Abstract
The loss of anti-proliferative responsiveness in prostate cancer cell lines toward ligands for vitamin D receptor, retinoic acid receptors/retinoid X receptors and peroxisome proliferator activated receptor (PPAR)α/γ may entail underlying epigenetic events, as ligand insensitivity reflects significantly altered messenger RNA expression of corepressors and histone-modifying enzymes. Expression patterns were dependent on phases of the cell cycle and associated with repressed basal gene expression of vitamin D receptor and PPARα/γ target genes, for example CDKN1A [encodes p21(waf1/cip1)]. Elevated nuclear corepressor 1 (NCOR1) and nuclear corepressor 2/silencing mediator of retinoic acid and thyroid hormone receptor protein levels were detected in prostate cancer cell lines compared with non-malignant counterparts. Knockdown of the corepressor NCOR1 significantly elevated basal expression of a cohort of target genes, including CDKN1A. Both chemical [histone deacetylases inhibitor (HDACi)] and NCOR1 knockdown targeting enhanced anti-proliferative sensitivity toward PPARα/γ ligands in prostate cancer cell lines. Pursuing PPARα/γ signaling, microarray approaches were undertaken to identify pathways and genes regulated uniquely by a combination of PPARα/γ activation and HDAC inhibition. Again, HDACi and knockdown approaches demonstrated that elevated NCOR1 expression and activity distorted PPARα/γ gene targets centered on, for example cell cycle control, including CDKN1A and TGFBRAP1. Quantitative real time polymerase chain reaction validation and chromatin immunoprecipitation assays both confirmed that elevated NCOR1 disrupted the ability of PPARα/γ to regulate key target genes (CDKN1A and TGFBRAP1). Interrogation of these relationships in prostate cancer samples using principal component and partial correlation analyses established significant interdependent relationships between NCOR1–PPARα/γ and representative target genes, independently of androgen receptor expression. Therefore, we conclude that elevated NCOR1 distorts the actions of PPARα/γ selectively and generates a potential epigenetic lesion with diagnostic and prognostic significance.
Introduction
Nuclear receptors (NRs) regulate gene targets that control cell growth and differentiation. The ability of NRs to exert these gene regulatory effects are intimately controlled by a number of corepressors including nuclear corepressor 1 (NCOR1), nuclear corepressor 2 (NCOR2)/silencing mediator of retinoic acid and thyroid hormone receptor (SMRT), COP9 constitutive photomorphogenic homolog subunit 2 (COPS2)/thyroid receptor interactin protein 15 (TRIP15)/Alien, nuclear receptor interacting protein 1/receptor interacting protein 140, ligand dependent corepressor, and SRA stem-loop-interacting RNA-binding protein (SLIRP) (reviewed in refs 1,2). The specificities of these interactions are beginning to emerge. Murine Ncor1 and Ncor2/Smrt knockout approaches have revealed tissue-specific interactions to govern NR-mediated differentiation, for example in neural cell lineages (3–5). Similarly, in adipocyte differentiation, these corepressors regulate peroxisome proliferator activated receptor (PPAR)γ actions (6), and interacting domains distinguish NR recognition (7).
The actions of NRs appear selectively distorted in malignancy (reviewed in ref. 8) and is associated with deregulated corepressors (reviewed in ref. 9). Overexpressed NCOR2/SMRT attenuates the ability of the vitamin D receptor (VDR) to regulate target genes that mediate its anti-proliferative actions in prostate cancer cell lines compared with non-malignant cultures, and primary cultures of prostate cancers compared with their matched normal controls (10,11). Similar events have been established in myeloid leukemias, where chimeric RARα fusion proteins inappropriately retain NCOR1 and thereby attenuate retinoic acid signaling (10–14). For other well-studied NRs, such as the androgen receptor (AR), signaling evolves during prostate cancer progression to govern a different cohort of target genes (15), with an emphasis on proliferative gene promoters (16). For example, the oncogenic effects of the TMPRSS2–ETS fusion are critical (17) precisely because the TMPRSS2 promoter is sustained in a AR-responsive state (15), whereas other AR targets, such as the prostate tumor suppressor NKX3.1, become silenced (17,18). Together these findings suggest that NR signaling is restricted in malignancy by epigenetic mechanisms.
It remains unknown to what extent the actions of corepressors, including NCOR1 and NCOR2/SMRT, are either unique or redundant, and how they commonly silence NRs. The current study specifically investigated the capacity for corepressors, notably NCOR1 and NCOR2/SMRT, to regulate NRs that control cell growth and have potential as chemotherapy agents in prostate cancer, namely the VDR, retinoic acid receptors (RARs)/retinoid X receptors (RXRs) and PPARα/γ. Understanding such mechanisms has therapeutic appeal to develop targeted epigenetic therapies and therefore a secondary aim was tested, namely the capacity to target corepressors by combining a clinically relevant HDAC inhibitor with ligands for VDR, RARs/RXRs and PPARα/γ.
Materials and methods
Agents
1α,25(OH)2D3 [gift of Dr Milan Uskokovic (BioXell S.p.A., Milan, Italy)] and all trans retinoic acid (ATRA) (Sigma–Aldrich, Bournemouth, UK) were stored as 1 mM stocks in ethanol. Suberoylanilide hydroxamic acid (vorinostat) (Merck, Whitehouse Station, NJ) and all other ligands (Sigma–Aldrich) were stored in dimethyl sulfoxide as 100 mM stocks.
Cell culture
RWPE-1 non-malignant prostate epithelial cells were maintained in KSF media supplemented with epidermal growth factor and bovine pituitary extract (Invitrogen, Paisley, UK). LNCaP, PC-3 and DU 145 prostate cancer cells were cultured as described previously (11).
Proliferation assays
Proliferation (ViaLight HS, LumiTech, Nottingham, UK) was measured as described previously (11) and optimized using different seeding densities to ensure exponential proliferation through the course of the experiment. Cancer cells (2 × 103 cells per well) and RWPE-1 (4 × 103 cells per well) were plated in 96-well, white-walled plates (Fisher Scientific Ltd, Loughborough, UK), dosed with agents to final volume of 100 μl per well and incubated for 96 h, with re-dosing after 48 h. Each treatment was performed in triplicate wells in triplicate experiments. The combination of the individual mean effects for each compound acting alone was the ‘predicted’ combined inhibition. The mean ‘observed’ combined inhibition was then compared with this value using the Student's t-test. Classification of the inhibitory effects were as follows: strong-additive effects were those with an observed value significantly greater than the predicted value, additive effects were those in which the observed value did not significantly differ from the predicted value and sub-additive effects were those in which the observed value was significantly less than the predicted value (10,11,13).
Sorting of cells dependent upon cell cycle status
Cells stained with Hoechst 33342 (H3570; Invitrogen), (1 μg/ml, 45 min) were fractionated using a MoFlo cell sorter (Beckman Coulter, High Wycombe, UK) and Summit V4.3 software (Beckman Coulter) and collected into Tri-reagent (Sigma–Aldrich).
Cell cycle analysis
Exponentially proliferating cultures were incubated with agents and re-dosed after 48 h. After 72 h, 1 × 106 cells (both those adhering and detached) were stained with propidium iodide buffer [10 μg/ml propidium iodide, 1% (wt/vol) tri-sodium citrate, 0.1% (vol/vol) Triton X-100 and 100 μM sodium chloride (Sigma–Aldrich)] and incubated on ice, in the dark, for 30 min. Cell cycle distribution was determined using a Becton-Dickinson Flow Cytometer and CellFIT Cell-Cycle Analysis software. Each condition was examined in triplicate experiments.
Primary prostate tumor material
All tumors were collected under Institutional Review Board approval at Roswell Park Cancer Institute. Total messenger RNA (mRNA) from local tumors and adjacent non-neoplastic tissue from the same patient were extracted from snap frozen radical prostatectomy samples with subsequent frozen section analysis for quality control. The frozen section hematoxylin and eosin was evaluated by a board-certified pathologist for prostatic adenocarcinoma versus benign tissue. Segments of tissue corresponding to prostatic adenocarcinoma with ≥70% neoplastic nuclei are submitted for RNA isolation. Macromolecule processing was done in a centralized core facility with standard operating procedures.
