Abstract
Background
Hypertension often coexists with hyperlipidemia, insulin resistance, and glucose intolerance in metabolic syndrome. Allylmercaptocaptopril is a conjugate of the angiotensin-converting enzyme inhibitor captopril with allicin, an active principle in garlic with multiple beneficial actions on metabolic-syndrome abnormalities. We sought to test the hypothesis that the conjugation of allicin to captopril may confer additional therapeutic actions in metabolic disease.
Methods
We compared allylmercaptocaptopril (53.5 mg/kg/day orally for 60 days) to an equimolar dose of captopril (40 mg/kg/day) in the spontaneously hypertensive, obese rat (SHROB) model.
Results
Allylmercaptocaptopril prevented progressive weight gain, without a detectable effect on food intake. Both captopril and allylmercaptocaptopril lowered blood pressure, but allylmercaptocaptopril was more effective. Allylmercaptocaptopril, but not captopril, improved cardiac hypertrophy, as indicated by heart weight and ventricular-wall thickness. Allylmercaptocaptopril improved, whereas captopril impaired, oral glucose tolerance after a fast. Triglycerides were decreased by both captopril and allylmercaptocaptopril. Total cholesterol and non-HDL cholesterol were reduced by captopril but not by allylmercaptocaptopril. The SHROB rats developed severe glomerulosclerosis and renal failure. Allylmercaptocaptopril showed significant nephro-protection, as indicated by reductions in urinary protein loss, urinary protein-to-creatinine ratio, and plasma creatinine. Captopril showed the same trends and also prevented the decline of creatinine clearance. Finally, both allylmercaptocaptopril and captopril reduced the basal level of lipolysis in isolated abdominal adipocytes, and restored the response to catecholamine stimulation.
Conclusions
Both captopril and allylmercaptocaptopril are effective in attenuating multiple abnormalities of metabolic syndrome. Allylmercaptocaptopril may have additional effectiveness on improving glucose tolerance, further lowering blood pressure, reducing cardiac hypertrophy, preventing weight gain, and protecting against renal disease.
Keywords: Angiotensin-converting enzyme inhibitors, obesity, glomerulosclerosis, adipocytes, lipolysis
Many therapeutic agents have established efficacy in hypertension. However, hypertension rarely occurs in isolation. Metabolic syndrome is a cluster of abnormalities including hypertension, insulin resistance, hyperlipidemia, glucose intolerance, and abdominal obesity. This syndrome frequently precedes type 2 diabetes and atherosclerosis. Antihypertensives differ in their metabolic effects. Human studies are not in agreement, but in general, thiazide diuretics and β-adrenergic antagonists have slightly adverse effects, long-acting calcium-channel antagonists have inconsistent effects, and α1-adrenergic antagonists and angiotensin-converting enzyme (ACE) inhibitors have positive effects on glucose and lipid homeostasis.1
The spontaneously hypertensive, obese rat (SHROB; the Koletsky rat) is a unique animal model of metabolic syndrome, with genetic obesity superimposed on a background of genetic hypertension.2,3 The obese phenotype results from a nonsense mutation in the leptin receptor gene, designated fak, which is a naturally occurring knockout of all forms of the leptin receptor.4 The SHROB rat develops severe glomerulosclerosis, which progresses to spontaneous renal failure and death between 10–14 months of age.2,5,6 The ACE inhibitor captopril was shown to have a protective effect in humans and animal models with glomerulosclerosis,7–9 although this agent has not been tested in the SHROB model.
Recent studies in both humans and animals identified positive effects on blood pressure, glucose, and lipid metabolism by garlic and its primary active principle allicin.10 –13 In particular, allicin ameliorated hypertension, insulin resistance, and hypertriglyceridemia, and prevented weight gain in a fructose-fed rat model of metabolic syndrome.11,12 Unfortunately, allicin is only produced by freshly crushed garlic and is temperature-sensitive, and its activity decays with time. Allicin can covalently react with captopril to form allylmercaptocaptopril, in which the allyl moiety is linked via a disulfide bond to captopril.14,15 Studies in Sprague–Dawley rats fed a high-fructose diet suggest that allylmercaptocaptopril combined some of the therapeutic actions of captopril with those of the natural product allicin. Thus, we compared the effects of chronic therapy with allylmercaptocaptopril and captopril on blood pressure, measures of glucose and lipid metabolism, and markers of renal function in the SHROB model of metabolic syndrome.
Methods
Adult male and female SHROB rats were obtained from a breeding colony maintained by continuous brother–sister mating since 1974,2 and were divided into three groups matched for age, sex, and body weight. Each group had roughly twice as many females as males. There were nine females and five males in the vehicle group, seven females and four males in the captopril group, and six females and three males in the allylmercaptocaptopril group. All drugs were dissolved in 20% ethanol, mixed with powdered chow, and pressed into pellets. Food intake was allowed ad libitum and monitored continuously to ensure dosage. Captopril was administrated at 40 mg/kg/day for 60 days. This dose was shown in the literature to be effective in lowering blood pressure in spontaneously hypertensive rats (SHR).16,17 Allylmercaptocaptopril was given at an equimolar dose of 53.5 mg/kg/day. Control vehicle-treated SHROB rats were fed normal chow. Food spillage, estimated at around 5%, was not taken into account. Every 2 weeks, tail–cuff blood pressure was determined between 12:00 PM and 4:00 PM after habituation of the animals to the testing procedure. Systolic blood pressures were averaged across three to five measurements.
