Abstract
Background
Thalidomide maintenance therapy after stem cell transplantation resulted in increased progression-free survival and overall survival in a few trials, but its role in non-transplant eligible patients with multiple myeloma remains unclear. This study assessed the impact of thalidomide-interferon in comparison to interferon maintenance therapy in elderly patients with multiple myeloma.
Design and Methods
Of 289 elderly patients with multiple myeloma who were randomized to thalidomide-dexamethasone or melphalan-prednisolone induction therapy, 137 finally completed 9 cycles of induction therapy with stable disease or better and thereby qualified for maintenance treatment. Of these, 128 have been randomized to either thalidomide-interferon or interferon alone. Primary study endpoints were progression-free survival and response rates; secondary endpoints were overall survival, toxicity and quality of life.
Results
Thalidomide-interferon maintenance therapy led to a significantly longer progression-free survival compared to interferon (27.7 vs. 13.2 months, P=0.0068), but overall survival was similar in both groups (52.6 vs. 51.4 months, P=0.81) and did not differ between patients aged 75 years or older, or younger patients (P=0.39). Survival after disease progression tended to be shorter in patients on thalidomide-interferon maintenance therapy (P=0.056). Progression-free survival and overall survival tended to be shorter in patients with adverse cytogenetic (FISH) findings compared to the standard risk group but differences were not significant (P=0.084 and P=0.082, respectively). Patients on thalidomide-interferon presented with more neuropathy (P=0.0015), constipation (P=0.0004), skin toxicity (P=0.0041) and elevated creatinine (P=0.026).
Conclusions
Thalidomide plus interferon maintenance therapy increased progression-free survival but not overall survival and was associated with slightly more toxicity than maintenance with interferon alone. (ClinicalTrials.gov Identifier: NCT00205751).
Keywords: thalidomide, thalidomide-interferon, progression-free survival, overall survival
Introduction
In multiple myeloma (MM), first-line therapy results in tumor response and improvement of quality of life1 in the majority of patients, and depth of response correlates with survival.2 In spite of substantial advances in therapy, relapse occurs in most patients with myeloma after a variable duration of remission. Many treatments have been tested to prolong the duration of the maintenance phase and to increase survival by postponing or avoiding relapse. Continuation of conventional cytostatic therapy as maintenance treatment did not result in longer survival.3 Corticosteroids in high doses were shown to prolong the duration of the maintenance phase, but results concerning overall survival (OS) were controversial.4,5 Interferon α-2b (IFN) exerts direct anti-myeloma activity6 but yielded inconsistent results when tested as maintenance treatment. Two meta-analyses, one on individual patient data7 and another on published results,8 showed a marginal but statistically significant gain in both maintenance duration and overall survival. Thalidomide is an immunomodulatory drug effective in newly diagnosed and relapsed/refractory patients with multiple myeloma. Up to now, only results of thalidomide maintenance therapy in younger patients after completion of autologous stem cell transplantation (ASCT) have been published9–13 while information on outcome in elderly patients is pending. In the posttransplant setting, maintenance with thalidomide resulted in increased progression-free survival in all trials but overall survival was found to be significantly increased in 2 of 5 trials only.9–13
In this study, we compared maintenance therapy with thalidomide plus interferon α-2b with interferon α-2b alone in elderly patients with multiple myeloma who had been pre-treated with either thalidomide-dexamethasone (TD) or melphalan-prednisolone (MP) induction therapy.14
Design and Methods
Two hundred and eighty-nine previously untreated patients with active myeloma not eligible for autologous stem cell transplantation had been enrolled between August 1, 2001 and October 31, 2007 in the induction phase, the outcome of which has been reported.14 Of the 249 patients evaluable for response to induction treatment with either thalidomide-dexamethasone or melphalan-prednisolone, 137 completed 9 cycles of therapy with stable disease or better (Figure 1) and thereby met the eligibility criteria for the maintenance phase. One hundred and twenty-eight patients were randomized to either thalidomide plus IFN or to IFN alone. Patients were stratified for quality of response, prior induction therapy, and treatment center. Recruitment for maintenance therapy had been continued until September 30, 2008. Patients were treated in 26 centers in Austria, the Czech Republic, Slovakia, Hungary and Croatia. A list of additional contributing members of the CEMSG (Central European Myeloma Study Group) is shown in the appendix. The study has been approved by the ethical committees responsible for all participating study centers and was carried out in accordance with the declaration of Helsinki and with an authorization by the Ministry of Health. All patients gave written informed consent before entering the study. In accordance with the ICMJE Guidelines, the trial is registered as NCT00205751 at http://ClinicalTrials.gov. The primary objectives were progression-free survival and response rates; secondary objectives were overall survival, quality of life (QOL) and toxicity.
