Abstract
Conjugated linoleic acid (CLA) is a class of commercially available fatty acids that have been associated with anticancer properties in rodent models of chemical carcinogenesis. We conducted a pilot study to examine the antitumor effect of dietary CLA in a polyoma virus-middle T antigen (PyMT) mouse model of invasive breast cancer. Virgin 4-week-old PyMT mice were administered a mixed-isomer CLA diet (1% wt/wt) or control AIN-93G diet for 4 weeks (N = 6 and 5, respectively) and tumor burden was assessed at 8 weeks of age. Thoracic mammary glands were prepared as whole mounts with other glands being formalin fixed and paraffin embedded for histology and immunohistochemistry (IHC). Total RNA was prepared for microarray and real-time reverse transcription–polymerase chain reaction analysis. Western blots were performed for protein expression analysis. Tumor incidence was significantly increased in CLA-treated animals compared with controls (P = 0.009) and occurred with extensive lobular–alveolar expansion and loss of mammary adipose tissue. More than 100 genes were downregulated ≥2-fold in the CLA-treated group compared with controls, including adipose-specific markers, as wells as cytoskeletal and adhesion-related genes. This was supported by dramatic decreases in the epithelial adherens E-cadherin and β-catenin as demonstrated by IHC. Taken together, these results suggest that dietary CLA affects the mammary stromal environment, leading to tumor progression and cellular expansion in the PyMT mouse model. Further studies of the potential for cancer promotion are needed, especially because mixed-isomer CLA formulations are sold commercially as a nutritional supplement.
Introduction
Despite recent decreases in overall breast cancer mortality, breast cancer remains the leading cause of cancer-related death in younger women (1). The success of tamoxifen for the prevention of hormonally responsive breast cancer provides the first evidence that breast cancer can be approached as a preventable disease (2). With the success of tamoxifen and related hormonal therapies, the testing of low-toxicity agents with potential prevention activity for the non-hormonal breast cancers such as the estrogen receptor negative and HER-2/neu breast cancers has emerged.
Conjugated linoleic acids (CLAs) are a class of fatty acid isomers of linoleic acid formed by the action of anaerobic bacteria in the rumen of ruminant animals (3). The two most biologically active CLA isomers are the cis-9, trans-11 (c9t11) and trans-10 and cis-12 (t10c12) isomers (4). Humans are exposed to trace amounts of CLA in dairy foods and some meats (5). Once ingested, CLAs are absorbed from the digestive tract with other lipids (5). Most of the CLA is stored in adipose, particularly the mammary gland, but small amounts are found in plasma with other circulating fatty acids (6). Humans have limited ability to metabolize CLA, though vaccenic acid, another CLA isomer obtained from dairy food, can be converted to the c9t11 isomer by desaturase enzymes in the mammary gland (4). The c9t11 CLA isomer is the most prominent form in foods, representing up to 90% of the CLA present in dairy foods, whereas the t10c12 isomer represents <5% of CLA in dairy foods (7). The t10c12 isomer has gained interest, however, based on reports of its activity in regulating body composition and weight loss, primarily in rodent models, and as a result, mixed-isomer dietary supplements of approximately equal amounts of the c9t11 and t10c12 isomers are commercially available and marketed as weight loss supplements (4,5).
CLA has received considerable attention as a putative antitumor compound (8). In rodent models of mammary carcinogenesis, CLA has been shown to block carcinogen-induced initiation, inhibit tumor growth and prevent metastasis (9–12). Likewise, CLA has been shown to exhibit antiproliferative and cytotoxic activity in cell culture models (13-15). In contrast to its reported benefits against chemically induced mammary tumorigenesis, Ip et al. reported an isomer-specific effect in HER2/neu overexpressing transgenic mice (16,17) in which a diet supplemented with the t10c12 CLA isomer promoted mammary tumor growth, increased tumor incidence and enhanced lung metastasis in mouse mammary tumor virus (MMTV) neu mice.
We sought to investigate the effect of a mixed-isomer CLA formulation on mammary tumorigenesis in a model system relevant to a subgroup of breast cancers unresponsive to hormone-targeted therapies. In a pilot study, we fed virgin 4-week-old polyoma virus-middle T antigen (PyMT) mice AIN-93G diet with or without 1% CLA supplementation (N = 5 control and 6 CLA supplemented) for 4 weeks. Despite the evidence supporting an antitumor activity of CLA in models of chemically induced carcinoma, we observed an increase in tumor incidence as was reported by Ip et al. with the t10c12 isomer diet (16,17). In our model system, the tumor-promoting effect of CLA was accompanied by a dramatic loss of mammary adipose and a significant decrease in expression of adipocyte-related and cytoskeletal genes. The data presented here represent novel findings suggesting a potential mechanism of CLA's tumor-promoting action on the mammary gland postinitiation that may be particularly relevant to women with an increased risk of breast cancer including those with a family history of the disease.
Materials and methods
Animals and diets
All protocols and procedures were approved by the University of Arizona Institutional Animal Care and Use Committee. FVB/N-Tg(MMTV-PyVT)634Mul/J (PyMT) and wild-type FVB mice were obtained from Jackson Laboratories (Bay Harbor, ME) and housed two to four per cage in micro insulator rooms. PyMT males were mated with FVB females to generate a PyMT female study population. Offspring were genotyped according to Jackson Laboratory protocols (http://jaxmice.jax.org/pub-cgi/protocols/protocols). At 4 weeks of age, animals were randomized to receive isocaloric diets providing 7% of calories from fat as either all soybean oil (AIN-93G diet) or 6% soybean oil + 1% CLA as mixed isomers (N = 5 and 6, respectively). CLA diets were replenished every 2 days to limit consumption of oxidized fatty acid. Control diets were replenished weekly. Food disappearance and animal weights were recorded weekly. Animals were palpated three times a week, and tumor area (length × width) was measured by caliper. Only palpable masses with an area ≥0.5 cm2 were considered established growths. Animals were euthanized by CO2 inhalation at 8 weeks of age. All 10 mammary glands from each animal were resected for tissue histology and analysis of gene and protein expression. Hematoxylin and eosin (H & E) staining was used to classify lesions as premalignant or malignant as described below. H & E analyses of multiple lesions from each animal were pulled to determine tumor burden per animal. One thoracic gland from each animal was reserved for whole-mount fixation, as described below. Mammary glands from a subset of 12-week-old animals receiving control or CLA-supplemented diet for 9 weeks were used for fatty acid extraction and analysis by gas chromatography as described below.
AIN-93G was obtained from Harlan Teklad (Madison, WI). CLA, in mixed-isomer formulation, was obtained from NuChek Prep (Elysian, MI), and preparation of the 1% CLA-supplemented chow was done by Harlan Teklad Laboratories. Mixed-isomer composition was c9t11: 39.1%, t10c12: 40.7%, other CLA isomers: 20.2%, as verified by high-pressure liquid chromatography analysis provided by the supplier. All diets were stored at −20°C until use.
Chemicals and reagents
Antibodies against Akt, phospho-Akt (Ser 473) and c-Src, phospho-c-Src (Tyr 527), p44/42 mitogen-activated protein kinase and phospho-p44/42 were purchased from Cell Signaling (Danvers, MA). Anti-glyceraldehyde 3-phosphate dehydrogenase and E-cadherin (#IHC2123 Clone 36B5) were purchased from Chemicon International (Temecula, CA). Goat polyclonal anti-beta catenin (sc 1496 C-18) and secondary antibodies were purchased from Santa Cruz Biotechnology (St Louis, MO). Anti-Ki67 was purchased from Vision Systems (Norwell, MA). Polymerase chain reaction primers for genotyping were obtained from Qiagen (Valencia, CA).
