Abstract
Physical exercise induces widespread neurobiological adaptations and improves learning and memory. Most research in this field has focused on hippocampus-based spatial tasks and changes in brain-derived neurotrophic factor (BDNF) as a putative substrate underlying exercise-induced cognitive improvements. Chronic exercise can also be anxiolytic and causes adaptive changes in stress reactivity. The present study employed a perirhinal cortex-dependent object recognition task as well as the elevated plus maze to directly test for interactions between the cognitive and anxiolytic effects of exercise in male Long Evans rats. Hippocampal and perirhinal cortex tissue was collected to determine whether the relationship between BDNF and cognitive performance extends to this non-spatial and non-hippocampal-dependent task. We also examined whether the cognitive improvements persisted once the exercise regimen was terminated. Our data indicate that 4 weeks of voluntary exercise every-other-day improved object recognition memory. Importantly, BDNF expression in the perirhinal cortex of exercising rats was strongly correlated with object recognition memory. Exercise also decreased anxiety-like behavior, however there was no evidence to support a relationship between anxiety-like behavior and performance on the novel object recognition task. There was a trend for a negative relationship between anxiety-like behavior and hippocampal BDNF. Neither the cognitive improvements nor the relationship between cognitive function and perirhinal BDNF levels persisted after 2 weeks of inactivity. These are the first data demonstrating that region-specific changes in BDNF protein levels are correlated with exercise-induced improvements in non-spatial memory, mediated by structures outside the hippocampus and are consistent with the theory that, with regard to object recognition, the anxiolytic and cognitive effects of exercise may be mediated through separable mechanisms.
Keywords: hippocampus, stress, anxiety, running wheel
Introduction
Physical exercise induces widespread neurobiological adaptations and has been shown to improve learning and memory in several tasks (Dishman, Berthoud, Booth, Cotman, Edgerton, Fleshner, Gandevia, Gomez-Pinilla, Greenwood, & Hillman 2006). In rodents, physical exercise increases neurogenesis, facilitates long-term potentiation and enhances synaptic plasticity and metabolic function in the hippocampus (Bjornbekk, Mathe, & Brene, 2005; Ding,Vaynman, Akhavan, Ying, & Gomez-Pinilla, 2006a, Ding, Vaynman, Souda, Whitelegge, & Gomez-Pinilla, 2006b; Vaynman, Ying, & Gomez-Pinilla, 2003; Vaynman, Ying, Wu, & Gomez-Pinilla, 2006). The tasks used to determine the functional significance of these changes have primarily tested spatial cognition and rely on hippocampal function, such as the Morris water maze (Adlard, Perreau, Engessar-Cesar, & Cotman, 2004; Albeck, Sano, Prewitt, & Dalton, 2006). From these studies, brain-derived neurotrophic factor (BDNF) has emerged as a putative substrate converting exercise-induced adaptations in energy metabolism into the neuroplastic changes thought to underlie improvements in cognitive function (Dishman et al., 2006). In fact, exercise-induced increases in hippocampal BDNF are necessary for improvements in the Morris water maze (Vaynman, Ying, & Gomez-Pinilla, 2004).
Physical exercise can also improve performance on non-spatial tasks that rely on other structures, including associative learning tasks (Burghardt, Pasumarthi, Wilson, & Fadel, 2006; Eisenstein & Holmes, 2007; Falls et al., 2010; VanHoomissen, Holmes, & Zellner, 2004) and object recognition (Fahey, Barlow, Day, & O’Mara, 2008; Griffin, Bechara, Birch, & Kelly, 2009; O’Callaghan, Ohle, & Kelly, 2007). For example, novel object recognition is mediated by the perirhinal cortex and can be designed such that hippocampal lesions have no effect on performance (Dere, Huston, & De Souza-Silva, 2007). To date, there has been very little research on the mechanisms that underlie exercise-induced improvements in tasks that do not test spatial cognition or rely on hippocampal function. Although there is evidence that forced physical exercise also increases perirhinal BDNF (Griffin et al., 2009), it is not known whether BDNF activity in the hippocampus, perirhinal cortex or elsewhere mediates exercise-induced improvements in object recognition. This is particularly important from a translational perspective since exercise studies in humans have reported the greatest improvements in non-spatial learning and memory (Colcomb & Kramer, 2003; Hillman, Erickson, & Kramer, 2008).
Novel object recognition is well-suited for studying the effects of exercise on cognition because it does not involve the strong aversive elements of other commonly used cognitive tasks, such as swimming-stress (Engelmann, Ebner, Landgraf, & Wotjak, 2006) or foot shock delivered during fear conditioning. Likewise, object recognition testing does not require food deprivation, which is a necessary component of many Pavlovian conditioning tasks and maze learning. The potentially confounding effect of these stressors is an important consideration due to the complex relationship between physical exercise and stress. For example, several weeks of regular exercise results in adaptive changes in stress-reactivity (Campbell, Rakhshani, Fediuc, Bruni, & Riddell, 2008; Droste, Chandromohan, Hill, Linthorst, & Reul, 2007) and can prevent the negative physiological and behavioral effects of uncontrollable stressors (Dishman,1997; Greenwood, Foley, Day, Campisis, Hammack, Campeau, Maier, & Fleshner, 2003; Greenwood, Foley, Day, Burhans Brooks, Campaeu, & Fleshner, 2005). Moreover, there is evidence that exercise can be anxiolytic in its own right, which could reflect these adaptive changes in stress-reactivity (Droste et al., 2007; Fulk, Stock, Lynn, Marshall, Wilson, & Hand, 2004) and confound interpretation of the effects of exercise on cognition per se.
