Abstract
Primary cilia are sensory organelles that have been shown to play a critical role in lineage commitment. It was our hypothesis that the primary cilium is necessary for chemically induced differentiation of human mesenchymal stem cells (MSC). To investigate this, polaris siRNA was used to inhibit the primary cilia and the mRNA levels of transcription factors Runx2, PPARγ were measured by RT PCR as markers of osteogenic, adipogenic and chondrogenic differentiation, respectively. MSCs with inhibited primary cilia had significantly decreased basal mRNA expression levels of all three lineages specific transcription factors indicating that primary cilia are critical in multiple differentiation pathways. Furthermore, to determine if primary cilia play a role in the differentiation potential of MSCs, progenitor cells transfected with either scrambled or polaris siRNA were cultured in osteo-inductive, chondro-inductive, or adipo-inductive media and lineage commitment was ascertained. Interestingly, within 24 h of culture, cells transfected with polaris siRNA in both osteogenic and adipogenic media lost adhesion and released from the slides; however MSCs in chondrogenic media as well as cells transfected with scrambled siRNA did not. These results suggest that the primary cilium is necessary for the normal progression of chemically induced osteogenic and adipogenic differentiation. As a control, the experiment was repeated with NIH3T3 fibroblasts and none of the effects of inhibited primary cilia were observed indicating that the loss of adhesion may be specific to MSCs. Furthermore after biochemically inducing the cells to differentiate, polaris knockdown resulted in abrogation of both Runx2 and PPARγ mRNA while SOX9 mRNA expression was significantly lower. These results suggest that primary cilia play an essential role not only in the initiation of both osteogenic and adipogenic differentiation, but also in maintaining the phenotype of differentiated cells. Interestingly, chondrogenic differentiation appeared less dependent on a functional primary cilium.
Keywords: Primary cilia, MSC, Stem cell
Introduction
Primary cilia are solitary, non motile microtubule based organelles emerging from the distal centriole of the centrosome of many mammalian cells.17 The construction of primary cilia requires intraflagellar transport, a coordinated process involving a system of motor proteins and adaptors.15 It has long been established that primary cilia have the potential to transduce both mechanical and biochemical signals and convert them into intracellular signals that control a wide variety of processes during development and tissue homeostasis.5 However, their potential role in mesechymal stem cell differentiation remains unknown.
Bone marrow derived mesenchymal stem cells (MSCs) are a popular progenitor cell source and are considered suitable candidates for applications in regenerative medicine due to their ability to differentiate into several mesenchymal cell lineages.10,12 A number of studies have convincingly shown the feasibility of MSC seeded grafts for tissue engineering purposes. Although human MSC offer considerable therapeutic potential, little is known about the molecular mechanisms that govern their differentiation.
Ciliary dysfunction or mutations affecting cilia formation have been shown to lead to developmental disorders.2,14 There are many families of signaling proteins that are regulated by primary cilia. These include signaling pathways that regulate growth, survival and differentiation of cells during embryonic development and maintenance of healthy tissues.4,17 Recently it was shown that the Hedgehog signaling pathway, which controls crucial aspects of development and stem cell function, is regulated through the transport of key signaling proteins into and out of the primary cilium.13 Furthermore, the response of cells to the family of secreted Wnt proteins has also been shown to be regulated by the primary cilium. Similar to Hedgehog proteins, Wnts have established roles in both cellular proliferation and differentiation, and thus, may be a central pathway through which cell fate is regulated by the primary cilium.
The purpose of this study was to determine whether MSCs derived from human bone marrow express primary cilia and determine their role in human MSC differentiation. It was our hypothesis that the primary cilium is necessary for biochemically induced differentiation of MSCs. To study this we examined Runx2, a transcription factor essential for bone formation and known to regulate expression of osteoblastic genes,8 Sox9, a homeodomain transcription factor expressed in chondrogenic differentiation,7 and PPARγ, a nuclear hormone receptor induced very early in the differentiation of several cultured adipocyte cell lines. We also determined whether, after inducing MSCs to differentiate, primary cilia play a role in maintenance of osteogenic, adipogenic and chondrogenic phenotypes. With NIH3T3 fibroblasts as controls, we also assessed whether these effects were specific to MSCs or perhaps a general property of all cells.
