Abstract
In an extension of our earlier studies, we examined the inhibitory effects of N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine (PEITC-NAC), myo-inositol (MI) and indole-3-carbinol (I3C) or 3,3′-diindolylmethane (DIM), alone and in combination, on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) plus benzo[a]pyrene (BaP)-induced A/J mouse lung tumorigenesis and proliferation of A549 cells and human bronchial epithelial cells (HBECs) and relevant potential mechanisms. Mice treated with NNK plus BaP and fed non-supplemented diet had 13.0 ± 4.1 lung tumors per mouse. Dietary feeding of mice with PEITC-NAC (5 μmol/g diet), I3C (5 μmol/g diet) or MI (56 μmol/g diet), beginning at 50% in the carcinogen treatment phase, significantly reduced tumor multiplicity to 8.2 ± 2.0, 8.4 ± 1.5 and 6.8 ± 1.7 tumors per mouse, respectively. In mice given combinations of the chemopreventive agents, lung tumor multiplicity was significantly reduced to 6.3 ± 2.2, 4.9 ± 1.8, 4.8 ± 1.9 and 3.6 ± 1.4 by PEITC-NAC plus I3C, PEITC-NAC plus MI, I3C plus MI or PEITC-NAC plus I3C plus MI, respectively. Post-carcinogen administration of combinations of the agents also caused significant but weaker effects. Assessment of the anti-proliferative effects of the individual agents or their combinations showed significant reductions in the proliferation of cigarette smoke condensate (CSC)-pretreated HBEC (reduction by 30–41% at 48 h and 41–58% at 72 h) and A549 cells (30–43% at 48 h and 40–59% at 72 h), but not in dimethyl sulfoxide-pretreated HBEC. Combinatorial treatment with the agents also caused marked reductions in the activation of Akt, extracellular signal-regulated kinase and nuclear factor-kappaB in lung tumor tissues, CSC-pretreated HBEC and A549 cells. In conclusion, our studies demonstrated the promise of combinations of PEITC-NAC, I3C/DIM and MI for the chemoprevention of lung carcinogenesis in current and former smokers.
Introduction
Lung cancer is the leading cause of cancer-related death in both men and women in the USA and the death rate for lung cancer exceeds the combined total for breast, prostate and colon cancer (1). The current 5 year survival rate of lung cancer is a discouraging 15% and survival advances seen in other common malignancies have not been realized in lung cancer. This is mainly because the majority of lung cancer patients present with late-stage disease. Cigarette smoking is the main risk factor for lung cancer, accounting for 90% of cases in men and 70–85% of cases in women (2). Although smoking cessation clearly reduces the risk of lung cancer, ex-smokers still carry a significant risk and, in the USA, the majority of lung cancers are diagnosed in former smokers (3). Therefore, additional strategies to reduce the burden of lung cancer are required. One promising approach is chemoprevention. Chemoprevention has been found to be effective in populations at high risk for prostate, colon and breast cancers (4–6). Many of the strategies that were successful in the chemoprevention of these malignancies may be applicable to lung cancer.
The human diet is known to contain agents, which have cancer preventive activities in laboratory animals. Among these are phenethyl isothiocyanate (PEITC) and indole-3-carbinol (I3C), which are released during the consumption of cruciferous vegetables and myo-inositol (MI), a constituent of a variety of foods such as whole grains, seeds and fruits. The cancer preventive activities of PEITC and I3C are mediated through alteration of carcinogen metabolism, modulation of signal transduction pathways, induction of cell cycle arrest and apoptosis and inhibition of cancer cell metastasis (7–10). Despite its potent chemopreventive activities, PEITC is highly pungent and irritant to mucus membranes. Therefore, N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine (PEITC-NAC), a prodrug of PEITC, which is less toxic and more water soluble but possesses comparable chemopreventive activities (11) has been introduced and used in a number of preclinical studies. I3C undergoes condensation reactions in the acidic milieu of the stomach to give rise to several products, the predominant one being 3,3′-diindolylmethane (DIM) (12). DIM is considered to be responsible for many of the physiological effects of I3C under in vivo conditions (7).
MI, a precursor in the phosphatidylinositol cycle and a source of several second messengers, is one of the safest chemopreventive agents available, being well tolerated even at a daily dose level of 18 g/day per person (13). MI inhibited tobacco carcinogen-induced lung tumorigenesis when administered at different temporal phases (14–16) and increased the rate of regression of preexisting dysplastic lesions in smokers (13). We and others have shown recently that MI inhibits signal transduction proteins related to cell proliferation (17,18).
Combinations of low doses of chemopreventive agents may be an effective strategy for cancer prevention since this approach could avoid toxic effects of high doses of individual agents. Moreover, additive or synergistic preventive effects could be possible. The use of a combination of chemopreventive agents is especially required against lung cancer since this disease is induced by a complex mixture of tobacco carcinogens and toxicants with different modes of action (19). In the present study, we examined inhibition by PEITC-NAC, I3C/DIM and MI, individually and in combination, of the following: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) plus benzo[a]pyrene (BaP)-induced lung tumors in A/J mice; proliferation of cigarette smoke condensate (CSC)-pretreated human bronchial epithelial cells (HBECs) and A549 cells and Akt, extracellular signal-regulated kinase (ERK) and nuclear factor-kappaB (NF-κB) activation in lung tumor tissues, CSC-pretreated HBEC and A549 cells. We found that the chemopreventive activities of the combinations were consistently stronger than that of the single compounds in both in vitro and in vivo systems.
Materials and methods
Chemicals, reagents and diets
I3C, MI, BaP and a cocktail of protease inhibitors were from Sigma (St Louis, MO). PEITC-NAC was synthesized as described previously (20). BioResponse-3,3′-diindolylmethane, hereafter termed as DIM, a formulated DIM with relatively higher bioavailability, was kindly provided by Dr Michael Zeligs (BioResponse, Boulder, CO). NNK was synthesized as described (21). CSC was from Murty Pharmaceuticals (Lexington, KY) and was prepared by machine smoking of University of Kentucky 1R3F research cigarettes under Federal Trade Commission conditions (35 ml puff volume, 2 s puff duration and 1 puff per min). Anti-phospho-Akt, anti-total Akt, anti-phospho-ERK, anti-total ERK, anti-phospho-NF-κB, anti-β-actin and goat anti-rabbit IgG were from Cell Signaling Technology (Beverly, MA). Mouse diets (AIN-93G and AIN-93M) were purchased from Harlan Teklad (Madison, WI). The AIN-93G diet, high in protein and fat, was used to support rapid growth of the mice during early age, whereas AIN-93M diet, low in protein and fat, was used for maintenance and growth.