Genotyping
DNA was obtained from mouse tail tips using Qiagen DNeasy™ protocol and amplified by polymerase chain reaction, then probed for the MMTV gene per Jackson Laboratory protocols (http://jaxmice.jax.org/pub-cgi/protocols/protocols). Polymerase chain reaction product (0.2 μg/μl) was separated by electrophoresis in 1.2% agarose gel in 0.5% Tris/Borate/EDTA and 10 μg/ml ethidium bromide and visualized by ultraviolet fluorescence.
Whole-mount fixation
Whole-mount fixation was performed on thoracic mammary glands as described previously (18). Briefly, glands were mounted onto glass slides, fixed with 1:3 glacial acetic acid:absolute ethanol and defatted in acetone. Slides were washed in 95% then 70% ethanol, stained with carmine aluminum overnight and then washed and destained and stored at room temperature in glycerol. Whole-mount images were obtained using the Leica MZFLIII dissection stereomicroscope with digital imaging (Leica Microsystems Inc., Bannockburn, IL).
Fat extraction and fatty acid analysis
In an independent analysis, mammary glands from nine 12-week-old animals receiving 1% CLA-supplemented (N = 5) or control AIN-93G (N = 4) diet for 9 weeks were analyzed by gas chromatography to estimate the tissue exposure in the 8-week-old animals. This subset analysis to compare CLA retention in the 4 week group is justified by an earlier report in rats in which CLA retention was reported to peak and plateau within 4 weeks of feeding (9). Using a modified Folch method as described in (ref. 19), total fat was extracted from the mammary tissue. Fatty acid methyl esters were prepared using a transmethylation procedure (20) and were quantified using gas chromatography (Hewlett Packard GC system 6890, Wilmington, DE) equipped with a flame ionization detector and a CP-7489 fused silica capillary column (100 m ± 0.25 mm internal diameter with 0.2 mm film thickness; Varian, Walnut Creek, CA). Peaks in the chromatogram were identified and quantified using pure methyl ester standards (GLC60, GLC68, cis-9, trans-11 and trans-10, cis-12 CLA; Nuchek Prep).
Immunohistochemistry and histology
Mouse mammary tissues were harvested, fixed in 10% neutral buffered formalin for 24 h, processed and embedded in paraffin. Routine H&E staining was performed on 3 μm sections of tissue cut from the formalin-fixed paraffin-embedded blocks. Detection of primary antibody was performed on a Discovery XT Automated Immunostainer (Ventana Medical Systems, Inc., Tucson, AZ). Deparaffinization and cell conditioning (antigen retrieval) using a borate–ethylenediaminetetraacetic acid buffer at 100°C were performed online using Ventana Medical Systems, Inc. validated reagents. Detection of primary antibody was accomplished using a biotinylated anti-Rat antibody (Vector) with streptavidin–horseradish peroxidase and diaminobenzidene. Hematoxylin counterstaining was also performed online. Following staining on the instrument, slides were dehydrated through graded alcohols to xylene and coverslipped with mounting medium (Richard Allan, #4112). Histologic images were captured on a Zeiss Axioskop light microscope and photographed with the Zeiss AxioCam digital camera. H & E and immunohistochemistry (IHC) staining were graded by a board-certified veterinary pathologist (D.G.B.). Immunostaining of E-cadherin and β-catenin was evaluated as percent of stained cells within a field as 0, <5%, 5–25%, 25–50% or >50%. Staining intensity was graded as 3, strong; 2, moderate and 1, weak.
Determination of tumor burden
Tumor burden per animal was determined by interpretations of H & E based upon a system described previously (21) and conformed to the guidelines for mouse models of mammary cancer (22). Briefly, sections were classified into one of the following categories: alveolar hyperplasia (basement membrane intact, no nuclear atypia or inflammation), adenoma/mammary intraepithelial neoplasia (basement membrane intact, minimal nuclear atypia, mild inflammation and no necrosis), early carcinoma (loss of intact basement membrane, neoplastic epithelial cell invasion of stroma and inflammation) and late carcinoma (nuclear atypia, apoptosis/necrosis, inflammation and increased mitotic index). Analyses of multiple lesions from each animal were pulled to determine tumor burden per animal using only classification of early or late carcinoma as indication of tumor.
Proliferative index
IHC for nuclear Ki67 staining was performed to determine proliferative index. The classification of either diffuse pattern (occurring throughout the section and scored as 1) or marginal pattern (occurring predominantly at the margins of the section and scored as zero) was used to interpret the proliferative index in each group. Probability of observing a grade of 1 within a group was determined with Fisher's Sign Test.
cDNA microarray
Total RNA from four control mammary glands and six CLA-treated mammary glands was hybridized to Affymetrix (Sacramento, CA) Mouse 430 2.0 GeneChips according to the manufacturer's instructions. Data analysis was performed using Bioconductor packages and R statistical tools. AffyQCReport package (http://www.bioconductor.org) was used for quality control analysis. Affymetrix cel files were read into R, and background adjustment and normalization of individual probe signals both within and between arrays were performed using the RMA method (23). A list of outlier genes that were changed between control and CLA-treated samples was determined by non-parametric rank order analysis. RankProd method in bioconductor was used to perform the analysis (24). This analysis identifies genes that are consistently ranked higher in replicate experiments. A rank product of each gene is calculated using all the experiments, and selection of upregulated and downregulated genes is based on the assigned P-value and the percentage of false positive prediction (pfp). Supplementary Table 1 (available at Carcinogenesis Online) provides the gene list of downregulated genes along with their fold change, pfp and P value. Lists were obtained using the pfp cutoff of 0.05. A pathway analysis of downregulated genes was performed using the Biorag Web site (http://www.biorag.org). All microarray data were described according to minimum information about microarray experiments guidelines. Microarray files are available through the National Center for Biotechnology Information Gene Expression Omnibus as series accession number GSE13553 (GEO, http://www.ncbi.nlm.nih.gov/geo/).
Western blot
Tissue (30–100 mg) was diced and placed in 200 μl ice-cold lysis buffer [20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.0, 150 mM NaCl, 1% Triton X-100, 2 mM ethylenediaminetetraacetic acid, pH 8.0, 2 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, pH 8.0] plus phosphatase inhibitors (2 mM sodium ortho vanadate, 50 μM ammonium molybdate, 10 mM sodium fluoride) and HALT proteinase inhibitor cocktail (Pierce, Rockford, IL, # 78415). Iced samples were sonicated for 10–15 s and incubated on ice for 30 min, centrifuged at 15 000g at 4°C for 10 min. Final supernatant was stored at −80°C.
Protein determination was performed using Pierce (St Louis, MO) micro BCA assay. Twenty-five micrograms of protein was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 8–10% acrylamide gel. 50 μg of protein was separated in 3–8% Tris-acetate precast gels (Bio-Rad, Hercules, CA). Western blots were performed according to standard protocols. Pierce Dura West Super Signal-enhanced chemiluminescence detection was used for visualization of protein bands. Blots were stripped for successive antibody probes using Pierce Restore™ stripping reagent with modifications to manufacturer's protocol. Removal of both primary and secondary antibodies was confirmed by enhanced chemiluminescence detection before reprobing. Densitometry of bands was performed using Scion Image version Alpha 4.0.3.2. Protein levels were expressed as a ratio to glyceraldehyde 3-phosphate dehydrogenase expression.
