Abstract
Background
Ischemic preconditioning is an important intrinsic mechanism for neuroprotection. Preconditioning can also be achieved by exposure of neurons to K+ channel–opening drugs that act on adenosine triphosphate–sensitive K+ (KATP) channels. However, these agents do not readily cross the blood–brain barrier. Inhalational anesthetics which easily partition into brain have been shown to precondition various tissues. Here, the authors explore the neuronal preconditioning effect of modern inhalational anesthetics and investigate their effects on KATP channels.
Methods
Neuronal–glial cocultures were exposed to inhalational anesthetics in a preconditioning paradigm, followed by oxygen–glucose deprivation. Increased cell survival due to preconditioning was quantified with the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide reduction test. Recombinant plasmalemmal KATP channels of the main neuronal type (Kir6.2/SUR1) were expressed in HEK293 cells, and the effects of anesthetics were evaluated in whole cell patch clamp recordings.
Results
Both sevoflurane and the noble gas xenon preconditioned neurons at clinically used concentrations. The effect of sevoflurane was independent of KATP channel activation, whereas the effect of xenon required the opening of plasmalemmal KATP channels. Recombinant KATP channels were activated by xenon but inhibited by halogenated volatiles. Modulation of mitochondrial K-ATP channels did not affect the activity of KATP channels, thus ruling out an indirect effect of volatiles via mitochondrial channels.
Conclusions
The preconditioning properties of halogenated volatiles cannot be explained by their effect on KATP channels, whereas xenon preconditioning clearly involves the activation of these channels. Therefore, xenon might mimic the intrinsic mechanism of ischemic preconditioning most closely. This, together with its good safety profile, might suggest xenon as a viable neuroprotective agent in the clinical setting.
Early ischemic stroke after heart surgery has a high incidence and is associated with substantial disability and mortality rates.1-3 Although volatile anesthetics have been found to initiate early phase ischemic tolerance in neurons, models of focal brain ischemia suggest that it can be expected to take 24 h for the preconditioning to develop its full effectiveness.4,5 Therefore, cardiac surgical patients are likely to be exposed to the greatest risk of cerebral ischemic damage in a phase where they are least protected. Consequently, neuroprotective strategies should be implemented well in advance of surgery to allow pathways to become fully activated. This stratagem is possible with the use of pharmacologic preconditioning.
The concept of preconditioning describes the phenomenon that an organ achieves protection against potentially lethal insults through preexposure to harmful stimuli.6 The term gained wide recognition in the late 1980s after the observation that brief episodes of reduced myocardial perfusion before the extended, harmful ischemic period, diminished tissue damage, and preserved cardiac function.7 Subsequently, preconditioning was also found in other organs, such as brain and kidneys, and this boosted clinical and scientific interest in this phenomenon.
Although a large body of studies thus far failed to unravel the entire cellular signaling pathways that lead to preconditioning, it became clear that adenosine triphosphate–sensitive K+ (KATP) channels can play a critical role in the process.6 This view received further support from recent studies demonstrating that genetic ablation of the pore-forming subunit Kir6.2 of the plasmalemmal KATP channel causes the loss of ischemic preconditioning of cardiac tissue8-10 and the loss of neuroprotection against acute hypoxia.11 Although these findings unequivocally demonstrated that the opening of plasmalemmal KATP channels is beneficial for cell survival, several drawbacks limit the scope for clinical exploration. For example, currently there is no convincing evidence that established KATP channel openers such as diazoxide readily cross the blood–brain barrier. This is different with anesthetics because penetration of the blood–brain barrier is one of the defining characteristics of this class of substances. Moreover, there is not only a vast amount of clinical experience in their safe use, but outcome parameters can also be directly monitored.
The noble gas xenon has been proposed as an alternative to classic anesthetics partly because of its suggested organ protective effects and its overall benign side effect profile.12 However, its high production cost has so far stopped it from being used widely.13
A number of reports suggest that other inhalational anesthetics have preconditioning effects on cardiac tissues, and a few studies suggest they have neuroprotective properties, though there is no consensus about their mode of action.14-16
Those studies that explored the involvement of KATP channels in anesthetic preconditioning in the brain have focused almost exclusively on the role of mitochondrial K-ATP (mito K-ATP) channels17,18; no study has ever examined the involvement of plasmalemmal KATP channels in neuronal preconditioning induced by either of the newer inhalational anesthetics sevoflurane or xenon. Furthermore, the majority of studies have not investigated the effects of drugs directly on KATP currents but simply relied on the perceived specificity of the drugs used for channel subtypes.19
With the current study, we aim to clarify the role of KATP channels in preconditioning induced by the inhalational anesthetics xenon and sevoflurane. We hypothesize that the opening of plasmalemmal ATP-sensitive K+ channels rather than mito K-ATP channels is required for neuronal preconditioning by these drugs. We also postulate that to verify the preconditioning action of these drugs via KATP channels, their direct stimulatory action on currents through these ion channels in a recombinant expression system must be evident.
Materials and Methods
Preconditioning Studies
Tissue culture neuronal–glial cell cocultures were prepared as described previously.20 All procedures involving animals were performed in accordance with the Animals (Scientific Procedures) Act 1986 and approved by the Home Office, London, United Kingdom. In brief, brains from 1- to 2-day-old BALB/c mouse pups were removed from the skull and microdissected to obtain whole cerebral neocortices. After trypsination and resuspension, cells were plated at a density of 6.25 × 104 cells/cm2 on four-well plates (Nunc, Roskilde, Denmark). They were cultured in Eagle's minimum essential medium supplemented with 20 mm glucose, 26 mm NaHCO3, 10% fetal bovine serum, 10% heat-inactivated horse serum, Antibiotic-Antimycotic Solution (AAS; Gibco, Paisley, United Kingdom), 2 mm glutamine (Sigma, Poole, United Kingdom), and 10 ng/ml murine epidermal growth factor (Gibco). Glial cells reached confluence approximately 5 days after plating. Using a similar procedure, cortical neuronal cells were obtained from fetal BALB/c mice at 14–16 days of gestation and were plated at a density of 1.25 × 105 cells/cm2 onto the confluent monolayer of glial cells. Neuronal cells reached mature morphology within 10 days after plating, and cocultures were used for preconditioning experiments after a total of 15 ± 1 day. Cultures were assigned to one of the following protocols: preconditioning, oxygen–glucose deprivation (OGD) only, or naive control. Cultures in the preconditioning group were exposed to either 1 minimum alveolar concentration (MAC) xenon, 2 MAC sevoflurane, or 10 μm diazoxide for 2 h in the presence or absence of the KATP channel inhibitor tolbutamide (0.1 mm) or the inhibitor of mito K-ATP channels 5-hydroxy-decanoic acid (5-HD, 0.5 mm).