After 60 days of treatment, an oral glucose tolerance test (OGTT; 6 g/kg glucose by oral gavage) was conducted after an 18-h fast, as previously described.2 Plasma insulin, glucagon, glucose, triglycerides, total cholesterol, free fatty acid (FFA), and creatinine were determined, as previously described.3 For determination of HDL and non-HDL fractions of cholesterol, HDL was selectively precipitated from plasma by the method of Vikari18 by adding 0.1 mL of a 10% polyethyleneglycol 6000 solution in glycine buffer (pH 10.0) to 0.1 mL plasma. After incubation at room temperature for 10 min and on ice for 20 min, samples were centrifuged at 3400 g for 20 min. Supernatants were assayed for cholesterol by the colorimetric enzymatic method. We defined HDL cholesterol as total cholesterol minus non-HDL cholesterol, determined after selective precipitation.
Urine was collected for 24 h in metabolic cages, immediately after the glucose tolerance test and immediately before sacrifice. To assure hydration and to eliminate food contamination of urine, rats were provided with a 5% sucrose solution plus a vitamin and mineral mix (AIN 76, ICI Chemicals, Cleveland, OH) in place of chow. Rats were exposed to this liquid diet overnight for 2 weeks prior to urine collection, to eliminate the novelty effect. For the drug-treated groups, the appropriate daily dose was added. Urine protein was assayed by the method of Peterson.19
At the end of the experiment, animals were anesthetized with enflurane, and a 5-mL blood sample was obtained by cardiac puncture. At autopsy, we recorded naso–anal length, left-ventricular wall thickness, and weight of the following organs: liver, heart, and kidneys. Fat pads were dissected and weighed as follows: three visceral depots consisting of retroperitoneal, mesenteric, and gonadal (epididymal and myometrial), and one subcutaneous depot (subscapular).
Adipose tissue (4 g) was minced and digested in 20 mL of Krebs’ buffer containing 1 mg/mL collagenase (Type VIII, Sigma Chemical Co., St. Louis, MO) at 37°C with gentle shaking for 1 h. Floating cells were filtered through 0.25-mm mesh into a Krebs’ buffer containing 4% bovine serum albumin. Adipocytes were maintained by continuous perifusion at 37°C, as previously described.20 Packed cells (0.2 mL) were sealed within 0.6-mL Teflon chambers and perfused at 0.25 mL/min with Krebs’ buffer containing 1% bovine serum albumin and 0.02% adenine deaminase. After a 45-min washout period, fractions were collected at 4-min intervals, with the first four fractions designated for baseline glycerol release. At Fraction 5, cells were exposed to 10.0 μM isoproterenol for 4 min. Samples were analyzed for glycerol with the use of a standard enzymatic assay kit (Sigma Chemical Co.).
Creatinine clearance was calculated according to the formula: Urine Creatinine × Urine volume (Plasma Creatinine × 1440). All results are presented as mean ± standard error of the mean. Comparisons between groups were made using one-way or two-way analysis of variance, or analysis of variance with repeated measures, using Prism (GraphPad Software, San Diego, CA), with post hoc analyses by Neuman-Keuls test.
Results
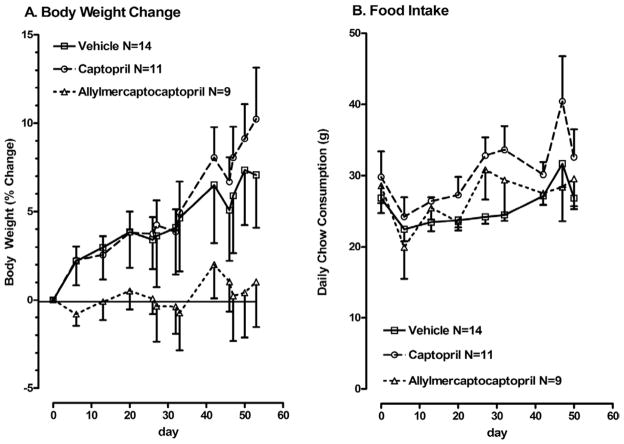
Each parameter in the vehicle control group was comparable to those in previous reports for SHROB rats of comparable age.2,3,6 Body weight and food intake are shown in Fig. 1. Allylmercaptocaptopril-treated SHROB rats did not gain weight during the treatment period, whereas the other two groups showed a significant increase in weight (P < .05, two-way analysis of variance with repeated measures), as expected from previous data for SHROB rats of this age. However, no corresponding change in daily food intake was observed, and there were no differences in food intake between groups (Fig. 1B). Cumulative food intake over the entire study was slightly increased in the captopril group relative to controls (6900 ± 135 kcal v 6160 ± 155 kcal, P < .05 according to Newman-Keuls test). Total cumulative caloric intake in the allylmercaptocaptopril group (6580 ± 260 kcal) did not differ significantly from either the vehicle control or the captopril group.
FIG. 1.
Body-weight changes (A) and daily food intake (B) in vehicle-fed SHROB rats and SHROB rats fed captopril or allylmercaptocaptopril. (A) Body weights are shown as percent change from baseline. Baseline body weights were 490 ± 17 SEM g for controls, 488 ± 13 SEM g for the captopril group, and 510 ± 26 SEM g for the allylmercaptocaptopril group. Two-way analysis of variance with repeated measures on body weight indicated a main effect of time (F = 8.8, P < .01) and a significant interaction between time and drug (F = 1.7, P = .02). (B) Food intake is shown in grams per day per animal. There were no changes over time and no differences between groups by two-way analysis of variance with repeated measures.