Statistics
Differences in tumor burden between groups were determined from interpretation of H & E stains, with early to late carcinoma being used as an indication of tumor. The total number of tumors per animal was pooled for a comparison between CLA-treated and untreated animals using the Student's t-test. Differences in protein densitometry were analyzed using Student's t-test. The binomial sign test was used to determine the relative probability of observing a grade of 1 for Ki67 staining. Level of significance for all tests was 0.05.
Results
Dietary CLA induces loss of mammary adipose while promoting lobular–alveolar expansion and tumor burden
To estimate tissue exposure to the dietary CLA, fatty acid analysis of mammary glands was performed in an independent group of 12-week-old animals receiving either 1% CLA-supplemented diet or AIN-93G control diet. A previous study using a rat model reported that CLA retention peak and plateau were achieved within 4 weeks of feeding (9). In the current study, average CLA concentration in the mammary glands of CLA-fed animals was 2.63% of total fatty acids (1.29% c9t11, 0.54% t10c12 and 0.89% other isomers) compared with 0.29% in the mice not exposed to dietary CLA (P > 0.005). The retention of CLA isomers in the mammary gland was in line with what has been reported in rats following a 1% dietary supplement of mixed isomer for 4 weeks (9).
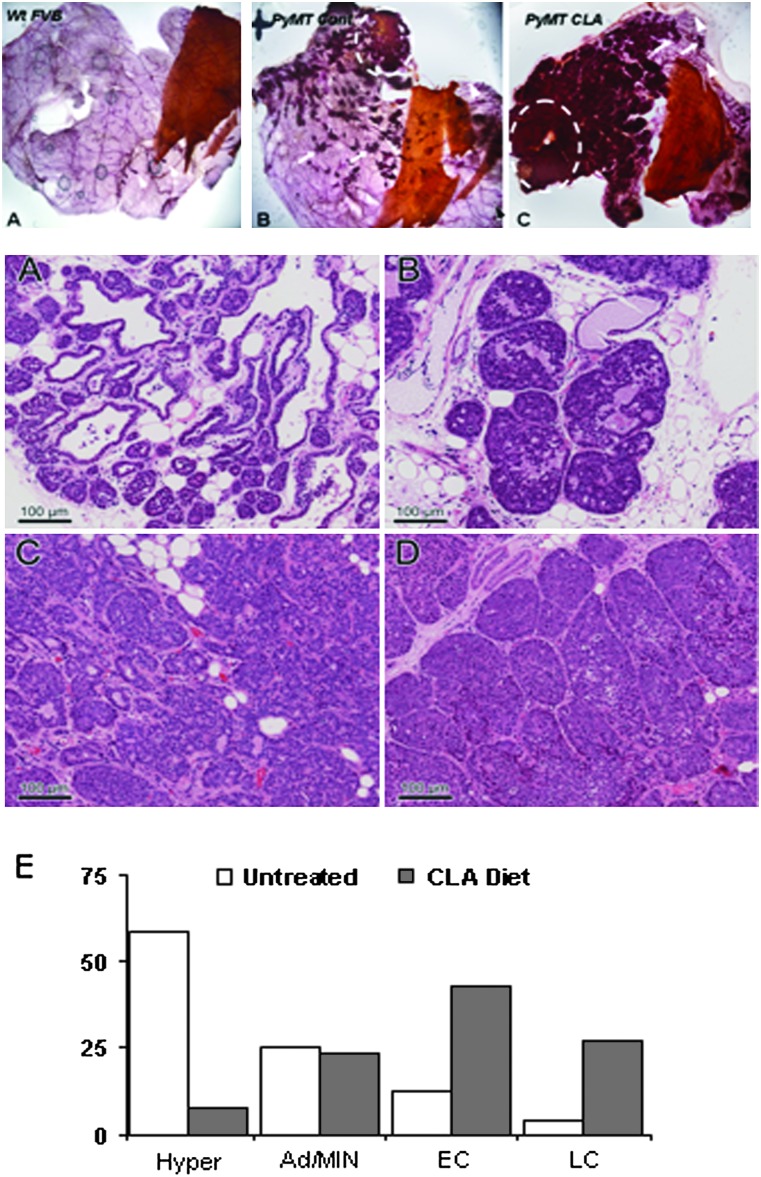
Time to tumor, as measured by palpation, did not differ between groups. However, carmine-stained mammary gland whole mounts revealed distinctive morphological differences between CLA- and control-fed animals that suggested a tumor-promoting effect of CLA (Figure 1, Top). Mammary glands of the PyMT mice in both control and CLA-fed cohorts developed normal mammary duct elongation and branching, which were secondarily expanded by progressive multifocal hyperplasia and neoplasias (Figure 1). In both cases, hyperplasias arose earliest from the proximal gland with additional foci developing progressively along the ductal tree as side branches and ductal expansions. In comparison, however, the CLA-fed animals developed a higher density of hyperplastic foci resulting from both increased numbers of foci and increase size of individual foci. The result is a much higher epithelium-to-stroma ratio, with very little residual mammary fat pad stroma in the proximal gland.
Fig. 1.
Top: alteration of mammary gland morphology by CLA (A) Wild-type nulliparrous FVB mouse at 8 weeks of age shows normal ductal architecture with nearly complete filling of the mammary stroma by the elongating ducts. Terminal end buds present and active where stromal filling is not yet complete. (B) Mammary gland from PyMT transgenic mouse maintained on control diet, nulliparrous 8 weeks of age, showing normal mammary gland development (distal) with proximal and mid gland hyperplastic and early neoplastic foci producing side buds and expansion. Highest density is seen most proximal, near the nipple (top middle). (C) Mammary gland of CLA-treated PyMT transgenic mouse, nulliparrous 8 weeks of age, showing normal to slightly increased branching of the ductal tree (right), with extensive proximal and mid gland hyperplasia and neoplastia with the highest density proximally near the middle nipple (left). Hyperplasias are larger and more numerous than those seen in the control. White arrowheads highlight terminal end buds. Solid arrows highlight early mammary intraepithelial neoplasia (MIN) lesions. Broken circles highlight advanced tumors. Thoracic mammary glands were selected for fixation and carmine staining as described in Materials and Methods. Slides are representative of general phenotypic characterizations within groups. Images were obtained using Leica MZFLIII dissection stereomicroscope with digital imaging at ×10 and optimized using Adobe Photoshop CS2 Version: 9.0.2. Bottom: (A–D) H & E-stained mammary glands from control and CLA-fed mice. (A) Hyperplastic lesion from control animal with minimal intraductal proliferation confined to the most distal aspects of the hyperbranched ducts with excess side budding. (B) Adenoma/MIN lesion from CLA-treated animal showing intraductal proliferation and ductal expansion. (C) Early carcinoma lesion from CLA-fed animal. An acinar and packeted proliferation are surrounded by a reactive and highly vascular stroma. (D) Late carcinoma lesion from CLA-fed animal. Acinar/ductule formation is lost, and the solid nodular proliferations develop central necrosis. Images were captured on a Zeiss Axioskop light microscope and photographed with the Zeiss AxioCam digital camera. (E) Quantification of tumor burden as determined by H & E interpretations. H & E stained sections from mammary gland tissue sections were used to determine tumor burden in each group. Multiple mammary sections were pooled for each animal. For each animal, a total of histologically confirmed early or late carcinomas (tumor burden) was determined and an average was calculated for each group. Statistical analysis indicated a significant increase in tumors/animal in CLA-fed versus control animals, P = 0.009. A total of 24 tissue sections from five control animals and 26 tissue sections from six CLA-fed animals were analyzed. Graph represents the percent of tissues analyzed from each treatment group in each stage of progression. Histology and staining interpretation were performed as described in Materials and Methods. Differences in tumor burden between groups were determined by Student's t-test with a significance level of 0.05.
