Abstract
Accumulating basic and clinical data support the hypothesis that angiotensin receptor blockers have beneficial effects on glucose and lipid metabolism that are not shared by other classes of antihypertensive agents. These metabolic actions might only partially be shared by angiotensin-converting enzyme inhibitors. Specific benefits beyond those of other angiotensin receptor blockers have been claimed for telemesartan and, to a lesser extent, irbesartan based on a partial agonist action on PPAR-γ receptors. Although the evidence is strong in vitro, specific actions not shared by other angiotensin receptor blockers have not yet been convincingly demonstrated in vivo or in clinical trials. In many cases, a full range of doses has not been compared, and the apparent superiority of telmesartan could be an artifact of its higher receptor binding affinity, greater tissue penetration owing to lipophilicity, and longer half life.
Introduction: importance of the metabolic actions of antihypertensive agents
Many antihypertensive agents are efficacious and lack serious side effects; however, hypertension rarely occurs in isolation, and there is increasing interest in the impact of antihypertensive agents on common accompanying conditions. Insulin resistance and hyperlipidemia commonly occur along with hypertension, a cluster of conditions known as metabolic syndrome or prediabetes that leads to increased cardiovascular disease independent of the development of type 2 diabetes [1]. Although there is controversy over whether the separate risk factors comprising this syndrome have multiplicative or additive effects, there is agreement that they commonly occur together[2,3].
Metabolic syndrome is commonly treated with multiple agents targeting separate abnormalities, with multiple agents being needed for the tight control of each risk factor. Antihypertensives with beneficial metabolic effects could improve control of other risk factors, notably plasma glucose and lipids. Generally, thiazide diuretics and β-adrenergic receptor antagonists have slight adverse effects, whereas α1-adrenergic receptor antagonists and inhibitors of the renin-angiotensin system (RAS) elicit significant benefits [4–7].
Clinical trials comparing different classes of antihypertensive agents have produced conflicting results. Limitations of clinical studies include marked heterogeneity between subjects regarding medical history, diet, exercise levels and levels of risk factors other than blood pressure, which include tobacco use, psychiatric conditions such as depression, and educational and socioeconomic background. These factors also influence compliance with the prescribed therapeutic plan. Few studies have focused on hypertensive patients with metabolic syndrome, who are the most likely to benefit from antihypertensive agents with additional pharmacological actions. Preclinical trials in animal models overcome almost all of these limitations. Together with mechanistic studies at the cellular and molecular level, these laboratory studies provide the clearest insight into distinct actions of drugs. Previous laboratory studies of the metabolic effects of antihypertensives are few in number and many have significant problems. Most studies compared one or two antihypertensive agents, and failed to characterize dose–response relationships that can lead to misleading results. Furthermore, most studies used hypertensive models or metabolically disturbed animals, but seldom studied animals that were both hypertensive and metabolically abnormal, a combination of abnormalities closer to the typical clinical picture [8].
Metabolic effects of inhibiting the RAS
Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are increasingly being seen as the treatments of choice for hypertensive patients with metabolic syndrome. ACE inhibitors and ARBs have been shown to slightly improve insulin resistance without affecting circulating lipids or body weight [9]. Both ACE inhibitors and ARBs reduce the incidence of new cases of type 2 diabetes [10,11]. Possible mechanisms for this apparent antidiabetic effect include hemodynamic changes improving substrate delivery, cross-talk between angiotensin and insulin receptor signaling pathways, and prevention of the adverse pancreatic actions of angiotensin [12•].
A major difference between ACE inhibitors and ARBs is that ACE inhibitors have the additional property of increasing levels of the vasodilator peptide bradykinin. Treatment with a bradykinin receptor antagonist blocked the beneficial effects of the ACE inhibitor ramapril on insulin resistance in the fructose-fed rat model, suggesting that bradykinin was responsible for the beneficial effect [13]. Bradykinin receptor antagonist treatment did not attenuate the antihypertensive effect, suggesting a separation between the hemodynamic and metabolic actions of bradykinin. If bradykinin mediates the actions of ACE inhibitors, then ARBs should not affect glucose and lipid metabolism. By contrast, some investigators have reported that ACE inhibitors and ARBs have equal effects on metabolism, and that blockade of bradykinin receptors has no influence [14]. Consistent with the latter result, angiotensin has been proposed to contribute to insulin resistance and diabetes [10,12•]. Thus, the majority of evidence favors angiotensin inhibition as the most significant mechanism in the improvement in glucose and lipid metabolism. Surprisingly, the metabolic effects of renin inhibitors are currently unknown.