Materials And Methods
Cell Culture
Human MSCs (Lonza) were cultured in MSC basal medium supplemented with the SingleQuot System (10% Growth Supplement, 2% l-glutamine and 0.1% penicillin–streptomycin mixture, Lonza) at 37 °C and 5% CO2 in a humidified incubator. The differentiation of MSCs was carried out using commercially available chondrogenic, adipogenic and osteogenic induction media according to the manufactures protocol (Lonza). Osteogenic induction medium consisted of l-glutamine, ascorbate, penicillin–streptomycin, mesenchymal cell growth supplement, β-glycerophosphate and dexamethasone. Adipogenic induction medium consisted of h-insulin, l-glutamine, dexamethasone, indomethacin, IBMX, penicillin–streptomycin, and mesenchymal cell growth supplement. Chondrogenic induction medium consisted of ascorbate, ITS + supplement, dexamethasone, penicillin–streptomycin, sodium pyruvate, proline, l-glutamine, and TGF-β3. All experiments were conducted with passage 3 or 4 cells. For gene expression experiments, cells were subcultured on fibronectin coated glass slides (76 × 48 × 1 mm). NIH3T3 fibroblasts (ATCC) were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum (Hyclone), 1% penicillin–streptomycin. Cells were maintained at 37 °C and 5% CO2. When the cells were 80–90% confluent they were subcultured onto glass slides.
Staining for Primary Cilia
Cells were fixed for 10 min in 10% paraformaldehyde and were processed for immunofluorescence as in Woods and Beier,18 using 6-11B-1 anti-acetylated α-tubulin (1:1000, Sigma) primary antibody followed by incubation with goat anti-mouse Alexa 594 (1:200, Molecular Probes). DNA was stained with DAPI (1:100,000 of a 5 mg/mL stock; Sigma). Cells were imaged on a Nikon C-1 confocal microscope.
Phenotypic Staining
Adipogenic and osteogenic differentiation was assayed by Oil Red O or alkaline phosphatase staining, respectively. Cells were fixed with a citrate concentrate (Sigma) in solution with acetone. To stain lipids, cells were exposed to a working solution of Oil Red O (3 mg/mL in 99% isopropanol, Sigma) and the background was cleared with 60% isopropanol. Early osteoblastic differentiation was determined by staining for alkaline phosphatase activity with a Diazonium salt solution containing 12 mg Fast Violet B Salt (Sigma) distilled water and Napthol AS-MX phosphate alkaline solution 0.25% (Sigma). Cells were counterstained with Mayer's hematoxylin solution (Sigma).
Inhibition of Primary Cilia
Primary cilia were inhibited by siRNA mediated depletion of the intraflagellar transport component polaris, which is required for primary cilium biogenesis and function.15 Polaris is the protein product of Tg737 the hypomorphic allele discovered in the Oak Ridge polycystic kidney disease mouse. It localizes to the primary cilia basal body and has no known activity outside of ciliogenesis. Briefly 20 μM siRNA targeting polaris (sequence: 5′-CCAGAAACAGATGAGGAC GACCTTT-3′) or 20 μM scrambled control siRNA (Invitrogen) was transfected into cells with the XtremeGEne transfection reagent (Roche Molecular Systems). After 4 h of incubation, growth medium was added. Depletion of polaris was confirmed by Western blotting. To determine the transfection efficiency, cells were transfected with Cy3-labeled siRNA. 24 h post-transfection cells were imaged. For the differentiation experiments, induction media was added 48 h after transfection following a PBS wash. In order to determine whether primary cilia played a role in continued lineage commitment after differentiation, cells were first differentiated following the manufactures protocol (Lonza). Once the differentiation was confirmed by staining, cells were transfected with siRNA directed against polaris or a scrambled sequence. On third day, total RNA was extracted.