To assess tumor burden, mammary glands from 8-week-old animals were evaluated histologically. Representative H & E stains from mammary glands of 8-week-old virgin control-fed and CLA-fed transgenic PyMT mice are presented in Figure 1 (middle A–D). Quantification of tumor burden by histological classification of multiple lesions per animal is presented in Figure 1 (graph) and Table 1. Based on the interpretation of H & E staining as described in Materials and Methods, the CLA-fed animals experienced a significantly higher number of histologically confirmed mammary tumors (indicated by early and late carcinoma) than did control animals with 3 versus 0.8 tumors per animal, respectively (Table 1). As illustrated by the graph in Figure 1, ∼70% of the sections analyzed from the CLA-treated group presented as malignancies, whereas only 15% of those analyzed in the control group had advanced to carcinoma consistent with the published progression rate of tumorigenesis in this model system (21).
Table I.
Food disappearance, tumor latency and tumor burden in CLA-fed versus control-fed mice
| Group | Food disappearance (g/day/animal) | Body weight (g) | TTT(days) | Tumor burden | P-value |
| Control (N = 5) | 2.66 ± 0.31 | 21.02 ± 0.9 | 48 ± 4.3 | 0.8 ± 0.37 | |
| CLA (N = 6) | 2.59 ± 0.88 | 22.7 ± 0.6 | 48 ± 1.0 | 3 ± 0.52 | 0.009 |
Statistics on food disappearance, body weight, tumor latency (TTT) and tumor burden. All animals were killed at 8 weeks of age. TTT: time to tumor represents time to first palpable mass measuring ≥0.5 cm2. Tumor burden represents the average number of malignant lesions per animal as determined by interpretation of H & E stain as described in Materials and Methods. Differences between groups were determined by Student's t-test with a significance level of 0.05.
Tumor promotion by CLA is independent of increased PyMT-induced signaling
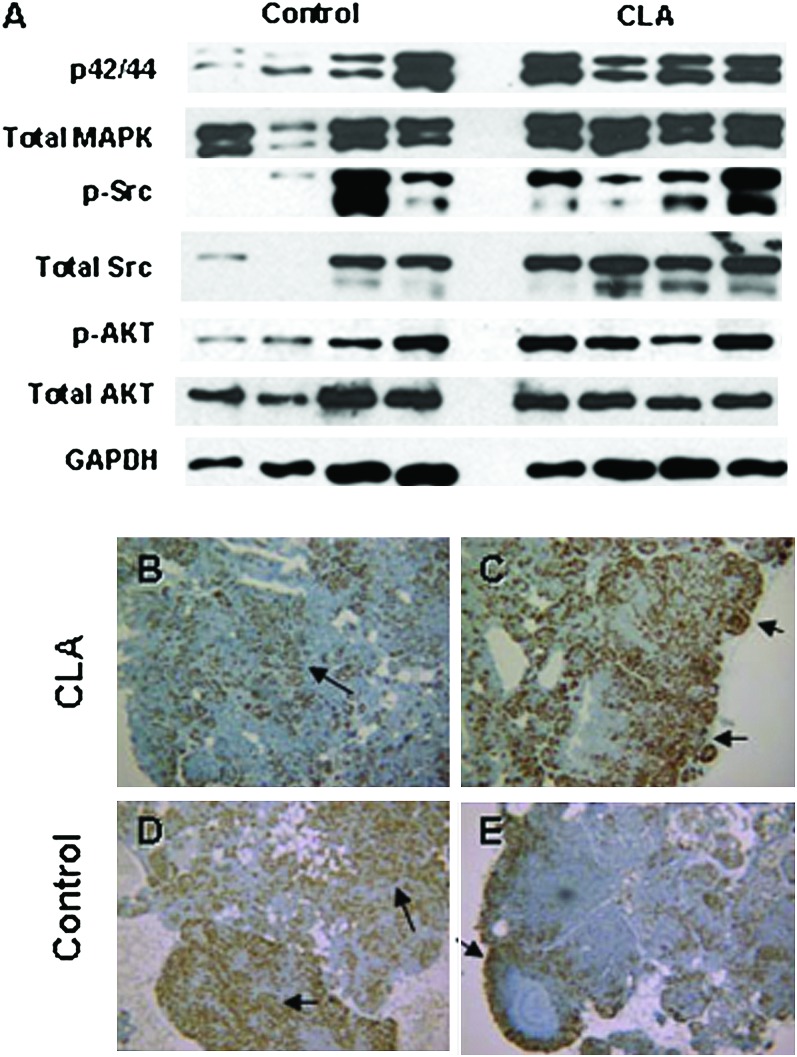
The middle T antigen is a membrane-bound protein that interacts with key mitogenic signaling molecules leading to constitutive tyrosine kinase activity and activation of c-Src, phosphoinositide 3-kinase and MAPK pathways (25–28). Protein expression of total and phosphorylated c-Src, Akt and extracellular signal-regulated kinase1/2 were examined by western blot. Though the blots suggested an activation of mitogen-activated protein kinase and c-Src signaling in the CLA-fed group, normalization to glyceraldehyde 3-phosphate dehydrogenase did not reveal any significant differences between the treated and untreated group (Figure 2).
Fig. 2.
Top: protein expression of PyMT-induced signaling factors. Protein expression of total and phosphorylated p42/44 mitogen-activated protein kinase, c-Src and Akt in control animals (lanes 1–4) and CLA-fed animals (lanes 5–8). Electrophoresis and immunoblot were performed as described in Materials and Methods. Bottom: Ki 67 staining in mammary glands from control and CLA-fed animals. Formalin-fixed paraffin-embedded mammary gland sections were immunostained for the proliferative marker, Ki67. Staining pattern was described as either marginal (predominantly on the tumor margins; B and D) or diffuse (occurring throughout the lesion; A and C), with diffuse staining being interpreted as a more aggressive phenotype. Images were selected to demonstrate that no difference in staining pattern was observed between groups. Panels A and B are from CLA-fed animals. C and D are from control animals. IHC was performed as described in Materials and Methods. Stains were graded as either 0 = marginal staining or 1 = diffuse staining. Probability of observing a grade of 1 within a group was determined with Fisher's Sign Test with a significance level of 0.05.
Though the mammary gland whole mounts in the CLA-treated group suggests increased cellularity, IHC analysis with the proliferative marker Ki67 resulted in patterns of nuclear staining that did not differ between groups. Both groups demonstrated high proliferation rates (Figure 2, Panels B–E). Failure to detect differences in Ki67 staining may be a consequence of the highly proliferative nature of the transgene in the mammary gland and suggests that the observed effect of CLA on tumor promotion was not mediated through proliferation.
