Abstract
Neural prosthetics capable of recording or stimulating neuronal activity may restore function for patients with motor and sensory deficits resulting from injury or degenerative disease. However, overcoming inconsistent recording quality and stability in chronic applications remains a significant challenge. A likely reason for this is the reactive tissue response to the devices following implantation into the brain, which is characterized by neuronal loss and glial encapsulation. We have developed a neural stem cell-seeded probe to facilitate integration of a synthetic prosthesis with the surrounding brain tissue. We fabricated parylene devices that include an open well seeded with neural stem cells encapsulated in an alginate hydrogel scaffold. Quantitative and qualitative data describing the distribution of neuronal, glial, and progenitor cells surrounding seeded and control devices is reported over four time points spanning three months. Neuronal loss and glial encapsulation associated with cell-seeded probes were mitigated during the initial week of implantation and exacerbated by six weeks post-insertion compared to control conditions. We hypothesize that graft cells secrete neuroprotective and neurotrophic factors that effect the desired healing response early in the study, with subsequent cell death and scaffold degradation accounting for a reversal of these results later. Applications of this biohybrid technology include future long-term neural recording and sensing studies.
Introduction
Neuroprosthetic devices have the capacity to record signals from a patient's uninjured cortex. These signals may then be used to place an exterior assistive device under the patient's control, thus restoring function while circumventing nervous tissue that has been damaged by injury or disease (Schwartz, Cui et al. 2006; Donoghue, Nurmikko et al. 2007; Fitzsimmons, Drake et al. 2007). In the last decade, monkeys were shown to be able to successfully control a cursor on a computer screen or a robotic arm using voluntary cortical signals (Taylor, Tillery et al. 2002; Carmena, Lebedev et al. 2003). These feats have been demonstrated more recently by tetraplegic humans implanted years after injury (Hochberg, Serruya et al. 2006; Donoghue, Nurmikko et al. 2007). Further, cortical control of a complex, multi-jointed robotic arm with a “hand” gripper has been achieved by monkeys during a self-feeding task (Velliste, Perel et al. 2008). Thus, neuroprostheses have the potential to restore some level of function to over 200,000 patients currently suffering from full or partial paralysis in the U.S. (Polikov, Tresco et al. 2005).
While such results are encouraging, these devices are currently plagued by inconsistent performance in terms of recording longevity and stability (Rousche and Normann 1998; Liu, McCreery et al. 1999; Williams, Rennaker et al. 1999; Nicolelis, Dimitrov et al. 2003; Polikov, Tresco et al. 2005; Liu, McCreery et al. 2006; Schwartz, Cui et al. 2006). In order for neuroprostheses to be useful in research and clinical settings, stable, long-term recordings from large populations of neurons in multiple brain areas must be reliably and reproducibly achieved (Lebedev and Nicolelis 2006). Following implantation into the brain, a reactive tissue response occurs to the prostheses, which is believed to be a major contributing factor to their inconsistent performance. An encapsulation layer composed of microglia and astrocytes isolates the device from the surrounding tissue, and neuronal density within the effective recording radius of the probe is reduced (Edell, Toi et al. 1992; Turner, Shain et al. 1999; Szarowski, Andersen et al. 2003; Kim, Hitchcock et al. 2004; Biran, Martin et al. 2005; Polikov, Tresco et al. 2005). The glial sheath creates a diffusion barrier to the transmission of ions through the extracellular space which may affect recording quality (Roitbak and Sykova 1999). Additionally, impedance magnitude at 1 kHz (the nominal bandwidth of an action potential) increases at recording sites with “extensive” glial reactivity (Williams, Hippensteel et al. 2007). While the relationship between recording quality and neuronal density has yet to be defined, it is reasonable to believe that neuronal loss would result in a reduction of signal quality. The reported 40% loss of neuronal density within 100 microns of the device surface in the first month after implantation is particularly concerning, given that this region produces the large amplitude, reliably separated spikes useful for neural prostheses (Henze, Borhegyi et al. 2000; Biran, Martin et al. 2005).
The tissue response to implanted prostheses is a characteristic reaction to central nervous system (CNS) damage (Silver and Miller 2004). Stem and progenitor cell therapy have shown promise as one approach to repair CNS injury, where the cause of recovery may be cell replacement and reinnervation. Alternatively, the “bystander” or “chaperone” effect, which describes the ability of transplanted cells to support host tissue by secreting therapeutic factors, correcting a biochemical deficit or inhibiting cytotoxic injury processes, may be the mechanism of healing (Teng, Lavik et al. 2002; Lindvall, Kokaia et al. 2004; Pluchino, Zanotti et al. 2005; Gaillard, Prestoz et al. 2007). In support of the bystander effect, several studies suggest that undifferentiated neural stem cells have an innate ability to promote healing and axonal regeneration of host neurons as well as a reduction in glial scar formation (Ourednik, Ourednik et al. 2002; Teng, Lavik et al. 2002; Lu, Jones et al. 2003; Heine, Conant et al. 2004; Llado, Haenggeli et al. 2004; Pluchino, Zanotti et al. 2005). This is believed to be due to constitutive secretion of multiple neurotrophic factors (Ourednik, Ourednik et al. 2002; Teng, Lavik et al. 2002; Lu, Jones et al. 2003; Llado, Haenggeli et al. 2004), as well as degrading molecules which are inhibitory to axonal growth (Heine, Conant et al. 2004). Additionally, the ability of neural precursor cells to protect injured CNS tissue by maintaining an undifferentiated state and displaying neuroprotective immune functions has been reported (Pluchino, Zanotti et al. 2005).
In recent years, reports of seeding synthetic materials with cells to improve the integration of implanted devices with living tissue have emerged (Stieglitz 2007). This “biohybrid” approach has been used to intervene in the foreign body response to implanted devices in a variety of experimental paradigms. Vascular endothelial growth factor-secreting cells have been used to induce angiogenesis surrounding implantable glucose sensors and improve their function (Klueh, Dorsky et al. 2005). Coating biosensor materials with adipose-derived stromal cells has been explored as a means of reducing fibrous capsule formation around prostheses (Prichard, Reichert et al. 2007; Prichard, Reichert et al. 2008). Seeding cochlear implants with brain derived neurotrophic factor (BDNF)-secreting fibroblasts enhanced the survival of neighboring spiral ganglion neurons (Rejali, Lee et al. 2007). Limited studies which describe biohybrid neural prostheses and their in vivo assessment exist. Kennedy reports the use of a segment of sciatic nerve placed inside a cone electrode to encourage axonal growth into the electrode (Kennedy 1989). Recordings were taken over 200 days in rats, and a later study revealed central myelinated axons and dendrites penetrating the device (Kennedy, Mirra et al. 1992). The “sieve electrode” is designed to potentially contain embryonic neurons to restore the interface between lesioned peripheral nerves and muscle (Stieglitz, Ruf et al. 2002). To our knowledge, no reports of in vivo evaluations of cell-seeded cortical neural prostheses are currently available.