Preconditioning
Preconditioning was performed as described previously.21 Anesthetic-enriched medium was obtained by bubbling xenon or sevoflurane for 15 min through experimental medium containing Eagle's minimal essential medium, 21.1 mm glucose, and 38 mm NaHCO3 in a Drechsel bottle. Neuronal–glial cell cocultures were washed twice with HEPES-buffered solution (120 mm NaCl, 5.4 mm KCl, 0.8 mm MgCl2, 15 mm glucose, and 2.0 mm HEPES, titrated to pH 7.4 using 1 m NaOH) and then incubated with anesthetic-enriched medium or with experimental medium containing 10 μm diazoxide. Tolbutamide (0.1 mm) or 5-HD (0.5 mm) were added to some dishes as appropriate. Cocultures were then transferred to an airtight, temperature-controlled gas exposure chamber for 2 h. The gas composition in the chamber for the xenon experiments consisted of 20% O2,5% CO2, and 75% xenon (approximately 1 MAC). The gas composition in the sevoflurane experiments was 20% O2, 5% CO2, 71.7% N2, and 3.3% sevoflurane (2 MAC). The atmosphere in the chamber for diazoxide preconditioning consisted of 20% O2, 5% CO2, and 75% N2. After 2 h, cultures were removed from the chamber, washed with HEPES-buffered solution, and finally incubated in culture medium until further use.
Cocultures of the OGD group underwent the same protocol as the cultures in the preconditioning group except that instead of being exposed to HEPES-buffered solution containing the preconditioning drugs, they were washed with HEPES-buffered solution only. Cultures of the naive control group received the same pattern of solution changes but were not exposed to the preconditioning drugs or OGD.
Oxygen–Glucose Deprivation
To model ischemic injury in the brain, neuronal cells were subjected to OGD.22 Twenty-four hours after preconditioning, cultures were washed twice with HEPES-buffered solution and once with prewarmed OGD medium. OGD medium contained 116 mm NaCl, 5.4 mm KCl, 0.8 mm MgSO4, 1.0 mm NaH2PO4, 1.8 mm CaCl2, and 26 mm NaHCO3 and was bubbled with 5% CO2 and 95% N2 in a Drechsel bottle at 37°C. Cultures were then placed into an airtight gas exposure chamber with an anaerobic environment consisting of 5% CO2 and 95% N2 at 37°C for 75 min. OGD was terminated by removing cultures from the chamber and changing the media back to the culture medium.
MTT Reduction Test
The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) reduction test is a quantitative measurement of cellular metabolic activity and therefore widely used for the assessment of cellular viability and cell proliferation.23,24 In the presence of active mitochondrial and cytosolic redox systems, the tetrazolium salts of MTT are reduced to intensely blue-colored formazans, which can be quantified in a spectrophotometer. The measured optical density corresponds directly to the viability of the cells in culture. Cell viability is stable between 6 and 24 h after OGD25; therefore, the MTT test was performed on the following day. Neuronal–glial cocultures were incubated for 2 h in 600 μl phosphate-buffered saline with 0.5 mg/ml MTT (Merck, Darmstadt, Germany) to allow uptake of MTT into the cells. After removing MTT from cultures, cells were dissolved and homogenized in 1 ml dimethyl sulfoxide (DMSO). Then, 0.3 ml of each sample was transferred to one well of a 96-well plate. The optical density (OD) was measured in triplicate at 570 nm in an MRX Microplate Reader (Dynex Technologies, Chantilly, VA).
The OD readings were then converted into normalized cell viability readings using equation 1:
| (1) |
where % Cvia is cell viability expressed in percent, ODdrug is the optical density reading of preconditioned cells, ODcontrol is the optical density reading of untreated naive cells, and ODOGD is the optical density reading of nonpreconditioned cells, which were exposed to OGD only.
Recombinant KATP Channel Studies
Tissue Culture
HEK293 cells were maintained in growth medium (minimum essential medium enriched with 10 ml per 500 ml penicillin–streptomycin solution, 5 ml per 500 ml minimum essential medium nonessential amino acids, 50 ml per 500 ml fetal bovine serum, and 2 mm l-glutamine) in 5% CO2–95% O2 in a humidified incubator at 37°C. When the cells were 80% confluent (after approximately 4 days), they were plated onto glass coverslips coated with poly-D-lysine (1 mg/ml), and after a 6-h resting period, transient transfection was performed using the calcium phosphate method.26 Cells were transfected with pcDNA3 vectors containing the coding sequence of mouse Kir6.2 (Genbank D5058127,28), rat SUR1 (Genbank L4062429), and green fluorescent protein. Cells were then incubated at 3% CO2–97% O2 for 6–8 h. Subsequently, cells were washed twice with phosphate-buffered saline and incubated in growth medium in 5% CO2–95% O2 in a humidified incubator at 37°C for 24–96 h until use for electrophysiologic recordings.
Electrophysiology
Stock solutions of tolbutamide (50 mm), diazoxide (20 mm), and pinacidil (10 mm) were prepared in 100 mm NaOH. Solutions containing sevoflurane, isoflurane, or halothane were prepared as volume fractions of a saturated aqueous solution. The concentration of these saturated solutions was taken as 11.8, 15.3, and 17.5 mm, respectively.30 Solutions for the xenon experiments were prepared by bubbling extracellular solution with pure gases (xenon, nitrogen, oxygen) at a rate of 30 ml/min for 20 min, producing saturated solutions for the individual gases.31 The test solutions with fractional gas concentrations were then obtained by mixing adequate volumes of the individual solutions with maintaining 20% oxygen–saturated solution and the balance (80%) being made up by varying amounts of nitrogen- and xenon-saturated solutions. Immediately after mixing, these solutions were transferred to glass syringe barrels containing a polypropylene float.31 All other drugs were added directly to the experimental solutions, which were prepared freshly each day. Xenon was obtained from Air Products (Basingstoke, United Kingdom), sevoflurane and isoflurane were obtained from Abbott (Queenborough, Kent, United Kingdom), and nitrogen and oxygen were obtained from BOC (Manchester, United Kingdom). All other chemicals were from Sigma (Poole, Dorset, United Kingdom).
For electrophysiologic recordings, a coverslip with transfected HEK cells was transferred into a recording chamber mounted under an upright microscope (Zeiss Axioskop 2FS; Carl Zeiss Ltd., Welwyn Garden City, United Kingdom) with epifluorescence. During the course of the experiment, cells were constantly superfused at 3–5 ml/min with extracellular solution of the following composition: 118 mm NaCl, 3 mm KCl, 1 mm MgCl2, 1.5 mm CaCl2, and 25 mm HEPES (pH adjusted to 7.4 with NaOH). Test drugs were added to this solution either directly or from stock solutions as described above. Only cells that were successfully transfected with green fluorescent protein as evident from green fluorescence (excitation: 395–440 nm, emission: 470–600 nm) were selected for electrophysiologic recordings.