Lastly, little work has been done to explore how long the effects of exercise persist once the exercise regimen has been terminated. Of the few studies available, there is evidence that the cognitive effects of exercise may be lost after just a few days (Alaei et al., 2007), as well as evidence suggesting that exercise-induced increases in BDNF persist longer (Berchtold et al., 2005). The present study was thus designed to address multiple gaps in the literature by testing whether the mechanisms underlying the cognitive effects of physical exercise generalize to a non-spatial and non-hippocampal-dependent task, and directly testing for interactions between the cognitive and anxiolytic effects of exercise. In addition, we examined whether these cognitive improvements persist once the exercise regimen is terminated.
Materials and Methods
Subjects
Thirty-two male Long Evans rats were obtained at 8 weeks of age (Harlan Sprague-Dawley, Indianapolis, IN). Rats were maintained on a 14:10 light-dark cycle throughout the study. For 7 days, rats were allowed to acclimate to the vivarium and had free access to food and water (Purina standard rat chow: Nestle Purina, St. Louis, MO) before being randomly assigned to either an exercise group (X) or non-exercise group (NX). All procedures were conducted in accordance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth College Institutional Animal Care and Use Committee.
Apparatus
Running wheels
Rats in the exercise group had access to an exercise wheel (Med Associates, St. Albans, VT) that was attached to the side of the home cage and accessible through an opening in the wall of the cage. The wheels were 35.6 cm in diameter and consisted of stainless steel rods (4.8 mm in length) spaced 1.6 cm apart. Every 1/4 revolution of the wheel was recorded by an automatic counter mounted on the side of the apparatus.
Elevated plus maze
Testing was conducted in a small dimly-lit room using a black Plexiglas platform with two open and two closed arms extending out from the center (10W × 75L cm). The maze arms were 45 cm above the floor, and the walls of the closed arms were 39 cm tall. A video camera was mounted above the maze and behavior was recorded on a DVD recorder.
Novel object recognition
Testing was conducted in the same room as the elevated plus maze procedure. The apparatus consisted of a plastic tub (30W × 4L × 38H cm) monitored by a video camera. The objects used were made of plastic building blocks (Learning Resources Inc, Vernon Hills, IL), constructed into distinct configurations with approximately the same dimensions (7W × 7L × 9H cm). One of the objects was purple and roughly similar in shape to a dumbbell and the other was green and shaped like a 3-dimensional axis.
Procedures
Physical exercise regimen
Exercising rats (n=16) alternated each day between individual cages containing a running wheel and pair-housing beginning 4 weeks before the start of the behavioral tasks and continuing throughout the first round of testing. Non-exercising rats (n=16) also alternated daily between single and pair-housing but did not have access to a running wheel at any point during the study. Importantly, this exercise regimen was chosen to allow us to record each subjects’ individual running distance, while minimizing any stress associated with individual housing (Ruis, Brake, Buwalda, De Boer, Meerlo, Korte, Blokhuis, & Koolhaas, 1999; Stranahan, Khalil, & Gould, 2006; Weiss, Pryce, Jongen-Relo, Nanz-Bahr, & Feldon, 2004). The counter that recorded wheel rotations was monitored daily and reset at the same time each afternoon.
Elevated plus maze
After 4 weeks in their respective conditions, rats began behavioral testing using the elevated plus maze to test anxiety-like behavior. Rats were individually placed in the center of the maze at which point the investigator left the room and the rat was allowed to explore freely for 5 minutes. The number of entries into open arms and time spent in the open arms were recorded as the dependent measures. Increased open arm exploration is thought to reflect lower levels of anxiety-like behavior (Carobrez & Bertoglio, 2005).
Novel object recognition
Twenty-four hours after elevated plus maze testing, cognitive function was tested using a 3 day novel object recognition task. The novel object recognition protocol was specifically designed to minimize spatial learning and hippocampal involvement (Bevins & Besheer, 2006; Dere et al., 2007). On day 1 (habituation session) each rat was exposed to the tub individually for 10 minutes to habituate to the testing environment. On day 2 (sample session), rats were placed in the tub and given 5 minutes to explore two identical sample objects. Twenty-four hours later (test session), rats were again placed in the tub with one familiar and one novel object (counter-balanced across subjects) and given 2 minutes to investigate the items. An investigator, who was blind to the experimental conditions, later scored the time spent exploring each object. Exploration was defined as direct sniffing or snout contact with the object. Twenty-four hours after completing the object discrimination task, 8 rats from each group were sacrificed for tissue collection and processing as described below. The remaining 16 rats continued to alternate between paired and single housing for an additional two weeks, however no rats had access to running wheels throughout this time. After 2 weeks these rats were tested again using the same object recognition protocol, but with a new set of objects. These rats were also sacrificed 24 hours following testing and their tissue collected as described below.
BDNF ELISA
Twenty-four hours after recognition memory testing, subjects were sacrificed by decapitation and their brains were rapidly dissected out. Neural tissue was collected bilaterally from the cortical surface along the caudal perirhinal cortex (from bregma, −5.0 −7.0 mm) and a 1 mm diameter × 1.5 mm length punch was removed bilaterally from the dorsal hippocampus. Fresh tissue samples were homogenized in RIPA lysis buffer (Promega, Madison, WI) with protease inhibitor cocktail added, and incubated on ice for 45 minutes. Homogenates were then centrifuged at 4°C for 10 minutes at 13,500RPM and supernatants collected. Total protein concentrations were measured by spectrophotometry using the BCA Protein Assay Kit (Pierce, Rockford IL). Mature BDNF protein levels were quantified using the BDNF Emax Immunoassay System according to the manufacturer’s protocol (Promega, Madison, WI). Briefly, 96-well plates were coated with anti-BDNF monoclonal antibody and carbonate coating buffer overnight at 4°C. The plates were then blocked for non-specific binding using Promega’s proprietary Block & Sample buffer for 1 hour at room temperature (RT). After washing the plate, BDNF standards and samples were added and incubated for 2 hours at RT with shaking. After 5 washes, Anti-Human BDNF polyclonal antibody was added to the plate and incubated for 2 hours at RT with shaking, followed by 5 washes. Anti-IgY HRP Conjugate was then added for a 1 hour incubation at RT followed by 5 more washes. TMB One Solution was added for a 10-minute incubation. The reaction was stopped with 1N HCL and absorbance measured at 450nM.
