Abstract
Thymomas are the most common tumors of the mediastinum. The introduction of multimodality treatment strategies, as well as novel approaches to the diagnosis of these tumors, has led to changes in the clinical management of thymomas. Here we review the literature for current clinical practice in the diagnosis, management, and treatment of thymomas.
Thymomas are rare neoplasms arising from tissue elements of the thymus and developing in the anterior mediastinum [1]. They can be associated with a variety of systemic and autoimmune disorders, such as pure red cell aplasia, pancytopenia, hypogammaglobulinemia, collagen-vascular disease, and most commonly with myasthenia gravis [2–5]. Thymomas are uncommon tumors with an annual incidence of only 0.15 cases per 100,000 person-years [6], yet representing the most frequently diagnosed tumor of the anterior mediastinum [7].
Classification of Thymomas
Several classification systems of thymomas have been developed and described. However, clinical, pathologic, and surgical classification of thymomas remains controversial. The histomorphologic variability and heterogeneity of cells within thymomas is a major factor guiding this intense debate [8–11]. The most commonly used classification systems are summarized in Table 1.
Table 1.
Thymoma Classification Systems
| Classification System | ||
|---|---|---|
| Lattes/Bernatz | Traditional description of the dominant cell type | |
| Muller-Hermelink | Presumed origin of the malignant cell (corticomedullary classification) | |
| WHO | Based on the traditional descriptive classification and the corticomedullary classification | |
| WHO type | Histologic criteria | |
| A | Bland spindle/oval epithelial tumor cells with few or no lymphocytes. (Synonyms: spindle cell thymoma; medullary thymoma) | |
| AB | Mixture of a lymphocyte-poor type A thymoma component and a more lymphocyte-rich type B-like component. (Synonyms: mixed thymoma) | |
| B1 | Histological appearance of normal thymus, composed predominantly of areas resembling cortex with epithelial cells scattered in a predominant population of immature lymphocytes, and areas of medullary differentiation. (Synonyms: lymphocyte-rich thymoma; predominantly cortical thymoma) | |
| B2 | Large, polygonal tumor cells that are arranged in a loose network and exhibit large vesicular nuclei with prominent large nucleoli – background population of immature T-cells always present. (Synonyms: cortical thymoma) | |
| B3 | Predominantly medium-sized round or polygonal cells with slight atypia. Epithelial cells are mixed with minor component of intraepithelial lymphocytes. (Synonyms: well-differentiated thymic carcinoma; epithelial thymoma; squamoid thymoma) | |
| C | Heterogeneous group of thymic carcinomas | |
| Masaoka | Presence of invasion and anatomic extent of involvement (clinically and histopathologically) | |
| I | Macroscopically completely encapsulated and microscopically no capsular invasion | |
| II | Microscopic invasion into capsule (IIa) or macroscopic invasion into surrounding fatty tissue or mediastinal pleura (IIb) | |
| III | Macroscopic invasion into neighboring organs (ie, pericardium, great vessels, or lung) | |
| IVa | Pleural or pericardial dissemination | |
| IVb | Lymphogenous or hematogenous metastasis | |
WHO = World Health Organization.
In 1961, Bernatz and colleagues [12] described thymomas according to their dominant cell type: predominantly spindle cell, predominantly lymphocytic, predominantly mixed, or predominantly epithelial.
In 1976, Levine and Rosai [13] emphasized the importance of presence or absence of invasiveness by classifying thymomas into (i) benign encapsulated thymomas, (ii) type 1 malignant thymomas (invasive thymomas), and (iii) type 2 malignant thymomas (thymic carcinomas).
However, in 1985, Marino and Müller-Hermelink [14], developed and described a classification system, which is based on the presumed origin of the malignant thymoma cells. It includes five subtypes as derivatives of the medullary and cortical cells typically seen in the different types of thymomas: medullary, mixed, predominantly cortical, and cortical thymomas, and well-differentiated thymic carcinomas.
Another frequently used thymoma classification system is the World Health Organization (WHO) histologic typing of tumors of the thymus (1999), based on cytologic similarities between normal thymic epithelial cells and neoplastic cells [15, 16].
The most widely used staging system acknowledging the presence of invasion and anatomic extent of involvement, both clinically and histopathologically, was defined by Masaoka and colleagues [17]. Derived from Masaoka, the French Study Group on Thymic Tumors (GETT) developed the GETT classification system of thymomas in 1991 relating to the extent of surgical resection to tumor histopathology [18]. However, it is rarely used in clinical practice.
Finally, another approach to classifying tumors of the thymus is based on a TNM-classification system by Yamakawa and colleagues [19] in 1994. This system was established by the authors and was applied to 207 patients with thymoma or thymic carcinoma. This classification system has not been widely used nor officially recognized by WHO [19, 20].
In 2005, Bedini and colleagues [21] published a novel TNM-based staging system called the Istituto Nazionale Tumori (INT), which is the Italian National Cancer Institute. It has been developed as a clinically applicable staging system to update the Masaoka system. Further assessment of the Italian National Cancer Institute system in larger multicenter series will be required to justify its clinical implementation.
Methods
To determine the state of the art in thymoma therapy, prognostic factors, and outcome, we searched the PubMed database for “thymoma” (with 6,981 hits listed). Only abstracts published in English were considered, and clinical studies with a case load with greater than 100 patients (n > 100) were selected for complete review. As no large prospective studies have been conducted as yet, all reviewed studies were retrospective. Due to the lack of larger studies evaluating diagnostic methods and the evidence of multimodality treatment approaches in thymomas, we considered studies with less than 100 thymoma patients to review these specific topics.
Published data have been reviewed, combined, and expressed as median or range, or both, for the purpose of describing the underlying patient population.