Animal studies
Female A/J mice, 5–6 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME). The experimental design is shown in Figure 1A and Table I. Upon arrival (week 0), the mice were housed in the specific pathogen-free animal quarters of Research Animal Resources, University of Minnesota Academic Health Center, and randomized into different groups and maintained on AIN-93G-pelleted diet. One week after arrival, the mice were switched to AIN-93G-powdered diet and treated by gavage with either a mixture of BaP plus NNK (2 μmol of each, groups 1–11) in 0.1 ml cottonseed oil or cottonseed oil alone (group 12), twice weekly for eight treatments. The chemopreventive agents were given in the diet beginning 1 day after the fourth treatment with the carcinogens (mice in groups 2, 3, 4, 5, 7, 8 and 10) or 1 week after the last dose of the carcinogens (mice in groups 6, 9 and 11) at the dose levels shown in Table I. The rationale for the lower doses of PEITC-NAC or I3C (5 μmol/g diet) was to minimize treatment-related reductions in body weight gain, which were observed in our earlier studies (17,22,23) at higher doses of the compounds. MI is free of adverse effects and, therefore, was used at a dose level that was effective in tumor prevention studies in earlier studies (56 μmol/g diet). Mice in group 1 (carcinogen control) and group 12 (untreated control) were maintained on non-supplemented diet. At week 10 of the study, the diet was changed from AIN-93G to AIN-93M. The experiment was terminated at week 23 by euthanizing the mice with an overdose of carbon dioxide. The lungs were harvested and tumors on the surface of the lung counted under a dissecting microscope. Normal lungs and microdissected lung tumors were kept at −80°C for subsequent western immunoblotting analysis.
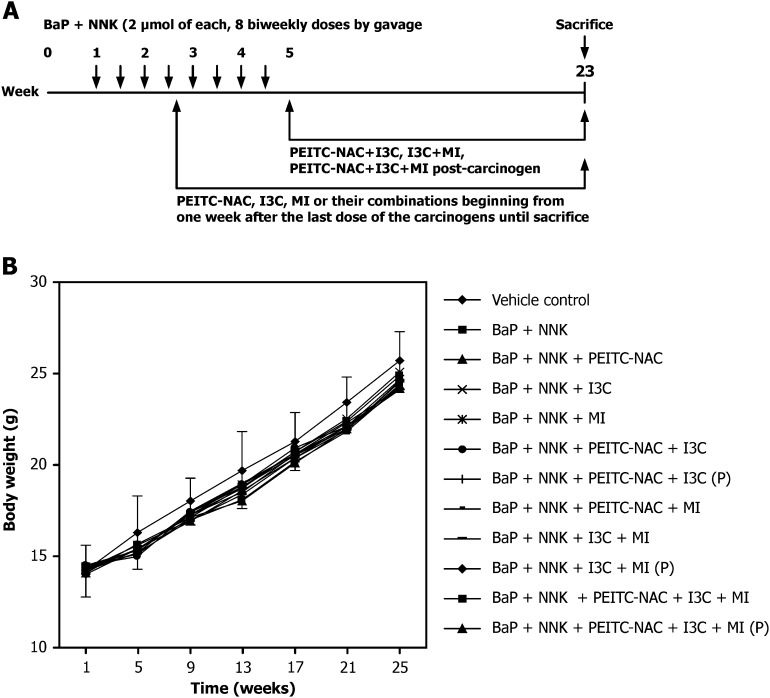
Fig. 1.
Effect of PEITC-NAC, I3C and MI on the body weight gain of female A/J mice. (A) Treatment protocol for the chemoprevention assay. (B) Mean body weight curves of mice treated with NNK plus BaP and PEITC-NAC, I3C and MI, alone or in combination. P, post-carcinogen administration of agents; otherwise, the chemopreventive agents were given beginning 1 day after 50% of the carcinogen treatment phase.
Table I.
Effects of PEITC-NAC, I3C and MI, alone or in combination, on lung tumor induction by a mixture of NNK plus BaP in A/J micea
| Group | Temporal sequence | Chemopreventive agent (dose, μmol/g diet) | No. of mice at termination | Mean body weight at termination | Lung tumors |
|||
| Tumor incidence (%) | Tumors per mouse (mean ± SD) | Reduction in tumor multiplicity (%) | Adjusted Pb | |||||
| 1 | — | None | 20 | 24.5 ± 1.8 | 100 | 13.0 ± 4.1 | ||
| 2 | A | PEITC-NAC (5) | 15 | 24.1 ± 2.2 | 100 | 8.2 ± 2.0 | 37 | 0.0051 |
| 3 | A | I3C (5) | 17 | 24.8 ± 1.6 | 100 | 8.4 ± 1.5 | 35 | 0.0051 |
| 4 | A | MI (56) | 17 | 25.2 ± 1.4 | 100 | 6.8 ± 1.7 | 48 | 0.0012 |
| 5 | A | PEITC-NAC (5) + I3C (5) | 15 | 24.0 ± 1.4 | 100 | 6.3 ± 2.2 | 51 | 0.0010 |
| 6 | B | PEITC-NAC (5) + I3C (5) | 17 | 24.2 ± 2.1 | 100 | 9.3 ± 2.7 | 29 | 0.0075 |
| 7 | A | PEITC-NAC (5) + MI (56) | 17 | 24.6 ± 1.8 | 100 | 4.9 ± 1.8 | 62 | <0.0001 |
| 8 | A | I3C (5) + MI (56) | 17 | 23.9 ± 2.0 | 94.2 | 4.8 ± 1.9 | 63 | <0.0001 |
| 9 | B | I3C (5) + MI (56) | 16 | 24.7 ± 1.6 | 100 | 6.2 ± 1.7 | 52 | 0.0006 |
| 10 | A | PEITC-NAC (5) + I3C (5) + MI (56) | 16 | 23.5 ± 1.4 | 100 | 3.6 ± 1.4 | 73 | <0.0001 |
| 11 | B | PEITC-NAC (5) + I3C (5) + MI (56) | 16 | 23.8 ± 1.7 | 100 | 5.8 ± 2.4 | 55 | <0.0001 |
| 12 | — | None | 10 | 26.6 ± 2.4 | 36.8 | 0.1 ± 0.3 | — | |
Beginning at age 6–7 weeks, groups of female A/J mice received a mixture of NNK plus BaP (2 μmol of each) orally by gavage twice a week for 4 weeks (groups 1–11). PEITC-NAC, I3C and MI, alone or in combination, were added to the diet in two temporal sequences, either beginning at 50% carcinogen treatment (A) or 1 week post-carcinogen (B). The experiment was terminated 19 weeks after the last dose of carcinogen.
Compared with group 1.
Cells and cell culture
Cyclin-dependent kinase 4/human telomerase reverse transcriptase -immortalized HBECs were kindly provided by Dr Naomi Fujioka (University of Minnesota). Human lung adenocarcinoma A549 cell line was obtained from American Type Culture Collection (Rockville, MD). HBEC were grown in serum-free bronchial epithelial cell growth medium (Clonetics, Walkersville, MD) in a humidified environment at 37°C with 5% CO2. A549 cells were grown in RPMI 1640 containing 10% fetal bovine serum under the same conditions used for HBEC.
