Abstract
In the presence of monoamines, L-type Ca2+ channels on the dendrites of motoneurons contribute to persistent inward currents (PICs) that can amplify synaptic inputs two- to sixfold. However, the exact location of the L-type Ca2+ channels is controversial, and the importance of the location as a means of regulating the input-output properties of motoneurons is unknown. In this study, we used a computational strategy developed previously to estimate the dendritic location of the L-type Ca2+ channels and test the hypothesis that the location of L-type Ca2+ channels varies as a function of motoneuron size. Compartmental models were constructed based on dendritic trees of five motoneurons that ranged in size from small to large. These models were constrained by known differences in PIC activation reported for low- and high-conductance motoneurons and the relationship between somatic PIC threshold and the presence or absence of tonic excitatory or inhibitory synaptic activity. Our simulations suggest that L-type Ca2+ channels are concentrated in hotspots whose distance from the soma increases with the size of the dendritic tree. Moving the hotspots away from these sites (e.g., using the hotspot locations from large motoneurons on intermediate-sized motoneurons) fails to replicate the shifts in PIC threshold that occur experimentally during tonic excitatory or inhibitory synaptic activity. In models equipped with a size-dependent distribution of L-type Ca2+ channels, the amplification of synaptic current by PICs depends on motoneuron size and the location of the synaptic input on the dendritic tree.
INTRODUCTION
There are several classes of voltage-dependent channels positioned on the dendrites of neurons that can amplify synaptic inputs and boost the output of the cell. In several classes of pyramidal cells, some of these voltage-dependent channels are distributed as a function of distance from the soma (Williams and Stuart 2003). As a consequence, the degree of amplification of synaptic inputs on these cells depends strongly on the region of the dendritic tree they innervate and the location of the voltage-dependent channels responsible for amplification (Oviedo and Reyes 2005). It is not known if this relationship applies to other types of neurons, in particular, spinal motoneurons.
One of the main classes of voltage-dependent channels that amplify synaptic input in motoneurons is the L-type Ca2+ channel (Cav1.3) (Heckman et al. 2005). In the presence of monoamines, L-type Ca2+ channels mediate persistent inward currents (PICs) that can amplify synaptic input two- to sixfold (Lee and Heckman 2000). Despite several anatomical studies showing that these channels are located on the dendritic tree, there is little consensus as to their precise location (soma and proximal dendrites, Simon et al. 2003; Westenbroek et al. 1998; Zhang et al. 2006; second- and third-order dendrites, Carlin et al. 2000; Davenport et al. 2003; patches throughout the entire dendritic tree, Ballou et al. 2006; Westenbroek et al. 2005). Alternative techniques such as direct recordings from the dendritic tree are largely precluded by the multipolar distribution and massive size of the dendritic, as well as the small diameter of dendrites that terminate >1,500 μm from the soma (Bras et al. 1987; Bui et al. 2003; Cameron et al. 1985; Cullheim et al. 1987; Fukunishi et al. 1999; Kernell and Zwaagstra 1989; Ulfhake and Kellerth 1981). As a consequence, the best electrophysiological evidence for L-type Ca2+ channels on the dendrites of motoneurons is based on recordings at the soma (Heckman et al. 2005).
Voltage commands elicited through somatic electrodes spread somatofugally and trigger the activation of PICs in dendritic regions that cannot be adequately voltage clamped (Müller and Lux 1993). When activated, the conductances responsible for PICs manifest themselves as abrupt increases in current reaching the soma, which impart a negative slope region in the I-V function (Carlin et al. 2000; Lee and Heckman 1998; Schwindt and Crill 1980). In feline lumbar motoneurons, the threshold for PIC activation, as seen from the soma during a resting state, ranges between approximately −55 and −45 mV (Lee and Heckman 1998). In motoneurons classified as low conductance, which typically innervate slow-twitch muscle fibers and have small dendritic surface areas (Cullheim et al. 1987), PICs are activated between −55 and −50 mV. Conversely, in motoneurons classified as high conductance, which typically innervate fast-twitch muscle fibers and have large dendritic surface areas (Cullheim et al. 1987), PICs are activated between −50 and −45 mV. Thus as the size of the motoneuron increases, a greater somatic depolarization is needed to activate PICs. This variation in PIC threshold may be caused by differences in the density and/or location of the channels underlying PICs or differences in the rate of voltage decay along the dendrites.
Recent studies have adopted a computational strategy to estimate the location of L-type Ca2+ channels on the dendrites of motoneurons (Bui et al. 2006; ElBasiouny et al. 2005). This approach was based on the experimental findings reported by Bennett et al. (1998), showing that the threshold for PIC activation, as recorded from the soma, varies in the presence or absence of excitatory synaptic activity. Using compartmental models, ElBasiouny et al. (2005) and Bui et al. (2006) mimicked these two synaptic states and compared the membrane potential throughout the dendritic tree at a somatic membrane potential just subthreshold to activation of PICs. Assuming that the threshold for PIC activation in the dendrites is the same in both synaptic states, Bui et al. (2006) predicted that the locations on the dendritic tree at which the membrane potential was the same in both states provided an estimate of the location of L-type Ca2+ channels. In keeping with this prediction, compartmental models containing localized “hotspots” of L-type Ca2+ channels, centered on these locations, replicated the experimental data reported by Bennett et al. (1998) (Bui et al. 2006; ElBasiouny et al. 2005). However, the exact location of these hotspots is controversial. ElBasiouny et al. (2005) estimated that the hotspots were located on dendrites 300–900 μm from the soma. In contrast, Bui et al. (2006) were only able to replicate the observations of Bennett et al. (1998) if the hotspots were located more proximally, 100 and 400 μm from the soma.
The total surface area of the dendritic trees of the three neck extensor motoneurons used by Bui et al. (2006) ranged between 390,000 and 450,000 μm2. The compartmental model constructed by ElBasiouny et al. (2005) was based on a single hindlimb motoneuron with a total dendritic surface area of 617,000 μm2. Based on the relationship between PIC threshold and motoneuron conductance described by Lee and Heckman (1998) and by inference, dendritic tree size (cf. Rall 1977), the smaller motoneurons should have a lower voltage threshold for PIC activation as seen from a somatic electrode. When considered in the context of the differences in the location of the hotspots as described by ElBasiouny et al. (2005) and Bui et al. (2006), the different threshold for PIC activation in high- and low-conductance motoneurons may be a consequence of the greater proximity of the hotspots to the somata of small motoneurons. In this study, we took advantage of a database consisting of five splenius motoneurons that ranged from small to large (surface areas ranging between 236,000 and 591,000 μm2) to test the hypothesis that the location of the hotspots is related to the size of the dendritic trees of motoneurons. Using the computational strategy developed by Bui et al. (2006), we show that, as the size of the motoneuron increases, the estimated locations of the L-type Ca2+ channels underlying PICs are located progressively more distal from the soma. Further simulations, based on this distribution, suggest that the magnitude of amplification of synaptic inputs by PICs depends on motoneuron size and the location of synaptic inputs.
METHODS
Construction of the compartmental models
The motoneurons used to construct the compartmental models are based on the results of three experiments (DVS 14, DVS 22, and DVS 25) described in detail by Grande et al. (2005). All procedures were approved by the Queen’s University Animal Care Committee. Briefly, all experiments were performed in adult cats weighing 3.0–3.4 kg. Splenius motoneurons were antidromically identified and intracellularly filled with 12% Neurobiotin (Vector Laboratories) and visualized with the fluorochrome Alexa 488 (1:100; Molecular Probes). Reconstructions of the dendritic trees were drawn with the aid of a Eutectic Neuron Tracing System (Eutectic Electronics) and a Leica microscope equipped with a ×40 objective (NA 0.70). The methods used to construct the compartmental models have been described in detail by Bui et al. (2003). The number of compartments ranged from 3,500 to 4,500. The specific resistivity of the cytoplasm was set at 70 Ω·cm in keeping with the experimental observation of Barrett and Crill (1974). Specific membrane resistivity was assigned a value of 15,000 Ω·cm2 for the entire motoneuron. This value was chosen to be consistent with the models described by ElBasiouny et al. (2005) and Bui et al. (2006). It is also very close to experimentally derived estimates of specific dendritic membrane resistivity of feline motoneurons (Fleshman et al. 1988). The membrane potential in each compartment, in the absence of synaptic activity or activation of L-type Ca2+ channels, was −64 mV. This value matches the average values of the resting membrane potential of motoneurons reported by Heckman and Binder (1988). Simulations were performed using the Saber Simulator software package (Synopsys, Mountain View, CA).
Synapses on neck motoneurons are uniformly distributed at a density of 7/100 μm2 (Rose and Neuber-Hess 1991; similar value reported for lumbar motoneurons by Brännström 1993). Based on the surface areas of the cells chosen for this study, the maximum number of synapses ranged from 16,500 for the small motoneuron to 41,500 for the largest motoneuron. The exact ratio of all excitatory to inhibitory synapses on the dendritic trees of motoneurons is unknown. However, observations based on selected regions of the dendritic tree suggest that this ratio is close or greater than 1 (Brännström 1993; Brännström and Kellerth 1998; Rose and Neuber-Hess 1991). For the purposes of our simulations, we assumed a ratio of 1:1. We also assumed that both classes of synapses were uniformly distributed on the dendritic tree. Although there may be exceptions to this assumption (e.g., more inhibitory synapses on the proximal dendrites of motoneurons innervating fast-twitch muscle fibers, Brännström 1993), more complex distributions are difficult to justify given the inherent sampling problems associated with electron microscopic observations.