In this study, we developed a neural stem cell (NSC)-seeded biohybrid probe hypothesized to improve device integration with brain tissue, and evaluated the tissue response to the probes in vivo in rats over a three month time period. These parylene probes contain a hollow well containing an NSC-seeded alginate hydrogel scaffold. Alginate is a biocompatible polysaccharide polymer which forms a hydrogel following cross-linking with divalent cations such as calcium. This material is commonly used to encapsulate secretory cells, localize them to an implant site, and provide an immuno-isolating barrier between the engrafted cells and the host tissue (Orive, Hernandez et al. 2003). We have previously demonstrated the release of neurotrophic and neuroprotective factors from NSCs encapsulated in alginate (Purcell, Singh et al. 2009). Here, we show that combining a cortical prosthesis with this scaffold diminishes the early tissue response and facilitates initial integration of the device with the surrounding brain tissue. Cell-seeding is associated with increased neuronal loss and glial encapsulation after six weeks. We hypothesize that the evolving response to neural stem cell-seeding may be related to changes in cell function and viability over time.
Materials and Methods
Probe Manufacture
The probes were microfabricated in the Lurie Nanofabrication Facility at the University of Michigan using methods previously described, and the process is illustrated in Figure 1 (Seymour and Kipke 2007). Briefly, a sacrificial release layer of SiO2 was grown on a Si wafer. Parylene-C (Specialty Coating, Indianapolis, IN) was deposited (5-microns thick) via chemical vapor deposition. SU-8 2025 (Microchem, Newton, MA) was spincoated and patterned to create the core of each tine. Parylene was etched using oxygen plasma RIE. Probes were released using hydrofluoric acid and then thoroughly rinsed in acetone, ethanol and DI water. Final dimensions of the probe were 2.6 mm long, 200 microns wide and 40 microns thick. An opening through the thickness of the device measuring 100 microns across and 2.1 mm in length was designed to carry scaffold material (Figures 1, 3a). Support arms to this feature had square cross-sections measuring 40 microns on each side.
Figure 1.
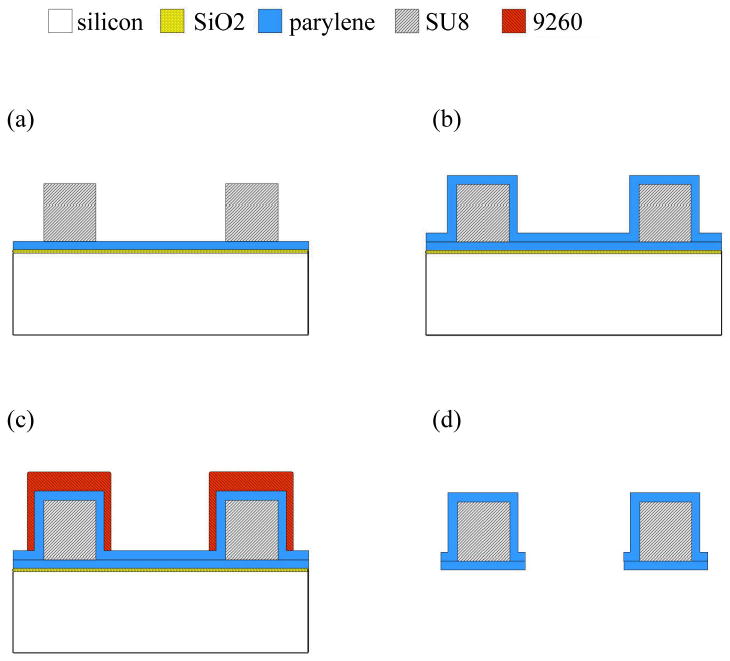
Cross-sectional view of wafer level fabrication. (a) Parylene deposited on SiO2 sacrificial layer and the SU-8 patterned shank. (b) Parylene encapsulated SU-8 structure. (c) 9260 resist patterned to form thick mask over shank. (d) Etched and released final structure. Photolithography masks used steps in (a, c).
Figure 3.
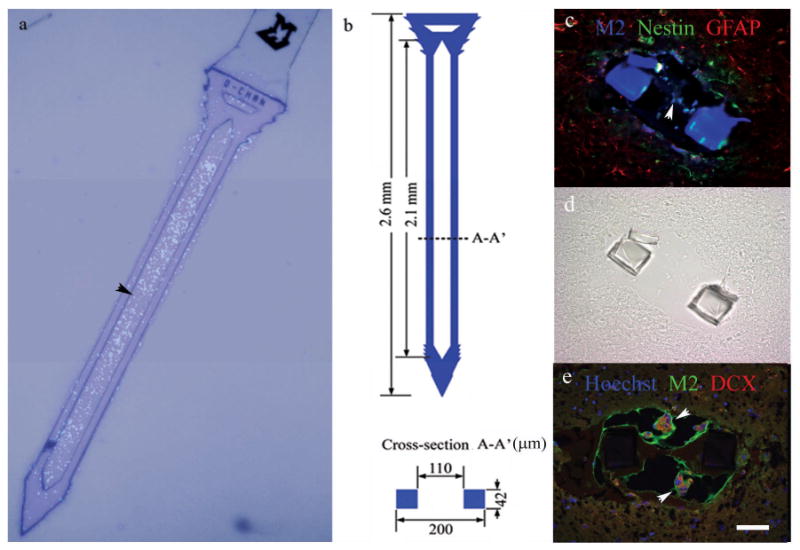
A neural stem cell-seeded probe (a), and associated dimensions (b). Cells are Hoechst-stained and nuclei appear fluorescent blue (a). Cross-section views of neural stem cell-seeded probes 1 day following implantation from two animals (c-e). The companion brightfield DIC image for (c) is shown in (d) for reference due to autofluorescence of the parylene probe. Graft cells (M2-labeled) were associated with nestin (c), GFAP (c), and doublecortin (DCX, e) expression. Alginate also autofluoresces green. Scale = 50 microns for (c)-(e).
Cell Culture and Probe Scaffolding
E14 murine cortical neural stem cells (NSCs) were obtained from StemCell Technologies (Vancouver, BC), cultured as neurospheres and expanded with 20 ng/mL epidermal growth factor (EGF) according to the supplier's protocol. The culture and stem cell characteristics of these cells have been described (Reynolds and Weiss 1992; Reynolds and Weiss 1996). The cells are a heterogeneous mixture of stem and progenitor cells (Reynolds and Weiss 1996). Probe scaffolding was achieved by mixing a cell slurry with alginate 50:50, rapidly dip-coating the probe in the mixture, and cross-linking with a 0.1 M calcium chloride solution. The alginate was highly purified with 68% guluronic acid content and MW = 219,000 g/mol from NovaMatrix (Drammen, Norway). This composition was chosen based on a previous study (Purcell, Singh et al. 2009). Probes had a final concentration of 10,000,000 cells/mL in 1% w/v alginate, resulting in an estimated 100 cells in the microscale probe well based on the well volume. This is an underestimate of the actual number of cells contained on the probe, as the cell-seeded volume appears to coat the surface of the probes as well (Figure 3a). This additional volume contains approximately 500 cells when the scaffold layer extended an average of 40 microns beyond the probe surface. Non-seeded probes were treated identically to NSC-seeded probes, with the exceptions that cells (“alginate” probes), or both cells and alginate (“probe alone”), were omitted from the coating procedure. Alginate coated probes were prepared by mixing alginate 50:50 with cell culture media. All probes were stored in media prior to implantation.
