Abstract
OBJECTIVE
To examine the relationship between duration of lactation and changes in maternal metabolic risk factors.
METHODS
This 3-year prospective study examined changes in metabolic risk factors among lactating women from preconception to postweaning and among nonlactating women from preconception to postdelivery, in comparison with nongravid women. Of 1,051 (490 black, 561 white) women who attended two consecutive study visits in years 7 (1992–1993) and 10 (1995–1996), 942 were nongravid and 109 had one interim birth. Of parous women, 48 (45%) did not lactate, and 61 (55%) lactated and weaned before year 10. The lactated and weaned women were subdivided by duration of lactation into less than 3 months and 3 months or more. Multiple linear regression models estimated mean 3-year changes in metabolic risk factors adjusted for age, race, parity, education, and behavioral covariates.
RESULTS
Both parous women who did not lactate and parous women who lactated and weaned gained more weight (+5.6, +4.4 kg) and waist girth (+5.3, +4.9 cm) than nongravid women over the 3-year interval; P<.001. Low-density lipoprotein cholesterol (+6.7 mg/dL, P<.05) and fasting insulin (+2.6 microunits, P= .06) increased more for parous women who did not lactate than for nongravid and parous women who lactated and weaned. High-density lipoprotein cholesterol decrements for both parous women who did not lactate and parous women who lactated and weaned were 4.0 mg/dL greater than for nongravid women (P<.001). Among parous, lactated and weaned women, lactation for 3 months or longer was associated with a smaller decrement in high-density lipoprotein cholesterol (−1.3 mg/dL versus −7.3 mg/dL for less than 3 months; P<.01).
CONCLUSION
Lactation may attenuate unfavorable metabolic risk factor changes that occur with pregnancy, with effects apparent after weaning. As a modifiable behavior, lactation may affect women’s future risk of cardiovascular and metabolic diseases.
In women, pregnancy contributes to excessive weight gain leading to obesity,1 accumulation of abdominal fat, and a more atherogenic lipid profile (ie, lower high-density lipoprotein cholesterol).2,3 This raises the question of whether childbearing contributes to future development of chronic disease later in life. The findings from previous research on this subject have been inconsistent. Multiparity (six or more births) has been associated with a 30–90% greater risk of cardiovascular disease events in some,4,5 but not all studies.6,7 Also, both multiparity and nulliparity have been associated with higher insulin levels many years after childbearing has ended. 8 The evidence that higher parity is associated with prevalence of type 2 diabetes is mixed in studies adjusting for body size. Three studies reported positive associations,9–11 three studies found no association, 12–14 and two studies of Native American women reported an inverse association of increasing risk with nulliparity.15,16 A single prospective study found that the increased risk of incident type 2 diabetes with higher parity was abolished after adjustment for age and obesity.17 The conflicting findings regarding parity and risk of cardiovascular disease and diabetes may be due to confounding from preconception risk factors (eg, obesity, infertility related to insulin resistance), glucose intolerance of pregnancy, and postpartum behaviors, including lactation, that affect these same metabolic risk factors.
Lactation has been associated with favorable effects on maternal glucose homeostasis, blood lipid profiles, body weight, and fat distribution. Lactating women exhibit lower plasma glucose and insulin levels, a less atherogenic lipid profile, and greater fat mass mobilization during the first year postpartum than nonlactating women.18,19 Prolonged, exclusive lactation after pregnancy has been associated with lower postpartum weight retention in some,20,21 but not all studies,22 and with lower risk of maternal overweight 10–15 years later.23,24 Cumulative months of lifetime lactation have been associated with lower incidence of type 2 diabetes among parous women later in life.25 However, very few prospective studies have evaluated whether lactation is associated with a return of metabolic risk factors to preconception levels within several months postweaning. In this prospective cohort study, we examined 3-year changes in metabolic risk factors among parous women who lactated compared with those who did not and in comparison with nongravid women. We hypothesized that lactation would attenuate the adverse changes in maternal metabolic risk factors that occur with pregnancy. Among parous women who lactated, we also examined whether changes in metabolic risk factors from preconception to postweaning varied by duration of lactation.
MATERIALS AND METHODS
The Coronary Artery Risk Development in Young Adults (CARDIA) Study is a multi-center, longitudinal, observational study designed to describe the development of risk factors for coronary heart disease in young black and white men and women. Descriptions of the prospective cohort study design and recruitment, methodology, and cohort characteristics have been previously reported.26,27 The study population was recruited in 1985–1986 from four geographic areas: Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California. A total of 5,115 subjects (2,787 women) aged 18–30 years were enrolled. Subjects were recruited to achieve equal numbers of each race; 52% black and 48% white. Study examinations at 3- to 5-year intervals included collection of blood specimens and a variety of self-reported measures.26 Retention rates were 81% and 79% for examinations at 7 and 10 years after baseline, respectively.28
To investigate the impact of pregnancy and lactation on changes in metabolic risk factors, we selected the first two consecutive CARDIA follow-up examinations which measured both fasting blood glucose and insulin levels. There were 1,993 women who attended examinations in 1992–1993 (year 7) and 1995–1996 (year 10). Women were excluded if they had a hysterectomy or removal of both ovaries (n = 12), reported a pregnancy within 12 months before the year 7 examination (n = 110), were currently pregnant or breastfeeding at the year 7 examination (n = 119), or were currently pregnant (n = 17) or currently breastfeeding (n = 21) at the year 10 examination. We also excluded women who had taken lipid-lowering medications (n = 8), had elevated plasma triglycerides (more than 400 mg/dL; n = 2), diabetes (n = 121), or were nonfasting (less than 8 hours) before venipuncture (n = 26) or missing covariates (n = 355) at years 7 or 10. We selected only women who delivered one singleton live birth or who were nonpregnant during the 3-year time interval. Women who delivered multiple fetuses, had two or more births (n = 9), or had any miscarriages or abortions (n = 95) during the interval were excluded. Finally, we excluded women who had the metabolic syndrome at year 7 (n = 47) defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria for women.29 The analytic sample included 1,051 CARDIA women (490 black, 561 white) who were either nongravid (n = 942) or gave birth once (n = 109) during the 3-year interval. The year 7 measurements are the preconception levels for women with interim births between 1992 and 1996 and the baseline levels for the referent group of nongravid women. Institutional review boards at each participating study center approved the study. Written informed consent was obtained from subjects for all study procedures.