Metabolic actions of AT1 receptor antagonists
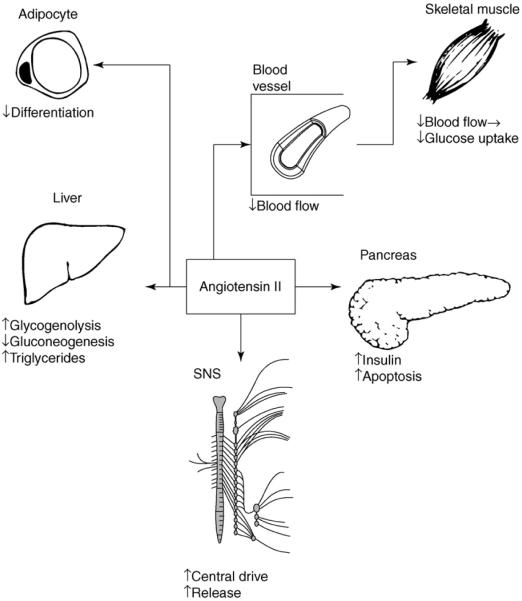
Angiotensin II affects glucose and lipid metabolism through multiple direct and indirect mechanisms, as shown in Figure 1 and discussed in detail below. Unfortunately, studies into the interactions between the RAS and glucose metabolism have produced an array of contradictory results. Angiotensin II appears to have opposing immediate or long-term effects. The major angiotensin receptor subtypes, AT1 and AT2, usually mediate opposite actions, such as AT1-mediated vasoconstricton and AT2-mediated vasodilation [15]. Blockade of AT1 receptors leads to compensatory increases in angiotensin II levels and the subsequent increased activation of AT2 receptors. Thus, some of the actions of AT1 antagonists might reflect increased AT2 receptor stimulation.
Figure 1.
Metabolic effects of AT1 receptors. The schematic shows the influence of angiotensin II on the adipose tissue, liver, skeletal muscle, pancreatic β-cells, and the SNS. Adipocytes are a major source of circulating angiotensinogen, along with the liver. In adipose tissue, AT1 receptors inhibit differentiation of new adipocytes, thereby reducing the ability of adipose tissue to take up glucose and lipid. In the liver, AT1 receptors increase glycogenolysis, which favors hyperglycemia, but have an opposing action of gluconeogenesis. AT1 receptors also promote secretion of triglycerides into the circulation. Hemodynamic effects link AT1 receptor-mediated vasoconstriction to reduced delivery of glucose and insulin to skeletal muscle. In the pancreas, AT1 receptors promote insulin secretion in the short-term, but chronic stimulation leads to apoptosis and a loss of function. Activation of the RAS ties into SNS overactivity through actions in the central nervous system and presynaptic effects to promote norepinephrine release. Increased catecholamines promote impairments in glucose and lipid metabolism through multiple mechanisms. Modified with permission from [7]; figure created using Smart-Draw 6 (Smart-Draw, San Diego, CA).
RAS in the endocrine pancreas
Acute application of angiotensin II can increase insulin secretion under both low glucose and high glucose [16,17]. By contrast, other reports on isolated rat and human pancreatic islets show that angiotensin II impairs the first phase of glucose-stimulated insulin secretion [18–20]. Angiotensin II also impairs insulin biosynthesis and promotes β cell apoptosis [21]. Thus, despite its acute action to stimulate release of insulin, the long-term effects of angiotensin II on the pancreas promote the development of diabetes. We speculate that, in vivo, vasoconstriction induced by angiotensin II acutely reduces the supply of glucose to the islets, thereby increasing insulin secretion, whereas, in the long-term, restricted blood flow promotes β cell apoptosis and limits β cell proliferation.
Consistent with the damaging long-term effects of angiotensin II on pancreatic function, inhibition of the RAS by either AT1 receptor antagonists or ACE inhibitors preserves insulin secretion and prevents the development of islet fibrosis and diabetes in the ZDF rat model of type 2 diabetes [12•]. Possible mechanisms include a reduction in oxidative damage and damaging cytokines, such as transforming growth factor-β. ACE inhibitors might yield additional effects by promoting insulin secretion through accumulation of bradykinin [22].