At the end of the study, body mass index as calculated from body weight and naso–anal length was reduced in the group treated with allylmercaptocaptopril relative to the other two groups (Table 1). However, there were no significant differences between groups in the weights of any of the individual fat pads, or the liver or kidney. In all three groups, the largest fat deposit was the subcutaneous fat from the subscapular region.
Table 1.
Characteristics of treated and untreated SHROB rats at termination of study
| Group | Control (N = 14) | Captopril (N = 11) | Allylmercaptocaptopril (N = 9) |
|---|---|---|---|
| Age (days) | 203 ± 9 | 198 ± 4 | 206 ± 9 |
| Final body weight (g) | 540 ± 17 | 530 ± 17 | 470 ± 28 |
| Body mass index** | 11.6 ± 0.24 | 11.1 ± 0.28 | 10.2 ± 0.50* |
| Retroperitoneal fat pad (g) | 27 ± 2 | 27 ± 2 | 29 ± 2 |
| Gonadal fat pad (g) | 17 ± 1 | 17 ± 1 | 18 ± 1 |
| Mesenteric fat (g) | 14 ± 1 | 14 ± 1 | 13 ± 1 |
| Subscapular fat pad (g) | 33 ± 2 | 35 ± 1 | 34 ± 4 |
| Liver weight (g) | 16 ± 1.3 | 17 ± 1.1 | 15 ± 0.8 |
| Kidney weight (g) | 2.25 ± 0.12 | 2.34 ± 0.54 | 2.33 ± 0.51 |
| Heart weight (g) | 1.29 ± 0.05 | 1.19 ± 0.02 | 1.08 ± 0.07* |
| Left-ventricular wall thickness (mm) | 4.11 ± 0.21 | 3.81 ± 0.11 | 3.38 ± 0.06* |
| Plasma creatinine (mg/dL) | 0.95 ± 0.027 | 0.89 ± 0.029 | 0.84 ± 0.033* |
| Creatinine clearance rate (mL/min) | 1.2 ± 0.10 | 1.5 ± 0.163 | 1.3 ± 0.22 |
Data are mean ± SEM.
Significantly different from control SHROB rats, P < .05 according to Newman-Keuls test;
Body mass index is defined as kg body weight/(m naso–anal length2). The mean naso–anal length was 216 mm and did not differ between groups.
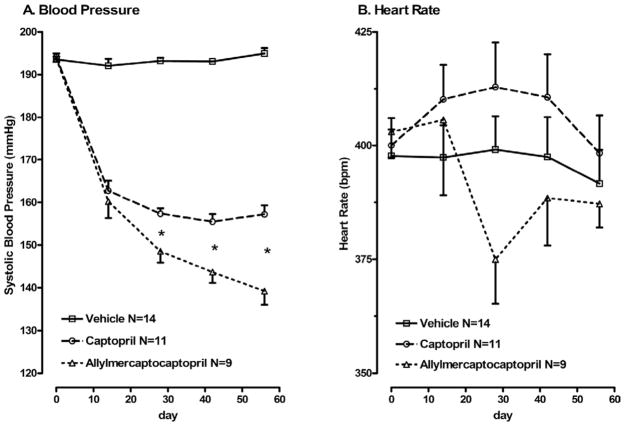
Blood pressure did not change over the course of treatment in control SHROB fed normal chow (Fig. 2A). In contrast, both captopril-treated and allylmercaptocaptopril-treated rats showed marked reductions in blood pressure. Blood pressure continued to fall with ongoing allylmercaptocaptopril treatment, so that by 30 days of treatment, blood pressure was lower in the allylmercaptocaptopril group than in the captopril group (P < .05, Newman-Keuls test). Heart rate showed no significant differences between any of the three groups (Fig. 2B).
FIG. 2.
Blood pressure (A) and heart rate (B) over the course of treatment in SHROB rats. Systolic blood pressure and heart rate were measured in lightly warmed, restrained SHROB rats by tail cuff, after habituation to the procedure using a Doppler detector built into the cuff. Two-way analysis of variance with repeated measures indicates significant effects of both drugs over time on systolic blood pressure. *Blood pressure in the allylmercaptocaptopril group was significantly lower than in the captopril group. There was no significant effect of either treatment on heart rate.
Treatment with allylmercaptocaptopril significantly improved measures of cardiac hypertrophy in SHROB rats (Table 1). Heart weight decreased, and left-ventricular wall thickness declined in parallel. Captopril tended to have similar effects, but these did not reach significance.
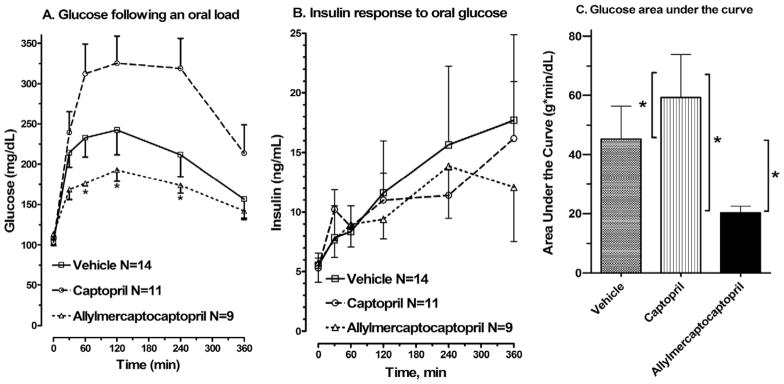
In response to an oral glucose load, vehicle control SHROB rats showed moderate glucose intolerance (Fig. 3A), relative to previously reported data for lean SHRs of the same age.2,6 Fasting glucose levels (Time 0) were within normal range and were not affected by treatment. Captopril produced a significant increase in the area under the curve compared with both control and allylmercaptocaptopril-treated SHROB rats. In contrast, SHROB rats treated with allylmercaptocaptopril showed a nearly twofold drop in area under the curve compared with control SHROB rats.
