Abstract
Institutional data are conflicting regarding the prognosis of breast cancer patients with extensive (≥10) axillary lymph node (ALN) metastases. We hypothesized that overall survival (OS) and disease specific survival (DSS) improved after the introduction of anthracycline-based therapy in 1997. We used the Surveillance, Epidemiology, and End results (SEER) database to identify breast cancer patients with ≥10 ALN metastases diagnosed between 1988 and 2004. Patients were categorized according to whether they were diagnosed prior to the FDA approval of anthracyclines (pre-anthracycline era, pre-AE) or after approval (post-anthracycline era, post-AE). Univariate analyses of OS and DSS were performed using the Kaplan–Meier method and differences assessed via the log rank test. Anthracycline era as an independent predictor of OS and DSS was evaluated using Cox proportional hazards models with patient age, hormone receptor status, tumor size, use of radiation therapy, and number of metastatic ALNs as covariates. Entry criteria were met by 12,653 patients. Of these, 5,655 (44.7%) and 6,998 (55.3%) were treated in the pre-AE and post-AE, respectively. On univariate analysis, post-AE patients experienced significantly improved rates of OS (P<0.001) and DSS (P<0.001) relative to pre-AE patients. On multivariate analysis, treatment in the post-AE favorably influenced both OS (Hazard Ratio [HR] 0.90, 95% Confidence Interval [CI] 0.84–0.96) and DSS (HR 0.84, CI 0.79–0.91). Both OS and DSS are poor in patients with extensive ALN metastases. Patients with advanced breast cancer treated in the post-AE demonstrated superior OS and DSS.
Keywords: Anthracycline, Advanced breast cancer, Survival, SEER
Introduction
The status of the axillary lymph nodes (ALN) is the most important predictor of overall survival (OS) and disease-specific survival (DSS) for patients with breast cancer [1, 2]. While the mere presence of ALN metastasis is prognostic, so too is the total number of ALN metastases. Studies of survival in breast cancer patients with ALN metastasis prior to the widespread adoption of adjuvant systemic chemotherapy demonstrated a correlation between the number of positive LN and DFS [3]. Patients with ≥4 positive ALN had a 5 year DFS of 39.7%, but those with ≥13 positive ALN had a DFS of only 16.4% [3]. The current 6th edition staging system of the American Joint Committee on Cancer (AJCC) takes into account the importance of the total number of ALN metastases to breast cancer outcomes. Patients with extensive ALN metastases (≥10) are categorized as having stage IIIc disease using current AJCC criteria. Survival rates for this select group of patients with extensive disease have been rather wide-ranging. Five year rates of OS have ranged from <30 to >70% [4–9].
In 1997 the United States Federal Drug Administration (FDA) approved anthracyclines for use an adjuvant systemic therapy agent for breast cancer. Subsequent randomized controlled trials have confirmed a DSS and OS benefit to the use of anthracyclines in patients with node positive breast cancer.
Our study objective was to estimate the true rate of OS and DSS in a large sample of breast cancer patients with ≥10 ALN metastases. We hypothesized that those patients treated prior to the FDA approval of anthracyclines for adjuvant therapy (pre-anthracycline era, pre-AE) would have poorer survival rates than those treated after anthracycline approval (post-anthracycline era, post-AE).
Methods
We used the Surveillance Epidemiology and End Results (SEER) database of the National Cancer Institute to identify patients with invasive ductal carcinoma (IDC), lobular carcinoma (ILC), ormixed ductal/lobular carcinoma (MDLC) of the breast associated with ≥10 metastatic lymph nodes diagnosed between 1988 and 2005. The geographic scope of the current SEER registry has been reported previously [10–12]. SEER registries routinely collect data on patient demographics, primary tumor site, tumor morphology, stage at diagnosis, first course of treatment, and follow-up vital status.
Patients were excluded if they had distant metastases, or the diagnosis of advanced BCa was documented only by death certificate or autopsy. Additional exclusions were made for patients with incomplete data. The final sample included 12,653 patients. Patients diagnosed with breast cancer prior to 1998 were categorized as being treated in the pre-AE era; those diagnosed in 1998 and beyond were categorized as being treated in the post-AE era. We compared differences among eras using Chi-square testing for categorical variables and proportions.
Survival was calculated as the number of completed months between the date of diagnosis and whichever occurred first: date of death, date last known to be alive, or December 31, 2004. The survival endpoints for the present study were OS and DSS. Patients who were lost to follow-up or survived beyond December 31, 2004 were coded as censored observations.
