 1.3, 1.3, and 2.1 MPa, respectively. Failure sites were mainly adhesive.
Table 2.
Tensile bond strength (MPa) and failure modes
| One Up Bond F Plus |
Adper Prompt L Pop |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Saliva | Control | SA | AS | ASW | ASWA | Control | SA | AS | ASW | ASWA |
| Nonsmoker’s | 21.7 (4.4) | 19.6 (5.5) | 23.1 (5.6) | 15.3 (5.6) | 20.8 (4.1) | 10.8 (2.2) | 9.3 (3.7) | 8.1 (2.1) | 8.3 (2.3) | 14.5 (3.7) |
| A (85%) | A (80%) | A (79%) | A (100%) | A (60%) | A (100%) | A (95%) | A (100%) | A (100%) | A (90%) | |
| C-CR (15%) | C-CR (20%) | C-CR (21%) | C-CR (40%) | C-CR (5%) | C-CR (10%) | |||||
| Smoker’s | 21.7 (4.4) | 22.2 (4.5) | 20.1 (5.5) | 23.1 (4.4) | 24.1 (5.0) | 10.8 (2.2) | 10.4 (4.8) | 9.3 (3.0) | 9.2 (3.5) | 13.4 (4.1) |
| A (85%) | A (61%) | A (92%) | A (94%) | A (67%) | A (100%) | A (100%) | A (100%) | A (100%) | A (80%) | |
| C-CR (15%) | C-CR (39%) | C-CR (8%) | C-CR (6%) | C-CR (33%) | C-CR (20%) | |||||
Tensile bond strength values are means (SD), n = 8
Fisher’s protected least significant differences (P = 0.05) intervals for critical adhesive, saliva, and surface condition differences were 1.3, 1.3, and 2.1 MPa, respectively
SA, saliva/air dry/adhesive; AS, adhesive/saliva/air dry; ASW, adhesive/saliva/water rinse/air dry; ASWA, adhesive/saliva/water rinse/air dry/adhesive; A, adhesive failure; C-CR, cohesive resin in composite resin
Table 3.
Analysis of variance of bond strength
| DF | Sum of squares | Mean square | F value | P value | Lambda | Power | |
|---|---|---|---|---|---|---|---|
| Bond agent | 1 | 4634.655 | 4634.655 | 265.374 | <0.001 | 265.374 | 1.000 |
| Smoker | 1 | 66.113 | 66.113 | 3.786 | 0.0537 | 3.786 | 0.475 |
| Condition | 4 | 316.258 | 79.065 | 4.527 | 0.0018 | 18.108 | 0.946 |
| Bond agent * smoker | 1 | 29.851 | 29.851 | 1.709 | 0.1932 | 1.709 | 0.240 |
| Bond agent * condition | 4 | 78.715 | 19.679 | 1.127 | 0.3464 | 4.507 | 0.339 |
| Smoker * condition | 4 | 128.576 | 32.144 | 1.841 | 0.1244 | 7.362 | 0.540 |
| Bond agent * smoker * condition | 4 | 143.160 | 35.790 | 2.049 | 0.0908 | 8.197 | 0.594 |
| Residual | 10 | 2445.051 | 17.465 |
When contaminated with nonsmoker’s saliva, specimens in the SA and ASW groups bonded with OUFP showed significant decreases in bond strength compared with the control group, and those in the AS group showed nonsignificant differences. When contaminated with smoker saliva, specimens in the ASWA group bonded with OUFP showed a significant increase in bond strength compared with the control group, and the other groups showed nonsignificant differences.
When contaminated with nonsmoker saliva, specimens in the AS and ASW groups bonded with APLP showed significant decreases in bond strength, and those in the ASWA group showed significantly increased bond strength, compared with the control, whereas those in the SA group showed no significant difference. When contaminated with smoker saliva, specimens in the ASWA group bonded with APLP showed a significant increase in bond strength compared with the control. The SA, AS, and ASW groups showed no significant differences. Bond strength of OUFP was greater than that of APLP under all conditions.
