Abstract
Background
The hierarchical organization of hematopoiesis with unidirectional lineage determination has become a questionable tenet in view of the experimental evidence of reprogramming and transdifferentiation of lineage-determined cells. Clinical examples of hematopoietic lineage plasticity are rare. Here we report on a patient who presented with an acute B-lymphoblastic leukemia and developed a Langerhans’ cell sarcoma 9 years later. We provide evidence that the second neoplasm is the result of transdifferentiation.
Design and Methods
B-cell acute lymphoblastic leukemia was diagnosed in an 11-year old boy in 1996. Treatment according to the ALL-BFM-1995 protocol resulted in a complete remission. Nine years later, in 2005, Langerhans’ cell sarcoma was diagnosed in a supraclavicular lymph node. Despite treatment with different chemotherapy protocols the patient had progressive disease. Finally, he received an allogeneic peripheral blood stem cell transplant and achieved a continuous remission. Molecular studies of IGH- and TCRG-gene rearrangements were performed with DNA from the Langerhans’ cell sarcoma and the cryopreserved cells from the acute B-lymphoblastic leukemia. The expression of PAX5 and ID2 was analyzed with real-time reverse transcriptase polymerase chain reaction.
Results
Identical IGH-rearrangements were demonstrated in the acute B-lymphoblastic leukemia and the Langerhans’ cell sarcoma. The key factors required for B-cell and dendritic cell development, PAX5 and ID2, were differentially expressed, with a strong PAX5 signal in the acute B-lymphoblastic leukemia and only a weak expression in the Langerhans’ cell sarcoma, whereas ID2 showed an opposite pattern.
Conclusions
The identical IGH-rearrangement in both neoplasms indicates transdifferentiation of the acute B-lymphoblastic leukemia into a Langerhans’ cell sarcoma. Loss of PAX5 and the acquisition of ID2 suggest that these key factors are involved in the transdifferentiation from a B-cell phenotype into a Langerhans’/dendritic cell phenotype. (Clinical trial registration at: Deutsches KrebsStudienRegister, http://www.studien.de, study-ID:8)
Keywords: hematopoiesis, IGH-rearrangement, neoplasms, PAX5, B-ALL
Introduction
Dendritic cells are part of the hematopoietic system and are derived from CD34-positive hematopoietic progenitor cells. They are a heterogeneous group of immune accessory cells with a pivotal role in the onset of innate immunity and peripheral tolerance. First described in 1868 by Paul Langerhans but misinterpreted as cutaneous nerve cells, they were discovered almost a century later in mouse spleen and termed “dendritic” cells based on their characteristic morphological appearance as described by Steinman and Cohn.1,2 There are four types of dendritic cells in the lymph node, each with distinct structural, functional and morphological features: follicular, interdigitating, Langerhans’ histiocytic/fibroblastic cells and plasmacytoid dendritic cells. The origin of dendritic cells long remained on the fence between stromal cell derivation and hematopoietic origin. First evidence for a hematopoietic origin was provided by epidemiological studies which found that dendritic cell-derived Langerhans’ cell histiocytosis and acute lymphoblastic leukemia occurred in the same individuals at a frequency greater than could be expected by chance alone suggesting a relation between the two diseases.3
The hematopoietic origin of dendritic cells and the relation between lymphoid and dendritic cell lineages was subsequently demonstrated in a mouse transplantation model with the reconstitution of dermal dendritic cells from myeloid and lymphoid progenitors.4 Further evidence of the relation was provided by studies of the malignant counterparts of Langerhans’ cell histiocytosis which displayed molecular features indicative of a clonal relationship between histiocytic/dendritic cell neoplasms and lymphoproliferative diseases.5–7 This raised the question of whether an acute B-lymphoblastic leukemia (B-ALL) and a malignant Langerhans’ cell sarcoma (LCS) occurring in the same patient are the result of a direct progression of the BALL into the LCS or whether both malignancies originate from the same hematopoietic precursor cell or from unrelated hematopoietic progenitors. To answer this question, we investigated the clinical course of a patient with a BALL and asynchronous occurrence of LCS 9 years later. Immunohistochemical studies were performed to verify the diagnosis of LCS. Molecular studies of cryopreserved blast cells and of biopsy specimens from the LCS were undertaken to elucidate the biological relationship between the two malignancies.