Probe Implantation
Sixteen male Sprague Dawley rats (300–350 g) were each implanted with four untethered probes (two containing NSC-seeded alginate, one with alginate only, and one untreated probe) using a surgical procedure similar to those previously reported (Vetter, Williams et al. 2004; Seymour and Kipke 2007). Anesthesia was achieved via a ketamine cocktail. A 3×3 mm craniotomy was centered over the somatosensory cortex (-2.0 mm AP, +/- 4.0 mm ML from Bregma) in each hemisphere. Dura was resected and two probes (one seeded, one unseeded) were manually inserted in each craniotomy with an approximate 2 mm span between them. Calcium alginate was used for duraplasty followed by surgical closure with silicone and dental acrylic. No immunosuppressive treatments were used. All procedures complied with the United States Department of Agriculture guidelines for the care and use of laboratory animals and were approved by the University of Michigan Animal Care and Use Committee.
Histology and Quantitative Analysis
After implantation, animals were deeply anesthetized and transcardially perfused with 4% paraformaldehyde at the appropriate time point (1 day, 1 week, 6 weeks, or 3 months, n=4 animals per time point). Brain tissue was explanted, postfixed overnight in 4% paraformaldehyde, and cryoembedded following sucrose protection. Probes were left in situ for histology. Serial 12 micron thick sections along the shank of the probes were collected, and eight tissue sections between cortical layers II and V were randomly selected and immunostained for NeuN (1:100, Millipore Corporation, Billerica, MA) and Neurofilament (1:1000, Novus Biologicals, Littleton,CO) and counterstained with Hoechst (1 μg/mL, Invitrogen Corporation, Carlsbad, CA) for quantitative analysis of tissue response. Six sections were stained with Ox-42 (1:100, Serotec, Oxford, UK), glial fibrillary acidic protein (GFAP, 1:100), and Hoechst (1 μg/mL) to assess glial encapsulation qualitatively. Six sections per probe were stained for nestin (1:100, StemCell Technologies, Vancouver, BC), GFAP (1:100, Sigma, St. Louis, MO), and M2 (1:10, Iowa Hybridoma Bank, Iowa City, Iowa) for identification of murine-derived NSCs and possible differentiation status. Six additional sections were stained with neuronal class III β-tubulin (TUJ-1) antibody (1:500, StemCell Technologies, Vancouver, BC), NG-2 (1:500, Millipore Corporation, Billerica, MA) and M2 (1:10, Iowa Hybridoma Bank, Iowa City, Iowa) to identify neuronal and oligodendrocyte precursors respectively. Six sections per probe were stained for doublecortin (1:1000, Abcam, Cambridge, UK) and M2, and counterstained with Hoechst. Where positive M2 staining was observed, a total of twelve sections were stained with nestin and GFAP and analyzed for a quantification of the expression of these markers as an indication of differentiation status. Approximately 60 cells per animal were counted in the two animals where positive staining was observed.
The immunohistochemistry procedure has been described (Cowell, Plane et al. 2003). Briefly, sections were hydrated in buffer (PBS or TBS), blocked with 10% normal goat serum, and incubated overnight with primary antibodies at 4°C. Sections were then rinsed, incubated in the appropriate secondary antibodies (1:200 Alexa-350, 488, and/or 568, Invitrogen), counterstained with Hoechst, and coverslipped with ProLong Gold (Invitrogen). Antibody solutions included 0.3% triton X-100 and 5% normal goat serum. Buffer was used in place of primary antibody for controls. Confocal images were collected with an Olympus FV500 microscope with a 20X or 40X objective.
A MatLAB graphical user interface was developed in-house to facilitate cell counting, using a method that has been previously described (Seymour and Kipke 2007). An outline of the exterior edge of the probe cross-section was delineated using a differential interference contrast (DIC) and UV fluorescence image. A blinded technician selected all nuclei as either neuronal (NeuN+, Hoechst+) or nonneuronal (Hoechst+ only) within a software-defined 175 micron radius of the probe surface. A representative image after cell counting and the resulting histograms are illustrated in Figure 2. Four optical images (3 mm step size) in each of eight physical sections per probe were analyzed. The software algorithm used the coordinates of the user-selected nuclei to calculate the shortest distance to the probe surface, bin the counts by distance, and calculate the sampling area of each bin to result in neuronal and non-neuronal densities as a function of distance from the probe.
Figure 2.
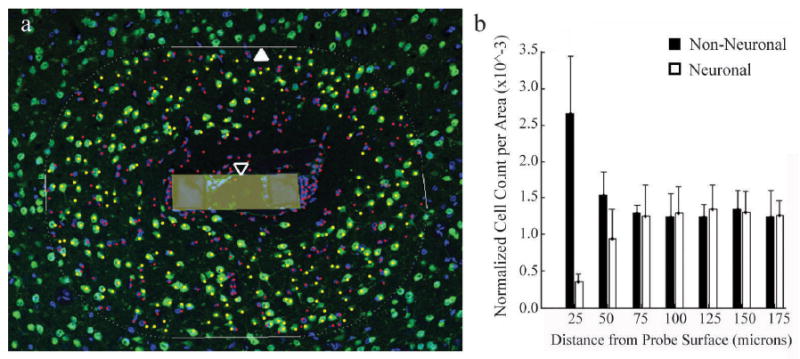
Neuronal (NeuN+ in green, Hoechst+ in blue) and non-neuronal (NeuN-, Hoechst+) nuclei counted by a blinded observer (a), and the resulting output (b). The counting boundary (closed arrow) was calculated based on the user-defined probe surface boundary (open arrow, shaded area), and a blinded observer manually selected neuronal and non-neuronal nuclei within this region (shown in yellow and red, respectively). The location of each nucleus relative to the probe surface was counted, binned in 25 micron increments, and normalized to area.
Statistics
For neuronal and non-neuronal density, eight sections were analyzed for each of four probes per animal, with four animals evaluated for each of four time points. Data were binned into seven 25-micron distance bins from the probe surface; a total of 2,688 individual data points for both neuronal and non-neuronal densities were used to generate the statistical models.
A linear mixed effects model was used to evaluate the responses of neuronal density and non-neuronal density due to the clustering of multiple probe evaluations within each animal. The fixed effects were the probe condition, distance, and time. The random effect was the animal subject. Analysis was performed using the statistical package SPSS (Chicago, IL). Results were assessed by Fisher's Least Significant Difference method and termed statistically significant at the P=0.05 level.