The current generated by a synapse is the product of the change in conductance generated by channel opening and the driving potential such that
| (1) |
where g is the conductance change, Erev is the reversal potential, and Vm is the membrane potential. Because the simulations were performed under steady-state conditions, we modeled the synaptic conductance changes for each compartment as a time-averaged conductance, g, as described by Bernander et al. (1991), and defined by the following equation
| (2) |
where gpeak is the peak conductance change, tpeak is the time-to-peak of the conductance change, f is the frequency of synaptic activity, and n is the number of synapses. The values assigned to gpeak and tpeak for excitatory and inhibitory synapses were similar to those used by ElBasiouny et al. (2005) and Bui et al. (2006).
Distribution of L-type Ca2+ channels
To determine the location of L-type Ca2+ channels on the motoneurons with different-sized dendritic trees, we modified the technique developed by Bui et al. (2006). The membrane potential along each dendritic path from the soma to a dendritic terminal was measured under two conditions. In the resting synaptic state, we voltage clamped the soma to −55, −50, and −45 mV for the small-, intermediate-, and large-sized motoneurons, respectively, to mimic the somatic membrane potential just subthreshold to PIC activation in low-, intermediate-, and high-conductance motoneurons as reported by Lee and Heckman (1998). Because it is likely that some level of synaptic activity is present at rest, a small number of excitatory and inhibitory synapses were activated at 50 and 25 Hz, respectively. This background synaptic activity was usually generated by activating 8% and 10% of the maximum number of excitatory and inhibitory synapses, respectively. These synapses were distributed according to the available surface area (i.e., uniformly). At a resting membrane potential of − 64 mV, this combination of synaptic activity generates 0 nA of current at the soma. For the excitatory synaptic state, we increased the number of active excitatory synapses to 15%. These additional synapses (i.e., from 8 to 15%) were also distributed uniformly to mimic the innervation pattern of Ia afferent synapses on lumbar motoneurons (Burke and Glenn 1996; data obtained from Fig. 13 in this study indicates there is no difference between the distribution of the total surface area of the dendritic tree and the distribution of Ia contacts, Kolmogorov-Smirnov test, P = 0.9). The activation frequency of these additional excitatory synapses was varied (40–85 Hz depending on the motoneuron; lowest in large cells and highest in small cell) to yield a somatic current of 4.2 nA at the soma at a membrane potential of − 64 mV. This corresponds to the average steady-state current reaching the soma of hindlimb motoneurons after the stimulation of triceps surae Ia afferents with little or no activation of PICs in all motoneuron types (Lee and Heckman 2000) (cf. Fig. 4, Minimal state). Higher values have also been reported (~6 nA by Lee et al. 2003). However, for the purposes of this study, whether we chose 4.2 or 6.0 nA was not critical. Estimates of the location of L-type Ca2+ channels based on the computational strategy developed by Bui et al. (2006) show little sensitivity to increasing the simulated excitatory synaptic current by 2–3 nA. Bennett et al. (1998) and Lee and Heckman (2000) have shown that the threshold at which PICs are activated is ~6 mV more hyperpolarized in the presence of excitatory synaptic activity compared with the absence of synaptic activity. Thus for each motoneuron during the excitatory synaptic state, we voltage clamped the soma at 6 mV more hyperpolarized than the resting synaptic state.
FIG. 4.

Location of the intersection points in a small (DVS 25–2) and large (DVS 14–1) motoneuron when somata are voltage clamped according to average PIC threshold values reported by Bennett et al. (1998). A: example of membrane potential profiles in a single dendritic path during the resting (R; solid lines) and excitatory (E; dashed lines) synaptic states. Black lines are based on average values reported by Bennett et al. (1998), and gray lines are based on somatic thresholds reported for low- and high-conductance motoneurons by Lee and Heckman (1998) and used in Fig. 1A. Arrows indicate location of intersection points. B: comparison of distribution of intersection points for all dendritic paths for small and large motoneurons for conditions described in A. Black circles are based on average values reported by Bennett et al. (1998), and gray circles are based on somatic thresholds reported for low- and high-conductance motoneurons by Lee and Heckman (1998). C: relationship between somatic voltage and current in responses to current ramps (5 nA/s) for small and large motoneurons when L-type Ca2+ channel hotspots are incorporated into models based on average PIC threshold values reported by Bennett et al. (1998). Arrows indicate somatic threshold for PIC activation. PIC threshold was measured as membrane potential at which derivative of somatic membrane potential with respect to time was 0.1 V/s.
The membrane potential along each dendritic path, for both the resting and excitatory synaptic states, were determined. In each state, this profile represents a snapshot of the membrane potential throughout the dendrites at the threshold for activating PICs by a somatic current injection. The common element between these snapshots is a dendritic region in which the membrane potential has been sufficiently raised to initiate PIC activation. The location of this is region is apparent when the membrane potential profiles from the resting and excitatory synaptic states are superimposed and provides an estimate of the location of the channels underlying PICs given the experimental constraints of the data reported by Lee and Heckman (1998). The site on the dendrite at which these profiles intersected was used as the midpoint of a 100- μm-long hotspot containing L-type Ca2+ channels. The methods for determining the dendritic region to place each hotspot and the parameters for modeling the L-type Ca2+ channels were the same as those described in Bui et al. (2006). For each motoneuron, the density of the L-type Ca2+ channels was adjusted until the somatic threshold for PIC activation in the resting synaptic state after a somatic voltage command was within 1 mV of the values reported by Lee and Heckman (1998).
RESULTS
The total surface area of the dendritic tree of the motoneurons used in this study ranged from small (DVS 25–2), to intermediate (DVS 25–3), to large (DVS 14–1, DVS 22–2, DVS 22–3; Table 1). Total dendritic surface area is well correlated with input conductance of motoneurons (Burke et al. 1982; Cullheim et al. 1987). Based on this correlation, we assumed that the small motoneuron corresponded to a low-conductance motoneuron and the large motoneurons corresponded to high-conductance motoneurons (Lee and Heckman 1998). The intermediate-sized motoneuron was assumed to have an intermediate conductance.
TABLE 1.
Comparison of motoneuron size, estimated location of L-type Ca2+ channel hotspots, and somatic threshold for PIC activation during resting, excitatory, and inhibitory synaptic states
| Cell | Surface Area of Dendritic Tree, μm2 | Median Hotspot Location From Soma, μm | Somatic PIC Threshold for Different Synaptic States, mV |
Threshold Change Between Resting and Excitatory States, mV | Threshold Change Between Resting and Inhibitory States, mV | ||
|---|---|---|---|---|---|---|---|
| Resting | Excitatory | Inhibitory | |||||
| DVS 25-2 | 236,414 | 180 | − 54.7 | − 58.7 | − 46.4 | − 4.0 | 8.3 |
| DVS 25-3 | 411,793 | 360 | − 49.8 | − 55.2 | − 42.9 | − 5.4 | 6.9 |
| DVS 14-1 | 578,589 | 790 | − 45.0 | − 49.9 | − 36.2 | − 4.9 | 8.8 |
| DVS 22-3 | 584,985 | 630 | − 44.6 | − 50.1 | − 38.9 | − 5.5 | 6.3 |
| DVS 22-2 | 591,671 | 845 | − 45.2 | − 49.4 | − 38.6 | − 4.2 | 6.0 |
PIC, persistent inward current.