Details of the methodology used to recruit subjects and data collection procedures have been previously reported.26,27 Participants were asked to fast before each examination and report the number of hours since their last intake of food or beverages before the blood draw. Blood samples were drawn in the morning after an overnight fast using a Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) containing ethylenediaminetetraacetic acid.30 Procedures followed in the collection and storage of plasma samples, as well as laboratory quality control procedures and methodology used to determine concentrations of plasma triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and total cholesterol have been reported in detail elsewhere.30 Fasting insulin was assayed by a radioimmunoassay using a unique antibody.31 Fasting glucose was measured using the hexokinase method. Blood pressure measurements were obtained after an initial 5-minute rest. Blood pressure was measured three times at 1-minute intervals using the Hawksley random-zero sphygmomanometer (Hawksley and Sons Ltd, Lancing, UK); the first and fifth phase Korotkoff sounds were recorded, and averages of the second and third measurements were used in the analyses.27 The protocol specified the appropriate cuff size (small, medium, large, extra large) based on the upper arm circumference, which was measured by the blood pressure technician at the midpoint between the acromion and the olecranon.
Weight, height, and waist circumference (waist girth) measurements were obtained by certified technicians according to a standardized protocol described previously.32 Body weight was measured to the nearest 0.2 kg using a calibrated balance beam scale in women wearing light clothing. Height (without shoes) was measured to the nearest 0.5 cm using a vertical ruler. Waist circumference was measured to the nearest 0.5 cm at the minimal abdominal girth.33 Body mass index (BMI) was computed as weight in kilograms divided by squared height in meters.
Pregnancies and births were self-reported at each examination. Participants were asked whether they were currently pregnant or breastfeeding, number of times they had been pregnant, including abortions, miscarriages, and live births or stillbirths since the previous examination, duration of gestation, and dates of delivery. Women also reported the amount of weight gained during the pregnancy (gestational weight gain) and whether they had breastfed their infant and for how long: none, less than 6 weeks, 6–11 weeks, 3–6 months, or more than 6 months.
Sociodemographic and behavioral data (medication use, alcohol intake [mL/d], cigarette smoking, education, marital status, oral contraceptive use, and physical activity) were collected at each examination using self- and interviewer-administered questionnaires. Categorical variables were as follows: smoking (never, former, or current), years of education (12 or less, 13–15, and 16 or more), marital status (never married, widowed, divorced, separated, or married), and oral contraceptive use (never, past, or current). Daily alcohol intake was categorized as 0 mL/d, 1–10 mL/d, 11–30 mL/d, and more than 30 mL/d. Assessments of physical activity were obtained using the interviewer-administered CARDIA Physical Activity History described previously.34 Race-specific quartiles for physical activity at each examination were formed because of the skewedness of the data to assess changes over time within race groups (data not shown). Dietary intake was measured at baseline in year 7. Dietary intake during the previous month was assessed using the CARDIA Dietary History administered by a trained interviewer.35 Daily dietary nutrient measures of total fat, saturated fat, total protein, total carbohydrate in grams per day, and energy intake in kilojoules per day were used to obtain the percentages of kilocalories from fat, protein, and carbohydrate.
Women included in the analytic sample were not pregnant and not lactating at examinations in years 7 or 10. Of these women, those who reported no pregnancies during the 3-year interval were classified as nongravid. Interim births were defined as delivery of a singleton live birth conceived after year 7 (baseline) and delivered before the year 10 examination. Women with interim births were further classified by lactation status into one of two groups: parous, no lactation, and parous, lactated and weaned before year 10. The lactated and weaned group was further dichotomized by duration of lactation into two groups: less than 3 months and 3 months or more. Time since delivery was calculated by subtraction of the date of delivery from the date of the year 10 examination. Among women who had lactated during the interval, time since weaning was calculated by subtracting the number of months of lactation from the time (number of months) since the delivery.
The metabolic risk factors are fasting plasma glucose, insulin, HDL-C, LDL-C, total cholesterol, triglycerides, and homeostasis model assessment of insulin resistance (HOMA-IR), blood pressure, weight, and waist girth measured at examinations in years 7 and 10. Change in each measure was calculated by subtracting the measurement in year 7 (baseline) from the measurement in year 10. For women who gave birth and did not lactate, the measurement at year 7 is at preconception and at year 10 is at postpartum. For women who lactated (lactated and weaned), the measurement at year 7 is at preconception and the measurement at year 10 is postweaning. Women were nonlactating at both years 7 and 10. The mean change in each metabolic risk factor was estimated across the nongravid, no lactation, and lactated and weaned groups, and by duration of lactation in the lactated and weaned group.
The most accurate and direct measures of insulin resistance are the euglycemic-hyperinsulinemic clamp and frequently sampled intravenous glucose tolerance test techniques. However, in large epidemiologic studies, surrogate indices of insulin sensitivity based on fasting glucose and insulin levels are commonly used.36,37 These indices include the homeostatic method (HOMA-IR), which is strongly correlated (ranging from 0.6 to 0.8) with physiologic measures (clamp or frequently sampled intravenous glucose tolerance test) across a range of glucose levels and among normoglycemic persons, and shown as receiver operating characteristics curves above 70%.36,37 We examined changes in indices of insulin resistance with the homeostatic method calculated using fasting glucose and insulin levels.
Definition: HOMA-IR = (G0×I0)/22.5, where G0 = fasting glucose (millimoles per liter) and I0 = fasting insulin (microunits per milliliter).
The metabolic syndrome was defined using the National Cholesterol Education Program criteria for women.29 Diagnosis of the metabolic syndrome is based on the presence of three of five of the characteristics: waist girth greater than 88 cm, fasting triglyceride of 150 mg/dL or more, HDL-C less than 50 mg/dL, systolic blood pressure 130 mmHg or greater or diastolic blood pressure 85 mmHg or greater or treatment with antihypertensive medication, and fasting glucose 110 mg/dL or greater or treatment with diabetes medication. At year 7, 47 women met these criteria for metabolic syndrome and were excluded from the sample.
