Abstract
The omega-3 fatty acid ethanolamides, docosahexaenoyl ethanolamide (DHEA) and eicosapentaenoyl ethanolamide (EPEA), displayed greater anti-proliferative potency than their parent omega-3 fatty acids, docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), in LNCaP and PC3 prostate cancer cells. DHEA and EPEA activated cannabinoid CB1 and CB2 receptors in vitro with significant potency, suggesting that they are endocannabinoids. Both LNCaP and PC3 cells expressed CB1 and CB2 receptors, and the CB1- and CB2-selective antagonists, AM281 and AM630, administered separately or together, reduced the anti-proliferative potencies of EPEA and EPA but not of DHEA or DHA in PC3 cells and of EPA but not of EPEA, DHEA or DHA in LNCaP cells. Even so, EPEA and EPA may not have inhibited PC3 or LNCaP cell proliferation via cannabinoid receptors since the anti-proliferative potency of EPEA was well below the potency it displayed as a CB1 or CB2 receptor agonist. Indeed, these receptors may mediate a protective effect because the anti-proliferative potency of DHEA in LNCaP and PC3 cells was increased by separate or combined administration of AM281 and AM630. The anandamide-metabolizing enzyme, fatty acid amide hydrolase (FAAH), was highly expressed in LNCaP but not PC3 cells. Evidence was obtained that FAAH metabolizes EPEA and DHEA and that the anti-proliferative potencies of these ethanolamides in LNCaP cells can be enhanced by inhibiting this enzyme. Our findings suggest that the expression of cannabinoid receptors and of FAAH in some tumour cells could well influence the effectiveness of DHA and EPA or their ethanolamide derivatives as anticancer agents.
Introduction
Our group, and others, have shown that the omega-3 long chain polyunsaturated fatty acids, docosahexaenoic acid [DHA; 22:6 (n-3)] and eicosapentaenoic acid [EPA; 20:5 (n-3)], elicit anti-proliferative anticancer effects both in cancer lines in vitro and in animals in vivo (1,2). There is also evidence that dietary omega-3 and omega-6 fatty acids can be converted to their ethanolamide derivatives in situ. Furthermore, the polyunsaturated omega-6 fatty acid ethanolamide, arachidonoyl ethanolamide (anandamide), is an endocannabinoid since it can activate cannabinoid CB1 and CB2 receptors when synthesized and released endogenously, as well as when administered exogenously (3,4). Endocannabinoids can have anticancer effects because there is evidence that anandamide can inhibit human breast cancer and prostate cancer cell proliferation (5,6).
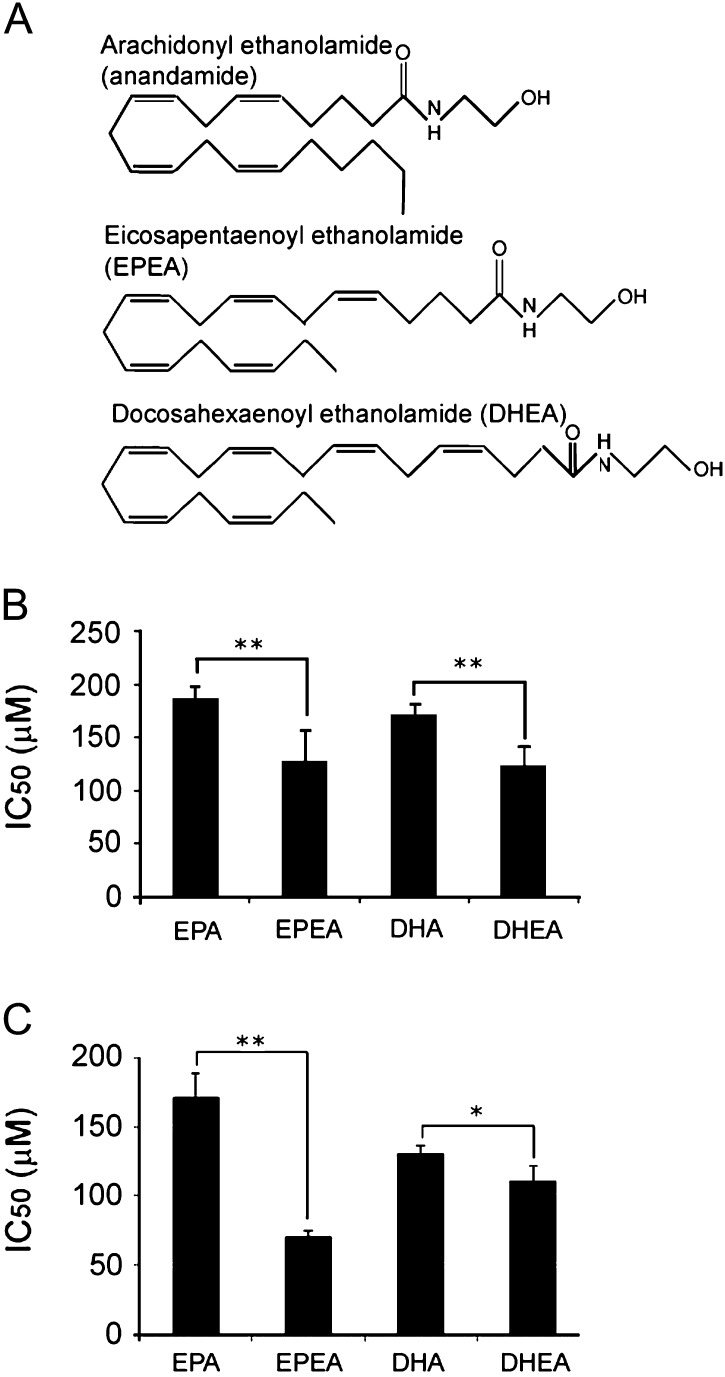
In this investigation, we explored the possibility that the anticancer effects of DHA and EPA depend, at least in part, on their conversion to their ethanolamides: DHA to docosahexaenoyl ethanolamide (DHEA) and EPA to eicosapentaenoyl ethanolamide (EPEA). The difference in the structures of EPEA, DHEA and anandamide can be seen in Figure 1A. Because there is evidence that anandamide acts through CB1 receptors to inhibit the proliferation of human breast and prostate cancer cells, we tested the hypothesis that DHA, EPA, DHEA and/or EPEA produce their anticancer effects by activating cannabinoid receptors in these cells (5,6). There is evidence that anandamide can be catabolized by fatty acid amide hydrolase (FAAH) and that inhibition of this enzyme can potentiate some effects of anandamide (7,8). Accordingly, we explored the possibility that the anticancer effects of DHA and EPA and/or of their ethanolamides can be potentiated by inhibiting this enzyme.
Fig. 1.
Structure of EPA and DHA ethanolamides and their effects on cell viability. (A) Chemical structure of EPEA and DHEA compared with anandamide. (B) IC50 values as determined from MTT assays in LNCaP and (C) PC3 cells treated with either fatty acids (EPA and DHA) or ethanolamides (EPEA or DHEA) for 24 h. Error bars represent standard error of mean. *P ≤ 0.05, **P ≤ 0.01 (n = 3).
Our experiments were performed in vitro, mainly with the human prostate cancer cell lines, LNCaP (androgen sensitive) and PC3 (androgen insensitive). To investigate whether DHEA or EPEA are cannabinoid CB1 or CB2 receptor agonists, we performed receptor-binding experiments with mouse whole-brain membranes and with membranes obtained from Chinese hamster ovary (CHO) cells that had been stably transfected with the human CB2 receptor.