RAS in the liver
Acutely, angiotensin II reduces both gluconeogenesis in hepatocytes and hepatic glucose output [23]. Conversely, blockade of AT1 receptors in insulin-resistant fructose-fed rats increased hepatic glucose output [24]. Thus, the actions of angiotensin II oppose those of glucagon [25]. By contrast, angiotensin II promotes glycogenolysis in perfused liver [26], which favors hyperglycemia. It is not clear which of these hepatic actions predominate in intact systems. It appears likely that the main effects of angiotensin II on glucose metabolism are mediated outside the liver. Angiotensin II has unequivocally adverse effects on lipid metabolism: infusions increase plasma triglycerides by promoting triglyceride synthesis [27]. Conversely, AT1 receptor antagonists ameliorate hypertriglyceridemia and fatty liver [27].
Systemic actions of the RAS
The RAS also demonstrates important interactions with the sympathetic nervous system (SNS) that might be relevant to metabolism [28]. Angiotensin II acts within the brain to increase SNS activity along with other unfavorable cardiovascular changes, and this mechanism is a primary factor in experimental models of hypertension [29]. Many ARBs are hydrophilic and do not cross the blood–brain barrier. However, telmisartan is sufficiently lipophilic to penetrate the brain and block the sympathoexcitatory effects of angiotensin II in the central nervous system. ARBs can also act directly on catecholaminergic nerve terminals to reduce the release of norepinephrine and on the adrenal medulla to reduce secretion of epinephrine [30]. Even a modest component of sympathoinhibition can contribute to favorable changes in glucose and lipid metabolism [31].
The hemodynamic effects of ARBs also contribute to their effects on glucose and lipid metabolism, because vasodilatation can facilitate disposal of glucose and lipids in peripheral tissues [32]. For example, telmisartan could have enhanced vascular effects owing to its lipophilicity. Relative to an equivalent dose of the hydrophilic ARB losartan, telmisartan improved nitric oxide release from the endothelium and reduced vascular remodeling in stroke-prone spontaneously hypertensive rats, a model of severe hypertension [33].
RAS and adipose tissue
Adipocytes are a major source of angiotensinogen in the circulation [34]. Angiotensinogen secretion increases with rising adipose mass, and thus the adipose-derived RAS might contribute to hypertension in obesity. Angiotensin II inhibits adipocyte differentiation through AT1 receptors while promoting differentiation via AT2 receptors [35]. Mice with a genetic deletion of AT2 receptors have decreased adiposity [36•]. Conversely, according to at least one report, blockade of AT1 receptors prevents differentiation of new adipocytes in obese rats [37].
A novel action of several AT1 antagonists is partial agonist activity at the peroxisome proliferator-activated receptor γ (PPAR-γ) — a nuclear receptor expressed primarily in adipose tissue [38•,39,40]. PPAR-γ subtypes are activated by fatty acids or prostaglandins in vivo, which allows them to act as transcription factors that regulate the expression of key genes by binding to peroxisome proliferator response elements within upstream DNA sequences in promoter regions. PPAR-γ regulates the gene expression of proteins involved in the differentiation of adipocytes from precursor fibroblast-like cells and the storage of fatty acids. All PPARs dimerize with the retinoid X receptor and bind to specific response elements within DNA to activate target genes.
The most potent PPAR-γ activity is displayed by telmisartan and, to a lesser extent, irbesartan [39,41]. In a mouse embryonic fibroblast cell line (3T3-L1), telmisartan was shown to activate two target genes of PPAR-γ receptors: phosphoenolpyruvate carboxykinase-1 (PEPCK-C) and acetyl coenzyme A carboxylase 2 (ACC2). These genes are markers of the differentiation of fibroblasts into adipocytes, which is promoted by PPAR-γ agonists. The metabolic actions of telmisartan in vivo are dependent upon the presence of an intact CD36 protein, a fatty acid transporter active in adipocytes and regulated by PPAR-γ [42]. This suggests that the PPAR-γ-regulated gene critically activated by telmisartan is CD36, resulting in enhanced clearance of fatty acids from the bloodstream into adipocytes. Presumably, reduced availability of free fatty acids will reduce ectopic storage of fat in non-adipose tissues, which could be a major contributor to insulin resistance [35].