Preliminary analyses involved description of the year 7 characteristics. Analysis of variance was used to assess baseline (year 7) differences in plasma lipids (HDL-C, LDL-C, total cholesterol, triglycerides), age, height, weight, BMI, waist girth, fasting glucose and insulin, index of insulin resistance, and behavioral characteristics (alcohol intake, dietary intake) among nongravid, no lactation, and lactated and weaned women. We also assessed year 7 characteristics among lactated and weaned women by duration of lactation (less than 3 months and 3 months or more). We assessed the average number of months since delivery between the no lactation and lactated and weaned groups and months since weaning by duration of lactation groups. Chi-square tests were used to assess associations with baseline demographic and behavioral categorical variables (race, overweight, smoking, education, oral contraceptive use, marital status, parity, gravidity, participating study center) within each group and across the lactated and weaned group by duration. Statistics from t tests were used to assess differences in baseline (year 7) age, height, weight, BMI, waist girth, plasma lipids, glucose, insulin, blood pressure, index of insulin resistance, and time since delivery between groups. Probability values were obtained from two-sided tests (significance was considered P<.05). The Kruskal-Wallis one-way test was used to assess differences in alcohol intake and physical activity due to skewedness in the distributions.
Multiple linear regression was used to examine differences in adjusted mean changes in metabolic risk factors (weight, BMI, waist girth, and fasting plasma lipids, glucose and insulin, index of insulin resistance) among the groups (nongravid, no lactation, lactated and weaned) adjusted for race and year 7 dependent measure and covariates: age, BMI, education, parity, smoking, and oral contraceptive use. In separate models we examined mean changes in the metabolic risk factors between the parous groups, no lactation and lactated and weaned women, adjusted for the above covariates plus time since delivery as a potential confounder. Other variables assessed as potential confounders included the participating study center, dietary intake, alcohol intake, and physical activity. However, these variables were not included in final models because they were not associated with metabolic risk factors independent of nongravid and parous groups based on criteria of less than 10% change in the coefficients for parous groups. In multivariable models examining duration of lactation, mean changes in the risk factors were adjusted for the year 7 measures of age, BMI, race, education, parity, smoking, and oral contraceptive use and time interval since weaning to the year 10 examination.
RESULTS
In year 7 (baseline), mean age of the cohort was 32 years, with a range of 24–42 years. The nongravid, no lactation, and lactated and weaned women (Table 1) did not differ significantly in race or baseline fasting plasma lipids, glucose and insulin, index of insulin resistance, or blood pressure. Nongravid women differed from no lactation and lactated and weaned women in that they were significantly older, more likely to be overweight, and had larger waist girths. Women who did not lactate were more likely to be parous at year 7 and more likely to be black than women who lactated (lactated and weaned). Women who did not lactate (no lactation) averaged a shorter time interval from delivery to the year 10 examination than lactated and weaned women. Gestational weight gain did not differ among parous women across the lactation groups (data not shown). No significant differences were found for any other metabolic risk factors at year 7 between no lactation and lactated and weaned women.
Table 1.
Baseline Year 7 (1992–1993) Characteristics for Nongravid and Parous Women by Lactation Status
| Baseline (1992–1993) Characteristics | Nongravid (n = 942) |
No Lactation (n = 48) |
Postweaning (n = 61) |
|---|---|---|---|
| Demographic/clinical | |||
| Race (black) | 437 (46.4) | 27 (56.3) | 26 (42.6) |
| Parity (nulliparous)* | 489 (51.9) | 19 (39.6) | 33 (54.1) |
| Gravidity(nulligravida) | 369 (39.2) | 11 (22.9) | 23 (37.7) |
| Overweight (BMI 25 kg/m2 or greater)* | 424 (45.0) | 17 (35.4) | 19 (31.2) |
| Smoker (current) | 212 (22.5) | 12 (25.0) | 10 (16.4) |
| Education (high school or less) | 239 (25.4) | 15 (31.3) | 10 (16.4) |
| OC use (current) | 218 (23.1) | 16 (33.3) | 15 (24.6) |
| Age (y)* | 32.4±3.6 | 29.8±3.4 | 31.0±2.9 |
| Waist girth (cm)* | 78.3±13.4 | 75.6±9.4 | 74.2±9.8 |
| Weight (kg) | 71.1±17.6 | 66.8±13.2 | 67.0±13.5 |
| Height (cm) | 164.7±6.9 | 164.4±6.6 | 164.9±6.2 |
| BMI (kg/m2) | 26.1±6.4 | 24.5±4.6 | 24.6±5.3 |
| DBP (mm Hg) | 67.0±8.9 | 66.4±11.0 | 64.9±8.0 |
| SBP (mm Hg) | 104.6±10.8 | 105.3±11.3 | 101.6±9.2 |
| Fasting plasma | |||
| Total cholesterol (mg/dL) | 172.1±29.9 | 175.3±32.4 | 173.0±30.0 |
| LDL-C (mg/dL) | 102.0±28.0 | 107.3±29.1 | 103.6±27.9 |
| HDL-C (mg/dL) | 56.2±12.8 | 54.6±13.9 | 56.8±13.0 |
| Triglycerides (mg/dL) | 67.1±36.1 | 64.1±32.0 | 60.9±30.0 |
| Insulin (microunits) | 12.4±6.3 | 11.7±4.1 | 11.8±6.4 |
| Glucose (mg/dL) | 85.5±7.1 | 84.3±5.8 | 84.8±5.8 |
| HOMA-IR | 2.7±1.5 | 2.5±0.9 | 2.5±1.5 |
| Time from delivery to follow-up (mo)* | — | 12.8±8.6 | 16.4±6.7 |
| Range | — | 1–34 | 3–27 |
OC, oral contraceptive; BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance.
Data are expressed as n (%) or mean±standard deviation.
Overall differences among groups; P<.05.