Data Analysis
Wheel running
The counters attached to the wheels recorded every ¼ turn of the running wheel, thus nightly running distance was calculated by dividing the count by 4 and multiplying by 1.12 to convert to meters.
Novel object recognition
The mean time spent exploring the identical objects during the sample session was analyzed using a two-sample t-test (α =0.05). For the test session data, a discrimination ratio served as the dependent variable of interest and was calculated as the time spent exploring the novel object divided by total time spent exploring both objects. This measure takes into account individual differences in total exploratory behavior. A one-sample t-test (expected value= 0.5; α=0.05) was used to determine whether each group was able to successfully discriminate between the two objects. A two-sample t-test was used to compare the exercise and no-exercise groups to one another (α=0.05).
Elevated plus maze
To measure anxiety-like behavior, the amount of time spent in the open arms of the maze, as well as the total number of open-arm entries, were each averaged for the two groups and analyzed using two-sample t-tests (α =0.05).
Locomotor activity
It was important to assess locomotor activity because any group differences that emerged in anxiety-like behavior or object exploration could be affected by exercise-induced changes in locomotor activity (e.g., fatigue, etc). To determine whether there were group differences in locomotor activity, the total number of arm entries made during exposure to the elevated plus maze was averaged for each group and analyzed using a two-sample t-test (α=.05). In addition, the DVD recording of the first day of novel object recognition testing was scored for locomotor activity. This was done by drawing a line on the video screen and dividing the testing apparatus into 2 equal areas. An observer blind to condition counted the number of times each rat crossed the center line. A line crossing was defined as placing all four paws on the other side of the line. Assessing locomotor activity during the object recognition task, rather than during a separate session in an open field apparatus, for example, provides a direct task-relevant measure of activity.
BDNF ELISA
Total protein levels in the hippocampus and perirhinal cortex were analyzed using a two-sample t-test (α=.05).
Correlations
Linear regression analyses were performed to test for correlations between individual subjects’ object recognition performance, anxiety-like behavior, and BDNF levels (α =0.05).
Results
Wheel running
Rats in the exercising condition ran an average of 3,982 ± 631 m/night overall, however average running distances increased steadily across the first 3 weeks before stabilizing at approximately 5,000 m/night.
Novel object recognition
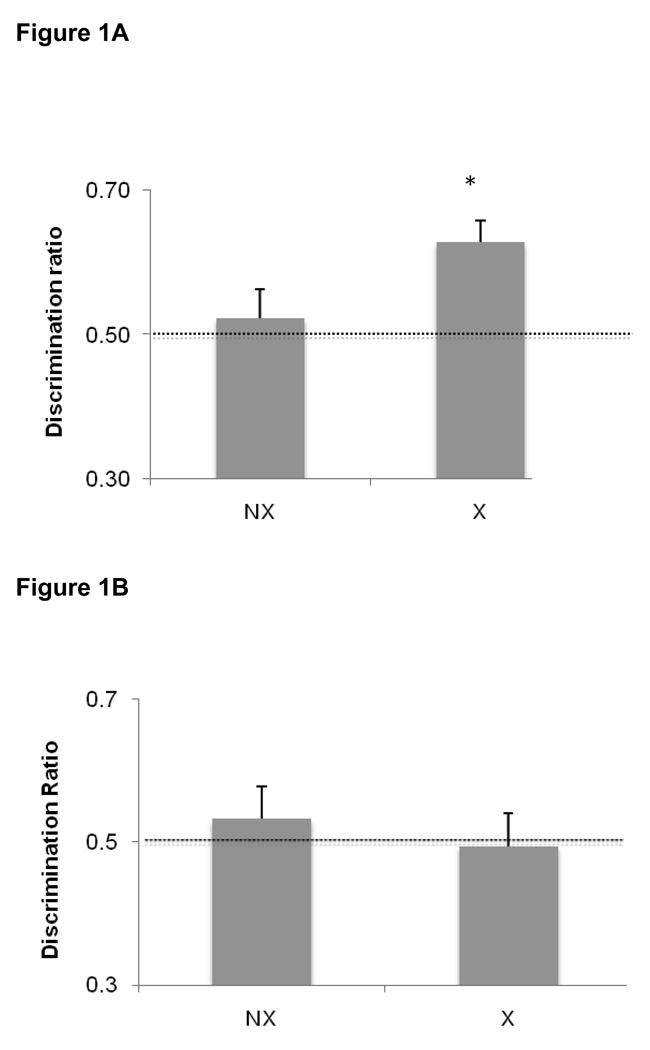
There were no differences between groups regarding the time spent exploring the objects during the first sample session. Mean exploration times were 26.9 ± 2.8 s and 26.6± 3.0 s for the exercising and non-exercising groups, respectively. In the first object recognition test, exercising rats were able to successfully discriminate between the novel and familiar items [t(15)= 4.27, P = 0.001] whereas the non-exercising rats failed to discriminate [t(15)= 0.54, P = 0.60]. For the exercising group, the mean time spent with the novel and familiar objects was 17.0± 2 s and 9.9± 1.3 s, respectively. For the non-exercising group, the meant time spent with the novel and familiar objects was 13.4 ± 1.8 s and 12.3 ± 1.8 s, respectively. Moreover, the discrimination ratio in the exercising group was significantly higher than that in the non-exercising group as shown in Figure 1A [t(31)= 2.12, P= 0.04].
Figure 1.