Results
Patient Characteristics
Twenty-eight studies that include 5,487 patients overall diagnosed with thymoma were identified, reviewed, and analyzed. Within these studies, patients’ median age ranged from 45 to 59.5 years with an average follow-up of 95.2 months.
Applying the Masaoka staging system, 36% of all study patients had a stage I thymoma, 26% were stage II, 22% were stage III, and 10% were stage IV. Applying the World Health Organization classification system, 8% were type A, 26% were type AB, 15% were type B1, 28% were type B2, 13% were type B3, and 8% were type C thymomas [7, 22–29] with 10-year survival rates of 100%, 90% to 100%, 78% to 94%, 33% to 85%, 35% to 41%, and 0 to 35%, respectively [7, 26, 27, 30].
Diagnosis of Thymoma
Radiological Imaging
Currently, computed tomography is the first choice technique to characterize a mediastinal mass with regard to its anatomic dissemination and invasion of neighboring structures, as well as possible distant metastases. Chest roentgenograms show merely a nonspecific broadened mediastinum [31], as shown and represented in Figure 1. Applying computed tomography, thymomas can often be distinguished from benign mediastinal lesions or from lymphoma in the case of multiple mediastinal abnormalities [32].
Fig 1.
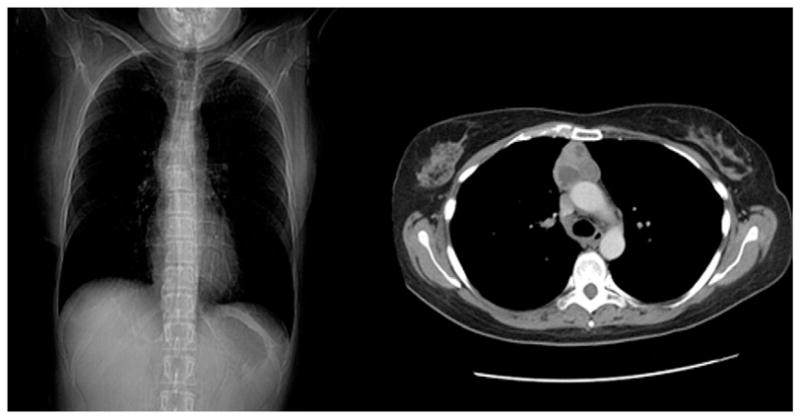
Chest roentgenogram (left) versus computed tomographic scan of a thymoma (right).
Magnetic resonance imaging is frequently implemented in the radiologic diagnosis of thymomas [33]. The suggested correlation with the WHO classification system is intensely discussed and needs to be further studied [34, 35]. The major role of the magnetic resonance image seems to lie in its value for surgical planning, especially if resection of thymomas is considered that invade neighboring structures such as the great vessels or the heart.
Recently, fluorodeoxyglucose positron emission tomographic scanning has been used in addition to computed tomography to diagnose thymomas. Data suggest that maximal standardized uptake values of thymic carcinomas are significantly higher than those of high-risk or low-risk thymomas [36, 37]. Moreover, a higher proportion of thymic carcinoma patients show homogeneous 18-French fluorodeoxyglucose uptake than low-risk or high-risk thymoma patients. The authors concluded from their data that positron emission tomographic scanning might be a useful tool for predicting the grade of malignancy in thymic epithelial tumors. Further clinical investigation is needed to determine whether positron emission tomographic scanning plays a role in the staging of thymomas, the distinction of thymomas from thymic carcinomas, or early detection of recurrent disease during follow-up.
The adoption of scintigraphy is an important tool in patients with myasthenia gravis, as thallium 201 single photon emission computed tomography can help to differentiate normal thymic tissue from hyperplastic tissue or thymoma [38].
Fine-Needle Aspirated Biopsy
A fine-needle aspiration (FNA) biopsy is an accepted and feasible method to differentiate mediastinal lesions and to diagnose or classify thymomas histopathologically [39–41]. However, as in other malignant tumors, needle track malignant cell seeding is feared, and therefore many programs do not routinely perform FNA biopsy in tumors suspicious of thymoma.
New approaches, such as the implementation of ultrasonically guided core needle biopsy rather than percutaneous needle biopsies have been adopted to obtain larger specimens for histologic examination. Annessi and colleagues [42] were able to establish a diagnosis in all patients that had undergone anterior mediastinal core needle biopsy by ultrasonic guidance with a sensitivity and specificity of 100%. They evaluated the procedure as superior in comparison with FNA, as surgical diagnostic procedures can be reduced, and the repetition of unsuccessful FNA is avoided. For the same reason, endoscopic ultrasound-guided FNA has become increasingly useful in obtaining cytologic specimens from mediastinal lesions [43, 44]. Larghi and colleagues [44] implemented an altered endoscopic ultrasound-guided FNA technique in a pilot study of 27 patients using continuous high, negative pressure suction for core tissue acquisition, and they compared this technique to standard endoscopic ultrasound-guided FNA. The diagnostic accuracy for both methods was 76.9%, and when combined, a correct diagnosis was achieved in 84.6% of the patients [44]. No superiority over standard endoscopic ultrasound-guided FNA could be shown in this study.
Percutaneous image-guided FNA biopsy of a mediastinal mass is diagnostic in up to 82% of cases [45]. However, histologic differentiation between thymomas, lymphomas, and thymic hyperplasia can be problematic for this form of biopsy, given the small tissue volumes obtained.