In Table 2, compared with nongravid women, no lactation, and lactated and weaned women had greater adjusted mean gains over 3 years in weight and waist girth and decrements in HDL-C (all P<.001). Increments in LDL-C levels were greater for women who did not lactate than for nongravid and lactated and weaned women (P<.05). Increments in fasting plasma insulin were higher in women who did not lactate than in nongravid and lactated and weaned women, but differences were only borderline statistically significant (P= .06). When changes in metabolic risk factors for parous groups (no lactation and lactated and weaned) were also adjusted for time since delivery to the year 10 visit, changes in HDL-C, fasting insulin, and index of insulin resistance continued to be more favorable for lactated and weaned women, but differences remained statistically nonsignificant (data not shown).
Table 2.
Multivariable Adjusted Mean (Standard Error) Three-Year Changes in Metabolic Risk Factors Among Nongravid and Parous Women by Lactation Status*
| Nongravid and Parous Women by Lactation Status | ||||
|---|---|---|---|---|
| Adjusted 3-Year Changes in Risk Factors | Nongravid (n = 942) |
No Lactation (n = 48) |
Postweaning (n = 61) |
P |
| Clinical | ||||
| Waist girth (cm) | 2.1 (0.2) | 5.3 (0.8)† | 4.9 (0.7)† | <.001 |
| Weight (kg) | 2.5 (0.3) | 5.6 (0.9)† | 4.4 (0.8)† | <.001 |
| BMI (kg/m2) | 0.9 (0.1) | 2.0 (0.3)† | 1.5 (0.3)† | <.001 |
| DBP (mm Hg) | 4.0 (0.4) | 4.5 (1.1) | 3.3 (1.0) | .67 |
| SBP (mm Hg) | 2.5 (0.4) | 1.8 (1.3) | 2.8 (1.2) | .82 |
| Fasting plasma | ||||
| Total cholesterol (mg/dL) | 0.8 (1.0) | 3.4 (3.0) | −2.1 (2.7) | .37 |
| LDL-C (mg/dL) | −0.1 (0.9) | 6.7 (2.8)† | −0.8 (2.5)‡ | .045 |
| HDL-C (mg/dL) | −0.4 (0.4) | −5.4 (1.3)† | −4.4 (1.2)† | <.001 |
| Triglycerides (mg/dL) | 8.0 (1.6) | 10.5 (5.1) | 15.5 (4.6) | .25 |
| Insulin (microunits) | 0.5 (0.3) | 2.6 (0.9)† | 0.9 (0.8) | .06 |
| Glucose (mg/dL) | −0.7 (0.4) | −1.3 (1.2) | −0.6 (1.2) | .86 |
| HOMA-IR | 0.16 (0.08) | 0.59 (0.24) | 0.24 (0.22) | .20 |
BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance.
Three-year change_follow-up (year 10)–baseline (year 7). Means were adjusted for baseline year 7 (1992–1993) measurement for each variable, BMI (except weight adjusted for height), age, race, parity (0 births, 1 or more births), smoking (never/past or current), oral contraceptive use (never/past or current), education (high school or less versus some college or more).
Group mean differs from the nongravid group (P <.05).
Group mean differs from the no-lactation group (P <.05).
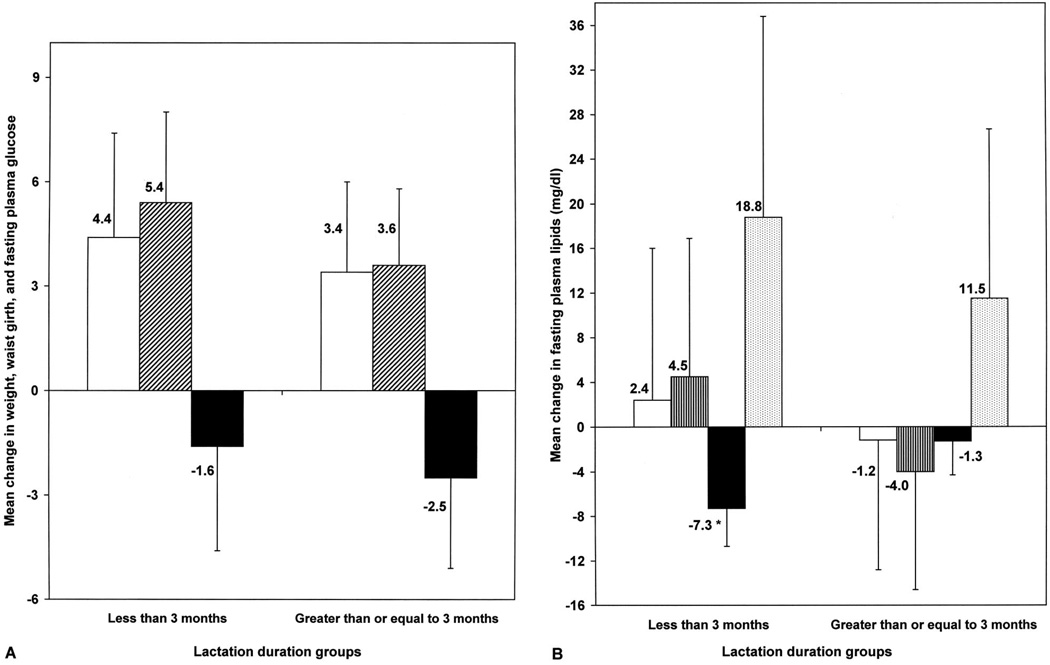
In the lactated and weaned group, 30% of women breastfed for less than 3 months, 44% for 3–6 months, and 26% for 6 months or longer. Overall, the average number of months since weaning until the year 10 visit was 13.2 (±6.8 standard deviation) and ranged from 6 weeks to 24 months. No statistically significant differences were found in baseline (year 7) clinical, anthropometric, or biochemical risk factors or in time since weaning by duration of lactation groups (Table 3). Longer duration of lactation was associated with white race and nulliparity at baseline. As shown in Figures 1A and 1B, mean changes in metabolic risk factors were more favorable in women who lactated for a longer duration, but only changes in HDL-C were statistically significant between the long- and short-duration lactation groups: mean HDL-C −1.3 mg/dL (95% confidence interval −4.3 to 1.7 mg/dL) for 3 months or more compared with −7.3 mg/dL (95% confidence interval −10.3 to −4.3 mg/dL) for less than 3 months; P<.01) (Fig. 1B).