Gene expression profiling reveals stromal and architectural gene shift in response to CLA diet
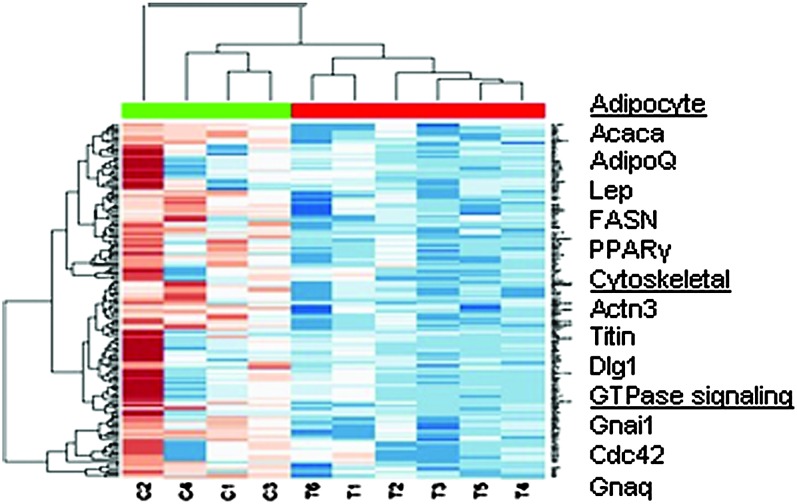
In an effort to gain a better understanding of molecular events that gave rise to the higher tumor burden in CLA-fed animals, we performed a cDNA microarray analysis on selected tissues from control and CLA-fed animals (N = 2 and 3, respectively). In the selection of RNA samples to be analyzed, the four histology stages of disease were used to identify gene changes that may have been a consequence of disease progression independent of treatment. The sample set included one hyperplastic lesion, two adenoma/mammary intraepithelial neoplasia lesions, four early carcinoma lesions and two late carcinoma lesions. Within this sample set, we did not detect a significant difference in gene expression associated with the stage of progression within a treatment group (data not shown). In contrast, comparing the control animals with the CLA treatment group identified >100 genes that were decreased by >2-fold in at least five out of the six CLA-treated samples compared with the median expression of controls. Supplementary Table 1 (available at Carcinogenesis Online) lists these downregulated genes by fold change, pfp and P-value. Lists were obtained using the pfp cutoff of 0.05. The heat map in Figure 3 illustrates the probe intensity of the genes within this group. Selected genes that were downregulated in the mammary gland of CLA-fed PyMT mice are highlighted in the margin of the figure. The downregulation of adipocyte-related genes, Fasn, Lep, AdipoQ and Pparg support a loss of adipose as observed in the mammary gland whole mounts in Figure 1.
Fig. 3.
Downregulation of genes in mammary tissue from CLA-fed mice compared with controls. Sample set includes mammary tissue from two untreated control (C1–4) and three CLA-treated (T1–6) mice. The genes represented in blue were downregulated and those in red were upregulated, with the intensity of color representing the magnitude of directional changes. The genes selected here were down at least 2-fold in five out of six CLA-treated samples. Genes listed in the margin represent the major classes of genes that were affected by CLA. Samples were selected for RNA quality and represent different stages of disease as determined by histological interpretation. The sample set included one hyperplastic lesion (C4), four adenoma/mammary intraepithelial neoplasia lesions (C2, C3, T1 and T4), three early carcinoma lesions (T2, T5 and T6) and two late carcinoma lesions (C1 and T3). Analysis by histology did not reveal differences in gene expression related to stage of disease. cDNA microarray hybridization and analysis were performed as described in Materials and Methods.
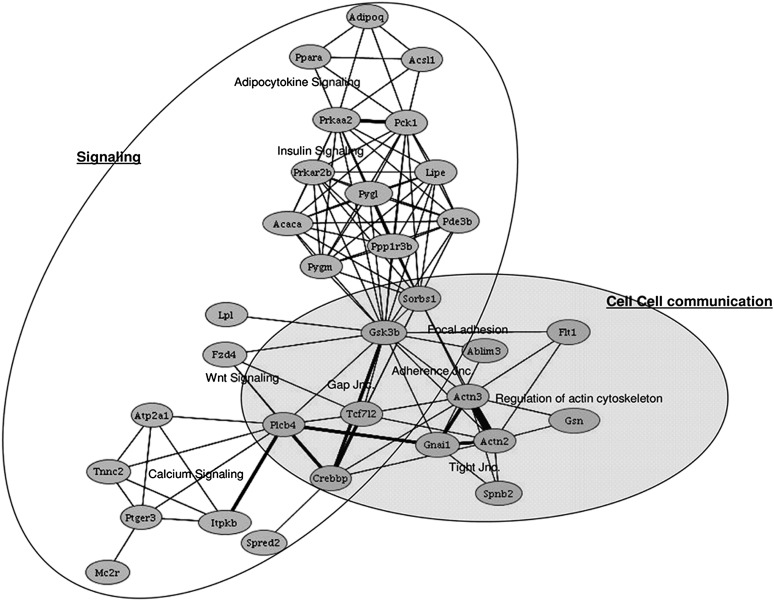
In addition to effects on what appeared to be largely adipocyte-specific genes, we observed a striking CLA-induced downregulation of a number of genes involved in cytoskeletal arrangement and tissue integrity, both structural (Actn3, Titin and Dlg1) and G-protein associated signaling pathways (Cdc42, Gnail and Gnaq). A pathway analysis presented in Figure 4 highlights the relationship between adhesion and cytoskeletal genes that were downregulated by CLA. As indicated by the bold lines, a number of these cytoskeletal genes (Figure 4 shaded area) are highly associated with Wnt and Ras signaling through membrane-bound phosphatidylinositol signaling molecules including Plcb4, Itpkb and Ptger3 (follow bold line from shaded area to calcium signaling gene cluster).
Fig. 4.
Gene pathway analysis. Pathway analysis of downregulated genes was performed using Pathway Miner software from Biorag Web site (http://www.biorag.org). Nodes represent genes that were downregulated by CLA. Lines connecting the nodes represent a relationship between the genes. Thickness of the lines reflects the number of shared pathways between the nodes. The shaded area highlights the genes involved in cytoskeletal arrangement and cell–cell communication referred to in the text. The bold lines connecting this area to Wnt and Calcium signaling emphasize the relative strength of the pathways shared by the selected groups of genes. Other targets of gene downregulation in the CLA-fed mice include genes involved in adipocyte signaling and energy metabolism, see top, both of which are known targets of CLA, particularly of the t10c12 isomer. Complementary DNA microarray hybridization and analysis were performed as described in Materials and Methods.
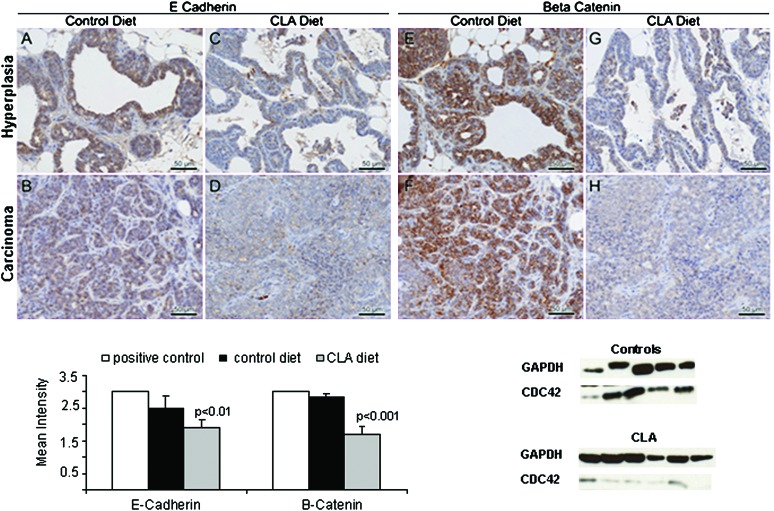
To verify the inhibition of CLA on cytoskeleton genes, we performed IHC to assess expression of β-catenin and E-cadherin in relation to CLA treatment. As shown in Figure 5 (C–D and G–H), CLA induced a global downregulation of both proteins that is apparent in both well-differentiated (C–G) and malignant lesions (D–H) with a noticeable loss of membrane staining that is replaced by diffuse cytoplasmic stain for both proteins. However, nuclear localization of β-catenin, a hallmark indication of Wnt signaling, was not observed. This may be a result of fixation artifact (antigen diffusion), which has been observed previously by one of the expert pathologists (A.D.B.) in mouse tissues fixed overnight or longer in neutral buffered formalin. Nevertheless, the cytoplasmic protein detection my IHC shows a clear quantitative difference (Figure 5 graph). For these tissue studies, scoring of staining intensity of E-cadherin and β-catenin was performed only on foci of well-differentiated cells as loss of differentiation was a prominent feature of the CLA-treated group and could represent a non-specific downregulation of both markers in advanced epithelial cancers (29-31). The treatment effect on these markers suggests that CLA may have had an early effect on tissue architecture leading to a more aggressive phenotype. Loss of Cdc42 has been associated with degradation of β-catenin and loss of E-cadherin in keratinocytes and embryoid bodies (32,33). In agreement with the IHC, Cdc42 protein was reduced by >5-fold in the CLA-treated group (Figure 5 lower right, P < 0.01).