Design and Methods
The patient’s characteristics
The 21-year old male patient was first diagnosed with a malignant LCS in 2005 and had a history of B-ALL 9 years previously. The diagnosis of a CD10-positive B-ALL was established with flow cytometry in 1996 (Figure 1). Informed consent was obtained from the guardians of the patient for treatment within the ALL-BFM95 trial. The study, registered at Deutsches KrebsStudien Register (http://www.studien.de) under study-ID 8, was performed in accordance with the Declaration of Helsinki and approved by the ethics review committee of the principal investigator’s institution. B-ALL cells from the bone marrow and peripheral blood were cryopreserved in liquid nitrogen in 1996.
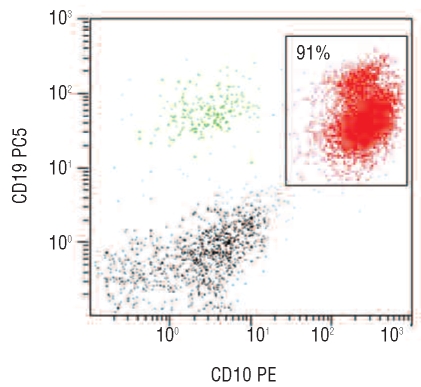
Figure 1.
Two-dimensional dot plot of the CD10 and CD19 staining of the bone marrow sample in 1996. Coexpression of CD19 and CD10 is compatible with a precursor B-cell acute leukemia (CD10 positive common-ALL).
Immunohistochemistry
Immunohistochemical staining of the supraclavicular lymph node and of an ileocecal biopsy was carried out with the following antibodies: CD45, vimentin, HLA-DR, CD68 (clone PGM-1), S-100, CD1a, lysozyme, langerin, CD21, CD23, clusterin, CD123, CD20, CD19, PAX-5, CD34, CD117, CD10, TdT, CD15, CD3, CD2, CD30, ALK, Melan A, HMB45 and Ki-67.
Molecular analyses
Analysis of IGH- and TCRG-gene rearrangements was performed by polymerase chain reaction (PCR) with DNA extracted from the supraclavicular lymph node, the ileocecal manifestation and the cryopreserved B-ALL blasts. IGH-PCR was carried out with the three different BioMed-2 primer combinations specific for IGH framework regions 1, 2 and 3, each in conjunction with the same JH primer. For TCRG-PCR the BioMed-2 primer combinations A and B were used covering all possible TCRG-gene rearrangements. Fluorescence-labeled PCR products were applied to capillary electrophoresis (GeneScan). DNA sequences of dominant IGH-PCR products derived from the B-ALL and the LCS (supraclavicular lymph node) were compared to each other and to the corresponding germ line segment.
Real-time reverse transcriptase polymerase chain reaction
TaqMan® reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) were applied to transcribe DNase I digested total RNA in cDNA with random hexamer primers. Real-time PCR for PAX5 and ID2 was carried out with SYBR® Green PCR-Master Mix on a GeneAmp®7000 sequence detection system employing the PCR parameters recommended by Applied Biosystems. The housekeeping gene succinate dehydrogenase complex, subunit A (SDHA) was amplified in parallel. Relative mRNA quantification was calculated using the comparative ΔΔCT method.
Results
Clinical course
In July 1996, an 11-year old boy presented with a CD10-positive B-ALL confirmed by flow cytometry (Figure 1). Cytogenetic and molecular analyses showed no evidence of chromosomal translocations or characteristic gene fusions. The patient was treated with chemotherapy according to the German ALL-BFM95 protocol for low-risk ALL and achieved a complete remission.8 He remained in complete remission and in good health until July 2005, when he started to complain of increasing abdominal pain. On admission to hospital for investigations he had a continuous fever of 39° C for 3–4 days, elevated liver enzymes and evidence of infection with a C-reactive protein of 278 mg/L. His blood count showed a leukocytosis of 23×109/L without any blast cells and thrombocytosis of 645×109/L. Imaging analysis by ultrasound and computed tomography scanning revealed enlarged mediastinal, pulmonary and abdominal lymph nodes. A biopsy was taken from a palpable supraclavicular lymphnode.