Current Treatment of Thymomas
Surgery
As the only curative treatment, surgery remains the baseline attempt in thymoma therapy. Complete or partial median sternotomy with complete thymectomy is the operative approach of choice [22, 23, 26, 46–49], as first described by Blalock in 1941 [50]. In advanced tumors, especially if the lung or pleural space is invaded, the extension of a sternotomy to a hemi-clamshell incision or a full clamshell incision can be suitable. Approaches like transcervical and video-assisted thoracoscopic thymectomy for thymoma are being intensely studied, but are generally viewed as contraindicated for the excision of thymomas [51, 52]. By other authors they are considered as effective alternative procedures for noninvasive thymomas if performed carefully in experienced hands [53, 54]. However, long-term studies are required to further evaluate these approaches in the therapy of thymomas. As a routine, we do not use the minimally invasive approach for the resection of thymomas in our programs given concern regarding oncologic equivalence with open approaches.
Whenever possible, completeness of resection has to be aimed for because it is an important prognostic factor for local control and survival, as illustrated in Table 2 [1, 22, 23, 25, 26, 30, 47, 49, 55]. According to a multicenter study on 1,320 patients by Kondo and Monden [55], total resection is the most important prognostic factor for survival; the 5-year survival rate of Masaoka stages III and IV thymomas is 92.9% after total resection versus 64.4% (p < 0.001) after subtotal resection versus 35.6% if inoperable. Although Rea and colleagues [26] confirmed these findings, they found no statistical difference in survival between patients who underwent incomplete resection (“debulking”) versus biopsy only.
Table 2.
Survival According to Extent of Resection
| Rea et al [26] | Zhu et al [49] | Nakagawa et al [30] | Regnard et al [47] | Kim et al [23] | Okumura et al [25] | |
|---|---|---|---|---|---|---|
| Surgery | 132 | 175 | 130 | 307 | 108 | 273 |
| Complete resection | 81.8% | 72.0% | 95.0% | 84.7% | 81.5% | 94.5% |
| 5-yr SR | 82.5% | 88.4% | 96.0% | — | c. 95% | c. 98% |
| 10-yr SR | 71.0% | — | 94.0% | 76.0% | c. 85% | c. 95% |
| Incomplete resection | 9.1% | 13.7% | 5.0% | 9.8% | 18.5% | 3.30% |
| 5-yr SR | 16.0% | 43.2% | 33.0% | — | c. 55% | — |
| 10-yr SR | 9.0% | — | 33.0% | 28.0% | c. 35% | c. 60% |
| Biopsy/gross disease | 9.1% | 14.4% | — | 5.5% | — | 1.6% |
| 5-yr SR | 33.0% | 73.5% | — | — | — | — |
SR = survival rate.
Recurrence rates after complete resection vary between 11% and 19% [29, 46] and correlated with stage: WHO tumor type A and AB 0%, B1 and B2 8%, B3 27% and C 50%, as shown by Wright and associates [29]. Haniuda and colleagues [46] followed 126 patients after complete resection and identified 24 recurrences (19%) with 83% relapses in Masaoka stage IVa thymomas and no recurrences in Masaoka stage I thymomas, which once again indicates that the recurrence rate increases with stage.
As reported by Cowen and associates [1], metastatic spread is significantly (p < 0.02) more often seen in patients who were not treated (biopsy only, 27%) or underwent subtotal resection (23%) than in patients who underwent complete resection (7.9%) [1].
Radiotherapy
Postoperative radiotherapy is currently not considered for completely resected Masaoka stage I thymomas, as no additional benefit on survival has been observed [30]. However, in incompletely resected or invasive thymomas (Masaoka stage II and III) adjuvant radiotherapy is frequently applied with the option of sole tumor bed versus extended field irradiation (entire mediastinum and supraclavicular fossae) [24, 49]. The applied radiation dose ranges from 40 to 60 Gy [1, 24, 49, 55].
Adjuvant radiation therapy for completely resected Masaoka stage II thymomas remains controversial. Some authors suggest that an R0 resection alone is adequate in the treatment of Masaoka stage I and II thymomas [56]. Yet, particularly in Masaoka stage II tumors of a high-risk WHO category such as B2, B3, or C, adjuvant radiotherapy should be considered as a significantly increased 5-year survival rate of 86% compared with 48% without adjuvant therapy has been reported (p < 0.002) [7, 24]. Recurrences and metastatic disease after resection of WHO B2 and B3 thymomas reflect their malignant behavior and suggest potential for intensified treatment [25, 47, 55].
According to several studies, neither increases of the irradiation dose nor extension of the radiation field (thymic bed vs entire mediastinum) seem to improve the outcome after resection [1, 24, 49]. As reported by Zhu and colleagues [49], the 5-year local control rate after irradiation of the tumor bed of 68% is comparable with that after extension of radiotherapy fields (67%) [49]. Comparing different radiation doses (≤ 50 Gy vs > 50 Gy) no significant differences on local control or survival were observed. A report by Kondo and Monden [55] suggest that the recurrence rate of completely resected Masaoka stage II and III thymomas is not significantly decreased by postoperative radiotherapy. Recurrence rates of Masaoka stage II and III thymomas were 5% and 23% in patients with postoperative radiotherapy, and 4% and 26% in patients without radiotherapy, respectively [55]. According to this study, even in patients after complete resection of Masaoka stage III and IV thymomas there is no significant difference in survival rates seen between surgery alone and surgery with postoperative radiotherapy (5-year and 10-year survival of 100% and 95% vs 93% and 78%, respectively). Furthermore, the use of routine adjuvant irradiation after complete resection of Masaoka stage III thymomas does not seem to prevent pleural recurrences and therefore needs to be re-addressed according to Mangi and colleagues [57]. However, other studies clearly consider adjuvant radiotherapy as an effective treatment in advanced thymomas (Masaoka stages III and IV) by inducing long-term complete or partial remissions, especially in Masaoka stage III thymomas [28, 58, 59, 11].
We have successfully used neoadjuvant radiotherapy in our programs to reduce the size of inoperable thymomas to achieve complete surgical resectability, as depicted in the computed tomographic images in Figure 2.
Fig 2.
