Abstract
Monogamous animals face an interesting dilemma: if and when to terminate a nonproductive relationship. To address this issue, we asked whether reproductive compatibility is a criterion for maintaining a monogamous pair-bond between mates. Prairie voles (Microtus ochrogaster), are small rodents that form long-term, monogamous pair-bonds evidenced by a strong preference for their familiar partners, and thus are an excellent model animal in which to study mate-fidelity. Accordingly, we examined partner-preferences by male prairie voles and related their behavior to the reproductive status of their mates. We found that, when given a choice between their familiar female partner and a stranger, male prairie voles differ in their responses depending on the pregnancy status of the partner. Males that were paired with females for two weeks displayed mate-fidelity only when mating had been initiated within ~48 hours after pairing. In contrast, males whose mates experienced a delay in the onset of sexual receptivity displayed non-selective affiliative behavior. We also found that, in addition to being appropriately timed, mating must be successful. Males that mated with ovariectomized females for whom pregnancy was precluded did not display mate-fidelity even though mating was initiated within the proper timeframe. These observations of mate-fidelity only when the partner’s pregnancy was sufficiently advanced relative to the duration of the pairing suggest that mating must be both successful and timely. Thus, reproductive compatibility can influence mate-fidelity.
Keywords: better options, divorce, incompatibility, mate choice, Microtus ochrogaster, monogamy, prairie vole, reproduction
Whether reproduction involves broadcast spawning, opportunistic encounters, harem formation, or other strategies, most animal species display promiscuous mating systems. Occasionally, however, selection pressures favor a monogamous mating system manifested by strong bonds between the members of a breeding pair. Exclusive mating between one male and one female increases relatedness of offspring, which may reduce the costs of pregnancy to the female (Trivers & Burt 1999). Further, remaining with the same partner can enhance lifetime reproductive success (Ribble 1992), often as a result of the male’s involvement in parental care (Wright & Brown 2002). Thus, it may be advantageous for a female to mate with one male exclusively, in turn allowing the male to be confident of the paternity of the offspring in which he is investing (Campbell et al. 2009). In short, both sexes may benefit from monogamous pairing (Emlen & Oring 1977). However, such pairings are not without costs. In fact, monogamous animals face an important challenge: if and when to abandon a partner with whom they may not be reproductively compatible.
Most studies that address animal divorce assume a prior breeding attempt on which an individual can base the divorce decision. This assumption is fine for long-lived species for which multiple breeding seasons are possible, and even for shorter-lived species where multiple broods within a season are likely, but it is poorly suited to species for which only a very few breeding attempts might be expected. For short-lived species, the costs of mate fidelity in the face of poor reproductive success likely are high (Getz et al. 2004). In fact, the benefits of separation may outweigh those of remaining together for some pairs (Ens et al. 1993; Rasmussen 1981); however, these benefits must be balanced by countervailing risks incurred during the search for a new mate (McNamara & Forslund 1996; Rasmussen 1981). In a study modeling the costs and benefits of divorce by female birds, McNamara and Forslund (1996), attempted to define circumstances under which a monogamous pair should divorce. They concluded that mate retention is favored when the mate exceeds some critical threshold for male quality, while divorce is favored below that threshold. This conclusion likely holds for both sexes as well as for other species.
Although common in birds, monogamous pair-bonding is rare among mammals (Kleiman 1977), but probably evolved independently several times (Komers & Brotherton 1997). Only about 3% of mammal species display such bonds, and even fewer exhibit obligate monogamy (Kleiman 1977). Prairie voles (Microtus ochrogaster) are among the few mammalian species that are monogamous. These voles form long-term pair-bonds with their partners that extend beyond the breeding season, share a common nest, and vigorously defend their mates against contact with conspecifics (Carter & Getz 1993). Both sexes provide parental care (Carter & Getz 1993), and females that lose their mates rarely form new pair-bonds (Pizzuto & Getz 1998) although they still may reproduce (Renfro et al. 2009; Thomas & Wolff 2004).
Prairie vole reproduction involves a complex interplay of social interactions and chemical communication between members of a potential breeding pair, all of which must be achieved before mating can occur. After the pair meet, they remain together for several days and each member of the pair appears to form a rudimentary preference for its partner. Since female prairie voles do not display an estrous cycle, this attraction may serve to keep the pair together long enough for activation of the female reproductive system, which requires an extended period of intimate contact with a male (Carter et al. 1980; Sawrey & Dewsbury 1985). During this period, olfactory/pheromonal signals from the male induce an increase in circulating estrogen in the female (Cohen-Parsons & Carter 1987) which, in turn, makes the female sexually receptive (Carter et al. 1987b). Absent this chemical communication, the female does not become receptive, and mating does not occur (Curtis et al. 2001; Williams et al. 1992b). In fact, olfactory bulbectomy or removal of the vomeronasal organ can impair partner preference formation and subsequent sexual behavior in either sex (Curtis et al. 2001; Kirkpatrick et al. 1994; Lepri & Wysocki 1987; Williams et al. 1992b).
Once the female becomes sexually receptive, mating is initiated and occurs in frequent bouts over the next 24–36 hours. Vaginal stimulation typically induces ovulation within about 10 hours after the onset of mating (Gray & Dewsbury 1973; Sawrey & Dewsbury 1985). Subsequent to the onset of mating, some combination of continued mating and/or the continued presence of the male after copulation can affect the probability of ovulation (Carter et al. 1986; Sawrey & Dewsbury 1985), as well as the number of embryos produced (Roberts et al. 1999).
It is clear that there are multiple points at which compatibility between the prairie vole partners is essential: 1) appropriate behaviors upon first meeting must be displayed; 2) both animals must develop a preference for close contact of sufficient duration for female sexual activation to occur; 3) the sender of pheromonal stimuli must produce the appropriate chemical signals and the receiver then must display the correct physiological response; 4) the appropriate sequence and timing of mating bouts must occur. All of these steps must take place even before pregnancy can occur. Thus, incompatibility between members of a pair at any of these points can side-track the mating process.
In studies of prairie voles in Illinois, the average survival time was about 40 to 65 days (Desy & Batzli 1989; Getz et al. 1997). Since prairie voles must be ~30 to 40 days of age prior to becoming sexually mature (body mass appears to dictate maturation more so than does a specific age; Nadeau 1985), many likely do not have more than one or two opportunities to reproduce. Given the relatively short life-span and the limited number of opportunities for reproduction, pair-bond formation in infertile pairs or in pairs with sub-optimal fertility may be counterproductive, and the costs of fidelity toward an incompatible mate should be high for prairie voles. As such, it might be expected that selection would favor individuals that best can assess the potential for successful reproduction. To test this possibility we exploited both natural and experimentally imposed inter-pair differences in mating success to examine whether variation in mating success between pairs of prairie voles would be reflected in their pair-bonding behavior.