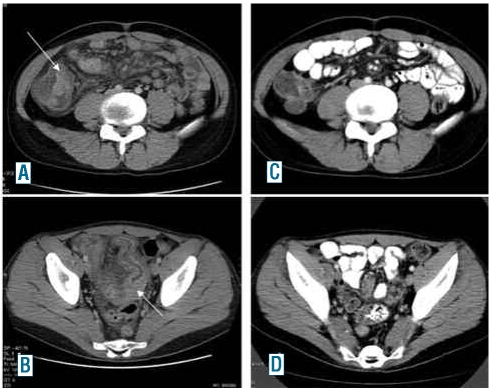
After the immunohistological diagnosis of a malignant LCS the patient was first treated with vinblastine and prednisolone but did not achieve a sustained remission. Restaging imaging was indicative of progressive disease with infiltration of the ileum (Figure 3A and B) and colonoscopy revealed an intraluminal tumor with infiltration of the terminal ileum histologically consistent with the previously diagnosed LCS. In a further effort to induce a durable remission one course of high-dose cytosine arabinoside and mitoxantrone (HAM) was administered with only minor effects on tumor regression and clinical symptoms. Since allogeneic stem cell transplantation remained the only curative therapeutic option, the patient was given a conditioning regimen of 2 x 60 mg/kg cyclophosphamide and 2 x 6 Gy of fractionated total body irradiation and allogeneic peripheral blood stem cells were transplanted from a matched unrelated donor in November 2006. The patient subsequently developed mild mucosal and hepatic graft-versus-host disease despite immunosuppressive therapy with cyclosporine and prednisolone. Computed tomography scans in January and August 2007 showed complete regression of the lymphadenopathy and the ileocecal manifestations except for a stationary residual lymph node of 1 cm adjacent to the celiac trunk (Figure 2C and 2D). Only 5 months later, in December 2007, the patient complained of recurrent abdominal pain and imaging analysis again revealed enlarged retroperitoneal lymph nodes. Repeated histological analysis was consistent with a relapse of the LCS and excluded a post-transplant lymphadenopathy. We stopped immunosuppressive therapy with consequent deterioration of the mucosal, conjunctival and hepatic graft-versus-host disease but, fortunately, simultaneous improvement of the abdominal symptoms. Repeated imaging analysis 2 months after cessation of immunosuppression showed a partial regression of the still enlarged abdominal lymph nodes. Currently, 3 years after bone marrow transplantation the patient is in a continuous remission with further regression of the enlarged retroperitoneal lymph nodes.
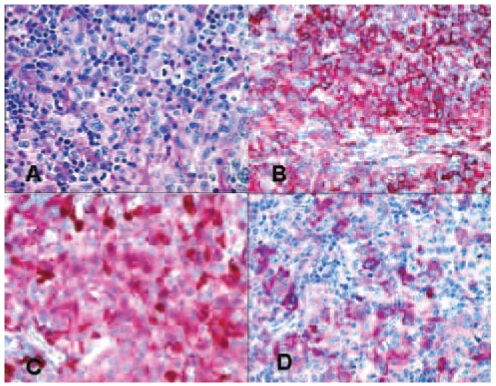
Figure 3.
Hematoxylin and eosin (H&E) staining and immunohistochemical (APAAP) labeling of the supraclavicular node for certain marker molecules. (A) H&E-staining, (B) labeling for CD45, (C) Labeling for S-100 and (D) labeling for CD1a.
Figure 2.
Computed tomography imaging of the abdominal manifestations before (A and B) and after (C and D) allogeneic peripheral blood stem cell transplantation.
