Abstract
Background
The use of high-dose chemotherapy and autologous stem-cell transplantation in patients with relapsed Hodgkin’s lymphoma is supported by two randomized clinical trials but its benefit in patients with primary refractory disease is less clear. Aiming to shed light on this issue, we analyzed and compared the outcomes of patients with relapsed or refractory Hodgkin’s lymphoma treated with second-line chemotherapy and planned autologous stem-cell transplantation.
Design and Methods
We retrospectively analyzed data on 157 consecutive patients with Hodgkin’s lymphoma referred to our institution for consideration of autologous stem-cell transplantation between 1999 and 2006. Of those, 73 met the definition of having primary refractory disease, ie. progressive disease during first line chemotherapy or within 3 months of completion of the treatment. Those patients achieving complete remission, partial remission and stable disease with symptomatic improvement after two or three cycles of salvage chemotherapy proceeded to stem cell mobilization and autologous transplantation.
Results
From first relapse/progression, the 3-year overall survival was 76% (95% CI: 66%−89%) for the refractory cohort and 91% (95% CI: 84%−98%) for the relapsed cohort (P=0.034); the overall response rate to second-line chemotherapy was 51% and 83% (P<0.0001), respectively. Three-year progression-free survival post-transplant was 49% in refractory patients and 67% in relapsed patients (P=0.21); overall survival was 75% and 91% (P=0.097), respectively.
Conclusions
Using the group with relapsed disease as a reference, we can conclude that the subset of patients with chemosensitive primary refractory Hodgkin’s lymphoma do benefit from autologous stem-cell transplantation.
Keywords: Hodgkin’s lymphoma, transplantation, refractory
Introduction
More than 80% of patients with Hodgkin’s lymphoma (HL) can be cured with multi-agent chemotherapy and/or radiotherapy.1–4 However, the outcome of patients for whom initial induction chemotherapy fails is poor.5,6 Despite a number of randomized controlled trials of primary therapy, very few prospective studies have assessed the outcomes of second-line treatments in patients with HL and thus, approaches in this setting are heterogeneous.
Two randomized trials support the use of high dose chemotherapy (HDCT) and autologous stem cell transplantation (ASCT) in patients with relapsed HL, having shown that this management improved disease-free survival.7,8 However, the benefit of this intervention in patients with primary refractory HL, defined as progressive disease during first-line chemotherapy or within 3 months of completion of the treatment9 is less clear. Encouraging results have been reported with ASCT in institutional reviews and cohort comparisons but no randomized trial has compared this approach with standard therapy and the impact of selection of patients on the reported outcomes is unclear.10–14
We, therefore, reviewed the outcomes of patients with primary refractory HL treated with second-line chemotherapy and planned ASCT and compare the results to those in patients who had previously attained a response lasting more than 3 months to initial therapy.
Design and Methods
Study design and selection of patients
Cases were identified from a prospectively collected electronic database that records all patients referred to our program for an opinion regarding second-line therapy and possible ASCT. We retrospectively analyzed data from 157 consecutive adult patients with relapsed or primary refractory HL referred to our institution for consideration of second-line chemotherapy followed by HDCT and ASCT between January 1999 and December 2006. Of those, 73 met the definition of having primary refractory disease and their characteristics and outcomes were compared to those of 84 patients with relapsed disease. Data were obtained from computerized records or from the patients’ charts as necessary. Incomplete information (where identified) have been noted in the tables summarizing the data. All patients provided written informed consent for HDCT, ASCT and related procedures according to institutional guidelines. The University Health Network Research Ethics Board approved this study.
Patients’ data were included in this retrospective review if the patients had a histological diagnosis of HL and presented with relapsed or primary refractory disease after receiving a single prior chemotherapy regimen, usually ABVD. All patients were staged according to the Ann Arbor staging system.15 Repeat biopsy was not mandatory in all patients but was performed if the original biopsy was unclear, if the relapse was late (4–5 years after primary therapy) or if clinically there was concern of an alternative diagnosis. Disease response was assessed according to the International Workshop Criteria.16 Primary refractory HL was defined according to the German Hodgkin Lymphoma Study Group (GHSG).9
At the time of recurrence or progression, patients were restaged with computed tomography scans of the chest, abdomen and pelvis. Gallium scintigraphy was recommended for those with large masses but was not required. Magnetic resonance imaging was performed if clinically indicated. Positron emission tomography scans were not performed routinely. Routine biopsy was not required at the time of disease progression but a bone marrow aspirate and biopsy were mandatory. Patients were ineligible if they had uncontrolled infection or significant organ dysfunction that would preclude safe administration of salvage chemotherapy and stem-cell transplantation.