Novel object recognition at the end of 4 weeks of exercise. The data presented are the average discrimination ratios (±SEM) of each group, calculated as the time spent exploring the novel object divided by total time spent exploring both objects. A discrimination ratio of 0.5 indicates no discrimination between the novel and familiar objects, as indicated by the dotted line. Values significantly greater than 0.5 reflect successful discrimination, i.e., rats spent more time exploring the novel vs. familiar object. A) Immediately following the exercise period, exercising rats (X) successfully discriminated between the novel and familiar objects (p=0.001) while non-exercising rats (NX) did not discriminate (p=0.73). In addition, the discrimination ratio for exercising rats was significantly higher than the ratio for non-exercising rats (p=0.04). B) Exercise-induced improvements in object discrimination did not persist after 2 weeks of inactivity (X = formerly exercising rats; NX = formerly sedentary rats). Data are average discrimination ratios ± SEM.
In contrast, when rats from each group were re-tested after 2 weeks of inactivity, they did not demonstrate successful discrimination between the objects, regardless of whether they were in the formerly exercising group [t(7)= −0.16, P =0.88] or the non-exercising group [t(7)= 0.72, P = 0.49]. During the sample session, mean exploration times were 38.8 ± 3.4 s and 40.1 ± 4.0 s for the formerly exercising and non-exercising groups, respectively. The mean time spent with the novel object versus the familiar object was 24.3 ± 2.9 s and 24.4 ± 2.6 s respectively for the formerly-exercising rats, and 22.3 ± 3.3 s and 19.1 ± 3.2 s for the novel and familiar objects (respectively) in the non-exercising group. Similarly, there was no group difference in the discrimination ratios [t(15)=0.63, P = 0.54], as shown in Figure 1B.
Anxiety-like behavior
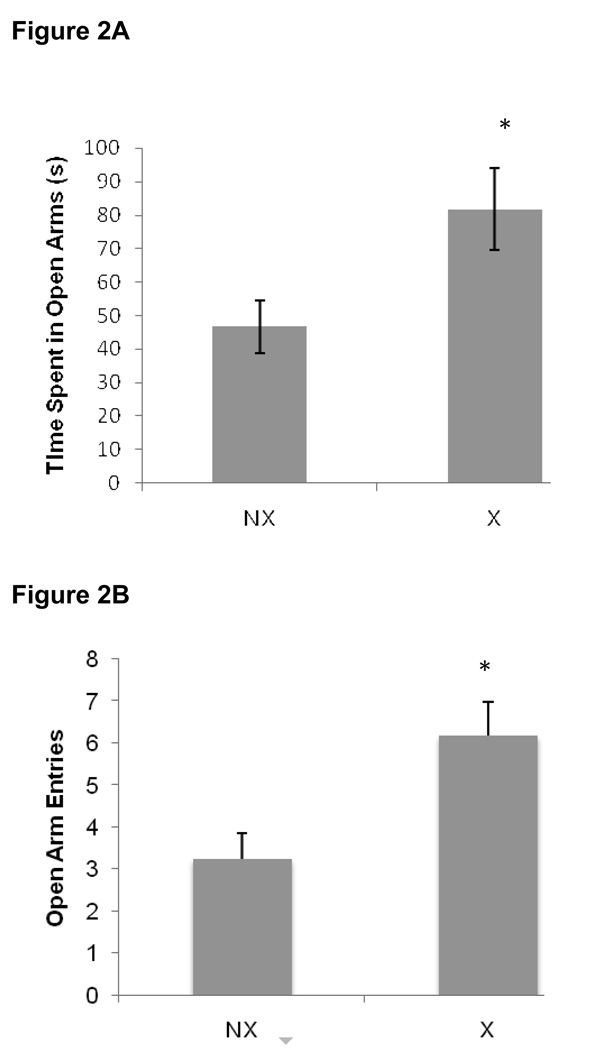
Data collected during the elevated plus maze session are shown in Figure 2. The exercising rats displayed significantly lower anxiety-like behavior compared to non-exercisers as evidenced by an increase in the amount of time spent in the open arms [t(31)= 2.40, P = 0.02], as well as the total number of open arm entries [t(31)= 2.95, P = 0.006] during exposure to the elevated plus maze.
Figure 2.
Four weeks of exercise significantly reduced anxiety-like behavior in exercising rats (X) compared to non-exercisers (NX; p=0.02). Data are A) mean time (sec) spent in the open arms ± SEM and B) mean number of open arm entries ± SEM.
Relationships between BDNF levels and behavior
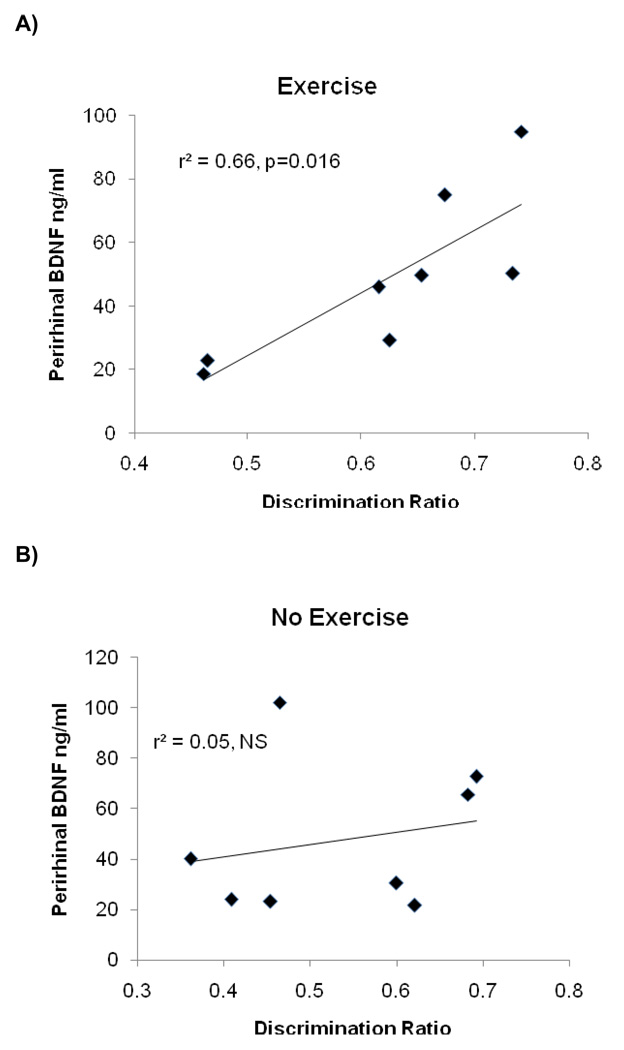
As shown in Figure 3, there was a significant positive correlation between the discrimination ratio during the first novel object recognition task and BDNF levels in the perirhinal cortex of the exercising rats [r2=0.66, P = 0.016], indicating that higher levels of BDNF in the perirhinal cortex were associated with better object recognition memory. In contrast, there was no relationship between novel object recognition and BDNF levels in the perirhinal cortex in non-exercising rats [r2=0.04, P = 0.59]. Moreover, neither group exhibited a relationship between novel object recognition and BDNF levels in the hippocampus [r2=0.16, P = 0.32 for exercising group; r2=0.02, P = 0.73 for non-exercising group]. We also did not find a significant correlation between BNDF levels in hippocampus and perirhinal cortex in either the exercising or non-exercising groups [r2=0.01, P = 0.79; r2=0.01, P = 0.82 respectively].