Patient, tumor, and treatment factors of known or potential prognostic importance were examined by univariate analysis for their influence on OS and DSS using the Kaplan–Meier method. Statistical differences among or between survival curves were assessed via the log-rank test. Variables subjected to univariate analysis included age (median split, ≤56 vs. >56), sex, tumor size (median split, ≤32 vs. >32 mm), estrogen receptor (ER) and progester-one receptor (PR) status (positive, negative, equivocal, unknown), use of radiation therapy (yes vs. no), and anthracycline era.
Significant factors from the univariate analysis were included in multivariate Cox proportional hazards models to assess significant predictors of OS and DSS. The covariates age and tumor size were analyzed as continuous variables in the multivariate models. Estimated risks of death were reported as hazard ratios (HR) with 95% confidence intervals (CI). Statistical significance was indicated by P<0.05. All analyses were conducted using STATA version 10 (StataCorp, College Station, Texas).
Results
Patient, tumor and treatment characteristics of the two study populations are detailed in Table 1. Briefly, the median age of patients in our study was 56 years. The median number of ALN metastases in our patients was 14. Women comprised the overwhelming majority of our study population (99.1%); 115 men (0.9%) were included. The median tumor size was 32 mm. The most common histology was IDC (75.3%); ILC and MDLC represented 14.5 and 10.2% of tumors, respectively. Overall, the majority of tumors were ER positive (50.5%); the remainder were ER negative (21%), or equivocal (0.7%). PR status was positive for 41.2% of tumors; the remainder were PR negative (28.8%), or PR equivocal (0.8%). Of note, ER and PR status were unknown in 27.9 and 29.2% of our patients, respectively. Pre-AE and Post-AE groups did not significantly differ from one another with respect to the total number of ALN metastases (<14 vs. ≥14) or sex. Statistically significant differences were noted for all other factors at the P<0.001 level, including age, tumor size, histology, hormone receptor status, and use of radiation therapy.
Table 1.
Patient, tumor and treatment characteristics of patients in the pre- and post-anthracycline eras
| Pre-AE | Post-AE | P-value | |
|---|---|---|---|
| Age (%) | <0.001 | ||
| <56 years | 2,618 (46.3) | 3,553 (50.8) | |
| ≥56 years | 3,037 (53.7) | 3,445 (49.2) | |
| ALN metastasis (%) | <0.07 | ||
| <14 | 2,707 (47.9) | 3,467 (49.5) | |
| ≥14 | 2,948 (52.1) | 3,531 (50.5) | |
| Sex (%) | 0.94 | ||
| Male | 51 (0.9) | 64 (0.9) | |
| Female | 5,604 (99.1) | 6,934 (99.1) | |
| Tumor size (%) | <0.001 | ||