Western Blot
Cells were transfected with scrambled and polaris siRNA respectively and total cellular protein was isolated on the third day post-transfection with RIPA lysis buffer (Santa Cruz Biotechnology). Protein concentration in the supernatant was determined using a Bradford protein assay kit (Bio-Rad Laboratories, Inc.). Equal amounts of protein from each sample were separated by electrophoresis in 4–12% pre-cast NuPAGE Bis–Tris gels (Invitrogen) and transferred onto Nitrocellulose membranes (Invitrogen). Membranes were probed with rabbit anti-polaris (1:5000) overnight at 4 °C with gentle rocking. Incubation with an HRP-conjugated anti-goat/mouse IgG (1:2000) was carried out for 1 h at room temperature with gentle rocking. Membranes were visualized with a chemilu-minescent visualization system (GE Healthcare). Den-sitometric analysis was performed using ImageQuant software. Protein levels were normalized using anti-actin (1:1000, Santa Cruz Biotechnology) antibody.
Gene Expression
Real time quantitative RT-PCR was performed using an ABI Prism 7900 Sequence Detection System. Primers for Runx2, SOX9, PPARγ and 18S were commercially obtained (Applied Biosystems). Total RNA was extracted with TriReagent (Invitrogen) and cDNA was synthesized using the TaqMan reverse transcription kit (Applied Biosystems). cDNA samples were then amplified by real time PCR. Amplification curves for control and experimental genes were recorded and relative gene levels between samples were quantified using the relative standard curve method (ABI Prism 7700 User Bulletin #2). All samples were normalized to endogenous control 18s rRNA levels. All samples and standards were run in triplicate.
Statistical Analysis
For two-sample comparisons, a student's t test was used. Statistical significance was accepted at p < 0.05 and data is reported as the mean ± standard error of the mean (SEM). A minimum sample size of eight was included in each comparison.
Results
To investigate if primary cilia play a role in MSC differentiation we first sought to determine whether human MSCs have primary cilia. When the cells were stained with acetylated α-tubulin and imaged, primary cilia were seen projecting from the surface of MSC cells (Fig. 1). Primary cilia were observed on more than 90% of the cells.
FIGURE 1.
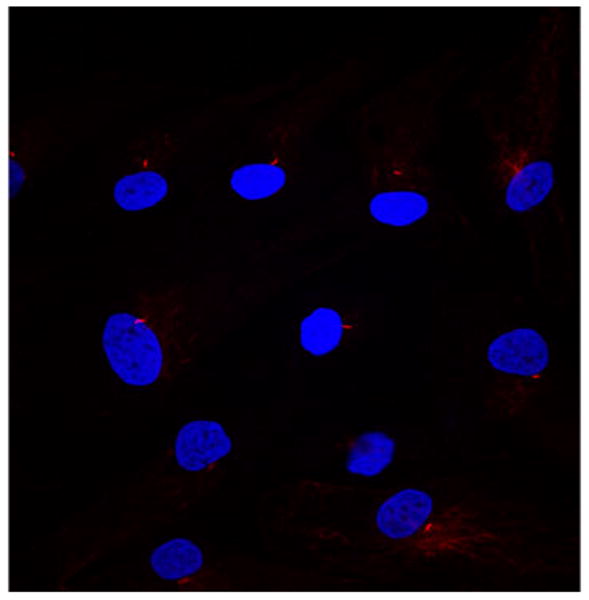
Primary cilia stained with anti acetylated tubulin (red) and DAPI (blue) in human mesenchymal stem cells. Cells were imaged on a Nikon C-1 confocal microscope (20×).
To determine the role of primary cilia in MSC behavior, they were inhibited by siRNA. In order to validate the transfection, a fluorescently labeled scrambled siRNA sequence was utilized. Transfection efficiency was found to be roughly 80–85% (data not shown). Also, depletion of polaris protein was confirmed by western blotting (Fig. 2).
FIGURE 2.
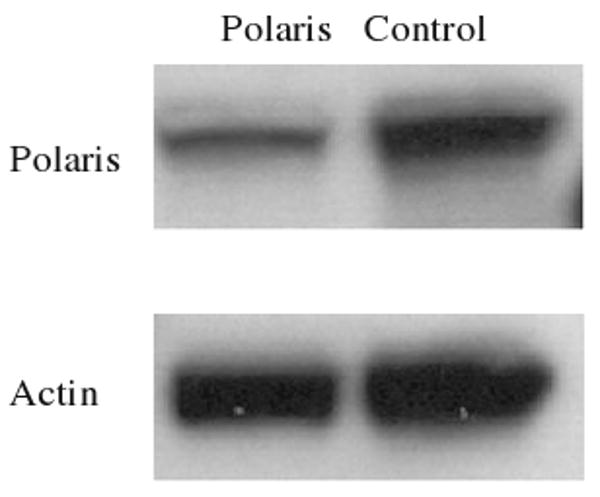
Western blot showing reduced levels of polaris protein in MSC cells transfected with polaris siRNA relative to cells transfected with control siRNA (right lane). Actin served as a loading control.
