Introduction
While laparoscopic approaches are used for many abdominal procedures and allow for faster recovery of bowel function, better immunologic response and overall accelerated recovery for the patient, the use of laparoscopy for cancer surgery is still a matter of debate. For patients with cancer, questions remain about the immunologic implications of laparoscopic surgery, the adequacy and standardization of laparoscopic techniques, the risk for disease recurrence, and the impact on survival. The safety and efficacy of laparoscopic surgery for colorectal cancer has certainly been established, but the same rigorous approach to other cancers has yet to be reported. In this article we review the current data and state of the art for laparoscopic approaches in abdominal cancer surgery.
Methods
Literature Review
An electronic search of the Medline database was performed using different key words that described abdominal cancer surgery. For each organ a search was conducted including as key words and phrases: cancer, laparoscopic versus open surgery, and the specific organ. The search terms were identified in the title, abstract, or medical subject heading. Abstracts of each identified publication were screened, and only publications that addressed the clinical questions of this analysis were further assessed. Each of these publications was independently and thoroughly reviewed by 2 authors (E.A. and O.J.H.).
Analysis
Relevant data, including authors, title, study design, methodology, main results, and conclusions, were extracted and documented on a separate data sheet for each publication. For every organ, the grade of recommendation based on the available literature was determined as proposed by Sackett (Table 1).1 Data for various malignancies are compared in Table 2.
TABLE 1.
Modified Level of Evidence and Grade of Recommendation According to Sackett1
| Level of Evidence |
Type of Trial | Grade of Recommendation |
|---|---|---|
| I | Large randomized trials with clear-cut results (and low risk of error) | A |
| II | Small randomized trials with uncertain results (and moderate to high risk of error) | B |
| III | Nonrandomized contemporaneous controls | C |
| IV | Nonrandomized, historical controls | none |
| V | No controls, case-series only, opinion of experts | none |
TABLE 2.
Evidence and Recommendations in the literature
| Organ | Level of Evidence |
Grade of Recommendation |
Literature |
|---|---|---|---|
| Colon | I | A | Veldkamp et al.9 Lacy et al.10,14 COSTSG8 Guillou et al.11 Hewett et al.12 Jayne et al.15 Buunen et al.16 Liang et al.18 |
| Rectum | II | B | Lujan et al.31 Pechlivadines et al.32 Zhou et al.34 Ng et al.35 Jayne et al.15 Guillou et al.11 Braga et al. 33 |
| Stomach | II | B | Hayashi et al.45 Huscher et al.46 |
| Esophagus | III | C | Bresadola et al.54 Benzoni et al.55 |
| Pancreas | III | C | Rotellar et al.58 Kooby et al.59 Palanivelu et al.63 |
| Liver | III | C | Cai et al.71 Lee et al.72 Topol et al.73 |
| Gastric GIST | IV | none | Hindmarsh et al.80 |
| Appendix | IV | none | Bucher et al.83 |
| Adrenal Gland | IV | none | Toniato et al.91 Walz et al.93 |
| Gall Bladder | V | none | Paoluccci et al.108 |
| Bile Duct | V | none | Weber et al.115 |
| Small Intestine | V | none | Tricarico et al.120 Eccher et al.122 Soeda et al.125 Kim et al.128 |
Colon
Level of Evidence: I Grade of Recommendation: A
Laparoscopic surgery for the colon was first described in the 1990's.2 In the initial reports of laparoscopic procedures for adenocarcinoma of the colon, a prohibitive port-site metastasis rate of 21% tempered the enthusiasm for this approach.3 Subsequent animal studies helped to elucidate the mechanism of port-site recurrences. Direct manipulation of the tumor, extraction of the tumor through the small wound without adequate wound protection, contamination of the laparoscopic instruments with cancer cells and inexperience of the surgeon were identified as important risk factors.4 As surgeons gained more experience with this approach, the rate of port-site metastasis declined to less than 1%, comparable to wound recurrences reported for open procedures.5-8
Several major randomized clinical trials were conducted to ultimately determine the efficacy of laparoscopic surgery for colon cancer, including COLOR (Colon Cancer Laparoscopic or Open Resection) in the Netherlands,9 the Barcelona trial in Spain,10 COSTSG (Clinical Outcomes of Surgical Therapy Study Group) in the United States,8 and CLASICC (Conventional versus Laparoscopic-assisted Surgery in Colorectal Cancer) in the United Kingdom.11 These studies demonstrated results comparable to open surgery with regard to number of lymph nodes resected, likelihood of a tumor free resection margin and size of specimen removed (Table 3).8-11 In groups undergoing a laparoscopic resection, operative times were longer, use of narcotics and analgesics was significantly less,8 and postoperative ileus was shorter combined with earlier resumption of oral intake9-12 and a shorter hospital stay.8-12
TABLE 3.
Prospective Randomized Trials on Laparoscopic versus Open Surgery for Colon Cancer
| Reference | Study | Resection Type | Exclusion | n | Technique | Operating time (min) |
Morbidity | Mortality | Lymph nodes (n) |
Median Follow-up (months) |
Recurrence | Overall 3-Year Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Veldkamp et al9 Buunen et al16 |
COLOR | Right Side Left Side Sigmoid Other |
BMI > 30 kg/m2 Transverse Splenic Flexure Metastasis Intestinal Obstrruction Multiple Primary Tumors Synchronous Abdominal Surgery Organ Invasion Previous Colon Surgery Previous Malignancy |
551 | open | 115 * | 20.0% | 2% | 10 | 53 | 17.0% | 84.2% |
| 544 | laparoscopy assisted |
145 * | 21.0% | 1% | 10 | 19.7% | 81.8% | |||||
| Lacy et al10,14 | Barcelona Trial |
Right Side Left Side Sigmoid Anterior Resection Subtotal Colectomy Hartmann Procedure |
Transverse Metastasis Organ Invasion Intestinal Obstruction Previous Colon Surgery |
102 | open | 118 * | 28.7% * | 2.9% | 11.1 | 95 | 28.0% | ~82% |
| 106 | laparoscopy assisted |
142 * | 10.8% * | <1% | 11.1 | 18.0% | ~86% | |||||
| Clinical Outcomes of Surgical Therapy Study Group8 |
COSTSG | Right Side Left Side Sigmoid |
Transverse Rectal Metastasis Emergency |
428 | open | 95 * | 20.0% | 1% | 12 | 53 | 19.6% | ~85% |
| 435 | laparoscopy assisted |
150 * | 21.0% | <1% | 12 | 17.5% | ~85% | |||||
| Guillou et al11 Jayne et al15 |
CLASICC | Right Side Left Side Sigmoid Anterior Resection Abdominoperineal Resection |
Transverse Intestinal Obstruction Previous Malignancy Multiple Primary Tumors Pregnancy Other GI Disease |
253 | open | _ | 42.0% | 5% | 13.5 | 36.8 | 22.2% | 66.7% |
| 484 | laparoscopy assisted |
_ | 47.0% | 4% | 12 | 23.8% | 68.4% |
significant in original publication
The most important goal of cancer surgery is cancer-free survival. As most recurrences occur in the first three years after operation,13 the primary endpoint for these prospective randomized controlled clinical trials was therefore the three-year survival. Cancer free survival was comparable or better in the laparoscopic groups. The Barcelona trial showed a survival advantage for the laparoscopic group after a median follow-up of 95 months.14 This difference was identified only in stage III cancer patients and has not been reproduced by any other study. The COSTSG trial reported a median follow-up of 52.8 months with similar survival and recurrence rates.8 Recently the three-year results of the CLASICC trial have been published and showed no difference in disease-free survival, local recurrence or quality of life.15 The COLOR trial failed to exclude the possibility that the laparoscopic procedure was inferior, but the differences were very small.16 Conversion rates varied between 11 and 30%. In the CLASICC trial, 143 (29.3%) of the 488 laparoscopic procedures were converted to open operations, with the risk factors for conversion including high BMI, male gender, ASA III grading and local tumor invasion.17
A recent meta-analysis of the published literature strengthens the justification for the use of laparoscopy for colon cancer surgery. In 10 randomized clinical trials including 2474 patients, there were no statistically significant differences in local cancer recurrence, port or wound site recurrence and distant metastases between laparoscopic and open surgery.18
Since the large randomized clinical trials excluded cancer located in the transverse colon, the corresponding data are not as strong as those for the left and right colon. Laparoscopic resection of transverse colon cancer is technically feasible,19 but the long-term outcome and results still need evaluation in prospective randomized trials.