Patch pipettes were pulled from thin-walled borosilicate glass (GC150TF; Harvard Apparatus, Edenbridge, Kent, United Kingdom) and had resistances of 3–5 MΩ when filled with pipette solution. The pipette solution contained 120 mm KCl, 1 mm NaOH, 1 mm MgCl2, 1 mm CaCl2, 5 mm EGTA, 5 mm HEPES, and 0.3 mm K2ATP (pH adjusted to 7.3 with KOH; final [K+] approximately 140 mm). Whole cell currents were recorded at a holding potential of −20 mV and at 20°–24°C. Currents were evoked by repetitive 700-ms hyperpolarizing voltage ramps from −20 mV to −120 mV every 15 s and recorded using an EPC9 patch clamp amplifier (HEKA Elektronik, Lambrecht, Germany). Recordings were digitized at 0.5 kHz and analyzed using Pulse/Pulsefit software (HEKA) running on a personal computer. To confirm that all effects on the holding current at −20 mV were indeed caused by modulation of K+ currents, reversal potentials were determined for each experimental condition and compared (table 1). There was no statistically significant difference in the reversal potential between the groups.
Table 1.
Reversal Potential of the Drug-modulated Current in HEK293 Cells
| Reversal Potential, mV | No. of Observations | |
|---|---|---|
| Xenon | −80 ± 3 | 10 |
| Sevoflurane | −83 ± 3 | 22 |
| Halothane | −76 ± 2 | 22 |
| Isoflurane | −77 ± 2 | 14 |
| Diazoxide | −77 ± 3 | 10 |
| Tolbutamide | −84 ± 3 | 10 |
| Washout | −80 ± 2 | 12 |
No significant difference was observed for any of the drugs tested.
Data Analysis
Concentration–response relations for the volatile anesthetics were obtained by alternating the control solution with a test concentration of anesthetic. The extent of inhibition by the anesthetic was then expressed as a fraction of the mean of the value obtained in the control solution before and after anesthetic application. Concentration–response curves were fitted to the Hill equation (equation 2):
| (2) |
where I is the holding current at −20 mV in the presence of the drug, Ic is the current in the absence of the drug, [Vol] is the volatile anesthetic concentration, Ki is the anesthetic concentration at which inhibition is half maximal, and h is the slope factor (Hill coefficient).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). All data were expressed as mean ± SEM. To test for differences between groups, two-tailed unpaired t test or one-way analysis of variance followed by the Tukey multiple comparison test were used as appropriate. A value of P < 0.05 was considered significant.
Results
Xenon but Not Sevoflurane Preconditioning Requires Functional Plasmalemmal KATP Channels
The current study was designed to analyze the role of KATP channels in preconditioning induced by xenon and sevoflurane using OGD in neuronal–glial cocultures as an in vitro model of ischemic brain injury (fig. 1). In this model, 70–80 min of OGD was sufficient to maximally damage neurons, with glial cells being left largely unharmed. The extent of the injury was found to remain unchanged from 6 to 24 h after OGD, with no further evidence for either a delayed second burst of damage or repair mechanisms taking place.32
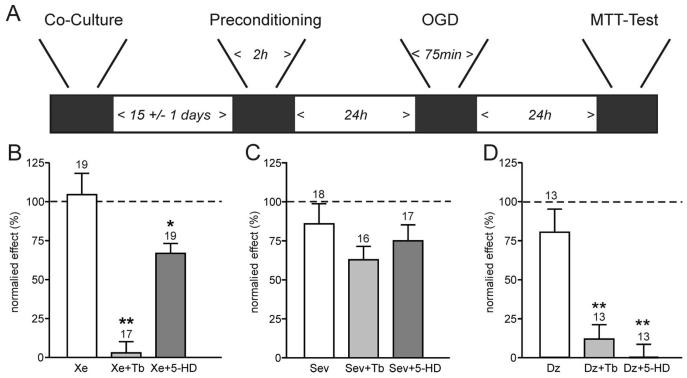
Fig. 1.
Preconditioning by xenon but not sevoflurane involves adenosine triphosphate–sensitive K+ (KATP) channels. A depicts the experimental timeframe adhered to. Neuronal and glial cells were cocultured for 2 weeks before a 2-h preconditioning stimulus with the anesthetics ± antagonists. After a further 24 h, cultures were exposed to oxygen–glucose deprivation (OGD) for 75 min, and a further 24 h later, damage was assessed using an 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. B, C, and D show the mean ± SEM data for preconditioning experiments. Cell viability was measured with MTT as optical density at 570 nm. The “normalized effect” describes the relative viability compared with maximal damage (OGD only, no preconditioning) and maximal survival (no OGD, naive cultures). Therefore, 100% cell viability represented the viability observed in naive cultures at the end of the protocol, whereas 0% cell viability indicated the viability found in cultures exposed to OGD only. B demonstrates that xenon preconditioning (Xe, 1 minimum alveolar concentration [MAC]) is effectively abolished by the KATP channel inhibitor tolbutamide (Xe + Tb, 0.1 mm), but not the mitochondrial K-ATP channel blocker 5-hydroxy-decanoic acid (5-HD, 0.5 mm). (C) In contrast, while sevoflurane (Sev, 2 MAC) effectively preconditions the cultures, this effect is not significantly reduced by tolbutamide (Tb) or 5-HD. (D) Preconditioning by the KATP channel opener diazoxide (Dz, 0.01 mm) is effectively blocked by either tolbutamide or 5-HD. Numbers (n) are given above the bars. *P < 0.05; **P < 0.01.
Exposure to the preconditioning anesthetics xenon or sevoflurane or to the potassium channel opener diazoxide 24 h before the OGD insult effectively prevented neuronal cell loss (survival: 80–100%; figs. 1B–D). The addition of the KATP channel inhibitor tolbutamide to the xenon group during preconditioning completely abolished the protective effect of xenon (fig. 1B). Xenon's protective effect was also slightly reduced by the addition of the mito K-ATP channel inhibitor 5-hydroxydecanoate (5-HD). Although statistically significant, the reversal was not complete, because cell viability remained high in cultures treated concomitantly with xenon and 5-HD (fig. 1B). In contrast, the preconditioning effect of sevoflurane could not be reversed with either the KATP channel inhibitor tolbutamide or the mito K-ATP channel inhibitor 5-HD (fig. 1C). Finally, diazoxide preconditioning was completely abolished by 5-HD and to a slightly lesser extent by tolbutamide (fig. 1D).
Diazoxide, at a concentration of 10 μm, is widely seen as specific for mito K-ATP channels. However, this is only true for cardiac tissue where KATP channels have a SUR2A subunit. The SUR1 subunit of neuronal KATP channels also responds to diazoxide. Therefore, it is not surprising that the preconditioning effect of diazoxide was sensitive to the KATP channel blocker tolbutamide as well as the mito K-ATP channel antagonist 5-HD, suggesting a role for both channels.
These results indicated that opening of plasmalemmal KATP channels, but not mito K-ATP channels, is essential for neuronal preconditioning by xenon, and demonstrated that sevoflurane preconditioning does not involve the opening of either plasmalemmal KATP channels or mito K-ATP channels.
