Abstract
Purpose
To determine the efficacy and toxicity of the combination of sorafenib, cytarabine, and idarubicin in patients with acute myeloid leukemia (AML) younger than age 65 years.
Patients and Methods
In the phase I part of the study, 10 patients with relapsed AML were treated with escalating doses of sorafenib with chemotherapy to establish the feasibility of the combination. We then treated 51 patients (median age, 53 years; range, 18 to 65 years) who had previously untreated AML with cytarabine at 1.5 g/m2 by continuous intravenous (IV) infusion daily for 4 days (3 days if > 60 years of age), idarubicin at 12 mg/m2 IV daily for 3 days, and sorafenib at 400 mg orally twice daily for 7 days.
Results
Overall, 38 (75%) patients have achieved a complete remission (CR), including 14 (93%) of 15 patients with mutated FMS-like tyrosine kinase-3 (FLT3; the 15th patient had complete remission with incomplete platelet recovery [CRp]) and 24 (66%) of 36 patients with FLT3 wild-type (WT) disease (three additional FLT3-WT patients had CRp). FLT3-mutated patients were more likely to achieve a CR than FLT3-WT patients (P = .033). With a median follow-up of 54 weeks (range, 8 to 87 weeks), the probability of survival at 1 year is 74%. Among the FLT3-mutated patients, 10 have relapsed and five remain in CR with a median follow-up of 62 weeks (range, 10 to 76 weeks). Plasma inhibitory assay demonstrated an on-target effect on FLT3 kinase activity.
Conclusion
Sorafenib can be safely combined with chemotherapy, produces a high CR rate in FLT3-mutated patients, and inhibits FLT3 signaling.
INTRODUCTION
The FMS-like tyrosine kinase-3 (FLT3) is a promising target in acute myeloid leukemia (AML).1,2 Activating mutations of the kinase occur in about one third of patients with AML and are associated with leukocytosis, higher marrow blast percentage, higher likelihood of relapse, and shorter survival.3–6 There are conflicting data on the association of these mutations with a lower complete remission (CR) rate and a higher induction death rate.3,6
An internal tandem duplication (ITD) in the juxtamembrane domain of the FLT3 gene occurs in approximately 25% of younger adult AML patients with the length of the duplicated DNA varying between 3 and > 400 base pairs.7 Such in-frame mutations produce functional proteins with constitutive kinase activity leading to the activation of downstream signaling pathways including the STAT5 and MAP kinase pathways.8 Variations in the ratio of wild-type (WT) to mutant allele levels, number of mutants of different sizes in the same patient, and the size of the inserted DNA have also been reported to be of prognostic significance.3,5,6,9–12
Constitutive phosphorylation of FLT3 in the absence of FLT3-ITD suggested the existence of other mechanisms of aberrant FLT3 signaling, including FLT3 tyrosine kinase domain (TKD) mutations and autocrine signaling by the FLT3 ligand and WT FLT3.13–16 Mutations affecting codons 835 and 836 in the FLT3 second kinase domain occur in approximately 7% of patients and lead to its constitutive activation.13,14 Other novel activating mutations within the activation loop of FLT3 kinase have been described.16–18 More recently, point mutations in the juxtamembrane domain of FLT3 have been reported as a new class of activating mutations.19 Prognostic significance of these less common mutations remains unclear, with conflicting reports on FLT3-TKD mutations being associated with either a poorer or more favorable outcome.20–23
A number of small-molecule kinase inhibitors such as lestaurtinib (CEP-701), midostaurin (PKC412), and tandutinib (MLN518) block the autophosphorylation of FLT3 and lead to inhibition of cell proliferation and induction of apoptosis; they have demonstrated clinical activity in patients with AML, in particular those with mutations.24–27 These drugs act synergistically with standard cytotoxic agents if used simultaneously with or after chemotherapy.28
Sorafenib is an oral small molecule, originally designed as an inhibitor of Raf-1 kinase targeting the RAF/MEK/ERK pathway; it has inhibitory properties against a number of other kinases including FLT3 and vascular endothelial growth factor receptor.29–31 In preclinical studies, sorafenib induced dephosphorylation of MEK1/2 and ERK and induced apoptosis in AML cells.32 Furthermore, sorafenib was 1,000- to 3,000-fold more potent in inducing apoptosis in Ba/F3 cells with FLT3-ITD or D835G mutations than those with WT FLT3.33 In a mouse model of AML with mutant FLT3, sorafenib reduced the leukemic burden and prolonged survival.33 It has been approved by the US Food and Drug Administration for the treatment of renal cell and hepatocellular carcinoma at a standard dose of 400 mg twice daily. In phase I studies and anecdotal use in patients with advanced AML, sorafenib was capable of producing significant clinical responses.33,34 The objectives of this study were to determine the feasibility, safety, and efficacy of combining sorafenib with induction chemotherapy.