The costs of laparoscopic surgery are higher than those of open procedures, but overall hospital stay appears to be one to two days shorter, allowing for an overall decrease in hospital costs.20-22 The learning curve remains an important issue. A single center study including 900 patients found that the learning curve for laparoscopic colon cancer surgery was 55 cases for the right colon and 62 cases for the left colon.23
In experienced hands laparoscopic surgery for colon cancer has results similar to open surgery and it has certain short-term advantages. Surgeons experienced in laparoscopic colon surgery can therefore safely offer this option, but open approaches are also acceptable. Data for transverse colon cancers are accumulating, and further studies should be performed within the context of clinical trials.
Rectum
Level of Evidence: II Grade of Recommendation: B
Total mesorectal excision, advocated by Heald et al., is the accepted best practice for adenocarcinoma of the rectum.24 As total mesorectal excision has been associated with lower recurrence rates and improved survival, alternative approaches for rectal cancer must be measured against total mesorectal excision. Several randomized controlled trials of laparoscopic rectal cancer surgery, among many retrospective studies, have shown the safety and feasibility of laparoscopic rectal cancer resection with short term benefits.25-27 A retrospective study of 86 patients with a mean follow-up of 6.5 years found no difference in local recurrence rates and incidence of distant metastases.28 A meta-analysis including 20 retrospective and prospective non-randomized studies identified short-term advantages for the laparoscopic group, including earlier stomal function, bowel movements and oral intake, and a shorter hospital stay. The procedures were found to have no differences in resection margin positivity or lymph node clearance.29 A Cochrane Review including 48 studies (randomized controlled trials, controlled clinical trials, case series and case reports) representing 4224 patients found no differences in disease-free survival rate, local recurrence rate, morbidity, mortality, anastomotic leakage, resection margin positivity and number of recovered lymph nodes. Advantages of the laparoscopic procedure included lower blood loss, earlier return to oral intake, lower use of narcotics and less inflammation measured by lower levels of interleukin-1, interleukin-6 and C-reactive protein.30,31 The disadvantages include longer operative time and higher procedural costs.31 Lymph node clearance and distribution also analyzed by prospective randomized trials showed no difference between the techniques.32-34 A prospective randomized trial comparing laparoscopic with open surgery for 171 patients with low or ultra-low rectal cancer demonstrated no statistical differences in operation time, analgesic use, oral intake or mortality for the two procedures. Moreover, the laparoscopy group had lower blood loss and shorter hospitalization.35
The CLASICC trial for colon cancer included rectal cancers and was followed by three other prospective randomized trials with long-term results.15,32,34,36 The CLASICC trial showed more positive margins and port-site metastases in the 3-year follow-up for the laparoscopic group,11 but the other three trials showed a similar local recurrence rate and disease free survival.15,32,34,36 These findings in the CLASSIC trial may be due to a specific lack of expertise for laparoscopic rectal surgery during the trial as evidenced by a high conversion rate, a preponderance of procedures differing from total mesorectal excision, and the multicentric recruitment of the patients to the trial. Bladder function was similar for laparoscopic and open rectal operations, while overall sexual function and erectile function were insignificantly worse after laparoscopic rectal surgery.
Although the evidence remains weaker than for colon cancer, laparoscopic surgery for rectal cancer seems comparable to that of open surgery and has short-term advantages. Due to the learning curve involved in this operation, surgeons experienced in both total mesorectal excision and laparoscopic surgery can therefore safely offer this option, but open surgery is also acceptable.
Stomach
Level of Evidence: II Grade of Recommendation: B
Gastric adenocarcinoma is a highly lethal form of malignancy, with a high incidence in Pacific Asian countries. Most studies of laparoscopic surgery include patients in Japan and Korea with early gastric cancer limited to the gastric mucosa or submucosa, regardless of lymph node involvement. Several retrospective studies have shown that laparoscopic distal gastrectomy for early gastric cancer is feasible and safe and associated with less pain, quicker recovery of gastrointestinal function, shorter hospital stay but longer operative time, compared with open surgery.37-42 However, the long-term quality of life after 5 years does not differ.43 The rate of positive resection margin has been found to be equivalent; the numbers of retrieved lymph nodes have been less than for open procedures in most of the studies, but above the required standard of 15 nodes.38,40,41 A meta-analysis of 4 randomized controlled trials and 12 retrospective studies of mainly early gastric cancer has confirmed a significantly lower number of lymph nodes in the laparoscopic group.44 Survival has been analyzed in retrospective and small prospective randomized studies and found to be similar between laparoscopic or open procedures, but these smaller studies have limited power to detect differences.45-47 A Korean study analyzing the learning curve for laparoscopic-assisted distal gastrectomy found that 50 cases are necessary for optimal operative performance.48
In the Western countries without mass population screening, only 10 - 20 % of the detected tumors are early gastric cancers.49 For advanced gastric cancer, the few published studies show the feasibility of laparoscopic gastrectomy with adequate oncological clearance.38,50 Only one small prospective randomized trial has demonstrated similar 5-year survival for laparoscopic compared to open gastric resection.47 This single study raises the evidence to level II.
At present, evidence is insufficient to justify laparoscopic procedure for gastric cancer outside the context of a clinical trial. In addition, a large experience with this approach is needed for adequate outcomes and requires advanced laparoscopic training.
Staging laparoscopy for gastric cancer
Laparoscopic staging of gastric cancer, especially with the addition of laparoscopic ultrasonography, has been shown to be more reliable than other imaging techniques. A staging laparoscopy is useful in cases of questionable stage or resectability and spares the patient an unnecessary laparotomy.51 It has been shown that staging laparoscopy can be performed safely and is not associated with port-site metastases.52
Esophagus
Level of evidence: III Grade of Recommendation: C
Both adenocarcinoma and squamous esophageal cancers have a poor prognosis, and the morbidity of surgical management approaches 50 %.53 One problem is the extent of the surgery, with abdominal and thoracic components. While it seems intuitive that laparoscopic approaches could mitigate the effects of this extensive operation and diminish perioperative morbidity, several trials have been conducted to demonstrate the feasibility of laparoscopic esophageal resection, and none has shown a major advantage with regard to morbidity and mortality.54 Two small non-randomized comparative studies have shown comparable operative times, shorter hospital stay, no difference in morbidity and mortality and comparable lymph node yield.55,56 Staging with laparoscopic or thoracoscopic ultrasonography and biopsy is feasible and prevents the need for explorations in patients with metastatic disease.57
Prospective randomized trials with large patient numbers to compare laparoscopic to open surgery are lacking; therefore, the open approach remains the standard for this disease. Laparoscopic procedures can be attempted, but should be performed in prospective randomized trials at this time.
