Abstract
Background
Bortezomib has been successfully used in the treatment of multiple myeloma and has been proposed as a potential treatment for chronic lymphocytic leukemia. In this study we investigated the mechanism by which bortezomib induces apoptosis in chronic lymphocytic leukemia cells.
Design and Methods
Using western blot analysis, we monitored the regulation of BCL2 family members, proteins of the unfolded protein response (endoplasmic reticulum stress response) and activation of caspases in relation to induction of apoptosis (measured by annexin-propidium iodide staining and loss of mitochondrial membrane potential) by bortezomib in chronic lymphocytic leukemia cells.
Results
Bortezomib induced apoptosis through activation of the mitochondrial pathway independently of changes associated with endoplasmic reticulum stress. Perturbation of mitochondria was regulated by a rapid and transcription-independent increase of NOXA protein, which preceded release of cytochrome c, HtrA2, Smac and activation of caspase-9 and −3. NOXA had a short half life (~ 1–2 h) and was ubiquitinated on at least three primary lysine residues, resulting in proteasomal-dependent degradation. Down-regulation of NOXA, using short interfering RNA in chronic lymphocytic leukemia cells, decreased bortezomib-induced apoptosis. Finally bortezomib when combined with seliciclib resulted in a stronger and earlier increase in NOXA protein, caspase-3 cleavage and induction of apoptosis in chronic lymphocytic leukemia cells.
Conclusions
These results highlight a critical role for NOXA in bortezomib–induced apoptosis in chronic lymphocytic leukemia cells and suggest that this drug may become more efficient for the treatment of chronic lymphocytic leukemia if combined with other agents able to interfere with the basal levels of MCL1.
Keywords: chronic lymphocytic leukemia, proteasome inhibitors, bortezomib, NOXA, MCL1
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in the western world, characterized by the clonal accumulation of CD5+ CD19+ and CD23+ cells.1 Unmutated IGHV gene segment usage, ZAP-70 expression, deletion of 17p, p53 mutation and high CD38-expression are each associated with poor prognosis.2 Novel therapies that may be effective, especially in the latter group, and are currently being tested in CLL and other hematologic malignancies include BCL2 inhibitors, such as ABT-263, histone deacetylase inhibitors, such as depsipeptide and LBH589, natural plant-derived products such as flavopiridol, and protea-some inhibitors, such as bortezomib.3–5 These agents induce apoptosis partly by modifying the balance between pro-apoptotic and anti-apoptotic BCL2 family members. Anti-apoptotic members include BCL2, BCL-XL, MCL1 and BCL2A1, whereas pro-apoptotic members include BAX and BAK as well as BH3-only proteins, such as BIM, PUMA, NOXA, BID and BIK.3–5
Proteasome inhibitors have emerged as promising new drugs for tumor therapy because of their selectivity towards transformed, highly proliferating cells.6,7 Bortezomib is currently the only proteasomal inhibitor used clinically and it has been successfully administered in the treatment of multiple myeloma, either as a single drug or in combination.8 These results encouraged an investigation of its potential in CLL.9 The results of this phase I clinical trial were rather disappointing.9 Previous studies had shown that the proteasome inhibitors, MG132 and lactacystin, induce apoptosis in cells by inducing a conformational change of BAX, mitochondrial perturbation and subsequent processing and activation of caspase-3.10,11 Recent studies have found that proteasome inhibitors induce NOXA protein in many cell types and occasionally BIM and BIK.12–16 Due to the accumulation of incompletely degraded proteins, proteasome inhibitors result in the activation of the unfolded protein response and consequent endoplasmic reticulum stress response, which is particularly evident in multiple myeloma and pancreatic cells.17–19 In this study, we explored the detailed mechanism of proteasome inhibitor-induced apoptosis in CLL cells.
Design and Methods
Chronic lymphocytic leukemia cells and cell lines
CLL cells were obtained from leukemic patients during routine diagnosis. Only patients with no previous treatment within the last 6 months were included in this study. Further details on the lymphocyte purification and culture are given in the Online Supplementary Design and Methods.
NALM6 (pre-B acute lymphoblastic leukemia), U266 (multiple myeloma) and Z138 (mantle cell lymphoma) cell lines were maintained in RPMI1640 supplemented with 10% fetal calf serum, penicillin, streptomycin (50 μg/mL), and L-glutamine (5 mM) and exposed to bortezomib (10 nM) or MG132 (1 μM). HEK293T (human embryonic kidney cells) were maintained in Dulbecco’s modified Eagle’s medium supplemented with penicillin/streptomycin and L-glutamine as above.