PATIENTS AND METHODS
Patient Eligibility
Patients with diagnosis of AML by WHO criteria who had relapsed after prior response (irrespective of number of prior salvage regimens) or were refractory to initial induction therapy with standard regimens were eligible to participate in the phase I part of the study. After the establishment of a safe dose of sorafenib in combination with chemotherapy, patients with previously untreated AML who were between the ages of 18 and 60 years were eligible. Patients older than age 60 years who had a low probability of 8-week mortality with intensive chemotherapy were also eligible, depending on the number of adverse risk factors (cytogenetics, performance status, antecedent hematologic disorder, organ function)35. For both phase I and II, patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1, or 2 and adequate cardiac, renal, and hepatic function with left ventricular ejection fraction ≥ 50%, creatinine ≤ 2.0 mg/dL, bilirubin ≤ 2.0 mg/dL, and liver transaminases less than three times the institutional upper limit of normal. All patients had to have reviewed and signed an appropriate informed consent approved by the institutional review board. Patients in the historical control group also signed an institutional review board–approved consent to participate in the study.
Treatment Regimen
The treatment included cytarabine at 1.5 g/m2 given by continuous intravenous (IV) infusion daily for 4 days (3 days for patients older than age 60 years) as well as idarubicin at 12 mg/m2 IV over 1 hour daily for 3 days. In phase I, sorafenib was administered during the first 7 days at escalating doses of 400 mg orally (PO) every other day, 400 mg PO daily, and 400 mg PO twice daily to cohorts of three patients. After the 400-mg twice daily dose was established as safe, all patients received this dose for the first 7 days of induction in phase II. Patients not achieving CR after one course could receive a second induction course if the treating physician determined this to be in the patients' best interest.
Patients achieving a CR could receive up to five cycles of consolidation (number chosen arbitrarily) with cytarabine at 0.75 g/m2 IV over 24 hours daily for 3 days, idarubicin at 8 mg/m2 IV over 1 hour daily for 2 days, and sorafenib at 400 mg PO twice daily for up to 28 days. Consolidation cycles were repeated every 4 to 6 weeks, depending on the recovery of neutrophil and platelet counts and toxicity. After the completion of consolidation courses, patients received maintenance sorafenib at 400 mg twice daily for a total of up to 1 year of sorafenib therapy (including the consolidation courses). Reductions to the doses of all three agents during consolidation and maintenance were allowed according to predetermined guidelines related to various adverse effects.
Response Criteria and Definitions
CR was defined by the presence of < 5% blasts in the bone marrow (BM) with > 1 × 109/L neutrophils and > 100 × 109/L platelets in the peripheral blood (PB). Relapse was defined by recurrence of > 5% blasts in a BM aspirate unrelated to recovery or by the presence of extramedullary disease. CR duration was calculated from the time of CR until relapse. Progression-free survival (PFS) was calculated from the beginning of treatment until an event including relapse, death during induction, or death in CR. Overall survival was calculated from the time of diagnosis until death.
Statistical Analysis
The overall trial objective was to provide an early assessment of efficacy of sorafenib when used in combination with idarubicin and cytarabine. Initially, escalating doses of sorafenib (up to the standard dose of 400 mg twice a day) were administered in cohorts of three patients. If grade 3 to 4 sorafenib-related toxicities were observed in more than two of six patients, the dose level would exceed the maximum tolerated dose. In the phase II study, we monitored the response rate and PFS as patients accrued and planned to stop the trial if we had evidence that the target CR of 70% or median PFS (7 months) could not be met. Formally, we planned to stop the study early (for futility) if there was less than a 2% chance that the median PFS rate was ≥ 7 months. On the basis of the above criterion, using simulations, we determined that if the true median PFS rate was ≥ 7 months, then there would be a ≤ 11% chance of declaring the treatment ineffective. Alternatively, if the true median PFS was ≤ 4 months, there would be a > 80% chance of declaring the treatment ineffective.
Survival curves were plotted by the Kaplan-Meier method and compared using the log-rank test. Differences in subgroups by different covariates were evaluated using the χ2 test for nominal values and the Mann-Whitney U test and Fisher's exact test for continuous variables.
RESULTS
Patient Characteristics
From October 2007 to February 2009, 61 patients with AML were enrolled, including 10 patients treated during phase I and 51 patients treated during phase II. Patient characteristics are summarized in Table 1. The median age of the patients treated in phase I was 34 years (range, 21 to 59 years). They had a median of two prior regimens (range, one to six prior regimens). Seven had FLT3 mutations, including one patient with FLT3-ITD and FLT3-TKD double mutants; in the phase I study, patients were targeted for the presence of FLT3 mutations.
Table 1.
Characteristics of Patients in Phase I and Phase II Trials
| Characteristic | Phase I |
Phase II |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 10 | 51 | ||
| Age, years | ||||
| Median | 34 | 53 | ||
| Range | 21-59 | 18-65 | ||
| > 60 | 0 | 0 | 11 | 22 |
| WBC at presentation, 109/L | ||||
| Median | 6.5 | 5.2 | ||
| Range | 0.9-28.4 | 0.6-122.7 | ||
| Antecedent HD | 0 | 0 | 11 | 22 |
| Cytogenetics | ||||
| Diploid | 2 | 22 | ||
| Intermediate risk | 7 | 24 | ||
| Unfavorable risk | 1 | 5 | ||
| No. of prior therapies | ||||
| Median | 2 | |||
| Range | 1-6 | 0 | ||
| FLT3-WT | 3 | 30 | 36 | 71 |
| FLT3-mutated | ||||
| ITD | 6 | 60 | 13 | 25 |
| TKD | 0 | 2 | 4 | |
| Both | 1 | 10 | 0 | |
| FLT3 mutation burden | ||||
| High (> 25%) | 5 | 10 | ||
| Low (≤ 25%) | 2 | 5 | ||
| NPM1-mutated | 3 of 7 | 43 | 12 of 50 | 24 |
Abbreviations: HD, hematologic disorder; FLT3-WT, FMS-like tyrosine kinase-3 wild type; ITD, internal tandem duplication; TKD, tyrosine kinase domain; NPM1, nucleophosmin-1.