Pancreas
Level of evidence: III Grade of Recommendation: C
Adenocarcinoma of the pancreas has an overall 5-year survival below 5%.58 Several studies have reported the feasibility of laparoscopic pancreatic resection, especially for left or distal pancreatectomy.59 A large retrospective multicenter comparison for left pancreatectomy yielded a similar rate of positive margin and pancreatic leak.60 Most of these studies have included benign neoplasms, while some reports included malignant cases. The long-term outcome and the oncologic results cannot be assessed at this time. The short term benefits include a shorter hospital stay and faster return to normal activity.61-63
The feasibility of laparoscopic pancreaticoduodenectomy has been demonstrated by several studies, among them an impressive series of 42 laparoscopic pancreaticoduodenectomies for several diseases with a mean follow-up of 36 months and an actuarial 5-year survival of 19.1% for the subgroup of the adenocarcinomas.64,65 The value of laparoscopy for pancreatic malignancy lies in its diagnostic and staging capabilities. The accuracy of assessment of resectability can be increased by laparoscopy and laparoscopic ultrasonography. The lesser sac can be assessed by laparoscopy but local involvement of the superior mesenteric vein cannot reliably be assessed independent of an open exploration.66 In contrast liver and peritoneal metastases can be found in most cases and this avoids a non-therapeutic laparotomy.66,67
Laparoscopic resection of pancreatic cancer should only be performed in the setting of prospective randomized trial with long-term survival and oncologic clearance as endpoints.
Palliation for pancreatic cancer
Level of Evidence: III Grade of Recommendation: C
Palliative gastric and biliary bypass both may be performed laparoscopically. However, a study examining 155 patients with unresectable pancreatic cancer diagnosed by laparoscopy found that jaundice was relieved by endoscopic or transhepatic decompression in all patients, and only 3% of the patients needed an operative decompression before death.68 The advantage of laparoscopic over open gastrojejunostomy was reported in a case-matched study showing reduced blood loss and shorter hospital stay in the laparoscopic group.69
Although prospective randomized trials are not available, laparoscopy can be used as an instrument to avoid non-therapeutic laparotomies in patients with pancreatic cancer.
Liver
Level of Evidence: III Grade of Recommendation: C
The theoretical concerns regarding laparoscopic surgery for liver cancer may be greater then for other organs because of the risks of bleeding and air embolism and the difficulties of exposure. As laparoscopic instrumentation and surgical skills have advanced, the staging of liver cancer by laparoscopy and laparoscopic ultrasonography has become a very useful tool. Resections of benign liver tumors including cysts in peripheral liver segments are technically simple and associated with earlier recovery when compared with open approaches.70 Diagnostic laparoscopy helps to avoid unnecessary exploratory laparotomies and does not appear to have an adverse effect on tumor recurrence in patients with ruptured hepatocellular carcinoma.71 Nevertheless, the resection of malignant liver tumors is much more challenging since these are often located close to the central vessels and bile ducts. While hemorrhage and bile leaks are therefore more likely than for peripheral resections, the feasibility of laparoscopic tumor resections has been demonstrated in pair-matched controlled trials for primary (hepatocellular carcinoma, cholangiocarcinoma) and secondary liver tumors located in segments II-VII.72-74 The follow-up of these patients showed outcomes similar to open surgery.
While these studies demonstrated the feasibility of left hepatic lobectomy and the more challenging segmental resections, concerns remain whether larger resections are justified if can be done laparoscopically. As these resections are performed for malignancy, mincing of the liver for extraction, as it is performed for splenic rescction, would impede pathological assessment. The large excision thus required for extraction, may undo the benefit of laparoscopy. As randomized clinical trials with prospective evaluation of survival have yet to be reported, laparoscopic surgery for malignant liver tumors should be undertaken within the confines of well designed trials.
Gastric gastrointestinal stromal tumor (GIST)
Level of evidence: IV Grade of Recommendation: none
GISTs are stromal tumors characterized by mutations in the tyrosine-kinase gene and they stain positive for CD117. They are often located in the stomach, but can occur in any portion of the alimentary tract. The goals of operation include a segmental resection with an intact pseudo-capsule and a thorough exploration of the abdomen for metastasis. Resection margins of 2 cm are sufficient.75 Lymphadenectomy is unnecessary, as nodal metastases are rare. Risk factors for tumor recurrence include high mitotic index >10 / 50 high power fields (HPF), larger tumor size (>5 cm), tumor rupture, ulceration and necrosis.76
Many retrospective and some prospective studies have shown the feasibility of laparoscopic resection of gastric GIST's with reasonable survival.77-80 Earlier studies included small tumors up to 2 cm. Later reports included larger tumors, but these are less easy to handle intraoperatively, and rupture of the tumor is a devastating complication. Comparative studies have demonstrated a shorter hospital stay for the laparoscopic group.81 A higher level of evidence is missing in the literature as these tumors are rare.
Given the simple surgical procedure required for resection of GISTs, we believe that laparoscopic surgery is justified for these tumors although prospective randomized trials comparing laparoscopic to open surgery are missing. Depending on the surgeon's experience, smaller tumors can be resected laparoscopically. For bulky tumors or surgeons with less experience in laparoscopic surgery, an open operation is technically simpler and less likely to result in rupture of the tumor during the procedure.
Appendix
Level of Evidence: IV Grade of Recommendation: none
Tumors of the appendix are rare and often discovered during or following appendectomy for appendicitis. Tumors are found in about 0.4-1% of appendectomy specimens, and synchronous colon cancers are present in 10 – 30% of these cases.82,83 Because of the rarity of this condition, the available literature is scarce. One retrospective study analyzed 43 appendiceal tumors (carcinoid and adenocarcinoma) treated by open or laparoscopic resection, with a higher rate of tumor positive resection margins in the laparoscopic group but comparable long-term survival.84
There is no evidence to support laparoscopic resection of appendiceal tumors by appendectomy. If these tumors are suspected preoperatively, a colonoscopy should be performed to exclude a synchronous colon cancer, and a formal right hemicolectomy should be planned, which may be performed laparoscopically.83
Adrenal Gland
Level of Evidence: IV Grade of Recommendation: none
Laparoscopic adrenalectomy was first described in 1992 by Gagner85 and since has been widely applied to different adrenal lesions. For benign conditions laparoscopic adrenalectomy has become the standard of care. Although large prospective studies are lacking, many retrospective reports and case-controlled studies as well as a small prospective study have shown excellent results for benign disease.86-93 Walz et al. reported an impressive prospective series of 560 adrenalectomies including tumors up to 7 cm. It is technically challenging but possible to remove tumors over 6-7 cm by the laparoscopic route, but tumors larger than 5 cm are more often malignant.92,94
Adrenocortical cancer and malignant pheochromocytomas are rare tumors with a poor prognosis, likely not well suited to laparoscopic approaches. Only a few small retrospective studies including malignant adrenal disease are available, and these studies agree with expert opinion that primary adrenal malignancy should be treated by an open approach, especially when invasion of adjacent organs is present. Laparoscopic procedures that identify adrenal tumors with local infiltration should be converted to an open technique.95-97
At this time there are no prospective randomized series to guide or endorse the use of laparoscopic resection for adrenocortical carcinoma or malignant pheochromocytoma.
Secondary adrenal tumors
Adrenal metastases occur in patients with melanoma and cancers of the lung, kidney, gastrointestinal tract, and breast.98 In a large series of abdominal CT scans, more than half of adrenal masses were secondary and often represented metastatic disease.99 Some authors have reported the laparoscopic resection of metastatic lesions in the adrenal gland and consider it safe.96,97,100,101 Paul et al. compared the outcome of laparoscopic resections in the literature to an unresected series and concluded that resection could result in prolonged survival in cases of favorable tumor biology.98 However, there are no data of quality to support this approach.