NOXA transfection and ubiquitination
HEK293T cells were transfected in 10 cm plates using the calcium phosphate method with empty vector (pMT25M), HA-tagged NOXA (in pMT25M vector) or untagged NOXA (in SPORT6 vector) and incubated for up to 24 h. The half-life of NOXA was determined in HEK293T cells transfected as above and then immediately exposed to cycloheximide (10 μM) for up to 24 h. For in vivo ubiquitination experiments, HEK293T cells were transfected for 24 h with NOXA (both HA-tagged and untagged plasmids) in the absence or presence of 6xHis-Ubiquitin plasmid (pM107, 8 ubiquitin moieties each attached to a 6xHis tag). HEK293T cells were also transfected with HA-NOXA plasmid concomitantly with either pM107 or 6xHis-Ubiquitin K-R plasmid (in each ubiquitin moiety all lysines are mutated to arginine, and are attached to a 6xHis tag) in order to determine the number of lysines targeted for ubiquitination in NOXA protein. Cells were then lysed in guanidine HCl buffer (6 M) and His-ubiquitin-tagged proteins were purified through Ni-NTA agarose (QIAGEN) and analyzed by western blotting. PM107 was kindly provided by Dr. D. Bohmann (University of Rochester, NY, USA) while the His-ubiquitin mutant plasmid (K-R mut) was kindly provided by Dr. L. Stevenson (University of Dundee, Dundee, Scotland).
Detailed of the other procedures and protocols are provided in the Online Supplementary Design and Methods.
Statistical analysis
All statistical analyses were performed using one-way ANOVA followed by Dunnett’s multiple test apart for the analysis of the data illustrated in Figure 4A, for which a paired Student’s t test was applied, and in Figure 6A, for which one-way ANOVA followed by Bonferroni’s multiple test was applied.
Figure 4.
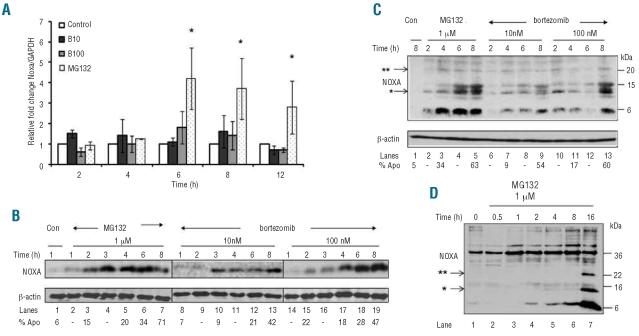
Regulation of NOXA in CLL cells. (A) Quantitative real time polymerase chain reaction results for NOXA mRNA levels from CLL cells, from seven patients, which were exposed to MG132 or bortezomib (B10: 10 nM, B100: 100 nM). Results represent the mean ± sem of the relative fold change in NOXA to GAPDH mRNA levels as calculated by the 2−ΔΔCt method and * denotes statistical significance with a P value <0.01 compared to unstimulated control cells. (B) CLL cells were exposed to MG132 or bortezomib for 1–8 h and NOXA induction assessed. (C) CLL cells were exposed to MG132 or bortezomib and examined for slower migrating bands immunoreactive with an anti-NOXA monoclonal antibody. (D) CLL cells were exposed to CD154L-expressing L cells plus interleukin-4 (15 ng/mL) overnight before being exposed to MG132. In panels (C–D), the presence of one or two asterisks indicates probable mono- and di-ubiquitinated NOXA, respectively.
Figure 6.
NOXA siRNA abrogates bortezomib-induced apoptosis in CLL cells. (A) CLL cells from nine patients were transfected with either NOXA siRNA or the negative control siRNA 1 before exposure to bortezomib (10 nM, B10) for 16 h. Apoptosis was measured by TMRE and phosphatidylserine externalization and the results shown are the mean ± sem; * denotes statistical significance with a P value <0.05 compared to bortezomib or bortezomib plus control siRNA exposed cells. (B) CLL cells from one patient were exposed as above and processed for western blot analysis. The proform and cleaved caspase-3 subunits are indicated. (C and D) CLL cells from seven patients were exposed to bortezomib (10 nM, B10) or seliciclib (10 μM) either alone or in combination for 4–16 h. Apoptosis was measured either by (C) TMRE or (D) annexin V-propidium iodide staining. Results shown are the mean ± sem and * denotes statistical significance with a P value <0.05 compared to untreated cells. (E) CLL cells from one representative patient were stimulated as in (C and D) and were processed for western blot analysis.
Results
Bortezomib induces apoptosis in chronic lymphocytic leukemia cells in a time- and concentration-dependent manner
Both bortezomib and MG132 (used as a positive control) caused a time-dependent induction of apoptosis in CLL cells, as assessed by increases in the percentage of phosphatidylserine-positive cells and in cells with low mitochondrial membrane potential (ΔΨm) (Figure 1A and B). Bortezomib and MG132 also efficiently inhibited the proteasome as indicated by a time-dependent accumulation of polyubiquitinated proteins (Online Supplementary Figure S1A).