Cell proliferation assay
First, HBEC were exposed to various concentrations of CSC for different time periods and cell proliferation was determined using the methylthiazoletetrazolium (MTT; Biotium, Hayward, CA) assay (described below). Subsequently, cells were treated with the concentration of CSC that did not affect proliferation (5 μg/ml) or the vehicle control, dimethyl sulfoxide (DMSO), for 12 weeks (culture media containing solutions of CSC were changed twice a week) and these cells were then used in subsequent studies with the chemopreventive agents in the absence of CSC. Morphological differences between DMSO- or CSC-treated cells are depicted in Figure 2A. Effects of PEITC-NAC, DIM and MI, alone or in combination, on the proliferation of HBEC and A549 cells were determined using MTT reagent as follows. Ten thousand HBEC (treated with DMSO or CSC, 5 μg/ml, for 12 weeks) were grown in a 96-well plate for 24 h and then exposed to PEITC-NAC (1 μM), DIM (2 μM) or MI (300 μM), alone or in combination, for 24, 48 and 72 h followed by MTT treatment (100 μl/well) for 3 h. A549 cells were grown in the same way as HBEC but were treated with higher doses of the chemopreventive agents (5 μM of PEITC-NAC, 10 μM of DIM or 300 μM of MI, alone or in combination). Subsequently, culture media were aspirated, 100 μl of DMSO was added to each well and absorbance at 570 nm was read with a plate reader. Each treatment with the chemopreventive agents and the corresponding solvent control (DMSO) was carried out in triplicate. The MTT assays were repeated three times.
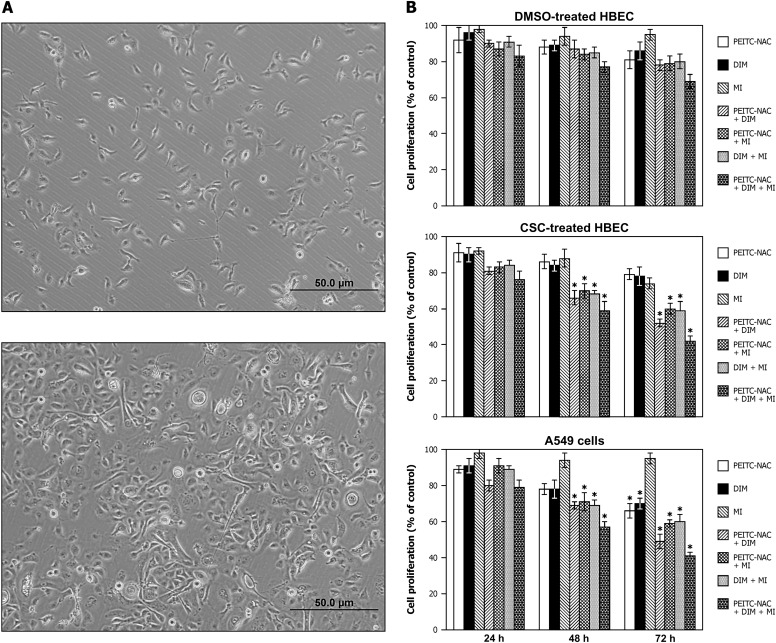
Fig. 2.
Effect of PEITC-NAC, DIM and MI, alone or in combination, on the proliferation of DMSO-pretreated HBEC, CSC-pretreated HBEC or A549 cells. (A) HBEC exposed to CSC (5 μg/ml, for 12 weeks, bottom) became elongated fibroblastoid-like cells instead of the normal epithelial cobblestone-like morphology of DMSO-treated HBEC (top). (B) Effect on cell growth. HBEC (treated with DMSO or 5 μg/ml CSC for 12 weeks) were exposed to PEITC-NAC (1 μM), DIM (2 μM) and MI (300 μM), alone or in combination, for 24, 48 and 72 h. A549 cells were treated with higher concentrations of PEITC-NAC (5 μM) and DIM (10 μM), but MI concentration was similar to that used for HBEC (300 μM). Cell proliferation was determined by MTT assay and the results were presented as mean ± SD of the percentage of the control.
Western immunoblot analyses
For the analysis of mouse lung tissues, aliquots of normal lungs (vehicle control mice, 30 mg per mouse) or microdissected tumors (carcinogen-treated mice, 30 mg per mouse) from six mice were pooled and processed as decribed previously (22,23). For the assay with HBEC, about 1 × 106 cells pretreated with DMSO or CSC for 12 weeks were exposed to PEITC-NAC (1 μmol/l), DIM (2 μmol/l) or MI (300 μmol/l), alone or in combination, for 2 weeks by replacing the culture media containing fresh solutions of the chemopreventive agents twice a week. Lysates were prepared by suspending the cells in lysis buffer on ice for 1 h. Subsequently, the preparations were centrifuged (14 000g for 25 min at 4°C), the supernatants collected, aliquoted and stored at −80°C. A549 cells were treated with the individual chemopreventive agents or their combinations for 1 week (by changing the media containing the agents twice a week) at concentrations similar to those used for MTT assay and cell lysates were prepared as for HBEC. For western immunoblotting, 60 μg of protein from lung tissues, HBEC or A549 cells were loaded onto a 4–12% Novex Tris-glycine gel (Invitrogen, Carlsbad, CA) and run for 60 min at 200 V. The proteins were then transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA) for 1 h at 30 V. Protein transfer was confirmed by staining membranes with BLOT-FastStain (Chemicon, Temecula, CA). Subsequently, membranes were blocked in 5% Blotto nonfat dry milk in Tris buffer containing 1% Tween 20 for 1 h and probed overnight with the following primary antibodies: anti-phospho-Akt (1:1000), anti-total-Akt (1:1000), anti-phospho-ERK (1:1000), anti-total ERK (1:1000) and anti-phospho-NF-κB (1:1000). After incubating, the membranes with a secondary antibody (goat anti-rabbit IgG; 1:5000) for 1 h, chemiluminescent immunodetection was used. Signal was visualized by exposing membranes to HyBolt CL autoradiography film. All membranes were stripped and reprobed with anti-β-actin (1:1000) to check for differences in the amount of protein loaded in each lane. For each protein, at least three western assays were carried out. Densitometric measurements of the bands were done with digitalized scientific software program UN-SCAN-IT purchased from Silk Scientific Corporation (Orem, UT).