Phenotypic characteristics of the malignant Langerhans’ cell sarcoma
Histologically, the supraclavicular lymph node showed greatly expanded interfollicular zones occupied by a population of large cells with a slightly eosinophilic, relatively abundant cytoplasm and pleomorphic, grooved, folded, or lobulated nuclei with fine chromatin and occasional small nucleoli (Figure 3A). A few neutrophils and eosinophils as well as occasional small lymphocytes were present within these areas. Immunohistochemical analysis revealed a consistent expression of CD45 (Figure 3B), vimentin, and HLA-DR as well as granular cytoplasmic expression of CD68 (clone PGM-1). Several of the neoplastic cells expressed S-100 (Figure 3C) and some of them also expressed CD1a with variable intensity (Figure 3D). Lysozyme was present in single neoplastic cells. Langerin was not detectable. The absence of CD21, CD23 and clusterin expression excluded a tumor of follicular dendritic cells, whereas the absence of CD123 excluded that the origin of the malignancy was plasmacytoid dendritic cells. There was no evidence of CD20 or CD19 expression, thus excluding any B-lymphocytes or residual B-lymphoblasts in the lymph node. The absence of CD34, CD117 and TdT excluded an origin from myeloid progenitor cells. The absence of CD3 and CD2 expression excluded a T-cell origin. Neither CD30 nor anaplastic lymphoma kinase was expressed. A malignant melanoma was excluded by the negative reaction of the tumor cells for MelanA and HMB45. On the basis of the nuclear morphology being highly consistent with that of Langerhans’ cells, the above described immunophenotype, and the high Ki-67 labeling index (50%), the diagnosis of a LCS was established without ultrastructural demonstration of Birbeck granules in the cytoplasm.
Molecular features of the Langerhans’ cell sarcoma and B-cell acute lymphoblastic leukemia
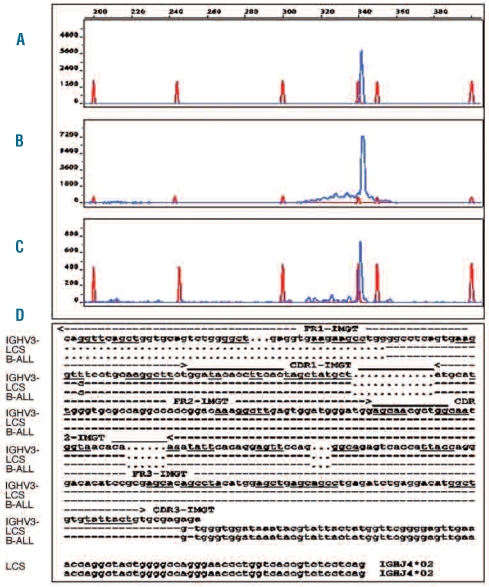
The asynchronous occurrence of both diseases in the same individual, although 9 years apart, raised the possibility of a common origin. We, therefore, investigated the status of the IGH- and TCRG-rearrangements with DNA extracted from the supraclavicular lymph node, the ileocecal manifestation and cryopreserved B-ALL blasts. Dominant amplification IGH-PCR products of the same size and identical DNA-sequence were detectable in all three manifestations. The dendritic cell origin of the clonal IGH-PCR products is highly conclusive given the absence of B cells in the lymph node, as proven by immunohistological staining. Moreover, the detection limit of the PCR assay requires at least 1% of clonal B cells in the sample to generate a dominant PCR product. Thus, a contamination of the IGH-PCR and the GeneScan analysis by residual blast cells or by B-lymphocytes was excluded. Examples of the results of the GeneScan analysis of the IGH-PCR products (primer combination specific for framework 1) are presented in Figure 4A–C. DNA sequences of dominant IGH PCR products (framework 1 and framework 2) of the B-ALL and LCS cells were compared to each other and to the corresponding VH germ line segment (V1-3). The single base substitution found by comparing the tumor-derived sequence with that of the corresponding VH germ line segment most likely represents an interindividual polymorphism rather than a true somatic mutation of the rearranged VH gene segment (Figure 4D). These findings unequivocally demonstrate that the B-ALL and the LCS are outgrowths of the same B-cell clone.