Table 3.
Baseline Year 7 (1992–1993) Characteristics Within the Postweaning Group by Duration of Lactation
| Duration of Lactation | ||
|---|---|---|
| Characteristics in Year 7 (1992–1993) | Less Than 3 Months (n=18) |
3 Months or More (n=43) |
| Race (black) | 10 (55.6) | 16 (37.2) |
| Parity (nulliparous) | 7 (38.9) | 26 (60.5) |
| Smoker (current) | 4 (22.2) | 6 (14.0) |
| Education (high school or less) | 4 (22.2) | 6 (14.0) |
| OC use (current) | 4 (22.2) | 11 (25.6) |
| Age (y) | 31.8±2.7 | 30.6±2.9 |
| BMI (kg/m2) | 24.6±5.4 | 24.6±5.4 |
| Waist (cm) | 76.3±11.3 | 73.3±9.0 |
| Fasting plasma | ||
| Insulin (microunits) | 10.4±4.0 | 12.4±7.1 |
| Ln insulin | 2.3±0.4 | 2.4±0.5 |
| Glucose (mg/dL) | 84.1±6.0 | 85.1±5.8 |
| HOMA-IR | 2.2±0.9 | 2.6±1.6 |
| Total cholesterol (mg/dL) | 168.2±30.8 | 175.0±29.8 |
| HDL-C (mg/dL) | 54.9±12.5 | 57.5±13.2 |
| LDL-C (mg/dL) | 99.0±31.0 | 105.5±26.5 |
| Triglycerides (mg/dL) | 68.4±44.5 | 57.7±21.1 |
| DBP (mm Hg) | 64.1±9.0 | 65.3±7.6 |
| SBP (mm Hg) | 101.2±8.1 | 101.7±9.7 |
| Time since weaned (mo) | 17.5 (2–25) | 11.6 (1.4–23) |
| Time interval since delivery to year 10 (mo) | 19.0 (6–28) | 16.8 (4–26) |
OC, oral contraceptive; BMI, body mass index; Ln, natural log; HOMA-IR, homeostasis model assessment for insulin resistance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data are expressed as n (%), mean±standard deviation, or median (range). There were no significant differences in risk factors at baseline or time intervals between groups.
Fig. 1.
Three-year changes in metabolic risk factor profiles from preconception to postweaning by duration of lactation (less than 3 months versus 3 months or more). A. White bar, weight (kilograms); black and white diagonal line bar, waist girth (centimeters); black bar, glucose (milligrams per deciliter). B. White bar, total cholesterol; black and white lines bar, low-density lipoprotein cholesterol; black bar, high-density lipoprotein cholesterol; dotted bar, triglycerides. Mean (95% confidence interval) adjusted for age, body mass index, race, education, parity, smoking, oral contraceptive use, time since weaning to year 10 examination. * P<.01.
Gunderson. Lactation and Maternal Metabolic Risk Factors. Obstet Gynecol 2007.
DISCUSSION
Our findings of greater adiposity and lower HDL-C levels with parity are consistent with previous studies. 2,3,38 The unique feature of this study is that data were obtained prospectively to examine the relationship between lactation and changes in maternal metabolic risk factors from the interval between preconception and an average of 13 months after weaning (range 2–24 months). Lactation was not associated with reduced pregnancy-related gains in weight and waist girth, but adverse changes in other metabolic risk factors (ie, plasma LDL-C and fasting insulin) were attenuated in women who had lactated (lactated and weaned women). In addition, lactation for at least 3 months was associated with a more favorable metabolic risk factor profile, in particular, a much smaller reduction in HDL-C levels.
The new finding of this study is that longer duration of lactation may attenuate the long-term 3- to 4-mg/dL decrements in HDL-C that occur with pregnancy. No lactation and lactation for less than 3 months were associated with HDL-C decrements of 5 and 7 mg/dL, respectively, compared with 1-mg/dL decrement with lactation for 3 months or more.
Each 1-mg/dL increase in HDL-C is estimated to decrease coronary heart disease (CHD) risk by 2–3% in women.39 A decrement of 7 mg/dL in HDL-C equates to an increase in CHD risk of 14–21%.
Average changes in LDL-C and triglycerides were also more favorable with longer duration of lactation (LDL-C −4.0 versus +4.5 mg/dL, triglycerides +11.5 versus +18.8 mg/dL), although not statistically significant due to the small sample size. These findings were adjusted for age, BMI, race, education, parity, smoking, oral contraceptive use, and time since weaning. Also, differences in gestational gain or weight gain during the 3-year interval did not explain the observed differences by duration of lactation.
Our study obtained metabolic risk factor measurements before conception and at postweaning, which enabled us to examine the influence of lactation. In studies that did not examine lactation status, lipid profile changes during the puerperium were reported to include rapid declines in triglyceride levels,40 with levels of total cholesterol and LDL-C remaining above40,41 and HDL-C remaining below42 preconception or early pregnancy levels for several months postpartum. Another study examined whether postpartum differences in blood lipid profiles were maintained postweaning43 among the 34 exclusively lactating women. Triglyceride, LDL-C, and total cholesterol levels declined between delivery and 6 months postpartum.43 The total cholesterol levels remained stable until 9 months postpartum, after which, at 2 months postweaning, the levels increased to delivery levels.43 These findings may be influenced by preconception lipid levels, postpartum weight loss, and other lifestyle behaviors that were not examined in the study.