Figure 1.
Study flow chart.
Treatment regimen
Investigators were requested to aim for a daily dose of 200 mg thalidomide (but could start at a lower dose if required because of previous exposure to thalidomide) in combination with interferon α-2b (Schering-Plough) at a dose of 3 Mega U, TIW or interferon α-2b at the same dose and schedule only. Maintenance treatment would be given until progression or intolerance, whichever came first. In the maintenance phase of the trial, thromboprophylaxis was not mandatory. All patients received zoledronate 4 mg, q four weeks continuously until the publication of guidelines recommending discontinuation of therapy in patients with better than very good partial response (VGPR) after two years.15
Assessments
At randomization to maintenance therapy, baseline assessments included standard hematologic and chemistry analysis plus bone marrow biopsy and aspiration. Response assessment included measurement of serum paraprotein calculated by multiplying the proportion of monoclonal protein in the serum electrophoresis by the total protein level or in case of baseline paraprotein concentrations of less than 0.2g/dL by immunological techniques. In addition, 24-hour urine paraprotein excretion was determined. Immunofixation (IF) was used to identify IF negative complete response. Radiological investigations were performed before enrolment for maintenance treatment.
For evaluation of response, the EBMT criteria,16 plus an additional category of ‘very good partial response’ (VGPR), were used. Progression-free survival was calculated from the time of randomization to the time of progressive disease or to death of any cause. Overall survival was calculated from randomization until the date of death of any cause or the date the patient was last known to be alive (censored). Survival after progressive disease on maintenance and survival after end of maintenance therapy was calculated from the respective event in the subgroup of patients in which this event had already occurred.
Adverse events were assessed at each visit and graded according to the National Cancer Institute Common Toxicity Criteria.17 Thromboembolism was assessed by clinically objective evidence of thrombosis and by ultrasound and, if indicated, by pulmonary CT scan.
Statistical analysis
The induction phase of the trial was originally designed to significantly detect a suspected superiority in progression-free survival after 12 months of 65% versus 50% of the standard MP regimen over the innovative chemotherapy-free regimen with a power of 85% and a one-sided alpha-error level of 0.025, requiring a total number of 194 evaluable patients. The same number of patients (n=194) had been calculated for the maintenance phase in order to detect a suspected improvement in progression-free survival from 50% to 65% with Thal-IFN over IFN alone at 12 months after randomization with a power of 85% and a one-sided alpha-error level of 0.025. In spite of the enrollment of more patients than had been initially calculated to be required into the induction phase (n=289), due to the requirement of completion of all 9 cycles of initial therapy, only 137 patients proved to be eligible for the maintenance phase of which 128 were finally randomized. By reducing the number of patients from 194 to 128 the power to detect the suspected improvement in progression-free survival as described above had been reduced from 85% to 69%.
Response and toxicity rates were analyzed by Fisher’s exact, Cochran-Armitage trend or Wilcoxon’s tests, as appropriate. Progression-free survival, overall survival, and time on maintenance treatment were estimated by the product limit method.18 Results are presented by intention-to-treat analysis. Univariate comparisons of these endpoints were performed using the log rank test.19 Cox’s proportional hazard model20 was applied for multivariate analyses of event-type data. All P values reported are two-sided. Except for the primary endpoint, all statistical tests are of exploratory nature and no adjustments for multiplicity were applied. Subgroup analyses of an the outcome in different age groups were not pre-specified prospectively.
Results
The study flow chart is shown in Figure 1. One hundred and thirty-seven patients qualified for maintenance therapy by completing 9 cycles and achieving stable disease or better, thus excluding patients who initially responded but progressed while continuing treatment, or discontinued therapy or died. Nine patients have not been included in the maintenance trial; 5 patients because of previous toxicity or general poor condition, and 4 patients did not give consent.