A univariate general linear model was used to assess the correlative relationship between neuronal density and non-neuronal density. Common correlation methods (such as Pearson's correlation) were not used as these techniques do not take in to account the fixed and random effects of the data, and may yield an inflated correlation coefficient. The fixed effects were the probe condition, distance, and time. The random effect was the animal subject. Non-neuronal density was defined as a covariate in order to assess it as a predictor of neuronal density.
Results and Discussion
Probe Design and In Vivo NSC Detection
Parylene devices were successfully designed and fabricated containing a hollow well for scaffold retention (Arrow, Figure 3a-b). In addition to filling the open channel of the neural probe, a thin layer of scaffold material covered coated probes, and cells were detected slightly outside of the well boundary in vivo (Figure 3a, c, e). This exterior scaffold layer varied in size along the shank of each probe, and appeared to be 40 microns thick on average upon visual inspection; probe coatings were similar between devices. Immunohistochemistry for the murine-specific marker M2 allowed for the in vivo detection of cortical neural stem cells derived from embryonic mice based on a previous report (Teng, Lavik et al. 2002). Alginate and parylene autofluoresced in green and blue respectively enabling visualization of these structures (Figure 3c-e). Neural stem cells were detected at the 1 day time point in vivo, and mainly expressed a combination of nestin and GFAP, with limited doublecortin (DCX, typically indicative of neuronal precursors) expression also observed (Figure 3c-e). Approximately 44% of M2-positive cells were associated with nestin, 5.5% expressed GFAP, and 36% displayed both of these markers, indicating undifferentiated cells, astrocytes, and glial progenitors respectively (Messam, Hou et al. 2000). There was no evidence of M2-positive oligodendrocyte precursors.
The markers observed were similar to those reported in the literature, where the majority of neural stem and progenitor cells express nestin and GFAP, and neuronal and oligodendrocyte markers are observed far less frequently (Reynolds and Weiss 1996; Ourednik, Ourednik et al. 2002; Teng, Lavik et al. 2002). The somewhat limited detection of NSCs (at the one day time point only) and identification of their differentiation status in vivo is likely due to the relatively small number of cells initially present in the microscale device and the reportedly large loss (>90%) in progenitor graft cell viability within the initial week following implantation (Sortwell, Pitzer et al. 2000; Bakshi, Keck et al. 2005). While scaffolds appeared relatively intact 24 hours after insertion (Figure 3c, e), the loss of graft cells upon insertion is also a possibility.
Scaffold Condition Effects on Neuronal and Non-Neuronal Densities
Quantitative and qualitative analysis of neuronal and non-neuronal densities within the first 175 microns of the probes revealed the degree of injury around the implants as a function of scaffold condition, time, and distance from the probe surface (Figures 4-6). Neural stem cell-seeded probes had a higher surrounding neuronal density compared to untreated probes at one day post-implantation (Figure 4a, P=0.023). Neuronal density was increased around NSC probes compared to both control conditions at the one week time point (Figure 4a, P≤0.002). The experimental probes had reduced non-neuronal encapsulation compared to alginate-only probes at one day and untreated probes at one week after insertion (Figure 4b; P≤0.001).
Figure 4.
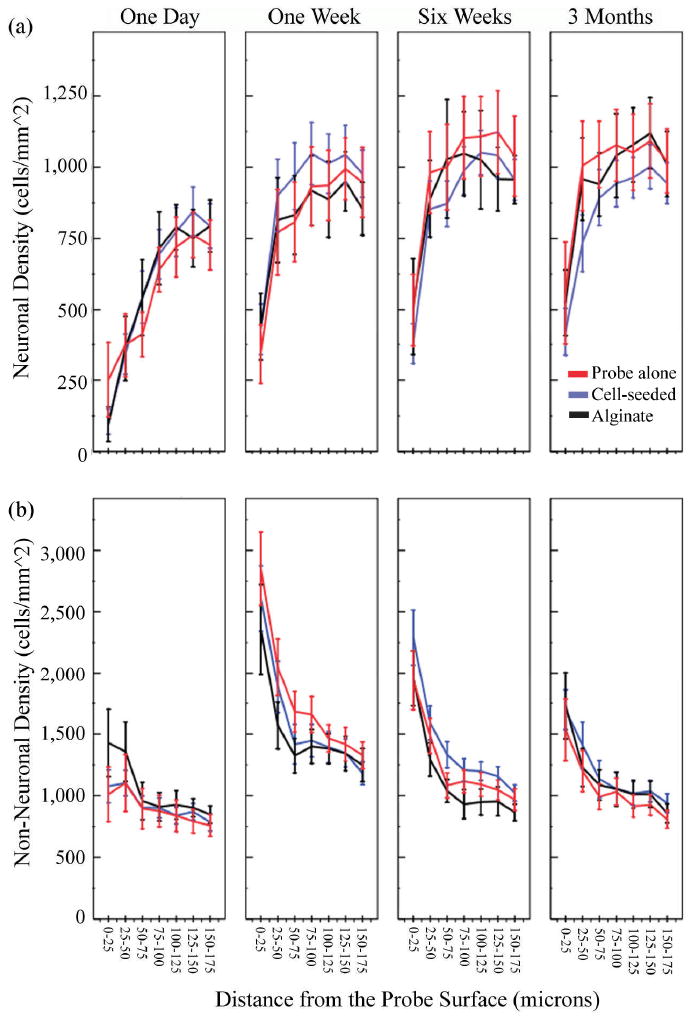
Mean neuronal density (a) and non-neuronal density (b) surrounding implants over time as a function of distance from the probe surface (± s.e.m). NSC-seeded probes had a higher neuronal density surrounding them compared to untreated probes at one day and both control conditions at one week post-implant (a, P<0.05). Cell-seeding reduced non-neuronal encapsulation compared to alginate-only probes at one day and untreated probes at one week post implant (P≤0.001). At six weeks and three months post implantation, the NSC-seeded probes had reduced neuronal density and increased non-neuronal density surrounding them in comparison to both control conditions (P<0.05).
Figure 6.