We first measured the baseline mRNA levels of Runx2, Sox9 and PPARγ in unstimulated human MSCs with and without primary cilia inhibition. The relative Runx2 mRNA levels for the polaris transfected cells was 0.41 vs. 1.61 for the control group, p < 0.001. The Sox-9 mRNA levels were 0.86 for the polaris transfected group vs. 1.53 for the control group, p < 0.01. The PPARγ mRNA levels for the polaris transfected cells were 0.61 vs. 1.69 for the control group, p < 0.001 (Fig. 3). We next determined whether inhibiting primary cilia has an effect on biochemically induced differentiation of MSC cells. When the cells were transfected with polaris siRNA and then placed in induction media 48 h later, the cells in the osteogenic and adipogenic environments lost their adhesion to the slides. This loss of adhesion did not occur with the scrambled siRNA sequence (Fig. 4) or for the cells placed in chondrogenic media. The detached cells were more than 95% viable by trypan blue exclusion. Also, in order to determine if this effect was specific to MSCs or a general property of many cell types, we repeated the experiment with fibroblasts. When fibroblasts were subjected to polaris knockdown and then placed in differentiation media, there was no loss of adhesion, indicating that this effect may be specific to MSCs. To further specify the role of primary cilia in MSC differentiation, we conducted an experiment in which the cells were differentiated first and then primary cilia formation was inhibited. The differentiation of the cells was confirmed by alkaline phosphatase and Oil Red O positive staining (results not shown). When relative gene expression levels of Runx2, Sox9 and PPARγ were quantified, the markers for bone, fat and cartilage were all reduced (Fig. 5).
FIGURE 3.
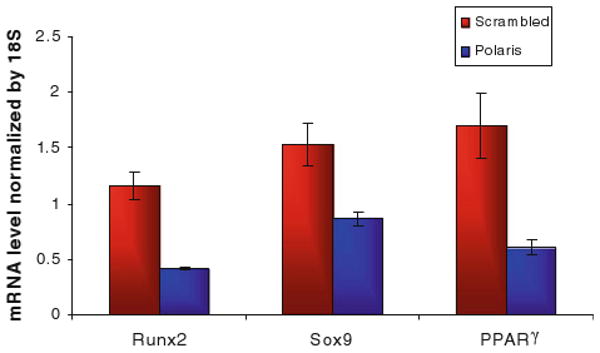
Baseline transcription factor mRNA levels in MSC cells with or without primary cilia. The relative gene expression levels were quantified by real time RT-PCR and normalized to 18s rRNA levels. Bars represent mean ± SEM (N ≥ 8 for all groups).
FIGURE 4.
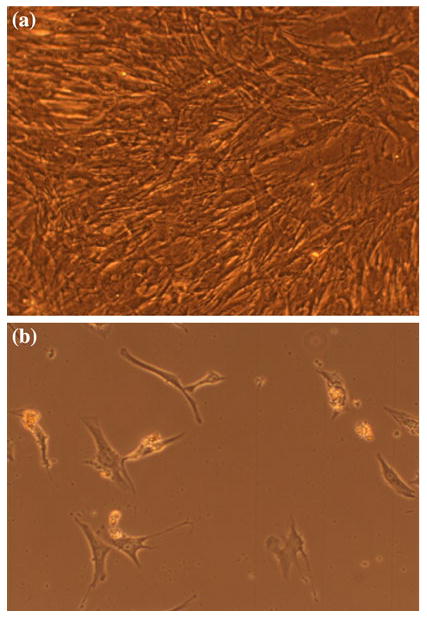
Loss of adhesion when MSCs were placed in adipogenic differentiation media. (a) Cells transfected with scrambled siRNA sequence. (b) Cells transfected with siRNA against polaris (20×).
