Abstract
Background
In patients with primary hyperaldosteronism, distinguishing between unilateral and bilateral adrenal hypersecretion is critical in assessing treatment options. Adrenal venous sampling (AVS) has been advocated by some to be the gold standard for localization of the responsible lesion however there remains a lack of consensus for the criteria and the standardization of technique.
Study Design
A retrospective study of 114 patients with a biochemical diagnosis of primary hyperaldosteronism who all underwent CT scan and AVS before and after ACTH stimulation. Univariate and multivariate analyses were performed to determine what factors were associated with AVS lateralization, and which AVS values were the most accurate criteria for lateralization.
Results
Eighty-five patients underwent surgery at our institution for unilateral hyperaldosteronism. Of the 57 patients that demonstrated unilateral abnormalities on CT, AVS localized to the contralateral side in 5 patients and revealed bilateral hyperplasia in 6 patients. Of the 52 patients who showed bilateral disease on CT scan, 43 lateralized with AVS. The most accurate criterion on AVS for lateralization was the post-ACTH stimulation values. Factors associated with AVS lateralization included a low renin value, high plasma aldosterone-to plasma-renin ratio, and adrenal mass ≥ 3cm on CT scan.
Conclusions
Because 50% of patients would have been inappropriately managed based on CT scan findings, patients with biochemical evidence of primary hyperaldosteronism and considering adrenalectomy should have AVS. The most accurate measurement for AVS lateralization was the post-ACTH stimulation values. Although several factors predict successful AVS lateralization, none are accurate enough to perform AVS selectively.
INTRODUCTION
The prevalence of primary hyperaldosteronism in the hypertensive population can be as high as 15%1,2. The majority of patients with primary hyperaldosteronism have either idiopathic bilateral hyperplasia (IHA) or an aldosterone producing adenoma (APA). Other rare causes include unilateral hyperplasia, aldosterone-producing adrenocortical carcinoma, aldosterone-producing ovarian tumor, or familial hyperaldosteronism including glucocorticoid-remediable hyperaldosteronism (GRA)3,4.
The goal of treatment is to minimize morbidity and mortality from aldosterone excess, which can result in adverse cardiovascular events independent of high blood pressure5. Therefore, in patients with primary hyperaldosteronism, distinguishing between unilateral and bilateral adrenal hypersecretion is critical in assessing treatment options. Diagnosis of unilateral hyperplasia or an APA may be amenable to surgical cure with a unilateral adrenalectomy, whereas a diagnosis of IHA should be managed with a mineralocorticoid receptor antagonist6.
Adrenal venous sampling was introduced in the late 1960’s as a test to differentiate unilateral from bilateral hyperaldosteronism7. Because of technical difficulties in cannulating both adrenal veins, and improved imaging modalities such as CT and MRI, AVS was not widely adopted. Although sensitivities with CT scan have been reported to be as high as 90%, recent studies highlight the pitfalls of noninvasive imaging techniques causing some to now advocate AVS as the gold standard for lateralization1,4,8–11. In a prospective study of 203 patients by Young and colleagues, 21.7% of patients would have been incorrectly excluded as candidates for adrenalectomy and an additional 24.7% may have had an unnecessary or inappropriate adrenalectomy based on CT scan findings alone4. In a meta-analysis of 950 patients by Kempers and associates, 37.8% of patients had CT/MRI results that were discordant with AVS.12 If only CT/MRI had been used, inappropriate adrenalectomy would have occurred in 14.6% of patients, inappropriate exclusion of adrenalectomy would have occurred in 19.1%, and incorrect side adrenalectomy in 3.9%. Zarnegar and colleagues found that CT scan can reliably diagnose adenomas larger than 1.0 cm and that AVS should be used selectively, when CT findings are either equivocal or both adrenal glands are abnormal13.
Although AVS may be re-emerging as the gold standard for differentiating between unilateral and bilateral primary hyperaldosteronism, there is a lack of standardization of technique and limited data on which AVS values to use to distinguish between unilateral and bilateral disease8–11, 13–15. The aims of this study were to determine if routine AVS is necessary in all patients, what factors predict AVS lateralization, and what technique for AVS should be utilized to determine lateralization.