In the phase II study, the median age was 53 years (range, 18 to 65 years). Eleven patients were older than 60 years of age, 11 patients had antecedent hematologic disorder, and five had unfavorable cytogenetics. Fifteen had FLT3 mutations, including 13 with FLT3-ITD (four with low mutation burdens) and two with FLT3-TKD (one with low mutation burden). The median presentation WBC was 5.2 × 109/L (range, 0.6 to 122.7 × 109/L). Eight patients were FLT3-ITD–positive/nucleophosmin-1 (NPM1) –negative. The median age of FLT3-mutated patients was 53 years (range, 20 to 65 years); nine had diploid karyotype, two had +8, one was −5/−7, and three were miscellaneous. Their median presentation WBC was 18.6 × 109/L (range, 1.9 to 122.7 × 109/L).
Response and Outcome
Among the 10 patients in the phase I part of the study, four (40%) achieved a CR (three of seven patients with FLT3-ITD mutation compared with one of three with FLT3-WT). The other six patients either had refractory disease or died from complications of therapy. All four patients achieving a CR proceeded to an allogeneic stem-cell transplantation and all are still alive.
In the phase II part of the study, 51 patients were available for response assessment and 38 (75%) patients have achieved a CR, including 12 (92%) of 13 patients with FLT3-ITD (the thirteenth patient had CRp), two (100%) of two patients with FLT3-TKD, and 24 (66%) of 36 patients with FLT3-WT disease (three patients with FLT3-WT had CRp; Table 2). The difference between CR rate of FLT3-mutated and FLT3-WT patients was statistically significant (P = .033). Three patients died at induction and six were resistant to therapy (all FLT3-WT). CR was achieved after one induction cycle in 34 patients and after two induction cycles in four patients. Fourteen FLT3-mutated patients achieved CR/CRp after one cycle and one achieved CR/CRp after two cycles of induction. Altogether, seven patients have proceeded to an allogeneic stem-cell transplantation in the first CR, including four FLT3-mutated patients (three FLT3-ITD, one FLT3-TKD).
Table 2.
Response in Phase II Study
| Response | FLT3 Mutational Status |
|||
|---|---|---|---|---|
| Negative | Low | High | Positive (All) | |
| CR | 24 | 4 | 10 | 14 |
| CRp | 3 | 0 | 1 | 1 |
| Early death | 3 | 0 | 0 | 0 |
| Resistant | 6 | 0 | 0 | 0 |
Abbreviations: FLT3, FMS-like tyrosine kinase-3; CR, complete remission; CRp, CR with incomplete platelet recovery.
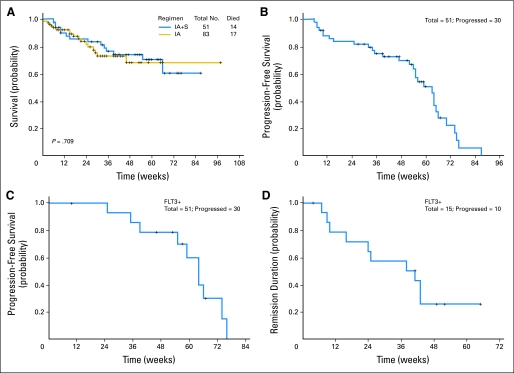
With a median follow-up of 54 weeks (range, 8 to 87 weeks) for all patients, the probability of survival at 6 months was 83%; at 12 months, it was 74% (Fig 1A). Figures 1B and 1C demonstrate the PFS for all patients and for patients with mutated FLT3; the CR duration for the latter is shown in Figure 1D. Among the patients with mutated FLT3, 10 have relapsed and five remain in CR with a median follow-up of 62 weeks (range, 10 to 76 weeks).
Fig 1.
(A) Survival for patients treated with idarubicin and cytarabine plus sorafenib (IA + S) and a historical IA alone, (B) progression-free survival for the patients treated with IA + S, (C) progression-free survival for FMS-like tyrosine kinase-3–mutated (FLT3+) patients treated with IA + S, and (D) duration of complete remission for FLT3+ patients treated with IA + S.
Toxicity
Assessment of toxicity and its attribution was based on baseline expectations and data available from sorafenib solid tumor studies.36 The regimen was reasonably well tolerated with adverse events being, in general, similar to those expected in patients receiving induction chemotherapy with the idarubicin + cytarabine (IA) combination. Grade ≥ 3 adverse events thought to be possibly related to the addition of sorafenib during induction included hyperbilirubinemia in four patients, elevated transaminases (five), diarrhea (four), rash (two), pancreatitis (one), colitis (one), pericarditis (one), hand and foot syndrome (two), and elevated creatinine (one; Table 3).
Table 3.