Single target quantitative real time plolymerase chain reaction
Complementary DNA was prepared using random primers (Promega, Madison, WI http://www.promega.com/) and target genes relative expression quantitated [ABI 7700; Applied Biosystems (Carlsbad, CA) (http://www.appliedbiosystems.com)] (11). NCOR1, NCOR2/SMRT, TRIP15/COPS2/Alien multiplex primer and probe sequences were described previously (11). Sequences for TGFBRAP-1 forward GCGGCTGTGTCCTTTCCATA, reverse GCGTCTGCTTCTGTTGCTGAT, probe AGCGCTCGATGACGAATTCATCACAG; CDC-2 forward CTAGCATCCCATGTCAAAAACTTG, reverse CAGTGCCATTTTGCCAGAAA, probe TTTGCTCTCGAAAATGTTAATCTATGATCCAGCC) and PTGS-2 forward GAATCATTCACCAGGCAAATTG, reverse TCTGTACTGCGGGTGGAACA, probe TGGCAGGGTTGCTGGTGGTAGGA (primers in italics recognize the mRNA of the gene) were designed and validated. The 18S VIC-labeled probe was used as an internal control (PE Biosystems, Warrington, UK). All other targets were detected using Assay-on-Demand primers and probes (Applied Biosystems). Measurements were carried in triplicate, in triplicate wells for each condition and fold changes calculated as described previously (11).
Multi-target microfluidic quantitative real time PCR
Measurement of multiple gene transcripts was undertaken on custom-designed TaqMan® Low Density Array (ABI 7900HT Fast Real-Time PCR System), as described previously (14); the full 95 gene list is available upon request. Briefly, the array included probes and primers for 18S and the gene targets in nine functional groups whose expression together reflects NR signaling capacity. These are (1) NRs (e.g. high and broad affinity such as VDR and PPARs); (2) NR cofactors [e.g. co-activators (p160 family, non-p160 members, members of the ‘bridging’ Vitamin D receptor interacting protein–thyroid hormone receptor-associated proteins complex), corepressors (NCOR1, NCOR2/SMRT and COPS2/TRIP15/Alien)]; (3) histone modifiers [histone deaceytlases (e.g. HDACs hSIRT1) and acetyltransferases (P300, CBP and PCAF), histone methyltyransferases (SUV39H1 and SUV39H2), demethylases (KDM1A/LSD1), histone deaminases (e.g. PAD14)]; (4) metabolic enzymes (e.g. CYP24 and SULTA1); (5) cell death regulators (e.g. CASP4 and BAX); (6) transcription factors (e.g. YY1 and ID1); (7) cell surface transporters (e.g. ABC transporters such as MRP3); (8) cell cycle regulators (e.g. CCND1, GADD45α, CDKN1A and TP53) and (9) signal transduction (e.g. IGFBPs, MAPKs and CDH1). The exact choices represented in classes (1)–(3) were guided by serial analysis of gene expression data from normal prostate tissue (19) and those in classes (4)–(9) included known direct target genes for NRs (20–27). mRNA from cell cycle sorted cells was quantified in triplicate samples measured in duplicate as described previously (14).
Fold changes were calculated as for single target gene expression and statistical analyses carried out using the TIGR MultiExperiment Viewer 4.0, MeV (www.tm4.org). A one sample t-test analysis based on permutation (Westfall Young stepdown (28)—MaxT correction) was used to identify genes significantly expressed in each phase of the cell cycle comparing PC-3 versus RWPE-1 cells and in PC-3 cells with stable knockdown of NCOR1. In this case, vectors containing gene expression values were tested against the mean of 18S-fold changes. One-way analysis of variance was used to identify genes that were differentially expressed across the three phases of the cell cycle for the short hairpin-NCOR1 (shNCOR1) knockdown experiment in PC-3 cells.
Western immunoblot analysis
Either total protein extracts, nuclear and cytoplasmic proteins, were resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred onto polyvinylidene difluoride membrane (Immobilon-P; Millipore, Bedford, MA) and blocked with (Tris-Buffered Saline Tween-20 containing 5% milk powder). Primary antibodies as follows: NCOR1 rabbit antibody (clone ab24552; AbCam, Cambridge, UK) diluted 1:500; NCOR2/SMRT rabbit polyclonal antibody (clone ab24551; AbCam) diluted 1:500. SLIRP rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA; clone sc-134011) diluted 1:500; VDR, rabbit antibody diluted 1:500 (clone, ab3508, AbCam); PPARα, mouse monoclonal antibody [Santa Cruz Biotechnology (http://www.scbt.com/); clone sc-7273] diluted 1/2000; PPARγ mouse monolconal (clone sc7273; Santa Cruz Biotechnology) diluted 1:5000. Secondary antibodies, sheep anti-rabbit/rabbit anti-mouse-horse radish peroxidase conjugate (Amersham, Amersham, UK) were diluted 1:1000. For control, membranes were washed for 15 min with Tris-Buffered Saline Tween-20 and incubated with either a mouse monoclonal β-actin antibody (AbCam 1/10 000 dilution or monoclonal nucleolin antibody (clone 13541; Abcam, 1/10 000 dilution) and anti-mouse-horseradish peroxidase secondary antibody (clone NA931; Amersham—1:5000). Proteins were detected using ECL (Amersham) and chemiluminescence. All experiments were performed in triplicate and a representative image is shown.
Short hairpin RNA targeted toward NCOR1
Oligos were designed [Qiagen RNAi design tool (www.qiagen.com)]. Short hairpin RNA targeting NCOR1 exon 38 was cloned into pcDNA3.1 vector (Invitrogen) with the human H1 promoter. Oligos were inserted in a hairpin loop construct flanked by XbaI and Acc65 restriction sites (29). Full sequences were exon 38: AAGGCAAATCAAGCGGAAAAT (6231—exon 38-39); forward: 5′-GTACC-AA(GGCAAATCAAGCGGAAAAT)-TTCAAGAGA-(ATTTTCCGCTTGATTTGCC)TT-TTTTT-GGAAAT-3′; reverse: 3′-G-TT(CCGTTTAGTTCGCCTTTTA)-AAGTTCTCT (TAAAAGGCGAACTAAACGG)AA-AAAAA-CCTTT-AGATC-5′. Cells (1 × 105) were transfected for 36 h with 6 μg of plasmid and 6 μl Fugene6 (Roche Diagnostic, http://www.roche.com/diagnostics/). Twenty-four hours later, media-containing G418 was substituted (1 μg/μl; Sigma–Aldrich) and individual clones selected.
Silencing RNA approaches to NCOR1
NCOR1 levels were transiently reduced in PC-3 cells using Silencing RNA approaches (14). Silencing RNA (100 nM) targeted toward NCOR1 (M-003518-01, Dharmacon, Lafayette, CO) was transfected into cells using Lipofectamine2000 (Invitrogen) at a 1:1 ratio for 48 h prior to quantitative real time polymerase chain reaction and chromatin immunoprecipitation assays.
Chromatin immunoprecipitation
To measure the H3K9ac levels X-ChIP protocols were used. Briefly, formaldehyde was added directly to 1.5 × 106 mid-exponential cells to a final concentration of 1% and halted by addition of 125 mM glycine. Cells are harvested in ice-cold phosphate-buffered saline with protease inhibitors, pelleted and washed, lysed prior to pre-optimized sonication to generate chromatin fragments (∼500 bp). Subsequently, samples were cooled on ice and the cleared supernatant retained for immunoprecipitation with chromatin immunoprecipitation grade H3K9ac antibody (30). Immunocomplexes were generated by rotation overnight at 4°C. Recovery and elution was optimized using immunocomplexed magnetic beads after sequential washing in ow Salt Immune Complex Wash Buffer, High Salt Immune Complex Wash Buffer, LiCl Immune Complex Wash Buffer and 1× TE buffer. Subsequent elution and reverse cross-linked allowed to DNA to be recovered by standard precipitation approaches. DNA (25 ng) was used per quantitative polymerase chain reaction using SYBRgreen method with optimized primers ensuring that primer product size was <200 bp. CDKN1A transcriptional start site (TSS) forward TATATCAGGGCCGCGCTG, CDKN1A TSS reverse GGCTCCACAAGGAACTGACTTC and TGFBRAP1 +261 forward, TGTGCGACTTGGTAGCTGTC, TGFBRAP1 +261 reverse GCACACTTCATCCCACACAC.