METHODS
Patients
A computerized medical record database was used to identify 114patients with the diagnosis of primary hyperaldosteronism who underwent CT scan and AVS under a clinical protocol approved by our Institutional Review Board. Medical records were retrospectively reviewed to obtain baseline demographic data, laboratory values, CT scan and AVS reports, pathology reports, and operative reports. All patients had comprehensive biochemical testing to confirm the diagnosis of primary hyperaldosteronism. In addition to aldosterone and renin levels, patients had confirmatory testing including either a sodium chloride loading test, a captopril test, or a posture test. Clinical outcome data included systolic and diastolic blood pressure, potassium level, aldosterone level, and number of antihypertensive medications at postoperative follow up. Follow up was performed either in the clinic or by telephone interview with the patient.
AVS Technique
Regardless of CT scan findings, all patients underwent AVS, which was performed by experienced interventional radiologists. Venous catheters were introduced via both femoral veins. Simultaneous catheterization of bilateral adrenal veins was performed in all patients using either 4 French Mickaelsson, Simmons 1, and Cobra 2 catheters with or without modifications to sample the right adrenal vein; and 4 French Simmon2 or 3 catheters and rarely a sub 3 French microcatheter to obtain samples from the left adrenal vein. Peripheral samples were obtained from the right iliac vein. Two sets of baseline blood samples were drawn 5 minutes apart from each adrenal vein and the iliac vein. After the baseline draws, an intravenous bolus of 0.25mg of ACTH followed by an infusion of ACTH (0.25mg in 250 mL normal saline) at a rate of 150cc/hr was administered. Blood samples were collected at 5 minutes, 10 minutes, and 15 minutes post ACTH infusion and levels for aldosterone and cortisol were measured. Appropriate placement of catheters in the adrenal veins was surmised by contrast venography and subsequently confirmed by appropriately elevated cortisol levels in the adrenal vein samples as compared to the peripheral samples (ratio > 2).
Sample Interpretation
The aldosterone-to-cortisol ratio (AC) was computed for each sample to correct for varying capture and dilution of adrenal venous effluent. Patients that demonstrated unilateral hypersecretion of aldosterone were referred for an adrenalectomy. Diagnosis of a unilateral hyperfunctioning adrenal gland was made if the AC ratio on one side was at least four times greater than on the contralateral side and the peripheral samples. An AC ratio that was lower than periphery on the unaffected side especially after stimulation suggested a suppressed gland and therefore a unilateral hyperfunctioning gland on the contralateral side. Diagnosis of bilateral hyperplasia was made if the AC ratio on both sides was elevated and the response to stimulation was similar with no gradient observed between the two sites. Twelve different ratios were derived from the aldosterone and cortisol levels pre- and post-ACTH stimulation for each side at the time intervals stated above. These were an AC ratio for each side, ratio of AC from one gland versus peripheral AC, and greater AC ratio from one gland versus the smaller AC ratio from the contralateral gland.
Statistical Methods
The association between CT and AVS was determined by cross classifying the two parameters. The initial screening to determine which ratios were associated with AVS lateralization to left vs. right side was performed using a Wilcoxon rank sum test. P-values presented are two-sided and have not been adjusted for multiple comparisons. Those ratios which were found to be statistically different according to left vs. right location of abnormality were further evaluated for their ability to classify correctly according to side individually, in pairs based on time of determination, and then jointly, using logistic regression analysis. The Mann-Whitney rank sum, or Kruskal-Wallis tests were used to identify factors associated with AVS lateralization. Statistical analysis was performed using standard statistical software (SAS, Inc., Cary, NC).
RESULTS
Of 114 patients, 85 patients underwent adrenalectomy at our institution for unilateral hyperaldosteronism. The demographic and clinical data are summarized in Table 1. Our group of 114 patients consisted of 63 men and 51 women with mean age of 50.6 years (range 3–73 years). The mean plasma aldosterone concentration was 41.1 ng/dL (range of 2 to 328 ng/dL), mean plasma renin activity value was 0.9 ng/mL (range 0.1 to 23 ng/mL), and mean plasma aldosterone concentration-to-plasma renin activity (PAC/PRA) ratio was 100 (range 7 to 640). Patients with a PAC/PRA of less than 14ng/dL had aldosterone levels greater than 30 ng/dL and all patients had confirmatory testing with either a sodium chloride loading test, a captopril test, or a postural test. The average duration of hypertension was 12.8 years and ranged from several months to a maximum of 40 years. Patients were on an average of 2 to 3 antihypertensive medications. The average creatinine and BMI were 1.1 (range 0.5 to 2.3) and 30.6 (range 19 to 47), respectively (Table 1).