Toxicity During Induction Course
| Toxicity | No. of Patients With Grade |
|
|---|---|---|
| 1 and 2 | 3 and 4 | |
| GI effects (nausea and vomiting) | 18 | |
| Mucositis | 7 | 2 |
| Colitis | 1 | |
| Anorexia | 2 | |
| Elevated liver enzymes | 3 | 5 |
| Elevated bilirubin | 5 | 4 |
| Rash | 15 | 2 |
| Bleeding | 3 | 1 |
| Diarrhea | 23 | 4 |
| Hand and foot syndrome | 4 | 2 |
| Cardiac/hypertension | 3 | 4 |
| Elevated creatinine | 1 | 1 |
| Weight gain/fluid overload | 2 | |
| Pulmonary effects | 2 | |
| Pancreatitis | 1 | |
Dynamics of Mutated FLT3 Levels During the Treatment
We examined the fate of the FLT3-mutated clone in BM aspirate samples taken 3 weeks after cycle 1 of treatment. Among 11 patients with available samples, six had complete regression of the FLT3-mutated clone, three had partial regression (as assessed by > two-fold change in the blast-normalized mutant allelic ratio), and two had no change in the ratio. Among the historical controls, 10 FLT3-mutated patients had available samples at 3 weeks, with six showing persistence of the FLT3-mutated clone, one with a decrease in the blast-normalized allelic ratio, and two with disappearance of the FLT3-mutated clone (P = .04, Fisher's exact test).
Plasma Inhibitory Assay and Comparison With Lestaurtinib and Midostaurin
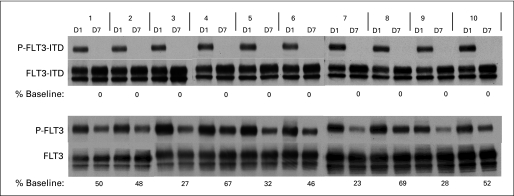
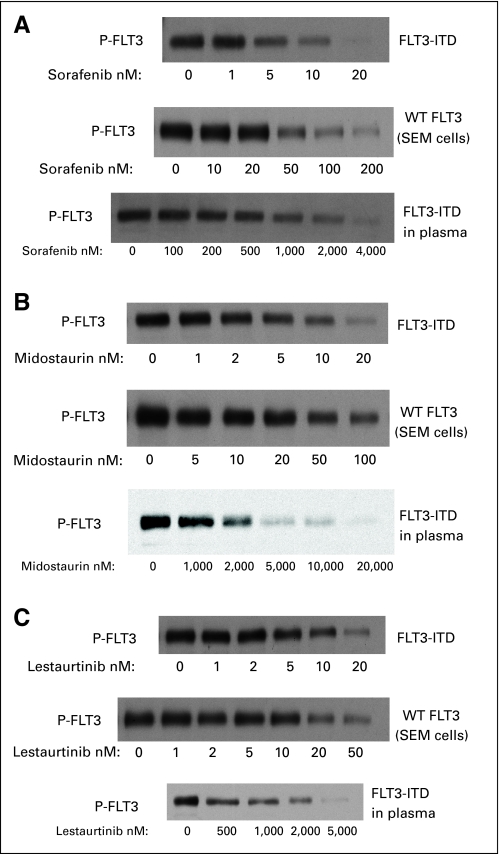
To determine the efficacy of in vivo FLT3 inhibition from sorafenib with this dosing regimen, we performed plasma inhibitory activity assays using blood samples obtained from trial patients 12 hours after dosing with sorafenib on day 7. Previous studies have shown the utility of this method for other FLT3 inhibitors.24,37,38 The results of plasma inhibitory activity assays for 10 trial patients are shown in Figure 2. All 10 patients displayed complete inhibition of phosphorylated FLT3 in this assay. This profound degree of in vivo FLT3 inhibition was not observed with other FLT3 inhibitors such as lestaurtinib, midostaurin, and KW-2449 (Fig 3).24,38
Fig 2.
Plasma samples from patients were incubated with TF-ITD cells (upper blots) and SEMK2 FLT3 wild type (FLT3-WT) cells (lower blots). The cells were then lysed and FLT3 was immunoprecipitated and subject to sodium dodecyl sulfate polyacrylamide gel electrophoresis. After transfer, the membranes were probed with antiphosphotyrosine (upper rows of each blot). The membranes were stripped and reprobed with anti-FLT3 to confirm equal loading (lower rows). The percent baseline refers to the densitometric measurement of the day 7 (D7) sample compared with the D1 sample for each patient. P-FLT3, phospho-FLT3.
Fig 3.
Effect of (A) sorafenib, (B) PKC412 (midostaurin), and (C) CEP-701 (lestaurtinib) on FMS-like tyrosine kinase-3 (FLT3) phosphorylation in TF-ITD cells (upper blots) and SEMK2 FLT3 wild type (FLT3-WT) cells (middle blots) in culture media (upper two blots) and plasma (lower blots).