Materials and methods
Cell lines
CHO, stably transfected with complementary DNA encoding human cannabinoid CB2 receptors, were cultured in Eagle’s medium nutrient mixture F-12 Ham supplemented with 1 mM L-glutamine, 10% (vol:vol) foetal bovine serum and 0.6% penicillin–streptomycin together with G418 (600 μg/ml). Human prostate cancer cell lines, LNCaP (androgen sensitive) and PC3 (androgen insensitive), were obtained from the European Collection of Animal Cell Cultures. These were cultured in RPMI 1640 medium (Sigma chemical, Dorset, UK) containing 10% (vol:vol) foetal bovine serum and 1% (vol:vol) Penicillin–Streptomycin Solution (10 000 U/ml penicillin and 10 mg/ml streptomycin in 0.9% sodium chloride; Sigma–Aldrich, Dorset, UK).
Animals
All animal care and experimental procedures complied with the UK Animals (Scientific Procedures) Act, 1986 and associated guidelines for the use of experimental animals. MF1 mice aged 6–7 weeks and weighing 30–35 g were purchased from Harlan UK Ltd (Blackthorn, UK). Mice were maintained on a 12/12 h light–dark cycle with free access to food and water. All experiments were performed with tissues obtained from adult male mice.
Biochemical reagents
DHA [22:6 (n-3)] and EPA [20:5 (n-3)] were purchased from Sigma–Aldrich. EPEA and DHEA were synthesized in Professor Mechoulam’s laboratory (9). Fatty acids and ethanolamides were each dissolved in 100% ethanol and FAAH inhibitor, JNJ1661010 (Tocris Bioscience, Bristol, UK), and the CB1-selective antagonist, AM281 (Insight Biotechnology, Middlesex, UK), were dissolved in dimethyl sulfoxide (DMSO) and all stored at 100 mM stock solutions, −80°C under nitrogen. The CB2-selective antagonist, AM630 (Tocris Bioscience), was stored in DMSO as a 10 mM solution. Appropriate concentrations were freshly prepared from stock solution using culture medium. DMSO and ethanol diluent controls were also in. For binding experiments, [3H]CP-55940 (160 Ci/mmol) and [35S] guanosine 5′-O-[gamma-thio]triphosphate (GTPγS) (1250 Ci/mmol) were obtained from PerkinElmer Life Sciences (Boston, MA), GTPγS and adenosine deaminase from Roche Diagnostic (Indianapolis, IN) and guanosine diphosphate (GDP) and phenylmethylsulfonyl fluoride (PMSF) from Sigma–Aldrich.
Cell viability assay
A standard 3-(4,-5-dimethylthiazol-2-yl)-2,-5-diphenyl tetrazolium bromide (MTT) dye reduction assay was used to assess the cytotoxicity of the respective compounds. Briefly, cells were plated in a flat-bottomed 96-well plate at seeding densities of 6 × 103 cells per well for LNCaP cells and 5 × 103 cells per well for PC3 cells. Cells were treated the following day with appropriate agents for 24 h. Following the incubation period, the MTT solution (5 mg/ml in phosphate-buffered saline) was added and incubated for 4 h. The contents of the wells were removed and replaced with 200 μl DMSO to dissolve the MTT formazan crystals. The plates were immediately read at 570 nM in a multiwell plate reader (DynaTech MR5000; Dynex Technologies Ltd, Worthing, UK).
Membrane preparation
Binding assays with [3H]CP-55940 and with [35S]GTPγS were performed with mouse whole brain membranes, prepared as described previously, or with CHO-hCB2 cell membranes (10,11). The hCB2-transfected cells were removed from flasks by scraping and then frozen as a pellet at −20°C until required (12). Before use in a radioligand-binding assay, cells were defrosted, diluted in Tris-buffer (50 mM Tris–HCl, 50 mM Tris-Base) and homogenized. Protein assays were performed using a Bio-Rad Dc kit (Hercules, CA).
[35S]GTPγS-binding assays
Measurement of agonist-stimulated [35S]GTPγS binding to cannabinoid CB1 receptors was adapted from methods described previously (12,13) The assays were carried out with GTPγS-binding buffer (50 mM Tris–HCl, 50 mM Tris-Base, 5 mM MgCl2, 1 mM ethylenediaminetetraacetic acid, 100 mM NaCl, 1 mM dithiothreitol and 0.1% bovine serum albumin) in the presence of [35S]GTPγS and GDP, in a final volume of 500 μl. Binding was initiated by the addition of [35S]GTPγS to the wells. Non-specific binding was measured in the presence of 30 μM GTPγS. The drugs were incubated in the assay for 60 min at 30°C. The reaction was terminated by a rapid vacuum filtration method using Tris-binding buffer, as described previously, and the radioactivity was quantified by liquid scintillation spectrometry. In all the [35S]GTPγS-binding assays, we used 0.1 nM [35S]GTPγS, 30 μM GDP and a protein concentration of 5 μg per well for mouse brain membranes and 33 μg per well for CHO-hCB2 cell membranes. Additionally, mouse brain membranes were pre-incubated for 30 min at 30°C with 0.5 U/ml adenosine deaminase (200 U/ml) to remove endogenous adenosine. Compounds were stored at −20°C in oil.
Protein analysis
Cells were homogenized in lysis buffer [20 mM Tris, 0.25 M sucrose, 10 mM ethyleneglycol-bis(aminoethylether)-tetraacetic acid, 2 mM ethylenediaminetetraacetic acid, 1 mM sodium orthovanadate, 25 mM sodium β-glycerophosphate and 50 mM sodium fluoride; pH 7.5]. Prior to use, 0.1% (vol:vol) Protease Inhibitor Cocktail (Sigma-Aldrich) was added. A total of 20 μg of protein was electrophoresed through a precast 16% polyacrylamide gel (Invitrogen, Paisley, UK) for 2 h and the separated proteins were transferred to nitrocellulose membranes (Bio-Rad, Hertfordshire, UK), then blocked with 5% (wt:vol) skimmed milk in Tris-buffered saline with 0.1% (vol:vol) Tween 20 (TBST solution) at room temperature and incubated with 1:200 dilution of anti-FAAH antibody, 1:100 CB1 antibody (Calbiochem, Nottingham, UK) or 1:200 CB2 antibody (Insight Biotechnology) at 4°C overnight. β-Actin (1:20 000) was used as an internal loading control to normalize between lanes during densitometry. Appropriate secondary antibody for FAAH (anti-mouse) and CB1 and CB2 (anti-goat) were used at a concentration of 1:5000 (Insight Biotechnology) and incubated at room temperature for 1 h. Proteins were visualized using ECL + plus™ chemiluminescent detection kit (Amersham Pharmacia, Buckinghamshire, UK), according to manufacturer’s instructions and a Fluor S phosphorimager (Bio-Rad). The experiments were performed with proteins isolated from three independent extractions.