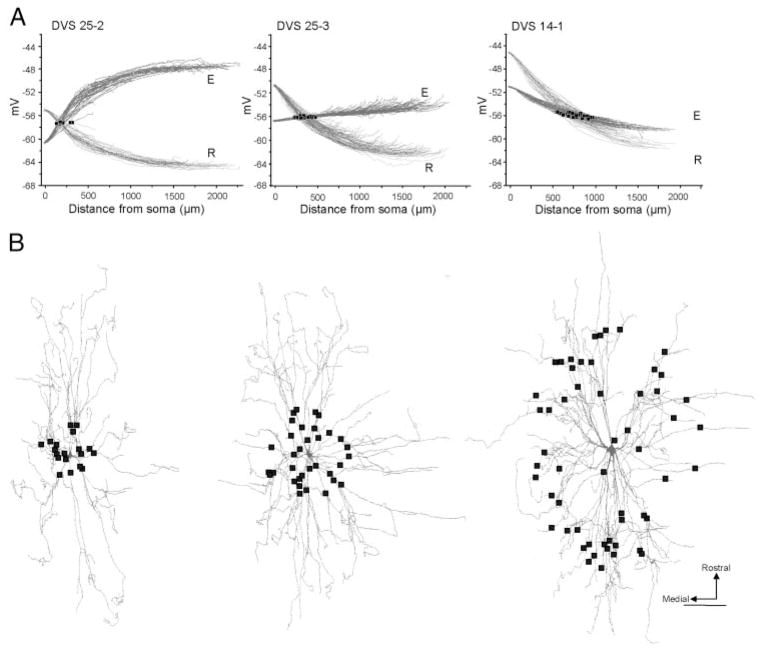
Figure 1A shows the membrane potential profiles for dendritic paths from the small, intermediate, and one of the large motoneurons (DVS 14–1). During the resting synaptic state, the membrane potential profiles decayed as a function of distance from the soma in all three motoneurons. The rate at which this decay occurred increased as the size of the motoneuron increased. The membrane potential profiles during the excitatory synaptic state were more variable. For the small motoneuron (DVS 25–2), the excitatory synaptic activity caused large depolarizations in the dendritic tree. Consequently, the membrane potential increased sharply in the proximal third of the dendritic tree. Compared with the small motoneuron, the depolarization generated by the excitatory synaptic activity in the intermediate-sized motoneuron (DVS 25–3) was smaller. As a result, the membrane potential profile increased slowly as a function of distance from the soma. For the large motoneuron (DVS 14–1), the membrane potential during the excitatory synaptic activity decayed slowly as a function of distance from the soma. Because of the differences in the level of depolarization caused by the excitatory synaptic activity between small, intermediate, and large motoneurons, the intersection points between the resting and excitatory membrane potential profiles occurred more distally as the motoneuron size increased (Fig. 1A, black squares). Despite the differences in the location of the intersection points on the dendritic tree, the membrane potential of the dendrites at which the intersection points occurred showed little variability (range between − 56 and − 57 mV). When viewed from the perspective of the entire dendritic tree, the intersection points on the small cell were tightly clustered around the soma (Fig. 1B). In contrast, the intersection points on the larger motoneuron were more widely dispersed forming an annulus on the outer one third of the dendritic tree. Intersection points on the intermediate-sized motoneuron were arranged between the distributions seen on the small and large motoneurons. Because there are more dendritic branches distally (Cullheim et al. 1987; Rose et al. 1985), there were a greater number of intersection points on the larger motoneurons.
FIG. 1.
A: membrane potential profiles as a function of distance from the soma for dendritic paths during the resting and excitatory synaptic states. Intersection points are indicated by black squares. Dendritic paths with voltage profiles that intersect (78/78, 122/125, and 96/150 for the small, intermediate-sized, and large motoneuron, respectively) are shown. R, resting state; E, excitatory synaptic state. B: reconstructions of corresponding dendritic trees of motoneurons examined in A. Each black square indicates midpoint of a 100- μm-long L-type Ca2+ channel hotspot.
Figure 2A and Table 1 summarize the dendritic distributions of the intersection points for all five motoneurons. For the small- (DVS 25–2) and intermediate-sized (DVS 25–3) motoneurons, the median locations of the intersection points were 180 and 360 μm from the soma, respectively. For the larger motoneurons, the median locations of the intersection points were more distal at 630, 790, and 845 μm from the soma. The location of these intersection points were linearly related to the total surface area of the dendritic tree (r2 = 0.89; P < 0.01; Fig. 2B).
FIG. 2.
A: box-plot of distribution of intersection points for all 5 motoneurons. B: median location of intersection points as a function of total surface area of dendritic tree (r2 = 0.89; P < 0.01).
A critical test of the use of the intersection points as a means of determining the location of L-type Ca2+ channels is the ability of the models with channels inserted at these intersection points to replicate the change in the threshold for PIC activation as a consequence of tonic synaptic activity (cf. Bennett et al. 1998 and Lee and Heckman 2000). Figure 3 shows the responses of the small, intermediate, and one of the large motoneurons to depolarizing current ramps (5 nA/s) injected into the soma during different synaptic states. These current ramps were performed after inserting the L-type Ca2+ channels into 100- μm hotspots on the dendritic tree centered at the intersection points. In each case, PICs were activated at a lower threshold during the excitatory synaptic state compared with the resting synaptic state (Fig. 3, arrows). The change in PIC threshold between the resting and excitatory synaptic states for all the motoneurons ranged between − 4.0 and − 5.5 mV (Table 1). On average, the thresholds in the excitatory synaptic state were 4.8 ± 0.7 mV more hyperpolarized than in the resting synaptic state. Bennett et al. (1998) showed that in the presence of tonic inhibitory synaptic activity, evoked by stimulation of the common peroneal nerve, the threshold for PIC activation is more depolarized than in the resting synaptic state. In previous simulations using models containing L-type Ca2+ channel hotspots located at the intersection points, Bui et al. (2006) replicated this finding by adding tonic inhibitory synaptic input to the model. We examined whether our models, which vary in dendritic tree size and L-type Ca2+ channel hotspot location, could also replicate the shift in PIC threshold to a more depolarized level in the presence of inhibitory synaptic activity. For the inhibitory synaptic state, we increased the number of inhibitory synapses to 15% and varied the activation frequency (21–45 Hz; lowest in large cells and highest in small cell) to generate − 2 to − 3 nA of current at the soma. These values are close to the effective synaptic current reaching the soma in motoneurons after stimulation of the common peroneal nerve (Powers and Binder 2000). For each motoneuron, PICs were activated at a higher threshold in the inhibitory synaptic state compared with the resting synaptic state (Fig. 3, arrows). The change in PIC threshold between the resting and inhibitory synaptic states ranged between 6.0 and 8.8 mV (Table 1). On average, the thresholds in the inhibitory synaptic state were 7.3 ± 1.2 mV more depolarized than in the resting synaptic state. These changes in somatic threshold for PIC activation for both the excitatory and inhibitory synaptic states are very similar to the experimental findings reported by Bennett et al. (1998) (mean change in threshold: excitatory synaptic state, −5.8 ± 4.5 mV; inhibitory synaptic state, 7.6 ± 1.2 mV).
FIG. 3.
Relationship between somatic voltage and current in response to current ramps (5 nA/s) for the small-, intermediate-, and large-sized motoneurons shown in Fig. 1 during the resting (R), excitatory (E), and inhibitory (I) synaptic states. Arrows indicate somatic threshold for persistent inward current (PIC) activation. PIC threshold was measured as membrane potential at which derivative of somatic membrane potential with respect to time was 0.1 V/s. Tops of plateaus truncated to highlight differences in onset of PICs.
Sensitivity of intersection points to changes in somatic PIC threshold
Bui et al. (2006) estimated that L-type Ca2+ channel hot-spots in intermediate-sized motoneurons are located on dendrites between 100 and 400 μm from the soma. In a large motoneuron, ElBasiouny et al. (2005) estimated that the hot-spots are located more distally, 300–900 μm from the soma. These results are consistent with our findings in that L-type Ca2+ channel hotspots are located progressively more distally on larger motoneurons. However, unlike this study, previous studies assumed that the somatic threshold for PIC activation is the same for all motoneurons (approximately −50 mV during a resting synaptic state and −56 mV in the presence of excitatory synaptic activity). Although these values reflect the experimental values reported by Bennett et al. (1998), according to the data collected by Lee and Heckman (1998), these values may only apply to intermediate-sized motoneurons. To determine if the intersection points depend on the somatic PIC threshold values assigned to the models, we recalculated the location of the intersection points in the small (DVS 25–2) and one large (DVS 14–1) motoneuron when the soma was clamped at −50 and −56 mV for the resting and excitatory synaptic states. Figure 4A compares the intersection points of the voltage profiles of a single dendritic path during the resting (solid lines) and excitatory (dashed lines) synaptic states according to the average PIC threshold values reported by Bennett et al. (1998) (black lines) and those reported for low- or high-conductance motoneurons (gray lines) (Lee and Heckman 1998). For the small motoneuron, DVS 25–2, there was a slight distal shift in the intersection point when the soma was clamped at − 50 and − 56 mV for the resting and excitatory synaptic states, respectively (arrows). Overall, the median location of the intersections points changed from 180 to 212 μm (Fig. 4B). Despite the fact that this change was small in terms of the absolute value, it was statistically significant (Mann-Whitney U test, P = 0.03). For the large motoneuron, DVS 14–1, the intersection points moved proximally (Mann-Whitney U test, P = 0.03). Once again, the absolute value of the change in the intersection points was small (difference in the medians: 35 μm). Repositioning the hotspots to the new intersection points had little effect on the change in PIC thresholds during the excitatory synaptic state compared with the resting synaptic state (− 4.5 and − 5.8 mV for the small and large motoneuron, respectively; Fig. 4C). Thus the relationship between motoneuron size and the estimated location of L-type Ca2+ channel hotspots was relatively independent of the somatic PIC threshold values chosen for the models.