| ≤32 mm | 2,808 (53.3) | 3,195 (48.8) | |
| >32 mm | 2,459 (46.7) | 3,353 (51.2) | |
| Histology (%) | <0.001 | ||
| IDC | 4,476 (79.2) | 5,047 (72.1) | |
| ILC | 756 (13.4) | 1,077 (15.4) | |
| MDLC | 423 (7.4) | 874 (12.5) | |
| ER status (%) | <0.001 | ||
| Positive | 2,767 (48.9) | 3,618 (51.7) | |
| Negative | 1,155 (20.4) | 1,503 (21.5) | |
| Equivocal | 71 (1.3) | 12 (0.2) | |
| Unknown | 1,662 (29.4) | 1,865 (26.6) | |
| PR status (%) | <0.001 | ||
| Positive | 2,907 (41.5) | 2,310 (40.9) | |
| Negative | 2,099 (30) | 1,543 (27.3) | |
| Equivocal | 34 (0.5) | 64 (1.1) | |
| Unknown | 1,958 (28) | 1,738 (30.7) | |
| Radiation (%) | <0.001 | ||
| No radiation | 3,263 (57.7) | 2,959 (42.3) | |
| Radiation | 2,392 (42.3) | 4,039 (57.7) |
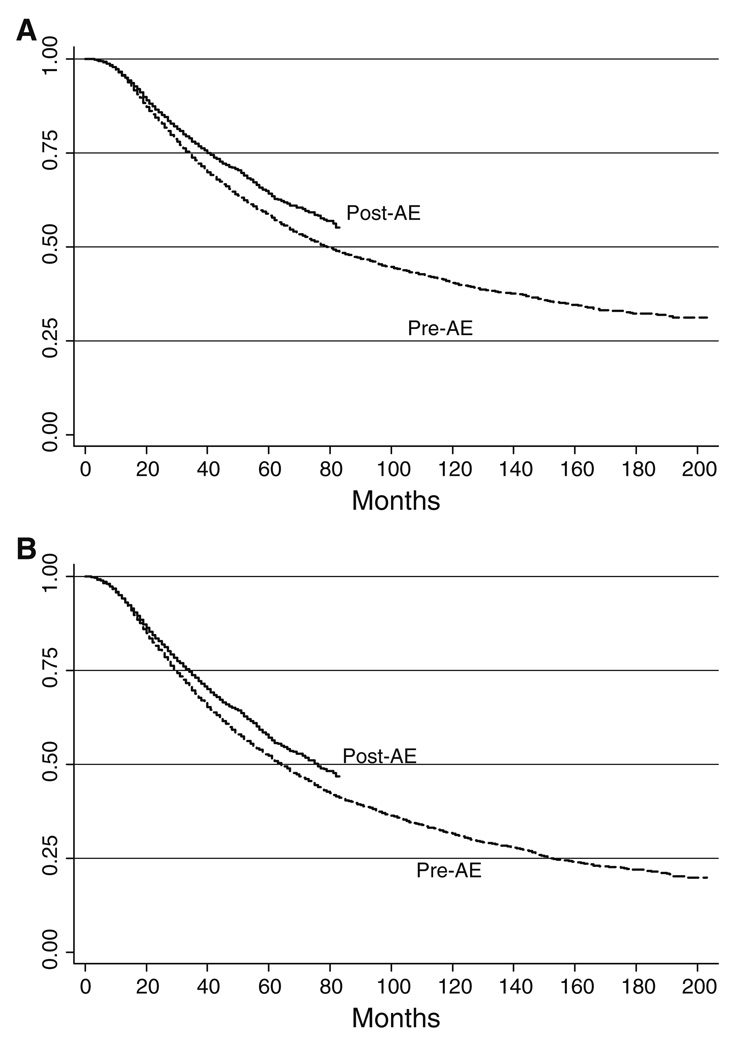
Significant predictors of DSS and OS are presented in Table 2. Only sex was not a significant predictor of DSS or OS. All other covariates were significant at the P<0.001 level. The median DSS and OS for the population as a whole were 86 and 68 months, respectively (Fig. 1). For the pre-AE group, the median DSS was 79 months, but the median DSS was not yet reached in the post AE group. Five-year DSS rates were 58 and 64% for pre-AE and post-AE groups, respectively. After 80 months of follow-up, DSS rates were 50 and 57% in the pre-AE and post-AE groups, respectively. For the pre-AE group, the median OS was 64 months, and the median OS was 76 months in the post AE group. Five-year OS rates were 52 and 57% for pre-AE and post-AE groups, respectively. After 80 months of follow-up, OS rates were 42 and 48% in the pre-AE and post-AE groups, respectively.
Table 2.
Univariate analysis
| Disease specific survival (P-value) |
Overall survival (P-value) |
|
|---|---|---|
| Age | <0.001 | <0.001 |
| <56 years | ||
| ≥56 years | ||
| ALN metastasis | <0.001 | <0.001 |
| <14 | ||
| ≥14 | ||
| Sex | <0.65 | <0.15 |
| Male | ||
| Female | ||
| Tumor size | <0.001 | <0.001 |
| ≤32 mm | ||
| >32 mm | ||
| Histology | <0.001 | <0.001 |
| IDC | ||
| ILC | ||
| MDLC | ||
| ER status | <0.001 | <0.001 |
| Positive | ||
| Negative | ||
| Equivocal | ||
| Unknown | ||
| PR status | <0.001 | <0.001 |
| Positive | ||
| Negative | ||
| Equivocal | ||
| Unknown | ||
| Radiation (%) | <0.001 | <0.001 |
| No radiation | ||
| Radiation |
Fig. 1.
a Disease specific survival. b Overall survival
We excluded sex from the multivariate models. The final Cox proportional hazards models for DSS and OS are presented in Table 3. Briefly, compared to pre-AE patients, post-AE patients demonstrated a decreased risk of death due to breast cancer (HR 0.84, CI 0.79–0.91; P<0.001) or any cause (HR 0.90, CI 0.84–0.96; P<0.001).