Evaluation of the Effects of Volatile Anesthetics on KATP Currents
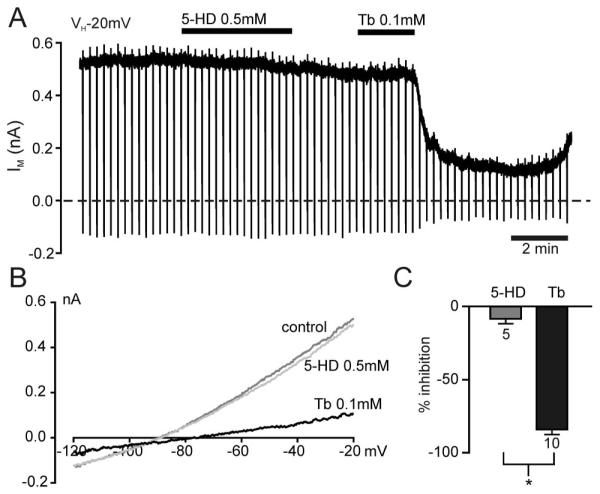
Plasmalemmal KATP channels exist in a wide variety of organs, but their subunit composition differs between tissues. For example, neurons and pancreatic β cells carry channels mostly composed of Kir6.2 and SUR1 subunits, whereas myocardial and skeletal muscle have channels made up of Kir6.2 and SUR2A.33-35 Consequently, it is not easily possible to extrapolate functional data and physiologic pathways related to KATP channel activation from one organ to the other (e.g. heart to brain). Here, we used Kir6.2 and SUR1 as the components for KATP channels because these are the subunits of KATP channels in most neurons. HEK293 cells were transiently transfected with Kir6.2 and SUR1 complementary DNA. In contrast to untransfected HEK293 cells, these cells exhibited a large standing outward current at −20 mV, had a resting potential more negative than −65 mV, and were sensitive to tolbutamide, consistent with the expression of KATP channels (fig. 2). During the first 5 min of whole cell voltage clamp recordings, an increase of outward current was observed consistent with the washout of adenosine triphosphate from the cytosol and subsequent progressive opening of plasmalemmal KATP channels. This was then followed by a stable holding current of 1.05 ± 0.12 nA (n = 30) in Kir6.2 and SUR1–transfected HEK293 cells (figs. 2A and B). This holding current was inhibited reversibly by the addition of the specific KATP channel blocker tolbutamide (100 μm; n = 17; figs. 2A and C). The zero-current potential for Kir6.2 and SUR1–transfected HEK293 cells was −77 ± 2 mV (n = 30), consistent with the fact that most of the whole cell conductance was made up by KATP channels. In contrast, untransfected HEK293 exhibited a zero-current potential of −40 ± 5 mV and a holding current of 22 ± 10 pA at −20 mV (n = 13). This current was not enhanced by the K+ channel opener diazoxide (200 μm; n = 6), consistent with the absence of functional KATP channels in untransfected HEK293 cells.
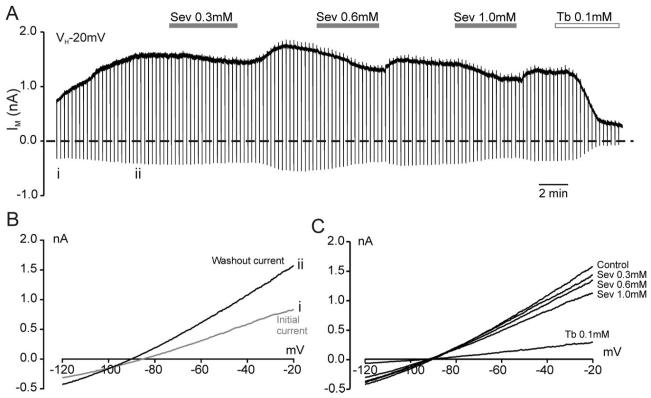
Fig. 2.
Adenosine triphosphate–sensitive K+ (KATP) channels expressed in HEK293 cells are mildly inhibited by sevoflurane. (A) Whole cell recording at a holding potential of −20 mV from an HEK293 cell transfected with Kir6.2 and SUR1. Sevoflurane (Sev) and tolbutamide (Tb) were bath applied as indicated by the horizontal bars. The dashed line indicates the zero-current level. Vertical deflections indicate the current response to a 700-ms voltage ramp from −20 mV to −120 mV. These current responses at points i and ii were plotted against the command voltage to obtain current–voltage relations (B). These demonstrate that the washout current has a reversal potential near the potassium equilibrium potential. (C) Current–voltage relations taken from A showing the concentration-dependent small inhibition of the KATP current by sevoflurane at clinically relevant concentrations, compared with the block by tolbutamide.
Addition of sevoflurane to the superfusate reduced the KATP current reversibly in a concentration-dependent manner (figs. 2A and C). This lack of activation of KATP current by sevoflurane at clinically relevant concentrations (0.3–2.5 mm = 0.8–7.5 MAC) was consistent with the lack of effect of KATP channel inhibitors on the preconditioning effect of sevoflurane. Next, the effects of sevoflurane were compared with those of two other volatile anesthetics: halothane and isoflurane. At a concentration of 1 mm, halothane had the strongest inhibitory effect on whole cell KATP currents, followed by isoflurane, and the smallest effect by sevoflurane (figs. 3A and B). This difference in potency was also reflected in the concentration–response curves for the different drugs (fig. 3C). Halothane inhibited KATP currents with an IC50 of 1.3 ± 0.4 mm, isoflurane had an IC50 of 2.1 ± 0.6 mm, and the effect of sevoflurane was extrapolated to an IC50 of approximately 50 mm.
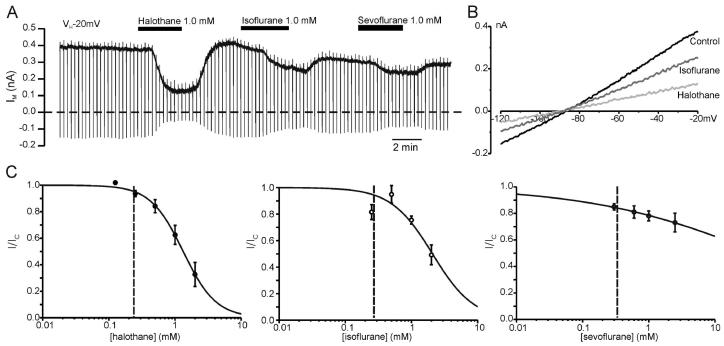
Fig. 3.
Halogenated volatile anesthetics inhibit adenosine triphosphate–sensitive K+ (KATP) channels. (A) Whole cell recording at a holding potential of −20 mV from an HEK293 cell transfected with Kir6.2 and SUR1. The dashed line indicates the zero-current level. Halothane, isoflurane, and sevoflurane (1 mm each) were bath applied as indicated by the horizontal bars. Each drug reversibly inhibited the whole cell KATP current. (B) Current–voltage relations taken from a recording as that shown in A demonstrating that the current inhibited by the volatile anesthetics has a reversal potential near EK. (C) Concentration–response curves of the inhibitory effect of halothane (n = 4–8), isoflurane (n = 3–5), and sevoflurane (n = 3–8) on whole cell KATP currents. The current in presence of the drug (I) is expressed as a fraction of the current in its absence (IC). The solid lines describe the best fit with a Hill equation using Ki = 1.32 mm and Hill coefficient (h) = 1.75 for halothane, Ki = 2.08 mm and h = 1.39 for isoflurane, and Ki = 47 mm and h = 0.34 for sevoflurane. In each case, it is assumed that complete inhibition of the KATP current could theoretically be achieved with high enough concentrations of the anesthetic. One minimum alveolar concentration is indicated by the vertical dashed line in each graph.