Sensitivity of PIC activation to changes in hotspot location, somatic Rm, and background synaptic activity
The previous simulations suggest that the intersection points of the voltage profiles provide a means of positioning L-type Ca2+ channels such that our models behave in a fashion that is consistent with the experimental observations of Bennett et al. (1998). However, the question of whether hotspots at these points are a unique means of mimicking the experimental data remains open. To address this question, we redistributed the L-type Ca2+ channel hotspots on the dendritic tree of the small (DVS 25–2) and large (DVS 14–1) motoneurons such that their distance from the soma was similar to that of the intermediate-sized motoneuron (DVS 25–3). For the small motoneuron, the hotspots were shifted ~160 μm distally (median location was 340 μm from the soma), and for the large motoneuron, the hotspots were shifted proximally by ~430 μm (median location was 360 μm from the soma). The density of the L-type Ca2+ channels was adjusted to produce a PIC threshold close to − 55 mV in the small motoneuron and − 45 mV in the large motoneuron in response to a somatic current ramp during the resting synaptic state. When the same current ramp was performed during the excitatory synaptic state, the PIC threshold for the small motoneuron decreased by 10 mV, and during the inhibitory synaptic state, the PIC threshold increased by 12 mV. The PIC threshold during the excitatory synaptic state in the large motoneuron decreased by 3.5 mV, and during the inhibitory synaptic state, the PIC threshold increased by 1.4 mV. Thus for the excitatory synaptic state, these values are outside the range of values obtained when the hotspots were positioned at the intersection points and outside most of the values obtained experimentally (Fig. 5) (Bennett et al. 1998). However, for the inhibitory synaptic state, these values were outside the range of values obtained when the hotspots were positioned at the intersection points and those obtained experimentally. We performed an additional simulation in the large motoneuron in which the L-type Ca2+ channels were distributed between 300 and 850 μm, similar to the wideband distribution used for the large motoneuron in ElBasiouny et al. (2005). To replicate the PIC threshold during the resting synaptic state with the hotspot length increased, the density of the L-type Ca2+ channels had to be lowered. In fact, the density of the L-type Ca2+ channels during these simulations was nearly identical to those used by ElBasiouny et al. (2005) (13.8 vs. 14.0 pS/μm2). In this instance, the excitatory synaptic activity activated the PICs when the soma was clamped at − 64 mV, indicating that the threshold for PIC activation was less than − 64 mV, a decrease of 19 mV.
FIG. 5.
Comparison of shift in somatic threshold of PIC activation from the resting to excitatory synaptic state and from the resting to inhibitory synaptic state. Open circle with cross-hairs indicates mean values and SD reported by Bennett et al. (1998). Closed circles indicate values for the 5 motoneurons used in this study when L-type Ca2+ channel hotspots are located at intersection points. Triangle and square indicate values for small (DVS 25–2) and large motoneurons (DVS 14–1), respectively, when L-type Ca2+ channel hotspots are located at same distance from soma as those in an intermediate-sized motoneuron (DVS 25–3).
Several studies have suggested that the Rm at the soma may be lower in comparison to the dendritic tree (Clements and Redman 1989; Fleshman et al. 1988; Maltenfort and Hamm 2004; Rose and Vanner 1988). All the simulations performed thus far assumed an Rm value of 15,000 Ω·cm2 throughout the entire motoneuron. To examine whether a lower somatic Rm affects the ability of the model to replicate the changes in PIC threshold, we repeated the simulations when the somatic Rm was lowered to 300 Ω·cm2; the midrange value reported by Fleshman et al. (1988), Clements and Redman (1989), and Maltenfort and Hamm (2004). The density of the L-type Ca2+ channels for the small (DVS 25–2), intermediate (DVS 25–3), and large (DVS 14–1) motoneurons was adjusted such that the PIC thresholds during the resting synaptic states were close to − 55, − 50, and − 45 mV, respectively. For each motoneuron, the PICs were activated at a lower threshold during the excitatory synaptic state compared with the resting synaptic state. The change in PIC threshold between the resting and excitatory synaptic states for all the motoneurons ranged between − 4.8 to − 5.9 mV. On average, the thresholds in the excitatory synaptic state were 5.4 mV more hyperpolarized than in the resting synaptic state. Thus the ability of our models to replicate the changes in PIC threshold based on the experimental data by Lee and Heckman (2000) and Bennett et al. (1998) is independent of the presence or absence of a somatic shunt.
In our models thus far, background synaptic activity in the dendritic tree was generated by activating 8% of the excitatory and 10% of the inhibitory synapses. To test the possibility that altering the level of background synaptic activity would affect the ability of the models to replicate the changes in PIC threshold, we repeated the simulations with the level of background synaptic activity reduced. In these simulations, the background synaptic activity was generated by activating 4% of the excitatory and 5% of the inhibitory synapses. Similar to the previous level of background synaptic activity, this activity generated 0 nA of current at the soma, assuming a resting membrane potential of − 64 mV. For the small (DVS25-2), intermediate (DVS 25–3), and large (DVS 14–1) motoneurons, we adjusted the density of the L-type Ca2+ channels to produce a PIC threshold close to − 55, − 50, and − 45 mV, respectively, during the resting synaptic state. When the simulations were repeated in the presence of excitatory synaptic activity, the PIC threshold was lower in all three motoneurons, and the difference in PIC threshold between the resting and excitatory synaptic states ranged between 4 and 5.5 mV; similar to the values when the background synaptic activity was generated by activating 8% of the excitatory and 10% of the inhibitory synapses. Based on this, the ability to replicate the changes in PIC threshold in the absence and presence of synaptic activity as reported by Lee and Heckman (2000) and Bennett et al. (1998) is not affected by the level of background synaptic activity used in our models.
Functional consequences
Lee et al. (2003) observed that PICs in low-conductance motoneurons increased the gain in firing rate (expressed as the increase in spikes/s per nA of current generated by synaptic activity alone) by a factor of 3 or 4. In contrast, the increase in gain caused by activation of PICs in large-conductance motoneurons was negligible. We therefore examined whether the amplification of synaptic inputs in our models of small-, intermediate-, and large-sized motoneurons was related to differences in hotspot location. Synaptic input was generated by activating excitatory synapses that were uniformly distributed throughout the entire dendritic tree. Because the total dendritic surface area of the motoneurons varied, the maximum number of excitatory synapses (i.e., 100%) ranged between ~8,000 and 20,000 synapses. The frequency of activation was fixed at 50 Hz, and the number of active excitatory synapses was varied from 4 to 100%. These synapses were activated in the presence of the same background synaptic activity used in the previous models (i.e., 8% of the excitatory and 10% of the inhibitory synapses).
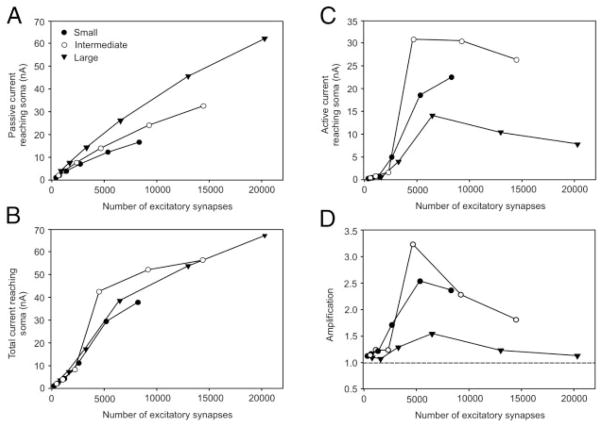
To determine the impact of PICs on the net current reaching the soma, we constructed two sets of models and voltage clamped the soma at − 64 mV. In one set of models, L-type Ca2+ channels were absent. In these models, the current reaching the soma increased as a function of motoneuron size (Fig. 6A). For example, after the activation of ~3,000 synapses, the current reaching the soma was nearly 70% greater in the large motoneuron (DVS 14–1) compared with the small motoneuron (DVS 25–2). In the other set of models, L-type Ca2+ channels were localized to hotspots centered in the intersection points. In these models, the differences in current reaching the soma observed under the passive condition for all three motoneurons was largely eliminated up to the point where ~3,000 synapses were activated (Fig. 6B). For the large and small motoneurons, these differences remained small as the level of synaptic activity was increased to 100% of all excitatory synapses on the small motoneuron. In the intermediate-sized motoneuron, there was an abrupt increase in the current reaching the soma after the activation of ~5,000 synapses. This was caused by the large increases in PICs (Fig. 6C). PICs contributed the smallest amount of current at the soma in the large motoneuron. To quantify these results, we calculated the degree of amplification of the synaptic current, defined as the ratio between the total current reaching the soma with and without L-type Ca2+ channels. In all three motoneurons, the magnitude of amplification peaked at the point where ~5,000 synapses were activated (Fig. 6D). The amplification was smallest in the large motoneuron reaching a maximum of 1.5-fold. In the small- and intermediate-sized motoneurons, the amplification was higher leading to an ~2.5- to 3.0-fold increase in the synaptic current reaching the soma. For all three motoneurons, amplification decreased at high levels of excitatory synaptic activity. This likely reflected saturation of L-type Ca2+ channel activation.
FIG. 6.
Current reaching soma caused by activation of PICs and excitatory synaptic activity. Somatic membrane potential was clamped at − 64 mV. Excitation levels were varied by activating 4, 8, 16, 32, 64, and 100% of maximum number of excitatory synapses. These excitation levels were converted to an absolute number of synapses to examine somatic current for a given amount of synaptic input. A: passive condition. Models do not contain voltage-dependent channels on dendritic tree. B: active condition. Models contain L-type Ca2+ channel hotspots on dendrites and are positioned according to Fig. 1. Total current reaching soma is comprised of both passive and active currents. C: current reaching soma from PICs. This was determined by taking difference between total and passive currents in A and B. D: amplification of synaptic of current caused by PIC activation as a function of excitation level. Gray dashed line at 1.0 indicates no amplification. Closed circles, DVS 25–2, small motoneuron; open circles, DVS 25–3, intermediate-sized motoneuron; triangles, DVS 14–1, large motoneuron.