Table 1.
Study Cohort Demographics and Clinical Characteristics
| Mean age ± SD, y | 50.6 ± 11.0 |
| Gender | |
| Male | 63 |
| Female | 51 |
| Duration of hypertension | 12.8 ± 8.9 |
| Number of antihypertensive medications | 2.7 ± 1.3 |
| Potassium (reference range 3.3–5.1 mmol/L) | 3.2 ± 0.5 |
| Creatinine (reference range 0.9–1.4 mg/dL) | 1.1 ± 0.3 |
| Body Mass Index (kg/m2) | 30.6 ± 6.1 |
| Aldosterone (reference range ≤ 21 ng/dL) | 41.4 (2–328) |
| Renin (reference range 0.6–3 ng/mL) | 0.92 (0.1–23) |
| Aldosterone/renin ratio | 99.6 (7–640) |
| Largest diameter on CT scan, cm | 2.4 ± 1.6 |
| History of MI/CHF | 11 |
| History of CVA/TIA | 8 |
| History of Thyroid dysfunction | 12 |
| Family history of Endocrinopathy | |
| Thyroid dysfunction | 8 |
| Hyperaldosteronism | 4 |
Data are presented as mean values ± standard deviation or range of values.
Twelve patients had a prior history of thyroid dysfunction. Eleven patients had a prior myocardial infarction or congestive heart failure and eight patients had suffered a prior cerebrovascular event or transient ischemic attack. Four patients had a first degree relative with primary hyperaldosteronism.
Both adrenal veins were catheterized successfully in all patients based on venography and elevated cortisol values compared to the periphery. Two patients had to have repeat procedures secondary to misplaced blood samples. One patient developed severe lumbar back pain that spontaneous resolved after an AVS procedure without radiographic evidence of adrenal hemorrhage or infarction. There were no other adverse events from AVS.
CT and AVS Results
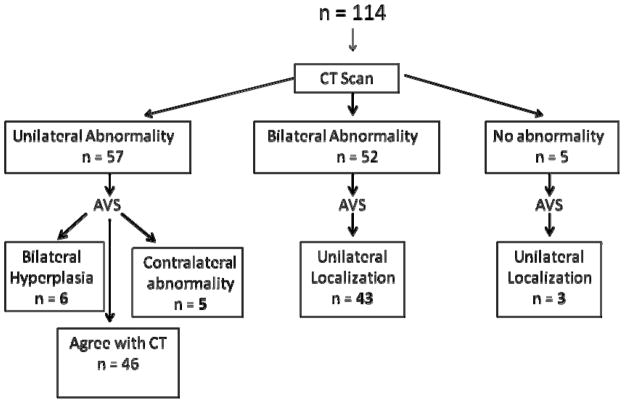
Of 57 patients that had a unilateral abnormality on CT scan, five patients lateralized to the contralateral side by AVS, and six patients had AVS consistent with bilateral hyperplasia (Figure 1). Therefore, 19.3% of patients would have had either an unnecessary adrenalectomy or adrenalectomy of the nonfunctioning adrenal gland if AVS was not performed. In 52 patients that showed bilateral disease on CT scan, AVS lateralized to one side in 43 patients and was concordant with the CT scan in 9 patients. In 5 patients that had no abnormality on CT scan, 3 lateralized with AVS. Of the group of 114 patients, 57 patients (50%) would have been inappropriately managed based on CT scan findings alone.
Figure 1.
Flow diagram of study cohort and localization by CT and adrenal venous sampling. Fifty percent of patients would have been inappropriately managed based on CT findings alone.