Salvage chemotherapy
Patients received second-line treatment to assess chemotherapy sensitivity most often using GDP (gemcitabine 1000 mg/m2 i.v. over 30 min on days 1 and 8, dexamethasone 40 mg p.o. in divided doses on days 1–4 and cisplatin 75 mg/m2 i.v. over 60 min on day 1 after gemcitabine) or mini-BEAM (carmustine 60 mg/m2 i.v. on day 1, etoposide 75 mg/m2 i.v. on days 2–5, cytarabine 100 mg/m2 i.v. twice daily on days 2–5 and melphalan 30 mg/m2 i.v. on day 5, up to a maximum dose of 50mg).17,18 Filgrastim was added during the second cycle in the event of febrile neutropenia during the first cycle or a low neutrophil count on a treatment day.
The patients’ response was evaluated by physical examination and computed tomography scans of the chest, abdomen and pelvis after two cycles of salvage chemotherapy. Gallium scintigraphy and bone marrow biopsy were repeated if abnormal at the start of salvage therapy. Patients who achieved a complete remission, partial remission or stable disease after salvage chemotherapy proceeded to stem-cell mobilization. Those with evidence of progressive disease or persistent abnormal gallium uptake could receive further chemotherapy as a second-line salvage regimen at the discretion of the treating physician. Patients with less than partial remission who experienced clinical improvement and normalization of gallium scans proceeded to stem-cell mobilization.
Stem-cell mobilization, high-dose chemotherapy and stem-cell reinfusion
Stem-cell mobilization was usually performed using cyclophosphamide 2 g/m2 on day 1, etoposide 200 mg/m2 on days 1–3 and filgrastim 10 μg/kg daily starting on day 6 until completion of the leukapheresis. Peripheral blood stem cell collection commenced when the peripheral blood CD34+ cell concentration was greater than 5/μL, usually on day 13. The target number of peripheral blood stem cells was 5×106 or greater CD34+ cells/kg. Patients who had insufficient peripheral blood stem cells for grafting (<2.0 ×106 CD34+ cells/kg) underwent autologous bone marrow harvesting after having been primed with filgrastim.19
High-dose chemotherapy consisted of etoposide (60 mg/kg over 8 hours on day -4) and melphalan (180 mg/m2 over 30 min on day -3). Stem cells were infused through a central venous line on day 0; supportive care was provided as described previously.20 Involved field radiation (35 Gy in 20 fractions) was typically administered to patients with bulky disease (defined as disease > 5 cm) at the time of disease progression/recurrence between 6–12 weeks post-ASCT.21
Re-staging was performed as above at 3 months and 1 year post-ASCT. Patients presenting with signs or symptoms suggestive of relapse or progression underwent appropriate work-up as required.
Statistical methods
The comparison between patients with primary refractory or relapsed disease was performed using either Fisher’s exact test or the Cochran-Armitage test when a trend was expected. Age was compared between the two groups using the Mann-Whitney test. Overall survival and progression-free survival were calculated as outcome measures, both since first relapse/progression and since ASCT. Percentages of overall and progression-free survival were estimated using the Kaplan-Meier method. The log-rank test was utilized to compare the curves. Multivariable analysis was performed using a Cox proportional hazards model.
Results
Patients
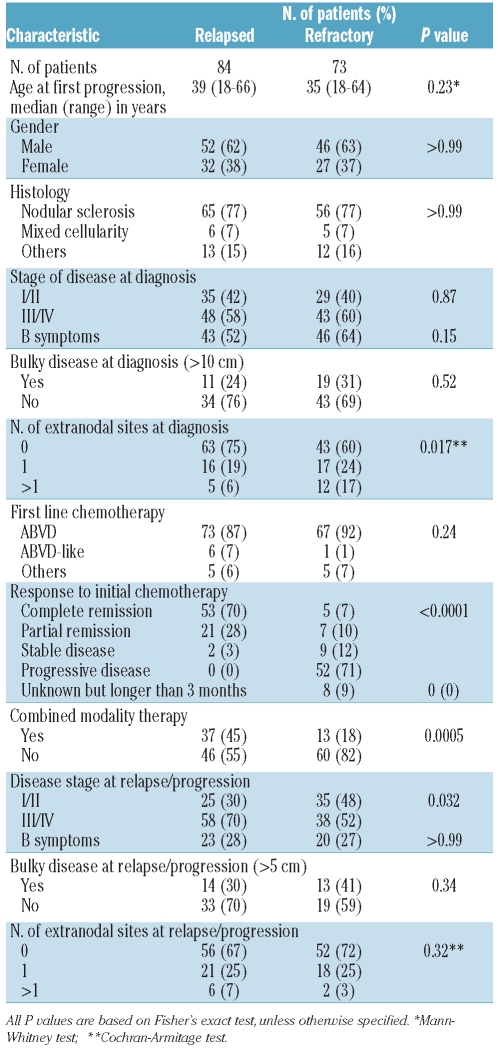
Seventy-three patients with primary refractory HL and 84 patients with relapsed HL were included in this study. The main characteristics of the patients are shown in Table 1. The two groups were similar in terms of age, gender, histology, advanced stage, bulky disease and B symptoms at diagnosis as well as for the type of first-line chemotherapy received. More patients in the group with primary refractory disease presented with one or more sites of extranodal disease (41% versus 25%, P=0.017). Combined modality primary treatment was administered to 13 (18%) of the patients with primary refractory disease and to 37 (45%) of the patients with relapsed HL (P=0.0005).