Flow cytometry analysis of the cell cycle
Effects of the fatty acid and ethanolamide treatments on the cell cycle were determined using the propidium iodide (PI) staining technique. Briefly, cells were grown in six-well plates at a density of 6 × 104 cells per well and treated with appropriate concentrations of reagents (IC50 values) for 24 h. Cells were collected by trypsinization, including the floating cells and fixed in ice-cold absolute alcohol and stained with PI for 30 min (50 μg/ml PI, 50 μg/ml ribonuclease A and 0.1% vol/vol Triton X-100 in phosphate-buffered saline). The percentage of cells in G1, S, G2, M and sub G1 phases was assessed by flow cytometry using FACSCalibur flow cytometer and analysed by Flowjo software (TreeStar, Oregon, OR).
Flow cytometry analysis of apoptosis by Annexin V staining
Cells were seeded and treated as for cell cycle analysis and harvested by brief trypsinization and washed in cold phosphate-buffered saline and then twice in Annexin-Binding Buffer (Beckton Dickinson, Oxford, UK). Cells were resuspended in 100 μl of binding buffer, and 5 μl fluorescein isothiocyanate-Annexin V solution (Beckton Dickinson) was added and cells were incubated for 15 min in the dark at room temperature. A further 400 μl of binding buffer was added prior to analysis, followed by 2 μl of 1 mg/ml solution of PI, where appropriate. A total of 10 000 cells were counted and analysed by Flowjo software (Teestar Inc). Jurkat cells treated with 6 μM camptothecin (Sigma–Aldrich) were used as a positive control of apoptosis to set the compensation and voltages.
Data analysis
Values have been expressed as means and variability as standard error of the mean or as 95% confidence limits. The concentration of the compounds under investigation that produced a 50% displacement of radioligand from specific binding sites (IC50 values) was calculated using GraphPad Prism and the corresponding Ki values were calculated using the equation of Cheng and Prusoff (14). Net agonist-stimulated [35S]GTPγS-binding values were calculated by subtracting basal binding values (obtained in the absence of agonist) from agonist-stimulated values (obtained in the presence of agonist) as detailed elsewhere (10). Values for half of the maximal effective concentration required to elicit a response (EC50), maximal effect (Emax) and standard error of the mean or 95% confidence limits of these values have been calculated by non-linear regression analysis using the equation for a sigmoid concentration–response curve (GraphPad Prism). Unpaired Student’s t-test or one-way analysis of variance (with Tukeys post-hoc analysis) was used where appropriate for MTT data and flow cytometry data. A value of P < 0.05 was taken as being significant.
Results
The ethanolamides of EPA and DHA induce cell death in LNCaP and PC3 cells
EPEA was more potent than EPA in inducing cell death in both LNCaP (P < 0.01) and PC3 cells (P < 0.01). Similarly, DHEA was more potent than DHA in both LNCaP (P < 0.01) and PC3 cells (P < 0.05, Figure 1B and C). Treatment of LNCaP cells with EPA or EPEA did not result in any significant changes in the G1, S or G2 phases of the cell cycle (Table I). However, DHA did induce a significant decrease in both G2 (P < 0.05) and S phases (P < 0.05), whereas DHEA caused a significant decrease in the G1 phase compared with untreated cells (P < 0.05) and also compared with DHA (P < 0.05). Treatment of PC3 cells with EPA resulted in a significant increase in G1 phase cells (P < 0.01), and a significant decrease in S phase (P < 0.01), when compared with untreated cells (Table I). EPEA treatment did not significantly alter the PC3 cell cycle in any phase but did induce a significant decrease in G1 when compared with its parent EPA counterpart (P < 0.01), (see supplementary Figure 1A, available at Carcinogenesis Online). DHA elicited a significant decrease in G2 phase PC3 cells (P < 0.01) but DHEA showed an increase in G2 compared with untreated cells, although this was of borderline significance (P = 0.057). However, DHEA did produce a significant increase in G2 in PC3 cells when compared with DHA (P < 0.01).
Table I.
Effects of ethanolamides on cell cycle and apoptosis
| Cell line | Untreated cells | EPA IC50 | EPEA IC50 | DHA IC50 | DHEA IC50 |
| LNCaP | |||||
| Cell cycle | |||||
| G1 | 76.13 ± 4.15 | 72.42 ± 1.57 | 78.84 ± 2.03 | 76.65 ± 3.89 | 66.83 ± 0.77*a/**a |
| S | 12.55 ± 2.63 | 10.40 ± 0.914 | 9.86 ± 1.74 | 7.45 ± 1.53*a | 9.63 ± 1.88 |
| G2 | 12.52 ± 1.67 | 9.57 ± 0.88 | 10.79 ± 1.17 | 8.21 ± 0.90*a | 10.07 ± 1.23 |
| PC3 | |||||
| Cell cycle | |||||
| G1 | 41.67 ± 4.61 | 62.26 ± 2.37*b | 45.18 ± 4.31 | 52.04 ± 6.67 | 43.49 ± 1.27 |
| S | 21.69 ± 5.02 | 6.12 ± 1.45*b | 16.69 ± 3.48 | 19.88 ± 7.48 | 13.65 ± 4.51 |
| G2 | 35.04 ± 0.55 | 28.75 ± 2.03 | 36.45 ± 7.40 | 27.73 ± 0.98*b | 41.81 ± 4.54**b |
| LNCaP | |||||
| Apoptosis | |||||
| Early | 10.94 ± 2.43 | 15.30 ± 3.66 | 16.19 ± 5.21 | 19.76 ± 3.71*a | 43.81 ± 4.70*b |
| Late | 14.94 ± 3.31 | 19.44 ± 10.99 | 17.01 ± 5.48 | 18.52 ± 5.86 | 29.59 ± 10.94 |
| PC3 | |||||
| Apoptosis | |||||
| Early | 9.47 ± 2.40 | 20.09 ± 10.57 | 31.63 ± 7.10*a | 20.41 ± 17.01 | 28.75 ± 0.10*a |
| Late | 5.84 ± 0.41 | 10.08 ± 6.44 | 27.52 ± 13.33 | 8.79 ± 4.41 | 24.36 ± 10.31*a |
*aP < 0.05, *bP < 0.01 against untreated cells, **aP < 0.05, **bP < 0.01 between fatty acid and corresponding ethanolamide. Cells treated with IC50 concentrations (see Figure 1) of each compound for 24 h. All experiments repeated three times.
Treatment of LNCaP cells with EPA or EPEA at IC50 concentrations did not increase early or late apoptosis significantly compared with untreated cells (Table I). However, treatment with either DHA or DHEA led to significantly higher levels of LNCaP cells in early apoptosis (P < 0.05 and P < 0.01, respectively), when compared with untreated cells (supplementary Figure 1B is available at Carcinogenesis Online). DHEA also induced significantly higher apoptosis scores than DHA (P < 0.05) in LNCaP cells, but not late apoptosis. In PC3 cells, neither DHA nor EPA induced significant increases in early or late apoptosis compared with untreated cells (Table I). Both EPEA and DHEA, however, did elicit significant increases in early apoptosis in PC3 cells compared with untreated cells (P < 0.01) and both caused significantly more late-stage apoptosis than in untreated cells (P < 0.05). Treatment of PC3 cells with DHEA showed a trend towards more apoptosis than with DHA, although this was not statistically significant.