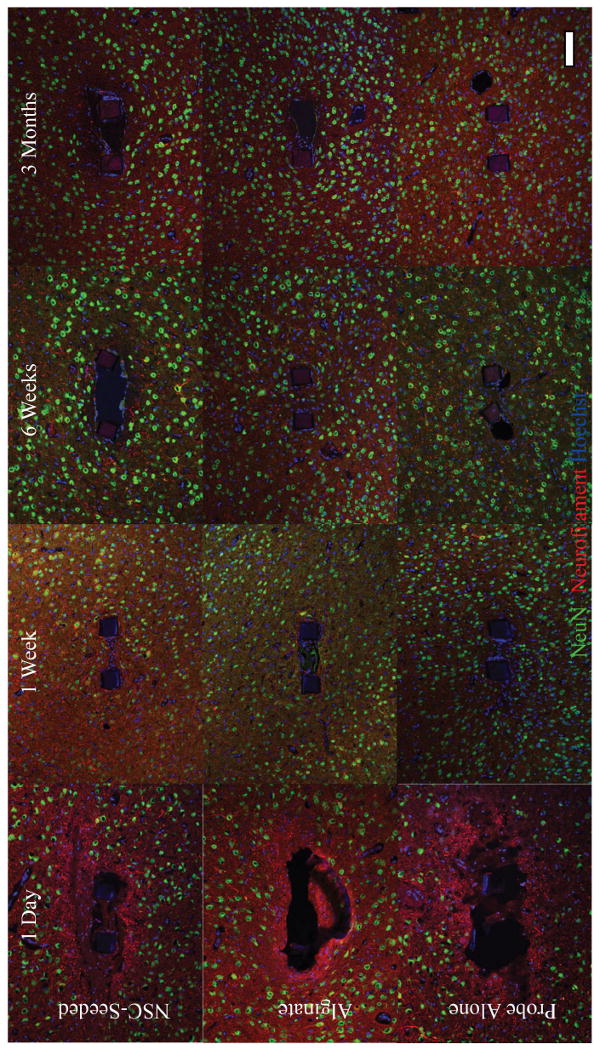
Immunohistochemistry for neuronal markers (NeuN, green; neurofilament, red) reveals an initial injury response in which neuronal soma are distanced from the probe, and neurofilament disruption indicative of damaged axons is seen one day after implantation. A marked improvement in the appearance of injury site occurs within the first week, with a significant increase in neuronal density surrounding probes evident at this time point (P≤0.006). NSC-seeding was associated with increased the neuronal density surrounding prostheses in the first week of implantation, with a reversal of this effect at later time points (P<0.05).
The mitigation of the early tissue response associated with NSC-seeded probes may be due to an initial bystander effect of the cells. The increased neuronal density surrounding seeded probes may result from the secretion of multiple neuroprotective factors by the grafted cells, both known and yet to be elucidated. We have previously demonstrated the release of brain derived neurotrophic factor (BDNF), glial derived neurotrophic factor (GDNF) and nerve growth factor (NGF) from alginate-encapsulated NSCs over a three week time period in vitro (Purcell, Singh et al. 2009). These neurotrophic factors are known to support neuronal survival and plasticity in various models of axonopathy and neuropathy (Kolb, Gorny et al. 1997; Han and Holtzman 2000; Nicole, Ali et al. 2001; Lu, Jones et al. 2003; Wilkins and Compston 2005). The neuroprotective effect of medium conditioned by NSC-seeded alginate beads was further verified using a serum-withdrawal mediated cell death model in PC-12 cells (Purcell, Singh et al. 2009). Alternatively, the increase in neuronal density could be due, in part, to the neuronal differentiation and integration of graft cells into the surrounding brain tissue. However, no direct evidence of this phenomenon was seen in the immunohistochemistry results, and the degree of neuronal density increase is unlikely to be largely explained by the number of cells seeded onto the microscale implant. The production of new neurons by endogenous stem cells originating in the host is an additional, and unexplored, possibility. This also seems improbable as a primary cause of results, as neural stem cells originating in the subventricular zone have been shown to contribute primarily to astrogliosis, with neuronal differentiation rarely observed following migration to the site of cortical trauma (Salman, Ghosh et al. 2004). However, it is worth noting that evidence of doublecortin positivity was observed in one graft (Figure 3e), as well as in host tissue one week after implantation (Supplementary Figure 1). Doublecortin (DCX) is a microtubule-associated protein expressed primarily in migrating neuronal precursors, and reportedly may also be associated with mature astrocytes according to Verwer et al. (Francis, Koulakoff et al. 1999; Verwer, Sluiter et al. 2007). Expression of DCX around neural implants has not been previously reported in the literature, and may represent an additional target for future strategies to improve device-tissue integration if its presence indicates a regenerative capacity of the injured brain, the grafted cells, or a combination of both.
At six weeks and three months post implantation, the neural stem cell-seeded probes had reduced neuronal density and increased non-neuronal encapsulation surrounding them in comparison to both control conditions (Figure 4a-b, P≤0.049 for neuronal density at six weeks, P≤0.001 at three months, P<0.001 for non-neuronal density at both time points). This effect may be due to reduced NSC viability over time, which has been reported previously and may be aggravated by degradation of the alginate scaffold (Sortwell, Pitzer et al. 2000; Bakshi, Keck et al. 2005). Alginate stability drops markedly during the first 24 hours in vivo, and is prone to fragmentation over the following three months (Nunamaker, Purcell et al. 2007). This may leave the grafted cells vulnerable to an immune reaction, and such a response could account for the exacerbation of the tissue response after six weeks. We hypothesize that reduced graft cell viability and function over time, followed by a secondary inflammatory reaction to cellular debris, may explain the deleterious effect of cell-seeding at later time points. It is also possible that an early reduction of glial activation by neural stem cells intensifies neuronal injury later (Hanisch and Kettenmann 2007). However, elucidating the mechanism behind the effects of neural stem cell-seeding is left as the subject of future studies that more directly assess graft cell viability and function over time.
Alginate coating (without cell seeding) had no effect on neuronal density surrounding probes over the course of the study (Figure 4a). However, alginate coating yielded mixed effects on non-neuronal density (Figure 4b). The evolving tissue response to alginate probes may be related to the degradation of the hydrogel over time. We have published a previous report in which alginate disks with various compositions were implanted subcutaneously in rats and assessed over time for mechanical stability and biocompatibility. The results indicated an initial collapse of all alginate samples within the first 24 hours of implantation, as evidenced by a dramatic 80% drop in the complex modulus. This metric was stable for the following three weeks (Nunamaker, Purcell et al. 2007). One day after implantation, alginate coated probes in the present study had a significantly higher non-neuronal density than NSC-seeded and control probes (Figure 4b, P≤0.001). This effect was especially pronounced within the first fifty microns of the probe surface. An initial loss of calcium ions and/or water to the surrounding tissue, causing a potentially injurious, non-isotonic environment resulting in glial activation, could explain this. Interestingly, cell-seeded alginate does not exhibit this initial activation of glia; the encapsulated cells may buffer the surrounding tissue from this effect (Figure 5). One week after implantation, alginate probes reduce non-neuronal density in comparison to untreated probes, likely due to the non-adhesive nature of the gel (P<0.001) (Rowley, Madlambayan et al. 1999). Alternatively, alginate may buffer the mechanical mismatch between the probe and surrounding brain tissue, and reduce glial encapsulation as a consequence. Alginate was stable during the first week in vivo according to histological evaluation in our previous report, after which it progressively fragmented, appearing to be largely infiltrated by tissue in-growth three months after implantation (Nunamaker, Purcell et al. 2007). By six weeks post-implantation, alginate and untreated probes have equivalent non-neuronal densities surrounding them. The alginate is likely to have significantly degraded by this point.