Network analyses of statistically significant gene changes
Networks were established based on known protein–protein interactions [HiMAP (http://www.himap.org/main/main.jsp) (31)]. Briefly, this approach was used to construct interaction maps between the protein products of differentially regulated gene targets (identified with microarray approaches) following the co-treatment of vorinostat plus bezafibrate in PC-3 cells (supplementary Table 2 is available Carcinogenesis Online).
Principal component analyses
The expression matrixes of 14 genes from 30 prostate cancer samples were used as the input for principal component analysis with the aim of identifying underlying co-expression based on the single value decomposition package under R environment. Principal component analysis is an exploratory data analysis method that reduces the multidimensional data sets to lower dimensions while remaining most of the variation in the data matrix (32). The first principal component (i.e. the direction along which the genes show largest variation) and the second principle component (i.e. the direction uncorrelated to the first component along which the genes show the largest deviation) were shown to capture the clustering structures. The Principal component analysis implementation here is based on the single value decomposition package (http://stat.ethz.ch/R-manual/R-patched/library/base/html/svd.html).
Partial correlation analysis of gene expression
Partial correlations (PC) (33,34), the correlation of two variables while controlling for a third or more other variables, were used to elucidate the pair-wise relationships between gene expression levels while considering the behavior of other genes. Significance was determined by comparing the controlled models to those of unconstrained correlation. For example, given rxw.y is the correlation of genes X and W, controlling for gene Y, we then compared rxw.y with the unconstrained correlation, rxw and given there is no significant difference in the pearson correlations (at the P < 0.05), the inference is that gene Y has no effect on the relationship between X and W. If the partial correlation (i.e. rxw.y) approaches a value of 0, the inference is that the unconstrained correlation (rxw) is spurious, such that there is no direct causal link between genes X and W. Given rxw.y < rxw, it can be concluded that Y suppresses the relationship between X and W. We determined pair-wise correlations between transcription factors and target genes and partial correlations between transcription factors and target genes controlling for either NCOR1 or NCOR2/SMRT.
Results
Insensitivity toward VDR, RAR and PPAR ligands is associated with elevated corepressor expression
RWPE-1 cells responded significantly to all ligands [high affinity—1α,25(OH)2D3 (VDR) and ATRA (RARs and RXRs as a consequence of metabolism) and broad affinity—eicosapentaenoic (PPARα), eicosatetraynoic acid (ETYA) (PPARγ) and Bezafibrate (PPARα/γ) (35)]; these effects were significantly reduced in PC-3 cells (Figure 1). Both cells were equally sensitive to the HDAC inhibitor vorinostat, suggesting that insensitivity toward the NR ligands was not a technical artifact.
Fig. 1.
Differential sensitivity to NR ligands in PC-3 and RWPE-1 cells. Cells were plated into 96-well plates and treated with the indicated ligands at the indicated dose. After 96 h, with a redose after 48 h, proliferation was measured according to Materials and Methods and expressed as a percentage of the same cells treated with vehicle-treated controls. Each data point represents the mean value of three separate experiments each undertaken in triplicate wells. Differences between inhibition of proliferation between the two cell lines were compared (***P < 0.001, **P < 0.01).
To illuminate the basis for differential ligand responsiveness, we profiled expression of the key NRs and cofactors using multi-target microfluidic quantitative real time PCR. Basal cell cycle distributions for the two cell lines differ significantly. RWPE-1 cells displayed 31 and 5% of cells in S and G2/M, whereas in S and G2/M distribution was 20 and 16% PC-3 cells (P < 0.05). Therefore to control for different proliferation rates between the cell lines, expression patterns were compared in separate phases of the cell cycle. All significant differences between PC-3 compared with RWPE-1 cells are shown in a heatmap (supplementary Figure 1 is available at Carcinogenesis Online) and key differences are summarized in Table I.
Table I.
Distorted expression of key NRs, epigenetic regulatory cofactors and target genes between PC-3 and RWPE-1 cells in different phases of the cell cycle.
| G1 | S | G2 | |
| NRs | |||
| PPARG | 4.1 | 3.0 | 29.3 |
| VDR | 3.4 | 15.5 | |
| PPARA | 2.2 | 5.6 | |
| PPARD | 0.4 | 0.4 | 2.3 |
| RARA | 0.2 | 0.2 | |
| RXRA | 0.2 | 0.1 | |
| RARG | 0.1 | 0.0 | 0.1 |
| RXRB | 3.1 | ||
| NR target genes | |||
| IGFBP1 | 4093.0 | 441.8 | 51.7 |
| G0S2 | 175.7 | 519.4 | 2378.0 |
| TGFB2 | 2.7 | 1.6 | 3.4 |
| CDKN1A | 0.4 | 0.1 | |
| PTGS2 | 8.0 | ||
| CDH1 | 0.04 | 0.08 | 0.07 |
| Co-activators | |||
| PPARGC1A | 18.6 | 9.4 | 43.3 |
| NCOA2 | 30.1 | 12.9 | |
| CRSP2 | 1.2 | 0.6 | 6.1 |
| NCOA4 | 0.3 | 2.7 | |
| CRSP6 | 1.0 | 0.3 | 2.4 |
| NCOA1 | 10.0 | ||
| Corepressors | |||
| COPS2/TRIP15/Alien | 0.6 | 8.6 | |
| NCOR2/SMRT | 7.9 | ||
| NCOR1 | 0.2 | 2.5 | |
| SIN3A | 0.4 | ||
| Histone modifiers | |||
| HDAC4 | 9.2 | 25.5 | |
| HDAC10 | 5.7 | 3.3 | 21.4 |
| SIRT2 | 4.6 | 2.7 | 19.0 |
| SIRT6 | 2.4 | 7.4 | |
| HDAC2 | 0.4 | 5.4 | |
| SUV39H1 | 1.5 | 0.7 | 4.5 |
| PADI4 | 11.1 | 8.5 | |
| KDM1A/LSD1 | 1.0 | 0.4 | 3.6 |
| HDAC3 | 0.4 | 2.6 | |
| CARM1/PRMT4 | 0.7 | 0.4 | 1.8 |
| SET7 | 0.5 | 0.2 | 1.4 |
| HDAC7A | 0.4 | 0.1 | |
| HDAC6 | 0.4 | 0.1 | |
| HDAC5 | 0.4 | ||
| HDAC1 | 0.2 | ||
Functional grouping of genes significantly (P < 0.01) differentially expressed between untreated RWPE-1 and PC-3 cells, compared with 18S RNA. The table is organized to highlight, at the top of the list, genes that change the most through the cell cycle toward genes that change the least, located at the end of the list. Blank wells indicate that the transcript concentration was too low and could not be detected by the polymerase chain reaction, although indicating differential expression between the cell lines. Exponentially growing cells were sorted in G1, S and G2/M phases; RNA was extracted, quantified and used for microfluidic quantitative real time PCR analysis. Listed in the table, the most biologically significant genes that are thought to contribute to the loss of responsiveness of NR towards their ligands. Levels of gene expression are indicated in fold changes; significance was calculated with a one-sample t-test comparing the expression levels with the value of 1, corresponding to no differences in gene expression between the two cell lines.
PC-3 cells upregulated a number of NRs compared with RWPE-1 cells, notably in G1 and G2/M, including those that displayed loss of anti-proliferative responsiveness (e.g. PPARG and VDR). In contrast, several RAR and RXR isoforms displayed suppressed expression in one or more phase (e.g. RARA and RXRA). NR co-regulators and histone-modifying enzymes were also significantly altered in PC-3 cells, in G1 and G2/M. For example NCOR1, NCOR2/SMRT and COPS2/TRIP15/Alien and the histone deactylases HDAC2/4/10 and SIRT2 were all upregulated in G2/M. However, co-activators such as PPARGC1A were also upregulated in G2/M. Reflecting these distortions, expression of VDR and PPAR target genes in PC-3 cells were both elevated and repressed (20–27). For example, IGFBP1 and G0S2 displayed significant elevation in all phases, whereas PTGS2 (encodes COX-2) was elevated in G2/M. CDKN1A [encodes p21(waf1/cip1)] and CDH1 (encodes E-cadherin) were repressed in G1 and S-phase and all phases, respectively. Other targets were undetected in PC-3 cells e.g. ALOX-5 (encodes 5-LO).