Our findings are consistent with studies showing that lactating women have more favorable lipid profiles during the postpartum period. A single longitudinal study examined preconception to postpartum changes in blood lipid profiles by lactation status in 34 women from preconception to 40 weeks (10 months) after delivery but did not examine postweaning lipid levels.44 Lactation was an important factor in reversal of gestational hyperlipidemia after delivery, with triglyceride levels declining significantly more rapidly in lactating (n = 22) than in nonlactating (n = 12) women within 20 weeks postpartum and stabilizing thereafter.44 Moreover, total cholesterol levels declined earlier, although not significantly.44 Two small longitudinal studies showed that lactating compared with nonlactating women (6 weeks to 6 months postpartum) had higher HDL-C levels,19 but no differences in LDL-triglyceride, LDL-C, or total cholesterol levels.45 Others reported significantly lower fasting serum triglyceride levels at 6 months postpartum46 and more rapid declines in plasma total cholesterol and triglyceride levels from delivery to 3–4 months postpartum in lactating women.47
Lactation is also associated with a more favorable metabolic risk factor profile among women with gestational diabetes and a favorable maternal glucose homeostasis. In a large cross-sectional study, Kjos and colleagues48 examined 809 women with recent gestational diabetes at 4–12 weeks postpartum (404 currently lactating and 405 nonlactating) and found HDL-C was 4 mg/dL higher for lactating women after adjusting for maternal age, BMI, and pregnancy insulin use. No differences in total cholesterol, LDL-C, or triglyceride levels were observed. In other cross-sectional studies, despite higher plasma prolactin levels, lactating women had lower fasting plasma glucose48 and insulin levels49 and lower insulin-glucose ratios46 than nonlactating women. In the postabsorptive state, lactating compared with nulliparous women had lower plasma insulin concentrations.50 In a small longitudinal study (n = 4) from preconception to postpartum with the euglycemic-hyperinsulinemic clamp method, insulin resistance increased above preconception levels during late pregnancy but declined to preconception levels at 8 weeks postpartum during lactation.51 Butte and colleagues46 reported significantly lower fasting insulin levels at 6 months postpartum for lactating compared with nonlactating women, although they found no difference in fasting glucose levels.
Whether lactation exerts persistent effects on maternal glucose homeostasis several months to years postweaning has been rarely studied. A cross-sectional study of Brazilian women at 12–18 months postpartum, of whom 67% were still lactating, reported that prolonged lactation was associated with a smaller area under the insulin curve and a “lasting protective effect” on insulin secretion.52 In the Nurse’s Health Study cohort, higher cumulative duration of lifetime lactation was associated with a 25% lower incidence of diabetes in women several years later.25 Although our study found no significant differences in changes in fasting plasma glucose and index of insulin resistance by lactation status, we observed a trend for larger increases in fasting insulin levels and index of insulin resistance in parous women who did not lactate compared with those who did (Table 2). Among women who lactated, we also found trends, although nonsignificant, for smaller increments in waist girth, body weight, LDL-C, and fasting glucose with longer duration of lactation from preconception to postweaning (Figs. 1A and 1B).
Mechanisms to explain the relationship between lactation and metabolic risk factors may be related to higher energy use or other effects on metabolism. Energy expenditure in lactating women is increased by 15–25%. However, greater weight losses of 2 kg have been generally associated with prolonged, exclusive lactation21,53,54 from 2.5–6 months postpartum21,55 and for the first year.20 Lactating women exhibit lower blood glucose and insulin concentrations along with the higher rates of glucose production and lipolysis compared with nonlactating women.56 Greater non–insulin-mediated use of glucose and reduction in trunk and femoral fat stores may improve the metabolic profiles.
Strengths of this study are the availability of preconception measurements of metabolic risk factor levels, its prospective nature, the ability to examine whether differences between groups exist postweaning, and whether differences exist by duration of lactation. Our nongravid reference group included nulligravidas and gravidas because the analysis using nulligravidas only did not alter the findings (results not shown). Limitations of our study include its small sample size of lactating women, lack of metabolic risk factor measurements during pregnancy, variable time interval postdelivery, and no information on lactation intensity or other residual confounders. Exclusive lactation would be expected to enhance differences in HDL-C.
The American Academy of Pediatrics recommends that all infants be exclusively breastfed through 6 months of age and that breastfeeding continue until the infant is 1 year of age.57 Although 80% of U.S. women initiate lactation, only 36% report “any” breastfeeding, and 14% report “exclusively” breastfeeding their infants at 6 months of age.58 Beyond the potential positive impact on maternal health, breastfeeding is an important health-related behavior that promotes healthy growth and development of the infant, and there is increasing evidence that breastfeeding prevents obesity during childhood. 59 As a lifestyle behavior, lactation may offer a practical low-cost intervention that may be more culturally acceptable, particularly to minority women, for promoting modest weight reduction. Yet, studies have rarely assessed the long-term effects of lactation on metabolic profiles among women in general or the duration necessary to achieve favorable effects. Lifetime lactation for 4 months or longer has been associated with a 25–35% reduction in the risk of type 2 diabetes in women,25 which is consistent with our finding that duration of lactation for 3 months or more was associated with a more favorable metabolic risk factor profile. Lactation is a modifiable behavior that may lower future risk of cardiovascular disease and diabetes in women.
Acknowledgments
This study was supported by contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095, from the National Heart, Lung, and Blood Institute, and by Career Development Award, 1 K01 DK059944, from the National Institute of Diabetes, Digestive and Kidney Diseases.