FIG. 3.
Results of oral glucose tolerance testing. Glucose (A), insulin (B), and cumulative area under the curve (AUC) for glucose (C) are shown. Tail blood was taken after an 18-h fast (Time 0) or at intervals after a glucose challenge. *In (A), significant differences from the captopril-treated group according to Newman-Keuls test; in (C), significant differences according to tests comparing all three groups.
Fasting insulin levels in all groups were about 10-fold higher than those previously reported for lean SHRs of the same age2 (Fig. 3B, Time 0). In response to an oral glucose load, all three groups showed a similar delayed and exaggerated rise in plasma insulin that was not affected by either of the drug treatments.
At the end of the study, blood samples were obtained under anesthesia stress. Under these conditions, plasma glucose was higher than in the conscious, mildly restrained state (Table 2; compare to Time 0 in Fig. 3A). The stress of anesthesia provoked a smaller rise in glucose in SHROB rats treated with allylmercaptocaptopril (4%) relative to vehicle controls (82% increase) or SHROB rats treated with captopril (37% increase). At the same time, anesthesia stress increased plasma insulin in SHROB rats treated with allylmercaptocaptopril, whereas insulin was lowered in vehicle controls and animals treated with captopril (Table 2; compare to Time 0 in Fig. 3B). Thus, the action of anesthesia to reduce insulin levels and thereby evoke hyperglycemia was prevented by allylmercaptocaptopril treatment.
Table 2.
Circulating factors in treated and untreated SHROB rats at termination of study
| Group | Vehicle (N = 14) | Captopril (N = 11) | Allylmercaptocaptopril (N = 9) |
|---|---|---|---|
| Glucose (mg/dL) | 188 ± 26 | 141 ± 15 | 119 ± 5* |
| Insulin (ng/mL) | 4.1 ± 0.9 | 5.0 ± 0.7 | 7.5 ± 1.3* |
| Glucagon (pg/mL) | 156 ± 10 | 147 ± 8 | 148 ± 12 |
| Triglycerides (mg/dL) | 329 ± 24 | 233 ± 27* | 210 ± 20* |
| Free fatty acids (mmol/L) | 0.91 ± 0.05 | 0.78 ± 0.12 | 0.64 ± 0.04* |
Data are mean ± SEM.
Significantly different from vehicle controls, P < .05 according to Newman-Keuls test.
Fasting glucagon levels were within the previously reported range21 and were approximately twofold elevated relative to lean SHRs of the same age (Table 2), confirming previous results. There was no effect of either drug treatment.
At the termination of the experiment, total cholesterol was slightly but significantly reduced in the captopril group relative to the vehicle control group but unchanged in the allylmercaptocaptopril group (Fig. 4). High-density lipoprotein cholesterol was not affected by either treatment, whereas non-HDL cholesterol was slightly reduced in SHROB rats treated with captopril.
FIG. 4.
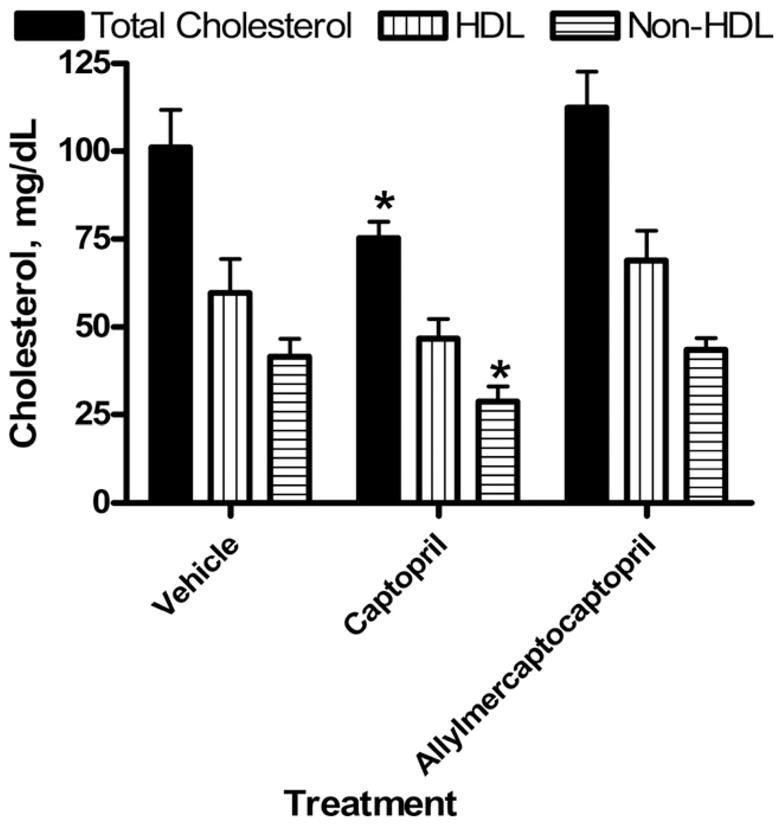
Cholesterol levels after 60 days of treatment with captropril or allylmercaptocaptopril. Total cholesterol was compared with non-HDL cholesterol, isolated after selective precipitation of HDL by standard methods. High-density lipoprotein cholesterol was estimated as the difference between total and non-HDL values. *Significant differences from control group, according to Newman-Keuls test.