Another study showed that telmisartan reduced triglyceride accumulation in muscle and liver, and reduced visceral adiposity, in dietary obese mice [43]. This latter effect was attributed to an increase in metabolic rate; however, there is no evidence for an anti-obesity action of telmisartan in humans.
Adiponectin is a major secretory product of adipocytes and acts to improve insulin sensitivity and lessen inflammation. Angiotensin II acting through AT1 receptors decreases circulating adiponectin [44], which contributes to insulin resistance and adipose tissue inflammation. Conversely, the AT1 receptor antagonist candesartan and the ACE inhibitor ramapril both increase adiponectin in humans, which might contribute to the metabolic impact of this class of drug [45,46]. Telmisartan has the same effect as other AT1 receptor antagonists [47], and so there is no reason to link the activity of telmisartan at PPAR-γ to its effects on adiponectin. However, the greater lipophilicity of telmisartan might allow it to penetrate more completely into adipose tissue, giving it improved access.
Conclusions: does a PPAR-γ agonist action of telmisartan account for its beneficial cardiometabolic effects?
The concept of an agent with dual PPAR-γ agonist and AT1 receptor antagonist actions is indeed promising [48], particularly from the standpoint of synergistic metabolic actions. However, there are several limitations that should be noted. Firstly, the major action of the glitazones and other antidiabetic PPAR-γ agonists in vivo is the differentiation of new adipocytes, particularly in subcutaneous regions, and a reduction in the size of visceral adipocytes.
Telmisartan appears to have the opposite action, reducing the formation of new fat cells [35] and leading, in some studies, to reduced subcutaneous fat [43]. Secondly, PPAR-γ effects are elicited only by micromolar concentrations of telmisartan, whereas low nanomolar concentrations are sufficient to block AT1 receptors [49]. Thus, telmisartan is >1000-fold more potent as an AT1 receptor antagonist than as a PPAR-γ agonist. Furthermore, the action of telmisartan to promote adipocyte differentiation is shared by other AT1 receptor antagonists. Thus, most of the effect of telmisartan on metabolism is likely to be mediated by blockade of AT1 receptors, together with compensatory overstimulation of AT2 receptors as angiotensin II levels rise during chronic AT1 blockade. Nonetheless, some contribution of a synergistic PPAR-γ effect seems likely.
However, another limitation at the present time is that the PPAR-γ-activating action of telmisartan has yet to be demonstrated in human cells [50]. This could be significant, as the role of the RAS might differ between rodent and human adipose tissues [50]. By contrast, the metabolic effects of angiotensin II and ARBs in the pancreas, liver and autonomic nervous system have been shown to operate in humans as well as in rodents. The effectiveness of telmisartan might be adequately explained by its greater potency, longer half-life and greater lipophilicity. However, the synergy between PPAR-γ activation and angiotensin receptor blockade is clear from preclinical data. Therefore, combined trials of thiazolidinediones and ARBs for the prevention or treatment of diabetes in hypertension might be warranted.
Update
Several important new articles have appeared in the past few months. Telmisartan increased leptin levels in diabetic hypertensives, which could have contributed to improved insulin sensitivity in the absence of changes in body weight [51]. Additional clinical trials confirmed the ability of telmisartan to reduce insulin resistance and fasting insulin levels [52,53], and improve pancreatic β cell function [53].
Telmisartan has now been shown for the first time to induce differentiation of human adipocytes [54••]. Furthermore, in human hepatoma cells, telmisartan has been shown to reduce levels of C-reactive protein expression through a specific PPAR-γ agonist action [55]. This action was not shared by candesartan, an ARB that lacks PPAR-γ effects, but was blocked by a selective PPAR-γ antagonist. Importantly, these are the first clear demonstrations of PPAR-γ actions of telmisartan in human cells. The data in hepatoma cells imply that telmisartan may have anti-inflammatory effects that reduce vascular injury, independent of blood pressure lowering. In addition, telmisartan might have a direct suppressive action on lymphocyte expression of pro-inflammatory surface proteins [56].
Telmisartan has now been shown to retard the progression of atherosclerosis in the apolipoprotein-E-deficient mouse model through an antioxidant action [57]. Conversely, other investigators have questioned the relevance of PPAR-γ agonist actions in the effects of telmisartan on insulin sensitivity, noting that effects are much greater in cell-free systems than in intact cells or in vivo [58].