Figure 1.
Induction of caspase-dependent apoptosis by bortezomib and MG132 in CLL cells. (A and B) CLL cells from 36 patients were left unstimulated or were exposed to 10 or 100 nM bortezomib (B10, B100, respectively) or MG132 (1 μM) for 4–16 h. Apoptosis was measured by (A) TMRE or (B) annexin V-propidium iodide (ANN-PI) staining. Results shown are the mean ± sem and * denotes statistical significance with a P value <0.01 compared to unstimulated control cells. (C) CLL cells from four patients were exposed to bortezomib (10 nM) or MG132 (1 μM) for 16 h in the presence or absence of z-VAD.fmk and apoptosis was monitored by TMRE and annexin V-propidium iodide (ANN-PI). Results shown are the mean ± sem and * denotes statistical significance with a P value <0.01. (D) Western blot analysis for caspase-9, −8 and −3 cleavage in cells exposed to DMSO, bortezomib or MG132 for 2–16 h. The proform as well as the cleaved products for each caspase are indicated. The percentage of phosphatidylserine-positive cells is shown below the blot. (E) Western blot analysis for cytochrome c, HtrA2 and Smac in cytosolic and mitochondrial fractions prepared from CLL cells exposed to either MG132 or bortezomib for 4–16 h. COX IV and β-actin served as loading controls for mitochondrial and cytosolic fractions, respectively.
Proteasome inhibitors activate the intrinsic pathway of apoptosis in chronic lymphocytic leukemia cells
The broad spectrum caspase inhibitor, z-VAD.fmk, completely abrogated the induction of apoptosis as assessed by annexin-propidium iodide staining but had no effect on the loss of ΔΨm (Figure 1C), demonstrating that phosphatidylserine externalization was a downstream event following caspase activation, whereas the loss in ΔΨm was largely caspase-independent. In cells exposed only to the solvent (dimethylsulfoxide), caspase-9, −8 and −3 were present predominantly as their unprocessed zymogens (Figure 1D, lanes 1–5). Following exposure to bortezomib (10 and 100 nM), caspase-9 was processed to its p37/p35 fragments at 6 or 8 h after treatment (Figure 1D, lanes 19–25) followed by further processing to two uncharacterized bands, p22/p20, as previously observed in CLL cells.20 Caspase-9 cleavage preceded cleavage of caspase-3 to its p20, p19 and p17 large subunits (Figure 1D, lanes 19–25). Bortezomib also induced processing of caspase-8 to its p43/41 subunits but only after 16 h of exposure (Figure 1D, lanes 20 and 25) suggesting that the main caspases activated following bortezomib treatment, responsible for induction of cell death, are caspase-9 and −3 (Figure 1D, lanes 20 and 25). Similar results were obtained following exposure of cells to MG132 (Figure 1D). Both bortezomib and MG132 caused time-dependent losses of mitochondrial cytochrome c, HtrA2 and Smac accompanied by increases in the cytosolic levels of these proteins (Figure 1E). Bortezomib (10–100 nM) also induced a time-dependent activation of both BAK and BAX (Online Supplementary Figure S1B and data not shown). Taken together these data demonstrate that bortezomib induces caspase-dependent apoptosis in CLL cells, primarily through the intrinsic pathway, with caspase-9 being the initiator caspase.
Proteasome inhibitors induce NOXA in chronic lymphocytic leukemia cells
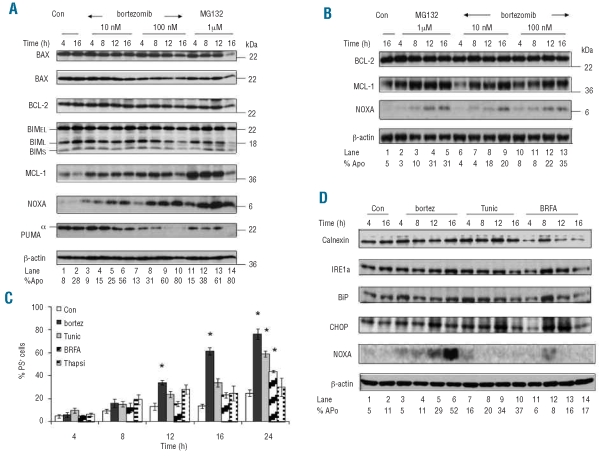
The intrinsic pathway is regulated by the levels of BCL-2 family members and their interactions. Exposure to bortezomib or MG132 revealed that BAX, BAK and BCL2 levels remained largely unchanged at all time points tested whereas the levels of MCL1 increased 4–8 h after exposure (Figure 2A and B), most probably due to inhibition of its rapid proteasomal degradation.21 At 12–16 h after stimulation, levels of MCL1 started to decline, most probably due to caspase-3-mediated cleavage corresponding to induction of extensive apoptosis.22 Some decrease was observed in BIM and PUMA, particularly when extensive apoptosis was observed (Figure 2A), compatible with PUMA being a caspase substrate.3 Both bortezomib and MG132 caused marked increases in NOXA protein as early as 4 h after stimulation (Figure 2A). The increase in NOXA protein correlated well with the efficiency of bortezomib and MG132 in inducing apoptosis because in a patient who responded very poorly to both drugs, there was a minimal increase in NOXA (Figure 2B).