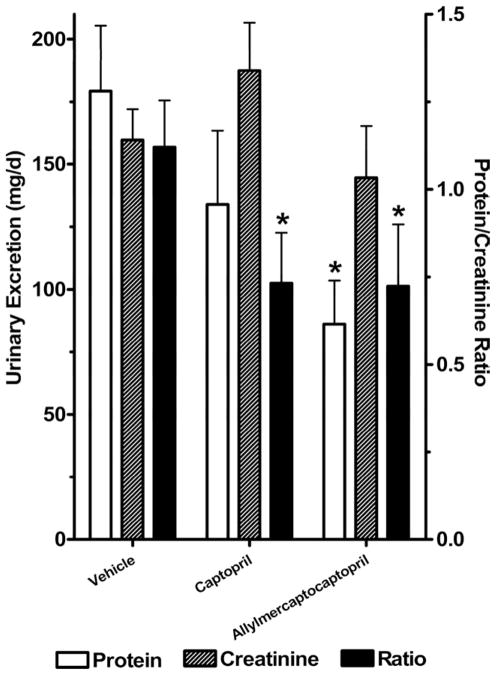
The SHROB rats develop severe glomerulosclerosis with proteinuria and die of renal failure at around 1 year of age.5,6 Therefore, we measured 24-h urinary excretion of protein and creatinine in SHROB rats (Fig. 5). Urinary protein excretion was very high in vehicle control SHROB rats (>175 mg/day), consistent with severe renal disease. Total urinary protein was significantly reduced by treatment with allylmercaptocaptopril but not by captopril treatment. Urinary creatinine was not significantly changed in the treatment groups. However, the ratio of protein to creatinine was reduced significantly by both treatments.
FIG. 5.
Urinary excretion of protein and creatinine over 24 h. Total urinary protein and creatinine were corrected for total urine volume and expressed as mg/day. The ratio of protein to creatinine is indicated on the scale of the right vertical axis. *Significant differences from control group, according to Newman-Keuls test.
Plasma creatinine was measured to further evaluate renal function. Plasma creatinine was reduced significantly by treatment with allylmercaptocaptopril but not by captopril treatment (Table 1). However, the estimated creatinine clearance rate was higher (P < .05) in the captopril group than in either vehicle controls or rats treated with allylmercaptocaptopril (Table 1).
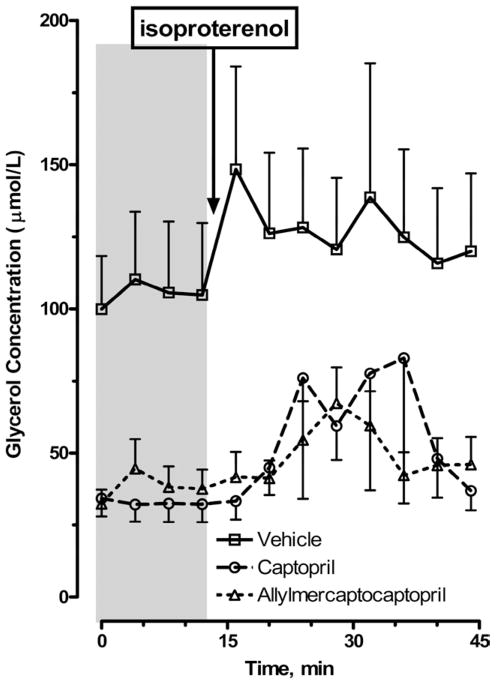
Adipocytes from SHROB rats showed a modest lipolytic response to catecholamine stimulation. Figure 6 depicts glycerol released from abdominal adipocytes from control SHROB rats or rats treated with either captopril or allylmercaptocaptopril. Control SHROB rats showed high basal glycerol release, and a peak response to 10 μM isoproterenol of <50%. Treatment with either captopril or allylmercaptocaptopril reduced basal release by approximately two-thirds and increased the amplitude of the response to β-adrenergic stimulation.
FIG. 6.
Lipolysis in abdominal adipocytes isolated from treated and untreated SHROB rats in response to isoproterenol stimulation. The baseline period is indicated by shaded area, and downward arrow indicates onset of isoproterenol stimulation (10 μM for 4 min). Abdominal adipocytes were isolated by collagenase digestion and filtration. Identical 200-μL aliquots of adipocytes were perifused with oxygenated Krebs3 buffer containing 0.1% bovine serum albumin and 0.025% adenosine deaminase at a rate of 0.25 mL/min, and 4-min fractions were collected and analyzed for glycerol.
Discussion
Based on previous results in the fructose-fed rat model,14,15 we predicted that allylmercaptocaptopril, a covalent conjugate of allicin with the ACE inhibitor captopril, would have a greater impact and therapeutic efficacy in correcting abnormalities in metabolic syndrome than captopril alone. In the current study, we showed that both captopril and the novel agent allylmercaptocaptopril had multiple therapeutic effects in an insulin-resistant rat model.