Gall Bladder
Level of Evidence: V Grade of Recommendation: none
Laparoscopic cholecystectomy has replaced conventional open approaches for benign gallbladder disease, because it has been shown to be safe and cost effective.102,103 In large series of laparoscopic cholecystectomies, adenocarcinoma of the gallbladder is reported at rates around 0.5%.104 Early reports claimed that laparoscopic cholecystectomy worsens the prognosis for patients with unsuspected gallbladder cancer because of intraabdominal spread and port-site metastasis. Retrospective studies have shown contradictory results,105-108 with little prospective data to resolve the question.109 To minimize the risk of intraabdominal dissemination and port-site metastasis, the surgeon should avoid bile spillage, which occurs in 20 - 44% of the laparoscopic cholecystectomies and has been correlated with poor survival and increased recurrences.110-112 Because of these concerns the surgeon should consider retrieving the specimen in a bag and opening the gallbladder for examination before the abdomen is closed.113
If gallbladder cancer is identified following laparoscopic cholecystectomy, additional treatment depends on the tumor stage.114 For patients with Tis and T1a tumors with negative resection margins, the laparoscopic approach is adequate.113 For these tumors the 5-year-survival after simple cholecystectomy is 95-100%.112,113 Other tumor stages may require a re-exploration with liver resection and lymphadenectomy. Current opinion states that en-bloc resection and portal node dissection are the best options for patients with more advanced gallbladder cancers.
There are no data to support the use of laparoscopic resection for advanced stages of gallbladder cancer. The importance of the excision of port-sites is debated. In the largest series, the occurrence of port-site metastasis is not higher than the wound recurrence after open operations with a rate of 5 – 6 %, but excision is advised by prominent reviews based on early studies. 105,109,115
Although there are no current indications for laparoscopic resection in advanced stages, laparoscopy is an excellent staging method for gallbladder cancer with low morbidity.115 Laparoscopic cholecystectomy should not be utilized if the diagnosis of cancer is known preoperatively for the risk of bile spilling.
Bile duct
Level of Evidence: V Grade of Recommendation: none
The location of an adenocarcinoma of the bile duct dictates the operative approach, including local resection, liver resection or pancreaticoduodenectomy. For the distal bile duct, the feasibility of laparoscopic pancreaticoduodenectomy has been demonstrated.64 In a series of 56 cases of hilar bile duct cancers, diagnostic laparoscopy detected peritoneal or liver metastases for the majority of patients who had metastatic disease, but failed to identify unresectability in locally advanced tumors.116
As for other organs, diagnostic laparoscopy is a valuable tool to avoid non-therapeutic laparotomies in patients with metastatic disease. Evidence is insufficient to recommend laparoscopic resection of bile duct cancers at this time.
Small Intestine
Level of Evidence: V Grade of Recommendation: none
Malignancies involving the small intestine are rare, with an estimated 6230 new cases and 1110 patient deaths in the US in 2009, thus accounting for fewer than 0.5 percent of all cancers.58 While laparoscopic surgery might seem advantageous for intestinal surgery, reports comparing outcome for intestinal malignancies are lacking. Studies are scarce even for benign disease and do not always show an advantage of laparoscopic over open procedures. We believe that laparoscopy is a useful tool for localization, resection of benign tumors, and exclusion of carcinomatosis, malignant ascites, and liver metastasis.
Leiomyoma
A few case reports show the feasibility of the laparoscopic or laparoscopic assisted procedure for bleeding and non-bleeding leiomyomas.117-121 Tumors with a mitotic index < 2 / 50 HPF may be locally excised.122 There is no evidence to sustain laparoscopic treatment for these tumors at this time.
GIST
GISTs are commonly found in the jejunum, followed by the ileum, duodenum, colon or rectum, and surgical resection is the best therapeutic option. Only a few reports of laparoscopic cases appear in the literature.123 Predictors for poor survival include high tumor cellularity, mitotic index > 5 / 50 HPF, and KI-67 index >=10%.124 Aggressive or advanced stages are treated with imatinib.123 Oncologic resection includes the inspection of the bowel and resection of the entire tumor with 2 cm margins, with efforts to avoid tumor rupture.125
As small tumors < 2 cm are unlikely to behave aggressively, we believe these may be resected laparoscopically, but there is no evidence to support use of the laparoscopic approach for larger tumors.
Carcinoid
Carcinoids account for up to 40 % of malignant tumors of the ileum, with synchronous tumors identified in 30% of the patients. Tumors < 1 cm rarely metastasize, but it appears that carcinoids of the small intestine metastasize earlier than similar tumors in the appendix. Treatment should include wide en-bloc resection including adjacent mesentery and lymph nodes. Virtually no data exist that support the laparoscopic resection of intestinal carcinoids.
Adenocarcinoma
No data exist for the use of a laparoscopic approach to resect adenocarcinoma of the small intestine. Wide surgical resection should include the tumor, mesentery and surrounding tissue at risk for contiguous spread. Right hemicolectomy is recommended for tumors of the distal ileum. Laparoscopic exploration followed by conversion to open resection was described in a single case report.126 The evidence to support laparoscopic resection is lacking.
Lymphoma
Risk factors for lymphoma of the small intestine include AIDS, permanent immunosuppression in transplant recipients, autoimmune disease and Crohn's disease. In the literature there are only a few case reports of laparoscopic diagnosis or resection.127-129 There are no data to support laparoscopic resection.
Palliative resection for metastasis
Metastases to the small intestine can arise from melanoma, cancers of the breast, lung and kidney, while direct invasion may occur with cervical, ovarian, gastric and colon cancer. Resection or bypass are mostly palliative except for melanoma where resection can possibly prolong survival.130 Laparoscopic or laparoscopic assisted resection/internal bypass may be considered to ameliorate obstruction and improve quality of life.131In the palliative situation we believe a laparoscopic therapy may be attempted, although there is no evidence to substantiate this.
Discussion
Appropriate trials for colorectal cancer have demonstrated that the laparoscopic approach is safe and at least equivalent to open techniques with regard to survival and recurrence rates.8-11 This allows the surgeon to offer the laparoscopic option to patients without restriction. To date the same cannot be assumed for other organs. In contrast to Pacific Asian countries early gastric cancers only makes up for a small percentage of all gastric cancers. Therefore the evidence to perform a laparoscopic procedure for our patients with gastric cancer is less strong (level II).49 Analogous prospective randomized clinical trials are necessary for all organs, comparing resection margin positivity, nodal harvests where appropriate, recurrence rates, survival, quality of life, and cost.
Innovation and the development of new techniques are critical to the advancement of surgery. But strictly speaking laparoscopic surgery for most gastrointestinal cancers must still be regarded as experimental. The learning curves for laparoscopic cancer surgery are considerable.11,23,48,94 These were mostly determined by indirect measures of surgical experience like conversion rates and operating time.11,94 Studies specifically designed to analyze the learning curve determined a vast panel of outcome measures including operative time, transfusion requirement, conversion rate, readmission and postoperative complication rates.23,48 For distal gastrectomy only operative time improved with the surgeons experience.48 For colon resection there was an additional benefit regarding the conversion rate which was also dependent on body mass index, ASA grade, type of resection and the presence of abscess or fistula.23 For the US colon cancer trials, cadre of surgeons across the country were evaluated for their skills and in some cases trained under the guidance of experts to ensure uniform operative technique and enough case expertise.8 The surgical community insisted on the completion of these randomized trials for colon and rectal cancer before it was determined that laparoscopic resection could be recommended.