Acknowledgements
The authors are supported by HL44514 from the NIH.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. The metabolic syndrome: requiescat in pace. Clin Chem. 2005;51:931–938. doi: 10.1373/clinchem.2005.048611. [DOI] [PubMed] [Google Scholar]
- 4.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study) J Hypertens. 2003;21:1563–1574. doi: 10.1097/01.hjh.0000084723.53355.76. [DOI] [PubMed] [Google Scholar]
- 5.Velliquette RA, Ernsberger P. Contrasting metabolic effects of antihypertensive agents. J Pharmacol Exp Ther. 2003;307:1104–1111. doi: 10.1124/jpet.103.054221. [DOI] [PubMed] [Google Scholar]
- 6.Brook RD. Mechanism of differential effects of antihypertensive agents on serum lipids. Curr Hypertens Rep. 2000;2:370–377. doi: 10.1007/s11906-000-0040-0. [DOI] [PubMed] [Google Scholar]
- 7.Ernsberger P, Koletsky RJ. Metabolic effects of antihypertensive agents: role of sympathoadrenal and renin-angiotensin systems. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:245–258. doi: 10.1007/s00210-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 8.Koletsky RJ, Velliquette RA, Ernsberger P. The role of I(1)-imidazoline receptors and alpha(2)-adrenergic receptors in the modulation of glucose and lipid metabolism in the SHROB model of metabolic syndrome X. Ann N Y Acad Sci. 2003;1009:251–261. doi: 10.1196/annals.1304.031. [DOI] [PubMed] [Google Scholar]
- 9.Bernobich E, De Angelis L, Lerin C, Bellini G. The role of the angiotensin system in cardiac glucose homeostasis: therapeutic implications. Drugs. 2002;62:1295–1314. doi: 10.2165/00003495-200262090-00002. [DOI] [PubMed] [Google Scholar]
- 10.De Champlain J. Do angiotensin II antagonists provide benefits beyond blood pressure reduction? Adv Ther. 2005;22:117–136. doi: 10.1007/BF02849883. [DOI] [PubMed] [Google Scholar]
- 11.Opie LH, Schall R. Old antihypertensives and new diabetes. J Hypertens. 2004;22:1453–1458. doi: 10.1097/01.hjh.0000133732.24501.9e. [DOI] [PubMed] [Google Scholar]
- 12•.Jandeleit-Dahm KA, Tikellis C, Reid CM, Johnston CI, Cooper ME. Why blockade of the renin-angiotensin system reduces the incidence of new-onset diabetes. J Hypertens. 2005;23:463–473. doi: 10.1097/01.hjh.0000160198.05416.72. [DOI] [PubMed] [Google Scholar]; Reviews clinical trial data as well as advancing possible mechanisms for the anti-diabetic actions of ARBs and ACE inhibitors in experimental models, including hemodynamic effects and alterations in glucose transport and insulin signaling mediated by inhibition of the RAS. The anti-inflammatory actions of ARBs and ACE inhibitors are also reviewed. All of these effects are accounted for in terms of interfering with the actions of angiotensin II.