METHODS
Animal husbandry
Experimental animals were obtained from an on-site captive breeding-colony descended from an Illinois prairie vole population, and housed in USDA inspected and approved facilities. General animal care is provided by Laboratory Animal Resources personnel. Animals are monitored daily and veterinary staff is available for consultation regarding animal health and welfare. All animals are maintained at ~21°C with a 14:10 light: dark cycle. Breeding pairs are housed in plastic cages (20 × 25 × 45 cm) containing corncob bedding with timothy hay as nesting material. Ad libitum food (Purina rabbit chow supplemented with black-oil sunflower seeds) and H2O are available. After weaning (~20 days of age), pups are housed as same-sex (typically sibling) pairs in shoebox style plastic cages (10 × 17 × 28 cm), again with ad libitum access to food and water. After weaning, females and breeder pairs are housed in a room separate from the males.
Subjects
Subjects were adult male prairie voles that were of the F3 and F4 generations relative to the most recent out-crossing of the colony with wild stock. Females were used in experimental pairings and as stimulus animals and were of similar age to that of the males with whom they were paired. Females also were from the F3 and F4 generations. All animals were at least 60 days of age (range 60–107) and sexually naïve at the start of the experiments.
In some experiments, ovariectomized (OVX) females were used in experimental pairings. Adult females were anesthetized with 1mg/kg pentobarbitol and the distal portion of the uterus and the ovaries were exposed through a small incision using a dorsal approach. Ovaries were tied off with suture and removed, and the incision closed with absorbable suture. Ovariectomized females were allowed at least two weeks of post-surgical recovery prior to being used in experiments.
Reproductive patterns
To minimize the number of animals used, data for overall mating success were gathered retrospectively from pairs (n = 92 pairs) used as unmanipulated controls in a variety of neurochemistry studies. Further, we examined the records from the first 25 pairs of our breeding stock to ascertain latency to first litter and the intervals between litters. Finally, we attempted to assess whether one or the other sex was predominantly responsible for a failure to reproduce by newly formed pairs. Some males from unproductive breeder pairs (defined as no pups born within 35 days after pairing), or from experimental pairings whose mates were not pregnant at termination two weeks after pairing, were paired with new females and the pairs (n = 14) were treated as typical breeder pairs. If, again, no pups were born within 35 days of pairing, the females were terminated and the conditions of their uteri were assessed for indication of sexual activation (Carter et al. 1987b). Males were considered to be responsible for mating failures if neither female with whom they were paired became pregnant. If the second female with whom the male was paired became pregnant, the original female was considered to be responsible for failed mating. A binomial test was used to assess whether the number of each sex assigned responsibility for failed mating differed from random (i.e., 50% for each sex).
Partner Preference testing
The apparatus for partner preference testing consisted of a central cage (17 × 12 × 22 cm) joined by tubes (7.5×16 cm) to two identical parallel cages (Williams et al. 1992a). Each of these latter cages contained a stimulus female (typically the familiar female partner with whom the male subject had been paired and a female that was completely unfamiliar to the male). The females were tethered to restrict their movements to their respective cages and thus had no direct contact with each other. Cage assignments were randomized for the first set of stimulus animals in each group tested; subsequent assignments alternated between the two cages. The male was released into the central cage and had unfettered access to all cages. All cages contained food and water. A customized computer program (R. Henderson, Florida State University) using a series of light beams across the connecting tubes was used to monitor the males’ movements among the cages. The computer program recorded the amount of time males spent in each cage and the number of crossings between cages. Throughout the test, the animals also were videotaped for detailed behavioral analysis. Each test lasted for three hours.
Final variables included the time spent by the male in each female’s cage and the amount of time the males spent in direct contact with each female. In addition, the number of cage crossings (a measure of activity to ensure that treatments did not affect locomotor behavior) and the amount of time males spent in the neutral cage (a measure of time spent in isolation) served as measures of non-social behavior. Finally, the total amount of time each male spent with both females combined was used to ensure that treatments did not alter social motivation. For each of the groups described below, comparisons of time spent in direct contact with the partner vs. with the stranger were made using paired t-tests. Significantly more time spent with the partners than with the strangers indicated a partner preference. Between groups treatment effects for other behavioral measures each were evaluated using one-way ANOVA.
For each group, the amount of time spent in contact with the partner by each male as a proportion of the total time that male spent in contact with both females was used to sort males into one of ten bins (≤ 10%, >10% and ≤ 20%, > 20% and ≤ 30%, … ≤ 90% and ≤ 100%), and the number of males in each bin was used as the observed value in Chi-squared analyses to test whether the data from individual animals in each group were uniformly distributed. The expected value for each bin was the number of animals in the group/10. A significant χ 2 value indicated a non-uniform distribution.
Effect of the partner’s pregnancy on male mate-fidelity
We used the partner preference test to examine whether the pregnancy status of his mate affected a male’s mate-fidelity. Experimental pairings were generated by randomly assigning unrelated males and females to be paired. Experimental pairs were housed for two weeks in cages of the same dimensions as those used to house weaned pups. Experimental animals were housed in the same room as the breeding pairs and were subjected to the standard cage maintenance applied to the colony as a whole. A separate group of males was exposed to a female for 6 hours without mating to serve as control males that had prior contact, but no pair-bond, with a stimulus female. At the end of the two-week or six-hour cohabitation periods, males were tested for a preference for contact with their familiar partners vs. with females that the male had not previously encountered. At the conclusion of the preference testing, both individuals of each pair were euthanized, the sexes were verified, and the females were examined to ascertain pregnancy status. Females were assigned to one of five pregnancy stages ranging from not reproductively active to “maximally pregnant” for the amount of time the pairs had been together (Table 1). Pairs in which the female was less than 10–12 days into her pregnancy or was not pregnant were considered to be incompatible.
Table 1.
Descriptions of uterine status at various stages of pregnancy for female prairie voles 14 days after pairing
| Pregnancy stage | Description | Number of females |
|---|---|---|
| 0 | No evidence of sexual activation, uterus white, thread-like | 4 |
| 0.5 | Uterus pink and swollen, no implantation sites evident | 5 |
| 1 | Implantation sites visible (~gestation day 3–5) | 7 |
| 1.5 | Individual fetuses identifiable (diameter ~ 5 mm) | 10 |
| 2 | Anterior–posterior axis evident (gestation days >10; length ~ 1 cm) | 66 |
The effect of timing of mating on male mate-fidelity
Experimental pairings were generated and treated as described above, except that wire mesh (~1 cm) barriers were placed in the cages during the first or second week of the cohabitation period to control the timing of mating onset. These barriers separated the animals, thus preventing mating, but allowed other types of social contact between the pair. Barriers were centered in the cages, providing both sexes with equal amounts of cage space. Pairs initially were assigned to one of two treatment groups: 1) barrier in place during the first week of the two-week cohabitation and then removed to allow normal contact between male and female for the remainder of the cohabitation period; or 2) male and female permitted to have normal interactions during the first week, but then separated by the barrier during the second week. At the end of the second week of cohabitation, males from each group were tested for a partner preference as outlined above. An additional group of males were allowed to interact normally with the females but were tested for a partner preference after one week of cohabitation. This group served as controls for the males in group 1 that interacted with females normally for only one week prior to preference testing. Together, these groups also served to control for experimental pairings that were generated by the experimenter, rather than by allowing the animals to choose their own mates. After behavioral testing, the pregnancy status of each female was assessed as outlined above, except that females from pairs that were never separated, but that were limited to one week of unfettered contact prior to behavioral testing were considered maximally pregnant if they were 3–5 days pregnant (pregnancy stage three in Table 1).