DISCUSSION
Dysregulated receptor tyrosine kinase function is implicated in the pathogenesis of a number of hematologic malignancies leading to a search for potential targets for kinase inhibition in AML.39–41 The role of FLT3 kinase in leukemogenesis and the identification of its mutant forms and their adverse influence on the outcome of patients with AML suggest that its inhibitors are of potential therapeutic benefit.1,42,43 This is further corroborated by the high level of expression of FLT3 receptor in most AML cells and demonstration of constitutive activation of FLT3 signaling not only through mutations but also by autocrine signaling in FLT3-WT AML.16,44,45 Mutations of the FLT3 receptor gene are among the most common molecular abnormalities in AML; the presence of FLT3-ITD has been associated with shorter duration of remission and overall survival by a number of groups, whereas information on the influence of FLT3-TKD on outcome has been conflicting.3–6,20,21,23
Inhibition of FLT3 kinase in vitro prevents its autophosphorylation and activation of downstream signaling pathways (including STAT5 and MEK/ERK MAP kinase pathways), leading to apoptosis.46–48 Small-molecule FLT3 kinase inhibitors have been developed with activity against FLT3-mutant cells. Furthermore, these agents act synergistically with chemotherapeutic agents such as idarubicin and cytarabine to induce cytotoxicity.28,49 The relative efficacy and toxicity of these agents alone or in combination with chemotherapy remains to be determined and is likely dependent on a number of factors such as plasma protein binding, degree of residual FLT3 phosphorylation and downstream STAT5 and MAP kinase signaling, specificity against target FLT3 and the number of other kinases inhibited, and presence or absence of de novo or induced resistance.37,46,48,50–52 For example, mutations in the kinase domain of FLT3 that generate FLT3-ITD-TKD double mutants have been shown to confer resistance to the small-molecule inhibitors.50,52,53
The variable potency of FLT3 inhibitors and demonstration of de novo and acquired resistance to FLT3 inhibitors justifies the identification of new agents active against mutant FLT3. The potential role of residual downstream signaling as a mechanism of resistance suggests that agents with dual activity against FLT3 and its downstream targets, such as the MAP kinase pathway, may be beneficial. Sorafenib induces the dephosphorylation of MEK1/2 and ERK proteins and leads to apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway.32 Furthermore, molecules that are less plasma protein bound may be more active in the clinical setting. Sorafenib is as potent as other available FLT3 inhibitors in culture media and is more potent in plasma possibly because of a lower degree of plasma protein binding (Fig 3 and Appendix Table A1, online only; data provided by M.L.).
The potential role of FLT3 tyrosine kinase inhibitors (TKIs) in the treatment of patients with AML and mutated FLT3 remains undefined. TKIs such as imatinib and dasatinib have been successfully combined with chemotherapy regimens to treat patients with Philadelphia chromosome–positive acute lymphoblastic leukemia.54,55 In that disease, the target gene and its protein product with enhanced kinase activity has been clearly implicated as a pivotal pathogenic factor. In AML, the mutations of FLT3 gene lead to enhanced kinase activity of the protein product and constitutive activation of proliferative and prosurvival signals. However, such mutations are insufficient to lead to the AML phenotype without the presence of cooperative mutations in genes involved in cellular differentiation. This multiple hit theory suggests that the inhibition of FLT3 signaling on its own may not be sufficient to completely reverse the AML phenotype and perhaps combination with other agents with activity against other deregulated pathways in the leukemic cells may be necessary to produce long-lasting remissions. Agents such as sorafenib with activity against downstream signaling pathways may have an advantage over other FLT3 inhibitors without such activity.
We compared the results with those in a matched population of 83 patients with AML treated at our institution with a regimen identical to the one described above but without the addition of sorafenib. Eleven had FLT3-ITD mutation (five with low mutation burden), four had FLT3-TKD mutations, and one had both. The response rates (CR and CRp) for patients with mutated FLT3 (including both FLT3-ITD and FLT3-TKD patients) treated with the sorafenib-containing regimen were higher than those seen with IA alone (15 [100%] of 15 v 11 [79%] of 14; P = .049). There was no statistically significant difference in response rate when comparing all treated patients and, so far, with small numbers and short follow-up, no significant difference in survival, PFS, or CR duration when comparing all patients (Fig 1A) or only FLT3-mutated patients.
In conclusion, we were able to achieve a universal response in patients with mutated FLT3 and clearly demonstrated the on-target effect of sorafenib on FLT3 signaling. However, with a relatively short follow-up, several patients with mutated FLT3 have already relapsed. Clearly, so far, the addition of the potent TKI in induction, consolidation, and maintenance does not appear to prevent relapse. This may be related to the schedule of administration of sorafenib, development of resistance to sorafenib, and other potential factors limiting the beneficial effect of sorafenib. Randomized clinical trials are needed to demonstrate any prolongation of CR duration and survival in patients receiving FLT3 inhibitors. Similarly, mechanisms of resistance to FLT3 inhibitors such as development of mutations or the protective effects of a microenvironment will need to be better defined.
Appendix
Polymerase Chain Reaction Assay of FLT3 and NPM1 Mutational Status
Genomic DNA from bone marrow samples was isolated using the Autopure extractor (QIAGEN/Gentra, Valencia, CA). FMS-like tyrosine kinase-3 internal tandem duplication (FLT3-ITD) and codon 835/836 point mutation levels were determined as described previously (Lin P: Am J Clin Pathol 126:530-533, 2006) by a semiquantitative DNA-based polymerase chain reaction capillary electrophoresis (PCR-CE) assay. The sensitivity of the assay for mutation detection was 1% as determined by dilution studies. Quantitation of the FLT3 allelic ratio in initial and follow-up samples was performed using GeneScan and/or Genemapper software (Applied Biosystems, Foster City, CA). Relative mutant allele burden was calculated by comparing the peak area of the mutant peak(s) with that of the wild-type/unmutated peak on the electropherogram. Following correction to blast count, allele burdens were categorized as high (> 25% of blast population) or low.