- 13.Erlich Y, Rosenthal T. Contribution of bradykinin to the beneficial effects of ramipril in the fructose-fed rat. J Cardiovasc Pharmacol. 1998;31:581–584. doi: 10.1097/00005344-199804000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Ura N, Higashiura K, Shimamoto K. The mechanisms of insulin sensitivity improving effects of angiotensin converting enzyme inhibitor. Immunopharmacology. 1999;44:153–159. doi: 10.1016/s0162-3109(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 15.Cosentino F, Savoia C, De Paolis P, Francia P, Russo A, Maffei A, Venturelli V, Schiavoni M, Lembo G, Volpe M. Angiotensin II type 2 receptors contribute to vascular responses in spontaneously hypertensive rats treated with angiotensin II type 1 receptor antagonists. Am J Hypertens. 2005;18:493–499. doi: 10.1016/j.amjhyper.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Gletsu N, Doan TN, Cole J, Sutliff RL, Bernstein KE. Angiotensin II-induced hypertension in mice caused an increase in insulin secretion. Vascul Pharmacol. 2005;42:83–92. doi: 10.1016/j.vph.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Ramracheya RD, Muller DS, Wu Y, Whitehouse BJ, Huang GC, Amiel SA, Karalliedde J, Viberti G, Jones PM, Persaud SJ. Direct regulation of insulin secretion by angiotensin II in human islets of Langerhans. Diabetologia. 2006;49:321–331. doi: 10.1007/s00125-005-0101-7. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson PO, Berne C, Jansson L. Angiotensin II and the endocrine pancreas: effects on islet blood flow and insulin secretion in rats. Diabetologia. 1998;41:127–133. doi: 10.1007/s001250050880. [DOI] [PubMed] [Google Scholar]
- 19.Leung PS, Carlsson PO. Pancreatic islet renin angiotensin system: its novel roles in islet function and in diabetes mellitus. Pancreas. 2005;30:293–298. doi: 10.1097/01.mpa.0000158028.76666.76. [DOI] [PubMed] [Google Scholar]
- 20.Tahmasebi M, Puddefoot JR, Inwang ER, Vinson GP. The tissue renin-angiotensin system in human pancreas. J Endocrinol. 1999;161:317–322. doi: 10.1677/joe.0.1610317. [DOI] [PubMed] [Google Scholar]
- 21.Chu KY, Lau T, Carlsson PO, Leung PS. Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes. 2006;55:367–374. doi: 10.2337/diabetes.55.02.06.db05-1022. [DOI] [PubMed] [Google Scholar]
- 22.Damas J, Garbacki N, Lefebvre PJ. The kallikrein-kinin system, angiotensin converting enzyme inhibitors and insulin sensitivity. Diabetes Metab Res Rev. 2004;20:288–297. doi: 10.1002/dmrr.489. [DOI] [PubMed] [Google Scholar]
- 23.Assimacopoulos-Jeannet FD, Blackmore PF, Exton JH. Studies of the interaction between glucagon and alpha-adrenergic agonists in the control of hepatic glucose output. J Biol Chem. 1982;257:3759–3765. [PubMed] [Google Scholar]
- 24.Hsieh PS, Tai YH, Loh CH, Shih KC, Cheng WT, Chu CH. Functional interaction of AT1 and AT2 receptors in fructose-induced insulin resistance and hypertension in rats. Metabolism. 2005;54:157–164. doi: 10.1016/j.metabol.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Morgan NG, Exton JH, Blackmore PF. Angiotensin II inhibits hepatic cAMP accumulation induced by glucagon and epinephrine and their metabolic effects. FEBS Lett. 1983;153:77–80. doi: 10.1016/0014-5793(83)80122-7. [DOI] [PubMed] [Google Scholar]
- 26.Reinhart PH, Taylor WM, Bygrave FL. Studies on alpha-adrenergic-induced respiration and glycogenolysis in perfused rat liver. J Biol Chem. 1982;257:1906–1912. [PubMed] [Google Scholar]
- 27.Ran J, Hirano T, Adachi M. Angiotensin II type 1 receptor blocker ameliorates overproduction and accumulation of triglyceride in the liver of Zucker fatty rats. Am J Physiol Endocrinol Metab. 2004;287:E227–E232. doi: 10.1152/ajpendo.00090.2004. [DOI] [PubMed] [Google Scholar]
- 28.Mancia G, Dell'oro R, Quarti-Trevano F, Scopelliti F, Grassi G. Angiotensin-sympathetic system interactions in cardiovascular and metabolic disease. J Hypertens Suppl. 2006;24:S51–S56. doi: 10.1097/01.hjh.0000220407.84363.fb. [DOI] [PubMed] [Google Scholar]