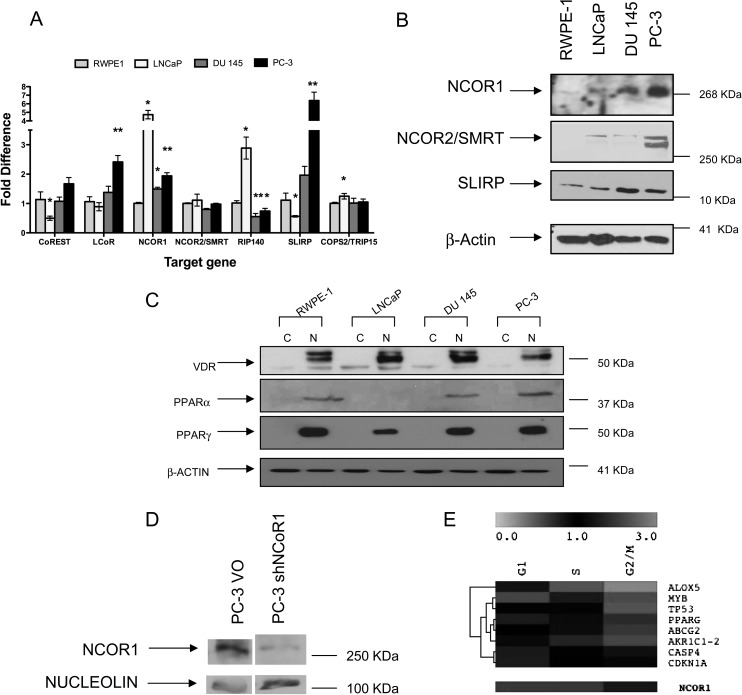
Together these finding suggest that distortion to corepressor complexes contributes to NR insensitivity. We therefore screened seven known corepressors in a wider panel of cell lines, namely RWPE-1, LNCaP, DU 145 and PC-3 cells (Figure 2A). Significantly elevated ligand dependent corepressor, NCOR1 and SLIRP mRNA levels were detected, notably in LNCaP and PC-3 cells, whereas nuclear receptor interacting protein 1/receptor interacting protein 140 was elevated in LNCaP cells only. In LNCaP cells, CoREST was significantly reduced as well. Elevated NCOR1, NCOR2/SMRT and SLIRP protein was detected in AR-independent DU 145 and PC-3 cells, an modestly elevated in AR-responsive LNCaP cells (Figure 2B). Expression of VDR and PPARγ appeared relatively constant between RWPE-1 cells and the cancer cell lines, whereas PPARα was diminished in LNCaP cells (Figure 2C).
Fig. 2.
Corepressor expression and manipulation in prostate cancer cell lines. Panel (A) differences in corepressor mRNA levels, compared with 18S expression, measured by quantitative real time plolymerase chain reaction in PC-3 and DU 145 cells compared with RWPE-1 cells. Each data point represents the mean of three separate experiments amplified in triplicate wells ±SEM (*P < 0.05, **P < 0.01). Panel (B) total cell proteins were subjected to western immunoblotting. The corepressors are indicated on the left with β-actin used as a control. Panel (C) nuclear and cytoplasmic cell proteins were subjected to western immunoblotting. The NRs are indicated on the left with β-actin used as a control. Panel (D) NCOR1 protein expression levels measured in PC-3 shNCOR1 or empty vector (PC-3 VO). Panel (E) heatmap representation of the significant expression changes through the cell cycle in red (upregulated) and green (downregulated) in PC-3 shNCOR1 compared with PC-3 VO cells. Triplicate biological samples were extracted and analyzed in duplicate and significant expression changes calculated (as measured by one-way analysis of variance analyses P < 0.05). The gene names are indicated on the right. The reduced levels of NCOR1 are indicated underneath.
Targeting corepressor complexes to enhance NR responses selectively
To reveal how critical altered corepressor expression was to drive NR insensitivity, we undertook two approaches. Specifically we used, chemical inhibition of class 1 and 2 HDACs by vorinostat that associate with corepressors, and knockdown of NCOR1 (Figure 2D and E) and SLIRP (supplementary Figure 2 is available at Carcinogenesis Online). Vorinostat inhibited prostate cancer cell proliferation (Figure 1 and data not shown) and upregulated p21(waf1/cip1) (data not shown), reflecting the findings of others (36). Subsequent studies utilized the ED25 (in vitro or in vivo dose of drug that produces 50% of its maximum response or effect) dose of vorinostat (0.5 μM) to establish combinatorial actions. Interestingly, this dose is achievable in vivo and approximates to the established IC50 (molar concentration of an agonist or antagonist which produces 50% of its maximum possible inhibition) for HDACs.
Stably reducing NCOR1 mRNA and protein levels (Figure 2D and E) changed neither the basal proliferation rate nor the gross morphology (data not shown), but did modestly shift the ED50 dose toward vorinostat from 0.24 μM in PC-3 VO cells to 0.16 μM in PC-3 shNCOR1 cells (P < 0.05) (data not shown). Multi-target microfluidic quantitative real time PCR analyses revealed knockdown of NCOR1 significantly altered expression of genes in specific phases including a cohort of established and putative target genes for VDR and PPARα/γ, for example CDH1 (37,38) in G2/M (data not shown). One-way analysis of variance analyses identified instead basal gene expression patterns that significantly varied across all phases (Figure 2E) and included PPARG, ALOX-5, CDKN1A and TP53. Interestingly, CDH1 and CDKN1A were repressed in parental PC-3 cells compared with RWPE-1 cells (Table I; supplementary Figure 1 is available at Carcinogenesis Online).
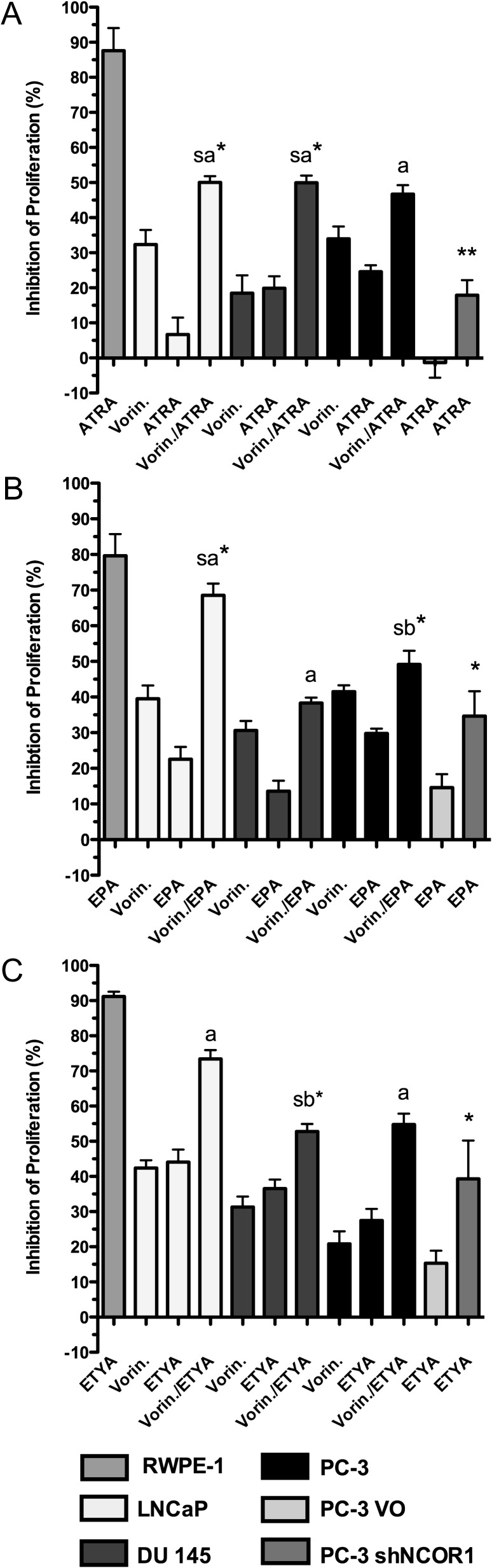
We next investigated the individual impact of either vorinostat or shNCOR1 on VDR, RAR and PPARs responsiveness (Figure 3). RARs/RXRs and PPARs ligand responsiveness was significantly enhanced by both co-treatment with vorinostat and reduction of NCOR1 levels. Cell lines treated with vorinostat plus either ATRA (RARs/RXRs), eicosapentaenoic (PPARα) or ETYA (PPARγ) displayed both additive and strong additive effects (P < 0.05). In several cases, the combinatorial effects approximated to ligand alone responses in RWPE-1 cells, most notably for co-treatments with ETYA (Figure 3C). By contrast, neither histone deacetylases inhibitor (HDACi) nor corepressor knock-down restored responses toward 1α,25(OH)2D3 (data not shown). The responses of 1α,25(OH)2D3, ATRA and ETYA were unaltered by the knockdown of SLIRP (supplementary Figure 2 is available at Carcinogenesis Online). Together these data suggest that deregulated NCOR1 significantly contributes to the suppression of anti-proliferative signaling via PPAR ligands and ATRA, but not 1α,25(OH)2D3.