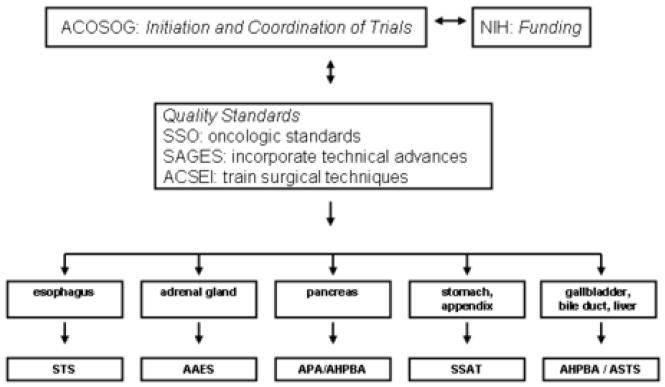
The same caution seems prudent for other cancers as well. The fundamental question is whether there is anything truly different about a laparoscopic approach or is it really just a different surgical instrument but the same operation? Do we really need to answer the question in randomized trials for each organ or can we extrapolate from open surgery? Approaching these operations requires immense laparoscopic skills, therefore the surgeon might short cut on the procedure making the operation different from an open procedure. The techniques for laparoscopic gastric and pancreatic resections currently are not uniform. While it is correct that a laparoscopic distal pancreatectomy can yield a nodal harvest similar to an open procedure, the laparoscopic approach currently used by many surgeons across the country often results in a skeletonization of the gland with few nodes retrieved. A randomized trial would address this deficiency. Randomized trials also stimulate technical innovation and provide a large bank of tumors for basic science research. As randomized trials for any of these conditions require financial resources, patient accrual, and surgical skill, a national organization will be needed to organize and complete this task. A way forward may be for the American College of Surgeons Surgical Oncology Group (ACOSOG) to engage specific surgical specialty organizations with the backing of the National Institutes of Health (NIH) for funding. As an example, both the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the Society for Surgery of the Alimentary Tract (SSAT) have members with expertise in both the laparoscopic and open treatment of intestinal tumors. Utilizing the resources and expertise of ACOSOG, the membership of these and other surgical organizations could work with ACOSOG to initiate and complete the trial. This process would allow adequate involvement of surgeons, patient accrual, assure sufficient medical and technical expertise, and reliably determine the utility of laparoscopic surgery for malignant disease that involves these organs (Figure 1).
Figure 1.
Schematic for proposed studies to establish laparoscopic surgery in abdominal cancer.
For the present, the surgeon is left to counsel the cancer patient regarding plans for operative treatment. Other than colon and rectal cancer, there are no data to support anything short of an open procedure for a known cancer, and the patient should be informed of this. As long as the patient understands the potential implications of this data void, the patient and surgeon may well come to the conclusion that a laparoscopic approach seems feasible. In selected cases a laparoscopic approach is reasonable, provided the surgeon has significant laparoscopic skill and experience. A better solution, though, would be a commitment to randomized trials so that we can be armed with reliable data that help to guide our surgical community and assure the best of patient care.
Acknowledgments
This work was supported by R21CA124609 (OJH), P01AT003960-01A1 (OJH), the Swiss National Science Foundation PBZHB-117008 (EA) and the Hirshberg Foundation.
Abbreviations
- AAES
American Association of Endocrine Surgeons
- ACOSOG
American College of Surgeons Surgical Oncology Group
- ACSEI
American College of Surgeons Education Institutes
- AHPBA
American Hepato-Pancreato-Biliary Association
- APA
American Pancreatic Association
- ASTS
American Society of Transplant Surgery
- CLASICC
Conventional versus Laparoscopic-assisted Surgery in Colorectal Cancer
- COLOR
Colon Cancer Laparoscopic or Open Resection
- COSTSG
Clinical Outcomes of Surgical Therapy Study Group
- GIST
Gastrointestinal stromal tumor
- HPF
High power field
- NIH
National Institute of Health
- SAGES
Society of American Gastrointestinal and Endoscopic Surgeons
- SSAT
Society for Surgery of the Alimentary Tract
- SSO
Society of Surgical Oncology
- STS
Society of Thoracic Surgeons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure information: Nothing to disclose.
Literature
- 1.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(2 Suppl):2S–4S. [PubMed] [Google Scholar]
- 2.Phillips EH, Franklin M, Carroll BJ, et al. Laparoscopic colectomy. Ann Surg. 1992;216(6):703–7. doi: 10.1097/00000658-199212000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berends FJ, Kazemier G, Bonjer HJ, et al. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344(8914):58. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 4.Are C, Talamini MA. Laparoscopy and malignancy. J Laparoendosc Adv Surg Tech A. 2005;15(1):38–47. doi: 10.1089/lap.2005.15.38. [DOI] [PubMed] [Google Scholar]
- 5.Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363(9416):1187–92. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 6.Lacy AM, Delgado S, Garcia-Valdecasas JC, et al. Port site metastases and recurrence after laparoscopic colectomy. A randomized trial. Surg Endosc. 1998;12(8):1039–42. doi: 10.1007/s004649900776. [DOI] [PubMed] [Google Scholar]
- 7.Kaiser AM, Kang JC, Chan LS, et al. Laparoscopic-assisted vs. open colectomy for colon cancer: a prospective randomized trial. J Laparoendosc Adv Surg Tech A. 2004;14(6):329–34. doi: 10.1089/lap.2004.14.329. [DOI] [PubMed] [Google Scholar]
- 8.A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350(20):2050–9. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 9.Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6(7):477–84. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 10.Lacy AM, Garcia-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359(9325):2224–9. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 11.Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365(9472):1718–26. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 12.Hewett PJ, Allardyce RA, Bagshaw PF, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248(5):728–38. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 13.Tominaga T, Sakabe T, Koyama Y, et al. Prognostic factors for patients with colon or rectal carcinoma treated with resection only. Five-year follow-up report. Cancer. 1996;78(3):403–8. doi: 10.1002/(SICI)1097-0142(19960801)78:3<403::AID-CNCR4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Lacy AM, Delgado S, Castells A, et al. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248(1):1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 15.Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25(21):3061–8. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 16.Buunen M, Veldkamp R, Hop WC, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10(1):44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 17.Thorpe H, Jayne DG, Guillou PJ, et al. Patient factors influencing conversion from laparoscopically assisted to open surgery for colorectal cancer. Br J Surg. 2008;95(2):199–205. doi: 10.1002/bjs.5907. [DOI] [PubMed] [Google Scholar]
- 18.Liang Y, Li G, Chen P, et al. Laparoscopic versus open colorectal resection for cancer: A meta-analysis of results of randomized controlled trials on recurrence. Eur J Surg Oncol. 2007 doi: 10.1016/j.ejso.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Schlachta CM, Mamazza J, Poulin EC. Are transverse colon cancers suitable for laparoscopic resection? Surg Endosc. 2007;21(3):396–9. doi: 10.1007/s00464-006-9042-6. [DOI] [PubMed] [Google Scholar]