Aldosterone-to-cortisol (AC) criteria for AVS lateralization
Logistic regression analysis including the above listed AC ratios pre- and post- ACTH stimulation showed that while 98% of patients could be lateralized based on the post-ACTH stimulation AC ratios with evidence of suppression of the contralateral gland from the periphery, 95% of patients could be lateralized with only pre-ACTH stimulation values (Table 2). The remaining 2% did not localize with AVS. The adrenal vein AC ratio on each side and the ratio of adrenal AC to periphery AC on each side were adequate for successful lateralization.
Table 2.
Aldosterone-to-Cortisol Ratio Criteria for Adrenal Venous Sampling Lateralization
| Ratio | AVS Lateralized to right | AVS Lateralized to left | P Value |
|---|---|---|---|
| Pre-ACTH Stimulation | |||
| AC Right | 126.70 ± 88.79 | 3.71 ± 0.68 | < 0.0001 |
| AC Left | 27.83 ± 21.92 | 33.26 ± 5.97 | < 0.0001 |
| AC Peripheral | 6.76 ± 2.64 | 4.95 ± 0.78 | 0.26 |
| AC Right:AC Peripheral | 22.82 ± 6.04 | 1.31 ± 0.27 | < 0.0001 |
| AC Left:AC Peripheral | 3.20 ± 1.12 | 10.7 ± 1.95 | < 0.0001 |
| AC Larger: AC Smaller | 23.57 ± 5.17 | 13.84 ± 2.14 | 0.67 |
| Post-ACTH Stimulation | |||
| AC Right | 22.32 ± 3.01 | 16.82 ± 15.18 | < 0.0001 |
| AC Left | 12.71 ± 10.75 | 31.95 ± 9.53 | < 0.0001 |
| AC Peripheral | 3.32 ± 0.56 | 3.90 ± 0.64 | 0.62 |
| AC Right:AC Peripheral | 8.71 ± 1.25 | 1.30 ± 0.61 | < 0.0001 |
| AC Left:AC Peripheral | 1.67 ± 0.65 | 8.95 ± 2.30 | < 0.0001 |
| AC Larger: AC Smaller | 24.99 ± 6.56 | 34.7 ± 10.03 | 0.83 |
Patients were divided into two groups based on which side they lateralized to by AVS. Mean values for each ratio are separately recorded for patients that lateralized to the left side vs. the right side. Data are reported as mean ± standard error of the mean.
Factors predicting lateralization
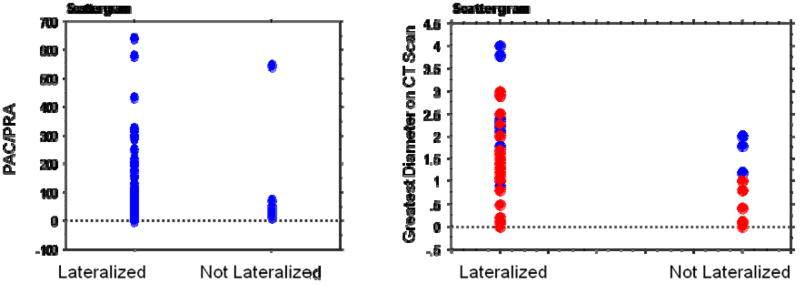
In our group of 114 patients, 17 patients were not localized with AVS indicating bilateral hyperplasia and the need for further medical management. Univariate analyses were performed to assess which factors may predict lateralization with AVS (Table 3). Age, gender, PAC, creatinine, BMI, duration of hypertension, number of antihypertensive medications, and ability to localize an enlarged adrenal gland or mass on CT were not associated with lateralization by AVS. However, PRA, PAC:PRA ratio, and size of a nodule on CT scan greater than or equal to 3cm were significantly associated with detection of unilateral disease by AVS (p ≤ 0.02) (Figure 2).
Table 3.