Allylmercaptocaptopril did not induce weight loss in SHROB rats but prevented the usual progression of weight gain. This effect was not shared by captopril, implicating the allicin moiety in this weight-stabilizing action. Allicin alone produced similar effects in normal Sprague–Dawley rats, preventing weight gain from a high-fructose diet.11 However, depot fat mass was unchanged by allylmercaptocaptopril treatment, a result that is not compatible with the specific antiobesity action of allylmercaptocaptopril. Food consumption and cumulative caloric intake were not decreased by allylmercaptocaptopril, suggesting an absence of adverse effects on health.
Blood-pressure responses to garlic have been inconsistent in normotensive humans and animals.10 Pure allicin and allylmercaptocaptopril both reportedly lower blood pressure in hypertensive rats.12,14,15 In the present study, we found that allylmercaptocaptopril is more potent than captopril alone in lowering blood pressure when given in equimolar doses adjusted for differences in molecular weight. The additional effect of allylmercaptocaptopril became more evident over time, and thus short-term studies may miss this slowly developing effect, which could account for some inconsistencies in the literature. The greater potency of allylmercaptocaptopril relative to captopril was previously suggested by the equivalent antihypertensive effects of 80 mg/kg/day captopril and 40 mg/kg/day allylmercaptocaptopril during 2 weeks of treatment in a fructose-fed model of hypertension and insulin resistance.15
Heart weight and left-ventricular wall thickness were decreased after allylmercaptocaptopril but not captopril treatment, consistent with the greater fall in blood pressure with allylmercaptocaptopril treatment. Thus, allylmercaptocaptopril might be particularly effective in reversing cardiac hypertrophy in obese hypertension.
In agreement with many clinical studies, blood pressure-lowering agents do not necessarily improve metabolic comorbidities. In fact, therapy for hypertension with hydralazine or α-methyldopa actually worsens glucose metabolism in SHROB rats.22 Similarly, in the present study, captopril significantly exacerbated glucose intolerance. Allylmercaptocaptopril improved glucose tolerance relative to captopril. Fasting glucose levels in the conscious state were not changed. Insulin levels in the fasted state or during an oral glucose challenge were also unchanged, in agreement with a recent study in fructose-fed rats.14 Thus, some factor other than insulin level must be responsible for the impairment and improvement of glucose tolerance by captopril and allylmercaptocaptopril, respectively. However, fasting glucagon levels were not affected by either drug treatment. Interestingly, under anesthesia stress, plasma glucose was lower in the allylmercaptocaptopril group compared with vehicle control and captopril-treated rats. This was explained by the improved maintenance of insulin levels under anesthesia in the allylmercaptocaptopril-treated group.
The adverse effect of captopril on glucose tolerance is surprising in light of previous findings of beneficial effects of captopril in rat models of insulin resistance.1 The improvement of glucose tolerance after ACE inhibitor treatment was attributed to hemodynamic effects, such as increased glucose delivery to skeletal muscle after vasodilatation. However, direct hemodynamic mechanisms may not apply in the SHROB model, since the direct vasodilator hydralazine worsens glucose tolerance.22 Long-term clinical trials showed that the ACE inhibitors can delay the onset of type 2 diabetes.1 One possible mechanism for the protective action of ACE inhibitors in humans is the preservation of pancreatic β cell mass. Because the SHROB model does not show loss of β cell mass and does not develop diabetes, this mechanism would not be applicable in this model.
Garlic and allicin were consistently found to lower total cholesterol and triglyceride levels in humans and in animals with dietary-induced hyperlipidemia.10,12 Allylmercaptocaptopril was found to reduce plasma triglycerides in dietary hyperlipidemia with 8 days of treatment at approximately the same dose used in the present study.1 Allylmercaptocaptopril lowered both plasma triglyceride and free fatty-acid levels in the present study, while captopril showed similar efficacy in lowering triglycerides only. Captopril, but not allylmercaptocaptopril, lowered total and non-HDL cholesterol without affecting levels of HDL cholesterol. It is noteworthy that triglycerides are the predominant blood lipid increased in the SHROB model, and cholesterol is only slightly elevated.2 Furthermore, as shown in the current study, most of the circulating cholesterol is associated with high-density lipoproteins, similar to other rodent models. Thus, the declines in triglyceride and free fatty-acid levels elicited by allylmercaptocaptopril represent a partial correction of the most prominent lipid abnormalities of the SHROB model.
The SHROB rat is a model of spontaneous end-stage renal disease due to focal segmental glomerulosclerosis, representing elements of both hypertensive and diabetic kidney disease.2 Treatment of either hypertension or metabolic abnormalities can delay or prevent the progression of renal disease, as indicated by urinary protein loss and confirmed by pathological changes.5,6 Captopril delays the onset of renal failure in humans even in the absence of hypertension and is also protective in SHR rats.23,24 In the present study, captopril did not lower 24-h urinary protein excretion, although after correction for urine creatinine, a protective effect was detected. However, captopril-treated animals maintained a higher level of creatinine clearance rate, relative to either controls or allylmercaptocaptopril-treated groups. Notably, allylmercaptocaptopril reduced total urinary protein excretion with or without correction for creatinine. One limitation of these studies is that the animals were maintained on a protein-free liquid diet during urine collection, and thus the level of proteinuria may be underestimated relative to the normal diet, which provides 24% of calories from protein. Consistent with these results, plasma creatinine tended toward improvement with captopril, but significant reduction in plasma creatinine within the plasma was only detected after allylmercaptocaptopril treatment.