Figure 2.
BCL2 family regulation following MG132 and bortezomib treatment in CLL cells. CLL cells from two representative patients (A and B) were exposed to MG132 or bortezomib for 4–16 h and processed for western blot analysis. (C) CLL cells from ten patients were exposed to bortezomib (10 nM), tunicamycin (10 μg/mL), brefeldin A (100 nM) or thapsigargin (1 μg/mL) for 4–24 h and apoptosis was measured by annexin V-propidium iodide staining. Results shown are mean ± sem and * denotes statistical significance with a P value <0.01 compared to unstimulated control cells. (D) CLL cells were stimulated as in (C) for 4–16 h and examined for induction of endoplasmic reticulum stress related proteins by western blot analysis.
Bortezomib and endoplasmic reticulum stress
Exposure to bortezomib results in induction of endoplasmic reticulum stress and the unfolded protein response, thereby inducing death of some types of cells.14,18 Accumulation of misfolded proteins in the endoplasmic reticulum results in activation of the three major signaling branches of the unfolded protein response, including inositol-requiring protein-1 (IRE1), leading to activation of genes such as CHOP/GADD153, to protect cells from endoplasmic reticulum stress; however, if homeostasis cannot be restored, the unfolded protein response can induce apoptosis.23 As endoplasmic reticulum stress may also result in up-regulation of NOXA,24 CLL cells were also exposed for up to 24 h to bortezomib (10 nM) as well as tunicamycin, brefeldin A and thapsigargin as positive controls. Tunicamycin induced apoptosis in a time-dependent manner, whereas brefeldin A was much less potent (Figure 2C and data not shown). Calnexin and IRE1α remained unchanged following exposure to bortezomib, tunicamycin or brefeldin A (Figure 2D), whereas CHOP and BiP were weakly increased in some but not all patients tested (Figure 2D and data not shown). Induction of NOXA was only observed in response to bortezomib, thus probably excluding the involvement of up-regulation of endoplasmic reticulum transcription factors in the induction of NOXA in CLL cells (Figure 2D). MG132 can induce the formation of vacuoles as an indicator of endoplasmic reticulum involvement.25 Finally, using electron microscopy, no abnormalities were detected in response to bortezomib after 4 h or 8 h but an increase in apoptosis was observed after 12 h and was much more common at 16 h (Figure 3B) when almost 50% of the cells contained swollen mitochondria with ruptured outer membranes. These ruptured membranes were largely restricted to cells with an electron-lucent cytoplasm and often coincided with the presence of flocculent densities within the mitochondrial matrix (Figure 3C), characteristic of secondary necrosis. In contrast, treatment with tunicamycin resulted in mild swelling of mitochondria within 4 h, which was usually associated with a slight decrease in the electron density of the mitochondrial matrix (Figure 3D). This change did not involve the loss of mitochondrial matrix granules and all other organelles, including the endoplasmic reticulum, maintained a normal morphology. Similar cells were present after treatment for 8–16 h but there was an increased incidence of apoptosis and secondary necrosis, particularly after 16 h. Thus none of the agents utilized in the present study induced significant endoplasmic reticulum stress in CLL cells and our data do not, therefore, support the hypothesis that bortezomib-induced endoplasmic reticulum stress is a major mechanism by which bortezomib induces either NOXA or apoptosis in CLL cells.
Figure 3.
CLL cells are resistant to endoplasmic reticulum stress. (A) Untreated CLL cells retained a normal ultrastructure, with compact mitochondria and few endoplasmic reticulum cisternae, even after incubation for 16 h. (B and C) Exposure to bortezomib (10 nM) for 16 h resulted in many apoptotic cells containing swollen mitochondria with ruptured outer membranes (arrowheads) and flocculent densities (arrows) within the mitochondrial matrix. (D) Treatment with tunicamycin (10 μg/mL) resulted in a mild swelling of mitochondria within 4 h and a slight decrease in the electron density of the mitochondrial matrix but no loss of matrix granules. The endoplasmic reticulum (arrowheads) was indistinguishable from that of control cells. [bars = 1μm].