Cannabinoid CB1 and CB2 receptors are expressed in LNCaP and PC3 cells
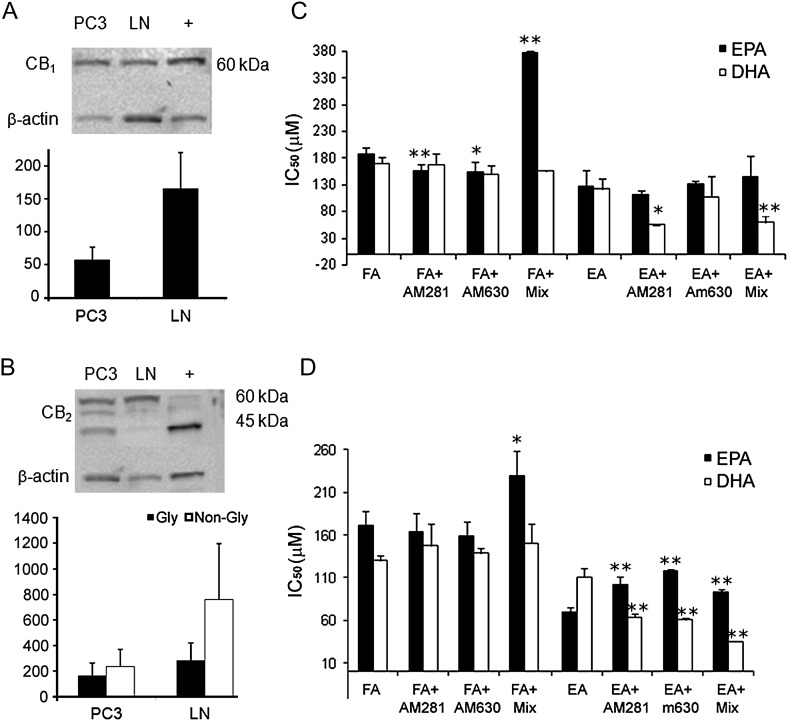
Western blotting was used to determine the relative expression of CB1 and CB2 receptors in the two cell lines. The results showed a considerable difference in the relative receptor distribution/density and form between the two cell lines. CB1 protein was expressed in both cell lines but the receptor was expressed more highly in LNCaP cells than in PC3 cells, reaching a borderline significant difference (P = 0.057; Figure 2A). CB2 protein was also expressed in both cell lines, but the antibody detected two prominent bands of different molecular weight, considered to reflect the presence of two different glycosylation states of the protein (15). In PC3 cells, the native non-glycosylated form was present in similar amounts as the glycosylated form (Figure 2B). However, in LNCaP cells, there was lower expression of the non-glycosylated form and higher expression of the glycosylated form.
Fig. 2.
(A and B) Cannabinoid receptor expression. A graphical representation of expression as a percentage relative to β-actin internal control and also a representative example of western blotting. (A) CB1 receptor protein expression in PC3 (PC) and LNCaP (LN) cells. β-Actin used as loading control and positive control is rat cerebellum lysate (+). (B) CB2 expression in PC3 (PC) and LNCaP (LN) cells. β-Actin used as loading control and positive control is HL-60 cells (+). Error bars represent standard error of mean (n = 3). (C and D) Effect of inhibiting cannabinoid receptors in (C) LNCaP cells and (D) PC3 cells. IC50 values as determined from MTT assays. (AM281, a CB1-selective antagonist; AM630, a CB2-selective antagonist; Mix, mixture of both antagonists; FA, fatty acid; EA, ethanolamide). AM251 and AM630 were each administered at a concentration of 1 μM. Error bars represent standard error of mean. *P ≤ 0.05, **P ≤ 0.01 comparing cells treated with a FA or EA alone and cells also treated with AM281 or AM630 or with both AM281 and AM630 (Mix), (n = 3–5).
Effects of cannabinoid receptor antagonists on the ability of DHA, EPA and their ethanolamides to inhibit proliferation of PC3 and LNCaP cells
In LNCaP cells (Figure 2C), combined but not separate administration of the CB1-selective antagonist, AM281, and CB2-selective antagonist, AM630, significantly reduced the anti-proliferative effect of EPA, whereas individual antagonists increased the effect, but there was no effect on EPEA. DHEA was, however, potentiated by AM281 when this was administered alone or together with AM630, but there was no significant effect on DHA.
In PC3 cells (Figure 2D), administration of AM281 and AM630, either separately or together, produced significant inhibition of the anti-proliferative effect of EPEA. Combined administration of these two antagonists also significantly reduced the anti-proliferative effect of EPA in this cell line, whereas AM281 or AM630 alone did not affect the potency of this omega-3 fatty acid. In contrast, separate or combined administration of AM281 and AM630 to PC3 cells did not significantly affect the anti-proliferative potency of DHA and actually potentiated that of DHEA.
DHEA and EPEA are cannabinoid CB1 and CB2 receptor agonists
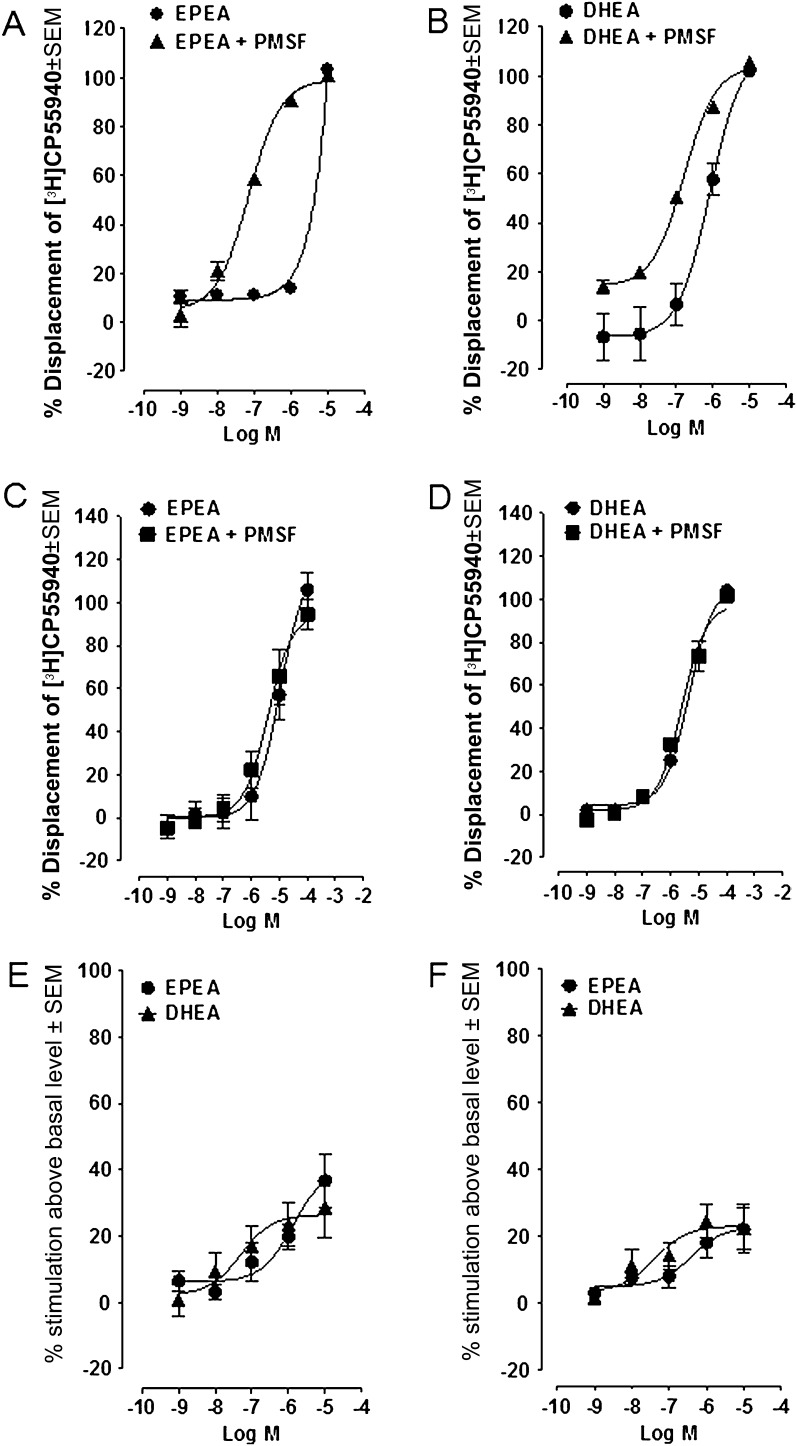
DHEA and EPEA each produced a concentration-related displacement of [3H]CP55940 from specific binding sites in mouse brain and CHO-hCB2 cell membranes (Figure 3A and B, respectively). The apparent mean Ki values of both compounds are shown in Table II. The two ethanolamides displaced [3H]CP55940 from brain membranes much more potently in the presence of the non-selective protease inhibitor, PMSF, than in its absence. This finding suggests that DHEA and EPEA may be susceptible to metabolism in these membranes, most probably by FAAH, the enzyme mainly responsible for the metabolism of the endocannabinoid, anandamide (see Introduction). In contrast, in CHO-hCB2 cell membranes, PMSF had very little (EPEA) or no statistically significant enhancing effect (DHEA), probably reflecting a lower expression of FAAH in CHO-hCB2 (Figure 3C and D) cells than mouse brain membranes (Figure 3A and B). The binding data we obtained also suggest that DHEA and EPEA bind to cannabinoid CB1 receptors with higher affinity than to CB2 receptors and that each of these compounds can fully displace [3H]CP55940 from both these receptors.