Figure 5.
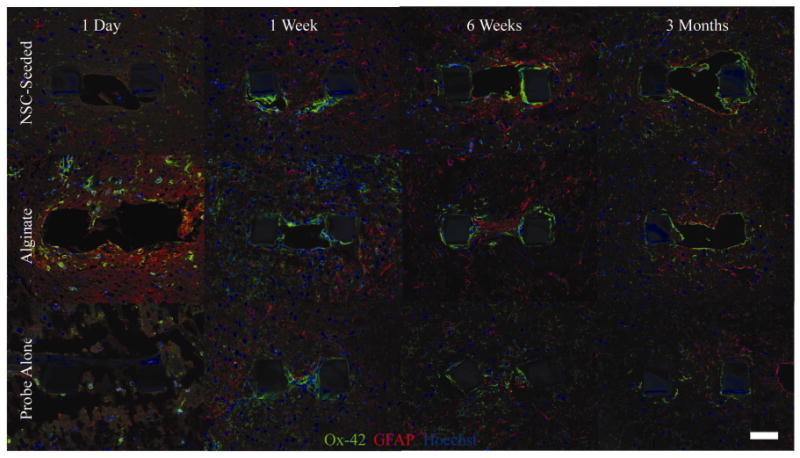
Glial encapsulation of each probe condition over the three month time course. Ox-42 labeled microglia (green) and GFAP labeled astrocytes (red) are shown. Images are taken from probes implanted in the same animal at each time point. NSC-seeding was associated with reduced non-neuronal density at one day post-implantation in comparison to alginate coated probes and at the one week time point in comparison to untreated probes (P<0.001). Glial activation is at its overall peak one week after insertion. A thin encapsulation layer surrounds probes at the six week and three month time points, with NSC-seeded probes having the greatest surrounding non-neuronal density (P<0.001). Interestingly, microglia appeared to have a ramified, or “surveilling,” morphology surrounding a neural stem cell-alginate probe initially, whereas activated cells with an amoeboid structure were found near an alginate probe in the same hemisphere of one animal (left panels).
Neuronal and Non-Neuronal Densities as a Function of Distance
Neuronal density increased as a function of distance from the probe, and non-neuronal density decreased reciprocally. Neuronal density was minimal in the first 25 microns of the probe, with significant incremental increases in the 25-50, 50-75, and 75-100 micron bins (Figure 4a, P<0.001). Neuronal density stabilized thereafter. The loss of neuronal soma within the first 100 microns of the probe is particularly concerning, considering that reliably sorted, large amplitude spikes are recorded when neurons are within 50 microns of recording sites, and no discernable signals are detected from cells greater than 140 microns from probes (Henze, Borhegyi et al. 2000). There was a significant decrease in non-neuronal density as a function of distance from the probe surface (Figure 4b). Non-neuronal density was highest in the 0-25 micron bin, and significantly decreased in the 25-50 micron bin, followed by a further decrease in the 50-75 micron bin (P<0.001). Non-neuronal density stabilized thereafter (P=0.001). Again, the greatest glial encapsulation of the device occurred in the region yielding the highest quality signals. The observation that the tissue response is the most severe in the recordable region surrounding the probe indicates that this phenomenon may result in recording instability, and corroborates previous reports (Szarowski, Andersen et al. 2003; Kim, Hitchcock et al. 2004; Biran, Martin et al. 2005).
The Correlation between Neuronal and Non-Neuronal Densities
There was a significant negative correlation between neuronal and non-neuronal densities (Figure 4a-b, P<0.001). This result expands upon previous work, which has demonstrated an inverse relationship between microglial and neurofilament staining as a function of distance from implanted probes (Biran, Martin et al. 2005). Possible reasons for this relationship include physical displacement of neurons by glia and deleterious effects on neuronal viability and axonal outgrowth by the glial scar. Our results indicate the latter, as clustering of displaced neurons was not observed in the density data. Further, reactive microglia involved in the injury response to probes are known to elute potentially neurotoxic inflammatory cytokines, and reactive astrocytes may produce proteoglycans inhibitory to axonal regeneration (Silver and Miller 2004; Biran, Martin et al. 2005).
Neuronal and Non-Neuronal Densities as a Function of Time
There are general trends in the distribution of neurons and non-neurons surrounding the devices as a function of time. Neurofilament disruption indicative of axonal damage was evident immediately after implantation for all conditions and accompanied the dramatic loss of neuronal density within the first 100 microns of the probe surface (Figure 6; Figure 4a, P≤0.036) (Chen, Meaney et al. 1999). This injury response appeared considerably improved one week after surgery, and neuronal density was significantly increased at this time (Figure 6; Figure 4a, P<0.01). Values stabilized thereafter. Non-neuronal density is lowest one day after transplantation, followed by a peak value of this metric one week after implantation (Figure 5; Figure 4b, P<0.05). By six weeks, a thin glial sheath surrounded all probes, with non-neuronal densities progressively decreasing through the three month time point (Figure 5; Figure 4b, P<0.001). These results corroborate a qualitative study of the glial encapsulation of silicon probes (Szarowski, Andersen et al. 2003). Sustained glial activation and neuronal density loss within the first 100 microns surrounding the insertion site of silicon probes was reported in a study which quantified these responses after two and four weeks in vivo (Biran, Martin et al. 2005). In these studies, the use of silicon probes necessitated the removal of the devices prior to tissue processing, potentially disrupting the interface. Our results are in general agreement with these reports in the literature, and expand upon them as a quantitative data set studying an intact device-tissue interface over multiple time points spanning three months.
Changes in neuronal density surrounding neural prostheses as a function of time have not been demonstrated previously in the literature, and the relationship between recording performance and neuronal density remains unknown. It should be noted that the devices in this study have inherently different geometries and material properties than those typically examined in the literature, potentially altering their interaction with the brain. Nonetheless, it is interesting to compare the time course of neuronal density changes observed in this study to the degradation of recording performance typically reported for microwires and silicon-based devices. The initially severe injury seen following insertion, followed by a return of neuronal soma near the probe at one week, corresponds well with the variation in recording quality observed with silicon probes; there is typically a loss and recovery of unit recordings generally corresponding to this time course (Ludwig, Uram et al. 2006). However, recording quality often diminishes over subsequent weeks and months, and this is not predicted by the general improvements in six week and three month neuronal and non-neuronal density data. It may be that any improvement in recorded signal derived from increased neuronal density is negated by the compact glial sheath formed at later time points. Astrogliosis is known to impede ionic transfer through tissue (Roitbak and Sykova 1999) Alternatively, the data raises the possibility of neuronal plasticity and the formation of silent synapses as additional explanations for recording instability. Henze et al. have reported detecting approximately six units per tetrode, despite estimations that 60-100 neurons should have sufficient amplitude to be discernible above noise (Henze, Borhegyi et al. 2000). Whether or not the neurons located within a recordable distance from electrodes are functionally normal or not is unknown. Understanding not only changes in the structure, but also the function, of tissue surrounding the probes may enable additional intervention strategies to improve recording stability.