Table 1.
Patients’ characteristics.
Survival after progression following primary treatment
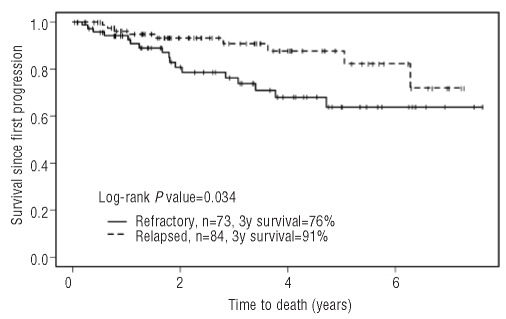
The median follow-up for all patients after first progression was 2.6 years (range, 0.04 – 7.6 years) while the median follow-up post-ASCT was 2.5 years (range, 0–7.2 years). The overall survival rate 3 years after first relapse/progression was 84% (95% CI: 78%–91%) for the entire group, being 76% (95% CI: 66%−89%) for the cohort with primary refractory disease and 91% (95% CI: 84%−98%) for the cohort with relapsed HL.
Salvage chemotherapy
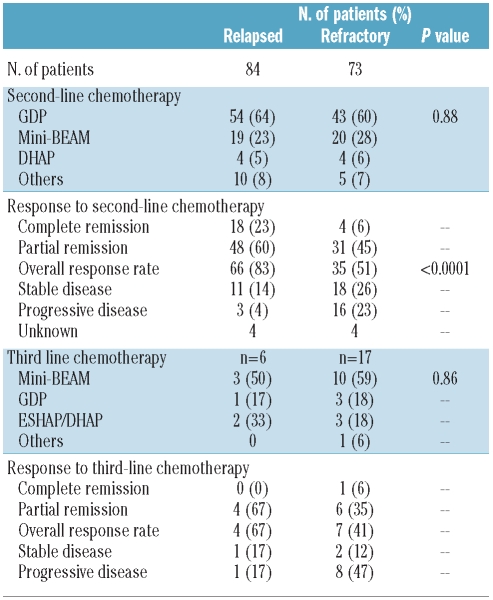
The overall response rate (i.e. percentage of patients with either complete or partial response) to second-line chemotherapy was 51% (35/73, 95% CI: 39%−63%) in the primary refractory group and 83% (66/84, 95% CI: 74%−91%) in the group with relapsed HL (P<0.0001). Four (6%) patients in the primary refractory group and 18 (23%) in the relapsed group achieved a complete remission prior to ASCT. Eighteen (26%) patients in the primary refractory cohort and 11 (14%) in the relapsed cohort had stable disease whereas 16 (23%) patients in the primary refractory group and 3 (4%) in the relapsed group had progressive disease. Seventeen patients with primary refractory disease and six with relapsed disease (a total of four - 1 with primary refractory disease and 3 with relapsed disease -because of a persistently positive gallium scan) required a second-line salvage chemotherapy regimen. The overall response rate to second-line salvage chemotherapy was similar between the two cohorts, although the numbers in each group are small (Table 2).
Table 2.
Salvage chemotherapy and outcome post-autologous stem-cell transplantation.
Overall survival and progression-free survival after high-dose chemotherapy and autologous stem-cell transplantation
One hundred and thirty-five patients had an adequate response (achievement of gallium-negative stable disease with symptomatic improvement after salvage chemotherapy as a minimum criterion to proceed) and were eligible for ASCT, including 77 (92%) in the relapsed group and 58 (79%) in the primary refractory group (P=0.028 χ2-square test and P=0.037 Fisher’s exact test). Twelve patients in the relapsed cohort and 24 in the primary refractory cohort received consolidation radiotherapy (P=0.015) for either bulky disease or localized relapse. For those patients who underwent HDCT and ASCT, the 3-year progression-free survival rate was 58% (95% CI: 50%−68%) while the 3-year overall survival was 84% (95% CI: 77%−92%).