FIGURE 5.
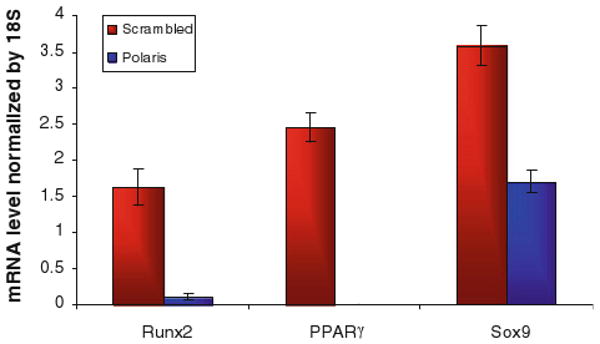
mRNA levels in MSC cells with or without primary cilia after differentiation. The relative gene expression levels were quantified by real time RT-PCR and normalized to 18s rRNA levels. Bars represent mean ± SEM (N ≥ 8 for all groups).
Discussion
In this study we determined that MSCs derived from human bone marrow do, indeed, posses primary cilia. We also show that primary cilia are critical to baseline MSC behavior, since MSCs lacking functional primary cilia had significantly decreased basal mRNA expression levels for osteogenic, chondrogenic and adipogenic lineage specific transcription factors. Furthermore, our results also suggest that the primary cilium is necessary for the normal progression of chemically induced osteogenic and adipogenic differentiation. Additionally, we show that primary cilia play an essential role not only in the initiation of both osteogenic and adipogenic differentiation, but also in maintaining the phenotype of differentiated cells. Interestingly, chondrogenic differentiation was less inhibited due to the lack of a functional primary cilium than osteogenic or adipogenic differentiation. This suggests that chondrogenic differentiation may be regulated by additional mechanisms other than signaling cascades involving the primary cilia.
It has been shown that primary cilia form highly specialized compartments that organize and coordinate the processing of several signaling pathways and potentially regulate stem cell turnover during tissue repair and regeneration.5 For example, recent discoveries have shown that the primary cilium plays an important function in regulating hedgehog signaling. Gli transcription factors are key effectors of the hedgehog signaling system and Scott and colleagues have shown that several components of the pathway, including the hedgehog receptor and the Gli proteins themselves, accumulate in primary cilia.14 Since hedgehog signaling through Gli proteins regulates the osteoblast vs. chondrocyte fate of mesenchymal progenitors, abrogating cilia may have its effect by interrupting hedgehog responsiveness.
Similarly, the family of Wnt secreted lipoproteins, which are involved in the regulation of both cell proliferation and differentiation, may be regulated by the primary cilium.6,16,20 Recent studies have demonstrated that the Wnt family of secreted proteins plays a vital role during differentiation down multiple mus-culoskeletal lineages including the adipogenic, chondrogenic and osteogenic cell fates.6,9 This correlates with our findings that MSCs lacking functional primary cilia had significantly decreased basal expression of the lineage specific transcription factors, PPARγ, Sox9 and Runx2.
Interestingly, Wnt signaling also has the potential to activate the small GTPases, RhoA and Rac1, both of which play key roles in actin cytoskeletal dynamics.1,11,19 This suggests that the Rho GTPase family may function as molecular switches regulating osteogenesis, adipogenesis and chondrogenesis by regulating unique aspects of cytoskeletal function. For example, the osteo-inductive or adipo-inductive media may produce alterations in Wnt signaling leading to abrogated RhoA activity, which, in turn, results in diminished adherence of focal adhesions to their extracellular environment. Additionally, there is evidence that supports the hypothesis that the primary cilium and the microtubular and actin networks are functionally linked and therefore defects in the primary cilium have a downstream effect on the cytoskeletal integrity of the entire cell.3,11 Thus, interfering with primary cilia may result in loss of cellular adhesion consistent with our observation of the detachment of MSCs with polaris knockdown. Furthermore, although inhibition of RHO/ROCK signaling enhances chondrogenesis it has also been shown that inhibition of the actin cytoskeleton has a negative effect on the expression of chondrogenic genes.18
Our study was conducted on a population of cells isolated from human bone marrow that has been shown to be multipotent. This cell population is a mixture of relatively rare stem cells as well as progenitor cells committed to varying degrees to the tissues of mesenchymal origin. Due to the heterogeneous nature of these cells, it is impossible to determine to what extent our observations are the result of primary cilia contributing to further lineage commitment of progenitor cells, selection of one lineage over another, differentiation of true stem cells, or all of these. Nonetheless, the mixed MSC population is currently exploited for therapeutic applications in tissue engineering and regenerative medicine. Thus, understanding the importance of primary cilia in the behavior of the mixed population of human MSCs has implications both for these applications as well as forming the foundation for future investigations of specific molecular events in clonally derived pure cell-types within the MSC population.