Nucleophosmin-1 (NPM1) insertion/deletion mutations in the coding region of exon 12 were detected similarly by DNA-based PCR-CE following amplification using primers described previously (Szankasi P: J Mol Diagn 10:236-241, 2008). Sensitivity for the mutation detection was 2% as determined by dilution studies using the NPM1-positive OCI-AML3 cell line.
Plasma Inhibitory Assay
The plasma inhibitory assay (PIA) using the TF-ITD cell line has been described previously (Levis M, Brown P, Smith BD, et al: Blood 108:3477-3483, 2006). Whole blood samples were collected into heparinized vacuum tubes and the plasma was separated by centrifugation. Frozen plasma samples were shipped via overnight mail to a central laboratory (The Johns Hopkins University, Baltimore, MD) on dry ice and stored frozen until used. Samples were thawed and clarified by centrifugation at 14,000 rpm for 2 minutes. All assays described herein were performed within 3 months of collection. For each time point, 3 × 106 TF-ITD cells were incubated, rocking gently, with 1 mL plasma at 37°C for 1 hour. The cells were washed twice with ice-cold phosphate-buffered saline and then lysed by resuspending them in lysis buffer (20 mmol/L Tris pH 7.4, 100 mmol/L NaCl, 1% Igepal (Sigma, St. Louis, MO), 1 mmol/L ethylenediaminetetra-acetate, 2 mmol/L NaVO4, plus Complete Protease Inhibitor (Roche, Indianapolis, IN) for 30 minutes while rocking. The lysate was clarified by centrifugation at 14,000 rpm. Anti-FLT3 antibody (S18; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the extract for overnight incubation; then protein A sepharose (Upstate Biotechnology, Lake Placid, NY) was added for an additional 2 hours. Following sodium dodecyl sulfate polyacrylamide gel electrophoresis, the immunoprecipitates were transferred to Immobilon membranes (Millipore, Bedford, MA), and immunoblotting was performed with antiphosphotyrosine antibody (4G10; Upstate Biotechnology) to detect phosphorylated FLT3. Blots were then stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins were visualized using chemiluminescence (Amersham, Piscataway, NJ), exposed on Kodak BioMax XAR Film, and developed and scanned using a BioRad GS800 densitometer (Bio-Rad Laboratories, Hercules, CA).
Table A1.
IC50 Estimates for Inhibition of Kinase Activity: Cell-Based Immunoblot Assays Using SEMK2 (FLT3-WT) or FLT3-ITD–Transfected Cells
| Drug (nM) | Culture Medium (10% serum) |
Plasma |
|
|---|---|---|---|
| FLT3-WT | FLT3-ITD | FLT3-ITD | |
| Sorafenib | 28 | 3 | 484 |
| Midostaurin (PKC412) | 21 | 3 | 1,700 |
| Lestaurtinib (CEP-701) | 13 | 3 | 700 |
Abbreviations: IC50, half maximal inhibitory concentration; FLT3-WT, FMS-like tyrosine kinase-3 wild type; FLT3-ITD, FLT3 internal tandem duplication.
Footnotes
Supported by a research grant from Bayer Pharmaceuticals and Onyx Pharmaceuticals, and in part by National Cancer Institute Leukemia Specialized Program of Research Excellence Grant No. P50 CA100632.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00542971.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Farhad Ravandi, Bayer Pharmaceuticals/Onyx Pharmaceuticals (C); Michael Andreeff, Bayer Pharmaceuticals/Onyx Pharmaceuticals (C) Stock Ownership: None Honoraria: Farhad Ravandi, Bayer Pharmaceuticals/Onyx Pharmaceuticals Research Funding: Farhad Ravandi, Bayer Pharmaceuticals/Onyx Pharmaceuticals; Hagop M. Kantarjian, Bristol-Myers Squibb, Novartis, Genzyme Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Farhad Ravandi, B. Nebiyou Bekele, Hagop M. Kantarjian
Provision of study materials or patients: Farhad Ravandi, Jorge E. Cortes, Daniel Jones, Stefan Faderl, Guillermo Garcia-Manero, Marina Y. Konopleva, Susan O'Brien, Zeev Estrov, Gautam Borthakur, Deborah Thomas, Rajyalakshmi Luthra, Mark Levis, Michael Andreeff, Hagop M. Kantarjian
Collection and assembly of data: Farhad Ravandi, Sherry R. Pierce, Mark Brandt, Anna Byrd
Data analysis and interpretation: Farhad Ravandi, Sherry R. Pierce, Mark Brandt, B. Nebiyou Bekele, Keith Pratz, Mark Levis
Manuscript writing: Farhad Ravandi, Daniel Jones, Mark Levis
Final approval of manuscript: Farhad Ravandi, Jorge E. Cortes, Daniel Jones, Stefan Faderl, Guillermo Garcia-Manero, Marina Y. Konopleva, Susan O'Brien, Zeev Estrov, Gautam Borthakur, Deborah Thomas, Sherry R. Pierce, Mark Brandt, Anna Byrd, B. Nebiyou Bekele, Keith Pratz, Rajyalakshmi Luthra, Mark Levis, Michael Andreeff, Hagop M. Kantarjian
REFERENCES
- 1.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 2.Small D. FLT3 mutations: Biology and treatment. Hematology Am Soc Hematol Educ Program. 2006:178–184. doi: 10.1182/asheducation-2006.1.178. [DOI] [PubMed] [Google Scholar]