The fractured specimens evaluated by SEM for in the OUFP control group (Fig. 1) showed some resin tags, no visible hybrid layer, and an adhesive layer about 13 μm thick. The APLP control group (Fig. 2) showed some resin tags with a hybrid layer about 5 μm thick.
Fig. 1.
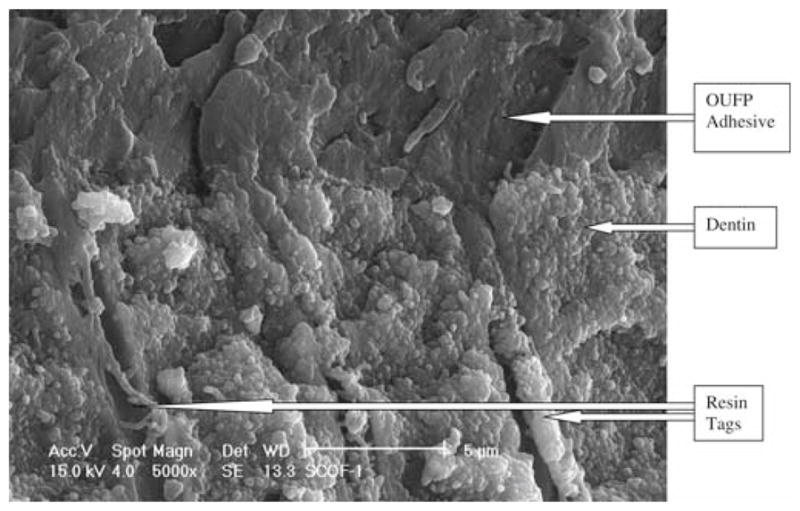
One Up Bond F Plus (OUFP) control group fracture, 5000× magnification
Fig. 2.
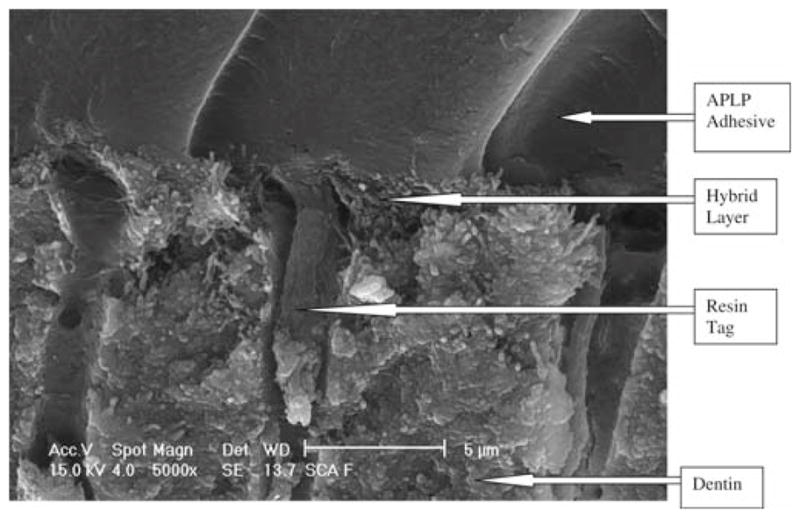
Adper Prompt L Pop (APLP) control group fracture, 5000× magnification
The OUFP nonsmoker’s saliva ASWA group (Fig. 3) showed short resin tags in poor contact with the subjacent dentin substrate. There was no visible hybrid layer, and the adhesive layer was 50 μm thick.
Fig. 3.