Figure 4.
GeneScan analysis demonstrates the same dominant IGH-PCR products (framework 1 primers) detectable in the LCS manifestations and the B-ALL. (A) B-ALL; (B) LCS in the supracalvicular lymph node and (C) LCS in the ileocecal ecal region; (D) Sequences of the IGH rearrangements of the BALL and the LCS manifestation were compared to each other as well as to their corresponding IGH germ line segments. The IGH rearrangements of both tumors were completely identical including their hypervariable CDR3 proportion. The comparison of the tumor specific IGH rearrangement to the corresponding germ line segments was done by employing the IMGT (http://www.imgt.org) algorithm and revealed an unmutated VH1-3/JH4 rearrangement. The single base substitution most likely represents an interindividual polymorphism.”
To further investigate the relationship between the two malignancies we analyzed the expression of key factors required for B-cell and dendritic cell development, PAX5 and ID2, respectively,9–12 by real-time reverse transcriptase PCR. The two factors were differentially expressed with a strong PAX5 signal in the cryopreserved B-ALL cells and only weak expression in the LCS cells of the neoplastic lymph node, whereas ID2 showed an opposite expression pattern (data not shown).
Discussion
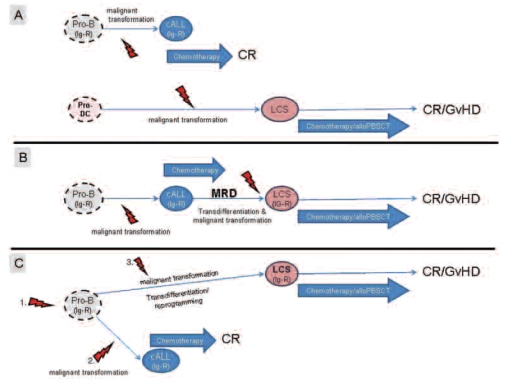
LCS and B-ALL represent two clinically, morphologically and immunophenotypically different hematopoietic diseases which originate from two different cell lineages, the dendritic cell lineage and the B-cell lineage, respectively. Here we report a patient with asynchronous occurrence of LCS 9 years after the diagnosis of childhood BALL. Basically, there are four possible explanations for the asynchronous occurrence of the two different malignancies in the same individual. The first possibility is that there is no relation between the malignancies, their occurrence is merely coincidental and the two diseases derive from two different hematopoietic cell types (Figure 5A). The other three possibilities include alternative pathways of cellular reprogramming.3 One possibility is a direct progression of the B-ALL into the LCS via a residual persisting cell of the B-ALL clone (minimal residual disease) which is induced by a second transforming event (Figure 5B). A further possibility is that a transformed residual progenitor B cell which gave rise to the B-ALL was in a dormant state until it was hit by another oncogenic event which induced its transdifferentiation into a LCS (Figure 5C). Finally, an additional mechanistic possibility exists with the “dedifferentiation” of a residual B-ALL cell to an uncommitted progenitor cell which eventually redifferentiates along the dendritic/Langerhans’ cell pathway (not depicted in Figure 5).14
Figure 5.
Possible cellular transdifferentiation mechanisms for the asynchronous occurrence of a B-ALL and a LCS in the same patient.
In order to determine which of these possibilities is valid we analyzed the IGH-rearrangements of the cryopreserved B-ALL cells and the LCS cells and we measured the expression of the key B-cell lineage factor PAX5 and the key dendritic cell lineage factor ID2 by real-time reverse transcriptase PCR. The molecular analyses of both malignancies displayed IGH-PCR products of the same size and identical DNA-sequences without somatic mutations. The lack of somatic mutations is in keeping with the immature differentiation state of the B-ALL. These findings exclude a merely coincidental occurrence (Figure 5A) of the two malignancies.
In view of the fact that a functional rearrangement of the IG loci is a conditio sine qua non for a committed B-cell precursor15 these findings also make it unlikely that cellular reprogramming occurred by lineage diversion of an uncommitted progenitor cell lacking the B-lineage specific IGH-rearrangement.