The differences in amplification observed between the different-sized motoneurons could be explained by two factors. First, because of the closer proximity of the L-type Ca2+ channel hotspots to the soma in the small- and intermediate-sized motoneurons, the current generated by these channels may suffer less attenuation en route to the soma compared with the more distally located channels in the large motoneurons. Second, the number of L-type Ca2+ channels may be greater in the smaller motoneurons. To achieve a PIC threshold near − 55 mV during the resting synaptic state in the small motoneuron and − 45 mV in the large motoneurons, the density of the L-type Ca2+ calcium channels within the hotspots was tailored for each motoneuron. For the small and large motoneurons, respectively, the conductance densities of the L-type Ca2+ channels hotspots were 89 and 40 pS/μm2, which was more than a twofold difference. However, there were a greater number of hotspots in the large motoneuron. These hotspots occupied ~3,000 μm2 of dendritic membrane area compared with 13,800 μm2 in the small motoneuron. As a result, the total conductance of the L-type Ca2+ channels was 1.2 μS in the small motoneuron and 1.4 μS in the large motoneuron. Thus assuming that the driving potential for Ca2+ ions is similar at hotspots on small and large motoneurons, under conditions of maximal PIC activation, the smaller PIC current reaching the soma in the large motoneuron was most likely caused by their distal location on the dendritic tree.
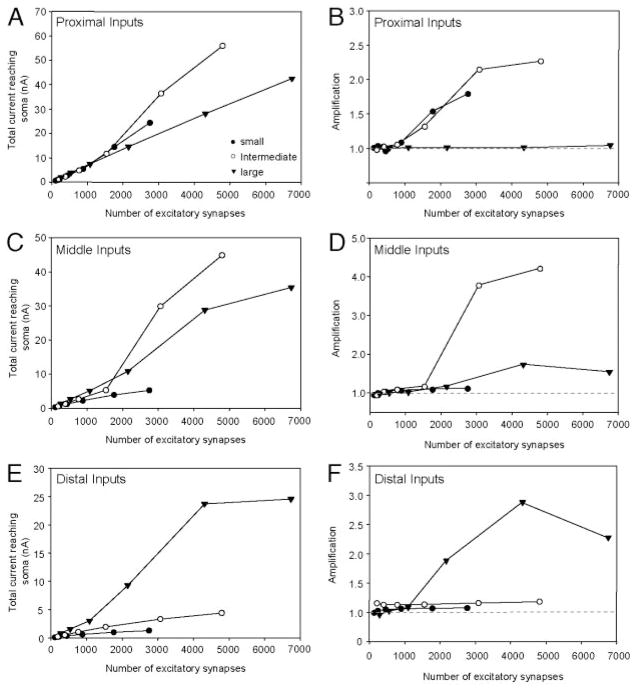
Thus far, we have measured amplification of synaptic input by PICs in models containing excitatory synapses distributed uniformly throughout the dendritic tree. In pyramidal neurons, the magnitude of amplification may vary depending on the location of voltage-dependent channels and the location of the synaptic inputs on the dendritic tree (Oviedo and Reyes 2005; Williams and Stuart 2003). Our results suggest that the channels responsible for PICs may be located in different regions of the dendritic tree for different motoneuron types. As a consequence, excitatory synaptic activity occurring in specific regions of the dendritic tree may be amplified differentially depending on the type of motoneuron. We tested this possibility by restricting the excitatory synaptic activity to the proximal, middle, and distal third of the dendritic tree based on the surface area. For the small motoneuron, the proximal third ranged between 0 and 490 μm from the soma, the middle third ranged between 491 and 980 μm from the soma, and the distal third ranged from 981 μm to the dendritic terminals. For the intermediate-sized motoneuron, the proximal third ranged between 0 and 480 μm from the soma, the middle third ranged between 481 and 880 μm from the soma, and the distal third ranged from 881 μm to the dendritic terminals. For the large motoneuron, the proximal third ranged between 0 and 415 μm from the soma, the middle third ranged between 416 and 745 μm from the soma, and the distal third ranged from 746 μm to the dendritic terminals. As before, the excitatory synapses were activated at 50 Hz.
When synaptic inputs were restricted to the proximal one third of the dendritic tree, the current reaching the soma was similar across all motoneurons after the activation of 1,500 synapses (Fig. 7A). At higher levels of synaptic excitation, the current reaching the soma was greater in the small- and intermediate-sized motoneuron compared with the large motoneuron. This difference was caused by the activation of PICs. For both the small- and intermediate-sized motoneurons, the PICs amplified the synaptic current reaching the soma by approximately twofold (Fig. 7B). When the synaptic inputs were restricted to the middle one third of the dendritic tree, the current reaching the soma was once again greatest in the intermediate-sized motoneuron (Fig. 7C). This result was a consequence of enhanced PIC activation (Fig. 7D). For the large motoneuron, the current reaching the soma was also amplified. However, in this instance, PICs were only slightly activated, and this only occurred at higher levels of synaptic excitation (~4,000 synapses). The synaptic inputs to the middle one third failed to activate PICs in the small motoneuron. As a consequence, very little current was delivered to the soma. When the synaptic inputs were moved to the distal one third of the dendritic tree, the current reaching the soma in the large motoneuron was substantially amplified in comparison to the small- and intermediate-sized motoneurons (Fig. 7E). This was because of the ability of the distal synaptic inputs to activate PICs in the large motoneuron but not in the other motoneurons (Fig. 7F). These results suggest that the magnitude of amplification that occurs in motoneurons is not only influenced by the dendritic location of the channels responsible for PICs, but also by the spatial relationship between these channels and the synaptic input on the dendritic tree.
FIG. 7.
Total current reaching soma and amplification of synaptic current caused by activation of PICs when excitatory synaptic inputs are restricted to proximal (A and B), middle (C and D), and distal (E and F) one third of dendritic tree. Closed circles, DVS 25–2, small motoneuron; open circles, DVS 25–3, intermediate-sized motoneuron; triangles, DVS 14–1, large motoneuron. Gray dashed line at 1.0 indicates no amplification.
DISCUSSION
Our primary goal was to examine the relationship between the location of L-type Ca2+ channels and motoneuron size. To reach this goal, we applied the computational strategy developed by Bui et al. (2006), taking into account applied differences in the PIC thresholds in a resting synaptic state for low-, intermediate-, and high-conductance motoneurons (− 55, − 50, and − 45 mV, respectively; see Lee and Heckman 1998). Our results indicate that the shift in PIC thresholds that occurs in response to tonic excitatory or inhibitory synaptic activity (Bennett et al. 1998) could only be replicated when L-type Ca2+ channel hotspots were positioned progressively more distal from the soma as the size of the motoneuron increased. We also examined whether these differences in hotspot location could explain the differences in PIC threshold reported by Lee and Heckman (1998) between low-, intermediate-, and high-conductance motoneurons. Contrary to our expectation, the relationship between PIC threshold and motoneuron conductance, observed by Lee and Heckman (1998), is not caused by differences in the location of the L-type Ca2+ channels. Instead, these differences were caused by the density of L-type Ca2+ channels. In models equipped with L-type Ca2+ channels based on the above results, the amplification of synaptic current by PICs depends on the size of the motoneuron and the location of the synaptic input on the dendritic tree.
Methodological considerations
SAMPLE SIZE
The location of L-type Ca2+ channels on the dendritic trees of different-sized motoneurons was derived from observations based on a small sample of motoneurons; one small-sized, one intermediate-sized, and three large-sized motoneurons. This raises the question of whether our sample of cells represents the characteristics of an entire pool of motoneurons. Ultimately, this question can only be answered with a larger sample of motoneurons, in particular small- and intermediate-sized motoneurons. However, in the absence of these data, there are several indications that our sample is a good estimate of the heterogeneity of motoneuron structure that is typical of a motoneuron pool. In addition to total surface area of the dendritic tree, the size of the motoneuron can also be described by the soma diameter, the axon diameter, the total dendritic length, and the number of dendritic terminals. In keeping with other studies, measurements of these parameters scaled with the surface area of the dendritic tree and their upper and lower limits were typical of much larger samples (Burke et al. 1982; Cullheim et al. 1987; Grande et al. 2005). The ability of our small sample to manifest the properties of a larger sample of cells is further emphasized by the results in ElBasiouny et al. (2005) and Bui et al. (2006). ElBasiouny et al. (2005) estimated in that L-type Ca2+ channel hotspots were located more than 500 μm away from the soma (customized distribution). This result was based on a model of a large motoneuron and fits with our results. For an intermediate-sized motoneuron, we estimated that the L-type Ca2+ channel hot-spots were located ~360 μm from the soma. This value is very similar to estimates based on the three intermediate-sized motoneurons used by Bui et al. (2006). Thus when viewed from the perspective of all computational estimates of the location of L-type Ca2+ channels on motoneurons, there is a remarkable consistency regarding the relationship between channel location and motoneuron size.