Patients’ characteristics and response status at baseline of maintenance of the 128 patients randomized are shown in Table 1. Median follow up after randomization to maintenance was 35 months and median duration of maintenance therapy was 13.2 months for patients randomized to Thal-IFN and 8.3 months for those randomized to IFN (log rank test P=0.20) (Figure 2A). The median daily dose of thalidomide was 75 mg (range 25–200 mg) with a median cumulative dose of 28,400 mg (range 1,300–235,200 mg). For IFN, the median weekly dose was 9 mega units (range 3–9 mega units) with a median cumulative dose of 258 mega units (range 3–938). The median cumulative dose of IFN was slightly but not significantly higher in patients on Thal-IFN (median 266 mega units) compared to those on IFN alone (median 216 mega units, P=0.69).
Table 1.
Patients’ characteristics and response status at randomization for maintenance therapy.
Figure 2.
(A) Time on maintenance therapy. (B) Progression-free survival by treatment arm. (C) Time to progression on treatment arm and induction group. (D) Overall survival by treatment arm. (E) Overall survival on treatment arm and induction group. (F) Overall survival by age group.
Maintenance therapy with Thal-IFN resulted in an improvement in the depth of response from partial response (PR) to VGPR or complete response (CR) in 5 (8%) and with IFN in 2 (3%) patients, respectively. Progression-free survival was significantly longer in patients randomized to Thal-IFN (27.7 months) compared to patients treated with IFN maintenance only (13.2 months, HR: 0.55; 95% CI, 0.36–0.86; log rank test, P=0.0068) (Figure 2B). Analysis of progression-free survival by either TD or MP induction therapy showed a significantly shorter progression-free survival in patients started on TD and subsequently randomized to IFN maintenance only (7.8 months, log rank test, P=0.037) compared to the 3 other treatment cohorts (Figure 2C). Progression-free survival was 27.7 months in patients started on TD and followed by Thal-IFN, 20.2 months in those with MP induction therapy followed by IFN maintenance, and 27.6 months in patients with Thal-IFN maintenance after MP induction therapy.
Overall survival was similar in both groups with 52.6 months in patients receiving Thal-IFN and 51.4 months in those being treated with IFN only (HR: 0.93, 95% CI: 0.53–1.66, log rank test; P=0.81) (Figure 2D). Overall survival by induction therapy did not vary significantly between the 4 treatment groups (log rank test, P=0.99) (Figure 2E). When patients were dichotomized according to age, no difference in overall survival was seen between patients younger than 75 years and those 75 years or older (Figure 2F). At the time of this analysis, 48 deaths have been observed. Of the 23 deaths noted in the Thal-IFN arm, 6 were non-myeloma related (2 cardiovascular, 2 infections, 2 other non-identified causes), while in the IFN group 7 of 25 deaths observed were due to causes other than myeloma (one cardiovascular, 4 infections, 2 other non-identified causes). Treatment withdrawals not related to progressive disease or death were noted in 18 patients of the Thal-IFN and in 10 patients of the IFN alone arm. Intolerance of the maintenance regimen or patient’s decision was noted in 9 and in 9 patients of the Thal-IFN group, and in 5 and 4 patients of the IFN alone arm, respectively.
Survival after progression of disease tended to be longer in patients who received IFN maintenance therapy only compared to those started on Thal-IFN (HR: 1.75, 95% CI, 0.97–3.14, log rank test P=0.056) (Figure 3A) while no difference was noted between both groups when overall survival was analyzed from termination of maintenance therapy (HR: 1.20, 95% CI, 0.65–2.20, log rank test P=0.57) (Figure 3B). Quality of life was similar in both arms and did not vary significantly during maintenance treatment; however, detailed data are not presented here due to space limitations.
Figure 3.
(A) Survival after progressive disease on maintenance. (B) Survival after end of maintenance therapy. (C) Progression-free survival by cytogenetic findings. (D) Overall survival by cytogenetic risk groups.
Cytogenetic data were available in 66 patients who were categorized according to their cytogenetic risk profile in those with high risk [t (4; 14), t (14; 16) Del 17p and abnormalities of 1q21] and those with standard risk (all others). Progression-free survival tended to be shorter in patients with adverse FISH findings compared to the standard risk group, but differences were not significant (median 31.5 vs. 21.6 months, HR: 1.69, 95% CI, 0.13–3.07, log rank test P=0.084) (Figure 3C). The median overall survival was 72.3 months in those with standard risk and 39.6 months in those with cytogenetic high-risk features (HR: 1.94, CI 0.91–4.13, log rank test P=0.082) (Figure 3D).