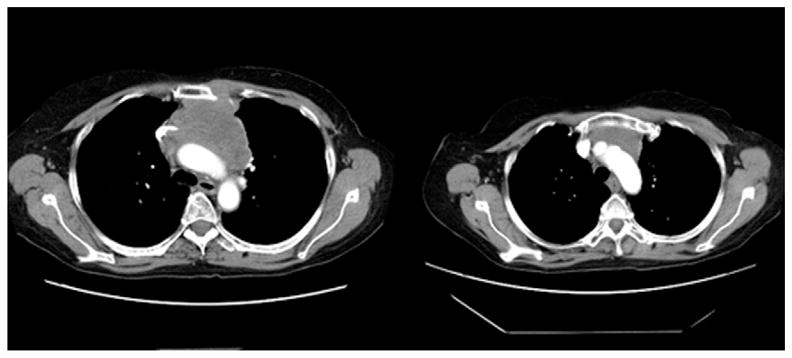
Computed tomographic scan of a thymoma before (left) and after (right) neoadjuvant radiotherapy.
Chemotherapy
Chemotherapy is not considered as a treatment of choice in localized, surgically resectable thymomas [60]. However, thymomas are often chemotherapy-sensitive tumors, and therefore chemotherapy is adopted in select patients with inoperable or gross residual disease after local treatment, mainly for Masaoka stage III or IV thymomas [27, 29, 49].
Frequently applied agents in the reviewed studies are cisplatin-based protocols consisting of doxorubicin, cyclophosphamide, cisplatin, vincristine, or cisplatin with etoposide and ifosfamide [22, 23]. Currently, however, no standardized regimen for chemotherapy in thymoma therapy exists.
In a study by Cowen and colleagues [1] the use of chemotherapy strongly correlates with Masaoka stage III and IVa thymomas (p < 0.01) and mediastinal compression on presentation (p < 0.01) [1]. The adjuvant platinum-based (cyclophosphamide, doxorubicin or adriamycin, cisplatin or carboplatin (CAP) regimen is associated with disease-free survival at 5 years of approximately 55% versus 20% if no chemotherapy was applied (p = 0.02).
Rea and colleagues [26] adopted the cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC) regimen neoadjuvantly in unresectable stage III or IVa, showing an improved resectability after neoadjuvant chemotherapy of 75% versus 58% if no neoadjuvant therapy was applied. Berruti and colleagues [61] have confirmed these positive effects.
Small prospective studies focusing on the use of chemotherapy also suggest a positive effect for the combination of cisplatin and etoposide [60, 62]. Loehrer and colleagues [60] concluded from their prospective study of 28 patients treated with the VIP regimen (cisplatin, iphosphamide and etoposide) that objective response rates and prolonged survival are achievable in patients with metastatic or locally progressive recurrent disease. In 32.1%, partial remission was achieved, although no patient experienced complete remission. Progressive disease developed in 7.1% of patients, whereas 60.1% of patients had stable disease. The 1-year and 2-year survival rates were 89% and 70%, respectively. In a phase II study conducted by Giaccone and colleagues [62], 16 patients with recurrent or metastatic thymoma were treated with a combination of cisplatin and etoposide, achieving a response rate in 56% of patients, of which 31% were complete responses with a median response duration of 3.4 years (median survival, 4.3 years; median progression-free survival, 2.2 years).
In summary, a number of studies have shown potential benefit for multiple chemotherapeutic regimens in the treatment of thymoma. The lack of prospective trials, however, with consistent regimens makes comparison across studies difficult. Larger controlled studies are clearly required for validation [55].
Multimodality Treatment
Multimodality treatment is an approach to manage primarily unresectable tumors as frequently seen in Masaoka III, IVa, and IVb thymomas [63, 64]. Bretti and colleagues [63] reported increased radical resection rates after neoadjuvant treatment from 46% to 65% in Masaoka stage III patients, and from 0% to 20% in those with Masaoka stage IVa disease, respectively. A total irradiation dose of 30 Gy was administered over 3 weeks and two chemotherapy regimens were adopted: four cycles of either ADOC or CDDP + VP16 (cisplatin and etoposide). Therefore, induction chemoradiotherapy can be regarded as an attempt to potentially downstage thymomas to improve surgical resectability and survival [63, 65, 66].
Lucchi and colleagues [67] reported reasonable long-term results in Masaoka stage III and IVa thymomas, applying neoadjuvant chemotherapy, surgery, and postoperative radiotherapy, or primary surgery followed by adjuvant chemotherapy or radiation therapy, or both. The neoadjuvant or adjuvant chemotherapy regimen consisted of three courses of cisplatin, epidoxorubicin, and etoposide every 3 weeks. Adjuvant irradiation consisted of 45 Gy for complete and 60 Gy for incomplete resections. Again, according to their data, neoadjuvant chemotherapy improved the resectability rate and survival. Kim and colleagues [68] confirmed these findings in a prospective study of 22 patients treated with induction chemotherapy (cyclophosphamide, doxorubicin, cisplatin and prednisone), surgery, and radiotherapy, plus consolidation chemotherapy. Eighteen of 19 patients who completed the multidisciplinary regimen were disease-free at a median follow-up of 50.3 months. The overall survival rate was 95% at 5 years and 79% at 7 years with a progression-free survival rate of 77% at 5 and 7 years. Low morbidity with promising long survival rates, supporting a multimodality approach in a select group of thymoma patients have been reported [48, 69, 70]. However, prospective multi-institutional studies are needed for further verification [48].
Prognostic Factors In Thymoma Treatment
Masaoka stage, WHO classification, and radical surgical resection are considered significant, independent prognostic factors on long-term disease-free survival in the majority of studies reviewed [7, 23, 24, 26, 28–30, 49, 55].