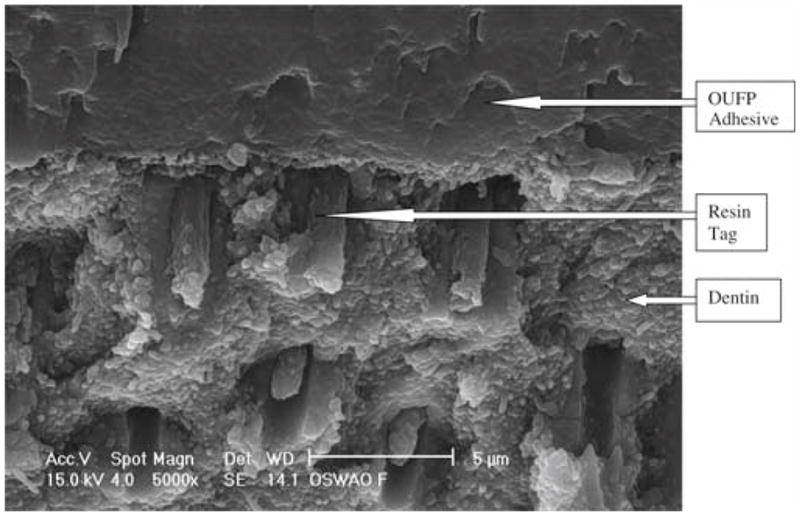
OUFP/nonsmoker’s saliva/water/air/OUFP (ASWA) treatment, 5000 × magnification
The APLP nonsmoker’s saliva ASWA group (Fig. 4) showed numerous long, fractured resin tags, a visible hybrid layer about 5 μm thick, and an adhesive layer about 14 μm thick. The OUFP smoker’s saliva ASWA group (Fig. 5) showed some short resin tags with no infiltration into the dentin substrate and no hybrid layer and an adhesive layer 94 μm thick. The APLP smoker’s saliva ASWA group (Fig. 6) showed long resin tags directed vertically and horizontally all over the interface, no hybrid layer, and a thick adhesive layer. We thus observed SEM differences among the groups reported and also between adhesive systems. The SEM micrographs of the different interfaces (OUFP) exhibited resin tags with morphological features consistent with low monomer/polymer conversion.21
Fig. 4.
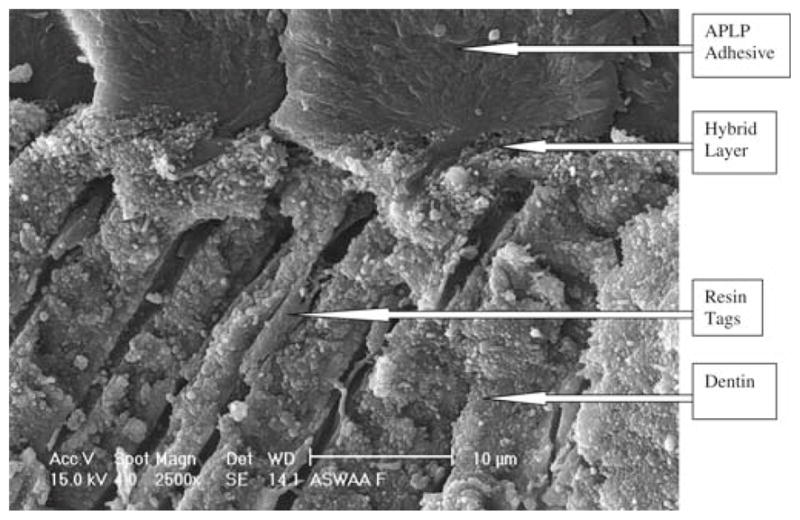
APLP with ASWA treatment, 5000× magnification
Fig. 5.
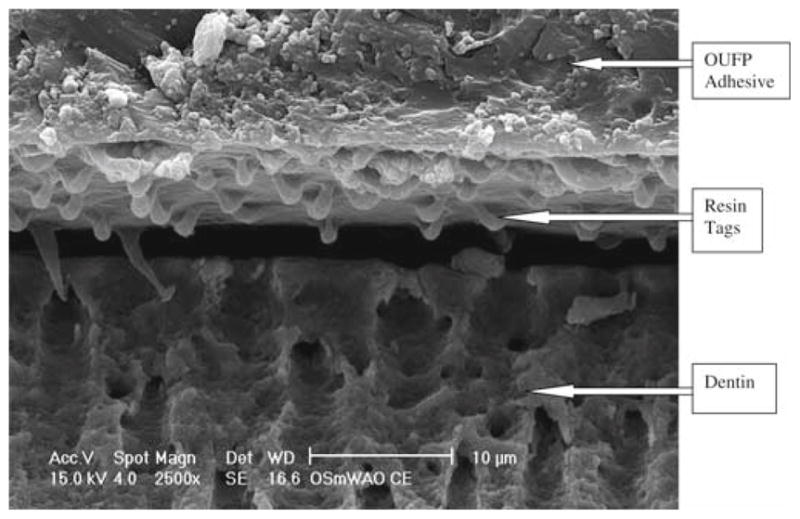
OUFP with ASWA treatment, 2500× magnification
Fig. 6.