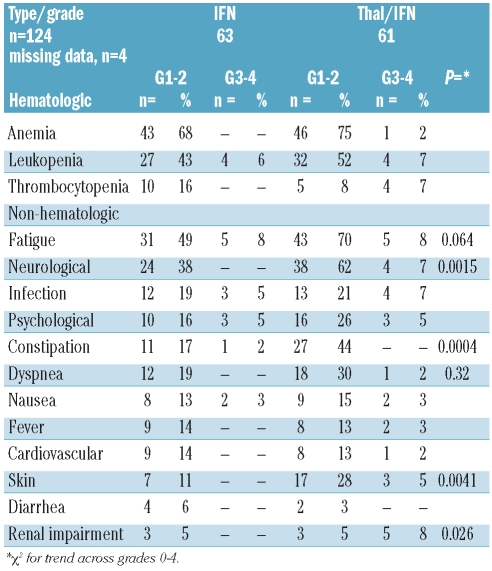
Hematologic toxicity was similar between both groups (Table 2), but patients on Thal-IFN maintenance experienced significantly more neuropathy (P=0.0015), constipation (P=0.0004), skin toxicity (P=0.0041) and renal impairment (P=0.026). In addition, there was a tendency for more dyspnea (P=0.32) and more fatigue (P=0.64) in patients on Thal-IFN maintenance therapy. Two thromboembolic events were observed in patients on Thal-IFN and one in a patient on IFN maintenance treatment. Grade 3 osteonecrosis of the jaw was noted in one patient of each group, with one still on bisphosphonates and the other one after discontinuation of bisphosphonate therapy one year before occurrence of the complication. Other non-hematologic toxicities were similarly distributed in both therapy arms.
Table 2.
Hematologic and non-hematologic toxicities.
Discussion
Inclusion into this maintenance protocol required 9 cycles of induction therapy, thus selecting mainly good-risk patients with initial tumor response. The proportion of patients who experienced an upgrade in their quality of response was low (8% and 3% of patients treated with Thal-IFN and IFN only, respectively). Higher conversion rates from less than VGPR to VGPR or CR have been reported in the French IFM 99-029 maintenance trial with thalidomide, and in the Australian study10 that employed thalidomide and prednisone maintenance therapy. In both of these studies, none of the patients had been exposed to thalidomide in the induction phase.
The most important finding of this study is the significant increase in progression-free survival by Thal-IFN compared to IFN maintenance therapy only, with no difference in overall survival. The power of overall survival analysis is, however, limited by the fact that only 48 deaths had been observed in the 128 patients at the time of this analysis. Overall survival after start of maintenance therapy was similar in patients younger than 75 years and those aged 75 years or older, in contrast to what was seen in the induction phase.14 Our observation of short progression-free survival in the subgroup of patients started on TD and randomized to IFN maintenance indicates that stopping thalidomide treatment after completion of a successful induction phase may result in earlier progression. Overall survival was remarkably long in both maintenance treatment groups; a further indication of the selection bias of patients eligible for maintenance therapy. Similar observations with thalidomide maintenance therapy have been presented by the MRC13 and the HOVON12 group with improvement of progression-free survival but not overall survival with thalidomide maintenance treatment. In contrast to our study, the MRC13 and the HOVON study,12 a significant improvement also in overall survival was seen in the IFM 99-029 and the Australian10 studies. In both trials, younger patients had been randomized to maintenance or to control after completion of conventional induction therapy followed by autologous stem cell transplantation. A significant increase in overall survival in addition to an improvement in progression-free survival with TD maintenance therapy was also reported in an Italian trial21 which randomized patients to either TD or to IFN-Dex maintenance therapy after prior treatment with TD and liposomal doxorubicin. Of note, this study included both patients with relapse to previous therapy and patients without prior therapy. Similar to our study, significantly more patients on IFN-Dex had to discontinue maintenance therapy compared to TD treated patients mostly because of progression of disease.