Table 3.
Multivariate analysis
| Disease-specific survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age | 1.01 | 1.01–1.01 | <0.001 | 1.02 | 1.01–1.02 | <0.001 |
| Tumor size (mm) |
1.00 | 1.00–1.00 | <0.001 | 1.00 | 1.00–1.00 | <0.001 |
| # ALN metastasis |
1.02 | 1.02–1.03 | <0.001 | 1.02 | 1.01–1.02 | <0.001 |
| ER status | ||||||
| Positive | Referent | |||||
| Negative | 1.84 | 1.67–2.02 | <0.001 | 1.71 | 1.57–1.86 | <0.001 |
| Borderline | 1.45 | 1.06–1.97 | <0.02 | 1.28 | 0.96–1.70 | <0.10 |
| Unknown | 1.62 | 1.25–2.10 | <0.001 | 1.62 | 1.29–2.05 | <0.001 |
| PR status | ||||||
| Positive | Referent | |||||
| Negative | 1.35 | 1.23–1.48 | <0.001 | 1.31 | 1.21–1.42 | <0.001 |
| Borderline | 1.92 | 1.44–2.56 | <0.001 | 1.71 | 1.30–2.23 | <0.001 |
| Unknown | 0.91 | 0.71–1.18 | <0.50 | 0.84 | 0.67–1.06 | <0.15 |
| Radiation | ||||||
| No | Referent | |||||
| Yes | 0.79 | 0.74–0.84 | <0.001 | 0.76 | 0.72–0.81 | <0.001 |
| Anthracycline era | ||||||
| Pre-AE | Referent | |||||
| Post-AE | 0.84 | 0.79–0.91 | <0.001 | 0.90 | 0.84–0.96 | 0.001 |
Covariates associated with an increased risk of breast cancer-related death included increasing patient age (HR 1.01, CI 1.01–1.01; P<0.001), increasing tumor size (HR 1.00, CI 1.00–1.00; P<0.001), increasing numbers of ALN metastases (HR 1.02, CI 1.02–1.03; P<0.001), ER negative status (HR 1.84, CI 1.67–2.02; P<0.001), ER status borderline (HR 1.45, CI 1.06–1.97; P<0.02), ER status unknown (HR 1.62, CI 1.25–2.10; P<0.001), PR negative status (HR 1.35, CI 1.23–1.48; P<0.001), and PR status borderline (HR 1.92, CI 1.44–2.56; P<0.001). Other than treatment in the post-AE, only use of radiation predicted a decreased risk of breast cancer-related mortality (HR 0.79, CI 0.74–0.84; P<0.001).
Covariates predicting an increased risk of death due to any cause included increasing age (HR 1.02, CI 1.01–1.02; P<0.001), increasing tumor size (HR 1.00, CI 1.00–1.00; P<0.001), increasing number of ALN metastases (HR 1.02, CI 1.01–1.02; P<0.001), ER negative status (HR 1.71, CI 1.57–1.86; P<0.001), ER status unknown (HR 1.62, CI 1.29–2.05; P<0.001), PR negative status (HR 1.31, CI 1.21–1.42; P < 0.001), and PR status borderline (HR 1.71, CI 1.30–2.23; P < 0.001). Other than treatment in the post-AE, a decreased risk of mortality due to any cause was predicted by the use of radiation (HR 0.76, CI 0.72–0.81; P < 0.001).
Discussion
Breast cancer with extensive ALN metastases has long been a clinical challenge. High-dose chemotherapy with bone marrow transplantation was conceived in an effort to provide these patients with an improvement in their otherwise dismal rates of survival. Although results were initially promising, no significant benefit in OS was ultimately demonstrated [13–17]. At a time when the merits of bone marrow transplantation and high-dose chemotherapy were being challenged, anthracyclines were approved for adjuvant use in breast cancer by the FDA. The basis for this approval was solid randomized controlled trial evidence demonstrating improvements in DSS and OS [18, 19].
Our data indicate that the 5 year survival rates for AJCC stage IIIc breast cancer patients with ≥10 ALN metastases is intermediate between the most pessimistic and optimistic values previously reported (approximate range, 30–70%). For our entire cohort, 5 year DSS and OS rates were 61 and 54.3%. We hypothesized that the introduction of anthracyclines into the adjuvant treatment regimen for breast cancer would lead to improvements to DSS and OS. Indeed, this is what we found. Post-AE patients had a 6 and 5% benefits in 5 year DSS and OS relative to their pre-AE counterparts.