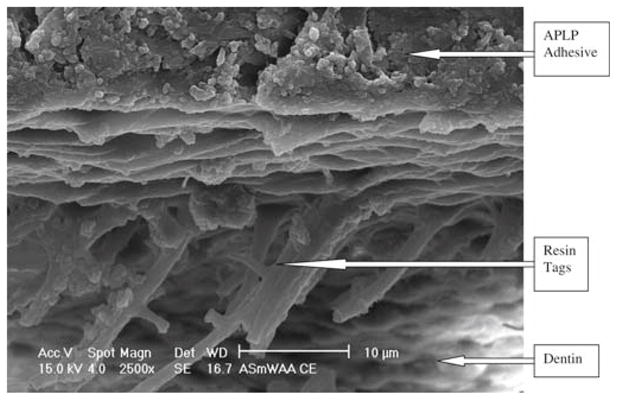
APLP with ASWA treatment, 2500× magnification
Discussion
The oral cavity should be adequately irrigated with saliva for reasons such as immunological defense and antioxidant and enzymatic functions. Saliva also protects the mucosa and promotes healing.22 A rubber dam is a useful tool for isolating the teeth during operative and endodontic treatments. First described in 1864,23,24 a rubber dam provides benefits during dental treatment; it was developed to isolate the teeth that will be restored from saliva, but not all dentists use it properly. Various researchers have discussed the potential detrimental effect of saliva on the bonding of some materials to the tooth surface for more than 40 years.
We found no differences in bond strength or interfacial structure between contamination with smoker’s and that with nonsmoker’s saliva. In addition, neither APLP or OUFP showed a significant decrease in bond strength under contaminated conditions. This result was somewhat surprising because saliva contamination has been reported to decrease the quality of the dentin–adhesive interface with some adhesive systems.12,25–28 However, self-etching systems are reported to not be sensitive to moisture and thus resistant to contamination with saliva.13–15
The self-etching adhesive systems used in this study might be less sensitive to moisture because they use a water-based solvent. Furthermore, different salivas were applied to the specimens, but the specimens were not stored for a long period in the different salivas. Different results might be observed if smokers’ teeth were used as well as smoker’s saliva or if the teeth were stored for a long period of time before testing with nonsmoker’s and smoker’s saliva.
There were significant differences between the adhesive systems in bond strength and in the dentin–adhesive interface microstructure. Several studies have suggested that 17 MPa is an acceptable bond strength when measuring the quality of bonding agents.29,30 We recorded acceptable bond strengths (17 MPa) for OUFP under different conditions and types of saliva. However, the SEM micrographs showed a very thin hybrid layer, and in some cases it was impossible to visualize any hybrid layer. Also, the resin tags were very thin and short. It is important to note that OUFP had a very compact interface structure strikingly different from that of APLP, and resulted in bond strengths that were twice as high. In fact, OUFP did not exhibit any hybrid layer under the SEM, whereas APLP exhibited a hybrid layer about 5 μm thick except under ASWA conditions.