- 3.Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: Analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 4.Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: A study of the AML Study Group Ulm. Blood. 2002;100:4372–4380. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 5.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 6.Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: Correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 7.Nakao M, Yokota S, Iwai T, et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 8.Hayakawa F, Towatari M, Kiyoi H, et al. Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–631. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 9.Gale RE, Green C, Allen C, et al. The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood. 2008;111:2776–2784. doi: 10.1182/blood-2007-08-109090. [DOI] [PubMed] [Google Scholar]
- 10.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 11.Kusec R, Jaksic O, Ostojic S, et al. More on prognostic significance of FLT3/ITD size in acute myeloid leukemia (AML) Blood. 2006;108:405–406. doi: 10.1182/blood-2005-12-5128. [DOI] [PubMed] [Google Scholar]
- 12.Ponziani V, Gianfaldoni G, Mannelli F, et al. The size of duplication does not add to the prognostic significance of FLT3 internal tandem duplication in acute myeloid leukemia patients. Leukemia. 2006;20:2074–2076. doi: 10.1038/sj.leu.2404368. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 15.Zheng R, Levis M, Piloto O, et al. FLT3 ligand causes autocrine signaling in acute myeloid leukemia cells. Blood. 2004;103:267–274. doi: 10.1182/blood-2003-06-1969. [DOI] [PubMed] [Google Scholar]
- 16.Ravandi F, Jilani I, Estey E, et al. Soluble phosphorylated fms-like tyrosine kinase III: FLT3 protein in patients with acute myeloid leukemia (AML) Leuk Res. 2007;31:791–797. doi: 10.1016/j.leukres.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Paez JG, Lee JC, et al. Identifying and characterizing a novel activating mutation of the FLT3 tyrosine kinase in AML. Blood. 2004;104:1855–1858. doi: 10.1182/blood-2004-02-0712. [DOI] [PubMed] [Google Scholar]
- 18.Kindler T, Breitenbuecher F, Kasper S, et al. Identification of a novel activating mutation (Y842C) within the activation loop of FLT3 in patients with acute myeloid leukemia (AML) Blood. 2005;105:335–340. doi: 10.1182/blood-2004-02-0660. [DOI] [PubMed] [Google Scholar]
- 19.Reindl C, Bagrintseva K, Vempati S, et al. Point mutations in the juxtamembrane domain of FLT3 define a new class of activating mutations in AML. Blood. 2006;107:3700–3707. doi: 10.1182/blood-2005-06-2596. [DOI] [PubMed] [Google Scholar]
- 20.Mead AJ, Linch DC, Hills RK, et al. FLT3 tyrosine kinase domain mutations are biologically distinct from and have a significantly more favorable prognosis than FLT3 internal tandem duplications in patients with acute myeloid leukemia. Blood. 2007;110:1262–1270. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 21.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: The combination matters—an analysis of 3082 patients. Blood. 2008;111:2527–2537. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 23.Mead AJ, Gale RE, Hills RK, et al. Conflicting data on the prognostic significance of FLT3/TKD mutations in acute myeloid leukemia might be related to the incidence of biallelic disease. Blood. 2008;112:444–445. doi: 10.1182/blood-2008-04-150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith BD, Levis M, Beran M, et al. Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood. 2004;103:3669–3676. doi: 10.1182/blood-2003-11-3775. [DOI] [PubMed] [Google Scholar]
- 25.Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood. 2005;105:54–60. doi: 10.1182/blood-2004-03-0891. [DOI] [PubMed] [Google Scholar]
- 26.DeAngelo DJ, Stone RM, Heaney ML, et al. Phase 1 clinical results with tandutinib (MLN518), a novel FLT3 antagonist, in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome: Safety, pharmacokinetics, and pharmacodynamics. Blood. 2006;108:3674–3681. doi: 10.1182/blood-2006-02-005702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knapper S, Burnett AK, Littlewood T, et al. A phase 2 trial of the FLT3 inhibitor lestaurtinib (CEP701) as first-line treatment for older patients with acute myeloid leukemia not considered fit for intensive chemotherapy. Blood. 2006;108:3262–3270. doi: 10.1182/blood-2006-04-015560. [DOI] [PubMed] [Google Scholar]
- 28.Levis M, Pham R, Smith BD, et al. In vitro studies of a FLT3 inhibitor combined with chemotherapy: Sequence of administration is important to achieve synergistic cytotoxic effects. Blood. 2004;104:1145–1150. doi: 10.1182/blood-2004-01-0388. [DOI] [PubMed] [Google Scholar]
- 29.Lowinger TB, Riedl B, Dumas J, et al. Design and discovery of small molecules targeting raf-1 kinase. Curr Pharm Des. 2002;8:2269–2278. doi: 10.2174/1381612023393125. [DOI] [PubMed] [Google Scholar]