REFERENCES
- 1.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord. 2000;24:1660–1668. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- 2.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004;28:525–535. doi: 10.1038/sj.ijo.0802551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith WD, Sidney S. Long-term plasma lipid changes associated with a first birth: the Coronary Artery Risk Development in Young Adults study. Am J Epidemiol. 2004;159:1028–1039. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ness RB, Schotland HM, Flegal KM, Shofer FS. Reproductive history and coronary heart disease risk in women. Epidemiol Rev. 1994;16:298–314. doi: 10.1093/oxfordjournals.epirev.a036155. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg L, Palmer JR, Rao RS, Adams-Campbell LL. Risk factors for coronary heart disease in African American women. Am J Epidemiol. 1999;150:904–909. doi: 10.1093/oxfordjournals.aje.a010098. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Willett WC, Stampfer MJ, Rosner B, Speizer FE, Hennekens CH. A prospective study of age at menarche, parity, age at first birth, and coronary heart disease in women. Am J Epidemiol. 1987;126:861–870. doi: 10.1093/oxfordjournals.aje.a114723. [DOI] [PubMed] [Google Scholar]
- 7.Steenland K, Lally C, Thun M. Parity and coronary heart disease among women in the American Cancer Society CPS II population. Epidemiology. 1996;7:641–643. doi: 10.1097/00001648-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Kritz-Silverstein D, Barrett-Connor E, Wingard DL, Friedlander NJ. Relation of pregnancy history to insulin levels in older, nondiabetic women. Am J Epidemiol. 1994;140:375–382. doi: 10.1093/oxfordjournals.aje.a117260. [DOI] [PubMed] [Google Scholar]
- 9.O’Sullivan J, Gordon T. Childbearing and diabetes mellitus: United States, 1960–62. Washington, DC: National Center for Health Statistics, U.S. Public Health Service; 1966. [PubMed] [Google Scholar]
- 10.Martin FI, Hopper JL, Dean B, Campbell DG, Hammond P. Glucose tolerance and mortality in diabetes mellitus in Maltese- born residents of Victoria. Med J Aust. 1984;141:93–97. doi: 10.5694/j.1326-5377.1984.tb132711.x. [DOI] [PubMed] [Google Scholar]
- 11.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The effect of parity on the later development of non-insulin-dependent diabetes mellitus or impaired glucose tolerance. N Engl J Med. 1989;321:1214–1219. doi: 10.1056/NEJM198911023211802. [DOI] [PubMed] [Google Scholar]
- 12.Boyko EJ. The effect of parity on the later development of diabetes. N Engl J Med. 1990;322:1320. [PubMed] [Google Scholar]
- 13.Collins VR, Dowse GK, Zimmet PZ. Evidence against association between parity and NIDDM from five population groups. Diabetes Care. 1991;14:975–981. doi: 10.2337/diacare.14.11.975. [DOI] [PubMed] [Google Scholar]
- 14.Alderman BW, Marshall JA, Boyko EJ, Markham KA, Baxter J, Hamman RF The San Luis Valley Diabetes Study. Reproductive history, glucose tolerance, and NIDDM in Hispanic and non-Hispanic white women. Diabetes Care. 1993;16:1557–1564. doi: 10.2337/diacare.16.12.1557. [DOI] [PubMed] [Google Scholar]
- 15.Charles MA, Pettitt DJ, McCance DR, Hanson RL, Bennett PH, Knowler WC. Gravidity, obesity, and non-insulin-dependent diabetes among Pima Indian women. Am J Med. 1994;97:250–255. doi: 10.1016/0002-9343(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 16.Hanley AJ, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever TM, Kwan J, et al. Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care. 2002;25:690–695. doi: 10.2337/diacare.25.4.690. [DOI] [PubMed] [Google Scholar]
- 17.Manson JE, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Arky RA, et al. Parity and incidence of non-insulin-dependent diabetes mellitus. Am J Med. 1992;93:13–18. doi: 10.1016/0002-9343(92)90674-z. [DOI] [PubMed] [Google Scholar]
- 18.McManus RM, Cunningham I, Watson A, Harker L, Finegood DT. Beta-cell function and visceral fat in lactating women with a history of gestational diabetes. Metabolism. 2001;50:715–719. doi: 10.1053/meta.2001.23304. [DOI] [PubMed] [Google Scholar]
- 19.Knopp RH, Walden CE, Wahl PW, Bergelin R, Chapman M, Irvine S, et al. Effect of postpartum lactation on lipoprotein lipids and apoproteins. J Clin Endocrinol Metab. 1985;60:542–547. doi: 10.1210/jcem-60-3-542. [DOI] [PubMed] [Google Scholar]
- 20.Janney CA, Zhang D, Sowers M. Lactation and weight retention. Am J Clin Nutr. 1997;66:1116–1124. doi: 10.1093/ajcn/66.5.1116. [DOI] [PubMed] [Google Scholar]
- 21.Dewey KG, Heinig MJ, Nommsen LA. Maternal weight-loss patterns during prolonged lactation. Am J Clin Nutr. 1993;58:162–166. doi: 10.1093/ajcn/58.2.162. [DOI] [PubMed] [Google Scholar]
- 22.Sichieri R, Field AE, Rich-Edwards J, Willett WC. Prospective assessment of exclusive breastfeeding in relation to weight change in women. Int J Obes Relat Metab Disord. 2003;27:815–820. doi: 10.1038/sj.ijo.0802285. [DOI] [PubMed] [Google Scholar]
- 23.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol. 2002;100:245–252. doi: 10.1016/s0029-7844(02)02125-7. [DOI] [PubMed] [Google Scholar]
- 24.Linne Y, Dye L, Barkeling B, Rossner S. Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res. 2004;12:1166–1178. doi: 10.1038/oby.2004.146. [DOI] [PubMed] [Google Scholar]
- 25.Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- 26.Cutter GR, Burke GL, Dyer AR, Friedman GD, Hilner JE, Hughes GH, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12 suppl:1S–77S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 27.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 28.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172–1181. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 29.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 30.Bild DE, Jacobs DR, Liu K, Williams OD, Hilner JE, Perkins LL, et al. Seven-year trends in plasma low-density-lipoproteincholesterol in young adults: the CARDIA Study. Ann Epidemiol. 1996;6:235–245. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 31.Haffner SM, Bowsher RR, Mykkanen L, Hazuda HP, Mitchell BD, Valdez RA, et al. Proinsulin and specific insulin concentration in high- and low-risk populations for NIDDM. Diabetes. 1994;43:1490–1493. doi: 10.2337/diab.43.12.1490. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: the CARDIA study. Am J Public Health. 1997;87:635–642. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- 34.Anderssen N, Jacobs DR, Sidney S, Bild DE, Sternfeld B, Slattery ML, et al. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Epidemiol. 1996;143:351–362. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 35.McDonald A, Van Horn L, Slattery M, Hilner J, Bragg C, Caan B, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104–1112. [PubMed] [Google Scholar]