It is not clear whether tumor size is an independent prognostic factor for outcome in thymoma patients, although it has been suggested by various studies. Nakagawa and colleagues [30] evaluated factors limiting the prognosis of thymomas, and rated tumor size as a significant predictor of outcome (p = 0.001). These results were supported by a single-center study of 179 patients by Wright and colleagues [29], finding a critical tumor size (≥ 8 cm) to be an independent predictor for recurrence.
The prognostic value of myasthenia gravis (MG) in thymomas is controversial in the reviewed literature. In a multicenter study of 1,089 patients, Kondo and Monden [55] found that thymomas associated with MG seem to behave less aggressively [71], as thymomas associated with MG are diagnosed at an earlier stage and have lower recurrence rates [22, 28, 71]. As a consequence, myasthenia gravis can be regarded as a positive prognostic factor for the outcome of thymomas. Myasthenia gravis is the most common disorder of neuromuscular transmission, defined clinically on the basis of weakness or fatigue after repetitive exercise that resolves with rest. Additional frequent primary symptoms include diplopia, ptosis, difficulties swallowing and chewing, and muscular pain (especially of the neck). The Osserman classification system subdivides the disease by its severity into ocular, mild generalized, moderate generalized, acute fulminating, and late severe MG [72]. Determination of serum levels of anti-acetylcholine receptor antibodies (AChR) and anti-muscle-specific tyrosine kinase antibodies (anti-MuSK) is applied to confirm the diagnosis of MG, especially if clinical symptoms do not seem typical for the disease [72–74]. Anti-titin and anti-ryanodine receptor antibodies are found in up to 95% of MG patients with thymoma; therefore, their detection can be valuable in diagnostics [72, 75, 76].
Comment
In summary, the Masaoka staging system seems to be the current state of the art in thymoma diagnostics and therapy. Computed tomography is the diagnostic tool of choice to image the mediastinal lesion and its anatomical extent. For histopathologic diagnosis, especially if the lesion is invading the neighboring structures, a preoperative specimen acquisition by FNA or core biopsy should be considered, especially if neoadjuvant therapy is considered.
Median sternotomy is the surgical approach of choice for complete surgical resection. It is effective for stage I thymomas without any further treatment. After surgical resection of stage II thymomas, adjuvant radiotherapy should be considered. Stage III thymomas may benefit from neoadjuvant therapy to achieve complete surgical resectability and to minimize the risk of recurrent disease. Postoperative radiotherapy may also be useful for stage III thymomas, especially if complete resection is not feasible. Patients with stage IV thymomas and unresectable stage III thymomas may benefit from a multimodality approach including radiotherapy and chemotherapy, and potentially surgery, if significant downstaging occurs. Therapy for advanced thymomas infiltrating neighboring structures with pericardial or pleural dissemination remains controversial.
Acquiring reliable data in prospective long-term studies to verify these findings is challenging and only feasible in multicenter trials given the overall low incidence of thymoma. Novel research approaches might improve the understanding of the disease and lead to new tools for diagnosis and treatment of thymomas.
Sasaki and colleagues [77] have shown that gene expression analysis of thymomas may provide a novel and promising approach for biologically classifying thymomas [77]. This idea has been further investigated by Lee and colleagues [78] observing genome-wide chromosomal aberrations in thymomas with correlation of specific alterations to thymoma subgroups.
The clinical impact of these findings is as of yet unknown, but may offer potential opportunities for complementing the current standard classification systems and improving prognosis and clinical outcomes for thymoma patients in the future.
References
- 1.Cowen D, Richaud P, Mornex F, et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le. Cancer Radiother Oncol. 1995;34:9–16. doi: 10.1016/0167-8140(94)01493-m. [DOI] [PubMed] [Google Scholar]
- 2.Hon C, Chui WH, Cheng LC, Shek TW, Jones BM, Au WY. Thymoma associated with keratoconjunctivitis, lichen planus, hypogammaglobinemia, and absent circulating B cells. J Clin Oncol. 2006;24:2960–1. doi: 10.1200/JCO.2005.04.3133. [DOI] [PubMed] [Google Scholar]
- 3.Miyakis S, Pefanis A, Passam FH, Christodulakis GR, Roussou PA, Mountokalakis TD. Thymoma with immunodeficiency (Good’s syndrome): review of the literature apropos three cases. Scand J Infect Dis. 2006;38:314–9. doi: 10.1080/00365540500372663. [DOI] [PubMed] [Google Scholar]
- 4.Murakawa T, Nakajima J, Sato H, Tanaka M, Takamoto S, Fukayama M. Thymoma associated with pure red-cell aplasia: clinical features and prognosis. Asian Cardiovasc Thorac Ann. 2002;10:150–4. doi: 10.1177/021849230201000213. [DOI] [PubMed] [Google Scholar]