Fig. 3.
Functional activity of ethanolamides. (A and D) Displacement of [3H]CP55940 by (A) EPEA and (B) DHEA from specific binding sites on MF1 mouse whole brain membranes (n = 4) and displacement of [3H]CP55940 by (C) EPEA and (D) DHEA from specific binding sites on CHO-hCB2 cell membranes (n = 8). Each experiment has been performed in the absence and presence of 100 μM PMSF. Each symbol represents the mean percent displacement ± standard error of the mean. Mean Ki values with 95% confidence limits shown in brackets are shown in Table II. (E and F) Mean log concentration–response curves of EPEA and DHEA. Each symbol represents the mean percentage change in binding of [35S]GTPγS to (E) MF1 mouse brain and (F) CHO-hCB2 cell (B) membranes ± standard error of the mean. Mean EC50 and Emax values are shown in Table II.
Table II.
[3H]CP55940 and [35S]GTP?S-binding assays
| Compound | CB1a |
CB1a |
CB2b |
CB2b |
CB1a |
CB1a |
CB2b |
CB2b |
| Ki (nM)c no PMSF (n = 4) | Ki (nM)c with PMSF (n = 4) | Ki (nM)c no PMSF (n = 8) | Ki (nM)c with PMSF (n = 8) | EC50 (nM)d (n = 4) | Emaxd | EC50 (nM)d (n = 4) | Emaxd | |
| EPEA | 2000 (989 and 4045) | 55 (37.5 and 80.9) | 9027 (6451 and 12630) | 3440 (2324 and 5092) | 1361 (223 and 8288) | 40.6 (24.7 and 56.6) | 397.1 (22.2 and 7085) | 22.9 (13.4 and 32.5) |
| DHEA | 633 (292 and 1372) | 124 (94.8 and 162) | 3843 (2995 and 4932) | 2141 (1320 and 3473) | 50 (2.74 and 911) | 26.5 (16.3 and 36.8) | 42 (2.6 and 676) | 23.1 (15.7 and 30.5) |
CB1 receptors have been investigated using MF1 mouse whole brain membranes.
CB2 receptors have been investigated using CHO-hCB2 cell membranes.
Ki values have been calculated for displacement of [3H]CP55940.
EC50 and Emax values have been calculated for stimulation of [35S]GTPγS binding.
We also found that DHEA and EPEA behaved as CB1 and CB2 receptor agonists, as indicated by their ability to produce a concentration-related stimulation of [35S]GTPγS binding to mouse brain (Figure 3E) and CHO-hCB2 cell membranes (Figure 3F). In both membrane preparations, DHEA displayed higher potency than EPEA. EC50 and Emax values with 95% confidence limits shown in brackets are listed in Table II.
FAAH expression and inhibition in LNCaP and PC3 cells
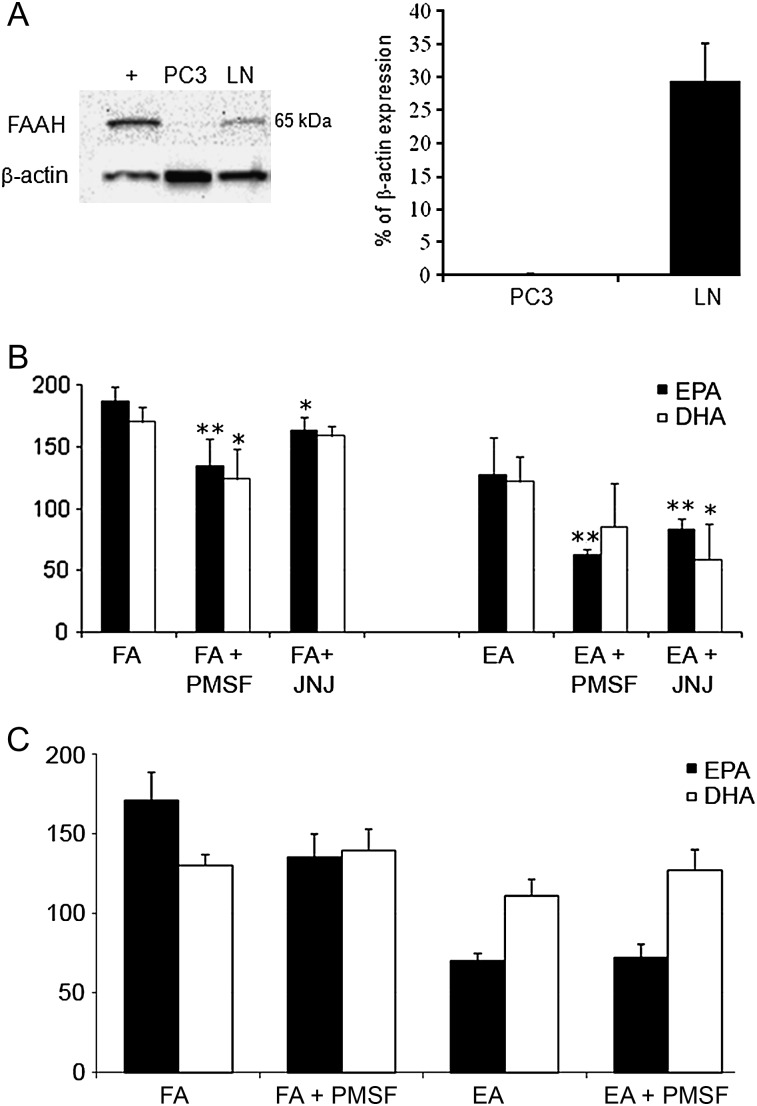
Because of the possibility that DHEA and EPEA may, like anandamide, be metabolized by FAAH, we went on to determine whether this enzyme is expressed by LNCaP or PC3 cells. We found that FAAH protein was indeed highly expressed in the androgen receptor-positive LNCaP cells. There was, however, little or no expression of FAAH protein in the androgen receptor-negative PC3 cells (Figure 4A). Since LNCaP cells were found to express FAAH, we went on to establish whether, as in mouse brain and CHO membranes, DHEA or EPEA could be potentiated by inhibiting FAAH in this cell line. We found that the potency of EPEA was indeed increased by PMSF, as indicated by a significant decrease in the IC50 in LNCaP cells (Figure 4B), as was that of EPA and DHA. The more selective FAAH inhibitor, JNJ1661010 (16), increased the anti-proliferative potency not only of EPEA but also of DHEA and EPA, although not of DHA. PMSF did not increase the anti-proliferative potencies of any compound in PC3 cells (Figure 4C).