Figure 3.
There was a significant positive correlation between object recognition performance and BDNF levels in the perirhinal cortex of exercising rats only.
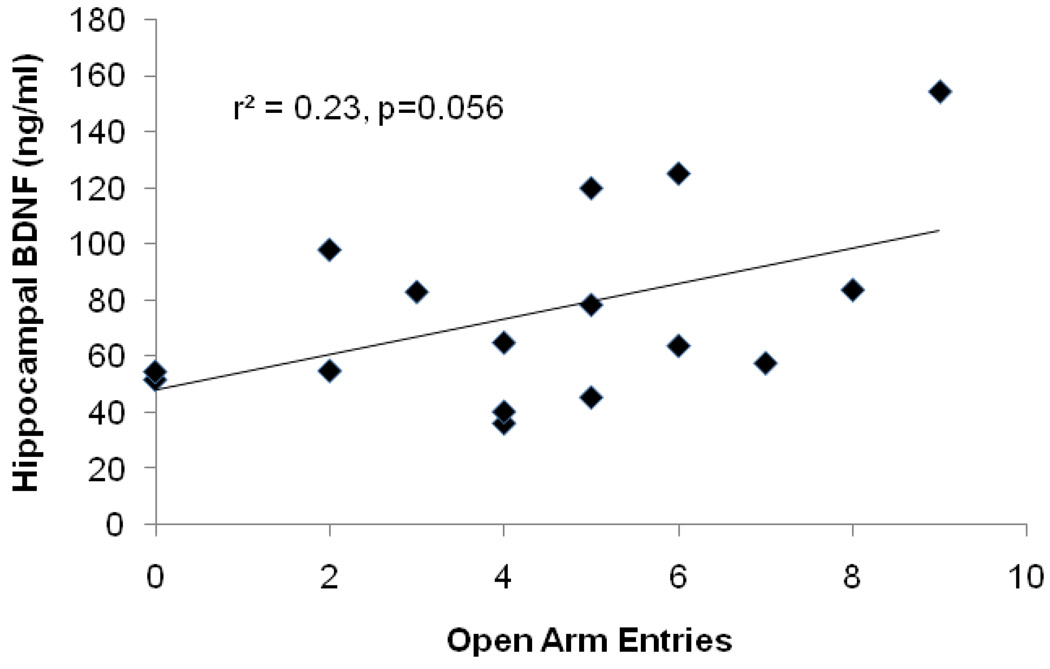
Interestingly, there was a trend toward a positive relationship between open arm entries and BDNF levels in the hippocampus across all rats (Figure 4), however this relationship did not reach statistical significance [r2=0.21 P = 0.056]. In contrast, there was no evidence of relationship between BDNF levels in perirhinal cortex and anxiety-like behavior [r2=0.02, P = 0.76; r2=0.17, P = 0.29 for exercise and non-exercise groups respectively]. Importantly, there was no relationship observed between object recognition memory and anxiety-like behavior in either condition [r2= 0.11, P= 0.41; r2= 0.12, P = 0.40 for exercising and non-exercising groups, respectively].
Figure 4.
There was a trend (p=0.056) toward a positive relationship between hippocampal BDNF and the number of open arm entries across both groups.
Locomotor activity
There were no differences in locomotor behavior between exercising and non-exercising rats as measured by the total number of arm entries made between groups during exposure to the elevated plus maze [t(31)= −0.19, P = 0.85]. Moreover, there were no differences in the number of center-line crosses during familiarization with the testing environment on the first day of the object recognition task [t(19)= 0.28; P= 0.79].
Discussion
A primary goal of the present study was to determine whether changes in BDNF expression are associated with exercise-induced improvement in a non-spatial, non-hippocampal dependent memory task. We found that 4 weeks of voluntary exercise every-other-day did indeed improve object recognition memory. In particular, exercising rats successfully discriminated between the novel and familiar items, whereas sedentary control rats did not. This result could not be attributed to exercise-induced changes in locomotor activity or enhanced novelty detection since there were no differences observed between the groups during either the habituation or sample sessions. While it might be argued that the lack of discrimination in the sedentary rats suggests that exercise induces, rather than enhances discrimination, this is unlikely due to the fact that the inherent propensity for rats to explore and attend more to novel objects and cues is a well-documented phenomenon (Dere et al., 2007). If anything, this innate tendency makes it difficult to interpret a magnitude difference in the degree to which one group discriminates relative to another. For this reason, we intentionally utilized a relatively short sample exposure and long delay between the sample and test sessions in order to increase the task difficulty. By adjusting the parameters in this way, we are able to make a categorical distinction between the groups, based on whether they are able to successfully discriminate the objects or not. Indeed, studies using similar training and testing parameters typically report results similar to ours in that rats in the control group did not exhibit discrimination between the objects (Griffin et al., 2009).