Fig. 5.
Downregulation of adhesion proteins by CLA. Top: immunostaining for E-cadherin (A–D) and β-catenin (E–H) in control (left) and CLA-fed (right) mice. Panels A, C, E and G are representative stains in hyperplastic lesions from each group. Panels B, D, F and H are representative stains from malignant lesions from each group and are presented to demonstrate that expression of both proteins was still present in the advanced lesions in the control group. Conversely, immunostaining was considerably diminished in the CLA-fed group, even in more differentiated foci (note differences in staining intensity between B and D and between F and H.). Bar represents 50 μm. Immunostaining was performed as described in Materials and Methods. Images were taken at ×40. Values (bottom left) represent the mean intensity score for each protein by group. Staining intensity was graded as 1 (weak), 2 (moderate) or 3 (strong). Multiple lesions were graded for each animal to obtain a mean intensity value per animal that was then used to calculate an average for treated versus control groups. A total of 24 sections (from five animals) were analyzed in the control group, and 23 sections (from six animals) were analyzed from the CLA group. Positive-staining controls (not shown): E-cadherin, human prostate; β-catenin; Mouse colon. Difference in staining intensity between groups was determined by Student's t-test with a significance level of 0.05. Bottom right: western blot from mammary gland tissue lysates from 8-week-old control (N = 5) and CLA-treated (N = 6) mice. Each lane represents one randomly selected mammary gland from each animal. Isolation of total protein, gel electrophoresis and immunoblot were performed as described in Materials and Methods. Expression of Cdc42 was normalized to glyceraldehyde 3-phosphate dehydrogenase (GADPH). Difference in normalized expression between groups was determined by Student's t-test with a significance level of 0.05 and indicated a significant decrease in Cdc42 expression in CLA versus control animals (P = 0.01).
Discussion
Studies in rodent models of chemically induced mammary carcinoma have demonstrated an antitumor effect of CLA. Similarly, investigations in a variety of cell types have identified a number of mechanisms to support these observations, including regulation of apoptosis, proliferation (34) and angiogenesis (35). Candidate CLA targets include cell cycle regulators (36,37), transcription factors (38,39), nuclear and hormone receptors (40,41), as well as growth factor receptors and their effectors (42,43). This pilot study was designed to test the hypothesis that CLA would have therapeutic potential against an aggressive tumor phenotype such as hormone insensitive tumors, based on the well-documented antitumor activity of CLA. We selected a highly aggressive model system with molecular characteristics similar to HER2-positive or ER-negative breast cancer in which to test this hypothesis. In contrast to studies in models of chemically induced carcinoma, we report a tumor-promoting effect in the PyMT model system. During preparation of this manuscript Ip et al., (16,17) reported tumor promotion by CLA in a HER2 transgenic model of mammary tumorigenesis, in which a 0.5% t10c12 CLA diet increased mammary tumor burden and lung metastasis in the MMTV/neu mouse model (16). Our results corroborate a potential mammary tumor-promoting role of CLA in genetically prone mouse models.
Inconsistent effects of CLA on tumor development between in vivo chemical carcinogenesis models and genetically engineered mouse models probably reflect differences in the action of CLA along the multistep carcinogenesis continuum. Models of chemically induced carcinoma support potentially protective effects of CLA on initiation and early events in tumor promotion (9,10,44). In contrast, transgenic models studied to date reflect tumor-promoting effects of CLA in the post-initiation and progression of tumors driven by potent oncogenes relevant to human disease. The present study and those described by Ip et al., (16,17) support the use of the PyMT and MMTV/neu models as preclinical models for screening potential chemopreventive agents that are relevant to the multistage process in human disease. The histopathology of the PyMT model resembles the multistage process of human breast cancer, with four morphologically distinct stages that include premalignant hyperplasia occurring as early as 4 weeks of age through adenoma/MIN, to carcinoma in situ, and finally invasive carcinoma (21). The membrane-associated polyoma viral middle T antigen activates oncogenic signaling cascades known to be upregulated in many human cancers, including c-Src (26), Shc (45), phosphoinositide 3-kinase (27) and Erb B2 (HER2 in humans), all of which appeared to be unchanged by CLA exposure. Although additional studies with larger sample sizes are necessary to confirm these results, our findings suggest that the effect of CLA on tumor progression was probably independent of the transgene, whose effects have been reported to be well established by 4 weeks in this model (21). Similarly, Ip et al. (16) reported that t10c12 CLA increased the expression of the proliferative marker, Ki67 in the mammary gland of wild-type mice, also suggesting that its effect was, at least in part, independent of the transgene. Our dietary intervention with mixed CLA isomers was begun at 4 weeks of age, a post-initiation timepoint that corresponds to the preneoplastic-to-carcinoma in situ transition (21). Consistent with our findings, Ip et al., reported the relevance of timing and duration of CLA activity in the MMTV/neu oncogene model, identifying a tumor-promoting effect of CLA in disease progression that was reversible with removal of the isomer (16,17).
The observed effect of CLA on tumor progression in the presence of a significant CLA effect on adipose tissue (e.g. loss of mammary adipose and downregulation of adipocyte-related genes) supports the known in vivo action of CLA on adipocytes (4,46). These results suggest that mammary adipose tissue may be acting as a potential barrier against mammary tumor progression. Adipose tissue is a major component of the mammary stroma in mice; however, its role in mammary tumorigenesis is unclear. Adipocyte-related factors such as peroxisome proliferator-activated receptor gamma, adiponectin and caveolin-1 have been reported to have protective properties in the mammary gland. For example, CLA-induced peroxisome proliferator-activated receptor gamma expression in the MCF-7 cell line has been associated with an upregulation and redistribution of E-cadherin and β-catenin proteins (47), whereas adiponectin has been inversely related to breast cancer risk (48,49). Similarly, loss of caveolin-1 has been associated with tumor invasion in mice (50). Here, we observed significantly lower expression of a number of adipocyte-related genes (Fasn, Lep, AdipoQ and Pparg), suggesting that direct effects of CLA on the adipocyte fraction of the mammary gland may have removed protective factors from the microenvironment. To better understand the role of mammary adipose in tumor promotion and progression, it would be more appropriate to study the effects of CLA in a model of diet-induced obesity but that was not a goal of this study.