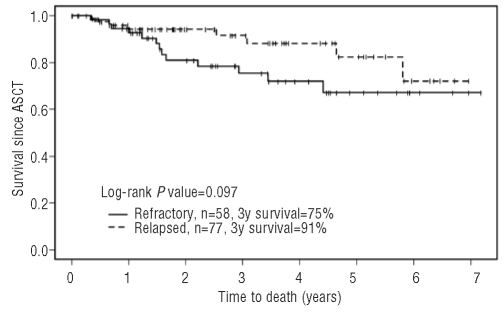
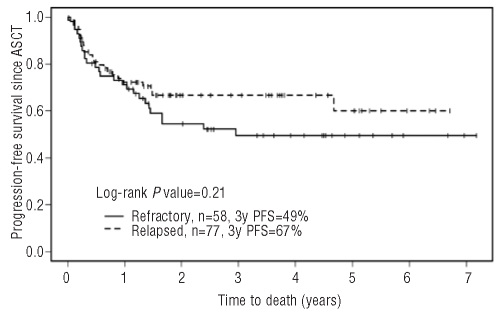
The estimated progression-free survival rates 3 years post-ASCT were 49% (95% CI: 37%−66%) for the primary refractory group and 67% (95% CI: 56%−79%) for the relapsed group (P=0.21), while the overall survival rates 3 years post-ASCT were 75% (95% CI: 63%−89%) for the primary refractory group and 91% (95% CI: 84%−99%) for the relapsed group (P=0.097). Response to salvage chemotherapy did not affect post-transplant progression-free survival or overall survival (log-rank P=0.22 and P=0.099, respectively). Forty-nine patients have relapsed since their transplant, 25 in the primary refractory group and 24 in the relapsed group. There have been 21 deaths (13 in the primary refractory cohort and 8 in the relapsed cohort), all but one of them in the setting of disease progression. Two patients have been diagnosed with myelodysplastic syndrome (1 after GDP and 1 after mini-BEAM) and one with acute myeloid leukemia (post-mini BEAM).
Figure 1.
Overall survival since progression after initiation of primary treatment.
Figure 2.
Overall survival post-autologous stem cell transplantation.
Figure 3.
Progression-free survival post-autologous stem cell transplantation.
Prognostic factors
The variables analyzed included status at salvage treatment (categorized as relapsed or primary refractory), clinical characteristics at relapse (stage, B symptoms, number of extranodal sites and bulky disease) and previously identified prognostic factors for progression-free survival (time to relapse: ≤12 months versus > 12 months) and for overall survival [age >50 years and failure to attain a temporary remission (progressive/stable disease)] on first-line treatment. 9,22
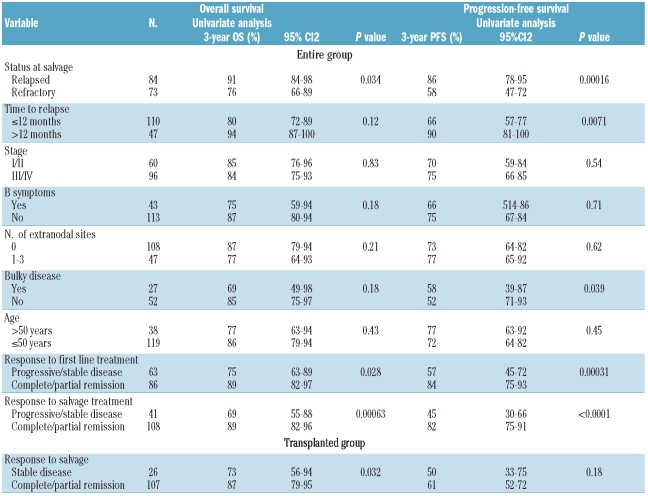
Results of the univariate analyses for overall and progression-free survival applied to the entire population are shown in Table 3. Variables associated with improved overall survival were status at salvage (relapsed versus primary refractory), response to first-line treatment (complete/partial response versus stable/progressive disease) and response to salvage chemotherapy (complete/partial response versus stable/progressive disease). No model was built with a combination of these three variables because of their obvious associations. The remaining variables were not significant in univariate analyses or in the models in which any of the three significant variables were tested. When only status at salvage (relapsed versus primary refractory) was included in the model, none of the variables analyzed achieved significance. Status at salvage, response to first line treatment, response to salvage chemotherapy, time to relapse and bulky disease (>10 cm) were found to be significant for progression-free survival. Bulky disease was significant in univariate analysis and remained significant when either time to relapse (P=0.04) or response to first-line treatment (P=0.015) was introduced into the model.