- 36.Hanson RL, Pratley RE, Bogardus C, Narayan KM, Roumain JM, Imperatore G, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol. 2000;151:190–198. doi: 10.1093/oxfordjournals.aje.a010187. [DOI] [PubMed] [Google Scholar]
- 37.Hanley AJ, Williams K, Gonzalez C, D’Agostino RB, Jr, Wagenknecht LE, Stern MP, et al. Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study [published erratum appears in Diabetes 2003;52:1306] Diabetes. 2003;52:463–469. doi: 10.2337/diabetes.52.2.463. [DOI] [PubMed] [Google Scholar]
- 38.Hubert HB, Eaker ED, Garrison RJ, Castelli WP. Life-style correlates of risk factor change in young adults: an eight-year study of coronary heart disease risk factors in the Framingham offspring [published erratum appears in Am J Epidemiol 1987;126:559] Am J Epidemiol. 1987;125:812–831. doi: 10.1093/oxfordjournals.aje.a114598. [DOI] [PubMed] [Google Scholar]
- 39.Gotto AM., Jr High-density lipoprotein cholesterol and triglycerides as therapeutic targets for preventing and treating coronary artery disease. Am Heart J. 2002;144 suppl:S33–S40. doi: 10.1067/mhj.2002.130301. [DOI] [PubMed] [Google Scholar]
- 40.Potter JM, Nestel PJ. The hyperlipidemia of pregnancy in normal and complicated pregnancies. Am J Obstet Gynecol. 1979;133:165–170. doi: 10.1016/0002-9378(79)90469-1. [DOI] [PubMed] [Google Scholar]
- 41.Knopp RH, Bergelin RO, Wahl PW, Walden CE, Chapman M, Irvine S. Population-based lipoprotein lipid reference values for pregnant women compared to nonpregnant women classified by sex hormone usage. Am J Obstet Gynecol. 1982;143:626–637. doi: 10.1016/0002-9378(82)90107-7. [DOI] [PubMed] [Google Scholar]
- 42.van Stiphout WA, Hofman A, de Bruijn AM. Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol. 1987;126:922–928. doi: 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- 43.Kallio MJ, Siimes MA, Perheentupa J, Salmenpera L, Miettinen TA. Serum cholesterol and lipoprotein concentrations in mothers during and after prolonged exclusive lactation. Metabolism. 1992;41:1327–1330. doi: 10.1016/0026-0495(92)90103-h. [DOI] [PubMed] [Google Scholar]
- 44.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol. 1982;89:211–215. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 45.Knopp RH, Bergelin RO, Wahl PW, Walden CE. Effects of pregnancy, postpartum lactation, and oral contraceptive use on the lipoprotein cholesterol/triglyceride ratio. Metabolism. 1985;34:893–899. doi: 10.1016/0026-0495(85)90134-9. [DOI] [PubMed] [Google Scholar]
- 46.Butte NF, Hopkinson JM, Mehta N, Moon JK, Smith EO. Adjustments in energy expenditure and substrate utilization during late pregnancy and lactation. Am J Clin Nutr. 1999;69:299–307. doi: 10.1093/ajcn/69.2.299. [DOI] [PubMed] [Google Scholar]
- 47.Qureshi IA, Xi XR, Limbu YR, Bin HY, Chen MI. Hyperlipidaemia during normal pregnancy, parturition and lactation. Ann Acad Med Singapore. 1999;28:217–221. [PubMed] [Google Scholar]
- 48.Kjos SL, Henry O, Lee RM, Buchanan TA, Mishell DR., Jr The effect of lactation on glucose and lipid metabolism in women with recent gestational diabetes. Obstet Gynecol. 1993;82:451–455. [PubMed] [Google Scholar]
- 49.Lenz S, Kuhl C, Hornnes PJ, Hagen C. Influence of lactation on oral glucose tolerance in the puerperium. Acta Endocrinol (Copenh) 1981;98:428–431. doi: 10.1530/acta.0.0980428. [DOI] [PubMed] [Google Scholar]
- 50.Motil KJ, Thotathuchery M, Montandon CM, Hachey DL, Boutton TW, Klein PD, et al. Insulin, cortisol and thyroid hormones modulate maternal protein status and milk production and composition in humans. J Nutr. 1994;124:1248–1257. doi: 10.1093/jn/124.8.1248. [DOI] [PubMed] [Google Scholar]
- 51.Stanley K, Fraser R, Bruce C. Physiological changes in insulin resistance in human pregnancy: longitudinal study with the hyperinsulinaemic euglycaemic clamp technique. Br J Obstet Gynaecol. 1998;105:756–759. doi: 10.1111/j.1471-0528.1998.tb10207.x. [DOI] [PubMed] [Google Scholar]
- 52.Diniz JM, Da Costa TH. Independent of body adiposity, breast-feeding has a protective effect on glucose metabolism in young adult women. Br J Nutr. 2004;92:905–912. doi: 10.1079/bjn20041288. [DOI] [PubMed] [Google Scholar]
- 53.Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. Int J Obes Relat Metab Disord. 2003;27:117–127. doi: 10.1038/sj.ijo.0802156. [DOI] [PubMed] [Google Scholar]
- 54.van Raaij JM, Schonk CM, Vermaat-Miedema SH, Peek ME, Hautvast JG. Energy cost of lactation, and energy balances of well-nourished Dutch lactating women: reappraisal of the extra energy requirements of lactation. Am J Clin Nutr. 1991;53:612–619. doi: 10.1093/ajcn/53.3.612. [DOI] [PubMed] [Google Scholar]
- 55.Ohlin A, Rossner S. Maternal body weight development after pregnancy. Int J Obes. 1990;14:159–173. [PubMed] [Google Scholar]
- 56.Motil KJ, Montandon CM, Garza C. Basal and postprandial metabolic rates in lactating and nonlactating women. Am J Clin Nutr. 1990;52:610–615. doi: 10.1093/ajcn/52.4.610. [DOI] [PubMed] [Google Scholar]
- 57.Breastfeeding and the use of human milk. American Academy of Pediatrics. Work Group on Breastfeeding. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 58.Center for Disease Control and Prevention, Department of Health and Human Services. [Retrieved December 9, 2006];National Immunization Survey. 2003 Available at: http://www.usbreastfeeding.org/breastfeeding/NIS_data/index.htm.
- 59.Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003;19:9–18. doi: 10.1177/0890334402239730. [DOI] [PubMed] [Google Scholar]