Importantly, we found that BDNF expression in the perirhinal cortex of exercising rats was strongly correlated with object recognition memory, supporting the hypothesis that exercise-induced effects on perirhinal BDNF are associated with improvement in both non-spatial tasks (present data) and spatial learning and memory procedures (Greenwood, Strong, Foley, & Fleshner, 2009; Vaynman et al., 2004). Moreover, there was no relationship between hippocampal BDNF and object recognition memory and no relationship between BDNF levels in the perirhinal cortex and hippocampus. Given the role of the perirhinal cortex in mediating object recognition (Dere et al., 2007), this finding is consistent with previous studies (involving other brain regions) demonstrating that task acquisition upregulates BDNF in a region-specific manner (Chen, Kitanishi, Ikeda, Matsuki, & Yamada, 2007; Harvey, McGuaran, Murphy, Burns, McMonagle, & Commins, 2008; Tokayuma, Okuno, Hashimoto, Xin Li, & Miyashita, 2000). However, the fact that the strong correlation between perirhinal BDNF and task performance was observed only in the exercising rats (and not in the non-exercising group, in which there were a few individuals who did demonstrate a strong preference for the novel object) suggests that there was something particular about the exercise regimen, perhaps a priming effect, underlying this correlation. In addition, these data provide further support for the general role of BDNF in mediating the effects of exercise on cognitive function by demonstrating that changes in BDNF in perirhinal cortex are associated with performance in a non-hippocampal-dependent task. Indeed, the vast majority of rodent studies exploring the cognitive effects of exercise have focused on hippocampus-based spatial tasks while very few have assessed whether the effects of exercise generalize to other types of tasks and other brain regions. This is particularly important from a translational standpoint given that in humans, exercise-induced cognitive improvements are most often reported in executive function and working memory tasks, which do not rely heavily on hippocampal function (Tomporowski, 2003). Designing studies to demonstrate the generalizability of the effects of physical exercise on cognitive function is an important step toward translating between human studies and rodent models in order to take full advantage of the potential benefits of exercise.
It has remained unclear whether exercise-induced cognitive improvements are due entirely to direct effects of exercise on learning and memory processes, or whether these effects are secondary to the stress-buffering, or anxiolytic effects of exercise. Therefore, a second goal of this study was to determine if the cognition enhancing effects of exercise could be dissociated from anxiolytic effects. Because of the anxiogenic effects of stress (Calvo, Martijena, Molina, & Volosin, 1998; Calvo & Volosin 2001; Martijena, Calvo, Volosin, & Molina,1997), we chose a task that is minimally stressful (Droste et al., 2007; Meuller, Dolgas, & Herman, 2004), and measured anxiety-like behavior as a covariate. We also implemented a new exercise regimen designed to reduce isolation stress by allowing rats to be group housed every other day. Indeed, stress has been shown to both improve (Sandi, Loscertales, & Guaza, 1997; Sandi 1998) and impair (McEwen, 1999) cognitive function, depending on the type of stressor, as well as its intensity and duration. Prolonged physical exercise results in adaptive changes in stress-reactivity. For example, mild stressors, such as exposure to novelty, result in an attenuated rise in circulating corticosterone in exercising rats, whereas more aversive stressors, such as forced swimming can cause an equal or greater corticosterone response in exercising rats relative to controls (Campbell et al., 2008; Droste et al., 2007). This relationship is critical to consider, given that the tasks most commonly used to measure physical exercise-induced cognitive improvements such as the Morris water maze and context fear conditioning are themselves robust stressors (Engelmann et al., 2006; Kant, Mougey, Pennington, & Meyerhoff, 1983), and task acquisition in these tasks is known to be influenced by circulating corticosterone levels during training (Cordero, Merino & Sandi,1998; Cordero & Sandi, 1998; Sandi, 1998).
Consistent with other published data demonstrating anxiolytic effects of physical exercise (Binder, Droste, Ohl, & Reul, 2004; Droste et al., 2007; Fulk et al., 2004), we found that exercise decreased anxiety-like behavior as measured by time spent in the open arms, as well as open arm entries in the elevated plus maze. This difference cannot be attributed to group differences in locomotor activity, since both groups made a similar number of total arm entries. An important new finding of the present study is that we did not find any evidence to support a relationship between anxiety-like behavior and novel object recognition. However, as stated above, the cognitive task used here does not engage the HPA-axis to the same degree as other common cognitive tasks, and therefore it cannot be concluded that similar results would be found using a more stressful task. It is also important to note here, that while the anxiolytic effects of exercise are reported quite consistently in humans, the rodent literature on this topic is quite mixed, with some researchers reporting ambiguous or null effects (Binder et al. 2004), and still others who have reported increased defensive behaviors following an exercise intervention (Burghardt, Fulk, Hand & Wilson, 2004). It may be that in the case of exercise and anxiety, several other experimental design factors play a modulatory role, such as housing conditions (group versus singly-housed), form of exercise (forced or voluntary), duration of exercise regimen, degree of experimenter handling, and the dependent variable used to measure anxiety-like behavior (García-Capdevila et al., 2009; Leisure and Decker, 2009; Leasure & Jones, 2008).