Fig. 3.
Enhancement of ligand responsiveness by targeting corepressor expression and activity. Anti-proliferative responses of RWPE-1, LNCaP, DU 145, PC-3, PC-3 VO and PC-3 shNCOR1 cells were measured in response to the indicated treatments. Panel (A) ATRA, panel (B) eicosapentaenoic (EPA) and panel (C) ETYA. The dose chosen was the ED25 values for each ligand that were calculated from the individual agent response curves, as follows: LNCaP, DU 145, PC-3—ATRA (10 nM), EPA (10 μM), ETYA (10 μM) vorinostat (0.5 μM). For comparison, RWPE-1 were treated at the same dose and PC-3 VO and PC-3 shNCOR1 treatments were also at the same doses except ATRA (10 μM), EPA (30 μM) and ETYA (20 μM). Each data point represents the mean value of three separate experiments each undertaken in triplicate wells. The significance of the difference between predicated combined effects versus observed combined effects was measured using a one-tailed Mann–Whitney test. (11). Strong additive (sa) effects were those with an experimental value significantly greater than the predicted value, additive (a) effects were those where the experimental value did not significantly differ from the predicted value, sub-additive (sb) effects were those where the experimental value was significantly less than the predicated value (*P < 0.05, **P < 0.01).
Voronistat plus bezafibrate induces cell cycle arrest and programmed cell death combined with distinct gene expression patterns
We pursued PPARs using bezafibrate, a dual PPARα/γ ligand that has been utilized clinically. The co-treatment of vorinostat (0.5 μM) and bezafibrate (0.5 μM) (PPARα/γ) displayed additive effects on inhibition of proliferation of LNCaP, DU 145 and PC-3 cells (Figure 4A) and significantly increased both G1 and G2M populations with a concomitant reduction in S phase. For example, co-treated PC-3 cells resulted in significant G2/M accumulation of 26 ± 1.2% (P < 0.01), whereas the single treatments did not differ from control (14 ± 0.9%) (data not shown). Programmed cell death processes increased uniquely by the co-treatment, for example, with a loss of mitochondrial transmembrane potential and increase in DNA fragmentation in PC-3 cells (supplementary Figure 3 is available at Carcinogenesis Online), reflecting previous findings (39).
Fig. 4.
NCOR1 disrupts PPAR transcriptional actions. Panel (A) Anti-proliferative responses of RWPE-1, LNCaP, DU 145 and PC-3 were measured in response to the indicated treatments. The ED25 values were calculated from the individual agent response curves, as follows: LNCaP, DU 145, PC-3—bezafibrate (0.5 μM) and vorinostat (0.5 μM). For comparison, RWPE-1 were treated at the same dose and additive effects calculated as Figure 3. Panel (B) changes in gene expression effects by targeting NCOR1 expression and activity in PC-3 cells treated with bezafibrate (0.5 μM) plus vorinostat (0.5 μM), and PC-3 VO and PC-3 shNCOR1 cells were treated with bezafibrate (0.5 μM) over a 6 h time course total mRNA was extracted and subjected to quantitative real time polymerase chain reaction. The fold changes at key time points are given. Left panel, mRNA levels at 3 h (CDKN1A), 5 h (ALOX-5) and 2 h (TGFBPAP1, CDC2 and PTGS2); right panel, mRNA levels at 3 h in PC-3 shNCOR1 cells and PC-3 VO cells. Each data point represents the mean of three separate experiments amplified in triplicate wells ±SEM (*P < 0.05). Panel (C) X-ChIP showing the acetylation levels of H3K9 in TGFBRAP1 and CDKN1A. Mid-exponential PC-3 cells were transfected with siNCOR or scramble silencing RNA (100 nM) as described in the Materials and Methods. The media was changed and cells were treated for 3 h with bezafibrate (0.5 μM). Cells were cross-linked, chromatin extracted and immunoprecipitated with H3K9ac antibody; DNA was isolated and used for qRT-PCR (SYBR Green) with primers for the indicated regions of the TGFBRAP1 and CDKN1A genes. A region at +261 of TGFBRAP1 was chosen because the TSS region is GC rich thus preventing reliable primer design. Data shown are mean of two biological replicates, amplified in technical triplicate ±SEM. Fold changes were calculated comparing the H3K9Ac enrichment levels in the corresponding immunoglobulin G immunoprecipitation controls.
We reasoned that these phenotypes reflected alleviation of NCOR1 transcriptional distortion. A micro-array approach revealed key changes in gene expression changes following individual and combined treatments (supplementary Table 1 is available at Carcinogenesis Online). Bezafibrate (0.5 μM) and voronistat (0.5 μM) co-treatment of PC-3 cells modulated 62 genes in an additive manner (28 and 34 elevated and repressed, respectively) (supplementary Table 2 is available at Carcinogenesis Online). Comparing these expression patterns to those in prostate cancer libraries (19,40) refined this list further. Fourteen of the 28 upregulated targets were repressed in prostate cancer, and 18 of the 34 downregulated targets were elevated in prostate cancer. Network construction (31) of the refined gene targets (32 genes) revealed a cell cycle regulatory hub focused around CDC2 and CDKN1A and a transforming growth factor-beta hub focused around TGFBRAP1, TGFB2 and ALOX5 interfaced by PTGS2. Interestingly CDKN1A and ALOX-5 were both repressed in PC-3 compared with RWPE-1 and were elevated by knockdown of NCOR1 (Table I; supplementary Figure 1 is available at Carcinogenesis Online). Therefore, a panel of genes including CDC2, CDKN1A, TGBRAP1, ALOX5 and PTGS2 (a PPARγ targets (41)) were chosen as candidate NCOR1 deregulated PPARα/γ gene targets.
Basal levels of CDC2, TGBRAP1 and PTGS2 were elevated whereas CDKN1A and ALOX-5 were repressed in PC-3 cells compared with RWPE-1 cells (data not shown). Either vorinostat co-treatment or knockdown of NCOR1 both enhanced bezafibrate-induced regulation of TGFBRAP1, CDKN1A, CDC2 ALOX5 and PTGS2 mRNA (Figure 4B), resulting in parallel and significant transrepression of TGFBRAP1 and CDC2 and transctivation of CDKN1A. ALOX-5 was significantly upregulated by only the co-treatment of bezafibrate with vorinostat and not in PC-3 shNCOR1 cells (Figure 4B).