It is known that the differentiation of MSC into different lineages depends on what local cues are present in their environment. In this work, we have shown that primary cilia are expressed on MSCs and may be one of the mechanisms of transducing environmental cues leading to lineage-specific differentiation. Interestingly, we also demonstrate that after lineage commitment, MSCs require primary cilia to maintain the phenotype of differentiated cells, suggesting a role in continued lineage commitment.
Acknowledgments
This work was funded by National Institute of Health Grants AR45989 and AR54156 and the United States Department of Veterans Affairs.
References
- 1.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation—the role of RhoA, ROCKII and cytoskeletal dynamics. J Cell Sci. 2009;122(Pt 4):546–553. doi: 10.1242/jcs.036293. Epub 2009 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 3.Bryan BA, Mitchell DC, Zhao L, Ma W, Stafford LJ, Teng BB, Liu M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol Cell Biol. 2005;25(24):11089–11101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christensen ST, Ott CM. Cell signaling. A ciliary signaling switch. Science. 2007;317(5836):330–331. doi: 10.1126/science.1146180. [DOI] [PubMed] [Google Scholar]
- 5.Christensen ST, Pedersen LB, Schneider L, Satir P. Sensory cilia and integration of signal transduction in human health and disease. Traffic. 2007;8(2):97–109. doi: 10.1111/j.1600-0854.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 6.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10(1):70–76. doi: 10.1038/ncb1670. Epub 2007 Dec 16 Erratum in. [DOI] [PubMed] [Google Scholar]; Nat Cell Biol. 2008 Apr;10(4):497. [Google Scholar]
- 7.de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19(5):389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- 8.Komori T. Mechanism of transcriptional regulation by Runx2 in osteoblasts. Clin Calcium. 2006;16(5):801–807. [PubMed] [Google Scholar]
- 9.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 10.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, Urabe M, Mizukami H, Kume A. Cell and gene therapy using mesenchymal stem cells (MSCs) J Autoimmun. 2008;30(3):121–127. doi: 10.1016/j.jaut.2007.12.008. Epub 2008 Jan 31. [DOI] [PubMed] [Google Scholar]
- 11.Pan J, You Y, Huang T, Brody SL. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci. 2007;120(Pt 11):1868–1876. doi: 10.1242/jcs.005306. Epub 2007 May 8. [DOI] [PubMed] [Google Scholar]
- 12.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317(5836):372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 14.Satir P, Christensen ST. Structure and function of mammalian cilia. Histochem Cell Biol. 2008;129(6):687–693. doi: 10.1007/s00418-008-0416-9. Epub 2008 Mar 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313(5787):629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 16.Torres VE, Harris PC. Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2(1):40–55. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 17.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20(1):73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 18.Woods A, Beier F. RhoA/ROCK signaling regulates chondrogenesis in a context-dependent manner. J Biol Chem. 2006;281(19):13134–13140. doi: 10.1074/jbc.M509433200. Epub 2006 Mar 24. [DOI] [PubMed] [Google Scholar]
- 19.Woods A, Wang G, Dupuis H, Shao Z, Beier F. Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis. J Biol Chem. 2007;282(32):23500–23508. doi: 10.1074/jbc.M700680200. Epub 2007 Jun 14. [DOI] [PubMed] [Google Scholar]
- 20.Yoder BK. Role of primary cilia in the pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2007;18(5):1381–1388. doi: 10.1681/ASN.2006111215. Epub 2007 Apr 11. [DOI] [PubMed] [Google Scholar]