Statistical analysis
A multivariate Poisson regression model was used to assess the effects of PEITC-NAC, MI and I3C, alone or in combination, on NNK plus BaP-induced lung tumor multiplicity in A/J mice. To investigate whether any synergistic effects exist when combinations were used, the two-way interactions of each pair of agents (PEITC-NAC plus I3C, PEITC-NAC plus MI and I3C plus MI) and the three-way interaction (PEITC-NAC plus I3C plus MI) were examined with the likelihood ratio test. A Pearson scaling factor was specified to adjust for the overdispersion of the tumor multiplicity. The results of the Poisson regression were qualitatively similar to a log-linear regression when the outcome was the log of tumor multiplicity plus one. Wilcoxon rank sum test was used for pair-wise comparisons, specifically, to compare each of the groups treated with carcinogens and chemopreventive agents against the group treated with the carcinogens only (Table I), to examine if the chemopreventive efficacy of combinations of agents was different from the effect of single agents or different combinations of agents (Table II) and to compare the effects of agents administered after 50% of carcinogen administration versus post-carcinogen administration (Table III). Bonferroni–Holm method was used to control the family-wise type-I error rate at 5% and the adjusted P-values are reported in each summary table. All analyses were conducted in SAS 9.1.3. For the analyses of results from the MTT assay and western immunoblotting, the two-sided Student’s t-test was used. Data are reported as mean ± SD of triplicate determinations. P values of <0.05 were considered statistically significant.
Table II.
Comparative efficacy of combination treatments to inhibit NNK plus BaP-induced lung tumorigenesisa
| Groups compared | Lung tumors per mouse | Adjusted P value |
| PEITC-NAC + I3C > PEITC-NAC | 6.3 ± 2.2 versus 8.2 ± 2.0 | 0.1976 |
| PEITC-NAC + MI > PEITC-NAC | 4.9 ± 1.8 versus 8.2 ± 2.0 | 0.0070 |
| PEITC-NAC + I3C > I3C | 6.3 ± 2.2 versus 8.4 ± 1.5 | 0.0888 |
| I3C + MI > I3C | 4.8 ± 1.9 versus 8.4 ± 1.5 | 0.0024 |
| PEITC-NAC + MI > MI | 4.9 ± 1.8 versus 6.8 ± 1.7 | 0.0714 |
| I3C + MI > MI | 4.8 ± 1.9 versus 6.8 ± 1.7 | 0.0688 |
| PEITC-NAC + MI > PEITC-NAC + I3C | 4.9 ± 1.8 versus 6.3 ± 2.2 | 0.1976 |
| PEITC-NAC + MI = I3C + MI | 4.9 ± 1.8 versus 4.8 ± 1.9 | 0.8622 |
| PEITC-NAC + I3C + MI > PEITC-NAC | 3.6 ± 1.4 versus 8.2 ± 2.0 | 0.0013 |
| PEITC-NAC + I3C + MI > I3C | 3.6 ± 1.4 versus 8.4 ± 1.5 | <0.0001 |
| PEITC-NAC + I3C + MI > MI | 3.6 ± 1.4 versus 6.8 ± 1.7 | 0.0033 |
| PEITC-NAC + I3C + MI > PEITC-NAC + I3C | 3.6 ± 1.4 versus 6.3 ± 2.2 | 0.0171 |
| PEITC-NAC + I3C + MI > PEITC-NAC + MI | 3.6 ± 1.4 versus 4.9 ± 1.8 | 0.1395 |
| PEITC-NAC + I3C + MI > I3C + MI | 3.6 ± 1.4 versus 4.8 ± 1.9 | 0.1976 |
Beginning at age 6–7 weeks, groups of female A/J mice received a mixture of NNK plus BaP (2 μmol of each) orally by gavage twice a week for 4 weeks. PEITC-NAC, I3C and MI, alone or in combination, were added to the diet from one day after 50% of carcinogen treatment phase until termination of the study at week 23.
Table III.
Summary of data on inhibition by PEITC-NAC plus MI and PEITC-NAC plus I3C plus MI of NNK plus BaP-induced lung tumorigenesis in A/J micea
| Temporal sequence of chemopreventive agent administration | Chemopreventive agent (dose, μmol/g diet) | Reduction in body weight gain (%) | Reduction in lung tumor multiplicity (%) | Reference |
| A | PEITC-NAC (3) + MI (56) | 2.5 | 58 | Hecht et al. (24) |
| A | PEITC-NAC (15) + MI (56) | 12.0 | 77 | Kassie et al. (22) |
| A | PEITC-NAC (9) + MI (56) | 7.6 | 70 | Kassie et al. (22) |
| A | PEITC-NAC (5) + MI (56) | No change | 62 | Present study |
| A | PEITC-NAC (5) + I3C (5) + MI (56) | 4.0 | 73 | Present study |
| B | PEITC-NAC (3) + MI (56) | 4.1 | 40 | Hecht et al. (24) |
| B | PEITC-NAC (15) + MI (56) | 13.0 | 55 | Kassie et al. (22) |
| B | PEITC-NAC (5) + I3C (5) + MI (56) | 3.0 | 55 | Present study |
Beginning at age 6–7 weeks, groups of female A/J mice received eight treatments of a mixture of NNK plus BaP (2 μmol of each) orally by gavage. Combinations of the chemopreventive agents were added to the diet in two temporal sequences, either beginning at 50% carcinogen treatment (A) or 1 week post-carcinogen (B). The experiment was terminated 19 weeks after the last dose of carcinogen.
Results
Effect of PEITC-NAC, I3C and MI on body weights of mice
None of the chemopreventive agents, alone or in combination, significantly reduced body weight gain of the mice (Figure 1B). The maximum reduction in body weight (4%) was caused by a combination of PEITC-NAC and I3C. During the first 4 weeks of the study (carcinogen treatment time), carcinogen-treated mice did not gain as much weight as the mice in the control group. This difference disappeared once carcinogen treatment was over.
Effects of PEITC-NAC, I3C and MI, alone and in combination, on NNK plus BaP-induced lung tumors
Our primary aim was to determine the lung tumor inhibitory effects of PEITC-NAC, I3C and MI when given in the diet to NNK plus BaP-treated mice individually or in combination. Diets were supplemented with the chemopreventive agents either beginning 50% in the carcinogen treatment phase or 1 week after the termination of carcinogen treatment. As shown in Table I, mice treated with NNK plus BaP and fed on non-supplemented diet had 13.0 ± 4.1 lung tumors per mouse. Carcinogen-treated mice given diets containing PEITC-NAC (5 μmol/g diet), I3C (5 μmol/g diet) or MI (56 μmol/g diet), beginning at 50% in the carcinogen treatment phase had 8.2 ± 2.0, 8.4 ± 1.5 and 6.8 ± 1.7 tumors per mouse, corresponding to reductions by 37, 35 and 48%, respectively. The effects of all three compounds were significant. The lung tumor multiplicity in the vehicle control group was within the range of historical controls (0.10 ± 0.30 tumors per mouse). None of the chemopreventive agents significantly reduced tumor incidence. This was not unexpected since the most sensitive indicator in the A/J mouse lung tumorigenesis model is tumor multiplicity.