When progression-free survival was analyzed in patients on maintenance therapy in relation to the previous induction regimen, a remarkably shorter progression-free survival was observed in the patient subgroup started on TD and randomized to IFN maintenance only (PFS 7.8 months) compared to the 3 other treatment sequences (PFS in TD followed by Thal-IFN 27.7 months, in MP followed by IFN 20.2 months, in MP followed by Thal-IFN 27.6 months, log rank test, P=0.037) (Figure 2C). TD pre-treatment for nine months during induction phase did not diminish the duration of progression-free survival with subsequent Thal-IFN maintenance therapy. Nevertheless, pre-exposure of myeloma cells and/or bone marrow stroma to thalidomide seems to significantly alter the sensitivity of the myeloma clone to subsequent therapy. Patients who progressed during or after maintenance therapy with Thal-IFN showed a marked tendency for shorter survival after progression compared to patients being exposed to IFN maintenance only (Figure 3A); a phenomenon that had been noted in several other,11–14,22 but not in all,23 studies that analyzed treatment outcome after failure to therapy containing thalidomide. Obviously, the dose and duration of thalidomide therapy, the type of concomitant chemotherapy, as well as patient characteristics may influence the degree of thalidomide induced treatment resistance.
Patients remained in a median almost five months longer on maintenance therapy with Thal-IFN than single agent IFN (13.2 vs. 8.3 months, P=0.2) (Figure 2A) which is likely due to the marked thalidomide induced delay in disease progression. In addition, thalidomide may have mitigated some IFN related side effects probably by suppressing IFN mediated cytokine expression as previously shown in murine granulomatous tissue24 or isolated human macrophages,25 enabling patients to stay longer on maintenance treatment. Survival after discontinuation of maintenance therapy was remarkably long and did not differ significantly between the maintenance groups (Figure 3B).
Expectedly, cytogenetic risk factors as defined by FISH [t (4; 14), t (14; 16), Del 17p, and 1q21 abnormalities] were found to be associated with a tendency for shorter progression-free and overall survival. The relevance of this finding is limited because FISH data could only be assessed in 66 of the 128 patients. We, therefore, had to refrain from analyzing the impact of cytogenetic risk factors by type of maintenance treatment. Previous data indicate that thalidomide does not overcome the negative impact of high-risk cytogenetics on outcome. Attal et al.9 previously reported no benefit of thalidomide maintenance therapy in patients with del 13 or t (4; 14) and recently Morgan et al.13 presented data from the MRC trial showing a significantly shorter survival in patients with del 17p treated with thalidomide maintenance therapy. Likewise, a significantly lower response rate has been reported in patients with unfavorable cytogenetics when thalidomide-based regimens have been compared with bortezomib-based combinations as induction therapy.26,27 The only exception to these rather uniform findings comes from the total therapy II study, where conventional cytogenetics was used for delineation of risk groups. Thalidomide therapy during induction and maintenance phase resulted in higher response rates and prolonged event-free survival, but similar overall survival compared to the same treatment protocol without the IMiD after a median follow up of 42 months. Unexpectedly, reanalysis after a long follow up of a median 72 months showed a significant prolongation of survival with thalidomide in the subgroup of patients with abnormal cytogenetics defined by metaphase karyotyping, but not in the entire patient cohort.28
Both maintenance treatments were well tolerated with grade 1 hematologic toxicity seen in up to 15% of patients. Non-hematologic toxicity was more common in patients on Thal-IFN. There was a tendency for more fatigue and dyspnea, and a significantly higher frequency of polyneuropathy, constipation, skin toxicity and decrease in glomerular filtration rate in the group treated with Thal-IFN. Patients on single agent IFN therapy discontinued treatment either because of tumor progression or intolerance, while in those on Thal-IFN maintenance, treatment was stopped more frequently because of toxicity of therapy, such as polyneuropathy, constipation and fatigue, and because of patient choice.
In conclusion, Thal-IFN maintenance therapy resulted in significantly longer progression-free survival but failed to improve overall survival in comparison to single agent IFN maintenance treatment. Patients tended to stay longer on Thal-IFN maintenance therapy in spite of more, mostly grade 1 and 2 polyneuropathy, constipation and skin toxicity. These data show only limited benefit of Thal-IFN maintenance treatment in elderly patients. For clinical practice, patients should be thoroughly informed about the advantages, limitations, and potential toxicities of thalidomide maintenance therapy in order to allow informed decisions to be made.
Acknowledgments
the authors would like to thank Ms. Silvia Bakos for assistance in manuscript preparation and Mr. Zoran Birovljev for data management.