Factors Predictive of Lateralization by Adrenal Venous Sampling
| Factor | Lateralized | Not lateralized | p Value |
|---|---|---|---|
| Gender, % | |||
| Male | 56 | 53 | 0.61 |
| Female | 44 | 47 | |
| Age ± SEM, y | 50.6 ± 1.0 | 52.9 ± 0.16 | 0.21 |
| Aldosterone | 38.5 ± 3.7 | 45.7 ±19.5 | 0.19 |
| Renin | 0.93 ± 0.03 | 0.87 ± 0.16 | 0.01 |
| PAC:PRA | 102.6 ± 13.4 | 61.9 ± 30.5 | 0.02 |
| Potassium | 3.2 ± 0.1 | 3.3 ± 0.1 | 0.77 |
| Creatinine | 1.1± 0.03 | 1.0 ± 0.04 | 0.84 |
| BMI | 30.6 ± 0.6 | 31.3 ± 1.5 | 0.58 |
| Duration of hypertension | 12.8 ± 0.9 | 12.8 ± 2.2 | 0.75 |
| No. of antihypertensives | 2.8 ± 0.1 | 2.4 ± 0.3 | 0.19 |
| CT Lateralization, % | 52.6 | 35.3 | 0.20 |
| Size on CT Scan ≥ 3 cm | n = 5 | n = 0 | < 0.0001 |
Mean values are presented for patients that either lateralized or did not lateralize by AVS. Data are reported as mean ± standard deviation or standard error of the mean.
Figure 2.
Scattergram showing the range of values for mean plasma aldosterone concentration-to-plasma renin activity (PAC/PRA) and greatest tumor diameter (cm) of lesion on CT scan versus patients that lateralized or did not lateralize by adrenal venous sampling. Blue and red dots refer to size of lesions on right and left sides, respectively.
Surgical Outcome
Of the 114 patients, 85 patients underwent surgery at our institution. Thirty-three patients underwent right adrenalectomy, and 51 patients underwent left adrenalectomy. One patient was identified as having massive macronodular adrenocortical disease (MMAD) and underwent bilateral adrenalectomy. Eighteen patients underwent open adrenalectomy and 67 patients had a laparoscopic adrenalectomy. Twenty patients experienced perioperative complications (Table 4). One patient had an intraoperative diaphragmatic injury and required placement of a chest tube. Another patient had a mallory weiss tear that was postoperative identified and embolized. The remaining patients had grade 1 and 2 complications. There were no perioperative deaths.
Table 4.
Postoperative Complications
| Complication | n |
|---|---|
| Fever with spontaneous resolution | 5 |
| Pneumonia treated with antibiotics | 3 |
| Arrhythmias/PVC | 3 |
| 1 episode of hemoptysis | 1 |
| Mallory Weiss tear requiring embolization | 1 |
| Pancreatitis | 2 |
| Transaminitis | 1 |
| Prolonged Ileus | 2 |
| Acute renal failure not requiring dialysis | 2 |
| Redman’s syndrome from preoperative Vancomycin | 1 |
| Intraoperative diaphragmatic injury | 1 |
| Hyperkalemia | 2 |
| Herpes Labialis | 1 |
Final pathologic findings confirmed a solitary cortical adenoma in 76% of patients and an additional 14% of patients had an adenoma within the background of cortical hyperplasia. Average adenoma size was 1.58 ± 0.81 cm (range 0.2cm to 4.5cm). Seven patients had nodular hyperplasia, and in one patient, no nodule was identified. This patient had persistent symptoms postoperatively, underwent further workup, and ultimately had a contralateral adrenalectomy likely secondary to a mislateralization. Her previous post ACTH AC ratio from the initial AVS was 48 and lateralized to the left adrenal gland. Pathology reports could not be located for seven patients. Eighty of 85 patients were either cured or had improved symptom control as evidenced by normal postoperative PAC levels or improvement or normalization of blood pressure. Postoperative aldosterone levels were available and normal in 27 patients. At postoperative follow up 17 patients were normotensive (blood pressure < 140/90) completely off all of their preoperative blood pressure medications and 48 patients were on less medication. The average reduction in blood pressure medication was 1.5 with a range of 1 to 3 medications discontinued per patient. Fifteen patients were on the same number of medications, but now normotensive. All but one patient came off potassium supplementation because of persistent disease. Median follow up was 8.8 months with a range from 1 month to 9 years.
DISCUSSION
The results of this study support the routine use of AVS in all patients with biochemical evidence of primary hyperaldosteronism, as 50% of patients would have been inappropriately managed based on CT scan findings alone. Moreover, the most accurate measurement for AVS lateralization was the post-ACTH stimulation AC ratio, although a majority of patients can be localized without ACTH stimulation. We also found several factors associated with successful AVS lateralization such as absolute renin value, aldosterone-to-renin ratio, and size of the lesion on CT scan but none were accurate enough to forgo the need for AVS.