In mice treated with the carcinogens and given combinations of the chemopreventive agents in the diet beginning 50% in the carcinogen treatment phase, the multiplicity of lung tumors was reduced from 13.0 ± 4.1 in the carcinogen only group to 6.3 ± 2.2, 4.9 ± 1.8, 4.8 ± 1.9 and 3.6 ± 1.4 in mice treated with the carcinogens and given mixtures of PEITC-NAC plus I3C, PEITC-NAC plus MI, I3C plus MI and PEITC-NAC plus I3C plus MI, respectively, corresponding to reductions by 51, 62, 63 and 73%. The effects of combinations of the three compounds were consistently significantly stronger than that of individual compounds (Table II), but according to the results of the likelihood ratio test, the effects of the individual compounds within the mixture were not synergistic. Also, administration of combinations of the chemopreventive agents during the post-carcinogen phase led to significant reductions of NNK plus BaP-induced lung tumor multiplicity (Table II). Mixtures of PEITC-NAC plus I3C, I3C plus MI and PEITC-NAC plus I3C plus MI reduced lung tumor multiplicity by 29, 52 and 55%, respectively. However, the effects were weaker compared with chemopreventive activities observed when the agents were given beginning 50% in the carcinogen treatment phase.
Effects of PEITC-NAC, DIM and MI, individually or in combination, on the proliferation of HBEC and A549 cells
As depicted in Figure 2B, in general, when added to the culture media individually, none of the chemopreventive agents significantly reduced the proliferation of HBEC. However, combinatorial treatment with the chemopreventive agents led to a higher anti-proliferative effect in CSC-pretreated HBEC as compared with DMSO-pretreated HBEC. Even after treatment with combinations of all three agents for 72 h, the proliferation of DMSO-pretreated HBEC was reduced non-significantly by 25% only (Figure 2B, upper panel). In CSC-pretreated HBEC, however, exposure of the cells to combinations of PEITC-NAC plus DIM, PEITC-NAC plus MI or DIM plus MI for 48 h reduced cell proliferation significantly by 34, 30 and 32%, respectively, whereas exposure to a combination of all three agents reduced cell proliferation by 41% (Figure 2B, middle panel). These effects were further enhanced upon treatment for 72 h (cell proliferation was reduced by 48, 40 and 41% by combinations of PEITC-NAC plus DIM, PEITC-NAC plus MI and DIM plus MI, respectively, and by 58% when cells were exposed to a combination of all three compounds).
In A549 cells, treatment with PEITC-NAC, DIM and MI, alone or in combination, for 24 h did not affect cell proliferation significantly (Figure 2B, lower panel). However, upon extending the treatment time to 48 h, combinatorial treatment with two and three agents significantly reduced cell proliferation by 30 and 43%, respectively; single agents did not induce significant effects. The anti-proliferative effects of the mixtures further increased upon treatment for 72 h (51, 41, 40 and 59% reduction by PEITC-NAC plus DIM, PEITC-NAC plus MI, DIM plus MI and PEITC-NAC plus DIM plus MI, respectively). At this time point, treatment with PEITC-NAC alone or DIM alone reduced cell proliferation significantly by 34 and 30%, respectively, but MI did not have any effect.
Effect of PEITC-NAC, DIM and MI on Akt, ERK and NF-κB activation in mouse lung tumor tissues, CSC-treated HBEC and human lung adenocarcinoma A549 cells
Activation of Akt, ERK and NF-κB is commonly observed during lung tumorigenesis and, therefore, these proteins could be important targets for lung cancer chemopreventive agents. To determine if the tumor inhibitory and anti-proliferative activities of PEITC-NAC, I3C/DIM and MI are paralleled by inhibition of Akt, ERK and NF-κB activation, we examined levels of p-Akt, p-ERK and p-NF-κB in lung tissues, HBEC and A549 cells. The results of these studies are shown in Figure 3.
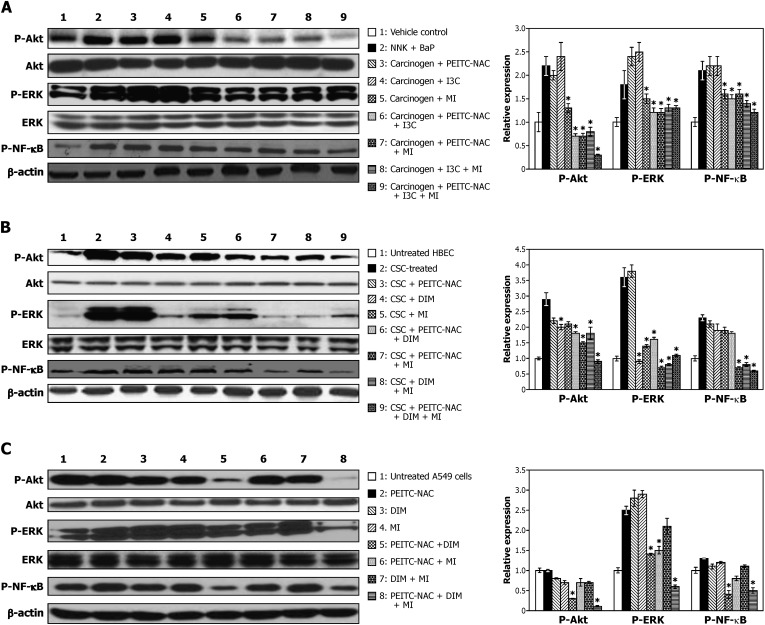
Fig. 3.
Modulation by PEITC-NAC, DIM and MI, alone and in combination, of p-Akt, p-ERK and p-NF-κB expression in mouse lung tissues (A) HBEC (B) and A549 cells (C). Lung tissue lysates were prepared from aliquots of normal lungs (lane 1) or lung tumors from mice treated with NNK plus BaP (lane 2) or NNK plus BaP plus chemopreventive agents, alone or in combination. (lanes 3–9). Samples from HBEC were prepared from cells pretreated with DMSO (lane 1) or CSC (lane 2) for 12 weeks or cells pretreated with CSC for 12 weeks and exposed to PEITC-NAC (1 μM), DIM (2 μM) and MI (300 μM), alone or in combination, for 2 weeks (lanes 3–9). Lysates from A549 cells were prepared after treatment with DMSO (lane 1) or PEITC-NAC (5 μM), DIM (10 μM) and MI (300 μM), alone or in combination, for 1 week (lanes 2–8). Samples (40 μg protein) were resolved on a 4–12% sodium dodecyl sulfate–polyacrylamide electrophoresis followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. Relative fold of expression of the proteins was determined by densitometry analysis. *P < 0.05.