LOCATION OF INTERSECTION POINTS
The distribution of L-type Ca2+ channel hotspots in our models was based on the membrane potential changes that occur in passive dendrites (from soma to terminal) in the resting and excitatory synaptic state. The point at which the membrane potential profiles intersected under these two conditions guided the placement of L-type Ca2+ channels. The results by ElBasiouny et al. (2005) and Bui et al. (2006), as well as ours, show that these intersection points provide a reliable means of estimating the location of L-type Ca2+ channels given the accuracy of the model assumptions. The location of these intersection points depend on the voltages at which the somata are clamped during the resting and excitatory synaptic states. This affects the rate at which the membrane potential decays along the dendritic paths (Fig. 4A). As a consequence, the location of the intersection points on the dendritic tree may change. We voltage clamped the somata during the resting synaptic states, just subthreshold to the activation of PICs, based on the threshold values reported by Lee and Heckman (1998) for low-, intermediate-, and high-conductance motoneurons (− 55, − 50, and − 45 mV, respectively). However, we also performed simulations in which the values used to clamp the somata of the small and large motoneurons were altered by 5 mV to match the average values reported experimentally by Bennett et al. (1998) and those used in the studies by ElBasiouny et al. (2005) and Bui et al. (2006). Despite these changes, the median location of the intersection points was altered, at most, by only 35 μm (Fig. 4). Based on this, the values selected to voltage clamp the somata during the resting and excitatory synaptic states does not affect the linear relationship between the location of the intersection points and the size of the motoneuron.
Most of the simulations performed in this study used the same value for the effective Rm of the dendritic tree. Several studies have suggested that the effective Rm may vary systematically with motoneuron type, such that the effective Rm of small motoneurons is higher than that of large motoneurons (Burke et al. 1982; Kernell and Zwaagstra 1989; but see, Fleshman 1988 for a different conclusion based on a low somatic Rm). We did not incorporate this feature in our models. However, in some simulations, we increased the effective value of Rm from 4,100 to 10,800 Ω·cm2 in all motoneurons by decreasing the level of background synaptic activity. The position of the hotspots was not changed. These models also replicated the changes in PIC threshold caused by synaptic activity as described by Bennett et al. (1998). Because our models, as well as those by Bui et al. (2006), could not replicate these experimental findings when the hotspots are positioned >100 μm from the intersection points, this suggests that the location of the intersection point does not depend on the exact value of Rm, at least over the range of Rm values typically reported for large and small motoneurons.
HOTSPOT LOCATION/LENGTH AND PIC THRESHOLD
For our models to satisfy the experimental data, the threshold at which PICs are activated had to be more hyperpolarized during the excitatory synaptic state and more depolarized during the inhibitory synaptic state relative to the resting synaptic state. In all five motoneurons, we were able to reproduce these shifts in PIC threshold when the hotspots were positioned at the location of the intersection points. Bui et al. (2006) showed that, if the hotspots are positioned >100 μm away from the intersection points, the models cannot replicate the shifts in PIC threshold reported by Bennett et al. (1998). Results from this study are consistent with this finding. When the hotspots in the small and large motoneuron were repositioned on the dendritic tree such that their median location was similar to that of the intermediate-sized motoneuron, a shift of >150 μm from intersection point, the changes in PIC threshold that occurred during the excitatory and inhibitory synaptic states were outside the range reported by Bennett et al. (1998) (Fig. 5). Because ElBasiouny et al. (2005) used a compartmental model of a large motoneuron and Bui et al. (2006) used compartmental models of smaller motoneurons, the discrepancy in the location of L-type Ca2+ channel hotspots reported in these studies could be reconciled by the differences in the size of the motoneuron.
L-type Ca2+ channel hotspots in our models were 100 μm in length. This is approximately the midrange value of the length of hotspots used in Bui et al. (2006) that could replicate the experimentally observed shifts in PIC threshold in response to synaptic activity. In contrast, the model constructed by ElBasiouny et al. (2005) contained 550-μm-long hotspots, located 300–850 μm from the soma (wideband distribution). In the study by ElBasiouny et al. (2005), three criteria were used to assess the validity of their model; the presence of hysteresis in the relationship between firing rate and injected current, the amplitude of the Ca2+ PIC, and the shift in PIC threshold that occurs in response to excitatory synaptic activity relative to a resting synaptic state. In this study, we verified our models based only on the latter criteria. When L-type Ca2+ hotspots were distributed in a similar fashion to the wideband distribution used by ElBasiouny et al. (2005) in one of our large motoneurons, PICs were activated at a somatic threshold below − 64 mV in response to excitatory synaptic activity. Thus we were unable to replicate the shift in PIC threshold that occurs during the excitatory synaptic state relative the resting synaptic state.
It is likely that this discrepancy can be explained by the different synaptic distribution patterns used to mimic the Ia synaptic input. In our models, the excitatory synapses were distributed uniformly throughout the dendritic tree based on the results of Burke and Glenn (1996), which showed that Ia homonymous and heteronymous synapses innervate motoneurons with no particular bias with respect to distance from the soma. Conversely, in the study by ElBasiouny et al. (2005), the synapses were distributed based on the Ia homonymous synaptic input (Segev et al. 1990) with a bias to the proximal regions of the dendritic tree. As a consequence, our models had a greater proportion of synapses located in the distal regions of the dendritic tree compared with the study by ElBasiouny et al. (2005). This is apparent in Fig. 7A in ElBasiouny et al. (2005), which shows an abrupt hyperpolarization of the membrane potential in the distal dendrites during excitatory synaptic activity. In contrast, in our simulations, the voltage profiles decayed monotonically (Fig. 1), and most of the intersection points were <850 μm from the soma. Inserting L-type Ca2+ channels more distally than the intersection points in our models creates a problem. In the resting synaptic state, the membrane potential 850 μm from the soma is hyperpolarized relative to the membrane potential at the intersection points and, most importantly, hyperpolarized relative to membrane potential during the excitatory synaptic state. We could adjust the density of L-type Ca2+ channels in the resting synaptic state to ensure that this membrane potential was just subthreshold for L-type Ca2+ channels 850 μm from the soma. However, in the excitatory synaptic state, the membrane potential at 850 μm from the soma would be suprathreshold at this location. To bring the membrane potential at 850 μm from the soma below threshold, the soma would have to be clamped at a more hyperpolarized value. Indeed, our simulations suggest that this clamp must be more negative than −64 mV, leading to a change in threshold that is much larger than seen experimentally. In the model developed by ElBasiouny et al. (2005), it may not have been necessary to clamp the somatic membrane potential at a more hyperpolarized value because the membrane potential in distal dendrites was more hyperpolarized because of the absence of excitatory synapses. It should be emphasized that the electrophysiological data that were used to constrain both models were based on activation of homonymous and heteronymous Ia synapses (Bennett et al. 1998). Thus we constructed models with a uniform distribution of excitatory synapses. The simulations based on these models suggest that the hotspots on large motoneurons must be restricted to a small region in the distal dendritic tree.
L-TYPE Ca2+ CHANNEL PROPERTIES
The half-activation voltage for L-type Ca2+ channels used in these simulations was − 33 mV. This value was selected to be consistent with the model used by Bui et al. (2006) and is consistent with other modeling studies of motoneurons (Carlin et al. 2000; Svirskis et al. 2001). However, recent studies of cloned Cav1.3 channel behavior in expression systems suggest a half-activation voltage of − 40 mV (Lipscombe et al. 2004), and some models use a half-activation voltage of − 40 to − 43 mV (Booth et al. 1997; ElBasiouny et al. 2005). We did not examine whether using other half-activation voltage values for L-type Ca2+ channels could replicate the shift in PIC threshold as reported by Bennett et al. (1998). However, Bui et al. (2006) showed that half-activation values of − 28 and − 33 mV could replicate the findings of Bennett et al. (1998), whereas a half-activation value of − 38 mV could not. This suggests that post-translational modifications and differences in subunit composition may affect channel behavior.
Voltage-dependent channels responsible for PICs
In motoneurons, the total PIC is mediated by Na+ and L-type Ca2+ channels (Heckman et al. 2005). Based on electrophysiological evidence, and their importance in spike initiation, it is likely that the channels mediating Na+ PICs are located on or near the soma (Lee and Heckman 2001; Li and Bennett 2003). More recent descriptions of the amplification of the high-frequency (180 Hz) component of Ia synaptic inputs to motoneurons provide indirect evidence that Na+ channels are distributed on more distal dendrites (Jones and Lee 2006). However, the exact location of these channels is unknown. In this study, we did not include these currents in our models. The average shifts in somatic PICs thresholds caused by excitatory synaptic activity (i.e., − 6 mV) are the same with and without Na+ channels (see Bui et al. 2006). The somatic PIC threshold in the resting synaptic state does change if Na+ channels are blocked (Lee and Heckman 1999). However, changes in this parameter had little effect on the location of the hotspots in this study. Thus the key experimental evidence on which this study was founded is applicable to models that lack Na+ channels. By using these models, we gained an important advantage: the ability to compare our results with those of ElBasiouny et al. (2005) and Bui et al. (2006). These models may be less suitable as a means of predicting the input-output properties of motoneurons. However, we could mimic the relationship between motoneuron size and amplification of synaptic inputs (Fig. 6), a property of motoneurons that was observed in circumstances that likely included Na+ channels currents (Lee et al. 2003).
In contrast to Na+ channels, there are numerous anatomical studies of the distribution of L-type Ca2+ channels on the dendrites of motoneurons (Ballou et al. 2006; Carlin et al. 2000; Davenport et al. 2003; Simon et al. 2003; Westenbroek et al. 1998, 2005; Zhang et al. 2006). However, there is little agreement as to their precise location. To further complicate this issue, a number of modeling studies have been able to replicate many of the electrophysiological properties of motoneurons using different distribution patterns L-type Ca2+channels on the dendrites of motoneurons (Booth et al.1997; Bui et al. 2006; Carlin et al. 2000; ElBasiouny et al. 2005; Svirskis et al. 2001; Taylor and Enoka 2004). The reasons for the variability reported in the anatomical and modeling studies are not known. What has emerged from this study is that, within motoneurons innervating the same muscle and using the same modeling procedures, the distribution L-type Ca2+channels may vary because of differences in the size of the dendritic trees.