The downregulation of adipose and cytoskeletal/adhesion components in the presence of tumor promotion is a novel finding of our study. Although the loss of E-cadherin and β-catenin may have been an indirect effect of CLA-induced peroxisome proliferator-activated receptor gamma downregulation (47), these observations are consistent with our overall finding that the CLA-fed animals experienced increased tumor burden compared with animals on the control diet. Complete loss of E-cadherin and β-catenin has previously been associated with more advanced disease in other epithelial cancers (51).
It should be noted that in spite of a striking loss of adipose in the mammary gland of the CLA-treated group, no differences in total body weight was observed between groups (Table 1). Although CLA has been reported to reduce body fat in mice (reviewed in ref. 4), this effect has not always been associated with changes in body weight (46).
Our short-term feeding protocol of a mixed-isomer CLA diet promoted mammary tumorigenesis in the PyMT model system, with the most striking feature being the loss of mammary adipose tissue. Although our small sample size and lack of endpoint data on metastasis limit interpretation of these finding, our findings corroborate findings reported by Ip et al. in the MMTV/neu transgenic model (16,17). Unlike their report, which identified an isomer-specific effect on tumor promotion and metastasis, our mixed-isomer diet precluded the identification of the responsible isomer but supports the presence of the tumor-promoting effect of CLA in a post-initiation state with mixed isomers in the diet (16,17). The remarkable loss of mammary adipose, however, might suggest that the activity of the t10c12 isomer was predominantly responsible for the tumor-promoting effect of the mixed-isomer diet in the PyMT model. This is supported by the report by Ip et al. (16), in which the c9t11 isomer had no effect on mammary gland hyperplasia or tumorigenesis in the MMTV/neu model. Although these effects may be strain-specific as both the PyMT and the MMTV/neu transgenic models are on the FVB background, they suggest that CLA may have strikingly different effects in oncogene-driven versus chemically induced carcinogenesis. Additional in vivo studies in other transgene tumor models would be informative regarding the potential interaction of CLA with oncogene activity.
The dietary exposure of CLA used here would be physiologically obtainable with supplementation and was maintained over a relatively short time [4 weeks, representing the stage from preneoplastic to carcinoma in situ in this model (21)]. For women who carry a higher risk of breast cancer or present with an aggressive tumor phenotype, an acute exposure to CLA supplements may have adverse effects on their risk or clinical outcome, respectively. Though the potential benefit of CLA in the prevention of breast cancer and other chronic diseases cannot be overlooked, given the inconsistent findings between model systems and the pleiotropic effects of CLA, use of over-the-counter CLA supplements should be discouraged, particularly in women until additional preclinical studies are conducted.
Supplementary material
Supplementary Table 1 can be found at http://carcin.oxfordjournals.org/
Funding
Mel and Enid Zuckerman and family to the Arizona Cancer Center; Zuckerman to M.F. and D.G.B. NIH (5 P30 CA023074).
Supplementary Material
Acknowledgments
The authors wish to acknowledge Dr Margot Ip, PhD, Department of Pharmacology and Therapeutics, Roswell Park Institute, Buffalo, NY, for her generous gift of MMTV/Neu mammary tumor tissue for use as Erb B2-positive controls and for her gracious and thoughtful critical review of aspects of this manuscript. We also wish to acknowledge the Arizona Cancer Center support grant for the shared services that were utilized in the completion of this work. These include the Genomics, Bioinformatics, Biometry, Experimental Animals, and the Tissue Acquisition and Cellular/Molecular Analysis Shared Services.
Author contributions: M.F. contributed to the design of the study, performed all animal husbandry and necropsis, molecular biology, data collection and drafted the manuscript; J.A.S. contributed to the design of the study, supervised the animal husbandry, assisted in data analysis and contributed to and approved the final manuscript; A.D.B. provided pathological interpretation of the mouse tissue histology and provided a second opinion to IHC interpretations and contributed to and approved the final manuscript; C.A.T. contributed to the design of the study, assisted in data interpretation and analysis and contributed to and approved the final manuscript; R.P. performed the microarray analysis and contributed to and approved the final manuscript; D.G.B. provided interpretation of IHC; P.A.T. designed and supervised the study, analyzed and interpreted the data and contributed to, edited and approved the final manuscript. This work was conducted at the University of Arizona, Arizona Cancer Center, Tucson, AZ, and has not been published elsewhere.
Additional data files: the microarray data discussed in this publication have been deposited in National Center for Biotechnology Information Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO Series accession number GSE13553.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CLA
conjugated linoleic acid
- fasn
fatty acid synthase
- H & E
hematoxylin and eosin
- IHC
immunohistochemistry
- MMTV
mouse mammary tumor virus
- pfp
percentage false positive
- PyMT
polyoma virus-middle T antigen
References
- 1.National Cancer Institute USA. National Cancer Institute Factbook. 2008. http://www.cancer.gov/cancertopics/types/breast. [Google Scholar]
- 2.Geller BA, et al. Chemoprevention of breast cancer in postmenopausal women. Breast Dis. 2005;24:79–92. doi: 10.3233/bd-2006-24107. [DOI] [PubMed] [Google Scholar]
- 3.Ip C, et al. Conjugated linoleic acid. A powerful anticarcinogen from animal fat sources. Cancer. 1994;74:1050–1054. doi: 10.1002/1097-0142(19940801)74:3+<1050::aid-cncr2820741512>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Pariza MW, et al. The biologically active isomers of conjugated linoleic acid. Prog. Lipid Res. 2001;40:283–298. doi: 10.1016/s0163-7827(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald HB. Conjugated linoleic acid and disease prevention: a review of current knowledge. J. Am. Coll. Nutr. 2000;19:111S–118S. doi: 10.1080/07315724.2000.10718082. [DOI] [PubMed] [Google Scholar]
- 6.Iversen SA, et al. Identification of a diene conjugated component of human lipid as octadeca-9,11-dienoic acid. FEBS Lett. 1984;171:320–324. doi: 10.1016/0014-5793(84)80512-8. [DOI] [PubMed] [Google Scholar]
- 7.Parodi PW. Distribution of isomeric octadecenoic fatty acids in milk fat. J. Dairy Sci. 1976;59:1870–1873. doi: 10.3168/jds.s0022-0302(76)84455-4. [DOI] [PubMed] [Google Scholar]
- 8.Kelley NS, et al. Conjugated linoleic acid isomers and cancer. J. Nutr. 2007;137:2599–2607. doi: 10.1093/jn/137.12.2599. [DOI] [PubMed] [Google Scholar]
- 9.Ip C, et al. Retention of conjugated linoleic acid in the mammary gland is associated with tumor inhibition during the post-initiation phase of carcinogenesis. Carcinogenesis. 1997;18:755–759. doi: 10.1093/carcin/18.4.755. [DOI] [PubMed] [Google Scholar]
- 10.Ip C, et al. Conjugated linoleic acid suppresses mammary carcinogenesis and proliferative activity of the mammary gland in the rat. Cancer Res. 1994;54:1212–1215. [PubMed] [Google Scholar]
- 11.Ip C, et al. Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J. Nutr. 1999;129:2135–2142. doi: 10.1093/jn/129.12.2135. [DOI] [PubMed] [Google Scholar]