Levels of p-Akt, p-ERK and p-NF-κB were higher in lung tumor tissues from NNK plus BaP-treated mice relative to the levels in normal lung tissues from vehicle-treated mice. Among NNK plus BaP-treated mice given PEITC-NAC, I3C or MI in the diet, individually, levels of p-Akt were reduced by MI only. On the other hand, in mice given combinations of the agents, Akt activation was significantly reduced by all mixtures, the greatest effect being in the group given all three agents. ERK activation was non-significantly increased by PEITC-NAC and I3C but significantly reduced by all combinatorial treatments. Neither the individual agents nor the combinations, except the combination of all three agents, led to a significant reduction in the level of p-NF-κB.
Compared with levels in DMSO-pretreated HBEC, expressions of p-Akt, p-ERK and p-NF-κB were significantly increased in HBEC exposed to CSC for 12 weeks. Although PEITC-NAC, DIM and MI failed to reduce CSC-induced activation of the proteins when added individually, their combinations were effective, the highest effect being induced by the mixture containing all three agents. Generally, the effects of the agents on activation of ERK were stronger in HBEC than in tumor tissues; also, unlike in tumor tissues, DIM did not cause ERK activation when added alone. Activation of NF-κB was inhibited by all mixtures except by combinations of PEITC-NAC plus DIM.
In A549 cells, combinations of PEITC-NAC plus DIM and PEITC-NAC plus DIM plus MI markedly inhibited Akt activation, but not the individual compounds or PEITC plus MI or DIM plus MI. ERK activation was enhanced by the individual agents but significantly reduced by the mixture containing all three agents. The trend for the effect of the chemopreventive agents on p-NF-κB activation was similar to that observed for ERK activation.
Discussion
In the present study, we showed that low doses of PEITC-NAC and/or I3C/DIM, products of commonly consumed cruciferous vegetables, when combined with MI, effectively reduced the multiplicity of tobacco smoke carcinogen-induced mouse lung tumors, proliferation of A549 cells and CSC-treated HBEC and levels of p-Akt, p-ERK and p-NF-κB in lung tumor tissues, A549 cells and HBEC. We reported in earlier studies that higher doses of PEITC-NAC (9 and 15 μmol/g diet) and I3C (30, 71 and 120 μmol/g diet) significantly reduced the multiplicity of carcinogen-induced lung tumors in mice (17,22,23). However, these effects were accompanied by reductions in body weight (∼10%). In the present study, body weights of the mice treated with the individual chemopreventive agents or their combinations were similar to those of the control animals. Also, in both in vitro and in vivo studies, the agents were used at concentrations that could be achieved physiologically in humans. For instance, plasma PEITC concentrations of 1.15 μmol/l could be achieved upon consumption of 100 g of watercress (25), which is equivalent to the concentration of PEITC-NAC (1 μmol/l) used in cell culture studies with HBEC. Similarly, the concentration of DIM used for cell proliferation and western assays in HBEC was similar to that achieved in the plasma of women who participated in a phase I clinical trial with I3C (2.46 μmol/l, 26). For the in vitro studies, we used DIM, a major in vivo condensation product of I3C, instead of the parent compound because I3C could not be optimally converted to DIM in cell culture conditions. MI is a promising chemopreventive agent with negligible toxic effects. In earlier studies, it has been administered to mice at a dose level of 168 μmol/g diet, which is three times the amount used in the present study (56 μmol/g diet), without causing any toxic effects (27). In phase I clinical trials of MI in smokers, a dose level of 18 g/day was well tolerated (13). Although tissue or plasma levels of MI were not determined in this study, in another investigation in which 100 mg/kg MI was given, plasma levels of 100 μmol/l MI were achieved (28). Given the fact that the dose level of MI used in phase I trials (18 g/day, 300 mg/kg for a person with a 60 kg body wt) was 3-fold higher than that used in the study by Groenen et al., it is plausible to speculate that the dose level of MI used in our in vitro studies (300 μmol/l) was relevant to the human situation.
Two important criteria for the development of chemopreventive agents are safety and efficacy. Potential approaches to reduce the toxicity of chemopreventive agents are the use of combinations of low doses of promising chemopreventive agents such as PEITC and I3C or mixtures of low doses of promising agents and high doses of relatively safe agents such as MI. Also, combinatorial treatment could increase the efficacy of cancer chemoprevention as it enables one to target multiple carcinogens or cancer-related signaling pathways. This is particularly justified in view of the fact that cigarette smoke, the main cause of lung cancer, is a complex mixture of >5000 compounds including >70 carcinogens (29,30) that contribute to lung carcinogenesis via different mechanisms. Moreover, the chronic and multi-step nature of carcinogenesis and the fact that lung cancer is a heterogenous group of diseases with alterations in several signaling proteins provide further support for a multi-agent approach to the chemoprevention of lung cancer (19).
In the present study, we showed that NNK plus BaP-treated mice given combinations of PEITC-NAC plus I3C plus MI had significantly lower tumor multiplicities than those mice given the compounds individually or a combination of PEITC-NAC plus I3C (Table II). However, there was no significant difference between the chemopreventive activity of mixtures of all three compounds and PEITC-NAC plus MI or I3C plus MI (Table II). Therefore, for chemoprevention trials in current-smokers/former smokers, the use of mixtures of PEITC-NAC plus MI or I3C plus MI would be more advisable than the combination of all three agents. Possible explanations for the greater efficacy of combinatorial treatment, especially when given beginning in the mid-phase of carcinogen treatment, could be that the chemopreventive agents cooperatively inhibit carcinogen metabolism and other aspects of carcinogenesis that lead to initiation in addition to their post-initiation effects. Indeed, PEITC and I3C modulate the metabolic activation of tobacco carcinogens via different mechanisms and also target separate cancer-related signaling pathways although cooperation in modulating a single pathway is also possible (7). Similar effects were observed in our previous study (24) in which a combination of PEITC-NAC plus MI showed the greatest lung tumor inhibitory activities when given during the entire experiment although additive effects were observed in all temporal sequences (carcinogen treatment phase, post-carcinogen treatment phase and 50 or 75% in the carcinogen treatment phase). Table III summarizes our present and earlier findings on inhibition of mouse lung tumorigenesis by combinations of PEITC-NAC plus MI/PEITC-NAC plus I3C plus MI.