Fig. 4.
Role of FAAH in LNCaP and PC3 cells. (A) A representative example of western blotting. β-Actin used as internal loading control. Positive control (+) is UM-84 cell line. Combined protein expression data shown as bar chart indicating ratio of expression of protein of interest to β-actin ± standard error of the mean. (B) Effect of inhibiting FAAH with PMSF or JNJ101660 (JNJ), in LNCaP, cells and (C) inhibition with PMSF in PC3 cells. (PMSF; JNJ, selective FAAH inhibitor, JNJ10660. FA, fatty acid, EA, ethanolamide). Error bars represent standard error of the mean. *P ≤ 0.05, **P ≤ 0.01 comparing cells treated with either FA only or EA only, against those treated with FAAH inhibitors.
Discussion
Our results indicate that the ethanolamide metabolites of two metabolically important omega-3 fatty acids, EPA and DHA, can activate CB1 and CB2 receptors in PC3 and LNCaP cells with significant potency. Since it has also been found that these ethanolamides, EPEA and DHEA, become detectable in vivo after consumption of diets rich in EPA and DHA (4,17), our results provide the first evidence that EPEA and DHEA may be endocannabinoids. We also showed that EPEA and DHEA are significantly more potent than their parent fatty acids at inhibiting prostate cancer cell growth/proliferation. This inhibition appears to result from changes in both cell cycle arrest and increased apoptosis. However, the precise mechanisms responsible for this inhibition are not clear at present and appear to differ between EPEA and DHEA and also between the two prostate cancer cell lines used in this study.
Although we show a statistically significant difference in potency of the ethanolamides compared with their fatty acid parent molecules (Figure 1), our data suggests higher IC50 values than studies have shown for other ethanolamides, such as the omega-6 ethanolamide, anandamide in prostate cancer cell lines (18). We did not investigate anandamide, and as this is the first study comparing the IC50 of EPEA and DHEA in prostate cancer cells, we have no other data to compare with, although our data is consistently reproducible. It is possible that DHEA and EPEA are less potent than anandamide, as they appear, from our other data, to also work through CB receptor-independent mechanisms. IC50 values for EPA and DHA in LNCaP cells are similar to those of Chung et al. 2001 (19). The EC50 value of DHEA for its activation of CB2 receptors was significantly less than its Ki value for the displacement of the CB1/CB2 receptor ligand, [3H]CP55940, from specific binding sites on these receptors (Table II). This was unexpected as DHEA exhibited rather low efficacy as a CB2 receptor agonist (Figure 3), suggesting that it is a CB2 receptor partial agonist; a type of agonist that is not expected to display greater potency in functional than in binding assays. Clearly, further experiments are required to explain this finding. In contrast, the EC50 value of EPEA for its activation of brain membrane receptors was significantly greater than its Ki value for the displacement of [3H]CP55940 from specific binding sites on these receptors. Furthermore, no statistically significant differences between EC50 and Ki values were detected for DHEA in brain membrane experiments or for EPEA in experiments with CHO-hCB2 cell membranes, when the binding assays were performed in the presence of PMSF, suggesting that inhibition of FAAH and/or increased substrate availability eliminated any differences. PMSF was included in our [3H]CP55940 binding experiments to prevent the metabolism of EPEA or DHEA by the fatty acid ethanolamide-metabolizing enzyme, FAAH. The presence of PMSF in the [35S]GTPγS-binding assays was deemed unnecessary as the concentration of protein, and hence of FAAH, was much less in these assays than in the ligand displacement assays.
In LNCaP cells, the significant decrease in S phase and in G1 arrest elicited by DHA and DHEA, respectively, was in marked contrast to the lack of any effect of EPA or EPEA on any of the cell cycle parameters compared with untreated cells. In PC3 cells, however, EPA did cause significant cell cycle arrest in the G1 phase (increase in G1 cells) compared with untreated cells, and DHA induced a marked decrease in S phase cells, suggesting both these fatty acids were able to reduce proliferation in the PC3 cells but only DHA was effective in the LNCaP cells, perhaps indicating a possible role for wild-type p53 because PC3 cells have a mutant p53 protein. These observations are in contrast to data obtained in some previous investigations in which the effects of different synthetic cannabinoid receptor agonists on cell cycle phases were explored. For example, Sarfaraz et al. (20) demonstrated G1 arrest with the CB1/CB2 receptor agonist, R-(+)-WIN55212. They are, however, in agreement with other studies that demonstrated a decreased G1 and increased G2 after treatment with R-(+)-methanandamide, a CB1-selective anandamide analogue and JWH-015, a CB2-selective agonist. This suggests that alternative mechanisms may account (21) for the effects of different natural and synthetic cannabinoids on cell cycle parameters. Indeed, in our studies, we have demonstrated differential effects between fatty acids and their ethanolamides on the cell cycle, which differed somewhat according to the cell line, but which still resulted in significant changes in tumour cell proliferation/growth. Why DHEA and EPEA should have such different effects on the cells is currently unclear. One possibility is that it reflects differences between the pharmacological properties of these ethanolamides or indeed of their respective metabolites and the possible presence of other receptors.
We found that, whereas DHEA could induce significant apoptosis in both LNCaP and PC3 cells, only EPEA increased apoptosis in PC3 cells, a further indication of differences between the two ethanolamides. Since PC3 cells, but not LNCaP cells, express the pro-apoptotic p53 oncogene mainly as the inactive, mutant form, our data suggest that the EPEA or DHEA can activate apoptosis through either p53-dependent or p53-independent mechanisms in different cell lines (22). This is interesting, as previous findings from our group showed that omega-3 LCPUFA could induce apoptosis, both dependently and independently of p53 activation and could actually alter the expression of mutant-p53 to reactivate/re-establish wild-type function in breast cancer cells (23). It remains to be determined if the omega-3 ethanolamides can influence apoptosis through a similar reactivation mechanism in either breast or prostate cancer cells. It is also interesting to note that despite multiple repetition, we continued to find variable results with using the EPA and DHA fatty acids alone, particularly with regards to early apoptosis, and only in PC3 cells. Perhaps the fatty acids interrupt the fluidity of PC3 more than LNCaP cell membrane, and interfere with Annexin binding to phosphatidyl serine on cell membranes, or affect membrane fluidity, giving spurious results. Other studies, however, have not discussed such a phenomenon with PC3 cells, and it may be a unique effect with our particular cell line? What is apparent from our observations is that the omega-3 ethanolamides generally affect cell cycle functions and apoptosis with greater potency than their parent fatty acids. This could form the basis for the development of novel therapeutic agents in cancer.