The detection of an identical IGH-rearrangement in both neoplasms indicates that the transdifferentiation of the B-ALL into a LCS occurred either by a direct progression of a persisting residual B-ALL cell (Figure 5B) or by reprogramming of a dormant B-lineage committed precursor from which the B-ALL originated (Figure 5C). Theoretically it cannot be excluded that a residual B-ALL-cell dedifferentiated into an uncommitted precursor cell which subsequently developed a dendritic/Langerhans’ cell phenotype. Unfortunately, the data available do not allow a definite conclusion on which of these possibilities was the actual mechanism for the transdifferentiation in the present case. With regards to reports describing that relapses of acute leukemia can occur many years following a complete remission it might well be that a dormant minimal residual disease clone of the B-ALL was hit by a second transforming event which led to the transdifferentiation from a B-ALL phenotype to a malignant LCS (Figure 5B).16 However, in favor of the possibility that the LCS originated from the same B-cell committed B-ALL precursor cell (Figure 5C) are previous findings in patients with classical Hodgkin’s lymphomas who asynchronously developed a follicular lymphoma or a diffuse large B-cell lymphoma.17,18 In these cases the different lymphoma types carried the same IGH rearrangement with common and different somatic mutations. This convincingly excluded a direct progression from one disease type to another and instead indicated a derivation of the different lymphoma types from a common precursor.17,18 Since somatic mutations are absent in the IGH rearrangement of both the B-ALL and the LCS the precise transdifferentiation pathway which led to a change of a B-ALL phenotype into a LCS phenotype in our patient must remain speculative.
We hypothesized that a change in the expression of the transcription factor PAX5 required for the development and maintenance of a B-cell phenotype19 and ID2, the inhibitor of E2A, required for silencing the B-cell phenotype and development of a dendritic cell phenotype were involved in the transdifferentiation from a B-ALL into a LCS.9,10,19–21 Our finding that PAX5 was lost and ID2 was acquired during the transdifferentiation of the B-ALL into the LCS is in keeping with this hypothesis. Similar results were reported by Feldman et al. who demonstrated that none of seven histiocytic/dendritic cell neoplasms that developed following a follicular lymphoma expressed PAX5.5
In conclusion, the clinical, pathological and molecular findings of the patient described here add a further clinical example of the plasticity of hematopoietic cell lineages.
Acknowledgments
we would like to thank Hans-Henning Müller and Elisabeth Oker for their excellent technical contribution.
Footnotes
Authorship and Disclosures
RR wrote the paper, planned the clinical strategy, treated the patient and analyzed the data. MH performed and analyzed molecular studies and wrote the paper. IA and DJ contributed immunohistological analyses. RA planned the clinical strategy, treated the patient and contributed clinical information. BD contributed to the design and data analysis. SM contributed to the design and data analysis. TB and OD contributed clinical details. W-DL contributed to the design and data analysis. HS discovered the case, designed the research, analyzed the data and wrote the paper.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Langerhans P. Über die Nerven der menschlichen Haut. Archiv für pathologische Anatomie und Physiologie, und für Klinische Medizin Berlin. 1868;44:325–37. [Google Scholar]
- 2.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egeler RM, Neglia JP, Aricò M, Favara BE, Heitger A, Nesbit ME, Nicholson HS. The relation of Langerhans cell histiocytosis to acute leukemia, lymphomas, and other solid tumors. The LCH-Malignancy Study Group of the Histiocyte Society. Hematol Oncol Clin North Am. 1998;12(2):369–78. doi: 10.1016/s0889-8588(05)70516-5. [DOI] [PubMed] [Google Scholar]