The effect of female fertility on male mate-fidelity
We examined whether the onset of mating or an actual pregnancy was responsible for changes in male fidelity. Experimental pairings were generated and treated as described above except that OVX females were used instead of intact females. Our goal was to mimic the naturally occurring pattern of reproductive activation and mating without the possibility of a successful pregnancy. Thus, eleven females were injected once daily with estradiol benzoate (EB, 10ug in 100ul sesame oil) beginning one day prior to pairing, and continuing for the first two days of cohabitation with the male. This treatment induces sexual receptivity in a majority of female voles. These pairs and a parallel set of pairs comprised of males and intact females were videotaped for the first 96 hours after pairing. Video recordings were later reviewed to ascertain the latency to mating after pairing and the duration of mating sequences for each pair. In the latter case, twelve hours without mating was assumed to indicate that mating was completed, and the last observed mating was used as the end point for mating duration. The video feed was lost during the mating sequence for one pair, preventing identification of the end of mating. Accordingly, we used only the data for onset of mating for that pair. Estrogen priming was not successful in 2 females, and these animals were not included in the preference testing. After two weeks of cohabitation, males were tested for a partner preference as outlined above.
The effect of the females’ presence on male mate-fidelity
We wished to examine male behavior in the absence of behavioral feedback from the female. Experimental pairings were generated and treated as in experiment 1 except that two days prior to preference testing, all pairs were transferred to special cages that allowed collection of bedding soiled by only one individual from a pair. This apparatus consisted of two identical parallel cages (17×12×22 cm) connected by a tube (7.5 × 16 cm). For each pair, the male was tethered in one cage while the female had free access to both cages. To collect bedding from sexually naive females, one female from a same-sex pair was tethered in one cage while the other female had free access to both cages. Females’ movements between cages were monitored for the first 24 hours as described for the partner preference test. Since voles tend to move away from the nest for waste elimination, it was expected that the females would use the unoccupied cages for waste elimination. During the first 24 hours, females averaged 341 ± 78 transits between cages and spent 21 ± 4% of their time in the empty cage. As expected, females’ use of the unoccupied cage was evident from the presence of fecal pellets throughout the cage, and in most cases, urine soaked bedding in one corner of the unoccupied cage. Thus, waste from only the female partner could be collected without the potential confound of forced separation from its cagemate. Soiled bedding for odor choice tests was collected over a 42 hour period. Immediately thereafter, the cages containing the soiled bedding were used in preference testing.
The apparatus, methods, and analysis for the bedding preference tests were identical to those described for previous partner preference tests except that cages containing bedding soiled by females were used instead of cages containing stimulus animals. Experimental males were tested in one of three conditions: (1) fresh bedding in all cages; bedding soiled by a sexually naïve female in one cage, and bedding soiled by either an unfamiliar pregnant female (2) or the pregnant mate of the subject male (3) in the other cage. The type of bedding assigned to each cage was counterbalanced to ensure that the various types of bedding were not always placed in the same cage. The center cage always contained fresh, unsoiled bedding and was designated as a neutral cage. The amounts of time males spent in each cage were compared using ANOVA.
Ethical note
All possible measures to ensure animal welfare were incorporated into the experimental design. Breeder pairs were housed in larger cages, and these cages included several inches of timothy hay as bedding material and to provide secluded nesting sites. In addition, after cage maintenance, sunflower seeds were scattered about the cage to provide the animals with an opportunity for more natural foraging activity. Voles experience stress responses when housed in isolation (DeVries et al. 1996); therefore, they are housed as same-sex (typically sibling) pairs after weaning. Finally, some experiments involved examining interactions between animals. Although extreme aggression is rare in our testing paradigm, we monitor the animals carefully, and are prepared to intercede if any animal appears to be suffering as a result of aggressive interactions. Animal handling procedures, experimental manipulations, and behavior testing are approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee (#’s 2006-03 and 2009-05).
RESULTS
Reproductive parameters
Twenty-one of the first 25 breeder pairs in our colony gave birth between 23 and 27 days after they were paired. The remaining 4 pairs gave birth at 35, 41, 49 and 51 days. Thus, the average time until birth of the typical first litter was 25.1 ± 0.3 days (n = 21 litters). The intervals to second and third litters were 22.4 ± 0.2 days (n = 36 litters).
We examined the pregnancy status of females from 92 pairs that had been together for fourteen days. Based on the stage of fetal development, 72% of females from these pairs were scored as being 10–12 days pregnant, while the remaining females ranged from no indication of reproductive activation to less than ten days pregnant (Table 1).
Fourteen males were re-paired after reproductive failure with their initial partner. In eight cases, the second pairing resulted in successful pregnancy, and thus the original female partners were considered to be responsible for the reproductive failure of the original pair. In the remaining 6 cases, neither the first nor the second female became pregnant, and in fact, none showed any indication of sexual activation (Carter et al. 1987b), and thus the male was considered to be at fault. This distribution was not statistically different from that expected by chance (z = 0.53, p = 0.79).
Effect of partner’s pregnancy on male mate-fidelity
The results of the basic preference test differed depending on the pregnancy stage of the female partners. Males whose partners were 10–12 days pregnant (n = 26) displayed a strong preference (t = 3.71, df = 25, p < 0.002) for their mates in the choice test (Fig 1A), while males (Fig 1C) that experienced only six hours of non-sexual cohabitation with a female (n = 17) did not display a partner preference (t = 0.17, df = 16, p = 0.86). Ninety-five percent (41 of 43) of males in these two groups spent ≥80% of their time with one or the other of the females (Fig 1A, C). The proportion of total contact time that was spent with the respective partners for these groups displayed non-uniform distributions (pair-bonded males - χ2 = 86.56, n = 26, p < 0.001; non-pair-bonded males - χ2 = 31.33, n = 17, p < 0.001). Males that remained with their partners for two weeks, but whose partners either were not pregnant or had experienced a delay in the onset of pregnancy (n = 17) did not display a partner preference (t = 0.44, df = 16, p = 0.66) (Fig 1B). In fact, these males often split their time between the females. Only about half (9 of 17) spent ≥80% of their contact time with one or the other female (Fig 1B). The remainder switched between huddling with the partner and huddling with the stranger one or more times during the test. Contact proportions for this group were uniformly distributed between 0% and 100% (χ2 = 7.67, n = 16, p = 0.96).