Traditionally, AVS has been considered a complex procedure associated with morbidities. The procedure itself is technically challenging and requires an experienced interventional radiologist. Historically, samples from the right adrenal vein could not be obtained in 30% of patients and complications such as bilateral adrenal infarction, adrenal vein dissection, and adrenal hemorrhage led to the use of CT and MRI as preferred methods to distinguish between unilateral and bilateral adrenal disease16. However, even high resolution thin section (2–3 mm) CT scans can miss small nodules and can also pick up small nonfunctional incidentalomas. Our findings support the routine use of AVS in all patients with biochemical evidence of primary hyperaldosteronism, as half of the patients would have been inappropriately managed based on CT findings alone. There were no technical complications associated with AVS in our study and our morbidity rate was 2.6%. Two patients required repeat AVS because a clerical error resulted in misplaced blood samples and one patient developed severe lumbar back pain that spontaneously resolved without radiographic evidence of adrenal hemorrhage or infarction.
No general consensus on the technique for AVS, the AC measurements taken, and the criteria used (with and without ATCH stimulation) for lateralization exists. While some investigators use sequential adrenal vein catheterization, others use simultaneous sampling of both adrenal veins. The decision to use ACTH for adrenal gland stimulation, the dose, and method of administration also varies across institutions. One of the strengths of our study is that AVS has been performed with a consistent and systematic protocol by experienced radiologists. At our institution AVS is performed by simultaneous catheterization of bilateral adrenal veins with and without ACTH stimulation given as a bolus and continuous infusion. However, upon univariate and multiple logistic regression analyses of twelve different ratios derived from the AC ratio, pre-ACTH stimulation values successfully lateralized in 95% of patients, and the addition of ACTH allowed 98% of patients to lateralize. ACTH stimulation was the most accurate method for lateralization, and also allows for confirmation of successful adrenal vein cannulation, and minimizes variability in cortisol secretion during sampling.
Factors that predicted successful AVS lateralization in univariate analyses included a low absolute renin value, high PAC/PRA, and size on CT scan greater than or equal to 3cm. To our knowledge, we are not aware of any studies that have addressed predictive factors for successful AVS lateralization using both demographic, clinical, laboratory and imaging data. It is not surprising to find that patients with a unilateral large adrenal mass on preoperative CT scan were more likely to have AVS lateralize the tumor, especially in the setting of a normal contralateral adrenal gland. None of these factors, however, in combination or alone could reliably predict unilateral hyperaldosteronism because the false positive rate of CT scan lateralization was 19%.
Prior studies have shown that unilateral adrenalectomy in patients with an aldosteronoma or unilateral hyperplasia results in complete surgical cure with normalization of blood pressure without the use of antihypertensives in about one-third of patients and improvement in blood pressure in the remaining15,16. Hypokalemia can be reversed in up to 100% of patients. Factors predictive of complete surgical cure include duration of hypertension less than 6 years, less than 3 antihypertensives to control blood pressure preoperatively, younger age (less than 50 years old), and female gender17,18. In this study, our surgical cure rate is slightly less than what has been previously reported in the literature. This may be due to a longer duration of hypertension (12.9 years) in our study cohort, and that 54% of patients were on three or more anti-hypertensives. Nonetheless, all the patients who had PAC levels available postoperatively had normal or undetectable levels. A majority of patients derived benefit from their operation in terms of hypokalemia resolution and number of blood pressure medications required. Postoperatively patients were on an average of 1.5 blood pressure medications (range 0–6).
The main limitation of our retrospective study is that our study cohort may not be reflective of most patients with primary hyperaldosteronism as we are a referral center. However, because all patients underwent comprehensive biochemical work up to confirm the diagnosis of primary hyperaldosteronism and underwent systematic approach for lateralization with both AVS and CT scanning in all cases, we believe our findings address the controversial issues of performing routine AVS, and determining if any clinical, imaging, and laboratory criteria can be used to avoid the need for AVS.