- 4.Mende I, Karsunky H, Weissman IL, Engleman EG, Merad M. Flk2+ myeloid progenitors are the main source of Langerhans cells. Blood. 2006;107(4):1383–90. doi: 10.1182/blood-2005-05-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman AL, Arber DA, Pittaluga S, Martinez A, Burke JS, Raffeld M, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111(12):5433–9. doi: 10.1182/blood-2007-11-124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldman AL, Berthold F, Arceci RJ, Abramowsky C, Shehata BM, Mann KP, et al. Clonal relationship between precursor T-lymphoblastic leukaemia/lymphoma and Langerhans-cell histiocytosis. Lancet Oncol. 2005;6(6):435–7. doi: 10.1016/S1470-2045(05)70211-4. [DOI] [PubMed] [Google Scholar]
- 7.Feldman AL, Minniti C, Santi M, Downing JR, Raffeld M, Jaffe ES. Histiocytic sarcoma after acute lymphoblastic leukaemia: a common clonal origin. Lancet Oncol. 2004;5(4):248–50. doi: 10.1016/S1470-2045(04)01428-7. [DOI] [PubMed] [Google Scholar]
- 8.Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14(12):2205–22. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 9.Hacker C, Kirsch RD, Ju XS, Hieronymus T, Gust TC, Kuhl C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol . 2003 Apr;4(4):380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 10.Heinz LX, Platzer B, Reisner PM, Jörgl A, Taschner S, Göbel F, Strobl H. Differential involvement of PU.1 and Id2 downstream of TGF-beta1 during Langerhans-cell commitment. Blood. 2006;107(4):1445–53. doi: 10.1182/blood-2005-04-1721. [DOI] [PubMed] [Google Scholar]
- 11.Tagoh H, Ingram R, Wilson N, Salvagiotto G, Warren AJ, Clarke D, et al. The mechanism of repression of the myeloid-specific c-fms gene by Pax5 during B lineage restriction. EMBO J. 2006;25(5):1070–80. doi: 10.1038/sj.emboj.7600997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiacci E, Pileri S, Orleth A, Pacini R, Tabarrini A, Frenguelli F, et al. PAX5 expression in acute leukemias: higher B-lineage specificity than CD79a and selective association with t(8;21)-acute myelogenous leukemia. Cancer Res. 2004;64(20):7399–404. doi: 10.1158/0008-5472.CAN-04-1865. [DOI] [PubMed] [Google Scholar]
- 13.Cobaleda C, Busslinger M. Developmental plasticity of lymphocytes. Curr Opin Immunol. 2008;20(2):139–48. doi: 10.1016/j.coi.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449(7161):473–7. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- 15.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112(5):1570–80. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vora A, Frost L, Goodeve A, Wilson G, Ireland RM, Lilleyman J, et al. Late relapsing childhood lymphoblastic leukemia. Blood. 1998;92(7):2334–7. [PubMed] [Google Scholar]
- 17.Küppers R, Sousa AB, Baur AS, Strickler JG, Rajewsky K, Hansmann ML. Common germinal-center B-cell origin of the malignant cells in two composite lymphomas, involving classical Hodgkin’s disease and either follicular lymphoma or B-CLL. Mol Med. 2001;7(5):285–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Marafioti T, Hummel M, Anagnostopoulos I, Foss HD, Huhn D, Stein H. Classical Hodgkin’s disease and follicular lymphoma originating from the same germinal center B cell. J Clin Oncol. 1999;17(12):3804–9. doi: 10.1200/JCO.1999.17.12.3804. [DOI] [PubMed] [Google Scholar]
- 19.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8(5):463–70. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 20.Mathas S, Janz M, Hummel F, Hummel M, Wollert-Wulf B, Lusatis S, et al. Intrinsic inhibition of transcription factor E2A by HLH proteins ABF-1 and Id2 mediates reprogramming of neoplastic B cells in Hodgkin lymphoma. Nat Immunol. 2006;7(2):207–15. doi: 10.1038/ni1285. [DOI] [PubMed] [Google Scholar]
- 21.Schebesta A, McManus S, Salvagiotto G, Delogu A, Busslinger GA, Busslinger M. Transcription factor Pax5 activates the chromatin of key genes involved in B cell signaling, adhesion, migration, and immune function. Immunity. 2007;27(1):49–63. doi: 10.1016/j.immuni.2007.05.019. [DOI] [PubMed] [Google Scholar]