- 20.Noblett SE, Horgan AF. A prospective case-matched comparison of clinical and financial outcomes of open versus laparoscopic colorectal resection. Surg Endosc. 2007;21(3):404–8. doi: 10.1007/s00464-006-9016-8. [DOI] [PubMed] [Google Scholar]
- 21.Lin KM, Ota DM. Laparoscopic colectomy for cancer: an oncologic feasible option. Surg Oncol. 2000;9(3):127–34. doi: 10.1016/s0960-7404(01)00002-0. [DOI] [PubMed] [Google Scholar]
- 22.Janson M, Bjorholt I, Carlsson P, et al. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. Br J Surg. 2004;91(4):409–17. doi: 10.1002/bjs.4469. [DOI] [PubMed] [Google Scholar]
- 23.Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242(1):83–91. doi: 10.1097/01.sla.0000167857.14690.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–6. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 25.Veenhof AA, Engel AF, Craanen ME, et al. Laparoscopic versus open total mesorectal excision: a comparative study on short-term outcomes. A single-institution experience regarding anterior resections and abdominoperineal resections. Dig Surg. 2007;24(5):367–74. doi: 10.1159/000107778. [DOI] [PubMed] [Google Scholar]
- 26.Laurent C, Leblanc F, Gineste C, et al. Laparoscopic approach in surgical treatment of rectal cancer. Br J Surg. 2007;94(12):1555–61. doi: 10.1002/bjs.5884. [DOI] [PubMed] [Google Scholar]
- 27.Laurent C, Leblanc F, Wutrich P, et al. Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg. 2009;250(1):54–61. doi: 10.1097/SLA.0b013e3181ad6511. [DOI] [PubMed] [Google Scholar]
- 28.Lezoche E, Guerrieri M, De Sanctis A, et al. Long-term results of laparoscopic versus open colorectal resections for cancer in 235 patients with a minimum follow-up of 5 years. Surg Endosc. 2006;20(4):546–53. doi: 10.1007/s00464-005-0338-8. [DOI] [PubMed] [Google Scholar]
- 29.Aziz O, Constantinides V, Tekkis PP, et al. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13(3):413–24. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 30.Leung KL, Lai PB, Ho RL, et al. Systemic cytokine response after laparoscopic-assisted resection of rectosigmoid carcinoma: A prospective randomized trial. Ann Surg. 2000;231(4):506–11. doi: 10.1097/00000658-200004000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;(4):CD005200. doi: 10.1002/14651858.CD005200.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Lujan J, Valero G, Hernandez Q, et al. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96(9):982–9. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 33.Pechlivanides G, Gouvas N, Tsiaoussis J, et al. Lymph node clearance after total mesorectal excision for rectal cancer: laparoscopic versus open approach. Dig Dis. 2007;25(1):94–9. doi: 10.1159/000099176. [DOI] [PubMed] [Google Scholar]
- 34.Braga M, Frasson M, Vignali A, et al. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50(4):464–71. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18(8):1211–5. doi: 10.1007/s00464-003-9170-1. [DOI] [PubMed] [Google Scholar]
- 36.Ng SS, Leung KL, Lee JF, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15(9):2418–25. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 37.Kim YW, Baik YH, Yun YH, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248(5):721–7. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 38.Pugliese R, Maggioni D, Sansonna F, et al. Total and subtotal laparoscopic gastrectomy for adenocarcinoma. Surg Endosc. 2007;21(1):21–7. doi: 10.1007/s00464-005-0409-x. [DOI] [PubMed] [Google Scholar]
- 39.Kim MC, Kim KH, Kim HH, et al. Comparison of laparoscopy-assisted by conventional open distal gastrectomy and extraperigastric lymph node dissection in early gastric cancer. J Surg Oncol. 2005;91(1):90–4. doi: 10.1002/jso.20271. [DOI] [PubMed] [Google Scholar]
- 40.Tanimura S, Higashino M, Fukunaga Y, et al. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc. 2008 doi: 10.1007/s00464-008-9786-2. [DOI] [PubMed] [Google Scholar]
- 41.Mochiki E, Nakabayashi T, Kamimura H, et al. Gastrointestinal recovery and outcome after laparoscopy-assisted versus conventional open distal gastrectomy for early gastric cancer. World J Surg. 2002;26(9):1145–9. doi: 10.1007/s00268-002-6286-8. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara M, Kodera Y, Kasai Y, et al. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection for early gastric carcinoma: a review of 43 cases. J Am Coll Surg. 2003;196(1):75–81. doi: 10.1016/s1072-7515(02)01539-9. [DOI] [PubMed] [Google Scholar]
- 43.Yasuda K, Shiraishi N, Etoh T, et al. Long-term quality of life after laparoscopy-assisted distal gastrectomy for gastric cancer. Surg Endosc. 2007;21(12):2150–3. doi: 10.1007/s00464-007-9322-9. [DOI] [PubMed] [Google Scholar]
- 44.Hosono S, Arimoto Y, Ohtani H, et al. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12(47):7676–83. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujiwara M, Kodera Y, Misawa K, et al. Longterm outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg. 2008;206(1):138–43. doi: 10.1016/j.jamcollsurg.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19(9):1172–6. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 47.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241(2):232–7. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11(47):7508–11. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Everett SM, Axon AT. Early gastric cancer in Europe. Gut. 1997;41(2):142–50. doi: 10.1136/gut.41.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber KJ, Reyes CD, Gagner M, et al. Comparison of laparoscopic and open gastrectomy for malignant disease. Surg Endosc. 2003;17(6):968–71. doi: 10.1007/s00464-002-8738-5. [DOI] [PubMed] [Google Scholar]
- 51.Goh PM, So JB. Role of laparoscopy in the management of stomach cancer. Semin Surg Oncol. 1999;16(4):321–6. doi: 10.1002/(sici)1098-2388(199906)16:4<321::aid-ssu7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 52.Deogracias ML, Rodriguez-Sanjuan JC, de la Torre F, et al. Absence of port-site metastases following staging laparoscopy for gastric carcinoma. Rev Esp Enferm Dig. 2006;98(10):755–9. doi: 10.4321/s1130-01082006001000005. [DOI] [PubMed] [Google Scholar]
- 53.Hulscher JB, Tijssen JG, Obertop H, et al. Transthoracic versus transhiatal resection for carcinoma of the esophagus: a meta-analysis. Ann Thorac Surg. 2001;72(1):306–13. doi: 10.1016/s0003-4975(00)02570-4. [DOI] [PubMed] [Google Scholar]
- 54.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003;238(4):486–94. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bresadola V, Terrosu G, Cojutti A, et al. Laparoscopic versus open gastroplasty in esophagectomy for esophageal cancer: a comparative study. Surg Laparosc Endosc Percutan Tech. 2006;16(2):63–7. doi: 10.1097/00129689-200604000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Benzoni E, Terrosu G, Bresadola V, et al. A comparative study of the transhiatal laparoscopic approach versus laparoscopic gastric mobilisation and right open transthoracic esophagectomy for esophageal cancer management. J Gastrointestin Liver Dis. 2007;16(4):395–401. [PubMed] [Google Scholar]
- 57.O'Brien MG, Fitzgerald EF, Lee G, et al. A prospective comparison of laparoscopy and imaging in the staging of esophagogastric cancer before surgery. Am J Gastroenterol. 1995;90(12):2191–4. [PubMed] [Google Scholar]
- 58.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 59.Rotellar F, Pardo F, Montiel C, et al. Totally laparoscopic Roux-en-Y duct-to-mucosa pancreaticojejunostomy after middle pancreatectomy: a consecutive nine-case series at a single institution. Ann Surg. 2008;247(6):938–44. doi: 10.1097/SLA.0b013e3181724e4a. [DOI] [PubMed] [Google Scholar]