We also explored the possibility that either or both EPEA and DHEA might act through cannabinoid CB1 and/or CB2 receptors to induce their inhibitory effects on the proliferation of LNCaP and PC3 prostate cancer cells. It is already known that anandamide, an endogenous omega-6 ethanolamide, can both activate cannabinoid receptors and inhibit cancer cell proliferation (24,25). Previous studies have shown that prostate cancer cells express cannabinoid receptors (18,26) and that LNCaP and PC3 cells express both CB1 and CB2 receptors (27). We confirmed this in our cell lines and then showed that EPEA and DHEA displayed significant potency in vitro as CB1 and CB2 receptor agonists. However, we also obtained evidence that the anti-proliferative effects of EPEA in LNCaP cells and of DHEA in LNCaP and PC3 cells are not CB1 or CB2 receptor-mediated. This was deduced from data obtained in experiments with the CB1-selective antagonist, AM281, and the CB2-selective antagonist, AM630, each applied at a concentration (1 μM) that has been used in other investigations to identify effects that are CB1 and/or CB2 receptor-mediated (28–30). Thus, we found that separate or combined administration of these antagonists had no effect on the anti-proliferative potencies of EPEA or DHA in LNCaP cells or of DHA in PC3 cells and increased rather than decreased the potency of DHEA in both cell lines (Figure 1). These findings are not altogether unexpected as it has been reported that anandamide-induced anti-proliferative effects in some cancer cell lines can be potentiated by CB1- or CB2-selective antagonists (24,25). In contrast, we did find that the anti-proliferative potencies of EPEA in PC3 cells and of EPA in PC3 and LNCaP cells were reduced by AM281 and AM630 when these antagonists were administered separately (EPEA) or in combination (EPEA and EPA). Even so, it is currently unclear whether EPA, possibly after its conversion to EPEA, or direct administration of EPEA were indeed acting through cannabinoid receptors to inhibit PC3 cell proliferation, as EPEA produced this effect with a potency well below the potency it displayed as a CB1 or CB2 receptor agonist (Figure 1 and Table I).
Further research is now needed to establish whether, as has been proposed for anandamide (23,24), the DHEA effect was potentiated by AM281 and AM630 in our experiments because blockade of cannabinoid receptors increased its ability to inhibit cancer cell proliferation through one or more cannabinoid receptor-independent mechanisms in the cancer cell lines we used. It will be important, therefore, to establish the extent to which DHEA targets non-CB1 and non-CB2 receptors, particularly transient receptor potential V1 cation channels. These channels can be activated by both anandamide and omega-3 polyunsaturated fatty acids and Maccarrone et al. have demonstrated that cannabinoid receptor activation can prevent apparent transient receptor potential V1-mediated apoptosis induced by anandamide (24,31). Consequently, it is possible that by blocking cannabinoid receptors, we reduced cannabinoid receptor-mediated protection of LNCaP and PC3 cells, thereby increasing the ability of DHEA to induce apoptosis through vanilloid receptors, which are expressed in both these cell lines (32). Interestingly, whereas DHA is a potent TRPV1 agonist, EPA inhibits the activation of this cation channel by various agonists (33). Whether DHEA and EPEA display this same difference in their pharmacology remains to be established. Since recent studies have demonstrated that CB1 and CB2 receptors do not mediate apoptosis in malignant astrocytomas if they are coupled to the prosurvival signal AKT (34), further research is also needed to establish the extent to which cannabinoid receptors couple to AKT in our cancer cell lines.
Our data suggest that EPEA and DHEA resemble the endocannabinoid, anandamide, not only in their ability to activate CB1 and CB2 receptors but also in their susceptibility to metabolism by FAAH. Thus, the potency with which each of these omega-3 ethanolamides displaced [3H]CP55940 from brain membranes was increased by the FAAH inhibitor, PMSF, as indeed was the potency of anandamide in this binding assay (data not shown). Moreover, PMSF increased the anti-proliferative potency of EPEA, as well as of EPA and DHA (but not DHEA), in LNCaP cells in which we found FAAH protein to be highly expressed. Although PMSF is not a selective inhibitor of FAAH, it is probably that it did produce this potentiation by inhibiting this enzyme. This is supported by the observations that, firstly, PMSF did not increase the anti-proliferative potencies of EPEA, DHEA, EPA or DHA in PC3 cells in which we detected little or no expression of FAAH protein, and secondly, a more selective FAAH inhibitor, JNJ1661010, also increased the anti-proliferative potencies of EPEA and EPA in LNCaP cells (16). Moreover, in contrast to PMSF, JNJ1661010 also increased the anti-proliferative potency of DHEA in LNCaP cells, though it failed to affect the potency of DHA. The observation that inhibition of FAAH in a prostate cancer cell line that expresses this enzyme could decrease the IC50 values of EPA and DHA supports the hypothesis that some of the many known effects of these omega-3 fatty acids, may actually arise as a result of their conversion in situ to their endocannabinoid metabolites in some cells, a process which has been shown to occur in some animal tissues (4,35). Our finding that PC3 cells do not seem to express FAAH conflicts with a previous report, however, this could be due to inherent differences in cell lines between different laboratories or to differential limits of detection by different antibodies/techniques (36). In our study, PC3 cells showed, in some replicates, a barely detectable level of expression, and in other replicates, no expression at all, whereas it was easily detected in LNCaP cells.
In conclusion, we show here for the first time, that the omega-3 ethanolamides, EPEA and DHEA, are more potent than their parent fatty acids, EPA and DHA, at inhibiting prostate cancer cell proliferation and that the anti-proliferative effects they produce appear to have different underlying mechanisms and may be cell specific. We also demonstrate that these ethanolamides activate CB1 and CB2 receptors with significant potency and that the potencies of both can be enhanced by inhibiting the anandamide-metabolizing enzyme, FAAH, both in brain tissue and in FAAH-expressing cancer cells. We propose, therefore, that EPEA and DHEA should be classified as endocannabinoids It has been shown previously that these omega-3 ethanolamides are generated in vivo after consumption of their parent fatty acids, EPA and DHA a finding that may explain some of the antitumour effects of these omega-3 fatty acids that have been observed in vivo in other studies (17,37,38). The enhancing effect of FAAH inhibition in FAAH-expressing LNCaP cells on the anti-proliferative effects of EPA and DHA also suggests, for the first time, that these cells can convert these fatty acids to ethanolamides in situ. It is also noteworthy that the results we obtained from our experiments with LNCaP and PC3 cells suggest that the omega-3 ethanolamides, EPEA and DHEA have different, albeit overlapping, pharmacological fingerprints, as has been shown for other cannabinoid receptor ligands (39). By identifying the particular pharmacological targets and physiological mechanisms through which EPEA and DHEA induce their inhibition of prostate cancer cell proliferation, it may be possible to identify tumours which are more liable to respond to treatment with either omega-3 fatty acids or their ethanolamides, perhaps in conjunction with a FAAH inhibitor or even with cannabinoid CB1 and/or CB2 receptor antagonists.