Subsequently, we measured H3K9 acetylation levels of genes that were either repressed (CDKN1A) or elevated (TGFBRAP1) in PC-3 cells. NCOR1 levels were knocked down to 0.4-fold lower than mock-transfected controls (14) and cells were treated with bezafibrate (0.5 μM) prior to measurement of H3K9ac levels either at, or immediately adjacent to, the TSS regions (Figure 4C). H3K9ac levels at the TGFBRAP1 TSS region were significantly reduced in siNCOR1 cultures treated with bezafibrate (P < 0.05) compared with treated control cells. An opposite effect was observed at the TSS of CDKN1A, with reduced H3K9ac levels upon bezafibrate treatment; this effect was attenuated by the knockdown of NCOR1. These findings support the concept that NCOR1 governs both repression and activation of a cohort of genes by direct regulation of acetylation levels. Enrichment for H3K9ac levels at the TSS of CDKN1A and matched immunoglobulin G controls is given in supplementary Figure 4 (available at Carcinogenesis Online) as a representative technical control.
Validation of PPARs, corepressor and target gene interrelationships in primary prostate cancer samples
Together these data support the concept that corepressors, notably NCOR1 and NCOR2/SMRT, through elevated expression selectively distort the capacity of PPARα/γ to control the regulation of key target genes. To test the significance of these interactions, we screened expression of key NRs, corepressors and target genes in a cohort of 30 primary prostate tumor normal matched pairs. Corepressor expression in localized androgen-dependent tumors displayed a broad spectrum of expression with both elevated levels in approximately one-third of samples (8 of 30). Principal component analyses were undertaken to address whether corepressor expression associated with expression patterns of either AR or PPARs (Figure 5A). These analyses revealed that NCOR1, PPARG, C/EBPA, PTGS2 and ALOX5 clustered separately from AR, NCOR2/SMRT and CDH1 (Figure 5A), suggesting corepressors selectivity in androgen-dependent prostate cancer.
Fig. 5.
NCOR1 and NCOR2/SMRT interactions in primary prostate cancer. Panel (A) PPARA, PPARG, NCOR1, NCOR2/SMRT, CDC2, ALOX-5, PTGS2, TGFBRAP1, CDKN1A CDH1, C/EBPA and AR mRNA levels were measured in 30 matched tumor normal pairs and principal component analyses was undertaken to reveal underlying co-expression patterns. This revealed three indicated clusters and suggested that NCOR1 expression was associated with PPARG. Panel (B) partial correlation analysis revealed relationships between PPARA, PPARG and C/EBPA and their respective targets ‘after’ removing the effects of NCOR1. Solid lines indicate that the pair-wise relationship was actually significantly dependent on NCOR1 expression and dashed lines indicate that the two genes are correlated independent of NCOR1-independent relationship.
Partial correlation analyses measured the significant dependence of corepressors on maintaining the relationships between PPARα/γ and target genes. These studies revealed that NCOR1, and to a lesser extent NCOR2/SMRT, played significant roles in maintaining the relationships between PPARA, PPARG and CEBP/A, and the representative targets (CDKN1A, CDC2, ALOX5 and PTGS2) (supplementary Table 3 is available at Carcinogenesis Online and Figure 5B). Of the two PPAR receptors, PPARA displayed stronger corepressor dependency with the target genes (CDKN1A, CDC2, ALOX5 and PTGS2) than PPARG (Figure 5B) reflecting, in part, the principal component analysis clustering. Although also true for NCOR2/SMRT, the magnitude of the influence was less (supplementary Table 3 is available at Carcinogenesis Online). PPARG displayed corepressor-dependent relationships with PTGS2 only, and again NCOR1 yielded the stronger regulatory influence.
Discussion
Prostate carcinoma remains a target for cancer for anticancer therapies that act upon the VDR, RARs/RXRs and PPARα/γ although de novo and acquired receptor resistance limits this application. Distorted corepressor functions appears to be an important component for this resistance (11,42,43) with similar events disrupting AR signaling (44,45) (reviewed in ref. 9) To date, ambiguity remains over the extent, timing and impact of corepressors expression changes in prostate cancer progression, and how they relate to different NRs. The current study investigated this ambiguity by examining the extent and contribution of deregulated corepressor complexes with regard to the actions of VDR, RARs/RXRs and PPARs. Cell line and tumor systems with differing AR expression and function were also included to address how the corepressor portfolio may evolve during cancer progression. For example, differences in nuclear receptor interacting protein 1/receptor interacting protein 140 changed between AR-responsive LNCaP cells and AR-negative PC-3 and DU 145 cells, reflecting how the co-repressor portfolio may evolve during cancer progression.
PC-3 prostate cancer cells demonstrated a progressive loss of NR responsiveness compared with RWPE-1 cells, a phenotype that is in keeping with other cancer cell lines (27,39,46,47). Suppressed ATRA responses were reflected by reduced RARs and RXRs, whereas VDR and PPARs were significantly elevated in G1 and G2/M phases despite insensitivity toward cognate ligand. Cell-phase-specific expression patterns were also displayed by a cohort of corepressors, histone-modifying enzymes and co-activators suggesting a combined effect on distortion of transcription. We investigated how NCOR1 determined NR responsiveness by both knockdown approaches and HDACi co-treatments. NCOR1 knockdown resulted in elevated basal expression of targets (CDH1 and CDKN1A) notably in G2/M adding weight to the emerging concept of the importance of the cell cycle to modulate epigenetic events and transcriptional outputs (48,49). Together the basal and regulated mRNA expression patterns suggest that distortion of NR signaling in G2/M maybe most critical to generate cellular insensitivity toward cognate ligands.
Elevated corepressor protein levels were also identified in DU 145 and PC-3, with NCOR1 mRNA and protein levels appearing to correlate clearly. The relationship between NCOR2/SMRT mRNA and protein was less direct, perhaps reflecting posttranslational mechanisms of stabilization (50,51). Furthermore, an additional NCOR2/SMRT band identified in PC-3 cells supports the findings of splice variants that potentially play separate functions (52,53).
Both HDACi and knockdown of NCOR1 enhanced cellular responsiveness toward ATRA and PPAR ligands, including the polyunsaturated fatty acids eicosapentaenoic and ETYA. Interestingly, the higher ATRA dose required for this effect is commensurate with being sensed by PPARδ (54). NCOR1 interacts with VDR (55) but the effects of 1α,25(OH)2D3 were unaltered in the presence of either vorinostat or shNCOR1 cell background. Furthermore, no ligand responses were altered by the knockdown of the NCOR1-associated cofactor SLIRP (56). Previously, we established that elevated levels of NCOR2/SMRT suppressed VDR responsiveness (10,11); other workers have re-enforced these concepts (57,58). Together these findings suggest shared and unique roles for NCOR1 and NCOR2/SMRT, with NCOR1 appearing to distort PPARα/γ principally, whereas NCOR2/SMRT is associated with VDR.
The co-treatment of the dual PPARα/γ ligand bezafibrate plus vorinostat resulted in strong additive anti-proliferative actions in three prostate cancer cells, altered genome-wide gene expression profiles selectively, and induced cell cycle arrest and programmed cell death profiles. Refining the uniquely regulated gene targets by vorinostat and bezafibrate co-treatment, by comparison with tumor-specific mRNA expression patterns, revealed that ∼50% of genes regulated by the voronistat and bezafibrate co-treatment were restored to comparable levels observed in normal prostate epithelial cells. Network analyses (31) of these genes revealed enrichment for several targets interfacing between cell cycle and lipid metabolism regulatory sub-networks. Representative gene targets between these functions (CDC2, CDKN1A, TGFBRAP1, PTGS2 and ALOX-5) were validated in PC-3 cells with both HDACi (vorinostat) and NCOR1 knock down approaches and revealed that deregulated NCOR1 preferentially alters the ability of PPARα/γ to govern H3K9ac and regulate gene expression. The NCOR1 complex suppressed upregulation of CDKN1A (60) and downregulation of TGFBRAP1, CDC2 and PTGS2. These data suggest elevated NCOR1 disrupts PPARα/γ signaling in three manners. Firstly, the basal levels of target genes are repressed in a cell cycle-specific manner; secondly, it dampens target genes transactivation and thirdly, and perhaps most surprising, the transrepression ability of PPARα/γ was also attenuated by elevated NCOR1.
The differential effects of NCOR1 may reflect its role on genes that are either considered to be active, repressed or fully silenced (reviewed in ref. 2). These actions appear guided in part by posttranslational modifications to receptor. Ligand-induced transrepression by PPARγ can result from sumoylation of the receptor, for example to suppress inflammatory genes, thereby retaining NCOR1 and preventing its ubiquitination (61). It remains to be established to what extent these mechanisms maybe corrupted in prostate cancer cells.