Appendix
The following members of the Central European Myeloma Study Group (CEMSG) also contributed by treating patients within the maintenance phase of the study and providing data. Austria: Michael Fridrik (General Hospital Linz), Werner Linkesch (University Clinic Graz), Josef Thaler (Klinikum Kreuzschwestern Wels), Alois Lang (LKH Feldkirch), Gunther Gastl (University Clinic Innsbruck), Werner Lin (LKH Villach), Hedwig Kasparu (A.ö. Krankenhaus der Elisabethinen Linz), Richard Greil (Private Medical University Hospital Salzburg); Czech Republic: Jaromir Gumulec (J.G. Mendel Cancer Center, Novy Jicin), Evzen Gregora. (Fac Hospital Kralovske Vinohrady, Prague); Slovakia: Martin Mistrik (Hospital of St. Cyril and Method, Bratislava); Croatia: Branimir Jaksic (Kl. Krank. Merkur, Zagreb), Rajko Kusec (Kl. Krank. Merkur, Zagreb).
Footnotes
Funding: this work was supported by the Austrian Forum against Cancer, by the Austrian Health authorities, the Association of Austrian Social Insurance Carriers, and partly by an investigational grant from Schering-Plough. Sample collections in the Czech Republic were supported by MSM0021622434 and LC06027.
Authorship and Disclosures
HL acts as principal investigator and takes primary responsibility for the paper, established study design with co-investigators, provided study material, treated patients, assembled and interpreted data, and wrote and approved the paper. ZA, ET, RH, BL, ME, IS, HG, JD provided study materials or recruited patients, collected and assembled data, commented on the manuscript and approved its final version. IK was responsible for conception and design, financial and administrative support, and approved the manuscript. AH conducted the statistical analysis, interpreted the data, and wrote and approved the manuscript. NZ provided study materials and recruited patients, collected and assembled data, performed data analysis and interpretation, wrote and approved the manuscript. IK has disclosed employment with Schering-Plough.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Wisløff F, Hjorth M. Health-related quality of life assessed before and during chemotherapy predicts for survival in multiple myeloma. Nordic Myeloma Study Group. Br J Haematol. 1997;97(1):29–37. doi: 10.1046/j.1365-2141.1997.222667.x. [DOI] [PubMed] [Google Scholar]
- 2.Quach H, Mileshkin L, Seymour JF, Milner A, Ritchie D, Harrison S, et al. Predicting durable remissions following thalidomide therapy for relapsed myeloma. Leuk Lymphoma. 2009;50(2):223–9. doi: 10.1080/10428190802663213. [DOI] [PubMed] [Google Scholar]
- 3.Belch A, Shelley W, Bergsagel D, Wilson K, Klimo P, White D, et al. A randomized trial of maintenance versus no maintenance melphalan and prednisone in responding multiple myeloma patients. Br J Cancer. 1988;57(1):94–9. doi: 10.1038/bjc.1988.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berenson JR, Crowley JJ, Grogan TM, Zangmeister J, Briggs AD, Mills GM, et al. Maintenance therapy with alternate-day prednisone improves survival in multiple myeloma patients. Blood. 2002;99(9):3163–8. doi: 10.1182/blood.v99.9.3163. [DOI] [PubMed] [Google Scholar]
- 5.Shustik C, Belch A, Robinson S, Rubin SH, Dolan SP, Kovacs MJ, et al. A randomised comparison of melphalan with prednisone or dexamethasone as induction therapy and dexamethasone or observation as maintenance therapy in multiple myeloma: NCIC CTG MY.7. Br J Haematol. 2007;136(2):203–11. doi: 10.1111/j.1365-2141.2006.06405.x. [DOI] [PubMed] [Google Scholar]
- 6.Mellstedt H, Ahre A, Björkholm M. Interferon therapy in myelomatosis. Lancet. 1979;1(8110):245–7. doi: 10.1016/s0140-6736(79)90770-0. [DOI] [PubMed] [Google Scholar]
- 7.Myeloma Trialists’ Collaborative Group. Interferon as therapy for multiple myeloma: an individual patient data overview of 24 randomized trials and 4012 patients. Br J Haematol. 2001;113:1020–34. doi: 10.1046/j.1365-2141.2001.02857.x. [DOI] [PubMed] [Google Scholar]
- 8.Fritz E, Ludwig H. Interferon-alpha treatment in multiple myeloma: meta-analysis of 30 randomised trials among 3948 patients. Ann Oncol. 2000;11(11):1427–36. doi: 10.1023/a:1026548226770. [DOI] [PubMed] [Google Scholar]