With APLP, bond strengths did not differ under the different conditions but they were well below values thought to be acceptable.29 Under SEM analysis, the APLP specimens showed a dentin–adhesive structure with long resin tags and a thick hybrid layer. This microstructure may be due to the acidity of the monomer present in the primer of the adhesive system. Although this acidity provided an interface with numerous resin tags and a thick hybrid layer, the interface was relatively weak. The hybrid layer is created by the penetration and polymerization of adhesive monomers after removal of the smear layer and superficial demineralization of the dentin in total etch systems.31 The main goal of a self-etching adhesive system is to infiltrate the resin monomer into the smear layer as well as to demineralize and infiltrate the dentin, simultaneously forming a hybrid layer.32 Self-etching adhesive systems are water based and mixed with acidic monomers, such as carboxylic acid or hydroxyethyl methacrylate and phosphate ester, and a co-solvent that can be ethanol or acetone.33
The hybrid layer is an important part of the bonding mechanism in total etch adhesive systems.30,31 Most self-etching adhesive systems yield a thin hybrid layer,34 but the bond strength in some cases is higher than with total etch systems.35 These and other reported results lead us to conclude that hybrid layer thickness is not necessarily correlated with bonding strength to dentin.36–40 The results of this study indicate that high bond strengths were not related to the presence of a hybrid layer, since OUFP, a self-etching adhesive system, did not exhibit a hybrid layer. Furthermore, we expected that the pellicle created by the smoker’s saliva would be different in protein composition or quantity than that created by nonsmoker’s saliva. If this were true, then the surface chemistry of the substrate would potentially be different. If these conditions were met, then the surfaces to which the adhesives were bonded would present with differences. The bond strength measurements did not support these expected differences in the surface chemistry.
Furthermore, the SEM evaluation showed tag fractures in the dentin–adhesive interface structure that might have been caused by inhibition of polymerization by some components of the saliva such as O2 or H2O. This finding is consistent with other reports in the literature that water has a detrimental effect on the monomer/polymer conversion of adhesives. 41–45
Conclusion
Differences in bond strength between contamination with smoker’s and nonsmoker’s saliva were not observed. Contamination with saliva reduced the bond strength in some treatment groups, but after contamination with saliva followed by washing and reapplication of adhesive (ASWA treatment), the bond strengths were the same as in the control group with both adhesive systems. There was a nearly twofold difference in bond strength between the adhesive systems, but only the APLP specimens exhibited hybrid layers. OUFP specimens exhibited resin tags with morphological features consistent with low monomer/polymer conversion.
Acknowledgments
We acknowledge UMKC-CRIS, Tokuyama Dental Corporation, and also NIH T32 DE015355-01 for supporting this study.
Contributor Information
Lilliam M. Pinzon, Email: Lilliam.Pinzon@ucsf.edu, Department of Preventive and Restorative Dentistry, 707 Parnassus Avenue, Room 3212, University of California, San Francisco, CA 94143-0758, USA, Tel. +1-415-476-2048; Fax +1-415-476-0858
Makoto Oguri, Tokuyama Dental Corporation, Tsukuba, Japan.
Kathy O’Keefe, Department of Restorative Dentistry and Biomaterials, University of Texas Dental Branch at Houston, Houston, TX, USA.
Vladimir Dusevish, Electron Microscopy Laboratory, University of Missouri, Kansas City, MO, USA.
Paulette Spencer, Department of Mechanical Engineering, University of Kansas, Lawrence, KS, USA.
John M. Powers, Department of Restorative Dentistry and Biomaterials, University of Texas Dental Branch at Houston, Houston, TX, USA
Grayson W. Marshall, Division of Biomaterials and Bioengineering and Department of Preventive and Restorative Dental Sciences, University of California, San Francisco, CA, USA