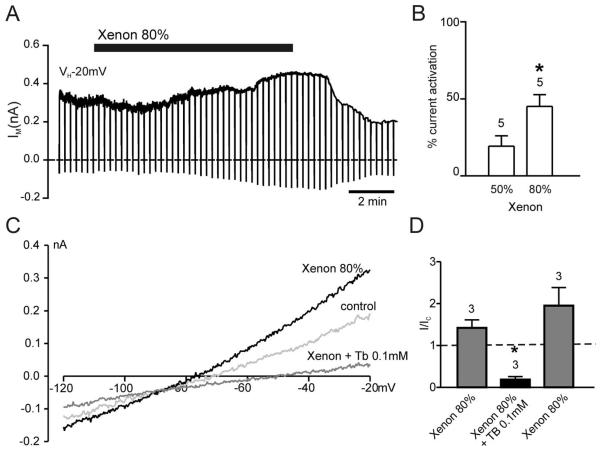
Next, the effects of xenon were tested on KATP currents. Xenon was applied at two concentrations: 50% and 80%. An 80% xenon solution is equivalent to 1 MAC or 3.4 mm xenon. Consequently, 50% xenon equates to 0.6 MAC and 2.1 mm. HEK293 cells transfected with Kir6.2 and SUR1 showed an increase in outward current by 19 ± 7% when superfused with a 50% xenon solution (n = 5) and by 44 ± 7% (n = 5) when exposed to 80% xenon solution as compared with control solution (80% N2; fig. 4). The reversal potential of this current (fig. 4C and table 1) indicated it being a K+ current. Furthermore, the current was inhibited by 0.1 mm tolbutamide (figs. 4C and D), and no increase in outward current in response to 80% xenon was observed in untransfected HEK293 cells (n = 5). These results strongly suggest that the xenon-induced K+ current was due to the opening of Kir6.2/SUR1 channels. This activation of plasmalemmal KATP channels was consistent with the preconditioning effect seen in the neuronal–glial cocultures being KATP channel dependent.
Fig. 4.
Xenon activates adenosine triphosphate–sensitive K+ (KATP) channels. (A) Whole cell recording from HEK293 cell transfected with Kir6.2 and SUR1 demonstrating reversible activation of the current by 80% xenon. (B) Mean effects of the two test concentrations of xenon on the holding current at −20 mV from KATP channel–transfected HEK293 cells. (C) Current–voltage relations in the absence and presence of 80% xenon and xenon + 0.1 mm tolbutamide. Xenon potentiated the KATP current, whereas tolbutamide completely inhibited the current, even in the presence of xenon. (D) Mean data from experiments as depicted in C. Mean holding current at −20 mV in the presence of the drug expressed as a fraction of that in the absence of any drug. Xenon led to an increase in holding current and tolbutamide blocked both the xenon-potentiated current and the current in the absence of xenon (dashed line). Numbers (n) are given above the bars. *P < 0.05.
Lack of Modulation of KATP Channels via Mitochondrial KATP Channels or by Drugs that Act on These
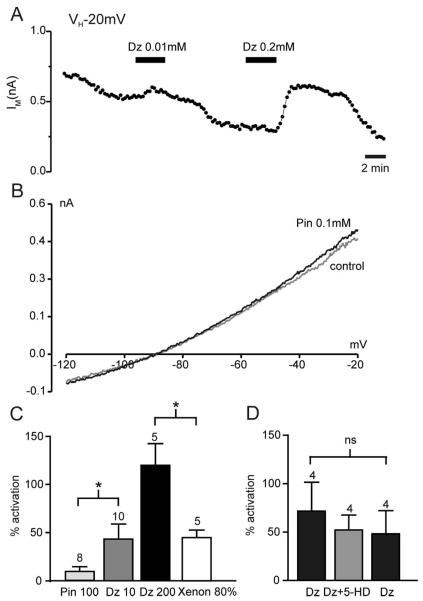
We next performed a series of experiments to demonstrate that the activity of KATP channels in HEK293 cells is not significantly modulated by mito K-ATP channels under our experimental conditions. First, whereas the KATP channel antagonist tolbutamide decreased current amplitude dramatically, no significant reduction in whole cell current was observed using the mito K-ATP channel inhibitor 5-HD (fig. 5). Equivalent results were obtained when using KATP channel openers (fig. 6). Diazoxide and pinacidil have both been reported to open mito K-ATP channels,36,37 but in addition, diazoxide activates KATP channels containing SUR1, and pinacidil activates KATP channels containing SUR2.38 Consequently, diazoxide is expected to be mito K-ATP channel specific in systems that do not contain SUR1 (e.g., heart muscle), and pinacidil would be specific for mito K-ATP channels in tissues not containing SUR2 (e.g., most neurons). In HEK293 cells expressing Kir6.2 and SUR1, pinacidil did not increase the whole cell current significantly (figs. 6B and C). We took this as an indication that opening of mito K-ATP channels does not modulate KATP currents in these cells. In contrast, diazoxide concentration-dependently increased the whole cell current (figs. 6A and C) in accord with its direct effect on KATP channels containing SUR1. In fact, at a concentration of 10 μm, diazoxide was able to stimulate the KATP current to a similar extend as 80% xenon (fig. 6C). Because both of these drugs effectively precondition at these concentrations, one might postulate that an increase in KATP current by approximately 50% is sufficient to initiate preconditioning. However, because diazoxide is known as a potent activator of mito K-ATP channels, it was explored whether mito K-ATP channel activation and subsequent alterations in mitochondrial function might contribute significantly to the KATP current seen with 10 μm diazoxide. To test this hypothesis, the KATP current was enhanced by 10 μm diazoxide and then 5-HD was added. No significant reduction of the diazoxide-elicited current was observed under these conditions (fig. 6D). These results established that 10 μm diazoxide activates KATP channels independently from its effects on mito K-ATP channels. Taken together, these results provide strong evidence that the effects of volatile anesthetics on KATP whole cell currents observed in this study are not secondary to the interaction of these drugs with mito K-ATP channels.
Fig. 5.
Adenosine triphosphate–sensitive K+ (KATP) currents in whole cell recordings are not affected by specific blockers of mitochondrial KATP (mito K-ATP) channels. (A) Whole cell recording demonstrating the lack of effect of the mito K-ATP channel blocker 5-hydroxy-decanoic acid (5-HD, 0.5 mm) on KATP channels made up of Kir6.2 and SUR1. In contrast, tolbutamide (Tb) strongly and reversibly inhibited the whole cell current. The drugs were applied as indicated by the bars above the recording. (B) Current–voltage relations in the absence (control) and presence of 0.5 mm 5-HD or 0.1 mm tolbutamide. (C) Mean data from experiments as that shown in A. *P < 0.05.