- 9.Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. for the Inter-Groupe Francophone du Myélome (IFM) Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–94. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 10.Spencer A, Prince HM, Roberts AW, Prosser IW, Bradstock KF, Coyle L, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27(11):1788–93. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 11.Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354(10):1021–30. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 12.Lokhorst HM, van der Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115(6):1113–20. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 13.Morgan GJ, Jackson GH, Davies FE, Drayson MT, Owen RG, Gregory WM, et al. Maintenance thalidomide may improve progression free but not overall survival; Results from the Myeloma IX Maintenance Randomisation. Blood. (ASH Annual Meeting Abstracts) 2008;112 abstract 656. [Google Scholar]
- 14.Ludwig H, Hajek R, Tóthová E, Drach J, Adam Z, Labar B, et al. Thalidomide-dexamethasone compared with melphalan-prednisolone in elderly patients with multiple myeloma. Blood. 2009;113(15):3435–42. doi: 10.1182/blood-2008-07-169565. [DOI] [PubMed] [Google Scholar]
- 15.Kyle RA, Yee GC, Somerfield MR, Flynn PJ, Halabi S, Jagannath S, et al. American Society of Clinical Oncology. American Society of Clinical Oncology 2007 clinical practice guideline update on the role of bisphosphonates in multiple myeloma. J Clin Oncol. 2007;25(17):2464–72. doi: 10.1200/JCO.2007.12.1269. [DOI] [PubMed] [Google Scholar]
- 16.Blade J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G, et al. Criteria for evaluating disease response and in patients with multiple myeloma treated with high dose chemotherapy and haematopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–23. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 17.Common Toxicity Criteria Manual: Version 2.0. Jun 1, 1999. Available from http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf.
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Ass. 1958;53:457–81. [Google Scholar]
- 19.Peto R, Peto J. Asymptotically efficient rank invariation test procedures (with discussion) J R Stat Soc A. 1972;135:185–206. [Google Scholar]
- 20.Cox DR. Regression models and life tables. J Roy Stat Soc (B) 1972;34:187–202. [Google Scholar]
- 21.Offidani M, Corvatta L, Polloni C, Piersantelli MN, Gentili S, Galieni P, et al. Thalidomide-dexamethasone versus interferon-alpha-dexamethasone as maintenance treatment after ThaDD induction for multiple myeloma: a prospective, multicentre, randomised study. Br J Haematol. 2009;144(5):653–9. doi: 10.1111/j.1365-2141.2008.07495.x. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112(8):3107–14. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 23.Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 24.Moreira AL, Tsenova-Berkova L, Wang J, Laochumroonvorapong P, Freeman S, Freedman VH, et al. Effect of cytokine modulation by thalidomide on the granulomatous response in murine tuberculosis. Tuber Lung Dis. 1997;78(1):47–55. doi: 10.1016/s0962-8479(97)90015-0. [DOI] [PubMed] [Google Scholar]
- 25.Rowland TL, McHugh SM, Deighton J, Dearman RJ, Ewan PW, Kimber I. Differential regulation by thalidomide and dexamethasone of cytokine expression in human peripheral blood mononuclear cells. Immunopharmacology. 1998;40(1):11–20. doi: 10.1016/s0162-3109(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Rosinol L, Cibeira T, Martínez J, Mateos MV, Terol MJ, de la Rubia J, et al. Thalidomide/Dexamethasone (TD) Versus Bortezomib (Velcade)/Thalidomide/Dexamethasone (VTD) Versus VB. International Myeloma Workshop; Washington, D.C. February 26 –March 1, 2009; abstract A160. [Google Scholar]
- 27.Cavo M, Testoni N, Terragna C, Marzocchi G, Durante S, Zambello R, et al. Superior rate of complete response with up-front Velcade-Thalidomide-Dexamethasone versus Thalidomide-Dexamethasone in newly diagnosed multiple myeloma is not affected by adverse prognostic factors, including high-risk cytogenetic abnormalities. Blood (ASH Annual Meeting Abstracts) 2008;112 abstract 1662. [Google Scholar]
- 28.Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112(8):3115–21. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]