- 29.Fink GD. Long-term sympatho-excitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- 30.Baiardi G, Bregonzio C, Jezova M, Armando I, Saavedra JM. Angiotensin II AT1 receptor blockade prolongs the lifespan of spontaneously hypertensive rats and reduces stress-induced release of catecholamines, glucocorticoids, and vasopressin. Ann N Y Acad Sci. 2004;1018:131–136. doi: 10.1196/annals.1296.015. [DOI] [PubMed] [Google Scholar]
- 31.Ernsberger P, Koletsky RJ, Friedman JE. Contribution of sympathetic nervous system overactivity to cardiovascular and metabolic disease. Rev Contemp Pharmacother. 1998;9:411–428. [Google Scholar]
- 32.Henriksen EJ, Jacob S. Angiotensin converting enzyme inhibitors and modulation of skeletal muscle insulin resistance. Diabetes Obes Metab. 2003;5:214–222. doi: 10.1046/j.1463-1326.2003.00265.x. [DOI] [PubMed] [Google Scholar]
- 33.Takai S, Kirimura K, Jin D, Muramatsu M, Yoshikawa K, Mino Y, Miyazaki M. Significance of angiotensin II receptor blocker lipophilicities and their protective effect against vascular remodeling. Hypertens Res. 2005;28:593–600. doi: 10.1291/hypres.28.593. [DOI] [PubMed] [Google Scholar]
- 34.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AM, Janke J, Gorzelniak K, Engeli S, Luft FC. Angiotensin blockade prevents type 2 diabetes by formation of fat cells. Hypertension. 2002;40:609–611. doi: 10.1161/01.hyp.0000036448.44066.53. [DOI] [PubMed] [Google Scholar]
- 36•.Yvan-Charvet L, Even P, Bloch-Faure M, Guerre-Millo M, Moustaid-Moussa N, Ferre P, Quignard-Boulange A. Deletion of the angiotensin type 2 receptor (AT2R) reduces adipose cell size and protects from diet-induced obesity and insulin resistance. Diabetes. 2005;54:991–999. doi: 10.2337/diabetes.54.4.991. [DOI] [PubMed] [Google Scholar]; This is convincing evidence of a role for AT2 receptors in the regulation of adipocyte function. This is significant because AT1 blockade increases AT2 receptor stimulation indirectly by elevating levels of angiotensin II. Thus, ARBs might affect adipocyte function not only through blockade of AT1 receptors, but also through indirect stimulation of AT2 receptors.
- 37.Di Filippo C, Lampa E, Tufariello E, Petronella P, Freda F, Capuano A, D'Amico M. Effects of irbesartan on the growth and differentiation of adipocytes in obese zucker rats. Obes Res. 2005;13:1909–1914. doi: 10.1038/oby.2005.235. [DOI] [PubMed] [Google Scholar]
- 38•.Kurtz TW. New treatment strategies for patients with hypertension and insulin resistance. Am J Med. 2006;119:S24–S30. doi: 10.1016/j.amjmed.2006.01.011. [DOI] [PubMed] [Google Scholar]; This is a thorough and up-to-date review of telmisartan as both a PPAR-γ agonist and as an ARB, and the possible role of this dual mechanism of action on the prevention of diabetes and cardiovascular disease in humans.
- 39.Kurtz TW. Treating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator. Acta Diabetol. 2005;42(Suppl 1):S9–S16. doi: 10.1007/s00592-005-0176-0. [DOI] [PubMed] [Google Scholar]
- 40.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 41.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 42.Li Y-Q, Ji H, Ding DY, Ye XL. Metabolic effects of telmisartan in spontaneously hypertensive rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 2006;373:264–270. doi: 10.1007/s00210-006-0069-y. [DOI] [PubMed] [Google Scholar]
- 43.Araki K, Masaki T, Katsuragi I, Tanaka K, Kakuma T, Yoshimatsu H. Telmisartan prevents obesity and increases the expression of uncoupling protein 1 in diet-induced obese mice. Hypertension. 2006;48:51–57. doi: 10.1161/01.HYP.0000225402.69580.1d. [DOI] [PubMed] [Google Scholar]