As discussed by Bui et al. (2006), the immunohistochemical studies may describe the anatomical distribution of L-type Ca2+channels, whereas the modeling studies describe the functional distribution of these channels: that is, the location of a subset of L-type Ca2+channels available for activation under the specific circumstances of the experimental conditions, e.g., activation of Ia afferent synapses in decerebrate cats (Bennett et al. 1998; Lee and Heckman 1998). If these circumstances change, the subset of L-type Ca2+channels available for activation may not be distributed as estimated in this study. Thus the results of this study do not preclude other distribution patterns. Such flexibility would enhance the computational power of the motoneuron and may be an important means by which the output of the motoneuron is matched to the demands of different motor tasks.
The primary objective in this study was to replicate the shifts in somatic PIC threshold as observed by Bennett et al. (1998). However, once PICs are activated, their amplitude and resulting plateau potential may be influenced by the activation of other channels such as K+ channels (Hounsgaard and Mintz 1988) and high-threshold Ca2+channels (Carlin et al. 2000). Similar to the results by Bui et al. (2006), the size of the plateau potentials in these simulations are larger than the experimentally reported values (Fig. 3). This is likely caused by the fact that our models did not contain K+ conductances. In the study by Bennett et al. (1998), the compound QX-314 was used block Na+ channels, which has been shown to reduce the amplitude of PICs (Lee and Heckman 1999). This may also explain the differences in the amplitude of the plateaus between our simulations and the experimentally reported values.
Functional implications
AMPLIFICATION OF SYNAPTIC INPUT BY PICS
Our results suggest that differences in the location of L-type Ca2+channels may underlie several functional differences between small and large motoneurons. In response to simulated increases in the activity of uniformly distributed excitatory synapses, PICs are more readily activated in smaller compared with larger motoneurons. This result is consistent with the experimental findings of Lee et al. (2003), which showed a negative correlation between input conductance and the amount of current reaching the soma due caused by PIC activation. Moreover, Lee et al. (2003) also reported that input from stretch-activated muscle afferents to small, low-conductance motoneurons, was more potent (i.e., greater gain in spikes/nA of current reaching the soma from synaptic current alone) in comparison with the same input to larger, presumably high-conductance motoneurons. This result fits with the greater amplification of simulated synaptic current in smaller versus larger motoneurons, although the possibility that low-conductance motoneurons possess smaller outward K+ currents compared with high conductance motoneurons should be considered (Lee and Heckman 1999).
There is one notable discrepancy between our results and the known differences between small and large motoneurons. The total current (i.e., synaptic and PIC) reaching the soma during muscle stretch is greater in low- versus high-conductance motoneurons (Lee et al. 2003). In contrast, our results show that the total current reaching the soma in the ranges reported by Lee et al. (2003) (i.e., 2–20 nA), will be similar for small-, intermediate-, and large-sized motoneurons for a given number of uniformly distributed excitatory synapses on the dendritic tree (Fig. 6B). The exact number of synapses activated on motoneurons of different sizes during muscle stretch is unknown. However, there is little difference in the passive component of the total current for different motoneuron types (Heckman and Binder 1988; Lee and Heckman 2000; Lee et al. 2003). Because the passive current/synapse reaching the soma is greater in the large motoneurons (Fig. 6A), this suggests that the number of synapses activated by muscle stretch may be inversely related to motoneuron size. Under this assumption, the total current reaching the soma in our models would be largest in small motoneurons because of the greater amplification of the passive current by PICs. Although this assumption has to be verified, it is a simple means of explaining the discrepancy between our simulations and the experimental data described by Lee et al. (2003). It is also possible that muscle stretch evokes a mixture of excitation and inhibition on the dendrites of large motoneurons (Lee et al. 2003). Under these circumstances, PIC activation in large motoneurons is likely to be reduced. Hence, under more physiological conditions, the greater amount of amplification observed in small motoneurons may also be caused by the presence of more inhibition on large motoneurons.
Amplification of synaptic currents in small-, intermediate-, and large-sized motoneurons is also dependent on the relative location of the hotspots and distributions of synapses. When synaptic inputs were restricted to the proximal or middle or distal one third of the dendritic tree, amplification of synaptic currents is greatest when the synaptic input and voltage-dependent channels are positioned in similar regions of the dendritic tree. This result is in agreement with similar studies of several classes of pyramidal cells, (Oviedo and Reyes 2005; Williams and Stuart 2003) and suggests that the same rules that govern the magnitude of amplification (i.e., the spatial relationship between the location of synaptic input and voltage-dependent channels on the dendritic tree) may apply to both multipolar and pyramidal-shaped neurons.
MOTONEURON RECRUITMENT
It is well established that, under most circumstances, increasing levels of excitatory synaptic drive to a motor pool leads to an orderly recruitment of motoneurons, from smallest to largest (Henneman and Mendell 1981). This orderly recruitment, commonly referred to as the Size Principle, is caused in part by differences in the amount of somatic current needed to activate motoneurons (rheobase). Small motoneurons, which typically innervate slow-twitch muscle fibers, require ~5 nA of current at the soma to be recruited, whereas larger motoneurons that typically innervate fast-twitch muscle fibers, require 15–20 nA of current at the soma (Gustafsson and Pinter 1984; Heckman and Binder 1988; Zengel et al. 1985). Lee et al. (2003) have suggested that larger PICs generated in small motoneurons compared with large motoneurons may contribute to the orderly recruitment of motoneurons. These simulations support this idea and suggest that the differences in hotspot location in motoneurons of different size may also contribute to the orderly sequence of motoneuron recruitment. At low levels of excitatory input generated by uniformly distributed synapses, small- and intermediate-sized motoneurons benefit the most from amplification of synaptic currents by PICs. Thus the bias toward recruitment of these motoneurons based solely on synaptic current (Fig. 6A) is enhanced. Our simulations also suggest that the orderly recruitment of motoneurons would occur when excitatory synaptic input is restricted to the proximal and middle one thirds of the dendritic tree (Fig. 7, A and C). This is in agreement with many studies that conclude that the orderly recruitment of motoneurons is highly preserved (Binder et al. 1996). However, there are some exceptions. Cutaneous stimulation can recruit fast-twitch motoneurons, presumably large motoneurons, without recruiting slow-twitch motoneurons, presumably small motoneurons (Garnett and Stephens 1981; Kanda et al. 1977; see however Clarke et al. 1993). The mechanisms responsible for this reversal in recruitment are partly caused by differences in the balance of inhibition and excitation received by motoneurons of different sizes. Our simulations suggest another mechanism. PICs are preferentially activated on large motoneurons in response to activation of distally distributed excitatory synapses. Although there is no anatomical evidence suggesting that cutaneous input preferentially innervates the distal dendrites of large motoneurons, the order in which motoneurons are recruited may depend on the distribution of the active synapses and their proximity to hotspots responsible for PICs.
Acknowledgments
The authors thank K. Fenrich for helpful comments on a previous version of the manuscript and D. Pace for programming assistance.
GRANTS
This work was supported by Canadian Institute for Health Research Grant 37765.