Improved outcomes for breast cancer patients with extensive ALN metastases have also been reported with the use of adjuvant radiation therapy. It is well-established that any patient undergoing lumpectomy should undergo radiation therapy to minimize rates of local recurrence. However, data from trials of post-mastectomy radiation therapy indicate that there is both a recurrence-free and OS advantage to the use of radiation therapy in patients with ≥4 ALN metastases. Current guidelines favor the use of radiation with ≥4 ALN metastases. Given current guidelines, all of our patients would be candidates for radiation therapy. Nevertheless, approximately 49% of our entire study population did not receive radiation therapy. Pre-AE patients received lower rates of radiation therapy than their post-AE counterparts (42.3 vs. 57.7%), which is consistent with the fact that the main trials supporting the use of radiation therapy for advanced nodal disease were published in 1997. The discrepancy between the use of radiation therapy between pre-and post-AE groups may partially explain the survival differences noted in our study.
Our study is further complicated by the fact that we make no direct measurement of the use of chemotherapy among our study patients. SEER data do not include information on use of hormonal therapy or chemotherapy. Furthermore, SEER does not provide information on health comorbidities that might otherwise preclude the use of adjuvant therapy. It was not our goal to measure the usage of chemotherapy in one time-period compared to another, however. Our objective was to provide an accurate estimate of the DSS and OS of breast cancer patients with extensive (≥10) ALN metastases based on “real world” population-based data. Furthermore, our objective was to demonstrate that survival rates have positively changed since anthracycline agents became available for adjuvant use.
ALN status remains the most important factor determining breast cancer outcome, but patient and tumor-specific factors such as age, tumor size and hormone receptor status are also important. In our study population, the number of ALN metastases beyond 10 had a modest impact on DSS and OS. For each ALN metastasis beyond 10, there was a 2% increased risk of death due to breast cancer or any cause. Advancing age and tumor size were associated with minimal, but likely clinically unimportant, increases in the risk of mortality. With regard to hormone receptor status, as expected patients that were ER or PR negative had a higher risk of mortality. ER negative patients had an 84 and 71% increased risk of mortality due to breast cancer or any cause, respectively. Similarly, PR negative patients had a 35 and 31% increased risk of mortality due to breast cancer or any cause, respectively.
Both DSS and OS are poor in patients with extensive ALN metastases. Patients with advanced breast cancer and extensive ALN metastases treated in the post-AE demonstrated superior DSS and OS.
Acknowledgments
Supported by Grant Number UL1 RR024146 from the National Center for Research Resources (NCRR) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NNCRR or NIH.
Footnotes
Information on NCRR is available athttp://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Contributor Information
Shannon H. Beal, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA
Steve R. Martinez, Email: steve.martinez@ucdmc.ucdavis.edu, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA; UC Davis Cancer Center, 4501 X Street, Suite 3010, Sacramento, CA 95817, USA.