Mechanisms of NOXA protein induction
Although NOXA was originally described as a p53-regulated gene,26 it can also be induced in a p53-independent manner. Neither apoptosis nor NOXA protein increase was significantly affected in a CLL patient with a frameshift deletion in one TP53 allele and a mutation in the second, suggesting that TP53 is not involved in the transcriptional regulation of NOXA (Online Supplementary Figure S2) in agreement with previous observations in cell lines.14 To determine whether NOXA protein induction was due to transcriptional induction or post-translational regulation, NOXA mRNA levels were measured by reverse transcriptase polymerase chain reaction. We considered that induction of transcription was indicated by a greater than two-fold increase in NOXA mRNA relative to GAPDH, which served as the control mRNA. No induction of NOXA transcription was observed for up to 12 h following exposure to bortezomib, whereas MG132 strongly increased NOXA mRNA after 6 h (Figure 4A). Neither bortezomib nor MG132 induced transcription of the TP53 gene at any time tested whereas they both induced transcription of MCL1 and Dnmt1 (DNA methyl-transferase 1) genes 2 h after stimulation (Online Supplementary Figure S3A-C). These results exclude experimental limitations being responsible for not detecting NOXA mRNA induction at early time points (2–4 h) (Figure 2A). Time-course studies revealed an increase in NOXA protein at 1 and 2–4 h following exposure to MG132 and bortezomib, respectively (Figure 4B), suggesting that NOXA protein was regulated at a post-translational level. Interestingly, exposure of CLL cells to protea-some inhibitors resulted in an increase in three slower migrating immunoreactive proteins of approximately 12–24 kDa (Figure 4C), indicating possible ubiquitination of NOXA. Similar higher mobility bands following exposure to bortezomib were also detected in highly proliferating cells such as NALM6 and Z138 (Online Supplementary Figure S4). The appearance of these higher mobility bands coincided with significant induction of apoptosis. To completely exclude the possibility that these slower migrating bands were caspase-mediated cleavage by-products, CLL cells were cultured with CD154-expressing L cells. CLL cells cultured in this way were completely resistant to both spontaneous and MG132 (1 μM)-induced apoptosis for up to 16 h. Exposure of these CLL cells to MG132 resulted in a time-dependent accumulation of NOXA and the appearance of slower migrating bands of approximately 14 and 22 kDa (Figure 4D). These results strongly support the suggestion that in the absence of any apoptosis, exposure of CLL cells to a proteasome inhibitor results in the accumulation of NOXA together with modified species of NOXA of a size compatible with mono- and di-ubiquitinated NOXA.
Ubiquitination of NOXA protein
Our data suggest that NOXA is ubiquitinated and degraded through the proteasome. Indeed when HEK293T cells were transfected with HA-tagged NOXA in the presence of cycloheximide, the half life of both endogenous and exogenous NOXA was approximately 1–2 h (Figure 5A). Although the majority of unstimulated CLL cells express low to undectable levels of NOXA protein, some patients show weak basal NOXA expression. In these latter cases, the half life of NOXA was also less than 2 h (Figure 5B). To prove that NOXA was ubiquitinated, HEK293T cells were co-transfected with a 6xHis-Ubiquitin-bearing plasmid (pM107) together with either untagged NOXA (NOXA-SP) or HA-tagged NOXA bearing plasmids. Both endogenous and exogenous NOXA were extensively ubiquitinated (Figure 5C, lanes 2, 4 and 6). Using an HA-specific antibody, bands corresponding to those of NOXA and its ubiquitinated products appeared only in cells transfected with HA-tagged NOXA (Figure 5D, lanes 4 and 5) but not with untagged NOXA (Figure 5D, lanes 6 and 7), further confirming that these are bona fide NOXA products. Further substantiation that these bands represented ubiquitinated NOXA was provided by the finding that these bands (especially the ones above 70 kDa representing polyubiquitinated NOXA) increased in intensity following exposure to MG132 (Figure 5C, lanes 3, 5 and 7). To determine how many primary sites for ubiquitination exist in NOXA, HEK293T cells were co-transfected with HA-tagged NOXA and pM107 or a ubiquitin-bearing plasmid in which all lysine residues had been mutated to arginine (K-R mut). These site-specific mutations allow ubiquitin to bind, via its C-terminus (G76), to NOXA forming only monoubiquitin conjugates, as the absence of lysines in ubiquitin (K-R) prevents the formation of polyubiquitin chains.27 Use of this mutated ubiquitin plasmid revealed that NOXA contains three principal lysines residues that are targeted for ubiquitination (Figure 5E, lane 4).
Figure 5.