- 60.Kooby DA, Gillespie T, Bentrem D, et al. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248(3):438–46. doi: 10.1097/SLA.0b013e318185a990. [DOI] [PubMed] [Google Scholar]
- 61.Melotti G, Butturini G, Piccoli M, et al. Laparoscopic distal pancreatectomy: results on a consecutive series of 58 patients. Ann Surg. 2007;246(1):77–82. doi: 10.1097/01.sla.0000258607.17194.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sa Cunha A, Rault A, Beau C, et al. A single-institution prospective study of laparoscopic pancreatic resection. Arch Surg. 2008;143(3):289–95. doi: 10.1001/archsurg.143.3.289. discussion 295. [DOI] [PubMed] [Google Scholar]
- 63.Velanovich V. Case-control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg. 2006;10(1):95–8. doi: 10.1016/j.gassur.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 64.Palanivelu C, Jani K, Senthilnathan P, et al. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205(2):222–30. doi: 10.1016/j.jamcollsurg.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 65.Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20(7):1045–50. doi: 10.1007/s00464-005-0474-1. [DOI] [PubMed] [Google Scholar]
- 66.Doran HE, Bosonnet L, Connor S, et al. Laparoscopy and laparoscopic ultrasound in the evaluation of pancreatic and periampullary tumours. Dig Surg. 2004;21(4):305–13. doi: 10.1159/000080885. [DOI] [PubMed] [Google Scholar]
- 67.White R, Winston C, Gonen M, et al. Current utility of staging laparoscopy for pancreatic and peripancreatic neoplasms. J Am Coll Surg. 2008;206(3):445–50. doi: 10.1016/j.jamcollsurg.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 68.Espat NJ, Brennan MF, Conlon KC. Patients with laparoscopically staged unresectable pancreatic adenocarcinoma do not require subsequent surgical biliary or gastric bypass. J Am Coll Surg. 1999;188(6):649–55. doi: 10.1016/s1072-7515(99)00050-2. discussion 655-7. [DOI] [PubMed] [Google Scholar]
- 69.Bergamaschi R, Marvik R, Thoresen JE, et al. Open versus laparoscopic gastrojejunostomy for palliation in advanced pancreatic cancer. Surg Laparosc Endosc. 1998;8(2):92–6. [PubMed] [Google Scholar]
- 70.Troisi R, Montalti R, Smeets P, et al. The value of laparoscopic liver surgery for solid benign hepatic tumors. Surg Endosc. 2008;22(1):38–44. doi: 10.1007/s00464-007-9527-y. [DOI] [PubMed] [Google Scholar]
- 71.Lang BH, Poon RT, Fan ST, et al. Influence of laparoscopy on postoperative recurrence and survival in patients with ruptured hepatocellular carcinoma undergoing hepatic resection. Br J Surg. 2004;91(4):444–9. doi: 10.1002/bjs.4450. [DOI] [PubMed] [Google Scholar]
- 72.Cai XJ, Yang J, Yu H, et al. Clinical study of laparoscopic versus open hepatectomy for malignant liver tumors. Surg Endosc. 2008 doi: 10.1007/s00464-008-9789-z. [DOI] [PubMed] [Google Scholar]
- 73.Lee KF, Cheung YS, Chong CN, et al. Laparoscopic versus open hepatectomy for liver tumours: a case control study. Hong Kong Med J. 2007;13(6):442–8. [PubMed] [Google Scholar]
- 74.Topal B, Fieuws S, Aerts R, et al. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22(10):2208–13. doi: 10.1007/s00464-008-0023-9. [DOI] [PubMed] [Google Scholar]
- 75.Matthews BD, Walsh RM, Kercher KW, et al. Laparoscopic vs open resection of gastric stromal tumors. Surg Endosc. 2002;16(5):803–7. doi: 10.1007/s00464-001-8319-z. [DOI] [PubMed] [Google Scholar]
- 76.Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243(6):738–45. doi: 10.1097/01.sla.0000219739.11758.27. discussion 745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Otani Y, Furukawa T, Yoshida M, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery. 2006;139(4):484–92. doi: 10.1016/j.surg.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 78.Catena F, Di Battista M, Fusaroli P, et al. Laparoscopic Treatment of Gastric GIST: Report of 21 Cases and Literature's Review. J Gastrointest Surg. 2008;12(3):561–8. doi: 10.1007/s11605-007-0416-4. [DOI] [PubMed] [Google Scholar]
- 79.Sexton JA, Pierce RA, Halpin VJ, et al. Laparoscopic gastric resection for gastrointestinal stromal tumors. Surg Endosc. 2008 doi: 10.1007/s00464-008-9807-1. [DOI] [PubMed] [Google Scholar]
- 80.Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc. 2007;21(6):875–8. doi: 10.1007/s00464-006-9065-z. [DOI] [PubMed] [Google Scholar]
- 81.Hindmarsh A, Koo B, Lewis MP, et al. Laparoscopic resection of gastric gastrointestinal stromal tumors. Surg Endosc. 2005;19(8):1109–12. doi: 10.1007/s00464-004-8168-7. [DOI] [PubMed] [Google Scholar]
- 82.Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. 1998;41(1):75–80. doi: 10.1007/BF02236899. [DOI] [PubMed] [Google Scholar]
- 83.Tchana-Sato V, Detry O, Polus M, et al. Carcinoid tumor of the appendix: a consecutive series from 1237 appendectomies. World J Gastroenterol. 2006;12(41):6699–701. doi: 10.3748/wjg.v12.i41.6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bucher P, Mathe Z, Demirag A, et al. Appendix tumors in the era of laparoscopic appendectomy. Surg Endosc. 2004;18(7):1063–6. doi: 10.1007/s00464-003-9255-x. [DOI] [PubMed] [Google Scholar]
- 85.Gagner M, Lacroix A, Bolte E. Laparoscopic adrenalectomy in Cushing's syndrome and pheochromocytoma. N Engl J Med. 1992;327(14):1033. doi: 10.1056/NEJM199210013271417. [DOI] [PubMed] [Google Scholar]
- 86.Tiberio GA, Baiocchi GL, Arru L, et al. Prospective randomized comparison of laparoscopic versus open adrenalectomy for sporadic pheochromocytoma. Surg Endosc. 2008;22(6):1435–9. doi: 10.1007/s00464-008-9904-1. [DOI] [PubMed] [Google Scholar]
- 87.Mutoh M, Takeyama K, Nishiyama N, et al. Systemic inflammatory response syndrome in open versus laparoscopic adrenalectomy. Urology. 2004;64(3):422–5. doi: 10.1016/j.urology.2004.04.042. [DOI] [PubMed] [Google Scholar]
- 88.Schell SR, Talamini MA, Udelsman R. Laparoscopic adrenalectomy for nonmalignant disease: improved safety, morbidity, and cost-effectiveness. Surg Endosc. 1999;13(1):30–4. doi: 10.1007/s004649900892. [DOI] [PubMed] [Google Scholar]
- 89.Bentrem DJ, Pappas SG, Ahuja Y, et al. Contemporary surgical management of pheochromocytoma. Am J Surg. 2002;184(6):621–4. doi: 10.1016/s0002-9610(02)01097-8. discussion 624-5. [DOI] [PubMed] [Google Scholar]
- 90.Jacobsen NE, Campbell JB, Hobart MG. Laparoscopic versus open adrenalectomy for surgical adrenal disease. Can J Urol. 2003;10(5):1995–9. [PubMed] [Google Scholar]
- 91.Imai T, Kikumori T, Ohiwa M, et al. A case-controlled study of laparoscopic compared with open lateral adrenalectomy. Am J Surg. 1999;178(1):50–3. doi: 10.1016/s0002-9610(99)00126-9. discussion 54. [DOI] [PubMed] [Google Scholar]
- 92.Toniato A, Boschin IM, Opocher G, et al. Is the laparoscopic adrenalectomy for pheochromocytoma the best treatment? Surgery. 2007;141(6):723–7. doi: 10.1016/j.surg.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 93.Thompson GB, Grant CS, van Heerden JA, et al. Laparoscopic versus open posterior adrenalectomy: a case-control study of 100 patients. Surgery. 1997;122(6):1132–6. doi: 10.1016/s0039-6060(97)90218-x. [DOI] [PubMed] [Google Scholar]
- 94.Walz MK, Alesina PF, Wenger FA, et al. Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery. 2006;140(6):943–8. doi: 10.1016/j.surg.2006.07.039. discussion 948-50. [DOI] [PubMed] [Google Scholar]