Robert J. Canter, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA
Steven L. Chen, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA
Vijay P. Khatri, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA
Richard J. Bold, Department of Surgery, Division of Surgical Oncology, University of California Davis, Sacramento, CA, USA
References
- 1.Beenken SW, Urist MM, Zhang Y, Desmond R, Krontiras H, et al. Axillary lymph node status, but not tumor size, predicts locoregional recurrence and overall survival after mastectomy for breast cancer. Ann Surg. 2003;237(5):732–738. doi: 10.1097/01.SLA.0000065289.06765.71. discussion 38–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavigne JC, Desaive CJ. Cancer of the breast. A study of prognostic factors as a guide in selecting cases for conservative treatment. Acta Chir Belg. 1975;74(1):63–81. [PubMed] [Google Scholar]
- 3.Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52(9):1551–1557. doi: 10.1002/1097-0142(19831101)52:9<1551::aid-cncr2820520902>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Nemoto T, Vana J, Bedwani RN, Baker HW, McGregor FH, Murphy GP. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45(12):2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::aid-cncr2820451203>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Moon TE, Jones SE, Bonadonna G, Valagussa P, Powles T, et al. Development and use of a natural history data base of breast cancer studies. Am J Clin Oncol. 1987;10(5):396–403. doi: 10.1097/00000421-198710000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Buzdar AU, Kau SW, Hortobagyi GN, Ames FC, Holmes FA, et al. Clinical course of patients with breast cancer with ten or more positive nodes who were treated with doxorubicin-containing adjuvant therapy. Cancer. 1992;69(2):448–452. doi: 10.1002/1097-0142(19920115)69:2<448::aid-cncr2820690229>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 7.Montero AJ, Rouzier R, Lluch A, Theriault RL, Buzdar AU, et al. The natural history of breast carcinoma in patients with > or =10 metastatic axillary lymph nodes before and after the advent of adjuvant therapy: a multiinstitutional retrospective study. Cancer. 2005;104(2):229–235. doi: 10.1002/cncr.21182. [DOI] [PubMed] [Google Scholar]
- 8.Walker MJ, Osborne MD, Young DC, Schneebaum S, La Valle GJ, Farrar WB. The natural history of breast cancer with more than 10 positive nodes. Am J Surg. 1995;169(6):575–579. doi: 10.1016/s0002-9610(99)80224-4. [DOI] [PubMed] [Google Scholar]
- 9.Hoehne F, Chen S, Mabry H, Giuliano AE. An update on prognosis in breast cancer patients with extensive axillary disease. Breast J. 2008;14(1):76–80. doi: 10.1111/j.1524-4741.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 10.Leggett MD, Chen SL, Schneider PD, Martinez SR. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Ann Surg Oncol. 2008;15(9):2493–2499. doi: 10.1245/s10434-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 11.Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg. 2008;196(4):495–499. doi: 10.1016/j.amjsurg.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Martinez SR, Robbins AS, Meyers FJ, Bold RJ, Khatri VP, et al. Racial and ethnic differences in treatment and survival among adults with primary extremity soft-tissue sarcoma. Cancer. 2008;112(5):1162–1168. doi: 10.1002/cncr.23261. [DOI] [PubMed] [Google Scholar]
- 13.Ayash LJ, Elias A, Schwartz G, Wheeler C, Ibrahim J, et al. Double dose-intensive chemotherapy with autologous stem-cell support for metastatic breast cancer: no improvement in progression- free survival by the sequence of high-dose melphalan followed by cyclophosphamide, thiotepa, and carboplatin. J Clin Oncol. 1996;14(11):2984–2992. doi: 10.1200/JCO.1996.14.11.2984. [DOI] [PubMed] [Google Scholar]
- 14.Farquhar C, Marjoribanks J, Basser R, Lethaby A. High dose chemotherapy and autologous bone marrow or stem cell transplantation versus conventional chemotherapy for women with early poor prognosis breast cancer. Cochrane Database Syst Rev. 2005;3 doi: 10.1002/14651858.CD003139.pub2. CD003139. [DOI] [PubMed] [Google Scholar]
- 15.Hortobagyi GN, Buzdar AU, Theriault RL, Valero V, Frye D, et al. Randomized trial of high-dose chemotherapy and blood cell autografts for high-risk primary breast carcinoma. J Natl Cancer Inst. 2000;92(3):225–233. doi: 10.1093/jnci/92.3.225. [DOI] [PubMed] [Google Scholar]
- 16.Leonard RC, Lind M, Twelves C, Coleman R, van Belle S, et al. Conventional adjuvant chemotherapy versus single-cycle, auto-graft-supported, high-dose, late-intensification chemotherapy in high-risk breast cancer patients: a randomized trial. J Natl Cancer Inst. 2004;96(14):1076–1083. doi: 10.1093/jnci/djh188. [DOI] [PubMed] [Google Scholar]
- 17.Rodenhuis S, Richel DJ, van der Wall E, Schornagel JH, Baars JW, et al. Randomised trial of high-dose chemotherapy and haemopoietic progenitor-cell support in operable breast cancer with extensive axillary lymph-node involvement. Lancet. 1998;352(9127):515–521. doi: 10.1016/S0140-6736(98)01350-6. [DOI] [PubMed] [Google Scholar]
- 18.Early Breast Cancer Trialists’ Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352(9132):930–942. [PubMed] [Google Scholar]
- 19.Bonadonna G, Zambetti M, Valagussa P. Sequential or alternating doxorubicin and CMF regimens in breast cancer with more than three positive nodes. Ten-year results. Jama. 1995;273(7):542–547. [PubMed] [Google Scholar]