Conclusions
We developed a novel, cell-seeded cortical neural prosthesis and evaluated its histological effects in vivo. Our data show that neural stem cell-seeding was related to an attenuation of the initial tissue response associated with the introduction of a foreign body into the brain. Given the physical isolation of the graft cells from the surrounding tissue, in vitro data demonstrating the secretion of neuroprotective factors by the scaffold (Purcell, Singh et al. 2009), and current evidence that the majority of cells express nestin initially in vivo, a bystander effect may be responsible. Doublecortin-positive immature neurons were associated primarily with seeded probes one week after implantation, possibly indicating a regenerative capacity of the injured brain, graft cells, or both. Degradation of the alginate scaffold may leave transplanted cells vulnerable to immune rejection at later time points. An inflammatory reaction to cellular debris could explain the detrimental effect of cell-seeding by six weeks post-implant. Future studies of this biohybrid device will include investigating these mechanisms further and functionalizing recording sites for neurophysiology studies.
Supplementary Material
Supplementary Figure S1. Doublecortin staining (DCX, red) was detected in three out of four animals one week post-implantation (a-c, d-f). Doublecortin (DCX) is a microtubule-associated protein expressed in migrating neuronal precursors, but may also be associated with mature astrocytes. DCX has been observed during glial scar formation following traumatic brain injury, with peak expression occurring at 1 week post-injury (Francis, Koulakoff et al. 1999; Itoh, Satou et al. 2007). Tissue was also labeled with NeuN (mature neuronal nuclei, green) and Hoechst (nuclear counterstain, blue). Expression appeared enhanced in NSC-seeded (a-c) probes compared to controls from the same animal (probe only, d-f). No staining was observed in the remaining animals. The origin and function of DCX-expressing cells are yet to be elucidated, and could possibly indicate a regenerative capacity of the injured brain, the grafted cells, or a combination of both. Scale = 50 microns.
Acknowledgments
The authors gratefully thank Aparna Singh for surgical assistance and Joe Kazemi from the Center for Statistical Consultation and Research at the University of Michigan for guidance with the mixed model development. The M2 antibody was obtained from the Developmental Studies Hybridoma Bank, where it was developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by the Center for Neural Communication Technology (NIBIB, P41-EB002030).
References
- Bakshi A, Keck CA, et al. Caspase-mediated cell death predominates following engraftment of neural progenitor cells into traumatically injured rat brain. Brain Res. 2005;1065(1-2):8–19. doi: 10.1016/j.brainres.2005.09.059. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, et al. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. doi: 10.1016/j.expneurol.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Carmena JM, Lebedev MA, et al. Learning to control a brain-machine interface for reaching and grasping by primates. PLoS Biol. 2003;1(2):E42. doi: 10.1371/journal.pbio.0000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XH, Meaney DF, et al. Evolution of neurofilament subtype accumulation in axons following diffuse brain injury in the pig. J Neuropathol Exp Neurol. 1999;58(6):588–96. doi: 10.1097/00005072-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Plane JM, et al. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J Neurosci. 2003;23(28):9459–68. doi: 10.1523/JNEUROSCI.23-28-09459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue JP, Nurmikko A, et al. Assistive technology and robotic control using motor cortex ensemble-based neural interface systems in humans with tetraplegia. J Physiol. 2007;579(Pt 3):603–11. doi: 10.1113/jphysiol.2006.127209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edell DJ, Toi VV, et al. Factors influencing the biocompatibility of insertable silicon microshafts in cerebral cortex. IEEE Trans Biomed Eng. 1992;39(6):635–43. doi: 10.1109/10.141202. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons NA, Drake W, et al. Primate reaching cued by multichannel spatiotemporal cortical microstimulation. J Neurosci. 2007;27(21):5593–602. doi: 10.1523/JNEUROSCI.5297-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–56. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Gaillard A, Prestoz L, et al. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10(10):1294–9. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- Han BH, Holtzman DM. BDNF protects the neonatal brain from hypoxic-ischemic injury in vivo via the ERK pathway. J Neurosci. 2000;20(15):5775–81. doi: 10.1523/JNEUROSCI.20-15-05775.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Heine W, Conant K, et al. Transplanted neural stem cells promote axonal regeneration through chronically denervated peripheral nerves. Exp Neurol. 2004;189(2):231–40. doi: 10.1016/j.expneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Henze DA, Borhegyi Z, et al. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J Neurophysiol. 2000;84(1):390–400. doi: 10.1152/jn.2000.84.1.390. [DOI] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Itoh T, Satou T, et al. Immature and mature neurons coexist among glial scars after rat traumatic brain injury. Neurol Res. 2007;29(7):734–42. doi: 10.1179/016164107X208086. [DOI] [PubMed] [Google Scholar]
- Kennedy PR. The cone electrode: a long-term electrode that records from neurites grown onto its recording surface. J Neurosci Methods. 1989;29(3):181–93. doi: 10.1016/0165-0270(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Kennedy PR, Mirra SS, et al. The cone electrode: ultrastructural studies following long-term recording in rat and monkey cortex. Neurosci Lett. 1992;142(1):89–94. doi: 10.1016/0304-3940(92)90627-j. [DOI] [PubMed] [Google Scholar]
- Kim YT, Hitchcock RW, et al. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25(12):2229–37. doi: 10.1016/j.biomaterials.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Klueh U, Dorsky DI, et al. Enhancement of implantable glucose sensor function in vivo using gene transfer-induced neovascularization. Biomaterials. 2005;26(10):1155–63. doi: 10.1016/j.biomaterials.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, et al. Nerve growth factor stimulates growth of cortical pyramidal neurons in young adult rats. Brain Res. 1997;751(2):289–94. doi: 10.1016/s0006-8993(96)01410-2. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006;29(9):536–46. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z, et al. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat Med. 2004;10(Suppl):S42–50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- Liu X, McCreery DB, et al. Evaluation of the stability of intracortical microelectrode arrays. IEEE Trans Neural Syst Rehabil Eng. 2006;14(1):91–100. doi: 10.1109/TNSRE.2006.870495. [DOI] [PubMed] [Google Scholar]
- Liu X, McCreery DB, et al. Stability of the interface between neural tissue and chronically implanted intracortical microelectrodes. IEEE Trans Rehabil Eng. 1999;7(3):315–26. doi: 10.1109/86.788468. [DOI] [PubMed] [Google Scholar]