Figure 1.
Partner preference expression by male prairie voles depended on the mate’s pregnancy status. Bars indicate group means (±SEM, left y-axis) for contact time with the partner (black bars) or with the stranger (grey bars). Open circles indicate the percentages of total contact time that individual males spent with their partners (right y-axis); numbers associated with open circles indicate the numbers of animals in each group that displayed either 100% or 0% (data from multiple animals may overlap at the extremes). Gray lines delineate 80% and 20% levels for contact with partner. (A) Males paired for two weeks with females that became pregnant within the typical 2–3 day timeframe after pairing (n=26). (B) Males paired with partners that were either not pregnant after two weeks of cohabitation or who required longer than the usual time to become pregnant (n = 17). Many of these males switched between the two stimulus females (open circles). (C) Males paired with females for six hours absent mating (n = 17). *p < 0.01.
There was a sex difference of seven days of age (males 92.6 ± 1.9 days, females 86.2 ± 1.3 days), and this age difference was reflected in the final groupings (pair bonded v. non-pair bonded 95.2 ± 3.4 v. 88.9 ± 2.4 days for males; 89.0 ± 2.2 v. 82.2 ± 1.3 days for females). However, the youngest male and the youngest female both were in pairs that mated successfully. Similarly, both the oldest male and the oldest female were in reproductively compatible pairs. These latter observations suggest that the small age difference did not account for the behavioral differences in the preference tests.
The effect of timing of mating on male mate-fidelity
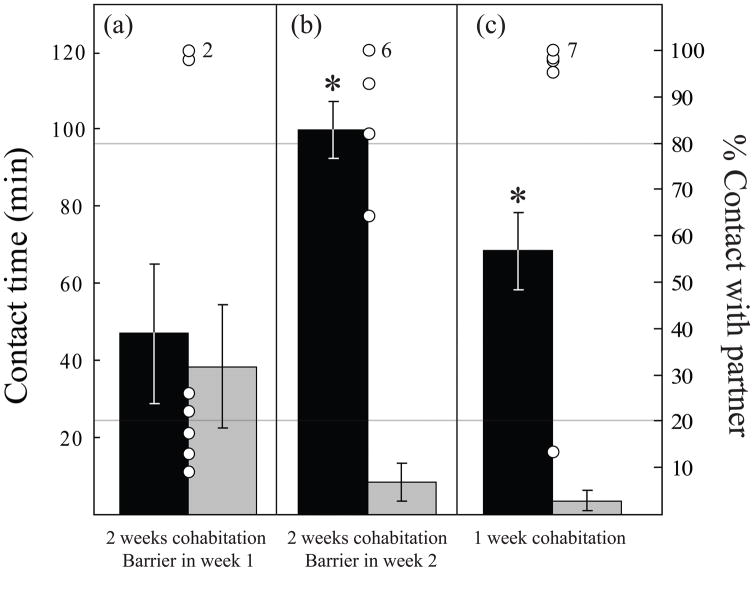
A potential confound in the present experiment is the fact that original pairings are experimenter generated, rather than allowing the animals to choose their own mates. To control for this possibility, a delay in mating was imposed on an entire group of male-female pairs. Eight pairs of voles were placed in cages in which the males and females were separated by a wire mesh barrier during week one of the two-week cohabitation period. The barrier allowed sufficient contact to induce behavioral estrous, but allowed us to control the timing of mating. When the barrier was removed, 5 of 8 pairs mated within minutes, and 7 of the 8 females were found to be one week pregnant at the time of the subsequent partner preference test. However, males from these pairs (Fig 2A) did not display a partner preference (t = 0.28, df = 7, p = 0.79). We controlled for potential stress associated with physical separation in a separate group (n = 9) by allowing normal patterns of sexual activation of the female and mating during the first week, but then separated the pair by putting the mesh barrier in place during week two of cohabitation. Despite separation during week two, males from this group displayed the typical partner preference (t = 9.20, df = 8, p < 0.001; Fig 2B). The lack of a preference by the group separated during the week one also might simply be due to the timing of the tests (one week after the earliest possible onset of mating vs. two weeks). Thus, we also tested males that experienced typical onset of sexual activity but after only one week of cohabitation with a female (n = 11). These males displayed a typical partner preference (t = 5.31, df = 10, p < 0.001; Fig 2C).
Figure 2.
Enforcing a delay in mating by separating prairie vole pairs with a wire mesh barrier during the first week of a two-week cohabitation (n = 8) eliminated partner preferences (A), while putting the barrier in place during the second week of cohabitation (n = 9) had no effect (B) suggesting that the timing of mating relative to pairing can impact pair-bonding. This difference was not simply the result of group differences in the time between mating and testing since males tested after one week of normal interactions with a female (n = 11) also displayed significant partner preferences (C). Bars indicate group means (±SEM, left y-axis) for contact time with partner (black bars) or with the stranger (grey bars). Open circles indicate the percentage of total contact time that individual males spent with their partners (right y-axis, numbers associated with open circles indicate the numbers of animals in each group that displayed either 100% or 0% of their time with the partner as appropriate). *p < 0.01
The effect of female fertility on male mate-fidelity
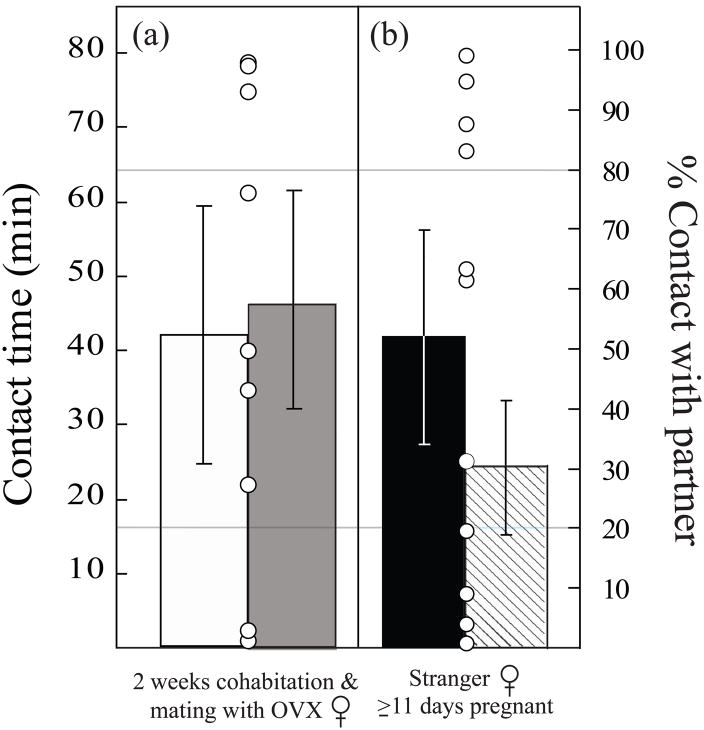
The behavioral differences between males described above may have been associated with copulation per se or with the mates’ pregnancies. To distinguish between these possibilities, males (n = 9) were paired with ovariectomized (OVX) females that were EB treated. The EB treatment successfully induced sexual receptivity in most OVX females (9 of 11), as expected (Carter et al. 1987a), and produced mating patterns similar to those of naturally-induced, reproductively-intact females (latency to mate: OVX 34.3 ± 6.7h, intact 39.5 ± 1.3h, t = 0.72, df = 14, p = 0.48; mating duration: OVX 17.8 ± 4.7h, intact 16.6 ± 2.5h, t = 0.82, df = 13, p = 0.82). However, despite normal mating patterns, males paired with OVX females for two weeks (Fig 3A) did not display a partner preference (t = 0.17, df = 8, p = 0.87). Rather, these males switched between stimulus females, similar to the behavior displayed by males whose mates were delayed in their pregnancies.