- 12.Visonneau S, et al. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997;17:969–973. [PubMed] [Google Scholar]
- 13.Maggiora M, et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int. J. Cancer. 2004;112:909–919. doi: 10.1002/ijc.20519. [DOI] [PubMed] [Google Scholar]
- 14.Kemp MQ, et al. Conjugated linoleic acid inhibits cell proliferation through a p53-dependent mechanism: effects on the expression of G1-restriction points in breast and colon cancer cells. J. Nutr. 2003;133:3670–3677. doi: 10.1093/jn/133.11.3670. [DOI] [PubMed] [Google Scholar]
- 15.Miglietta A, et al. Conjugated linoleic acid induces apoptosis in MDA-MB-231 breast cancer cells through ERK/MAPK signalling and mitochondrial pathway. Cancer Lett. 2006;234:149–157. doi: 10.1016/j.canlet.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Ip MM, et al. The t10, c12 isomer of conjugated linoleic acid stimulates mammary tumorigenesis in transgenic mice over-expressing erbB2 in the mammary epithelium. Carcinogenesis. 2007;28:1269–1276. doi: 10.1093/carcin/bgm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X, et al. t10, c12-Conjugated linoleic acid stimulates mammary tumor progression in Her2/ErbB2 mice through activation of both proliferative and survival pathways. Carcinogenesis. 2008;29:1013–1021. doi: 10.1093/carcin/bgn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pochampalli MR, et al. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res. 2007;67:6591–6598. doi: 10.1158/0008-5472.CAN-06-4518. [DOI] [PubMed] [Google Scholar]
- 19.Folch J, et al. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Sanders SR, et al. Effects of specific conjugated linoleic acid isomers on growth characteristics in obese Zucker rats. Lipids. 2004;39:537–543. doi: 10.1007/s11745-004-1260-0. [DOI] [PubMed] [Google Scholar]
- 21.Lin EY, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am. J. Pathol. 2003;163:2113–2126. doi: 10.1016/S0002-9440(10)63568-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardiff RD, et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–988. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Breitling R, et al. Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett. 2004;573:83–92. doi: 10.1016/j.febslet.2004.07.055. [DOI] [PubMed] [Google Scholar]
- 25.Courtneidge SA, et al. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 26.Guy CT, et al. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 1994;8:23–32. doi: 10.1101/gad.8.1.23. [DOI] [PubMed] [Google Scholar]
- 27.Webster MA, et al. Requirement for both Shc and phosphatidylinositol 3′ kinase signaling pathways in polyomavirus middle T-mediated mammary tumorigenesis. Mol. Cell. Biol. 1998;18:2344–2359. doi: 10.1128/mcb.18.4.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuevas BD, et al. MEKK1 controls matrix degradation and tumor cell dissemination during metastasis of polyoma middle-T driven mammary cancer. Oncogene. 2006;25:4998–5010. doi: 10.1038/sj.onc.1209507. [DOI] [PubMed] [Google Scholar]
- 29.Oka H, et al. Immunohistochemical evaluation of E-cadherin adhesion molecule expression in human gastric cancer. Virchows Arch. A Pathol. Anat. Histopathol. 1992;421:149–156. doi: 10.1007/BF01607048. [DOI] [PubMed] [Google Scholar]
- 30.Murant SJ, et al. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur. J. Cancer. 1997;33:263–271. doi: 10.1016/s0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- 31.Krishnadath KK, et al. Reduced expression of the cadherin-catenin complex in oesophageal adenocarcinoma correlates with poor prognosis. J. Pathol. 1997;182:331–338. doi: 10.1002/(SICI)1096-9896(199707)182:3<331::AID-PATH860>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, et al. Cdc42 is crucial for the establishment of epithelial polarity during early mammalian development. Dev. Dyn. 2007;236:2767–2778. doi: 10.1002/dvdy.21309. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, et al. Cdc42 controls progenitor cell differentiation and beta-catenin turnover in skin. Genes Dev. 2006;20:571–585. doi: 10.1101/gad.361406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ou L, et al. Conjugated linoleic acid induces apoptosis of murine mammary tumor cells via Bcl-2 loss. Biochem. Biophys. Res. Commun. 2007;356:1044–1049. doi: 10.1016/j.bbrc.2007.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masso-Welch PA, et al. Inhibition of angiogenesis by the cancer chemopreventive agent conjugated linoleic acid. Cancer Res. 2002;62:4383–4389. [PubMed] [Google Scholar]
- 36.Lim DY, et al. Inhibition of colon cancer cell proliferation by the dietary compound conjugated linoleic acid is mediated by the CDK inhibitor p21CIP1/WAF1. J. Cell. Physiol. 2005;205:107–113. doi: 10.1002/jcp.20380. [DOI] [PubMed] [Google Scholar]
- 37.Kim EJ, et al. trans-10, cis-12 conjugated linoleic acid inhibits the G1-S cell cycle progression in DU145 human prostate carcinoma cells. J. Med. Food. 2006;9:293–299. doi: 10.1089/jmf.2006.9.293. [DOI] [PubMed] [Google Scholar]
- 38.Degner SC, et al. Conjugated linoleic acid attenuates cyclooxygenase-2 transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast cancer cells. J. Nutr. 2006;136:421–427. doi: 10.1093/jn/136.2.421. [DOI] [PubMed] [Google Scholar]
- 39.Lee SH, et al. Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis. 2006;27:972–981. doi: 10.1093/carcin/bgi268. [DOI] [PubMed] [Google Scholar]
- 40.Moya-Camarena SY, et al. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J. Lipid Res. 1999;40:1426–1433. [PubMed] [Google Scholar]
- 41.Liu J, et al. Anti-estrogenic effects of conjugated linoleic acid through modulation of estrogen receptor phosphorylation. Breast Cancer Res. Treat. 2005;94:161–169. doi: 10.1007/s10549-005-6942-4. [DOI] [PubMed] [Google Scholar]
- 42.Miglietta A, et al. Conjugated linoleic acid induces apoptosis in MDA-MB-231 breast cancer cells through ERK/MAPK signalling and mitochondrial pathway. Cancer Lett. 2006;234:149–157. doi: 10.1016/j.canlet.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Cho HJ, et al. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G996–G1005. doi: 10.1152/ajpgi.00347.2002. [DOI] [PubMed] [Google Scholar]
- 44.Ip C, et al. Mammary cancer prevention by conjugated dienoic derivative of linoleic acid. Cancer Res. 1991;51:6118–6124. [PubMed] [Google Scholar]
- 45.Davol PA, et al. Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer. Cancer Res. 2003;63:6772–6783. [PubMed] [Google Scholar]
- 46.Park Y, et al. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- 47.Bocca C, et al. Involvement of PPAR gamma and E-cadherin/beta-catenin pathway in the antiproliferative effect of conjugated linoleic acid in MCF-7 cells. Int. J. Cancer. 2007;121:248–256. doi: 10.1002/ijc.22646. [DOI] [PubMed] [Google Scholar]
- 48.Korner A, et al. Total and high-molecular-weight adiponectin in breast cancer: in vitro and in vivo studies. J. Clin. Endocrinol. Metab. 2007;92:1041–1048. doi: 10.1210/jc.2006-1858. [DOI] [PubMed] [Google Scholar]
- 49.Mantzoros C, et al. Adiponectin and breast cancer risk. J. Clin. Endocrinol. Metab. 2004;89:1102–1107. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 50.Sotgia F, et al. Caveolin-1 deficiency (-/-) conveys premalignant alterations in mammary epithelia, with abnormal lumen formation, growth factor independence, and cell invasiveness. Am. J. Pathol. 2006;168:292–309. doi: 10.2353/ajpath.2006.050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aresu L, et al. Dog as model for down-expression of E-cadherin and β-catenin in tubular epithelial cells in renal fibrosis. Virchows Arch. 2008;453:617–625. doi: 10.1007/s00428-008-0684-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.