References
- 1.Powers JM, O’Keefe KL, Pinzon LM. Factors affecting in vitro bond strength of bonding agents to human dentin. Odontology. 2003;91:1–6. doi: 10.1007/s10266-003-0026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thornton DJ, Davies JR, Carlstedt I, Sheehan JK. In: In air way mucus: basic mechanisms and clinical perspectives. Rogers DF, Lethem MI, editors. Basel, Switzerland: Birkhauser; 1997. pp. 41–66. [Google Scholar]
- 3.Eiriksson SO, Pereira PN, Swift EJ, Jr, Heymann HO, Sigurdsson A. Effects of saliva contamination of resin-resin bond strength. Dent Mater. 2004;20:37–44. doi: 10.1016/s0109-5641(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 4.Ellison SA. Handbook of physiology, section 6, alimentary canal. Baltimore: Williams and Wilkins; 1967. Proteins and glycoproteins of saliva. [Google Scholar]
- 5.Gupta R, Jentoft N, Jamieson AM, Blackwell J. Structural analysis of purified human tracheobronchial mucins. Biopoly. 1990;29:347–55. doi: 10.1002/bip.360290207. [DOI] [PubMed] [Google Scholar]
- 6.Matos AB, Oliveira DC, Vieira SN, Netto NG, Powers JM. Influence of oil contamination on in vitro bond strength of bonding agents to dental substrates. Am J Dent. 2008;21:101–4. [PubMed] [Google Scholar]
- 7.Itoh T, Fukushima T, Inoue Y, Arita S, Miyazaki K. Effect of water, saliva and blood contamination on bonding of metal brackets with a 4-META/MMA/TBB resin to etched enamel. Am J Dent. 1999;12:299–304. [PubMed] [Google Scholar]
- 8.Benderli Y, Gokce K, Buyukgokcesu S. In vitro shear bond strength of adhesive to normal and fluoridated enamel under various contaminated conditions. Quintessence Int. 1999;30:570–5. [PubMed] [Google Scholar]
- 9.Yoo HM, Pereira PN. Effect of blood contamination with 1-step self-etching adhesives on microtensile bond strength to dentin. Oper Dent. 2006;31:660–5. doi: 10.2341/05-1279. [DOI] [PubMed] [Google Scholar]
- 10.Van Schalkwyk JH, Botha FS, van der Vyver PJ, de Wet FA, Botha SJ. Effect of biological contamination on dentin bond strength of adhesive resins. J S Afr Dent Assoc. 2003;58:143–7. [PubMed] [Google Scholar]
- 11.Bishara SE, Oonsombat C, Ajlouni R, Denehy G. The effect of saliva contamination on shear bond strength of orthodontic brackets when using a self-etch primer. Angle Orthod. 2002;72:554–7. doi: 10.1043/0003-3219(2002)072<0554:TEOSCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.O’Keefe KL, Pinzon LM, Rivera B, Powers JM. Bond strength of composite to astringent-contaminated dentin using self-etching adhesives. Am J Dent. 2005;18:168–72. [PubMed] [Google Scholar]
- 13.Park JW, Lee KC. The influence of salivary contamination on shear bond strength of dentin adhesive systems. Oper Dent. 2004;29:437–442. [PubMed] [Google Scholar]
- 14.Yoo HM, Oh TS, Pereira PN. Effect of saliva contamination on the microshear bond strength of one self-etching adhesive systems to dentin. Oper Dent. 2006;31:127–34. doi: 10.2341/04-206. [DOI] [PubMed] [Google Scholar]
- 15.Sattabanasuk V, Shimada Y, Tagami J. Effects of saliva contamination on dentin bond strength using all-in-one adhesives. J Adhes Dent. 2006;8:311–8. [PubMed] [Google Scholar]
- 16.Irani S, Pinzon LM, Powers JM. Effect of saliva contamination on bond strength of self-etching adhesives to dentin. J Dent Res. 2003;82 AADR Abstract #571. [Google Scholar]
- 17.Pinzon LM, O’Keefe KL, Powers JM. Adhesion of composite with self-etching primer to saliva-contaminated moist and dry dentin. Trans Acad Dent Mater. 2002;16 Abstract #P2. [Google Scholar]
- 18.Schaneveldt S, Foley TF. Bond strength comparison of moisture-insensitive primers. Am J Orthod Dentofacial Orthop. 2002;122:267–73. doi: 10.1067/mod.2002.126594. [DOI] [PubMed] [Google Scholar]
- 19.Hitmi L, Attai JP, Degrange M. Influence of the time-point of salivary contamination on dentin shear bond strength of 3 dentin adhesive systems. J Adhes Dent. 1999;1:219–32. [PubMed] [Google Scholar]