- 95.Henry JF, Defechereux T, Raffaelli M, et al. Complications of laparoscopic adrenalectomy: results of 169 consecutive procedures. World J Surg. 2000;24(11):1342–6. doi: 10.1007/s002680010222. [DOI] [PubMed] [Google Scholar]
- 96.Kebebew E, Siperstein AE, Clark OH, et al. Results of laparoscopic adrenalectomy for suspected and unsuspected malignant adrenal neoplasms. Arch Surg. 2002;137(8):948–51. doi: 10.1001/archsurg.137.8.948. discussion 952-3. [DOI] [PubMed] [Google Scholar]
- 97.Gill IS. The case for laparoscopic adrenalectomy. J Urol. 2001;166(2):429–36. [PubMed] [Google Scholar]
- 98.Paul CA, Virgo KS, Wade TP, et al. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol. 2000;17(1):181–7. [PubMed] [Google Scholar]
- 99.Herrera MF, Grant CS, van Heerden JA, et al. Incidentally discovered adrenal tumors: an institutional perspective. Surgery. 1991;110(6):1014–21. [PubMed] [Google Scholar]
- 100.Bendinelli C, Lucchi M, Buccianti P, et al. Adrenal masses in non-small cell lung carcinoma patients: is there any role for laparoscopic procedures? J Laparoendosc Adv Surg Tech A. 1998;8(3):119–24. doi: 10.1089/lap.1998.8.119. [DOI] [PubMed] [Google Scholar]
- 101.Elashry OM, Clayman RV, Soble JJ, et al. Laparoscopic adrenalectomy for solitary metachronous contralateral adrenal metastasis from renal cell carcinoma. J Urol. 1997;157(4):1217–22. [PubMed] [Google Scholar]
- 102.A prospective analysis of 1518 laparoscopic cholecystectomies. The Southern Surgeons Club. N Engl J Med. 1991;324(16):1073–8. doi: 10.1056/NEJM199104183241601. [DOI] [PubMed] [Google Scholar]
- 103.Legorreta AP, Silber JH, Costantino GN, et al. Increased cholecystectomy rate after the introduction of laparoscopic cholecystectomy. Jama. 1993;270(12):1429–32. [PubMed] [Google Scholar]
- 104.Akatsu T, Ueda M, Shimazu M, et al. Long-term survival of patients with gallbladder cancer detected during or after laparoscopic cholecystectomy. World J Surg. 2005;29(9):1106–9. doi: 10.1007/s00268-005-7886-x. discussion 1110. [DOI] [PubMed] [Google Scholar]
- 105.Fong Y, Heffernan N, Blumgart LH. Gallbladder carcinoma discovered during laparoscopic cholecystectomy: aggressive reresection is beneficial. Cancer. 1998;83(3):423–7. doi: 10.1002/(sici)1097-0142(19980801)83:3<423::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 106.Kang CM, Choi GH, Park SH, et al. Laparoscopic cholecystectomy only could be an appropriate treatment for selected clinical R0 gallbladder carcinoma. Surg Endosc. 2007;21(9):1582–7. doi: 10.1007/s00464-006-9133-4. [DOI] [PubMed] [Google Scholar]
- 107.Shih SP, Schulick RD, Cameron JL, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245(6):893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Onoyama H, Yamamoto M, Tseng A, et al. Extended cholecystectomy for carcinoma of the gallbladder. World J Surg. 1995;19(5):758–63. doi: 10.1007/BF00295925. [DOI] [PubMed] [Google Scholar]
- 109.Paolucci V, Neckell M, Gotze T. Unsuspected gallbladder carcinoma--the CAE-S/CAMIC registry. Zentralbl Chir. 2003;128(4):309–12. doi: 10.1055/s-2003-38795. [DOI] [PubMed] [Google Scholar]
- 110.Weiland ST, Mahvi DM, Niederhuber JE, et al. Should suspected early gallbladder cancer be treated laparoscopically? J Gastrointest Surg. 2002;6(1):50–6. doi: 10.1016/s1091-255x(01)00014-2. discussion 56-7. [DOI] [PubMed] [Google Scholar]
- 111.Sarli L, Contini S, Sansebastiano G, et al. Does laparoscopic cholecystectomy worsen the prognosis of unsuspected gallbladder cancer? Arch Surg. 2000;135(11):1340–4. doi: 10.1001/archsurg.135.11.1340. [DOI] [PubMed] [Google Scholar]
- 112.Ouchi K, Mikuni J, Kakugawa Y. Laparoscopic cholecystectomy for gallbladder carcinoma: results of a Japanese survey of 498 patients. J Hepatobiliary Pancreat Surg. 2002;9(2):256–60. doi: 10.1007/s005340200028. [DOI] [PubMed] [Google Scholar]
- 113.Kim EK, Lee SK, Kim WW. Does laparoscopic surgery have a role in the treatment of gallbladder cancer? J Hepatobiliary Pancreat Surg. 2002;9(5):559–63. doi: 10.1007/s005340200074. [DOI] [PubMed] [Google Scholar]
- 114.Greene FL. AJCC Cancer Staging Manual. sixth ed. Springer; New York: 2003. [Google Scholar]
- 115.Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol. 2003;4(3):167–76. doi: 10.1016/s1470-2045(03)01021-0. [DOI] [PubMed] [Google Scholar]
- 116.Weber SM, DeMatteo RP, Fong Y, et al. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235(3):392–9. doi: 10.1097/00000658-200203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ehrmantraut W, Sardi A. Laparoscopy-assisted small bowel resection. Am Surg. 1997;63(11):996–1001. [PubMed] [Google Scholar]
- 118.Ueda Y, Tominaga M, Nishijima N, et al. Laparoscopy for adult intussusception caused by leiomyoma of the jejunum. J Clin Gastroenterol. 1998;27(3):255–6. doi: 10.1097/00004836-199810000-00015. [DOI] [PubMed] [Google Scholar]
- 119.Chung RS. Laparoscopy-assisted jejunal resection for bleeding leiomyoma. Surg Endosc. 1998;12(2):162–3. doi: 10.1007/s004649900621. [DOI] [PubMed] [Google Scholar]
- 120.Beajow M, Singh HK, Wiese DA, et al. Bleeding jejunal leiomyoma: a new approach. Am J Gastroenterol. 1995;90(1):131–3. [PubMed] [Google Scholar]
- 121.Tricarico A, Cione G, Sozio M, et al. Digestive hemorrhages of obscure origin. Surg Endosc. 2002;16(4):711–3. doi: 10.1007/s004640090074. [DOI] [PubMed] [Google Scholar]
- 122.Morgan BK, Compton C, Talbert M, et al. Benign smooth muscle tumors of the gastrointestinal tract. A 24-year experience. Ann Surg. 1990;211(1):63–6. doi: 10.1097/00000658-199001000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eccher C, Fama R, Berlanda G, et al. Gastrointestinal stromal tumors. Suppl Tumori. 2005;4(3):S102–3. [PubMed] [Google Scholar]
- 124.Wu TJ, Lee LY, Yeh CN, et al. Surgical treatment and prognostic analysis for gastrointestinal stromal tumors (GISTs) of the small intestine: before the era of imatinib mesylate. BMC Gastroenterol. 2006;6:29. doi: 10.1186/1471-230X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heinrich MC, Corless CL. Gastric GI stromal tumors (GISTs): the role of surgery in the era of targeted therapy. J Surg Oncol. 2005;90(3):195–207. doi: 10.1002/jso.20230. discussion 207. [DOI] [PubMed] [Google Scholar]
- 126.Soeda J, Sekka T, Hasegawa S, et al. A case of primary small intestinal cancer diagnosed by laparoscopy. Tokai J Exp Clin Med. 2004;29(4):159–62. [PubMed] [Google Scholar]
- 127.Chiu CC, Wei PL, Huang MT, et al. Laparoscopic treatment of ileocecal intussusception caused by primary ileal lymphoma. Surg Laparosc Endosc Percutan Tech. 2004;14(2):93–5. doi: 10.1097/00129689-200404000-00010. [DOI] [PubMed] [Google Scholar]
- 128.Joyce AM, Burns DL, Marcello PW, et al. Capsule endoscopy findings in celiac disease associated enteropathy-type intestinal T-cell lymphoma. Endoscopy. 2005;37(6):594–6. doi: 10.1055/s-2005-861322. [DOI] [PubMed] [Google Scholar]
- 129.Kim J, Kim YS, Chun HJ, et al. Laparoscopy-assisted exploration of obscure gastrointestinal bleeding after capsule endoscopy: the Korean experience. J Laparoendosc Adv Surg Tech A. 2005;15(4):365–73. doi: 10.1089/lap.2005.15.365. [DOI] [PubMed] [Google Scholar]
- 130.Elsayed AM, Albahra M, Nzeako UC, et al. Malignant melanomas in the small intestine: a study of 103 patients. Am J Gastroenterol. 1996;91(5):1001–6. [PubMed] [Google Scholar]
- 131.Felsher J, Brodsky J, Brody F. Laparoscopic small bowel resection of metastatic pulmonary carcinosarcoma. J Laparoendosc Adv Surg Tech A. 2003;13(6):397–400. doi: 10.1089/109264203322656478. [DOI] [PubMed] [Google Scholar]