Of the different signal transduction pathways that are deregulated during lung carcinogenesis, Akt, ERK and NF-κB pathways have been proposed to play a central role (31–33). Akt and ERK are involved in cell survival and proliferation, respectively, whereas NF-κB regulates the expression of inflammation-related genes. All three pathways were found to be activated in normal bronchial and alveolar cells and non-small cell lung cancer cells treated with tobacco smoke constituents (18,34,35), human lung cancer lesions (36–39) and lung tumors from tobacco carcinogen-treated mice or transgenic mice (17,22,40,41). There is also a close interaction between Akt, ERK and NF-κB. For instance, the function of both phosphatidylinositol 3-kinase–Akt and RAS–ERK pathways was required to transform human melanocytes by v-SEA and neither of the genes was sufficient to transform the cells (42). Also, a cross talk was shown between the RAF–MAPK/ERK kinase–ERK and phosphatidylinositol 3-kinase–Akt pathways, mediated by the direct interaction of Akt with and its phosphorlyation of RAF that switched the biological response of breast cancer cells from growth arrest to proliferation (43). One of the mechanisms by which Akt promotes cell survival is via induction of IkappaB degradation that results in the release of NF-κB from the cytoplasm to the nucleus (44). The expression of p-Akt, p-ERK and p-NF-κB in transformed and malignant lung cells as well as the close interaction among the proteins makes them attractive targets for lung cancer chemoprevention. Indeed, in the present study, levels of p-Akt, p-ERK and p-NF-κB were reduced by PEITC-NAC, I3C/DIM and MI or their combinations, combinatorial treatment being more effective than treatment with a single agent, and the trend in p-Akt, p-ERK and p-NF-κB modulation is similar to the effects observed in the tumor bioassay and MTT assay. Thus, the higher chemopreventive activities of the mixtures may be explained, at least partly, by their additive effects in abrogating Akt, ERK and NF-κB activation. We and others have previously reported that PEITC-NAC, I3C and MI, when given individually to mice at relatively higher doses in the diet, inhibited carcinogen-induced lung tumorigenesis through modulation of activities of cell proliferation- and apoptosis-related proteins (17,22,23,45–47). In the present study, the individual effects of PEITC-NAC and I3C on lung tumor multiplicity and activation of Akt, ERK and NF-κB were weaker than those observed in the above studies. This discrepancy could be due to differences in the dose levels of the chemopreventive agents. Our findings with MI are in line with the reports of Han et al. (18) in which the compound significantly decreased Akt activation in dysplastic lesions from heavy smokers and endogenous and tobacco carcinogen-induced treated immortalized HBEC but not in lung cancer cell lines. Therefore, for MI to be effective against lung tumorigenesis, it should be administered during the relatively early phase of the carcinogenic process.
Although data on the effect of combinatorial chemopreventive treatments on lung tumor multiplicity and the associated lung cancer-related proteins are lacking, studies in other models are in line with our results. For instance, combination treatment with PEITC and curcumin caused additive apoptotic effects in PC-3 human prostate cancer cells through simultaneous targeting of epiderma growth factor receptor, Akt and NF-κB signaling pathways (48). The same group showed that a combination of curcumin and PEITC significantly reduced the growth of tumor xenografts through inhibition of Akt and NF-κB signaling pathways, whereas PEITC or curcumin alone had little effect (49).
In conclusion, we showed that the efficacy of combinations of PEITC-NAC, I3C/DIM and MI was higher than the individual compounds in inhibiting NNK plus BaP-induced lung tumor multiplicity in mice and proliferation of A549 cells and CSC-treated HBEC. Also, combinatorial treatment with the chemopreventive agents led to a stronger abrogation of Akt, ERK and NF-κB activation in lung tumor tissues, A549 cells and HBEC, indicating that the higher chemopreventive activities of the mixtures could be mediated, at least partly, via the combined inhibitory effects of the agents on activation of these crucial mitogenic and anti-apoptotic proteins. Moreover, combinatorial treatment exerted differential toxicity toward CSC-treated HBEC as compared with DMSO-treated cells and did not affect body weight gains in mice. The higher chemopreventive efficacy and safety of combinations of PEITC-NAC, I3C/DIM and MI indicates the promise of mixtures for prevention of lung cancer in current and former smokers.
Funding
National Institutes of Health/National Cancer Institute (CA-128801 to F.K., CA-102502 to S.S.H.).
Acknowledgments
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- BaP
benzo[a]pyrene
- CSC
cigarette smoke condensate
- DIM
3,3′-diindolylmethane
- DMSO
dimethyl sulfoxide
- ERK
extracellular signal-regulated kinase
- HBEC
human bronchial epithelial cell
- I3C
indole-3-carbinol
- NNK
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- MTT
methylthiazoletetrazolium
- MI
myo-inositol
- PEITC-NAC
N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine
- NF-κB
nuclear factor-kappaB
- PEITC
phenethyl isothiocyanate
References
- 1.Jemal A, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 83. Lyon: IARC Press; 2004. Tobacco smoke and involuntary smoking; pp. 53–119. [PMC free article] [PubMed] [Google Scholar]
- 3.Tong L, et al. Lung carcinoma in former smokers. Cancer. 1996;78:1004–1010. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, et al. The influence of finasteride on the development of prostate cancer. N. Engl. J. Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- 5.Meyskens FL, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsinger LS, et al. Chemoprevention of breast cancer: a summary of the evidence for the US Preventive Services Task Force. Ann. Intern. Med. 2002;137:59–69. doi: 10.7326/0003-4819-137-1-200207020-00017. [DOI] [PubMed] [Google Scholar]
- 7.International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Vol. 9. Lyon: IARC Printing Press; 2004. Cruciferous vegetables, isothiocyanates and indoles; pp. 171–176. [Google Scholar]
- 8.Rahman KM, et al. Therapeutic intervention of experimental breast cancer bone metastasis by indole-3-carbinol in SCID-human mouse model. Mol. Cancer Ther. 2006;5:2747–2756. doi: 10.1158/1535-7163.MCT-06-0221. [DOI] [PubMed] [Google Scholar]
- 9.Kim EJ, et al. Oral administration of 3,3'-diindolylmethane inhibits lung metastasis of 4T1 murine mammary carcinoma cells in BALB/c mice. J. Nutr. 2009;139:2373–2379. doi: 10.3945/jn.109.111864. [DOI] [PubMed] [Google Scholar]
- 10.Hwang ES, et al. Phenylethyl isothiocyanate and its N-acetylcysteine conjugate suppress the metastasis of SK-Hep1 human hepatoma cells. Nutr. Biochem. 2006;17:837–846. doi: 10.1016/j.jnutbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Jiao D, et al. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis. 1997;18:2143–2147. doi: 10.1093/carcin/18.11.2143. [DOI] [PubMed] [Google Scholar]