Fig. 6.
Whole cell adenosine triphosphate–sensitive K+ (KATP) currents are not affected by specific openers of mito K-ATP channel openers. (A) Whole cell recording from HEK293 cell expressing Kir6.2 and SUR1. Plotted is the mean holding current at −20 mV every 15 s. The SUR1-specific K+ channel opener diazoxide (Dz) is applied as indicated by the solid bars and concentration-dependently potentiated the whole cell current. (B) Current–voltage relations demonstrating the lack of effect of the cardiac K+ channel opener pinacidil (Pin, 0.1 mm) on Kir6.2/SUR1 currents in HEK293 cells. (C) Mean effects of the various K+ channel openers on Kir6.2/SUR1 currents. (D) Diazoxide at 10 μm increases the plasma KATP current independently of its potential effect on mitochondrial KATP channels, as demonstrated by the lack of effect of the mitochondrial KATP channel inhibitor 5-hydroxy-decanoic acid (5-HD) on the diazoxide activation of the whole cell current. Numbers (n) are given above the bars. *P < 0.05.
Discussion
Neuronal Preconditioning by Anesthetics
The current study demonstrated that the mechanisms by which neuronal preconditioning is achieved can vary between individual inhalational anesthetics. More precisely, we were able to identify for xenon a pathway which was dependent on opening of plasmalemmal KATP channels. Conversely, sevoflurane preconditioning was plasmalemmal KATP channel independent. Importantly, neither of these anesthetics showed a substantial involvement of mito K-ATP channels in its preconditioning action. This lack of effect cannot be due to pathologic changes in our preparation because the control preconditioning with diazoxide, which acts in neurons on both plasmalemmal KATP channels and on mito K-ATP channels, was indeed impaired by both the KATP channel antagonist tolbutamide and the mito K-ATP channel blocker 5-HD. Given the dependence of ischemic preconditioning on functional plasmalemmal KATP channels, as demonstrated by the lack of this preconditioning in cardiac muscle of Kir6.2−/− mice,8 it seems as if (1) xenon mimics ischemic preconditioning and (2) sevoflurane acts on a different target. Whether this target is downstream from the activation of KATP channels or involves an entirely independent pathway for preconditioning is unclear. A similar observation has been made in cerebellar brain slices, where blocking KATP channels had no effect on isoflurane preconditioning.39 Similarly to these results, Marinovic et al. 40 reported that cardiac cytoprotection did not involve sarcolemmal KATP channel opening during the preconditioning phase with isoflurane.
Anesthetic Effects on Recombinant Neuronal Plasmalemmal KATP Channels
Contribution of Mitochondrial K-ATP Channels
Activation of mito K-ATP channels would be expected to dissipate the mitochondrial membrane potential, thus causing a reduction in adenosine triphosphate production.37,41 With plasmalemmal KATP channels being archetypal adenosine triphosphate sensors,38 it would be expected that this decrease in adenosine triphosphate production would lead to channel activation and an increase in outward current (e.g., as seen with cyanide exposure). However, our observation that neither pinacidil nor 5-HD had any effect on the activity of plasmalemmal KATP channels indicates that modulation of mito K-ATP cannot influence plasmalemmal KATP channels in our system. Therefore, the observed effects of anesthetics on these channels cannot be explained by an indirect effect via mito K-ATP channels.
Direct Effects on Plasmalemmal KATP Channels
Probably the most interesting finding of this study was that the noble gas xenon acts as a K-channel opener. In previous studies that were primarily aimed at identifying the target for xenon's anesthetic action, this noble gas has been identified as an antagonist for the glycine binding site on N-methyl-d-aspartate receptors,42 and it has been shown that xenon activates selective two-pore-domain potassium channels31 (for review, see Preckel et al.43). Here, we have demonstrated that the tolbutamide-sensitive preconditioning with xenon is mirrored by the activation of plasmalemmal KATP channels by xenon at 1 MAC. At this concentration, xenon activated KATP channels as efficiently as the specific KATP channel opener diazoxide at its preconditioning concentration of 10 μm. Our results can be seen as indication that an increase in KATP channel activity by 50% is sufficient to trigger preconditioning responses. They also clearly demonstrate that the neuroprotective effect of xenon is achieved at anesthetic or even subanesthetic concentrations and thus can be accomplished with clinically safe doses.
In contrast to xenon, sevoflurane did not precondition in a KATP channel–dependent manner, and sevoflurane also did not activate plasmalemmal KATP channels. In fact, slight inhibition was observed. Similar results were also found for isoflurane and halothane. Both of these inhibited the plasmalemmal KATP channel mildly at 1 MAC, but more substantially than sevoflurane at higher concentrations. Similarly, it has previously been shown that barbiturates inhibit neuronal KATP channels at supraclinical concentrations.44
A recent study investigating the acute effects of 4% sevoflurane on the membrane potential of hippocampal CA1 pyramidal cells also did not see an activation of KATP channels despite the fact that hypoxia elicited such a hyperpolarization, which was also enhanced after preexposure to sevoflurane.45 These results probably reflect our finding that sevoflurane is able to precondition, but not in a KATP channel–dependent manner. Inhibition by halogenated anesthetics might be a general feature of inwardly-rectifying K+ channels, because several members of this ion channel family were inhibited to varying degrees by these agents.46 In the case of the KATP channel, the SUR subunit might have an additional modulatory influence on the anesthetic sensitivity, because Bienengraeber et al.47 reported that the SUR2A subunit of cardiac sarcolemmal KATP channels conferred isoflurane activation of the K+ current under acidic conditions.
Physiologic Significance of KATP Channel Inhibition
Here, we have shown that halogenated anesthetics inhibit plasmalemmal KATP channels. This should have little effect because KATP channels are closed under physiologic conditions in most tissues. They would only open under conditions of metabolic stress, such as ischemia. However, a notable exception is the pancreatic β cell, where KATP channel opening controls the glucose-dependent insulin release.48 Consequently, inappropriate KATP channel inhibition could potentially lead to hyperinsulinemia and hypoglycemia. In addition, patients who take antianginal medication such as the K-channel opener nicorandil or antihypertensives such as cromakalim or pinacidil might lose some protection if exposed to drugs that inhibit KATP channels.49,50
The halogenated volatiles tested here had inhibitory effects on KATP currents at 1 MAC (fig. 3), but currently there are not sufficient data to allow a critical evaluation of potential interactions between anesthetics and K-channel openers. Furthermore, because it is unknown whether these drugs interact with the potassium channel subunit or the sulfonylurea receptor (SUR1 in this case), it is not clear whether the cardiac or the smooth muscle plasmalemmal KATP channel is affected at all.