Figure 3.
Both timely mating with the partner and pregnancy are required for pair-bond formation in prairie voles. (A) Males paired for two weeks with estrogen-primed (and thus sexually receptive) ovariectomized (OVX) females (n = 9) did not display a preference for these females (white bar) vs. naïve females (grey bar) in a subsequent choice test. (B) Males (n = 13) given a choice between a pregnant female (black bar) who was not their partner and an intact, sexually naïve female (grey bar) did not display a preference for either class of female. In both cases, males also tended to switch between the two stimulus females (open circles). Bars indicate group means (±SEM, left y-axis) for contact time with OVX female partner or unfamiliar pregnant female (black bars) or with the stranger (grey bars). Open circles indicate the percentage of total contact time that individual males spent with their partners (right y-axis).
The effect of the females’ presence on male mate-fidelity
We also examined the effects of cues associated with the onset and development of a female’s pregnancy on male fidelity. We earlier established that males prefer their pregnant partners vs. unfamiliar females (Fig 1A). Therefore, in this test, males (n = 13) were presented with a choice of two unfamiliar females, one of which was sexually naïve, while the other was 11–12 days pregnant by a different male. Given these choices, males did not display a preference for either female (t = 0.91, df = 12, p = 0.38), but again showed the switching behavior displayed by males with incompatible mates (Fig 3B).
A final group of males was given a choice between cages containing fresh bedding and bedding soiled by sexually naïve females, or by either familiar (n = 10) or unfamiliar (n = 8) pregnant females. Regardless of the choices available, these males showed no preference for any cage position or any of the bedding choices (Fig 4; bedding effect, F1,15 = 1.83, p = 0.20; cage position effect, F2,30 = 0.56, p = 0.57; interaction, F4,30 = 2.11, p = 0.11). There were no differences in locomotor behavior (F2,15 = 0.67, p = 0.52).
Figure 4.
Males did not prefer cages containing stimuli associated with a pregnant partner (n = 10) or an unfamiliar pregnant female (n = 8) vs. a sexually naïve female. Bars indicate group means (±SEM) for time spent in the cage containing bedding from a pregnant female (black bars) or a naïve female (grey bars).
Male behavior during preference testing
Male behavior during the preference testing after two weeks of cohabitation produced unexpected patterns of movements between female stimulus animals that differed depending on the partners’ pregnancy status. Data from all males are combined in Figure 5 to facilitate comparison of these differences. Typical behavior of non-pair-bonded males during a choice test is shown in Fig 5, column A, and typical behavior by pair-bonded males is shown in Fig 5, column C. In both cases, males spend the majority of their contact time with one or the other of the females, and rarely switch between females. Fig 5, column B shows the contrasting behavior displayed by males that had extensive social and/or sexual contact with their female partners prior to the choice testing, but whose partners, either naturally or via experimental manipulations, did not become pregnant within a few days after pairing. Included in these data is male behavior toward a female who is at the appropriate stage of pregnancy, but that is not the male’s partner. Males in these latter three groups were much more likely to switch between females.
Figure 5.
Summary of males’ behaviors during the various preference tests. Male behaviors toward the stimulus females varied depending on the females’ pregnancy status. Typical non-pair-bonded (A) and pair-bonded males (C) tended to settle with one or the other of the female stimulus animals (i.e., > 80% of contact with one female), while males paired with females with unusual pregnancy conditions (B) were more likely to switch between stimulus females (<80% of contact time with one female). Numbers associated with symbols indicate the number of animals with either 100% or 0% of their contact with the partner.
Overall activity and total contact measures
No statistically significant effects among groups (Table 2) were found for the combined time spent in contact with the two females (F1,111 = 1.46, p = 0.19), cage crossings (a measure of locomotor activity; F7,111 = 0.53, p = 0.81), or for time spent in the neutral cage (F7,111 = 0.93, p = 0.49).
Table 2.
Measures of social and nonsocial behaviours of male prairie voles during choice-of-female testing
| Cohabitation and ♀ pregnancy status | N | Mean ± SE | ||
|---|---|---|---|---|
| Total contact (min) | Cage crossings (no.) | Neutral cage (min) | ||
| 10 days pregnant | 19 | 88.2±6.3 | 194.4±27.6 | 19.9±3.2 |
| >10 days pregnant | 27 | 79.6±6.1 | 252.4±25.2 | 27.1±2.6 |
| 6 h cohabitation | 16 | 94.8±9.8 | 252.0±36.7 | 19.6±3.1 |
| Barrier during week 1 | 8 | 85.3±15.3 | 224.0±42.0 | 27.7±4.7 |
| Barrier during week 2 | 9 | 108.1±8.1 | 254.4±69.5 | 21.6±4.5 |
| 1 week cohabitation | 11 | 72.0±8.6 | 260.4±36.8 | 26.8±2.7 |
| 2 weeks w/OVX female | 9 | 88.5±17.3 | 290.9±55.4 | 28.4±4.9 |
| Pregnant vs naïve preference | 13 | 65.4±14.6 | 233.1±48.2 | 26.9±5.4 |
| F7,111 | 1.46 | 0.53 | 0.93 | |
| P | 0.19 | 0.81 | 0.49 | |
F and P values represent the outcomes of one-way ANOVAs for each variable. OVX = ovariectomized.
DISCUSSION
We used differences in partner-fidelity to test whether reproductive compatibility was a pre-requisite for pair-bond expression by male prairie voles. Males in reproductively compatible pairs displayed a strong preference for their mates vs. unfamiliar females in a choice test, whereas males from incompatible pairs, (pairs with delayed or failed pregnancies), did not display this preference.