In conclusion, distinguishing between unilateral and bilateral adrenal hypersecretion in patients with primary hyperaldosteronism is critical in assessing treatment options. This study supports the routine use of AVS in patients with biochemical evidence of primary hyperaldosteronism and who want to proceed with adrenalectomy. Although the majority of patients can successfully be lateralized with only pre-ACTH stimulation values, the most accurate method for AVS lateralization is post ACTH stimulation. Furthermore, utilizing renin values, PAC/PRA ratios, and size of the lesion on CT scan may guide the clinician in determining which patients will successfully lateralize with AVS, but none of these predictive factors are accurate enough to determine which patients need AVS.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McKenzie TJ, Lillegard JB, Young WF, Jr, Thompson GB. Aldosteronomas—state of the art. Surg Clin North Am. 2009;89:1241–1253. doi: 10.1016/j.suc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. doi: 10.1210/jc.2003-031337. [DOI] [PubMed] [Google Scholar]
- 3.Young WF., Jr Minireview: primary aldosteronism-changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208–2213. doi: 10.1210/en.2003-0279. [DOI] [PubMed] [Google Scholar]
- 4.Young WF, Jr, Stanson AW, Thompson GB, et al. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 5.Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 7.Melby JC, Spark RF, Dale SL, et al. Diagnosis and localization of aldosterone-producing adenomas by adrenal-vein catheterization. N Engl J Med. 1967;277:1050–1056. doi: 10.1056/NEJM196711162772002. [DOI] [PubMed] [Google Scholar]
- 8.Stewart PM, Allolio B. Adrenal vein sampling for primary aldosteronism: Time for a reality check. Clin Endocrinol. 2009 doi: 10.1111/j.1365-2265.2009.03714.x. Accepted article. [DOI] [PubMed] [Google Scholar]
- 9.Mulatero P, Bertello C, Sukor N, et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension. 2010 doi: 10.1161/HYPERTENSIONAHA.109.146613. Accepted article. [DOI] [PubMed] [Google Scholar]
- 10.Magill SB, Raff H, Shaker JL, et al. Comparison of adrenal vein sampling and computed tomography in the differentiation of primary aldosteronism. J Clin Endocrinol Metab. 2001;86:1066–1071. doi: 10.1210/jcem.86.3.7282. [DOI] [PubMed] [Google Scholar]
- 11.Phillips JL, Walther MM, Pezzullo JC, et al. Predictive value of preoperative tests in discriminating bilateral adrenal hyperplasia from an aldosterone-producing adenoma. J Clin Endocrinol Metab. 2000;85:4526–4533. doi: 10.1210/jcem.85.12.7086. [DOI] [PubMed] [Google Scholar]
- 12.Kempers MJ, Lenders JW, van Outheusden L, et al. Systematic Review: Diagnostic Procedures to Differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Zarnegar R, Bloom AI, Lee J, et al. Is adrenal venous sampling necessary in all patients with hyperaldosteronism before adrenalectomy? J Vasc Interv Radiol. 2008;19:66–71. doi: 10.1016/j.jvir.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Kline GA, Harvey A, Jones C, et al. Adrenal vein sampling may not be a gold-standard diagnostic test in primary aldosteronism: final diagnosis depends upon which interpretation rule is used. Int Urol Nephrol. 2008;40:1035–1043. doi: 10.1007/s11255-008-9441-9. [DOI] [PubMed] [Google Scholar]
- 15.Harvey A, Kline G, Pasieka JL. Adrenal venous sampling in primary hyperaldosteronism: Comparison of radiographic with biochemical success and the clinical decision-making with “less than ideal” testing. Surgery. 2006;140:847–855. doi: 10.1016/j.surg.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Doppman JL, Gill JR., Jr Hyperaldosteronism: Sampling the adrenal veins. Radiology. 1996;198:309–312. doi: 10.1148/radiology.198.2.8596821. [DOI] [PubMed] [Google Scholar]
- 17.Sawka AM, Young WF, Jr, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135:258–261. doi: 10.7326/0003-4819-135-4-200108210-00010. [DOI] [PubMed] [Google Scholar]
- 18.Zarnegar R, Young WF, Jr, Lee J, et al. The aldosteronoma resolution score. Predicting complete resolution of hypertension after adrenalectomy for aldosteronoma. Ann Surg. 2008;247:511–518. doi: 10.1097/SLA.0b013e318165c075. [DOI] [PubMed] [Google Scholar]