- 44.Ran J, Hirano T, Fukui T, Saito K, Kageyama H, Okada K, Adachi M. Angiotensin II infusion decreases plasma adiponectin level via its type 1 receptor in rats: an implication for hypertension-related insulin resistance. Metabolism. 2006;55:478–488. doi: 10.1016/j.metabol.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Koh KK, Quon MJ, Han SH, Chung WJ, Lee Y, Shin EK. Anti-inflammatory and metabolic effects of candesartan in hypertensive patients. Int J Cardiol. 2006;108:96–100. doi: 10.1016/j.ijcard.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 46.Yenicesu M, Yilmaz MI, Caglar K, Sonmez A, Eyileten T, Acikel C, Kilic S, Bingol N, Bingol S, Vural A. Blockade of the renin-angiotensin system increases plasma adiponectin levels in type-2 diabetic patients with proteinuria. Nephron Clin Pract. 2005;99:c115–c121. doi: 10.1159/000083929. [DOI] [PubMed] [Google Scholar]
- 47.Clasen R, Schupp M, Foryst-Ludwig A, Sprang C, Clemenz M, Krikov M, Thone-Reineke C, Unger T, Kintscher U. PPARgamma-activating angiotensin type-1 receptor blockers induce adiponectin. Hypertension. 2005;46:137–143. doi: 10.1161/01.HYP.0000168046.19884.6a. [DOI] [PubMed] [Google Scholar]
- 48.Doggrell SA. Telmisartan – killing two birds with one stone. Expert Opin Pharmacother. 2004;5:2397–2400. doi: 10.1517/14656566.5.11.2397. [DOI] [PubMed] [Google Scholar]
- 49.Kakuta H, Sudoh K, Sasamata M, Yamagishi S. Telmisartan has the strongest binding affinity to angiotensin II type 1 receptor: comparison with other angiotensin II type 1 receptor blockers. Int J Clin Pharmacol Res. 2005;25:41–46. [PubMed] [Google Scholar]
- 50.Sharma AM. Telmisartan: the ACE of ARBs? Hypertension. 2006;47:822–823. doi: 10.1161/01.HYP.0000215184.00915.62. [DOI] [PubMed] [Google Scholar]
- 51.Usui I, Fujisaka S, Yamazaki K, Takano A, Murakami S, Yamazaki Y, Urakaze M, Hachiya H, Takata M, Senda S, Iwata M, Satoh A, Sasaoka T, Ak ND, Temaru R, Kobayashi M. Telmisartan reduced blood pressure and HOMA-IR with increasing plasma leptin level in hypertensive and type 2 diabetic patients. Diabetes Res Clin Pract. 2007 doi: 10.1016/j.diabres.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Benndorf RA, Rudolph T, Appel D, Schwedhelm E, Maas R, Schulze F, Silberhorn E, Boger RH. Telmisartan improves insulin sensitivity in nondiabetic patients with essential hypertension. Metabolism. 2006;55:1159–1164. doi: 10.1016/j.metabol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 53.Nagel JM, Tietz AB, Goke B, Parhofer KG. The effect of telmisartan on glucose and lipid metabolism in nondiabetic, insulin-resistant subjects. Metabolism. 2006;55:1149–1154. doi: 10.1016/j.metabol.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 54••.Janke J, Schupp M, Engeli S, Gorzelniak K, Boschmann M, Sauma L, Nystrom FH, Jordan J, Luft FC, Sharma AM. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activation. J Hypertens. 2006;24:1809–1816. doi: 10.1097/01.hjh.0000242405.68461.84. [DOI] [PubMed] [Google Scholar]; This article is of outstanding interest because it demonstrates for the first time that telmisartan regulates the differentiation of human adipocytes.
- 55.Yoshida T, Yamagishi S, Nakamura K, Matsui T, Imaizumi T, Takeuchi M, Koga H, Ueno T, Sata M. Telmisartan inhibits AGE-induced C-reactive protein production through downregulation of the receptor for AGE via peroxisome proliferator-activated receptor-gamma activation. Diabetologia. 2006 doi: 10.1007/s00125-006-0437-7. [DOI] [PubMed] [Google Scholar]
- 56.Link A, Lenz M, Legner D, Bohm M, Nickenig G. Telmisartan inhibits beta2-integrin MAC-1 expression in human T-lymphocytes. J Hypertens. 2006;24:1891–1898. doi: 10.1097/01.hjh.0000242415.73406.17. [DOI] [PubMed] [Google Scholar]
- 57.Takaya T, Kawashima S, Shinohara M, Yamashita T, Toh R, Sasaki N, Inoue N, Hirata K, Yokoyama M. Angiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Atherosclerosis. 2006;186:402–410. doi: 10.1016/j.atherosclerosis.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Erbe DV, Gartrell K, Zhang YL, Suri V, Kirincich SJ, Will S, Perreault M, Wang S, Tobin JF. Molecular activation of PPARgamma by angiotensin II type 1-receptor antagonists. Vascul Pharmacol. 2006;45:154–162. doi: 10.1016/j.vph.2006.05.002. [DOI] [PubMed] [Google Scholar]