- 5.Skeie GO, Apostolski S, Evoli A, et al. Guidelines for the treatment of autoimmune neuromuscular transmission disorders. Eur J Neurol. 2006;13:691–9. doi: 10.1111/j.1468-1331.2006.01476.x. [DOI] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM. Malignant thymoma in the United States: demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer. 2003;105:546–51. doi: 10.1002/ijc.11099. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Marx A, Wen-Hu C, et al. New WHO histologic classification predicts prognosis of thymic epithelial tumors: a clinicopathologic study of 200 thymoma cases from China. Cancer. 2002;95:420–9. doi: 10.1002/cncr.10665. [DOI] [PubMed] [Google Scholar]
- 8.Harris NL, Müller-Hermelink HK. Thymoma classification: a siren’s song of simplicity. Am J Clin Pathol. 1999;112:299–303. doi: 10.1093/ajcp/112.3.299. [DOI] [PubMed] [Google Scholar]
- 9.Kornstein MJ. Thymoma classification: my opinion. Am J Clin Pathol. 1999;112:304–7. doi: 10.1093/ajcp/112.3.304. [DOI] [PubMed] [Google Scholar]
- 10.Suster S, Moran CA. Thymoma classification. The ride of the valkyries? Am J Clin Pathol. 1999;112:308–10. doi: 10.1093/ajcp/112.3.308. [DOI] [PubMed] [Google Scholar]
- 11.Kuo TT. Classification of thymic epithelial neoplasms: a controversial issue coming to an end? J Cell Mol Med. 2001;5:442–8. doi: 10.1111/j.1582-4934.2001.tb00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernatz PE, Harrison EG, Clagett OT. Thymoma: a clinicopathologic study. J Thorac Cardiovasc Surg. 1961;42:424–44. [PubMed] [Google Scholar]
- 13.Rosai J, Levine GD. Atlas of Tumor Pathology 2nd series, Fasc 13. Armed Forces Institute of Pathology; Washington, DC: 1976. Tumors of the thymus. [Google Scholar]
- 14.Marino M, Müller-Hermelink HK. Thymoma and thymic carcinoma: Relation of thymoma epithelial cells to the cortical and medullary differentiation of thymus. Virchows Arch A Pathol Anat Histopathol. 1985;407:119–49. doi: 10.1007/BF00737071. [DOI] [PubMed] [Google Scholar]
- 15.Müller-Hermelink HK, Ströbel P, Zettl A, et al. Combined thymic epithelial tumours. In: Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart (WHO classification of tumours series) Lyon, France: IARC Press; 2004. pp. 196–8. [Google Scholar]
- 16.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. Pathology and genetics of tumours of the lung, pleura, thymus and heart (WHO classification of tumours series) Lyon, France: IARC Press; 2004. pp. 152–171. [Google Scholar]
- 17.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–92. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Gamondès JP, Balawi A, Greenland T, et al. Seventeen years of surgical treatment of thymoma: factors influencing survival. Eur J Cardiothorac Surg. 1991;5:124–31. doi: 10.1016/1010-7940(91)90210-b. [DOI] [PubMed] [Google Scholar]
- 19.Yamakawa Y, Masaoka A, Hashimoto T, et al. A tentative tumor-node-metastasis classification of thymoma. Cancer. 1991;68:1984–7. doi: 10.1002/1097-0142(19911101)68:9<1984::aid-cncr2820680923>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya R, Koga K, Matsuno Y, Mukai K, Shimosato Y. Thymic carcinoma: proposal for pathological TNM and staging. Pathol Int. 1994;44:505–12. doi: 10.1111/j.1440-1827.1994.tb02600.x. [DOI] [PubMed] [Google Scholar]
- 21.Bedini AV, Andreani SM, Tavecchio L, et al. Proposal of a novel system for the staging of thymic epithelial tumors. Ann Thorac Surg. 2005;80:1994–2000. doi: 10.1016/j.athoracsur.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Evoli A, Minisci C, Di Schino C, et al. Thymoma in patients with MG: characteristics and long-term outcome. Neurology. 2002;59:1844–50. doi: 10.1212/01.wnl.0000032502.89361.0c. [DOI] [PubMed] [Google Scholar]
- 23.Kim DJ, Yang WI, Choi SS, Kim KD, Chung KY. Prognostic and clinical relevance of the World Health Organization schema for the classification of thymic epithelial tumors: a clinicopathologic study of 108 patients and literature review. Chest. 2005;127:755–61. doi: 10.1378/chest.127.3.755. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa K, Uno T, Toita T, et al. Postoperative radiotherapy for patients with completely resected thymoma. A multi-institutional, retrospective review of 103 patients. Cancer. 2002;94:1405–13. doi: 10.1002/cncr.10373. [DOI] [PubMed] [Google Scholar]
- 25.Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94:624–32. doi: 10.1002/cncr.10226. [DOI] [PubMed] [Google Scholar]
- 26.Rea F, Marulli G, Girardi R, et al. Long-term survival and prognostic factors in thymic epithelial tumours. Eur J Cardiothorac Surg. 2004;26:412–8. doi: 10.1016/j.ejcts.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 27.Rena O, Papalia E, Maggi G, et al. World Health Organization histologic classification: an independent prognostic factor in resected thymomas. Lung Cancer. 2005;50:59–66. doi: 10.1016/j.lungcan.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Ströbel P, Bauer A, Puppe B, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22:1501–9. doi: 10.1200/JCO.2004.10.113. [DOI] [PubMed] [Google Scholar]
- 29.Wright CD, Wain JC, Wong DR, et al. Predictors of recurrence in thymic tumors: importance of invasion, World Health Organization histology, and size. J Thorac Cardiovasc Surg. 2005;130:1413–21. doi: 10.1016/j.jtcvs.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg. 2003;126:1134–40. doi: 10.1016/s0022-5223(03)00798-0. [DOI] [PubMed] [Google Scholar]
- 31.Yang WT, Lei KI, Metreweli C. Plain radiography and computed tomography of invasive thymomas: clinicoradiologic-pathologic correlation. Australas Radiol. 1997;41:118–24. doi: 10.1111/j.1440-1673.1997.tb00695.x. [DOI] [PubMed] [Google Scholar]