Table 3.
Significant prognostic factors for overall survival and progression-free survival for the entire cohort and for the transplanted group.
Univariate analysis for progression-free survival and overall survival applied to the patients who underwent ASCT identified response to salvage chemotherapy (stable disease versus complete/partial response) as a significant prognostic factor for overall survival.
Discussion
The outcome of HL patients with early treatment failure managed with standard-dose salvage chemotherapy is known to be very poor. In a retrospective analysis of the GHSG, no patient with primary progressive disease survived more than 5 years23 and the overall survival rate at 8 years ranged between 0 and 8% in several other series.24,25 In an attempt to improve the outcome of these patients, HDCT and ASCT have been increasingly used in the setting of patients with primary refractory HL.12,26,27 Based on the results of two randomized trials, HDCT and ASCT is now considered the standard of care for patients with relapsed or primary refractory HL.7,8 However, only the BNLI trial, a small study underpowered to report outcomes on any subsets of patients, included patients with primary refractory disease. Older reports from registry and institutional series mainly focused on transplanted patients did not examine the larger denominator of patients who undergo second-line chemotherapy prior to a planned transplant. The major strength of our study is that it reports the response to second-line chemotherapy and prognostic factors in a group of patients who were planned to undergo ASCT treated within the past decade.
We evaluated a cohort of relapsed and primary refractory HL patients treated with second-line chemotherapy and planned ASCT. Analysis of the entire group from the time of relapse/progression demonstrated that the overall survival in patients with refractory disease was inferior to that of patients who relapsed 3 months or later after the completion of initial therapy (76% versus 91%, P=0.034). As the majority of prognostic factors were found to be balanced between the two groups, the most likely explanation for this finding is the lower rate of chemosensitivity observed in patients with primary refractory HL which translated into a small proportion of patients proceeding to ASCT in this group. Furthermore, primary refractory disease was identified in univariate analysis and confirmed in multivariate analysis as an independent adverse prognostic factor.
The characteristics at diagnosis of the 157 patients analyzed were similar between the relapsed and primary refractory groups except for the presence of extranodal disease which was more frequent in the cohort with primary refractory disease. Extranodal disease was also identified by the GHSG as an unfavorable prognostic factor in patients with limited stage HL.27 Although most patients in both groups received ABVD as a first line treatment, a significantly higher proportion of patients in the group with primary refractory disease received ABVD alone, likely because of rapidly progressive disease prior to the start of planned consolidative radiation. Moskowitz et al. also found that the majority (79%) of 75 refractory patients had no exposure to radiotherapy at the time of salvage chemotherapy.28
Different variables have been identified as prognostic factors at relapse in HL, including response to salvage chemotherapy.29–31 This was confirmed in the present study when univariate analyses for progression-free and overall survival were applied to the transplanted group. Bulky disease was significant for progression-free survival in univariate analysis applied to the entire cohort and remained significant in the Cox model. This is in accordance with the known predictive value of bulky disease at the time of transplantation, as reported by several groups in the past.20,25 The significance of this finding (P=0.039) needs to be interpreted with caution given that data were incomplete for a considerable number of patients. However, given the typically poor outcomes associated with bulky disease in HL, we feel that this finding is of significance.
The overall response rate to second-line chemotherapy was lower among the primary refractory cohort than among the relapsed cohort (51% versus 83%, P<0.0001). This result appears similar to the response rate of 43% reported by the GHSG for a group of 206 patients with primary refractory disease, the majority of whom (51%) received dexa-BEAM.9 Response rates in patients with primary refractory HL in other published series range between 32% and 84%; this wide range is likely a reflection of imbalances in prognostic factors between groups and small sample sizes.9,11,12,28,32–34
As a consequence of the differences in chemosensitivity observed between the two groups, a significantly lower proportion of patients with primary refractory disease was able to proceed to ASCT (79% in the primary refractory group versus 92% in the relapsed group, P=0.028). The outcome was, however, similar in the two groups post-ASCT suggesting some benefit from HDCT in patients with primary refractory disease.