- 12.Bjeldanes LF, et al. Aromatic hydrocarbon receptor agonist generated from indole-3-carbinol in vitro and in vivo: comparisons with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Proc. Natl Acad. Sci. USA. 1991;88:9543–9547. doi: 10.1073/pnas.88.21.9543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam S, et al. A phase I study of myo-inositol for lung cancer chemoprevention. Cancer Epidemiol. Biomarkers Prev. 2006;15:1526–1531. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 14.Wattenberg LW, et al. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis. 2000;21:179–182. doi: 10.1093/carcin/21.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Estensen RD, et al. Studies of chemopreventive effects of myo-inositol and benzo(a)pyrene induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14:1975–1977. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 16.Hecht SS, et al. Dose-response study of myo-inositol as an inhibitor of lung tumorigenesis induced in A/J mice by benzo[ a]pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Lett. 2001;167:1–6. doi: 10.1016/s0304-3835(01)00454-2. [DOI] [PubMed] [Google Scholar]
- 17.Kassie F, et al. Dose-dependent inhibition of tobacco smoke carcinogen-induced lung tumorigenesis in A/J mice by indole-3-carbinol. Cancer Prev. Res. 2008;1:568–576. doi: 10.1158/1940-6207.CAPR-08-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han W, et al. The chemopreventive agent myo-inositol inhibits Akt and extracellular signal-regulated kinase in bronchial lesions from heavy smokers. Cancer Prev. Res. 2009;2:370–376. doi: 10.1158/1940-6207.CAPR-08-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS, et al. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat. Rev. Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mennicke WH, et al. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983;13:203–207. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- 21.Hecht SS, et al. Effects of-deuterium substitution on the mutagenicity of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Carcinogenesis. 1983;4:305–310. doi: 10.1093/carcin/4.3.305. [DOI] [PubMed] [Google Scholar]
- 22.Kassie F, et al. Combinations of N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol inhibit tobacco carcinogen-induced lung adenocarcinoma in mice. Cancer Prev. Res. 2008;1:285–297. doi: 10.1158/1940-6207.CAPR-08-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassie F, et al. Inhibition of vinyl carbamate-induced pulmonary adenocarcinoma by indole-3-carbinol and myo-inositol in A/J mice. Carcinogenesis. 2009;31:239–245. doi: 10.1093/carcin/bgp174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecht SS, et al. Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–1461. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y, et al. Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal. Biochem. 2003;323:39–47. doi: 10.1016/j.ab.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Reed GA, et al. Single-dose and multiple-dose administration of indole-3-carbinol to women: pharmacokinetics based on 3,3-diindolylmethane. Cancer Epidemiol. Biomarkers Prev. 2006;15:2477–2481. doi: 10.1158/1055-9965.EPI-06-0396. [DOI] [PubMed] [Google Scholar]
- 27.Estensen RD, et al. Studies of chemopreventive effects of myo-inositol on benzo(a)pyrene-induced neoplasia of the lung and forestomach of female A/J mice. Carcinogenesis. 1993;14:1975–1977. doi: 10.1093/carcin/14.9.1975. [DOI] [PubMed] [Google Scholar]
- 28.Groenen PM, et al. Kinetics of myo-inositol loading in women of reproductive age. Ann. Clin. Biochem. 2003;40:79–85. doi: 10.1258/000456303321016213. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann D, et al. The changing cigarette, 1950–1995. J. Toxicol. Environ. Health. 1997;50:307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- 30.Rodgman A, et al. The Chemical Components of Tobacco Smoke. CRC press; 2009. [Google Scholar]
- 31.Brognard J, et al. Akt/protein kinase b is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001;61:3986–3997. [PubMed] [Google Scholar]
- 32.Brognard J, et al. Variable apoptotic response of NSCLC cells to inhibition of the MEK/ERK pathway by small molecules or dominant negative mutants. Cell Death Differ. 2002;9:893–904. doi: 10.1038/sj.cdd.4401054. [DOI] [PubMed] [Google Scholar]
- 33.Tang X, et al. Nuclear factor-kappaB (NF-kappaB) is frequently expressed in lung cancer and preneoplastic lesions. Cancer. 2006;107:2637–2646. doi: 10.1002/cncr.22315. [DOI] [PubMed] [Google Scholar]
- 34.West KA, et al. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J. Clin. Invest. 2003;111:81–90. doi: 10.1172/JCI16147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laag E, et al. NNK activates ERK1/2 and CREB/ATF-1 via β-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cells. Int. J. Cancer. 2006;119:1547–1552. doi: 10.1002/ijc.21987. [DOI] [PubMed] [Google Scholar]
- 36.Tsao AS, et al. Increased phospho-AKT (Ser 473)) expression in bronchial dysplasia: implications for lung cancer prevention studies. Cancer Epidemiol. Biomarkers Prev. 2003;12:660–664. [PubMed] [Google Scholar]
- 37.Balsara BR, et al. Frequent activation of AKT in non-small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 38.Tsurutani J, et al. Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells. Carcinogenesis. 2005;26:1182–1195. doi: 10.1093/carcin/bgi072. [DOI] [PubMed] [Google Scholar]
- 39.Vicent S, et al. ERK1/2 is activated in non-small cell lung cancer and associated with advanced tumors. Br. J. Cancer. 2004;90:1047–1052. doi: 10.1038/sj.bjc.6601644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan N, et al. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007;67:3475–3482. doi: 10.1158/0008-5472.CAN-06-3941. [DOI] [PubMed] [Google Scholar]
- 41.Stathopoulos GT, et al. Epithelial NF-kappaB activation promotes urethane-induced lung carcinogenesis. Proc. Natl Acad. Sci. USA. 2007;104:18514–18519. doi: 10.1073/pnas.0705316104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agazie Y, et al. Concomitant activation of the PI3K-Akt and the Ras-ERK signaling pathways is essential for transformation by the V-SEA tyrosine kinase oncogene. Oncogene. 2002;21:697–707. doi: 10.1038/sj.onc.1205115. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann S, et al. Phosphorylation and regulation of Raf by Akt (protein kinase B) Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 44.Burow S, et al. PI3-K/AKT regulation of NF-kappaB signaling events in suppression of TNF-induced apoptosis. Biochem. Biophys. Res. Commun. 2000;271:342–345. doi: 10.1006/bbrc.2000.2626. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, et al. Inhibition of benzo(a)pyrene-induced lung tumorigenesis in A/J mice by dietary N-acetylcysteine conjugates of benzyl and phenethyl isothiocyanates during the postinitiation phase is associated with activation of mitogen-activated protein kinases and p53 activity and induction of apoptosis. Cancer Res. 2002;62:2–7. [PubMed] [Google Scholar]
- 46.Ye B, et al. Induction of lung lesions in Wistar rats by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and its inhibition by aspirin and phenethyl isothiocyanate. BMC Cancer. 2007;7:90. doi: 10.1186/1471-2407-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conaway CC, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 48.Kim J, et al. Inhibition of EGFR signaling in human prostate cancer PC-3 cells by combination treatment with β-phenylethyl isothiocyanate and curcumin. Carcinogenesis. 2006;27:475–482. doi: 10.1093/carcin/bgi272. [DOI] [PubMed] [Google Scholar]
- 49.Khor TO, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer Res. 2006;66:613–621. doi: 10.1158/0008-5472.CAN-05-2708. [DOI] [PubMed] [Google Scholar]