- 32.Maher MM, Shepard JA. Imaging of thymoma. Semin Thorac Cardiovasc Surg. 2005;17:12–9. doi: 10.1053/j.semtcvs.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Tomiyama N, Honda O, Tsubamoto M, et al. Anterior mediastinal tumors: Diagnostic accuracy of CT and MRI. Eur J Radiol. 2009;69:280–8. doi: 10.1016/j.ejrad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Han J, Lee KS, Yi CA, et al. Thymic epithelial tumors classified according to a newly established WHO scheme: CT and MR findings. Korean J Radiol. 2003;4:46–53. doi: 10.3348/kjr.2003.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue A, Tomiyama N, Fujimoto K, et al. MR imaging of thymic epithelial tumors: correlation with World Health Organization classification. Radiat Med. 2006;24:171–81. doi: 10.1007/s11604-005-1530-4. [DOI] [PubMed] [Google Scholar]
- 36.Endo M, Nakagawa K, Ohde Y, et al. Utility of 18FDG-PET for differentiating the grade of malignancy in thymic epithelial tumors. Lung Cancer. 2008;61:350–5. doi: 10.1016/j.lungcan.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Sung YM, Lee KS, Kim BT, Choi JY, Shim YM, Yi CA. 18F-FDG PET/CT of thymic epithelial tumors: usefulness for distinguishing and staging tumor subgroups. J Nucl Med. 2006;47:1628–34. [PubMed] [Google Scholar]
- 38.Higuchi T, Taki J, Kinuya S, et al. Thymic lesions in patients with myasthenia gravis: characterization with thallium 201 scintigraphy. Radiology. 2001;221:201–6. doi: 10.1148/radiol.2211001047. [DOI] [PubMed] [Google Scholar]
- 39.Ali SZ, Erozan YS. Thymoma. Cytopathologic features and differential diagnosis on fine needle aspiration. Acta Cytol. 1998;42:845–54. doi: 10.1159/000331958. [DOI] [PubMed] [Google Scholar]
- 40.Chhieng DC, Rose D, Ludwig ME, Zakowski MF. Cytology of thymomas: emphasis on morphology and correlation with histologic subtypes. Cancer. 2000;90:24–32. [PubMed] [Google Scholar]
- 41.Shin HJ, Katz RL. Thymic neoplasia as represented by fine needle aspiration biopsy of anterior mediastinal masses. A practical approach to the differential diagnosis. Acta Cytol. 1998;42:855–64. doi: 10.1159/000331959. [DOI] [PubMed] [Google Scholar]
- 42.Annessi V, Paci M, De Franco S, et al. Diagnosis of anterior mediastinal masses with ultrasonically guided core needle biopsy. Chir Ital. 2003;55:379–84. [PubMed] [Google Scholar]
- 43.Kramer H, Sanders J, Post WJ, Groen HJ, Suurmeijer AJ. Analysis of cytological specimens from mediastinal lesions obtained by endoscopic ultrasound-guided fine-needle aspiration. Cancer. 2006;108:206–11. doi: 10.1002/cncr.21914. [DOI] [PubMed] [Google Scholar]
- 44.Larghi A, Noffsinger A, Dye CE, Hart J, Waxman I. EUS-guided fine needle tissue acquisition by using high negative pressure suction for the evaluation of solid masses: a pilot study. Gastrointest Endosc. 2005;62:768–74. doi: 10.1016/j.gie.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Assaad MW, Pantanowitz L, Otis CN. Diagnostic accuracy of image-guided percutaneous fine needle aspiration biopsy of the mediastinum. Diagn Cytopathol. 2007;35:705–9. doi: 10.1002/dc.20738. [DOI] [PubMed] [Google Scholar]
- 46.Haniuda M, Kondo R, Numanami H, Makiuchi A, Machida E, Amano J. Recurrence of thymoma: clinicopathological features, re-operation, and outcome. J Surg Oncol. 2001;78:183–8. doi: 10.1002/jso.1146. [DOI] [PubMed] [Google Scholar]
- 47.Regnard JF, Magdeleinat P, Dromer C, et al. Prognostic factors and long-term results after thymoma resection: a series of 307 patients. J Thorac Cardiovasc Surg. 1996;112:376–84. doi: 10.1016/S0022-5223(96)70265-9. [DOI] [PubMed] [Google Scholar]
- 48.Wright CD, Choi NC, Wain JC, Mathisen DJ, Lynch TJ, Fidias P. Induction chemotherapy followed by resection for locally advanced Masaoka Stage III and IVa thymic tumors. Ann Thorac Surg. 2008;85:385–9. doi: 10.1016/j.athoracsur.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 49.Zhu G, He S, Fu X, Jiang G, Liu T. Radiotherapy and prognostic factors for thymoma: a retrospective study of 175 patients. Int J Radiat Oncol Biol Phys. 2004;60:1113–9. doi: 10.1016/j.ijrobp.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 50.Blalock A, McGehee AH, Ford FR. The treatment of myasthenia gravis by removal of the thymus. JAMA. 1941;18:1529–33. [Google Scholar]
- 51.Aubert A, Chaffanjon P, Brichon PY. Video-assisted extended thymectomy in patients with thymoma by lifting the sternum: is it safe? Ann Thorac Surg. 2004;77:1878. doi: 10.1016/j.athoracsur.2003.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Ferguson MK. Transcervical thymectomy. Semin Thorac Cardiovasc Surg. 1999;11:59–64. doi: 10.1016/s1043-0679(99)70021-3. [DOI] [PubMed] [Google Scholar]
- 53.Cheng YJ, Kao EL, Chou SH. Videothoracoscopic resection of stage II thymoma: prospective comparison of the results between thoracoscopy and open methods. Chest. 2005;128:3010–2. doi: 10.1378/chest.128.4.3010. [DOI] [PubMed] [Google Scholar]
- 54.Iablonski PK, Pishchik VG, Nuraliev SM. Comparative assessment of the effectiveness of traditional and videothoracoscopic thymectomies in complex treatment of myasthenic thymomas. Vestn Khir Im I I Grek. 2005;164:38–42. [PubMed] [Google Scholar]
- 55.Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg. 2003;76:878–84. doi: 10.1016/s0003-4975(03)00555-1. [DOI] [PubMed] [Google Scholar]