Transplant procedures are associated with an inherent selection bias that our study aimed to reduce by including all patients who were potential candidates for ASCT at the time of first relapse/progression. To our knowledge, data regarding the outcome of patients undergoing chemotherapy prior to ASCT are scarce. This analysis, however, has potential limitations. Our program did not require routine re-biopsy of patients with an adequate histological specimen at primary diagnosis, with remission lasting less than 4–5 years and a presentation typical of HL. We accept that a very small proportion of patients may have had a different diagnosis but we felt that it was impractical to mandate biopsy unless the clinical picture was suggestive of an alternate problem. A variety of second-line chemotherapy regimens were employed in this cohort reflecting the lack of randomized controlled trials in this area. The majority of patients in the study received mini-BEAM18 or, more recently, GDP based on our institutional data.35
The role and timing of consolidative radiotherapy peri-ASCT has not been tested in controlled trials. Historically pre-ASCT consolidative radiation has been a source of concern because of potentially high rates of pulmonary toxicity if mediastinal radiation is required.21 In a recent, prospective study from Australia, the rate of pneumonitis in 19 patients treated post-ASCT was 5%,37 contrasting with the 21% found in the phase I trial by Dawson et al. of involved field radiotherapy pre-ASCT.38 In the absence of phase III data, the timing of consolidative radiotherapy is likely to be based on toxicity considerations. The role of radiation itself remains another area of controversy. The GHSG/EBMT randomized ASCT trial recommended radiotherapy in all patients with residual lesions “judged to represent active Hodgkin’s disease” in the absence of randomized data.7 Our current practice reflects our historical practice of employing post-ASCT radiotherapy for masses larger than 5 cm and potentially to treat localized recurrence if radiotherapy was not previously employed (an extrapolation from combined modality therapy in the primary treatment setting).39 As a higher proportion of patients with primary refractory disease received post-ASCT radiation, (24 versus 12, P=0.015), radiotherapy may be contributing to the outcome of these chemosensitive patients.
Our results highlight the inferior survival and higher rate of chemoresistant disease in patients with primary refractory compared to relapsed HL. Given the lack of randomized controlled trials in the field, they also suggest the potential benefit of ASCT-based strategies which may include consolidative radiotherapy in the subset of chemosensitive patients with primary refractory disease. We recommend the ongoing use of ASCT in patients with primary refractory HL and encourage investigators to evaluate these ASCT-based strategies prospectively.40 Future studies should try to understand the unique biology and behavior of primary refractory HL in order to improve survival in this group of patients.
Footnotes
Authorship and Disclosures
NP wrote the manuscript; MP analyzed the data; TS, KF, TN, NF, RT, AK and MC reviewed the manuscript and JK designed the research and wrote the manuscript.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Duggan DB, Petroni GR, Johnson JL, Glick JH, Fisher RI, Connors JM, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin’s disease: a report of an intergroup trial. J Clin Oncol. 2003;21(4):607–14. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 2.Connors JM, Klimo P, Adams G, Burns BF, Cooper I, Meyer RM, et al. Treatment of advanced Hodgkin’s disease with chemotherapy : comparison of MOPP/ABV hybrid regimen with alternating courses of MOPP and ABVD – A report from the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1997;15(4):1638–45. doi: 10.1200/JCO.1997.15.4.1638. [DOI] [PubMed] [Google Scholar]
- 3.Horning SJ, Williams J, Bartlett NL, Bennett JM, Hoppe RT, Neuberg D, et al. Assessment of the Stanford V regimen and consolidative radiotherapy for bulky and advanced Hodgkin’s disease: Eastern Cooperative Oncology Group pilot study E1492. J Clin Oncol. 2000;18(5):972–80. doi: 10.1200/JCO.2000.18.5.972. [DOI] [PubMed] [Google Scholar]
- 4.Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–95. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 5.Longo DL, Duffey PL, Young RC, Hubbard SM, Ihde DC, Glatstein E, et al. Conventional dose salvage combination chemotherapy in patients relapsing with Hodgkin’s disease after combination chemotherapy: the low probability for cure. J Clin Oncol. 1992;10(2):210–8. doi: 10.1200/JCO.1992.10.2.210. [DOI] [PubMed] [Google Scholar]