Based on data from our captive breeding colony, we expected that females in reproductively compatible pairs would be 10–12 days pregnant after two weeks with a male. This expectation was borne out as nearly ¾ of the females were at that stage, while the remaining females ranged from no indication of reproductive activation to less than ten days pregnant (Table 1). These data, along with previous reports that ~30% of females fail to mate within 72 hours after pairing (Witt et al. 1988), show that the onset of mating differs among pairs of prairie voles. Our observation that approximately half of failed pairings appeared to arise from problems with the male and half from the female, suggests that neither sex bears sole responsibility for reproductive incompatibility within a pairing.
We next tested whether pair-bond expression by male voles was related to differential mating success. Previous studies have shown that when given a choice after mating, prairie voles display a robust preference for their partner vs. a stranger (Williams et al. 1992a). Although they may interact with an unfamiliar individual, mated prairie voles typically spend very little time in close contact with a stranger, choosing instead to huddle closely with their partner (Williams et al. 1992a). However, using a detailed assessment of natural reproductive compatibility, we found that the behavior of males during a partner-preference test actually reflected the reproductive status of their mates. Males whose partners became pregnant within three days after pairing displayed a strong preference for their mate in the choice test. In contrast, males with partners that were not pregnant or with delayed onset of pregnancy did not display a partner-preference. Indeed, these latter males differed from more typical non-pair-bonded voles in their behaviors during the choice test in an interesting and unexpected way.
Prairie voles that are paired for only six hours without mating often are used as the non- bonded baseline in studies examining pair-bond formation (Curtis & Wang 2003). Under these conditions, both sexes display non-selective affiliation and are equally likely to huddle with a stranger as with the familiar partner (Aragona & Wang 2004). Importantly, although as a group, the mean amounts of contact time with partners and with strangers do not differ, individual voles rarely switch between the two stimulus animals during a test (unpublished observations). Instead, they typically spend the majority of their time with one stimulus animal or the other: half settle down with the stranger, the other half with the partner. In contrast, we found that males whose partners were delayed in pregnancy onset often split their time between females. Over half of these males switched between huddling with the partner and then with the stranger one or more times during the test. It is important to note that locomotor activity did not differ between groups. Thus, differences in overall activity do not account for the differences in social contact. It also is important to note that the preference tests in the present study were performed after the voles were together for two weeks, whereas 1–2 day cohabitation periods are more commonly used (Williams et al. 1992a). Thus, the switching behavior may arise only after the pair has had sufficient time to assess reproductive compatibility.
There are several possible explanations for the differences in preference expression. First, assigning animals to pairs instead of allowing the animals to choose their own partners may by-pass species-typical mate-choice mechanisms. Thus, the lack of a partner preference by some males might simply reflect pairs that would not have formed pair-bonds under any circumstances. To test this, we enforced a delay in mating/conception for all pairs in one group by separating the males and females with a mesh barrier during the first week of the two-week cohabitation period. This allowed sufficient contact to induce behavioral estrous, but precluded mating during week one of cohabitation. Despite the fact that all but one of the females were at a stage of pregnancy appropriate for the amount of time that sexual contact was possible at the time of the subsequent partner preference test, males from these pairs did not display a partner preference. It is possible that the stress accompanying enforced separation (DeVries et al. 1996) may have interfered with the males’ behaviors during the subsequent preference test. We controlled for this possibility in another group of males by allowing normal patterns of behavior during the first week of cohabitation, but then separated the pair during the second week. Despite separation during week two, these males displayed the typical partner preference. Finally, the lack of a preference also might simply reflect the timing of the tests (one week vs. two weeks after mating). However, males that experienced normal mating patterns, but that were tested after only one week of cohabitation, also displayed a typical partner preference. Together, these results show that the lack of pair-bond expression by males with incompatible mates is not simply an artifact of artificial pairings, nor is the timing of testing relative to pairing a decisive factor. Rather, a delay in pregnancy relative to pairing appears to contribute to atypical male behavior.
The physiology and behavior of males may change in association with the reproductive status of their mates. Male California mice (Peromyscus californicus) begin to display paternal behavior soon after mating (Gubernick et al. 1994), and vasopressin fiber density changes in male prairie voles through the course of their mates’ pregnancies (Bamshad et al. 1993). It is unclear whether these behavioral and physiological changes are associated with copulation per se or with the mates’ pregnancies. Mating was sufficient to alter the behavior of male mice toward pups three weeks later, despite the absence of the female during the intervening 21 days (Perrigo et al. 1990), but cues from gestating females were necessary for the onset of paternal behaviors in male voles (Terleph et al. 2004). Thus, either time since mating or cues from the female could inform the male about his mate’s reproductive status.
We examined the contribution of the time since mating to pair-bond expression by pairing males with estrogen-primed, ovariectomized females. This treatment induced female sexual receptivity and mating within the typical 24–48 hour timeframe (Carter et al. 1987a), but precluded pregnancy. Despite experiencing normal mating patterns, males paired with OVX females for two weeks did not display a partner preference. Instead, these males displayed the switching behavior similar to that of males whose mates were delayed in their pregnancies. These data suggest that, in and of itself, the appropriate timing of mating relative to initial pairing is not sufficient for long-term pair-bonding in prairie voles in the absence of pregnancy.
We also sought to determine whether the pregnant partner was the relevant cue or if any female at the appropriate stage of pregnancy would be preferred. Male voles can differentiate between females based on their reproductive status (Ferguson et al. 1986; Ferkin et al. 2008). When given a choice between two unfamiliar females, one of which was sexually naïve, while the other was 10–12 days pregnant but by a different male, males in the present study did not display a preference for either female, but did show the switching behavior displayed by males with incompatible mates. An important consideration in interpreting these results is that the behavior of pregnant females toward unfamiliar males could influence the males’ behaviors in this test. Thus, we repeated the test but gave the males a choice between bedding that had been soiled by a naïve female, and bedding soiled by either an unfamiliar pregnant female or by the pregnant mate of the subject male. The males showed no bedding preferences, suggesting that cues associated with intimate physical contact with the female partner are important for male fidelity. The fact that males did not appear to distinguish between the bedding from the pregnant partner vs from other females suggests that non-volatile compounds (Dulac & Torello 2003) associated with the pregnant mate influence pair-bond expression by male prairie voles. A lack of preference for one or the other of the types of bedding would be consistent with earlier reports that meadow vole males preferred scents from females that were close to, and just past, parturition, but not during earlier stages of pregnancy (Ferkin & Johnston 1995) and that pheromonal input is critical for partner preference expression in prairie voles(Curtis et al. 2001). It is important to note that the lack of a preference does not necessarily mean the males cannot distinguish between the various types of soiled bedding. Rather the results of this test emphasize the importance of the female herself, and not simply female cues, in dictating male preference behavior.