Physiologic Consequence of KATP Channel Activation
Conversely, drugs that open KATP channels can potentially interfere with the sulfonylurea-induced insulin secretion in diabetic patients. However, our finding that tolbutamide prevents the opening of KATP channels by xenon argues that on the contrary diabetes treatment might interfere with the organ-protective effect afforded by xenon. This is reminiscent of the interference of sulfonylurea drugs with the antianginal drug nicorandil.49 Although this is a valid concern for peripheral tissue, the fact that tolbutamide does not easily partition into the brain51,52 makes xenon a viable choice even for diabetic patients.
Most KATP channel openers do not cross the blood–brain barrier under physiologic conditions. Nevertheless, studies have reported neuroprotective effects of these drugs in vivo. However, rather than exerting their effects directly on the neuronal plasma membrane, the observed effect might be secondary to increased cerebral blood flow due to the vasodilatory effect of these drugs. They open KATP channels in vascular smooth muscle cells.53 Similarly, xenon has been shown to stimulate cerebral blood flow54-56 and to dilate pial vessels.57 This might indicate that xenon activates KATP channels of the smooth muscle subtype Kir6.1/SUR2B and raises the question of whether xenon differs from classic KATP channel openers by having no, or a reduced, tissue specificity and thus works via a novel mechanism.
In summary, we have established xenon as a novel plasmalemmal KATP channel opener (that can readily cross the blood–brain barrier) on both a molecular and a functional basis. Furthermore, we have demonstrated two distinct preconditioning pathways, one that is KATP channel dependent and one that is not. Further studies are likely to reveal subtle differences in the protection afforded by the KATP channel–dependent pathway that probably matches the naturally occurring ischemic preconditioning most closely, and the protection via the KATP channel–independent pathway. This might open new doors for the treatment of stroke patients. Finally, xenon, with its favorable safety profile, might be the drug of choice to preoptimize patients before surgery, especially for procedures that carry some risk of inducing ischemic stroke, such as heart surgery or carotid endarterectomy.
Acknowledgments
The authors thank Mahmuda Hossain, Ph.D. (Technician, Department of Anaesthetics, Pain Medicine and Intensive Care, Chelsea and Westminster Hospital, Imperial College London, London, United Kingdom), and Cleoper Paule, Ph.D. (Research Student, Department of Anaesthetics, Pain Medicine and Intensive Care, Chelsea and Westminster Hospital, Imperial College London), for their technical support.
Supported by the Medical Research Council, London, United Kingdom, and the Westminster Medical School Research Trust, London, United Kingdom.
Footnotes
Presented at the Annual Meeting of the American Society of Anesthesiologists, Chicago, Illinois, October 17, 2006, and the Annual Meeting of the Society for Neuroscience, San Diego, California, November 6, 2007.
References
- 1.Selim M. Perioperative stroke. N Engl J Med. 2007;356:706–13. doi: 10.1056/NEJMra062668. [DOI] [PubMed] [Google Scholar]
- 2.Toumpoulis IK, Anagnostopoulos CE, Chamogeorgakis TP, Angouras DC, Kariou MA, Swistel DG, Rokkas CK. Impact of early and delayed stroke on in-hospital and long-term mortality after isolated coronary artery bypass grafting. Am J Cardiol. 2008;102:411–7. doi: 10.1016/j.amjcard.2008.03.077. [DOI] [PubMed] [Google Scholar]
- 3.Lisle TC, Barrett KM, Gazoni LM, Swenson BR, Scott CD, Kazemi A, Kern JA, Peeler BB, Kron IL, Johnston KC. Timing of stroke after cardiopulmonary bypass determines mortality. Ann Thorac Surg. 2008;85:1556–62. doi: 10.1016/j.athoracsur.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitano H, Kirsch JR, Hurn PD, Murphy SJ. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–28. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: Temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–50. doi: 10.1161/01.str.29.9.1937. [DOI] [PubMed] [Google Scholar]
- 6.Hanley PJ, Daut J. K(ATP) channels and preconditioning: A re-examination of the role of mitochondrial K(ATP) channels and an overview of alternative mechanisms. J Mol Cell Cardiol. 2005;39:17–50. doi: 10.1016/j.yjmcc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Murray CE, Jennings RB, Reimer KA. Preconditioning with ischaemia: A delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 8.Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, Miki T, Seino S, Terzic A. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol. 2003;284:H2106–13. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- 9.Nakaya H, Miki T, Seino S, Yamada K, Inagaki N, Suzuki M, Sato T, Yamada M, Matsushita K, Kurachi Y, Arita M. Molecular and functional diversity of ATP-sensitive K+ channels: The pathophysiological roles and potential drug targets [in Japanese] Nippon Yakurigaku Zasshi. 2003;122:243–50. doi: 10.1254/fpj.122.243. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, Shimizu T, Seino S, Inagaki N. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 12.Preckel B, Schlack W. Inert gases as the future inhalational anaesthetics? Best Pract Res Clin Anaesthesiol. 2005;19:365–79. doi: 10.1016/j.bpa.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Sanders RD, Maze M. Xenon: From stranger to guardian. Curr Opin Anaesthesiol. 2005;18:405–11. doi: 10.1097/01.aco.0000174957.97759.f6. [DOI] [PubMed] [Google Scholar]
- 14.Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034:147–52. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Ma D, Hossain M, Pettet GK, Luo Y, Lim T, Akimov S, Sanders RD, Franks NP, Maze M. Xenon preconditioning reduces brain damage from neonatal asphyxia in rats. J Cereb Blood Flow Metab. 2006;26:199–208. doi: 10.1038/sj.jcbfm.9600184. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Traystman RJ, Murphy SJ. Inhalational anesthetics as preconditioning agents in ischemic brain. Curr Opin Pharmacol. 2008;8:104–10. doi: 10.1016/j.coph.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehl F, Payne RS, Roewer N, Schurr A. Sevoflurane-induced preconditioning of rat brain in vitro and the role of KATP channels. Brain Res. 2004;1021:76–81. doi: 10.1016/j.brainres.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 18.Kaneko T, Yokoyama K, Makita K. Late preconditioning with isoflurane in cultured rat cortical neurones. Br J Anaesth. 2005;95:662–8. doi: 10.1093/bja/aei228. [DOI] [PubMed] [Google Scholar]