Ubiquitination of NOXA. (A) HEK293T cells were left untransfected or were transfected with HA-tagged NOXA for 24 h and the half-life of both endogenous and exogenous NOXA was determined in the presence of cycloheximide (10 μM). (B) Freshly isolated CLL cells from a patient who expressed basal levels of NOXA were exposed to cycloheximide and the half-life of NOXA was determined by western blotting. (C and D) HEK293T cells were left untransfected (lane 1) or were transfected with empty vector (Vector), HA-tagged NOXA, or untagged NOXA (NOXA-SP) concomitantly with 6xHis-tagged wild type ubiquitin (pM107) for 24 h as indicated. MG132 (1 μM) was added 8 h before harvesting as indicated. Lysates were purified through Ni-NTA agarose beads and analyzed by western blotting using either (C) anti-NOXA or (D) anti-HA antibody. Black arrows indicate ubiquitination of endogenous NOXA. (E) HEK293T cells were left untransfected or were transfected with HA-tagged NOXA (HA-NOXA) concomitantly with pM107 or with 6xHis-tagged ubiquitin bearing plasmid in which all lysines were mutated to arginine (K–R mut) for 24 h. Lysates were purified through Ni-NTA agarose beads and analyzed by western blotting using anti-NOXA antibody. Black arrows indicate mono-ubiquitinated NOXA protein.
Effect of NOXA short interfering RNA on bortezomib-induced apoptosis in chronic lymphocytic leukemia cells
To ascertain, more precisely, the role of NOXA in bortezomib-induced apoptosis in CLL cells, we used NOXA short interfering RNA (siRNA). Transfection of NOXA siRNA but not a negative control siRNA caused a decrease in bortezomib-induced apoptosis (Figure 6A) and this inhibition may be an underestimate due to the cytotoxicity caused by nucleofection alone. NOXA siRNA but not the control siRNA caused a marked decrease in bortezomib-induced accumulation of NOXA and processing of cas-pase-3 (Figure 6B). When NOXA siRNA was present, higher levels of MCL1 protein were observed in the presence of bortezomib (Figure 6B), possibly due to inhibition of either caspase-3-mediated cleavage or proteasomal-mediated degradation of MCL1 in the absence of NOXA.21,22,28 Similar stabilization of MCL1 was observed following exposure of CLL cells to MG132 in the presence of NOXA siRNA (Online Supplementary Figure S5A and B). We, therefore, investigated whether down-regulation of MCL1 using seliciclib29,30 would enhance bortezomib-induced apoptosis in CLL cells. Bortezomib (10 nM), when combined with seliciclib (10 μM), induced apoptosis as early as 4 h after stimulation (Figure 6C and D). In the presence of seliciclib, bortezomib caused an earlier and/or stronger induction of both NOXA protein and caspase-3 cleavage and resulted in weaker stabilization of MCL1 protein at all times tested (Figure 6E, compare lanes 4–6 to 8–10). In conclusion, down-regulation or inhibition of MCL1 further increases the sensitivity of CLL cells to bortezomib.
Discussion
In this study we found that bortezomib induces apoptosis in CLL cells exclusively by perturbation of mitochondria and activation of the intrinsic pathway. In contrast to multiple myeloma cells, bortezomib failed to significantly induce proteins involved in endoplasmic reticulum stress, such as BiP and CHOP (Figure 2).19 In our study CLL cells appeared relatively resistant to endoplasmic reticulum stress, possibly because of their rather limited endoplasmic reticulum network, their low rates of protein synthesis and low levels of death associated protein kinase (DAPK),31 which is required for endoplasmic reticulum stress-induced apoptosis.32 The refractory nature of CLL cells to endoplasmic reticulum stress may also be due to their lack of XBP-1 expression. XBP1 is cleaved by IRE1 to XBP1s, which transcriptionally regulates several proteins involved in the unfolded protein response.33 Our results support the hypothesis that CLL cells are markedly resistant to endoplasmic reticulum stress and that the protea-some inhibitors induce apoptosis primarily by activating the mitochondrial pathway.
Activation of this pathway in CLL cells was found to be regulated through an increase in NOXA protein, which preceded the release of cytochrome c, HtrA2 and Smac and caspase-3 activation. Induction of NOXA was largely independent of transcription, since increases in NOXA protein occurred in the absence of or earlier than increases in NOXA mRNA levels (Figure 4A and B). Because we found that CLL cells expressed higher basal levels of NOXA mRNA compared with PUMA or MCL1 mRNA as previously described,34,35 our data are compatible with the hypothesis that there is sufficient NOXA mRNA present to account for new protein synthesis and that bortezomib inhibits the continuous turnover of NOXA protein. We found that NOXA is a shortlived protein (t1/2~1–2 h), extensively ubiquitinated and rapidly degraded by the protea-some (Figure 5). To our knowledge, this is the first demonstration of NOXA ubiquitination and proteasome-mediated degradation in intact cells. NOXA binds preferentially to MCL1 and promotes its degradation, possibly by the E3 ubiquitin ligase, Mule.36,37 BH3-only proteins are critical for the initiation of apoptosis and their activity is known to be tightly controlled both by transcriptional and post-translational mechanisms.38 Prior to this study, BIM was the only BH3-only protein known to be regulated post-translationally by the ubiquitin proteasome system.39 Our results now demonstrate that NOXA is also regulated by the ubiquitin proteasome system, highlighting an additional tier of regulation of BH3-only proteins.