These results suggest that prairie vole pair-bonds likely are formed via a two-step process. Partner-preference expression can occur within 24h after pairing (usually, although not always, with ad libitum mating) (Carter & Getz 1993). Thus, it is likely that basic preferences for each other are established within the first day after a vole pair meet. Under natural circumstances, these basic preferences keep the pair in close proximity until pheromonal signals from the male induce behavioral estrous in the female and initiate the natural mating sequence. However, it is clear that some pairs (~30%) do not mate within the typical period, and thus may not be reproductively compatible. Studies in other species have shown that delayed reproduction can reduce lifetime reproductive fitness in small rodents (Ribble 1992). Thus, if a prairie vole pair is not reproductively compatible, pair-bonding may reduce the chances of successful reproduction for both members of the pair. We propose that a “clock” starts when a pair of prairie voles first meet. If pregnancy subsequently occurs within a prescribed time, the initial partner preference is reinforced and may become a permanent pair-bond. If, however, pregnancy does not occur within the prescribed period, the initial preference begins to decay, and the male’s preference for the partner is weakened. Consistent with this idea, males expressed preferences for their mates only if the females were at an appropriate stage of pregnancy relative to the duration of pairing. Together, these data suggest that it is not mating per se (witness the results with the OVX females), but rather timely and successful mating that is important for long-term pair-bond formation in voles. Absent these factors, the initial preferences may weaken and the pair “divorces”. Given this interpretation, the fact that some males impregnated the second of their two female companions after their original mate failed to become pregnant should not be interpreted as evidence of a lack of reproductive monogamy.
The majority of analyses of animal divorce have been conducted on birds (Choudhury 1995). The present study is one the few analyses of divorce behavior in non-human monogamous mammals. Given the relatively few monogamous mammalian species, this is not surprising. Even among mammals, most monogamous species studied are monestrous, whereas voles are polyestrous and may reproduce year-round (Gavish et al. 1981). This is an important contrast since many monogamous bird species are single brooded, and thus, like monestrous mammals, attempt reproduction only once per year (Bried et al. 2003; Ralls et al. 2007). As such, divorce decisions for many birds and most mammals often are related to a given year’s reproductive success. In other words, the question becomes “was my reproductive effort last year successful enough that I should remain with the same mate this year?” The results of the present experiments suggest that voles may make “divorce decisions” even prior to validation of reproductive success. This “haste” may be related to the relatively short reproductive life of voles.
Prairie voles display a variety of characteristics that are consistent with monogamy: extensive paternal care, relatively little sexual dimorphism, shared defense of a common territory, and typically, the rejection even of opposite-sex individuals by both members of a pair (Carter & Getz 1993; Gavish et al. 1981; Getz & Hofmann 1986). Despite these monogamous traits, occasional studies produce results that call into question a monogamous mating system. For example, some studies have found evidence of extra-pair copulations or evidence for mate switching (Ophir et al. 2008; Solomon et al. 2004; Wolff et al. 2002). Thus, the argument has been made that, while prairie voles are socially monogamous, they are not sexually monogamous. The present results may help to address this issue. Specifically, our finding that the strength of the partner-preference that underlies monogamy can vary depending on the reproductive compatibility of the pair shows that very detailed knowledge of a pair’s history must be taken into account before discounting sexual monogamy as the modal mating system for prairie voles. These data extend those showing that, in the field, a similar percentage of poorly performing pairings are dissolved (Getz et al. 2004). Perhaps it would be prudent to describe the species as being monogamous, but with facultative polygamy or facultative promiscuity possible under circumstances where a pair may not be reproductively compatible.
Acknowledgments
I would like to thank Dr. Zuoxin Wang for generously providing the animals used to start our prairie vole colony and to the OSU Center for Health Sciences laboratory animal resources staff for excellent animal maintenance. I also would like to thank Dr. Kathleen Curtis for critical evaluation of earlier drafts of this manuscript. This research was supported by the National Institute for Child Health and Development (HD-48462) and an intramural research grant from the Oklahoma State University Center for Health Sciences (CHS-0806).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR Journal. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ. Sex and species differences in the vasopressin innervation of sexually naive and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. Journal of Neuroendocrinology. 1993;5:247–255. doi: 10.1111/j.1365-2826.1993.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Bried J, Pontier D, Jouventin P. Mate fidelity in monogamous birds: a reexamination of the Procellariiformes. Animal Behaviour. 2003;65:235–246. [Google Scholar]
- Campbell JC, Laugero KD, Van Westerhuyzen JA, Hostetler CM, Cohen JD, Bales KL. Costs of pair-bonding and paternal care in male prairie voles (Microtus ochrogaster) Physiology & Behavior. 2009;98:367–373. doi: 10.1016/j.physbeh.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Getz LL. Monogamy and the prairie vole. Scientific American. 1993;268:100–106. doi: 10.1038/scientificamerican0693-100. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, Cohen-Parsons M. Relationships between social organization and behavioral endocrinology in a monogamous mammal. Advances in the Study of Behavior. 1986;16:109–145. [Google Scholar]
- Carter CS, Getz LL, Gavish L, McDermott JL, Arnold P. Male-related pheromones and the activation of female reproduction in the prairie vole (Microtus ochrogaster) Biology of Reproduction. 1980;23:1038–1045. doi: 10.1095/biolreprod23.5.1038. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Auksi T, Casten L. Estrogen and the induction of lordosis in female and male prairie voles (Microtus ochrogaster) Hormones and Behavior. 1987a;21:65–73. doi: 10.1016/0018-506x(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Hormones and Behavior. 1987b;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Choudhury S. Divorce in birds: a review of the hypotheses. Animal Behaviour. 1995;50:413–429. [Google Scholar]
- Cohen-Parsons M, Carter CS. Males increase serum estrogen and estrogen receptor binding in brain of female voles. Physiology & Behavior. 1987;39:309–314. doi: 10.1016/0031-9384(87)90227-7. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Liu Y, Wang Z. Lesions of the vomeronasal organ disrupt mating-induced pair bonding in female prairie voles (Microtus ochrogaster) Brain Research. 2001;901:167–174. doi: 10.1016/s0006-8993(01)02343-5. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. The neurochemistry of pair bonding. Current Directions in Psychological Science. 2003;12:49–53. [Google Scholar]