As a key test of the biological significance of this corepressor deregulated PPARα/γ network, we interrogated its expression patterns in primary prostate cancer tumors. Principal component analyses in primary prostate tumor-normal matched pairs revealed significant clustering between NCOR1, PPARG and C/EBPA expression and target genes including CDKN1A, TGFBRAP1 and CDC2. This cluster was separated from AR, again suggesting a specificity in prostate tumor biology of NCOR1 to target PPARα/γ. Partial correlation analyses also revealed the significant dependence of PPARA and PPARG on NCOR1 to determine expression levels of representative target genes, including CDKN1A and CDC2. These functions were augmented by the pioneer factor C/EBPA (41).
While HDACs inhibitors have shown some promise, their application as single agents has been limited in prostate cancer. Potentially, these findings concerning corepressor expression have strong prognostic significance, for example to stratify patients for responsiveness to HDAC-centered combination therapies with PPAR ligands. The ligands for PPARs can either be dietary-derived polyunsaturated fatty acids or clinically relevant bezafibrate, which has an extensive and safe clinical history exists. The strong links that have emerged in the current study between epigenetic deregulation of PPARγ and the regulation of PTGS2 (encodes COX-2) also support a dual anticancer targeting of PPARγ and COX-2 (62,63).
In summary, convergent lines of evidence support a role for NCOR1 to disrupt and restrict PPARα/γ signaling. Thus, corepressors and associated HDACs were dysregulated through the cell cycle and reflect loss of sensitivity to VDR, RARs/RXRs and PPARs ligands. Critically, only co-treatment with HDAC inhibitors and knock down of NCOR1 both enhanced PPARα/γ signaling and epigenetically governed expression of key genes, such as CDKN1A and TGFBRAP1. These associations were not restricted to cancer cell lines but also were significantly detected in primary prostate tumors. The resultant NCOR1–PPAR epigenetic lesion can be targeted by co-treatments of ligands plus the HDAC, vorinostat to restore PPARα/γ functions.
Supplementary material
Supplementary Materials and Methods, Figures 1–3 and Tables 1–3 can be found at http://carcin.oxfordjournals.org/
Funding
National Institute of Health (RO1 CA095367-06, 2R01-CA-095045-06); National Cancer Institute Cancer Center support grant to the Roswell Park Cancer Institute (CA016056).
Supplementary Material
Acknowledgments
M.J.C. acknowledges the support of NucSys, a European Community FP6-Marie Curie Research Training Network, the Biotechnology and Biological Sciences Research Council; and in part, support from National Institute of Health; National Cancer Institute Cancer Center Support Grant to the Roswell Park Cancer Institute.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- AR
androgen receptor
- ATRA
all trans retinoic acid
- COPS2
COP9 constitutive photomorphogenic homolog subunit 2
- ETYA
eicosatetraynoic acid
- HDACi
histone deacetylases inhibitor
- mRNA
messenger RNA
- NCOR1
nuclear corepressor 1
- NCOR2
nuclear corepressor 2
- NR
nuclear receptor
- PC
partial correlation
- PPAR
peroxisome proliferator activated receptor
- RARs
retinoic acid receptors
- RXRs
retinoid X receptors
- shNCOR1
short hairpin-NCOR1
- SLIRP
SRA stem-loop-interacting RNA-binding protein
- SMRT
silencing mediator of retinoic acid and thyroid hormone receptor
- TRIP15
thyroid receptor interactin protein 15
- TSS
Transcriptional Start Site
- VDR
vitamin D receptor
References
- 1.Rosenfeld MG, et al. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–1428. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 2.Perissi V, et al. Deconstructing repression: evolving models of co-repressor action. Nat. Rev. Genet. 2010;11:109–123. doi: 10.1038/nrg2736. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen K, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature. 2007;450:415–419. doi: 10.1038/nature06270. [DOI] [PubMed] [Google Scholar]
- 4.Astapova I, et al. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc. Natl Acad. Sci. USA. 2008;105:19544–19549. doi: 10.1073/pnas.0804604105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nofsinger RR, et al. SMRT repression of nuclear receptors controls the adipogenic set point and metabolic homeostasis. Proc. Natl Acad. Sci. USA. 2008;105:20021–20026. doi: 10.1073/pnas.0811012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, et al. The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. J. Biol. Chem. 2005;280:13600–13605. doi: 10.1074/jbc.M409468200. [DOI] [PubMed] [Google Scholar]
- 7.Sutanto MM, et al. SMRT recruitment by PPARgamma is mediated by specific residues located in its carboxy-terminal interacting domain. Mol. Cell. Endocrinol. 2007;267:138–143. doi: 10.1016/j.mce.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Thorne JL, et al. Transcription factors, chromatin and cancer. Int. J. Biochem. Cell Biol. 2009;41:164–175. doi: 10.1016/j.biocel.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Battaglia S, et al. Transcription factor co-repressors in cancer biology; roles and targeting. Int. J. Cancer. 2010;126:2511–2519. doi: 10.1002/ijc.25181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashid SF, et al. Synergistic growth inhibition of prostate cancer cells by 1 alpha,25 Dihydroxyvitamin D(3) and its 19-nor-hexafluoride analogs in combination with either sodium butyrate or trichostatin A. Oncogene. 2001;20:1860–1872. doi: 10.1038/sj.onc.1204269. [DOI] [PubMed] [Google Scholar]
- 11.Khanim FL, et al. Altered SMRT levels disrupt vitamin D3 receptor signalling in prostate cancer cells. Oncogene. 2004;23:6712–6725. doi: 10.1038/sj.onc.1207772. [DOI] [PubMed] [Google Scholar]
- 12.Lin RJ, et al. Role of the histone deacetylase complex in acute promyelocytic leukaemia. Nature. 1998;391:811–814. doi: 10.1038/35895. [DOI] [PubMed] [Google Scholar]
- 13.Banwell CM, et al. Altered nuclear receptor corepressor expression attenuates vitamin D receptor signaling in breast cancer cells. Clin. Cancer Res. 2006;12:2004–2013. doi: 10.1158/1078-0432.CCR-05-1218. [DOI] [PubMed] [Google Scholar]
- 14.Abedin SA, et al. Elevated NCOR1 disrupts a network of dietary-sensing nuclear receptors in bladder cancer cells. Carcinogenesis. 2009;30:449–456. doi: 10.1093/carcin/bgp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hendriksen PJ, et al. Evolution of the androgen receptor pathway during progression of prostate cancer. Cancer Res. 2006;66:5012–5020. doi: 10.1158/0008-5472.CAN-05-3082. [DOI] [PubMed] [Google Scholar]
- 17.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 18.Bowen C, et al. Loss of NKX3.1 expression in human prostate cancers correlates with tumor progression. Cancer Res. 2000;60:6111–6115. [PubMed] [Google Scholar]
- 19.Lal A, et al. A public database for gene expression in human cancers. Cancer Res. 1999;59:5403–5407. [PubMed] [Google Scholar]
- 20.Zandbergen F, et al. The G0/G1 switch gene 2 is a novel PPAR target gene. Biochem. J. 2005;392:313–324. doi: 10.1042/BJ20050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matilainen M, et al. Regulation of multiple insulin-like growth factor binding protein genes by 1alpha,25-dihydroxyvitamin D3. Nucleic Acids Res. 2005;33:5521–5532. doi: 10.1093/nar/gki872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Towsend K, et al. Identification of VDR-responsive gene signatures in breast cancer cells. Oncology. 2006;71:111–123. doi: 10.1159/000100989. [DOI] [PubMed] [Google Scholar]
- 23.Degenhardt T, et al. The insulin-like growth factor binding protein 1 gene is a primary target of peroxisome proliferator-activated receptors. J. Biol. Chem. 2006;281:39607–39619. doi: 10.1074/jbc.M605623200. [DOI] [PubMed] [Google Scholar]
- 24.Chene G, et al. n-3 and n-6 polyunsaturated fatty acids induce the expression of COX-2 via PPARgamma activation in human keratinocyte HaCaT cells. Biochim. Biophys. Acta. 2007;1771:576–589. doi: 10.1016/j.bbalip.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 25.Janabi N. Selective inhibition of cyclooxygenase-2 expression by 15-deoxy-Delta(12,14)(12,14)-prostaglandin J(2) in activated human astrocytes, but not in human brain macrophages. J. Immunol. 2002;168:4747–4755. doi: 10.4049/jimmunol.168.9.4747. [DOI] [PubMed] [Google Scholar]