- 30.Mori S, Cortes J, Kantarjian H, et al. Potential role of sorafenib in the treatment of acute myeloid leukemia. Leuk Lymphoma. 2008;49:2246–2255. doi: 10.1080/10428190802510349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Konopleva M, Ruvolo VR, et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia. 2008;22:808–818. doi: 10.1038/sj.leu.2405098. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Konopleva M, Shi YX, et al. Mutant FLT3: A direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–198. doi: 10.1093/jnci/djm328. [DOI] [PubMed] [Google Scholar]
- 34.Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: Sustained regression before and after allogeneic stem cell transplantation. Blood. 2009;113:6567–6571. doi: 10.1182/blood-2009-03-208298. [DOI] [PubMed] [Google Scholar]
- 35.Kantarjian H, O'Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 36.Atallah E, Cortes J, O'Brien S, et al. Establishment of baseline toxicity expectations with standard frontline chemotherapy in acute myelogenous leukemia. Blood. 2007;110:3547–3551. doi: 10.1182/blood-2007-06-095844. [DOI] [PubMed] [Google Scholar]
- 37.Levis M, Brown P, Smith BD, et al. Plasma inhibitory activity (PIA): A pharmacodynamic assay reveals insights into the basis for cytotoxic response to FLT3 inhibitors. Blood. 2006;108:3477–3483. doi: 10.1182/blood-2006-04-015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2009;113:3938–3946. doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wadleigh M, DeAngelo DJ, Griffin JD, et al. After chronic myelogenous leukemia: Tyrosine kinase inhibitors in other hematologic malignancies. Blood. 2005;105:22–30. doi: 10.1182/blood-2003-11-3896. [DOI] [PubMed] [Google Scholar]
- 40.Sawyers CL. Finding the next Gleevec: FLT3 targeted kinase inhibitor therapy for acute myeloid leukemia. Cancer Cell. 2002;1:413–415. doi: 10.1016/s1535-6108(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 41.Ravandi F, Talpaz M, Estrov Z. Modulation of cellular signaling pathways: Prospects for targeted therapy in hematological malignancies. Clin Cancer Res. 2003;9:535–550. [PubMed] [Google Scholar]
- 42.Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 43.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 44.Birg F, Courcoul M, Rosnet O, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80:2584–2593. [PubMed] [Google Scholar]
- 45.Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–1096. [PubMed] [Google Scholar]
- 46.Barry EV, Clark JJ, Cools J, et al. Uniform sensitivity of FLT3 activation loop mutants to the tyrosine kinase inhibitor midostaurin. Blood. 2007;110:4476–4479. doi: 10.1182/blood-2007-07-101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown P, Meshinchi S, Levis M, et al. Pediatric AML primary samples with FLT3/ITD mutations are preferentially killed by FLT3 inhibition. Blood. 2004;104:1841–1849. doi: 10.1182/blood-2004-03-1034. [DOI] [PubMed] [Google Scholar]
- 48.Knapper S, Mills KI, Gilkes AF, et al. The effects of lestaurtinib (CEP701) and PKC412 on primary AML blasts: The induction of cytotoxicity varies with dependence on FLT3 signaling in both FLT3-mutated and wild-type cases. Blood. 2006;108:3494–3503. doi: 10.1182/blood-2006-04-015487. [DOI] [PubMed] [Google Scholar]
- 49.Yee KW, Schittenhelm M, O'Farrell AM, et al. Synergistic effect of SU11248 with cytarabine or daunorubicin on FLT3 ITD-positive leukemic cells. Blood. 2004;104:4202–4209. doi: 10.1182/blood-2003-10-3381. [DOI] [PubMed] [Google Scholar]
- 50.Heidel F, Solem FK, Breitenbuecher F, et al. Clinical resistance to the kinase inhibitor PKC412 in acute myeloid leukemia by mutation of Asn-676 in the FLT3 tyrosine kinase domain. Blood. 2006;107:293–300. doi: 10.1182/blood-2005-06-2469. [DOI] [PubMed] [Google Scholar]
- 51.Piloto O, Wright M, Brown P, et al. Prolonged exposure to FLT3 inhibitors leads to resistance via activation of parallel signaling pathways. Blood. 2007;109:1643–1652. doi: 10.1182/blood-2006-05-023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bagrintseva K, Schwab R, Kohl TM, et al. Mutations in the tyrosine kinase domain of FLT3 define a new molecular mechanism of acquired drug resistance to PTK inhibitors in FLT3-ITD-transformed hematopoietic cells. Blood. 2004;103:2266–2275. doi: 10.1182/blood-2003-05-1653. [DOI] [PubMed] [Google Scholar]
- 53.Bagrintseva K, Geisenhof S, Kern R, et al. FLT3-ITD-TKD dual mutants associated with AML confer resistance to FLT3 PTK inhibitors and cytotoxic agents by overexpression of Bcl-x(L) Blood. 2005;105:3679–3685. doi: 10.1182/blood-2004-06-2459. [DOI] [PubMed] [Google Scholar]
- 54.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 55.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: A phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol. 2006;24:460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]