- 19.Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–7. doi: 10.1097/00000539-200301000-00047. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm S, Ma D, Maze M, Franks NP. Effects of xenon on in vitro and in vivo models of neuronal injury. Anesthesiology. 2002;96:1485–91. doi: 10.1097/00000542-200206000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Ma D, Hossain M, Rajakumaraswamy N, Franks NP, Maze M. Combination of xenon and isoflurane produces a synergistic protective effect against oxygen-glucose deprivation injury in a neuronal-glial co-culture model. Anesthesiology. 2003;99:748–51. doi: 10.1097/00000542-200309000-00034. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: Calcium-dependent and calcium-independent mechanisms of neuronal injury. J Neurosci. 1993;13:3510–24. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch Biochem Biophys. 1993;303:474–82. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi S, Abe T, Gotoh J, Fukuuchi Y. Substrate-dependence of reduction of MTT: A tetrazolium dye differs in cultured astroglia and neurons. Neurochem Int. 2002;40:441–8. doi: 10.1016/s0197-0186(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 25.Rajakumaraswamy N, Ma D, Hossain M, Sanders RD, Franks NP, Maze M. Neuroprotective interaction produced by xenon and dexmedetomidine on in vitro and in vivo neuronal injury models. Neurosci Lett. 2006;409:128–33. doi: 10.1016/j.neulet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–52. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki N, Inazawa J, Seino S. cDNA sequence, gene structure, and chromosomal localization of the human ATP-sensitive potassium channel, uKATP-1, gene (KCNJ8) Genomics. 1995;30:102–4. doi: 10.1006/geno.1995.0018. [DOI] [PubMed] [Google Scholar]
- 28.Sakura H, Ammala C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–44. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: A regulator of insulin secretion. Science. 1995;268:423–6. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 30.Jenkins A, Franks NP, Lieb WR. Effects of temperature and volatile anesthetics on GABA(A) receptors. Anesthesiology. 1999;90:484–91. doi: 10.1097/00000542-199902000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–52. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 32.Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 33.Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci. 2000;21:439–45. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- 34.Fujita A, Kurachi Y. Molecular aspects of ATP-sensitive K+ channels in the cardiovascular system and K+ channel openers. Pharmacol Ther. 2000;85:39–53. doi: 10.1016/s0163-7258(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 35.Liss B, Roeper J. Molecular physiology of neuronal K-ATP channels. Mol Membr Biol. 2001;18:117–27. [PubMed] [Google Scholar]
- 36.Garlid KD, Paucek P, Yarov YV, Sun X, Schindler PA. The mitochondrial K-ATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–9. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 37.Holmuhamedov EL, Jovanovic S, Dzeja PP, Jovanovic A, Terzic A. Mitochondrial ATP-sensitive K+ channels modulate cardiac mitochondrial function. Am J Physiol. 1998;275:H1567–76. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- 38.Trapp S, Ashcroft FM. A metabolic sensor in action: News from the ATP-sensitive K+-channel. News Physiol Sci. 1997;12:255–63. [Google Scholar]
- 39.Zheng S, Zuo Z. Isoflurane preconditioning reduces Purkinje cell death in an in vitro model of rat cerebellar ischemia. Neuroscience. 2003;118:99–106. doi: 10.1016/s0306-4522(02)00767-4. [DOI] [PubMed] [Google Scholar]
- 40.Marinovic J, Bosnjak ZJ, Stadnicka A. Distinct roles for sarcolemmal and mitochondrial adenosine triphosphate-sensitive potassium channels in isoflurane-induced protection against oxidative stress. Anesthesiology. 2006;105:98–104. doi: 10.1097/00000542-200607000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Ashcroft FM, Trapp S. The ATP-sensitive potassium channel: A metabolic sensor. In: Storey KB, editor. Molecular Mechanisms of Metabolic Arrest: Life in Limbo. BIOS Scientific Publishers; Oxford: 2001. pp. 43–76. [Google Scholar]
- 42.Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-d-aspartate receptor by the anesthetics xenon and isoflurane: Evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–67. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 43.Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology. 2006;105:187–97. doi: 10.1097/00000542-200607000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Ohtsuka T, Ishiwa D, Kamiya Y, Itoh H, Nagata I, Saito Y, Yamada Y, Sumitomo M, Andoh T. Effects of barbiturates on ATP-sensitive K channels in rat substantia nigra. Neuroscience. 2006;137:573–81. doi: 10.1016/j.neuroscience.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Lei B, Popp S, Meng F, Cottrell JE, Kass IS. Sevoflurane immediate preconditioning alters hypoxic membrane potential changes in rat hippocampal slices and improves recovery of CA1 pyramidal cells after hypoxia and global cerebral ischemia. Neuroscience. 2007;145:1097–107. doi: 10.1016/j.neuroscience.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 46.Yamakura T, Lewohl JM, Harris RA. Differential effects of general anesthetics on G protein–coupled inwardly rectifying and other potassium channels. Anesthesiology. 2001;95:144–53. doi: 10.1097/00000542-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 47.Bienengraeber M, Warltier DC, Bosnjak ZJ, Stadnicka A. Mechanism of cardiac sarcolemmal adenosine triphosphate–sensitive potassium channel activation by isoflurane in a heterologous expression system. Anesthesiology. 2006;105:534–40. doi: 10.1097/00000542-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Ashcroft FM. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J Clin Invest. 2005;115:2047–58. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reimann F, Ashcroft FM, Gribble FM. Structural basis for the interference between nicorandil and sulfonylurea action. Diabetes. 2001;50:2253–9. doi: 10.2337/diabetes.50.10.2253. [DOI] [PubMed] [Google Scholar]
- 50.Gribble FM, Reimann F. Sulphonylurea action revisited: The post-cloning era. Diabetologia. 2003;46:875–91. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 51.Takanaga H, Murakami H, Koyabu N, Matsuo H, Naito M, Tsuruo T, Sawada Y. Efflux transport of tolbutamide across the blood-brain barrier. J Pharm Pharmacol. 1998;50:1027–33. doi: 10.1111/j.2042-7158.1998.tb06918.x. [DOI] [PubMed] [Google Scholar]
- 52.Murakami H, Takanaga H, Matsuo H, Ohtani H, Sawada Y. Comparison of blood-brain barrier permeability in mice and rats using in situ brain perfusion technique. Am J Physiol Heart Circ Physiol. 2000;279:H1022–8. doi: 10.1152/ajpheart.2000.279.3.H1022. [DOI] [PubMed] [Google Scholar]
- 53.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 54.Gur D, Yonas H, Jackson DL, Wolfson SK, Jr, Rockette H, Good WF, Maitz GS, Cook EE, Arena VC. Measurement of cerebral blood flow during xenon inhalation as measured by the microspheres method. Stroke. 1985;16:871–4. doi: 10.1161/01.str.16.5.871. [DOI] [PubMed] [Google Scholar]
- 55.Junck L, Dhawan V, Thaler HT, Rottenberg DA. Effects of xenon and krypton on regional cerebral blood flow in the rat. J Cereb Blood Flow Metab. 1985;5:126–32. doi: 10.1038/jcbfm.1985.16. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann A, Dettmers C, Schuier FJ, Wassmann HD, Schumacher HW. Effect of stable xenon on regional cerebral blood flow and the electroencephalogram in normal volunteers. Stroke. 1991;22:182–9. doi: 10.1161/01.str.22.2.182. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda T, Nakayama H, Yanagi K, Mizutani T, Miyabe M, Ohshima N, Toyooka H. The effects of 30% and 60% xenon inhalation on pial vessel diameter and intracranial pressure in rabbits. Anesth Analg. 2001;92:1245–50. doi: 10.1097/00000539-200105000-00030. [DOI] [PubMed] [Google Scholar]