- 20.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;2:S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 21.Zappacosta B, Persichilli S, De Sole P, Mordente A, Giardina B. Effect of smoking one cigarette on antioxidant metabolites in the saliva of healthy smokers. Arch Oral Biol. 1999;44:485–8. doi: 10.1016/s0003-9969(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 22.Stewart BW, Kleihues P. World cancer report. Lyon, France: IAR-CPress; 2003. [Google Scholar]
- 23.Guerin MR, Jenskins RA, Tomkins BA. Mainstream and side-stream cigarette smoke. In: Eisberg M, editor. The chemistry of environmental tobacco smoke: composition and measurement. Chelsea, MI: Lewis Publisher; 1992. [Google Scholar]
- 24.Wang Y, Spencer P. Continuing etching of an all-in-one adhesive in wet dentin tubules. J Dent Res. 2005;84:350–4. doi: 10.1177/154405910508400411. [DOI] [PubMed] [Google Scholar]
- 25.Barakat MM, Powers JM. In vitro bond strength of cements to treated teeth. Aust Dent J. 1986;31:415–9. doi: 10.1111/j.1834-7819.1986.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 26.Nagler RM, Klein I, Zarzhevsky N, Drigues N, Reznick AZ. Characterization of the differentiated antioxidant profile of human saliva. Free Radic Biol Med. 2002;32:268–77. doi: 10.1016/s0891-5849(01)00806-1. [DOI] [PubMed] [Google Scholar]
- 27.Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8:306–35. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 28.Barnum SC. History of the discovery of the dam. Can J Dent Sci. 1877;4:88–9. [Google Scholar]
- 29.Ireland L. The rubber dam: its advantages and application. Texas Dent J. 1962;80:6–15. [Google Scholar]
- 30.Davidson CL, de Gee AJ. Relaxation of polymerization contraction stresses by flow in dental composites. J Dent Res. 1984;63:146–8. doi: 10.1177/00220345840630021001. [DOI] [PubMed] [Google Scholar]
- 31.Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16:265–73. doi: 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- 32.Nakabayashi N. Dentinal bonding mechanisms. Quintessence Int. 1991;22:73–4. [PubMed] [Google Scholar]
- 33.Haller B. Recent developments in dentin bonding. Am J Dent. 2000;13:44–50. [PubMed] [Google Scholar]
- 34.Vaidyanathan TK, Vaidyanathan J. Recent advances in the theory and mechanism of adhesive resin bonding to dentin: a critical review. J Biomed Mater Res B Appl Biomater. 2009;88:558–78. doi: 10.1002/jbm.b.31253. [DOI] [PubMed] [Google Scholar]
- 35.Luz MA, Arana-Chavez VE, Netto NG. Scanning electron microscopy examination of 3 different adhesive systems. Quintessence Int. 2005;36:687–94. [PubMed] [Google Scholar]
- 36.Strydom C. Self-etching adhesives: review of adhesion to tooth structure part I. SADJ. 2004;59:413, 415–7. [PubMed] [Google Scholar]
- 37.Pashley DH, Ciucchi B, Sano H, Homer JA. Permeability of dentin to adhesive agents. Quintessence Int. 1993;24:618–31. [PubMed] [Google Scholar]
- 38.Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- 39.Spencer P, Swafford JR. Unprotected protein at the dentin adhesive interface. Quintessence Int. 1999;30:5001–7. [PubMed] [Google Scholar]
- 40.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79:1458–63. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Spencer P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J Biomed Mater Res. 2002;59:46–55. doi: 10.1002/jbm.1215. [DOI] [PubMed] [Google Scholar]
- 42.Spencer P, Wang Y, Katz JL, Misra A. Physicochemical interactions at the dentin/adhesive interface using FTIR chemical imaging. J Biomed Optics. 2005;10:031104. doi: 10.1117/1.1914844. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Spencer P, Hager C, Bohaty B. Comparison of interfacial characteristics of adhesive bonding to superficial versus deep dentin using SEM and staining techniques. J Dent. 2006;34:26–34. doi: 10.1016/j.jdent.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Spencer P, Yao X, Ye Q. Effect of Co-initiator and water on the photoreactivity and photopolymerization of HEMA/camphoroquinone-based reactant mixtures. J Biomed Mater Res A. 2006;78:721–8. doi: 10.1002/jbm.a.30733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye Q, Spencer P, Wang Y, Misra A. Relationship of solvent to the photopolymerization process, properties and structure in model dentin adhesives. J Biomed Mater Res A. 2007;80:342–50. doi: 10.1002/jbm.a.30890. [DOI] [PMC free article] [PubMed] [Google Scholar]