- 56.Singhal S, Shrager JB, Rosenthal DI, LiVolsi VA, Kaiser LR. Comparison of stages I–II thymoma treated by complete resection with or without adjuvant radiation. Ann Thorac Surg. 2003;76:1635–41. doi: 10.1016/s0003-4975(03)00819-1. [DOI] [PubMed] [Google Scholar]
- 57.Mangi AA, Wain JC, Donahue DM, Grillo HC, Mathisen DJ, Wright CD. Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg. 2005;79:1834–9. doi: 10.1016/j.athoracsur.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 58.Cesaretti JA. Adjuvant radiation with modern techniques is the standard of care for stage III thymoma. Ann Thorac Surg. 2006;81:1180–1. doi: 10.1016/j.athoracsur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 59.Sugie C, Shibamoto Y, Ikeya-Hashizume C, et al. Invasive thymoma: postoperative mediastinal irradiation, and low-dose entire hemithorax irradiation in patients with pleural dissemination. J Thorac Oncol. 2008;3:75–81. doi: 10.1097/JTO.0b013e31815e8b73. [DOI] [PubMed] [Google Scholar]
- 60.Loehrer PJ, Sr, Jiroutek M, Aisner S, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer. 2001;91:2010–5. [PubMed] [Google Scholar]
- 61.Berruti A, Borasio P, Gerbino A, et al. Primary chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide in locally advanced thymomas: a single institution experience. Br J Cancer. 1999;81:841–5. doi: 10.1038/sj.bjc.6690773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giaccone G, Ardizzoni A, Kirkpatrick A, Clerico M, Sahmoud T, van Zandwijk N. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma: a phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. J Clin Oncol. 1996;14:814–20. doi: 10.1200/JCO.1996.14.3.814. [DOI] [PubMed] [Google Scholar]
- 63.Bretti S, Berruti A, Loddo C, et al. Multimodal management of stages III–IVa malignant thymoma. Lung Cancer. 2004;44:69–77. doi: 10.1016/j.lungcan.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 64.Shin DM, Walsh GL, Komaki R, et al. A multidisciplinary approach to therapy for unresectable malignant thymoma. Ann Intern Med. 1998;129:100–4. doi: 10.7326/0003-4819-129-2-199807150-00006. [DOI] [PubMed] [Google Scholar]
- 65.Jacot W, Quantin X, Valette S, Khial F, Pujol JL. Multimodality treatment program in invasive thymic epithelial tumor. Am J Clin Oncol. 2005;28:5–7. doi: 10.1097/01.coc.0000138963.01562.d3. [DOI] [PubMed] [Google Scholar]
- 66.Venuta F, Rendina EA, Pescarmona EO, et al. Multimodality treatment of thymoma: a prospective study. Ann Thorac Surg. 1997;64:1585–91. doi: 10.1016/s0003-4975(97)00629-2. [DOI] [PubMed] [Google Scholar]
- 67.Lucchi M, Ambrogi MC, Duranti L, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Ann Thorac Surg. 2005;79:1840–4. doi: 10.1016/j.athoracsur.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 68.Kim ES, Putnam JB, Komaki R, et al. Phase II study of multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer. 2004;44:369–79. doi: 10.1016/j.lungcan.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 69.Huang J, Riely GJ, Rosenzweig KE, Rusch VW. Multimodality therapy for locally advanced thymomas: state of the art or investigational therapy? Ann Thorac Surg. 2008;85:365–7. doi: 10.1016/j.athoracsur.2007.10.098. [DOI] [PubMed] [Google Scholar]
- 70.Huang J, Rizk NP, Travis WD, et al. Feasibility of multimodality therapy including extended resections in stage IVA thymoma. J Thorac Cardiovasc Surg. 2007;134:1477–83. doi: 10.1016/j.jtcvs.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 71.Kondo K, Monden Y. Thymoma and myasthenia gravis: a clinical study of 1,089 patients from Japan. Ann Thorac Surg. 2005;79:219–24. doi: 10.1016/j.athoracsur.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 72.Grehl H, Reinhardt F. Checkliste Neurologie. 3. Georg Thieme Verlag; Stuttgart, Germany: 2005. pp. 670–8. [Google Scholar]
- 73.Bartoccioni E, Scuderi F, Minicuci GM, Marino M, Ciaraffa F, Evoli A. Anti-MuSk antibodies: correlation with myasthenia gravis severity. Neurology. 2006;67:505–7. doi: 10.1212/01.wnl.0000228225.23349.5d. [DOI] [PubMed] [Google Scholar]
- 74.Morgan BP, Chamberlain-Banoub J, Neal JW, Song W, Mizuno M, Harris CL. The membrane attack pathway of complement drives pathology in passively induced experimental autoimmune myasthenia gravis in mice. Clin Exp Immunol. 2006;146:294–302. doi: 10.1111/j.1365-2249.2006.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romi F, Bø L, Skeie GO, Myking A, Aarli JA, Gilhus NE. Titin and ryanodine receptor epitopes are expressed in cortical thymoma along with costimulatory molecules. J Neuroimmunol. 2002;128:82–9. doi: 10.1016/s0165-5728(02)00145-5. [DOI] [PubMed] [Google Scholar]
- 76.Li YF, Li YH, Cui LY. Thymus CT scan and thymoma associated antibodies in myasthenia gravis with thymoma. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:517–9. [PubMed] [Google Scholar]
- 77.Sasaki H, Ide N, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Gene expression analysis of human thymoma correlates with tumor stage. Int J Cancer. 2002;101:342–7. doi: 10.1002/ijc.10624. [DOI] [PubMed] [Google Scholar]
- 78.Lee GY, Yang WI, Jeung HC, et al. Genome-wide genetic aberrations of thymoma using cDNA microarray based comparative genomic hybridization. BMC Genomics. 2007;8:305. doi: 10.1186/1471-2164-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]