- Llado J, Haenggeli C, et al. Neural stem cells protect against glutamate-induced excitotoxicity and promote survival of injured motor neurons through the secretion of neurotrophic factors. Mol Cell Neurosci. 2004;27(3):322–31. doi: 10.1016/j.mcn.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–29. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Ludwig KA, Uram J, et al. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiphene) (PEDOT) film. J Neural Eng. 2006;3(1):59–70. doi: 10.1088/1741-2560/3/1/007. [DOI] [PubMed] [Google Scholar]
- Messam CA, Hou J, et al. Coexpression of nestin in neural and glial cells in the developing human CNS defined by a human-specific anti-nestin antibody. Exp Neurol. 2000;161(2):585–96. doi: 10.1006/exnr.1999.7319. [DOI] [PubMed] [Google Scholar]
- Nicole O, Ali C, et al. Neuroprotection mediated by glial cell line-derived neurotrophic factor: involvement of a reduction of NMDA-induced calcium influx by the mitogen-activated protein kinase pathway. J Neurosci. 2001;21(9):3024–33. doi: 10.1523/JNEUROSCI.21-09-03024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Dimitrov D, et al. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci U S A. 2003;100(19):11041–6. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunamaker EA, Purcell EK, et al. In vivo stability and biocompatibility of implanted calcium alginate disks. J Biomed Mater Res A. 2007;83(4):1128–37. doi: 10.1002/jbm.a.31275. [DOI] [PubMed] [Google Scholar]
- Orive G, Hernandez RM, et al. Cell encapsulation: promise and progress. Nat Med. 2003;9(1):104–7. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- Ourednik J, Ourednik V, et al. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat Biotechnol. 2002;20(11):1103–10. doi: 10.1038/nbt750. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436(7048):266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, et al. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Prichard HL, Reichert WM, et al. Adult adipose-derived stem cell attachment to biomaterials. Biomaterials. 2007;28(6):936–946. doi: 10.1016/j.biomaterials.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard HL, Reichert WM, et al. IFATS Collection: Adipose-Derived Stromal Cells Improve the Foreign Body Response. Stem Cells. 2008;26(10):2691–95. doi: 10.1634/stemcells.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell EK, Singh A, et al. In Vitro Development and Characterization of a Cortical Neural Stem Cell-Seeded Alginate Scaffold. Tissue Eng C. 2009 in revision. [Google Scholar]
- Rejali D, Lee VA, et al. Cochlear implants and ex vivo BDNF gene therapy protect spiral ganglion neurons. Hear Res. 2007;228(1-2):180–7. doi: 10.1016/j.heares.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–10. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175(1):1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Roitbak T, Sykova E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia. 1999;28(1):40–8. doi: 10.1002/(sici)1098-1136(199910)28:1<40::aid-glia5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Normann RA. Chronic recording capability of the Utah Intracortical Electrode Array in cat sensory cortex. J Neurosci Methods. 1998;82(1):1–15. doi: 10.1016/s0165-0270(98)00031-4. [DOI] [PubMed] [Google Scholar]
- Rowley JA, Madlambayan G, et al. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- Salman H, Ghosh P, et al. Subventricular zone neural stem cells remodel the brain following traumatic injury in adult mice. J Neurotrauma. 2004;21(3):283–92. doi: 10.1089/089771504322972077. [DOI] [PubMed] [Google Scholar]
- Schwartz AB, Cui XT, et al. Brain-controlled interfaces: movement restoration with neural prosthetics. Neuron. 2006;52(1):205–20. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Seymour JP, Kipke DR. Neural probe design for reduced tissue encapsulation in CNS. Biomaterials. 2007;28(25):3594–607. doi: 10.1016/j.biomaterials.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sortwell CE, Pitzer MR, et al. Time course of apoptotic cell death within mesencephalic cell suspension grafts: implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165(2):268–77. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- Stieglitz T, Ruf HH, et al. A biohybrid system to interface peripheral nerves after traumatic lesions: design of a high channel sieve electrode. Biosens Bioelec. 2002;17(8):685–696. doi: 10.1016/s0956-5663(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Stieglitz T. Restoration of neurological functions by neuroprosthetic technologies: future prospects and trends towards micro-, nano-, and biohybrid systems. Acta Neurochir Suppl. 2007;97(Pt 1):435–42. doi: 10.1007/978-3-211-33079-1_57. [DOI] [PubMed] [Google Scholar]
- Szarowski DH, Andersen MD, et al. Brain responses to micro-machined silicon devices. Brain Res. 2003;983(1-2):23–35. doi: 10.1016/s0006-8993(03)03023-3. [DOI] [PubMed] [Google Scholar]
- Taylor DM, Tillery SI, et al. Direct cortical control of 3D neuroprosthetic devices. Science. 2002;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- Teng YD, Lavik EB, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–9. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JN, Shain W, et al. Cerebral astrocyte response to micromachined silicon implants. Exp Neurol. 1999;156(1):33–49. doi: 10.1006/exnr.1998.6983. [DOI] [PubMed] [Google Scholar]
- Velliste M, Perel S, et al. Cortical control of a prosthetic arm for self-feeding. Nature. 2008 doi: 10.1038/nature06996. [DOI] [PubMed] [Google Scholar]
- Verwer RW, Sluiter AA. Mature astrocytes in the adult human neocortex express the early neuronal marker doublecortin. Brain. 2007;130(Pt 12):3321–35. doi: 10.1093/brain/awm264. [DOI] [PubMed] [Google Scholar]
- Vetter RJ, Williams JC, et al. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans Biomed Eng. 2004;51(6):896–904. doi: 10.1109/TBME.2004.826680. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Compston A. Trophic factors attenuate nitric oxide mediated neuronal and axonal injury in vitro: roles and interactions of mitogen-activated protein kinase signalling pathways. J Neurochem. 2005;92(6):1487–96. doi: 10.1111/j.1471-4159.2004.02981.x. [DOI] [PubMed] [Google Scholar]
- Williams JC, Hippensteel JA, et al. Complex impedance spectroscopy for monitoring tissue responses to inserted neural implants. J Neural Eng. 2007;4(4):410–23. doi: 10.1088/1741-2560/4/4/007. [DOI] [PubMed] [Google Scholar]
- Williams JC, Rennaker RL, et al. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res Brain Res Protoc. 1999;4(3):303–13. doi: 10.1016/s1385-299x(99)00034-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Doublecortin staining (DCX, red) was detected in three out of four animals one week post-implantation (a-c, d-f). Doublecortin (DCX) is a microtubule-associated protein expressed in migrating neuronal precursors, but may also be associated with mature astrocytes. DCX has been observed during glial scar formation following traumatic brain injury, with peak expression occurring at 1 week post-injury (Francis, Koulakoff et al. 1999; Itoh, Satou et al. 2007). Tissue was also labeled with NeuN (mature neuronal nuclei, green) and Hoechst (nuclear counterstain, blue). Expression appeared enhanced in NSC-seeded (a-c) probes compared to controls from the same animal (probe only, d-f). No staining was observed in the remaining animals. The origin and function of DCX-expressing cells are yet to be elucidated, and could possibly indicate a regenerative capacity of the injured brain, the grafted cells, or a combination of both. Scale = 50 microns.