Supplementary material
Supplementary Figure 1 can be found at http://carcin.oxfordjournals.org/
Funding
National Institutes of Health (DA-009789); TENOVUS Scotland; NHS Grampian Endownments fund.
Supplementary Material
Acknowledgments
The authors wish to thank Mrs Lesley A.Stevenson for technical support, and the Aberdeen University Flow Cytometry facility.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
- CHO
Chinese hamster ovary
- DMSO
dimethyl sulfoxide
- MTT
3-(4,-5-dimethylthiazol-2-yl)-2,-5-diphenyl tetrazolium bromide
- DHA
docosahexaenoic acid
- DHEA
docosahexaenoyl ethanolamide
- EPA
eicosapentaenoic acid
- EPEA
eicosapentaenoyl ethanolamide
- FAAH
fatty acid amide hydrolase
- EC50
half of the maximal effective concentration required to elicit a response
- PMSF
phenylmethylsulfonyl fluoride
- PI
propidium iodide
References
- 1.Berquin IM, et al. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaikh IA, et al. Docosahexaenoic acid enhances the efficacy of docetaxel in prostate cancer cells by modulation of apoptosis: the role of genes associated with the NF-kappaB pathway. Prostate. 2008;68:1635–1646. doi: 10.1002/pros.20830. [DOI] [PubMed] [Google Scholar]
- 3.Howlett AC. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68-69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 4.Wood JT, et al. Dietary docosahexaenoic acid supplementation alters select physiological endocannabinoid-system metabolites in brain and plasma. J. Lipid Res. 2010;51:1416–1423. doi: 10.1194/jlr.M002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Petrocellis L, et al. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl Acad. Sci. USA. 1998;95:8375–8380. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mimeault M, et al. Anti-proliferative and apoptotic effects of anandamide in human prostatic cancer cell lines: implication of epidermal growth factor receptor down-regulation and ceramide production. Prostate. 2003;56:1–12. doi: 10.1002/pros.10190. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch DG, et al. Enzymatic synthesis and degradation of anandamide, a cannabinoid receptor agonist. Biochem. Pharmacol. 1993;46:791–796. doi: 10.1016/0006-2952(93)90486-g. [DOI] [PubMed] [Google Scholar]
- 8.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 9.Sheskin T, et al. Structural requirements for binding of anandamide-type compounds to the brain cannabinoid receptor. J. Med. Chem. 1997;40:659–667. doi: 10.1021/jm960752x. [DOI] [PubMed] [Google Scholar]
- 10.Ross RA, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas A, et al. 6“-Azidohex-2”-yne-cannabidiol: a potential neutral, competitive cannabinoid CB1 receptor antagonist. Eur. J. Pharmacol. 2004;487:213–221. doi: 10.1016/j.ejphar.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Kurkinen KM, et al. Gamma-35S]GTP autoradiography allows region-specific detection of muscarinic receptor-dependent G-protein activation in the chick optic tectum. Brain Res. 1997;769:21–28. doi: 10.1016/s0006-8993(97)00663-x. [DOI] [PubMed] [Google Scholar]
- 13.Breivogel CS, et al. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- 14.Cheng Y, et al. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 15.Gong JP, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 16.Keith JM, et al. Thiadiazolopiperazinyl ureas as inhibitors of fatty acid amide hydrolase. Bioorg. Med. Chem. Lett. 2008;18:4838–4843. doi: 10.1016/j.bmcl.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 17.Artmann A, et al. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta. 2008;1781:200–212. doi: 10.1016/j.bbalip.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Melck D, et al. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology. 2000;141:118–126. doi: 10.1210/endo.141.1.7239. [DOI] [PubMed] [Google Scholar]
- 19.Chung BH, et al. Effects of docosahexaenoic acid and eicosapentaenoic acid on androgen-mediated cell growth and gene expression in LNCaP prostate cancer cells. Carcinogenesis. 2001;22:1201–1206. doi: 10.1093/carcin/22.8.1201. [DOI] [PubMed] [Google Scholar]
- 20.Sarfaraz S, et al. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]
- 21.Olea-Herrero N, et al. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R(+)-Methanandamide and JWH-015: involvement of CB2. Br. J. Cancer. 2009;101:940–950. doi: 10.1038/sj.bjc.6605248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carroll AG, et al. P53 oncogene mutations in three human prostate cancer cell lines. Prostate. 1993;23:123–134. doi: 10.1002/pros.2990230206. [DOI] [PubMed] [Google Scholar]
- 23.Majumder B, et al. Conjugated linoleic acids (CLAs) regulate the expression of key apoptotic genes in human breast cancer cells. FASEB J. 2002;16:1447–1449. doi: 10.1096/fj.01-0720fje. [DOI] [PubMed] [Google Scholar]
- 24.Maccarrone M, et al. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- 25.Mnich K, et al. Inhibition by anandamide of 6-hydroxydopamine-induced cell death in PC12 cells. Int. J. Cell. Biol. 2010;2010 doi: 10.1155/2010/818497. ID:818497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligresti A, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez MG, et al. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell. Signal. 2003;15:851–859. doi: 10.1016/s0898-6568(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 28.Jan CR, et al. Novel effect of CP55,940, a CB1/CB2 cannabinoid receptor agonist, on intracellular free Ca2+ levels in bladder cancer cells. Chin. J. Physiol. 2002;45:33–39. [PubMed] [Google Scholar]
- 29.Ryan D, et al. Interactions of cannabidiol with endocannabinoid signalling in hippocampal tissue. Eur. J. Neurosci. 2007;25:2093–2102. doi: 10.1111/j.1460-9568.2007.05448.x. [DOI] [PubMed] [Google Scholar]
- 30.Williams EJ, et al. The FGF receptor uses the endocannabinoid signaling system to couple to an axonal growth response. J. Cell Biol. 2003;160:481–486. doi: 10.1083/jcb.200210164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bifulco M, et al. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez MG, et al. Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur. J. Pharmacol. 2005;515:20–27. doi: 10.1016/j.ejphar.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Matta JA, et al. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007;578:397–411. doi: 10.1113/jphysiol.2006.121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cudaback E, et al. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS One. 2010;5:e8702. doi: 10.1371/journal.pone.0008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger A, et al. Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc. Natl Acad. Sci. USA. 2001;98:6402–6406. doi: 10.1073/pnas.101119098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Llorente L, et al. Characterization of an anandamide degradation system in prostate epithelial PC-3 cells: synthesis of new transporter inhibitors as tools for this study. Br. J. Pharmacol. 2004;141:457–467. doi: 10.1038/sj.bjp.0705628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose DP, et al. Effect of omega-3 fatty acids on the progression of metastases after the surgical excision of human breast cancer cell solid tumors growing in nude mice. Clin. Cancer Res. 1996;2:1751–1756. [PubMed] [Google Scholar]
- 38.Wu M, et al. Omega-3 polyunsaturated fatty acids attenuate breast cancer growth through activation of a neutral sphingomyelinase-mediated pathway. Int. J. Cancer. 2005;117:340–348. doi: 10.1002/ijc.21238. [DOI] [PubMed] [Google Scholar]
- 39.Pertwee RG. Receptors and channels targeted by synthetic cannabinoid receptor agonists and antagonists. Curr. Med. Chem. 2010;17:1360–1381. doi: 10.2174/092986710790980050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.