Using NOXA siRNA in CLL cells, we demonstrated a critical role for NOXA in proteasome inhibitor-induced apoptosis in CLL cells, in agreement with data from other cell types including melanoma and mantle cell lymphoma.14,17 Thus, with regards to CLL cells, we propose that NOXA protein binds to MCL1 and displaces it from BAK.36 BAK will then undergo a conformational change resulting in the release of cytochrome c and caspase activation. The displaced MCL1 may be susceptible to proteasomal degradation or caspase cleavage21,22 leading to the observed decrease in MCL1 protein at later times (Figure 2A). Given the critical role of NOXA in mediating bortezomib-induced apoptosis, our data suggest that the efficiency of bortezomib in treating CLL could be significantly enhanced if it were to be combined with novel agents, such as histone deacetylase inhibitors and cyclin-dependent kinase inhibitors, such as seliciclib or flavopiridol, which increase NOXA or down-regulate MCL1.29,30,40
Acknowledgments
the authors thank the Leukaemia & Lymphoma Research for supporting this study and Millennium Pharmaceuticals for providing bortezomib. We thank Judy McWilliam for preparation of samples for electron microscopy. We also thank Drs. Bohmann and Stevenson for providing the ubiquitin bearing plasmids, pM107 and Ubiquitin mutant (K-R mut), respectively.
Footnotes
Funding: this work was supported by the Medical Research Council and a grant from the Leukaemia & Lymphoma Research.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
MB performed research and contributed to the data analysis and manuscript writing. SLK, MB and MV performed research and analyses. DD performed electron microscopy. RW and AM contributed with patients’ samples and analyses. EE provided some analytic tools and helped to write the manuscript. MJSD contributed with patients’ data and manuscript writing. GMC designed research and contributed to data analysis and manuscript writing.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Damle RN, Ghiotto F, Valetto A, Albesiano E, Fais F, Yan XJ, et al. B-cell chronic lymphocytic leukemia cells express a surface membrane phenotype of activated, antigen-experienced B lymphocytes. Blood. 2002;99(11):4087–93. doi: 10.1182/blood.v99.11.4087. [DOI] [PubMed] [Google Scholar]
- 2.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue S, Riley J, Gant TW, Dyer MJ, Cohen GM. Apoptosis induced by histone deacetylase inhibitors in leukemic cells is mediated by Bim and Noxa. Leukemia. 2007;21(8):1773–82. doi: 10.1038/sj.leu.2404760. [DOI] [PubMed] [Google Scholar]
- 4.Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113(18):4403–13. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 5.Vogler M, Dinsdale D, Sun XM, Young KW, Butterworth M, Nicotera P, et al. A novel paradigm for rapid ABT-737-induced apoptosis involving outer mitochondrial membrane rupture in primary leukemia and lymphoma cells. Cell Death Differ. 2008;15(5):820–30. doi: 10.1038/cdd.2008.25. [DOI] [PubMed] [Google Scholar]
- 6.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59(11):2615–22. [PubMed] [Google Scholar]
- 7.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. Br J Haematol. 2007;137(5):429–35. doi: 10.1111/j.1365-2141.2007.06585.x. [DOI] [PubMed] [Google Scholar]
- 9.Faderl S, Rai K, Gribben J, Byrd JC, Flinn IW, O’Brien S, et al. Phase II study of single-agent bortezomib for the treatment of patients with fludarabine-refractory B-cell chronic lymphocytic leukemia. Cancer. 2006;107(5):916–24. doi: 10.1002/cncr.22097. [DOI] [PubMed] [Google Scholar]
- 10.Almond JB, Snowden RT, Hunter A, Dinsdale D, Cain K, Cohen GM. Proteasome inhibitor-induced apoptosis of B-chronic lymphocytic leukaemia cells involves cytochrome c release and caspase activation, accompanied by formation of an approximately 700 kDa Apaf-1 containing apoptosome complex. Leukemia. 2001;15(9):1388–97. doi: 10.1038/sj.leu.2402201. [DOI] [PubMed] [Google Scholar]
- 11.Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22(17):2643–54. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Bougie P, Wuilleme-Toumi S, Menoret E, Trichet V, Robillard N, Philippe M, et al. Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma. Cancer Res. 2007;67(11):5418–24. doi: 10.1158/0008-5472.CAN-06-4322. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Li R, Grandis JR, Johnson DE. Bortezomib induces apoptosis via Bim and Bik up-regulation and synergizes with cisplatin in the killing of head and neck squamous cell carcinoma cells. Mol Cancer Ther. 2008;7(6):1647–55. doi: 10.1158/1535-7163.MCT-07-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Galan P, Roue G, Villamor N, Montserrat E, Campo E, Colomer D. The proteasome inhibitor bortezomib induces apoptosis in mantle-cell lymphoma through generation of ROS and Noxa activation independent of p53 status. Blood. 2006;107(1):257–64. doi: 10.1182/blood-2005-05-2091. [DOI] [PubMed] [Google Scholar]