- 6.Yuen AR, Rosenberg SA, Hoppe RT, Halpern JD, Horning SJ. Comparison between conventional salvage therapy and high-dose therapy with autografting for recurrent or refractory Hodgkin’s disease. Blood. 1997;89(3):814–22. [PubMed] [Google Scholar]
- 7.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomized trial. Lancet. 2002;359(9323):2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 8.Linch DC, Goldstone AH, McMillan A, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomized trial. Lancet. 1993;341(8852):1051–4. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 9.Josting A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic factors and treatment outcome in primary progressive Hodgkin lymphoma: a report from the German Hodgkin Lymphoma Study Group. Blood. 2000;96(4):1280–6. [PubMed] [Google Scholar]
- 10.Schmitz N, Haverkamp H, Josting A, et al. Long term follow up in relapsed Hodgkin’s disease: updated results of the HD-R1 study comparing conventional chemotherapy to high-dose chemotherapy with autologous haemopoetic stem cell transplantation of the German Hodgkin Study Group (GHSG) and the Working Party Lymphoma of the European Group for Blood and Marrow Transplantation (EBMT) J Clin Oncol 2005ASCO Annual Meeting Proceedings2316SPart I of II (June 1 Supplement)650816170160 [Google Scholar]
- 11.Andre M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, et al. Comparison of high-dose therapy and autologous stem cell transplantation with conventional therapy for Hodgkin’s disease induction failure: a case-control study. Societe Francaise Greffe de Moelle. J Clin Oncol. 1999;17(1):222–9. doi: 10.1200/JCO.1999.17.1.222. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, et al. Autotransplants for Hodgkin’s disease in patients never achieving a remission: a report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999;17(2):534–45. doi: 10.1200/JCO.1999.17.2.534. [DOI] [PubMed] [Google Scholar]
- 13.Sweetenham JW, Carella AM, Taghipour G, Cunningham D, Marcus R, Della Volpe A, et al. High-dose chemotherapy and autologous stem cell transplantation for adult patients with Hodgkin’s disease who do not enter remission after induction chemotherapy: results in 175 patients reported to the European Group for Blood and Marrow Transplantation. J Clin Oncol. 1999;17(10):3101–9. doi: 10.1200/JCO.1999.17.10.3101. [DOI] [PubMed] [Google Scholar]
- 14.Josting A, Reiser M, Rueffer U, Salzberger B, Diehl V, Engert A. Treatment of primary progressive Hodgkin’s and aggressive non-Hodgkin’s lymphoma: is there a chance for cure? J Clin Oncol. 2000;18(2):332–9. doi: 10.1200/JCO.2000.18.2.332. [DOI] [PubMed] [Google Scholar]
- 15.Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–6. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Hornig SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphoma. J Clin Oncol. 1999;17(4):1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 17.Baetz T, Belch A, Couban S, Imrie K, Yau J, Myers R, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin’s disease: a phase II study by the National Cancer Institute of Canada Clinical Trails Group. Ann Oncol. 2003;14 (12):1762–7. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 18.Colwill R, Crump M, Couture F, Danish R, Stewart AK, Sutton DM, et al. Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease before intensive therapy and autologous bone marrow transplantation. J Clin Oncol. 1995;13(2):396–402. doi: 10.1200/JCO.1995.13.2.396. [DOI] [PubMed] [Google Scholar]
- 19.Mollee P, Pereira D, Nagy T, Song K, Saragosa R, Keating A, et al. Cyclophosphamide, etoposide and G-CSF to mobilize peripheral blood stem cells in patients with lymphoma. Bone Marrow Transplant. 2002;30(5):273–8. doi: 10.1038/sj.bmt.1703653. [DOI] [PubMed] [Google Scholar]
- 20.Crump M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, et al. High-dose etoposide, melphalan and autologous bone marrow transplantation for patients with advanced Hodgkin’s disease: importance of disease status at transplant. J Clin Oncol. 1993;11(4):704–11. doi: 10.1200/JCO.1993.11.4.704. [DOI] [PubMed] [Google Scholar]
- 21.Tsang RW, Gospodarowicz MK, Sutcliffe SB, Crump M, Keating A. Thoracic radiation therapy before autologous bone marrow transplantation in relapsed or refractory Hodgkin’s disease. PMH Lymphoma Group and the Toronto Autologous BMT Group. Eur J Cancer. 1999;35(1):73–8. doi: 10.1016/s0959-8049(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 22.Josting A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin’s lymphoma registered in the database of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2002;20(1):221–30. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 23.Cabanillas F, Velasquez WS, McLaughlin P, Jagannath S, Hagemeister FB, Redman JR, et al. Results of recent salvage chemotherapy regimens for lymphoma and Hodgkin’s disease. Semin Hematol. 1988;25 (suppl 2):47–50. [PubMed] [Google Scholar]