- Desy EA, Batzli GO. Effects of food availability and predation on prairie vole demography: a field experiment. Ecology. 1989;70:411–421. [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences USA. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature Reviews Neuroscience. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- Emlen ST, Oring LW. Ecology, Sexual Selection, and Evolution of Mating Systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Ens BJ, Safriel UN, Harris MP. Divorce in the long-lived and monogamous oystercatcher, Haematopus ostralegus: incompatibility or choosing the better option. Animal Behaviour. 1993;45:1199–1217. [Google Scholar]
- Ferguson B, Fuentes SM, Sawrey DK, Dewsbury DA. Male preferences for unmated versus mated females in two species of voles (Microtus ochrogaster and Microtus montanus) Journal of Comparative Psychology. 1986;100:243–247. [Google Scholar]
- Ferkin MH, Combs A, delBarco-Trillo J, Pierce AA, Franklin S. Meadow voles, Microtus pennsylvanicus, have the capacity to recall the “what”, “where”, and “when” of a single past event. Animal Cognition. 2008;11:147–159. doi: 10.1007/s10071-007-0101-8. [DOI] [PubMed] [Google Scholar]
- Ferkin MH, Johnston RE. Effects of pregnancy, lactation and postpartum estrus on odor signals and the attraction to odors in female meadow voles, Microtus pennsylvanicus. Animal Behaviour. 1995;49:1211–1217. [Google Scholar]
- Gavish L, Carter CS, Getz LL. Further evidences for monogamy in the prairie vole. Animal Behaviour. 1981;29:955–957. [Google Scholar]
- Getz LL, Hofmann JE. Social organization in free-living prairie voles, Microtus ochrogaster. Behavioral Ecology and Sociobiology. 1986;18:275–282. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T. Does mate choice take place in free-living prairie voles Microtus ochrogaster? Evidence from field data. Acta Zoologica Sinica. 2004;50:527–534. [Google Scholar]
- Getz LL, Simms LE, McGuire B, Snarski ME. Factors affecting life expectancy of the prairie vole, Microtus ochrogaster. Oikos. 1997;80:362–370. [Google Scholar]
- Gray GD, Dewsbury DA. A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster) Brain, Behavior and Evolution. 1973;8:426–452. [PubMed] [Google Scholar]
- Gubernick DJ, Schneider KA, Jeannotte LA. Individual differences in the mechanisms underlying the onset and maintenance of paternal behavior and the inhibition of infanticide in the monogamous biparental California mouse, Peromyscus californicus. Behavioral Ecology and Sociobiology. 1994;34:225–231. [Google Scholar]
- Kirkpatrick B, Williams JR, Slotnick BM, Carter CS. Olfactory bulbectomy decreases social behavior in male prairie voles (M. ochrogaster) Physiology & Behavior. 1994;55:885–889. doi: 10.1016/0031-9384(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Komers PE, Brotherton PNM. Female space use is the best predictor of monogamy in mammals. Proceedings of the Royal Society of London Series B-Biological Sciences. 1997;264:1261–1270. doi: 10.1098/rspb.1997.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepri JJ, Wysocki CJ. Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiology & Behavior. 1987;40:349–355. doi: 10.1016/0031-9384(87)90058-8. [DOI] [PubMed] [Google Scholar]
- McNamara JM, Forslund P. Divorce rates in birds: predictions from an optimization model. American Naturalist. 1996;147:609–640. [Google Scholar]
- Nadeau JH. Ontogeny. In: Tamarin RH, editor. Biology of New World Microtus. American Society of Mammalogists; 1985. pp. 254–285.pp. 254–285. Special Publication No. 8. [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, Wolff JO. Social but not genetic monogamy is associated with greater breeding success in prairie voles. Animal Behaviour. 2008;75:1143–1154. [Google Scholar]
- Perrigo G, Bryant WC, Saal FSV. A unique neural timing system prevents male mice from harming their own offspring. Animal Behaviour. 1990;39:535–539. [Google Scholar]
- Pizzuto T, Getz LL. Female prairie voles (Microtus ochrogaster) fail to form a new pair after loss of mate. Behavioural Processes. 1998;43:79–86. doi: 10.1016/s0376-6357(97)00091-0. [DOI] [PubMed] [Google Scholar]
- Ralls K, Cypher B, Spiegel LK. Social monogamy in kit foxes: formation, association, duration, and dissolution of mated pairs. Journal of Mammalogy. 2007;88:1439–1446. [Google Scholar]
- Rasmussen DR. Pair-bond strength and stability and reproductive success. Psychological Review. 1981;88:274–290. [Google Scholar]
- Renfro CA, Pesek DW, Bobeck K, Solomon NG. Does time after pair bond disruption affect subsequent reproduction in the socially monogamous woodland vole (Microtus pinetorum)? Behavioural Processes. 2009;81:60–64. doi: 10.1016/j.beproc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribble DO. Lifetime reproductive success and its correlates in the monogamous rodent, Peromyscus californicus. Journal of Animal Ecology. 1992;61:457–468. [Google Scholar]
- Roberts RL, Wolf KN, Sprangel ME, Rall WF, Wildt DE. Prolonged mating in prairie voles (Microtus ochrogaster) increases likelihood of ovulation and embryo number. Biology of Reproduction. 1999;60:756–762. doi: 10.1095/biolreprod60.3.756. [DOI] [PubMed] [Google Scholar]
- Sawrey DK, Dewsbury DA. Control of ovulation, vaginal estrus, and behavioral receptivity in voles (Microtus) Neuroscience and Biobehavioral Reviews. 1985;9:563–571. doi: 10.1016/0149-7634(85)90003-x. [DOI] [PubMed] [Google Scholar]
- Solomon NG, Keane B, Knoch LR, Hogan PJ. Multiple paternity in socially monogamous prairie voles (Microtus ochrogaster) Canadian Journal of Zoology. 2004;82:1667–1671. [Google Scholar]
- Terleph TA, Jean-Baptiste N, Bamshad M. Mechanisms and time course for induction of paternal behavior in prairie voles (Microtus ochrogaster) Journal of Mammalogy. 2004;85:1124–1129. [Google Scholar]
- Thomas SA, Wolff JO. Pair bonding and “the widow effect” in female prairie voles. Behavioural Processes. 2004;67:47–54. doi: 10.1016/j.beproc.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Trivers R, Burt A. Kinship and genomic imprinting. In: Ohlsson R, editor. Genomic Imprinting; an Interdisciplinary Approach. Springer; Heidelberg: 1999. pp. 1–21. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior. 1992a;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Williams JR, Slotnick BM, Kirkpatrick BW, Carter CS. Olfactory bulb removal affects partner preference development and estrus induction in female prairie voles. Physiology & Behavior. 1992b;52:635–639. doi: 10.1016/0031-9384(92)90390-n. [DOI] [PubMed] [Google Scholar]
- Witt DM, Carter CS, Carlstead K, Read LD. Sexual and social interactions preceding and during male-induced estrus in Prairie Voles, Microtus ochrogaster. Animal Behaviour. 1988;36:1465–1471. [Google Scholar]
- Wolff JO, Mech SG, Dunlap AS, Hodges KE. Multi-male mating by paired and unpaired female prairie voles (Microtus ochrogaster) Behaviour. 2002;139:1147–1160. [Google Scholar]
- Wright SL, Brown RE. The importance of paternal care on pup survival and pup growth in Peromyscus californicus when required to work for food. Behavioural Processes. 2002;60:41–52. doi: 10.1016/s0376-6357(02)00101-8. [DOI] [PubMed] [Google Scholar]