Adipocytes are key participants in the abnormalities of metabolic syndrome. The renin–angiotensin system in the circulation and locally within adipose tissue may contribute to changes in lipid and glucose metabolism.25 Recent evidence suggests that angiotensin II has a direct lipolytic action.26 The present study indicates that elevated basal lipolysis and impaired response to β-adrenergic stimulation in SHROB rats can be corrected by in vivo treatment with either captopril or allylmercaptocaptopril, implicating ACE inhibition as the likely mechanism. One limitation of this study was that only the cell volume was controlled. Correction for cell number, cell size, or lipid content per cell might have yielded slightly different results. However, an alternative data analysis using the percent increase in glycerol release over baseline yielded nearly identical results (not shown).
The mechanisms for the augmentation of captopril’s metabolic and nephro-protective effects by chemical modification with an allyl moiety are not known. Antioxidant effects are often invoked to account for the actions of garlic and allicin,10 and oxidative stress may be a major contributor to insulin resistance.27 The effects of allicin12 and allylmercaptocaptopril14 in the fructose-fed rat model of metabolic syndrome are mimicked by the antioxidant lipoic acid.28 Lipoic acid also lowers blood pressure in Dahl hypertensive rats.29 In addition, antioxidant therapy delays the progression of diabetic renal disease in humans with type 2 diabetes.30 Future studies should test the hypothesis that allylmercaptocaptopril works through combined antioxidant and ACE-inhibiting actions.
The present results suggest that the allyl moiety of allylmercaptocaptopril is responsible for its additional therapeutic benefits relative to captopril. However, most of the beneficial effects were shared between captopril and allylmercaptocaptopril, and in some cases captopril was more effective, notably in improving the cholesterol profile. Nonetheless, the modification of captopril to form allylmercaptocaptopril maintains most of its actions and augments some of its therapeutic effects, most remarkably nephro-protection.
Acknowledgments
Supported by a grant from Bioline Pharmaceuticals, Jerusalem, Israel.
The sponsor (Bioline Pharmaceuticals, Jerusalem, Israel) contributed to the design of the study and review of the manuscript. The authors were responsible for the conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation and approval of the manuscript.
References
- 1.Ernsberger P, Koletsky RJ. Metabolic effects of antihypertensive agents: role of sympathoadrenal and renin-angiotensin systems. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:245–258. doi: 10.1007/s00210-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 2.Ernsberger P, Koletsky RJ, Friedman JE. Molecular pathology in the obese spontaneous hypertensive Koletsky rat: a model of syndrome X. Ann NY Acad Sci. 1999;892:272–288. doi: 10.1111/j.1749-6632.1999.tb07801.x. [DOI] [PubMed] [Google Scholar]
- 3.Velliquette RA, Kossover R, Previs SF, Ernsberger P. Lipid-lowering actions of imidazoline antihypertensive agents in metabolic syndrome X. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:300–312. doi: 10.1007/s00210-005-0024-3. [DOI] [PubMed] [Google Scholar]
- 4.Friedman JE, Ishizuka T, Liu S, Farrell CJ, Bedol D, Koletsky RJ, Kaung HL, Ernsberger P. Reduced insulin receptor signaling in the obese spontaneously hypertensive Koletsky rat. Am J Physiol. 1997;273:E1014–E1023. doi: 10.1152/ajpendo.1997.273.5.E1014. [DOI] [PubMed] [Google Scholar]
- 5.Koletsky RJ, Boccia J, Ernsberger P. Acceleration of renal disease in obese SHR by exacerbation of hypertension. Clin Exp Pharmacol Physiol. 1995;22(Suppl):S254–S256. doi: 10.1111/j.1440-1681.1995.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 6.Ernsberger P, Koletsky RJ, Collins LA, Bedol DL. Sympathetic nervous system in salt-sensitive and obese hypertension: amelioration of multiple abnormalities by a central symaptholytic agent. Cardiovasc Drugs Ther. 1996;10:275–282. doi: 10.1007/BF00120497. [DOI] [PubMed] [Google Scholar]
- 7.Deedwania PC. Diabetes and hypertension, the deadly duet: importance, therapeutic strategy, and selection of drug therapy. Cardiol Clin. 2005;23:139–152. doi: 10.1016/j.ccl.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Duarte J, Martinez A, Bermejo A, Vera B, Gamez MJ, Cabo P, Zarzuelo A. Cardiovascular effects of captopril and enalapril in obese Zucker rats. Eur J Pharmacol. 1999;365:225–232. doi: 10.1016/s0014-2999(98)00879-6. [DOI] [PubMed] [Google Scholar]
- 9.Westenend PJ, Nooyen YA, van der Krogt JA, Van Brummelen P, Weening JJ. The effect of a converting enzyme inhibitor upon renal damage in spontaneously hypertensive Fawn Hooded rats. J Hypertens. 1992;10:417–422. doi: 10.1097/00004872-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Tattelman E. Health effects of garlic. Am Fam Physician. 2005;72:103–106. [PubMed] [Google Scholar]