References
- Ballou EW, Smith WB, Anelli R, Heckman CJ. Measuring dendritic distribution of membrane proteins. J Neurosci Methods. 2006;156:257–266. doi: 10.1016/j.jneumeth.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Barrett JN, Crill WE. Specific membrane properties of cat motoneurons. J Physiol. 1974;239:301–324. doi: 10.1113/jphysiol.1974.sp010570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bernander Ö, Douglas RJ, Martin KAC, Koch C. Synaptic background activity influences spatiotemporal integration in single pyramidal cells. Proc Natl Acad Sci USA. 1991;88:11569–11573. doi: 10.1073/pnas.88.24.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MD, Heckman CJ, Powers RK. The physiological control of motoneuron activity. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. New York: Oxford; 1996. pp. 1–53. [Google Scholar]
- Booth V, Rinzel J, Kiehn O. Compartmental model of vertebrate motoneurons for Ca2+-dependent spiking and plateau potentials under pharmacological treatment. J Neurophysiol. 1997;78:3371–3385. doi: 10.1152/jn.1997.78.6.3371. [DOI] [PubMed] [Google Scholar]
- Brännström T. Quantitative synaptology of functionally different types of cat medial gastrocnemius α-motoneurons. J Comp Neurol. 1993;330:439–454. doi: 10.1002/cne.903300311. [DOI] [PubMed] [Google Scholar]
- Brännström T, Kellerth JO. Changes in synaptology of adult cat spinal α-motoneurons after axotomy. Exp Brain Res. 1998;118:1–13. doi: 10.1007/s002210050249. [DOI] [PubMed] [Google Scholar]
- Bras H, Destombes J, Gogan P, Tyc-Dumont S. The dendrites of single-brainstem motoneurons intracellularly labeled with horseradish peroxidase in the cat. An ultrastructural analysis of the synaptic covering and the microenvironment. Neuroscience. 1987;22:971–981. doi: 10.1016/0306-4522(87)92973-3. [DOI] [PubMed] [Google Scholar]
- Bui TV, Cushing S, Dewey D, Fyffe RE, Rose PK. Comparison of the morphological and electrotonic properties of Renshaw cells, Ia inhibitory interneurons, and motoneurons in the cat. J Neurophysiol. 2003;90:2900–2918. doi: 10.1152/jn.00533.2003. [DOI] [PubMed] [Google Scholar]
- Bui TV, Ter-Mikaelian M, Bedrossian D, Rose PK. Computational estimation of the distribution of L-type Ca2+ channels in motoneurons based on variable threshold of activation of persistent inward currents. J Neurophysiol. 2006;95:225–241. doi: 10.1152/jn.00646.2005. [DOI] [PubMed] [Google Scholar]
- Burke RE, Dum RP, Fleshman JW, Glenn LL, Lev-Tov A, O’Donovan MJ, Pinter MJ. An HRP study of the relation between cell size and motor unit type in cat ankle extensor motoneurons. J Comp Neurol. 1982;209:17–28. doi: 10.1002/cne.902090103. [DOI] [PubMed] [Google Scholar]
- Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol. 1996;372:465–485. doi: 10.1002/(SICI)1096-9861(19960826)372:3<465::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Averill DB, Berger AJ. Quantitaive analysis of the dendrites of cat phrenic motoneurons stained intracellularly with horseradish peroxidase. J Comp Neurol. 1985;230:91–101. doi: 10.1002/cne.902310108. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Clarke BD, Dacko SM, Cope TC. Cutaneous stimulation fails to alter motor unit recruitment in the decerebrate cat. J Neurophysiol. 1993;70:1433–1439. doi: 10.1152/jn.1993.70.4.1433. [DOI] [PubMed] [Google Scholar]
- Clements JD, Redman SJ. Cable properties of cat spinal motoneurones measured by combining voltage clamp, current clamp and intracellular staining. J Physiol. 1989;409:63–87. doi: 10.1113/jphysiol.1989.sp017485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol. 1987;255:68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- Davenport C, Margolis D, Powers RK, Detwiler P, Binder MD. Multiphoton fluorescence imaging of calcium fluxes in the dendrites of rat hypoglossal motoneurons. Soc Neurosci Abstr. 2003;499:6. [Google Scholar]
- ElBasiouny SM, Bennett DJ, Mushahwar VK. Simulation of dendritic Cav1.3 channels in cat lumbar motoneurons: spatial distribution. J Neurophysiol. 2005;94:3961–3974. doi: 10.1152/jn.00391.2005. [DOI] [PubMed] [Google Scholar]
- Fleshman JW, Segev I, Burke RE. Electrotonic architecture of type-identified α-motoneurons in the cat spinal cord. J Neurophysiol. 1988;60:60–85. doi: 10.1152/jn.1988.60.1.60. [DOI] [PubMed] [Google Scholar]
- Fukunishi Y, Nagase Y, Yoshida A, Moritani M, Honma S, Hirose Y, Shigenaga Y. Quantitative analysis of the dendritic architectures of cat hypoglossal motoneurons stained intracellularly with horseradish peroxidase. J Comp Neurol. 1999;405:345–358. [PubMed] [Google Scholar]
- Garnett R, Stephens JA. Changes in the recruitment threshold of motor units produced by cutaneous stimulation in man. J Physiol. 1981;311:463–473. doi: 10.1113/jphysiol.1981.sp013598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Armstrong S, Neuber-Hess M, Rose PK. Distribution of contacts from vestibulospinal axons on the dendrites of splenius motoneurons. J Comp Neurol. 2005;491:339–351. doi: 10.1002/cne.20699. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Pinter MJ. Relations among passive electrical properties of lumbar α-motoneurons of the cat. J Physiol. 1984;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman CJ, Binder MD. Analysis of the effective synaptic currents generated by homonymous Ia afferent fibers in motoneurons of the cat. J Neurophysiol. 1988;60:1946–1966. doi: 10.1152/jn.1988.60.6.1946. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2005;31:153–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell LM. Functional organization of motoneuron pool and its inputs. In: Brooks VB, editor. Handbook of Physiology, The Nervous System, Motor Control. Bethesda, MD: American Physiological Society; 1981. pp. 423–507. [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurons in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Lee RH. Fast amplification of dynamic synaptic inputs in spinal motoneurons in vivo. J Neurophysiol. 2006;96:2200–2206. doi: 10.1152/jn.00537.2006. [DOI] [PubMed] [Google Scholar]
- Kanda K, Burke RE, Walmsley B. Differential control of fast and slow twitch motor units in the decerebrate cat. Exp Brain Res. 1977;29:57–74. doi: 10.1007/BF00236875. [DOI] [PubMed] [Google Scholar]
- Kernell D, Zwaagstra B. Size and remoteness: two relatively independent parameters of dendrites, as studied for spinal motoneurones of the cat. J Physiol. 1989;413:233–254. doi: 10.1113/jphysiol.1989.sp017651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behaviour in spinal motoneurons in vivo. J Neurophysiol. 1999;82:2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. J Neurophysiol. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Lee RH, Kuo JJ, Jiang MC, Heckman CJ. Influence of active dendritic currents on input-output processing in spinal motoneurons in vivo. J Neurophysiol. 2003;89:27–39. doi: 10.1152/jn.00137.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, Xu W. L-type calcium channels: the low down. J Neurophysiol. 2004;92:2633–2641. doi: 10.1152/jn.00486.2004. [DOI] [PubMed] [Google Scholar]
- Maltenfort MG, Hamm TM. Estimation of the electrical parameters of spinal motoneurons using impedance measurements. J Neurophysiol. 2004;92:1433–1444. doi: 10.1152/jn.00875.2003. [DOI] [PubMed] [Google Scholar]
- Müller W, Lux HD. Analysis of voltage-dependent membrane currets in spatially extended neurons from point-clamp data. J Neurophysiol. 1993;69:241–247. doi: 10.1152/jn.1993.69.1.241. [DOI] [PubMed] [Google Scholar]
- Oviedo H, Reyes AD. Variation of input-output properties along the somatodendritic axis of pyramidal neurons. J Neurosci. 2005;25:4985–4995. doi: 10.1523/JNEUROSCI.0562-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Summation of effective synaptic currents and firing rate modulation in cat spinal motoneurons. J Neurophysiol. 2000;83:483–500. doi: 10.1152/jn.2000.83.1.483. [DOI] [PubMed] [Google Scholar]
- Rall W. Handbook of Physiology. The Nervous System. Cellular Biology of Neurons. Bethesda, MD: American Physiological Society; 1977. Core conductor theory and cable properties of neurons; pp. 39–97. [Google Scholar]
- Rose PK, Keirstead SA, Vanner SJ. A quantitative analysis of the geometry of cat motoneurons innervating neck and shoulder muscles. J Comp Neurol. 1985;238:89–107. doi: 10.1002/cne.902390108. [DOI] [PubMed] [Google Scholar]
- Rose PK, Neuber-Hess M. Morphology and frequency of axon terminals on the somata, proximal dendrites, and distal dendrites of dorsal neck motoneurons in the cat. J Comp Neurol. 1991;307:259–280. doi: 10.1002/cne.903070208. [DOI] [PubMed] [Google Scholar]
- Rose PK, Vanner SJ. Differences in somatic and dendritic specific membrane resistivity of spinal motoneurons: an electrophysiological study of neck and shoulder motoneurons in the cat. J Neurophysiol. 1988;60:149–166. doi: 10.1152/jn.1988.60.1.149. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Properties of persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980;43:1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Segev I, Fleshman JW, Jr, Burke RE. Computer simulation of group Ia EPSPs using morphologically realistic models of cat α-motoneurons. J Neurophysiol. 1990;64:648–660. doi: 10.1152/jn.1990.64.2.648. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Gutman A, Hounsgaard J. Electrotonic structure of motoneurons in the spinal cord of the turtle: inferences for the mechanisms of bistability. J Neurophysiol. 2001;85:391–398. doi: 10.1152/jn.2001.85.1.391. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Enoka RM. Quantification of the factors that influence discharge correlation in model motor neurons. J Neurophysiol. 2004;91:796–814. doi: 10.1152/jn.00802.2003. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Kellerth JO. A quantitative light microscopic study of the dendrites of cat spinal α-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1981;202:571–583. doi: 10.1002/cne.902020409. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Newkirk GS, Powers RK, Binder MD. Distribution of Cav 1.2, Cav 1.3, Cav 2.1, and Cav 2.2 channels on the somata and dendrites of rat hypoglossal motoneurons. Soc Neurosci Abstr. 2005;750:11. [Google Scholar]
- Williams SR, Stuart GJ. Role of dendritic synapse location in the control of action potential output. Trends Neurosci. 2003;206:147–154. doi: 10.1016/S0166-2236(03)00035-3. [DOI] [PubMed] [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]
- Zhang M, Sukiasyan N, Møller M, Bezprozvanny I, Zhang H, Wienecke J, Hultborn H. Localization of L-type calcium channels Cav 1.3 in cat lumbar spinal cord—with emphasis on motoneurons. Neurosci Lett. 2006;407:42–47. doi: 10.1016/j.neulet.2006.07.073. [DOI] [PubMed] [Google Scholar]