- 26.Seuter S, et al. Functional characterization of vitamin D responding regions in the human 5-Lipoxygenase gene. Biochim. Biophys. Acta. 2007;1771:864–872. doi: 10.1016/j.bbalip.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Campbell MJ, et al. Inhibition of proliferation of prostate cancer cells by a 19-nor-hexafluoride vitamin D3 analogue involves the induction of p21waf1, p27kip1 and E-cadherin. J. Mol. Endocrinol. 1997;19:15–27. doi: 10.1677/jme.0.0190015. [DOI] [PubMed] [Google Scholar]
- 28.Qiu X, et al. Assessing stability of gene selection in microarray data analysis. BMC Bioinformatics. 2006;7:50. doi: 10.1186/1471-2105-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunath T, et al. Transgenic RNA interference in ES cell-derived embryos recapitulates a genetic null phenotype. Nat. Biotechnol. 2003;21:559–61. doi: 10.1038/nbt813. [DOI] [PubMed] [Google Scholar]
- 30.O'Neill LP, et al. Immunoprecipitation of chromatin. Methods Enzymol. 1996;274:189–197. doi: 10.1016/s0076-6879(96)74017-x. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes DR, et al. Probabilistic model of the human protein-protein interaction network. Nat. Biotechnol. 2005;23:951–959. doi: 10.1038/nbt1103. [DOI] [PubMed] [Google Scholar]
- 32.Joliffe IT, et al. Principal component analysis and exploratory factor analysis. Stat. Methods Med. Res. 1992;1:69–95. doi: 10.1177/096228029200100105. [DOI] [PubMed] [Google Scholar]
- 33.de la Fuente A, et al. Discovery of meaningful associations in genomic data using partial correlation coefficients. Bioinformatics. 2004;20:3565–3574. doi: 10.1093/bioinformatics/bth445. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, et al. Studying alternative splicing regulatory networks through partial correlation analysis. Genome Biol. 2009;10:R3. doi: 10.1186/gb-2009-10-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willson TM, et al. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–50. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 36.Butler LM, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 37.Palmer HG, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J. Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell MJ, et al. Synergistic inhibition of prostate cancer cell lines by a 19-nor hexafluoride vitamin D3 analogue and anti-activator protein 1 retinoid. Br. J. Cancer. 1999;79:101–107. doi: 10.1038/sj.bjc.6690018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler R, et al. Nonapoptotic cell death associated with S-phase arrest of prostate cancer cells via the peroxisome proliferator-activated receptor gamma ligand, 15-deoxy-delta12,14-prostaglandin J2. Cell Growth Differ. 2000;11:49–61. [PubMed] [Google Scholar]
- 40.Rhodes DR, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lefterova MI, et al. PPAR{gamma} and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang TH, et al. Enhanced growth inhibition by combination differentiation therapy with ligands of peroxisome proliferator-activated receptor-gamma and inhibitors of histone deacetylase in adenocarcinoma of the lung. Clin. Cancer Res. 2002;8:1206–1212. [PubMed] [Google Scholar]
- 43.Annicotte JS, et al. Peroxisome proliferator-activated receptor gamma regulates E-cadherin expression and inhibits growth and invasion of prostate cancer. Mol. Cell. Biol. 2006;26:7561–7574. doi: 10.1128/MCB.00605-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao G, et al. Regulation of androgen receptor activity by the nuclear receptor corepressor SMRT. J. Biol. Chem. 2003;278:5052–5061. doi: 10.1074/jbc.M206374200. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson MC, et al. The androgen receptor recruits nuclear receptor CoRepressor (N-CoR) in the presence of mifepristone via its N and C termini revealing a novel molecular mechanism for androgen receptor antagonists. J. Biol. Chem. 2005;280:6511–6519. doi: 10.1074/jbc.M408972200. [DOI] [PubMed] [Google Scholar]
- 46.Campbell MJ, et al. Expression of retinoic acid receptor-beta sensitizes prostate cancer cells to growth inhibition mediated by combinations of retinoids and a 19-nor hexafluoride vitamin D3 analog. Endocrinology. 1998;139:1972–1980. doi: 10.1210/endo.139.4.5943. [DOI] [PubMed] [Google Scholar]
- 47.Kaeding J, et al. Activators of the farnesoid X-receptor negatively regulate androgen glucuronidation in human prostate cancer LNCaP cells. Biochem. J. 2008;410:245–253. doi: 10.1042/BJ20071136. [DOI] [PubMed] [Google Scholar]
- 48.Jin F, et al. A novel androgen receptor-binding element modulates Cdc6 transcription in prostate cancer cells during cell-cycle progression. Nucleic Acids Res. 2009;37:4826–4838. doi: 10.1093/nar/gkp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okada M, et al. Switching of chromatin-remodelling complexes for oestrogen receptor-alpha. EMBO Rep. 2008;9:563–568. doi: 10.1038/embor.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Yoon HG, et al. Purification and functional characterization of the human N-CoR complex: the roles of HDAC3, TBL1 and TBLR1. EMBO J. 2003;22:1336–1346. doi: 10.1093/emboj/cdg120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Majada V, et al. Aberrant cytoplasmic localization of N-CoR in colorectal tumors. Cell Cycle. 2007;6:1748–1752. doi: 10.4161/cc.6.14.4429. [DOI] [PubMed] [Google Scholar]
- 52.Malartre M, et al. Alternative splicing generates multiple SMRT transcripts encoding conserved repressor domains linked to variable transcription factor interaction domains. Nucleic Acids Res. 2004;32:4676–4686. doi: 10.1093/nar/gkh786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cote S, et al. Expression of SMRT{beta} promotes ligand-induced activation of mutated and wild-type retinoid receptors. Blood. 2004;104:4226–4235. doi: 10.1182/blood-2003-10-3583. [DOI] [PubMed] [Google Scholar]
- 54.Schug TT, et al. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saramaki A, et al. Cyclical chromatin looping and transcription factor association on the regulatory regions of the p21 (CDKN1A) gene in response to 1alpha ,25-dihydroxyvitamin D3. J. Biol. Chem. 2009;284:8073–8082. doi: 10.1074/jbc.M808090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatchell EC, et al. SLIRP, a small SRA binding protein, is a nuclear receptor corepressor. Mol. Cell. 2006;22:657–668. doi: 10.1016/j.molcel.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Kim JY, et al. Involvement of SMRT corepressor in transcriptional repression by the vitamin D receptor. Mol. Endocrinol. 2008;23:251–264. doi: 10.1210/me.2008-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ting HJ, et al. Increased expression of corepressors in aggressive androgen-independent prostate cancer cells results in loss of 1alpha, 25-dihydroxyvitamin D3 responsiveness. Mol. Cancer Res. 2007;5:967–980. doi: 10.1158/1541-7786.MCR-06-0318. [DOI] [PubMed] [Google Scholar]
- 59.Campbell MJ, et al. A Role for the PPARgamma in Cancer Therapy. PPAR Res. 2008;2008:314974. doi: 10.1155/2008/314974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Copland JA, et al. Novel high-affinity PPARgamma agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25:2304–17. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 61.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun WH, et al. Inhibition of COX-2 and activation of peroxisome proliferator-activated receptor gamma synergistically inhibits proliferation and induces apoptosis of human pancreatic carcinoma cells. Cancer Lett. 2008;275:247–255. doi: 10.1016/j.canlet.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Mustafa A, et al. Suppression of tumor formation by a cyclooxygenase-2 inhibitor and a peroxisome proliferator-activated receptor gamma agonist in an in vivo mouse model of spontaneous breast cancer. Clin. Cancer Res. 2008;14:4935–4942. doi: 10.1158/1078-0432.CCR-08-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.