There was also a trend for a negative relationship between anxiety-like behavior and hippocampal BDNF, with higher BDNF levels corresponding to a greater number of open arm entries. Although this trend was not apparent using total time spent in the open arms, it is consistent with a growing body of literature demonstrating that increased hippocampal BDNF levels are associated with decreased anxiety, and that interfering with BDNF activity in the hippocampus can increase anxiety levels (Bergami, Rimondini, Santi, Blum, Gotz, & Canossa, 2008; Chen, Jing, Bath, Ieraci, Khan, Siao, Herrera, Toth, Yang, McEwen, Hempstead, & Lee, 2006; Cirulli, Berry, Chiarotti, & Alleva, 2004; Koponen, Voikar, Riekki, Saarelainen, Rauramaa, Rauvala, Taira, & Castren, 2004; Siuciak, Lewis, Wiegand, & Lindsay, 1997). Given this relationship, and the fact that task acquisition upregulates BDNF in a region-specific manner (Chen et al., 2006; Kesslak, So, Choi, Cotman, & Gomez-Pinilla, 1998; Naimark et al., 2007; Tokuyama et al., 2000), an interesting syllogism emerges in which simply learning a hippocampus-based cognitive task could lead to subsequent reductions in anxiety-like behavior via increases in hippocampal BDNF activity in rodents. While the present study was not designed to test this hypothesis, such a relationship between hippocampal-dependent learning and anxiety could have intriguing implications for the treatment of anxiety-related mood disorders. In future studies designed to determine the mechanisms by which exercise improves mental health it would be advantageous to assess task-induced stress-reactivity in exercising and non-exercising rats, particularly in those tasks that are known to be highly stressful.
The final goal of the present study was to fill a gap in the literature regarding how long exercise will continue to affect cognitive function. The data indicate that the cognitive improvements resulting from the exercise regimen used here did not persist after 2 weeks of inactivity. Likewise, the relationship between cognitive function and perirhinal BDNF levels was no longer present after 2 weeks of inactivity. There is very little published research exploring the durability of cognitive improvements resulting from physical exercise, however one study, testing the effects of a 30 day forced (treadmill) exercise on spatial learning reported that the exercise-induced cognitive improvements were lost after 3 days if the exercise was discontinued (Alaei, Moloudi, Sarkaki, Azizi-Malekabadi, & Hanninen, 2007). Combined with the results of the present study, these data suggest that exercise-induced cognitive improvements are fairly short-lived, however it is possible that manipulating some element of the exercise regimen such as the duration of the regimen or the rats’ developmental status might influence the persistence of these effects (Patterson, Dunn-Meynell, & Levin, 2008; Wright, Hebert, & Perrot-Sinal, 2008).
In sum, the present study provides the first data to indicate that changes in BNDF levels are associated with voluntary exercise-induced improvements in non-spatial memory that are mediated by structures outside the hippocampus. These findings support the general role of physical exercise as a means to improve learning and memory processes and provide evidence that BDNF plays an important role in mediating these effects in a region-specific manner. In addition, despite the potentially confounding relationship between task stress, anxiety, and cognitive performance, the results of this study are consistent with the theory that the effects of exercise on anxiety-like behavior and object recognition memory are mediated through separable mechanisms. Although this result is compelling, future studies will be helpful in further differentiating the possible interactions between the stress-buffering and cognitive benefits of exercise. The findings from this study also provide some of the first data regarding the longevity of the effects of exercise on cognitive function and offer a model for future work to systematically manipulate the parameters of exercise to maximize the persistence of its effects on cognition.
Acknowledgements
Research supported by NIH Grant MH082893 and a Dartmouth College Rockefeller Center Research Grant
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adlard PA, Perreau VM, Engessar-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neuroscience Letters. 2004;363:43–48. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behavioral Brain Research. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Alaei H, Moloudi R, Sarkaki A, Azizi-Malekabadi H, Hanninen O. Daily running promotes spatial learning and memory in rats. Pathophysiology. 2007;14:105–108. doi: 10.1016/j.pathophys.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–861. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, Götz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Procedings of the National Academy of Science. 2008;105(40):15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial nonmatching-to-sample learning task to study 'recognition memory'. Nature Protocols. 2006;1(3):1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Binder E, Droste S, Ohl F, Reul JM. Regular voluntary exercise reduces anxiety-related behavior and impulsiveness in mice. Behavioral Brain Research. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Mathé AA, Brené S. The antidepressant effect of running is associated with increased hippocampal cell proliferation. International Journal of Neuropsychopharmacology. 2005;8(3):357–368. doi: 10.1017/S1461145705005122. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Research. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- Burghardt PR, Pasumarthi RK, Wilson MA, Fadel J. Alterations in fear conditioning and amygdalar activation following chronic wheel running in rats. Pharmacology Biochemistry and Behavior. 2006;84:306–312. doi: 10.1016/j.pbb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Research. 1998;800(2):227–235. doi: 10.1016/s0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- Calvo N, Volosin M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology. 2001;73(4):261–271. doi: 10.1159/000054643. [DOI] [PubMed] [Google Scholar]
- Campbell JE, Rakhshani N, Fediuc S, Bruni S, Riddell MC. Voluntary wheel running initially increases adrenal sensitivity to adrenocorticotrophic hormone, which is attenuated with long-term training. Journal of Applied Physiology. 2008;106(1):66–72. doi: 10.1152/japplphysiol.91128.2008. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neuroscience and Biobehavioral Reviews. 2005;29(8):1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kitanishi T, Ikeda T, Matsuki N, Yamada MK. Contextual learning induces an increase in the number of hippocampal CA1 neurons expressing high levels of BDNF. Neurobiology of Learning and Memory. 2007;88(4):409–415. doi: 10.1016/j.nlm.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14(7):802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Merino JJ, Sandi C. Correlational relationship between shock intensity and corticosterone secretion on the establishment and subsequent expression of contextual fear conditioning. Behavioral Neuroscience. 1998;112(4):885–891. doi: 10.1037//0735-7044.112.4.885. [DOI] [PubMed] [Google Scholar]