- 11.Elkayam A, Mirelman D, Peleg E, Wilchek M, Miron T, Rabinkov A, Oron-Herman M, Rosenthal T. The effects of allicin on weight in fructose-induced hyperinsulinemic, hyperlipidemic, hypertensive rats. Am J Hypertens. 2003;16:1053–1056. doi: 10.1016/j.amjhyper.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 12.Elkayam A, Mirelman D, Peleg E, Wilchek M, Miron T, Rabinkov A, Sadetzki S, Rosenthal T. The effects of allicin and enalapril in fructose-induced hyperinsulinemic hyperlipidemic hypertensive rats. Am J Hypertens. 2001;14:377–381. doi: 10.1016/s0895-7061(00)01298-x. [DOI] [PubMed] [Google Scholar]
- 13.Kannar D, Wattanapenpaiboon N, Savige GS, Wahlqvist ML. Hypocholesterolemic effect of an enteric-coated garlic supplement. J Am Coll Nutr. 2001;20:225–231. doi: 10.1080/07315724.2001.10719036. [DOI] [PubMed] [Google Scholar]
- 14.Oron-Herman M, Rosenthal T, Mirelman D, Miron T, Rabinkov A, Wilchek M, Sela BA. The effects of S-allylmercaptocaptopril, the synthetic product of allicin and captopril, on cardiovascular risk factors associated with the metabolic syndrome. Atherosclerosis. 2005;183:238–243. doi: 10.1016/j.atherosclerosis.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Miron T, Rabinkov A, Peleg E, Rosenthal T, Mirelman D, Wilchek M. Allylmercaptocaptopril: a new antihypertensive drug. Am J Hypertens. 2004;17:71–73. doi: 10.1016/s0895-7061(03)01035-5. [DOI] [PubMed] [Google Scholar]
- 16.Giudicelli JF, Freslon JL, Glasson S, Richer C. Captopril and hypertension development in the SHR. Clin Exp Hypertens. 1980;2:1083–1096. doi: 10.3109/10641968009037162. [DOI] [PubMed] [Google Scholar]
- 17.Swislocki AL, LaPier TL, Khuu DT, Fann KY, Tait M, Rodnick KJ. Metabolic, hemodynamic, and cardiac effects of captopril in young, spontaneously hypertensive rats. Am J Hypertens. 1999;12:581–589. doi: 10.1016/s0895-7061(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 18.Vikari J. Precipitation of plasma lipoproteins by PEG-6000 and its evaluation with electrophoresis and ultracentrifugation. Scand J Clin Lab Invest. 1976;36:265–268. doi: 10.1080/00365517609055259. [DOI] [PubMed] [Google Scholar]
- 19.Peterson GL. A simplification of the protein assay method of Lowry et al which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 20.Edwards L, Ernsberger P. Assay of arachidonic acid release coupled to alpha 1- and alpha 2-adrenergic receptors. Methods Mol Biol. 2000;126:375–390. doi: 10.1385/1-59259-684-3:375. [DOI] [PubMed] [Google Scholar]
- 21.Velliquette RA, Koletsky RJ, Ernsberger P. Plasma glucagon and free fatty acid responses to a glucose load in the obese spontaneous hypertensive rat (SHROB) model of metabolic syndrome X. Exp Biol Med. 2002;227:164–170. doi: 10.1177/153537020222700303. [DOI] [PubMed] [Google Scholar]
- 22.Velliquette RA, Ernsberger P. The role of I(1)-imidazoline and alpha(2)-adrenergic receptors in the modulation of glucose metabolism in the spontaneously hypertensive obese rat model of metabolic syndrome X. J Pharmacol Exp Ther. 2003;306:646–657. doi: 10.1124/jpet.103.050468. [DOI] [PubMed] [Google Scholar]
- 23.Cao Z, Bonnet F, Davis B, Allen TJ, Cooper ME. Additive hypotensive and anti-albuminuric effects of angiotensin-converting enzyme inhibition and angiotensin receptor antagonism in diabetic spontaneously hypertensive rats. Clin Sci. 2001;100:591–599. [PubMed] [Google Scholar]
- 24.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 25.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 26.Cabassi A, Coghi P, Govoni P, Barouhiel E, Speroni E, Cavazzini S, Cantoni AM, Scandroglio R, Fiaccadori E. Sympathetic modulation by carvedilol and losartan reduces angiotensin II-mediated lipolysis in subcutaneous and visceral fat. J Clin Endocrinol Metab. 2005;90:2888–2897. doi: 10.1210/jc.2004-1995. [DOI] [PubMed] [Google Scholar]
- 27.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 28.Thirunavukkarasu V, Anitha Nandhini AT, Anuradha CV. Lipoic acid attenuates hypertension and improves insulin sensitivity, kallikrein activity and nitrite levels in high fructose-fed rats. J Comp Physiol [B] 2004;174:587–592. doi: 10.1007/s00360-004-0447-z. [DOI] [PubMed] [Google Scholar]
- 29.Vasdev S, Gill V, Parai S, Gadag V. Dietary lipoic acid supplementation attenuates hypertension in Dahl salt sensitive rats. Mol Cell Biochem. 2005;275:135–141. doi: 10.1007/s11010-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 30.Endo K, Miyashita Y, Sasaki H, Ohira M, Saiki A, Koide N, Otsuka M, Oyama T, Takeyoshi M, Ito Y, Shirai K. Probucol delays progression of diabetic nephropathy. Diabetes Res Clin Pract. 2006;71:156–163. doi: 10.1016/j.diabres.2005.05.012. [DOI] [PubMed] [Google Scholar]