- 24.Bonfante V, Santoro A, Viviani S, Devizzi L, Balzarotti M, Soncini F, et al. Outcome of patients with Hodgkin’s disease failing after primary MOPP-ABVD. J Clin Oncol. 1997;15(2):528–34. doi: 10.1200/JCO.1997.15.2.528. [DOI] [PubMed] [Google Scholar]
- 25.Horning SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89(3):801–13. [PubMed] [Google Scholar]
- 26.Chopra R, McMillan AK, Linch DC, Yuklea S, Taghipour G, Pearce R, et al. The place of high dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin’s disease: a single centre 8-year study of 155 patients. Blood. 1993;81(5):1137–45. [PubMed] [Google Scholar]
- 27.Sieber M, Engert A, Diehl V. Treatment of Hodgkin’s disease: results and current concepts of the German Hodgkin’s Lymphoma Study Group. Ann Oncol. 2000;11 (Suppl 1):81–5. [PubMed] [Google Scholar]
- 28.Moskowitz CH, Kewalramani T, Ninmer SD, Gonzalez M, Zelenetz AD, Yahalom J, et al. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin’s disease. Br J Haematol. 2004;124(5):645–52. doi: 10.1111/j.1365-2141.2003.04828.x. [DOI] [PubMed] [Google Scholar]
- 29.Lohri A, Barnett M, Fairey RN, O’Reilly SE, Phillips GL, Reece D, et al. Outcome of first relapse of Hodgkin’s disease after primary chemotherapy: identification of risk factors from the British Columbia experience 1970 to 1980. Blood. 1991;77(10):2292–8. [PubMed] [Google Scholar]
- 30.Martín A, Fernández-Jiménez MC, Caballero MD, Canales MA, Pérez-Simón JA, García de Bustos J, et al. Long-term follow-up in patients treated with mini-BEAM as salvage therapy for relapsed or refractory Hodgkin’s disease. Br J Haematol. 2001;113 (1):161–71. doi: 10.1046/j.1365-2141.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 31.Brice P, Bastion Y, Divine M, Nedellec G, Ferrant A, Gabarre J, et al. Analysis of prognostic factors after the first relapse of Hodgkin’s disease in 187 patients. Cancer. 1996;78(6):1293–9. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1293::AID-CNCR18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Czyz J, Szydlo R, Knopinska-Posluszny W, Hellmann A, Gozdzik J, Hansz J, et al. Treatment for primary refractory Hodgkin’s disease: a comparison of high-dose chemotherapy followed by ASCT with conventional therapy. Bone Marrow Transplant. 2004;33(12):1225–9. doi: 10.1038/sj.bmt.1704508. [DOI] [PubMed] [Google Scholar]
- 33.Akhtar S, El Weshi A, Abdelsalam M, Hussaini H, Janabi I, Rahal M, et al. Primary refractory Hodgkin’s lymphoma: outcome after high-dose chemotherapy and autologous SCT and impact of various prognostic factors on overall survival and event-free survival. A single institution result of 66 patients. Bone Marrow Transplant. 2007;40(7):651–8. doi: 10.1038/sj.bmt.1705792. [DOI] [PubMed] [Google Scholar]
- 34.Ferme C, Mounier N, Divine M, Brice P, Stamatoullas A, Reman O, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin’s disease in relapse or failure after initial chemotherapy: results of the Group D’Etudes des Lymphomes de l’Adulte H89 Trial. J Clin Oncol. 2002;20(2):467–75. doi: 10.1200/JCO.2002.20.2.467. [DOI] [PubMed] [Google Scholar]
- 35.Kuruvilla J, Nagy T, Pintilie M, Tsang R, Keating A, Crump M. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106(2):353–60. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]
- 37.Wirth A, Prince HM, Wolf M, Stone JM, Matthews J, Gibson J, et al. Optimal scheduling to reduce morbidity of involved field radiotherapy with transplantation for lymphomas: a prospective Australasian Leukaemia and Lymphoma Group Study. Bone Marrow Transplant. 2005;35(3):291–8. doi: 10.1038/sj.bmt.1704759. [DOI] [PubMed] [Google Scholar]
- 38.Dawson LA, Saito NG, Ratanatharathorn V, Uberti JP, Adams PT, Ayash LJ, et al. Phase I study of involved field radiotherapy preceding autologous stem cell transplantation for patients with high-risk lymphoma or Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2004;59(1):208–18. doi: 10.1016/j.ijrobp.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Meyer RM, Gospodarowicz MK, Connors JM, Pearcey RG, Bezjak A, Wells WA. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited stage Hodgkin lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(21):4634–42. doi: 10.1200/JCO.2005.09.085. [DOI] [PubMed] [Google Scholar]
- 40.Morschhauser F, Brice P, Fermé C, Diviné M, Salles G, Bouabdallah R, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin’s lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008;26(36):5980–7. doi: 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]