- 15.Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced killing of melanoma cells by simultaneously targeting Mcl-1 and NOXA. Cancer Res. 2006;66(19):9636–45. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- 16.Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65(14):6282–93. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 17.Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, et al. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281(42):31440–7. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 18.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr, Huang P, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005;65(24):11658–66. doi: 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 19.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107(12):4907–16. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inoue S, Snowden RT, Dyer MJ, Cohen GM. CDDO induces apoptosis via the intrinsic pathway in lymphoid cells. Leukemia. 2004;18(5):948–52. doi: 10.1038/sj.leu.2403328. [DOI] [PubMed] [Google Scholar]
- 21.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17(12):1475–86. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowden RT, Sun XM, Dyer MJ, Cohen GM. Bisindolylmaleimide IX is a potent inducer of apoptosis in chronic lymphocytic leukaemic cells and activates cleavage of Mcl-1. Leukemia. 2003;17(10):1981–9. doi: 10.1038/sj.leu.2403088. [DOI] [PubMed] [Google Scholar]
- 23.Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318(5852):944–9. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Mora-Jensen H, Weniger MA, Perez-Galan P, Wolford C, Hai T, et al. ERAD inhibitors integrate ER stress with an epigenetic mechanism to activate BH3-only protein NOXA in cancer cells. Proc Natl Acad Sci USA. 2009;106(7):2200–5. doi: 10.1073/pnas.0807611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ding WX, Ni HM, Yin XM. Absence of Bax switched MG132-induced apoptosis to non-apoptotic cell death that could be suppressed by transcriptional or translational inhibition. Apoptosis. 2007;12(12):2233–44. doi: 10.1007/s10495-007-0142-0. [DOI] [PubMed] [Google Scholar]
- 26.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 27.Ciechanover A. Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6(1):79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- 28.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA. 2007;104(15):6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvi AJ, Austen B, Weston VJ, Fegan C, MacCallum D, Gianella-Borradori A, et al. A novel CDK inhibitor, CYC202 (R-roscovitine), overcomes the defect in p53-dependent apoptosis in B-CLL by down-regulation of genes involved in transcription regulation and survival. Blood. 2005;105(11):4484–91. doi: 10.1182/blood-2004-07-2713. [DOI] [PubMed] [Google Scholar]
- 30.Inoue S, Walewska R, Dyer MJ, Cohen GM. Downregulation of Mcl-1 potentiates HDACi-mediated apoptosis in leukemic cells. Leukemia. 2008;22(4):819–25. doi: 10.1038/leu.2008.1. [DOI] [PubMed] [Google Scholar]
- 31.Raval A, Tanner SM, Byrd JC, Angerman EB, Perko JD, Chen SS, et al. Downregulation of death-associated protein kinase 1 (DAPK1) in chronic lymphocytic leukemia. Cell. 2007;129(5):879–90. doi: 10.1016/j.cell.2007.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15(12):1875–86. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 33.Reimold AM, Iwakoshi NN, Manis J, Vallabhajosyula P, Szomolanyi-Tsuda E, Gravallese EM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412(6844):300–7. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 34.Mackus WJ, Kater AP, Grummels A, Evers LM, Hooijbrink B, Kramer MH, et al. Chronic lymphocytic leukemia cells display p53-dependent drug-induced Puma upregulation. Leukemia. 2005;19(3):427–34. doi: 10.1038/sj.leu.2403623. [DOI] [PubMed] [Google Scholar]
- 35.Smit LA, Hallaert DY, Spijker R, de Goeij B, Jaspers A, Kater AP, et al. Differential Noxa/Mcl-1 balance in peripheral versus lymph node chronic lymphocytic leukemia cells correlates with survival capacity. Blood. 2007;109(4):1660–8. doi: 10.1182/blood-2006-05-021683. [DOI] [PubMed] [Google Scholar]
- 36.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121(7):1085–95. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103(6):839–42. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 39.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278(21):18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 40.Hallaert DY, Spijker R, Jak M, Derks IA, Alves NL, Wensveen FM, et al. Crosstalk among Bcl-2 family members in B-CLL: seliciclib acts via the Mcl-1/Noxa axis and gradual exhaustion of Bcl-2 protection. Cell Death Differ. 2007;14(11):1958–67. doi: 10.1038/sj.cdd.4402211. [DOI] [PubMed] [Google Scholar]