- Cordero MI, Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Research. 1998;786(1–2):11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza-Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and Biobehavioral Reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F. Insulin-like growth factor I interfaces with brain-derived neurotrophic factor to modulate aspects of exercise-induced cognitive function. Neuroscience. 2006a;140:823–833. doi: 10.1016/j.neuroscience.2006.02.084. [DOI] [PubMed] [Google Scholar]
- Ding Q, Vaynman S, Souda P, Whitelegge JP, Gomez-Pinilla F. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. European Journal of Neuroscience. 2006b;24:1265–1276. doi: 10.1111/j.1460-9568.2006.05026.x. [DOI] [PubMed] [Google Scholar]
- Dishman RK. Brain monoamines, exercise and behavioral stress: animal models. Medicine & Science in Sports and Exercise. 1997;29(1):63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton R, Fleshner M, Gandevia S, Gomez-Pinilla F, Greenwood BN, Hillman CH. Neurobiology of Exercise. Obesity. 2006;14(3):345–356. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- Droste SK, Chandramohan Y, Hill LE, Linthorst AC, Reul JM. Voluntary exercise impacts on the rat hypothalamic-pituitary-adrenocortical axis mainly at the adrenal level. Neuroendocrinology. 2007;86(1):26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacology, Biochemistry and Behavior. 2007;86(4):607–615. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Hormones and Behavior. 2006;50(3):496–501. doi: 10.1016/j.yhbeh.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Fahey B, Barlow S, Day JS, O'Mara SM. Interferon-α-induced deficits in novel object recognition are rescued by chronic exercise. Physiology and Behavior. 2008;95:125–129. doi: 10.1016/j.physbeh.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Falls WA, Fox JH, MacAulay CM. Voluntary exercise improves both learning and consolidation of cued conditioned fear in C57 mice. Behavioral Brain Research. 2010;207(2):321–331. doi: 10.1016/j.bbr.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. International Journal of Sports Medicine. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Foley T, Day HE, Campisi J, Hammack SH, Campeau S, Maier S, Fleshner M. Freewheel running prevents learned helplessness/ behavioral depression: Role of dorsal raphe serotonergic neurons. Journal of Neuroscience. 2003;23(7):2889–2898. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley T, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT1a, 5-HT1b, and alpha1b-adrenergic receptor mRNA in the rat raphe nuclei. Biological Psychiatry. 2005;57:559–568. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Strong PV, Foley TE, Fleshner M. A Behavioral Analysis of the Impact of Voluntary Physical Activity on Hippocampus-Dependent Contextual Conditioning Hippocampus. Hippocampus. 2009;19(10):988–1001. doi: 10.1002/hipo.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EW, Bechara RG, Birch AM, Kelly AM. Exercise enhances hippocampal-dependent learning in the rat: Evidence for a BDNF-related mechanism. Hippocampus. 2009;19(10):973–980. doi: 10.1002/hipo.20631. [DOI] [PubMed] [Google Scholar]
- Harvey DR, McGauran AM, Murphy J, Burns L, McMonagle E, Commins S. Emergence of an egocentric cue guiding and allocentric inferring strategy that mirrors hippocampal brain-derived neurotrophic factor (BDNF) expression in the Morris water maze. Neurobiology of Learning and Memory. 2008;89(4):462–479. doi: 10.1016/j.nlm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Mougey EH, Pennington LL, Meyerhoff JL. Graded footshock stress elevates pituitary cyclic AMP and plasma beta-endorphin, beta-LPH corticosterone and prolactin. Life Sciences. 1983;33(26):2657–2663. doi: 10.1016/0024-3205(83)90350-8. [DOI] [PubMed] [Google Scholar]
- Koponen E, Võikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, Taira T, Castrén E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Molecular and Cellulsr Neurosciemce. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Martijena ID, Calvo N, Volosin M, Molina VA. Prior exposure to a brief restraint session facilitates the occurrence of fear in response to a conflict situation: behavioral and neurochemical correlates. Brain Research. 1997;752(1–2):136–142. doi: 10.1016/s0006-8993(96)01465-5. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Reviews in Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology. 2004;145(8):3763–3768. doi: 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- Naimark A, Barkai E, Matar MA, Kaplan Z, Kozlovsky N, Cohen H. Upregulation of neurotrophic factors selectively in frontal cortex in response to olfactory discrimination learning. Neural Plasticity. 2007;2007:13427. doi: 10.1155/2007/13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behavioral Brain Research. 2007;176:362–366. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. American Journal of Physiological Regulation and Integrated Comparitive Physiology. 2008;294:R290–R301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24(3):285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Sandi C, Loscertales M, Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. European Journal of Neuroscience. 1997;9(4):637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Sandi C. The role and mechanisms of action of glucocorticoid involvement in memory storage. Neural Plasticity. 1998;6(3):41–52. doi: 10.1155/NP.1998.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology, Biochemistry and Behavior. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyama W, Okuno H, Hashimoto T, Xin Li Y, Miyashita Y. BDNF upregulation during declarative memory formation in monkey inferior temporal cortex. Nature Neuroscience. 2000;3(11):1134–1142. doi: 10.1038/80655. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychologia (Amst) 2003;112(3):297–324. doi: 10.1016/s0001-6918(02)00134-8. [DOI] [PubMed] [Google Scholar]
- Van Hoomissen JD, Holmes PV, Zellner AS. Effects of B-adrenoreceptor blockade during chronic exercise on contextual fear conditioning and mRNA for galanin and brain-derived neurotrophoc factor. Behavioral Neuroscience. 2004;118(6):1378–1390. doi: 10.1037/0735-7044.118.6.1378. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Wu A, Gomez-Pinilla F. Coupling energy metabolism with a mechanism to support brain-deived neurotrophic factor mediated synaptic plasticity. Neuroscience. 2006;139:1221–1234. doi: 10.1016/j.neuroscience.2006.01.062. [DOI] [PubMed] [Google Scholar]
- Weiss IC, Pryce CR, Jongen-Rêlo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behavioral Brain Research. 2004;152(2):279–295. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Wright LD, Hébert KE, Perrot-Sinal TS. Periadolescent stress exposure exerts long-term effects on adult stress responding and expression of prefrontal dopamine receptors in male and female rats. Psychoneuroendocrinology. 2008;33(2):130–142. doi: 10.1016/j.psyneuen.2007.10.009. [DOI] [PubMed] [Google Scholar]