Abstract
Background
Type G immunoglobulins against ADAMTS13 are the primary cause of acquired (idiopathic) thrombotic thrombocytopenic purpura. However, the domains of ADAMTS13 which the type G anti-ADAMT13 immunoglobulins target have not been investigated in a large cohort of patients with thrombotic thrombocytopenic purpura.
Design and Methods
Sixty-seven patients with acquired idiopathic thrombotic thrombocytopenic purpura were prospectively collected from three major U.S. centers. An enzyme-linked immunosorbent assay determined plasma concentrations of anti-ADAMTS13 type G immunoglobulins, whereas immunoprecipitation plus western blotting determined the binding domains of these type G immunoglobulins.
Results
Plasma anti-ADAMTS13 type G immunoglobulins from 67 patients all bound full-length ADAMTS13 and a variant truncated after the eighth TSP1 repeat (delCUB). Approximately 97% (65/67) of patients harbored type G immunoglobulins targeted against a variant truncated after the spacer domain (MDTCS). However, only 12% of patients’ samples reacted with a variant lacking the Cys-rich and spacer domains (MDT). In addition, approximately 37%, 31%, and 46% of patients’ type G immunoglobulins interacted with the ADAMTS13 fragment containing TSP1 2-8 repeats (T2-8), CUB domains, and TSP1 5-8 repeats plus CUB domains (T5-8CUB), respectively. The presence of type G immunoglobulins targeted against the T2-8 and/or CUB domains was inversely correlated with the patients’ platelet counts on admission.
Conclusions
This multicenter study further demonstrated that the multiple domains of ADAMTS13, particularly the Cys-rich and spacer domains, are frequently targeted by anti-ADAMTS13 type G immunoglobulins in patients with acquired (idiopathic) thrombotic thrombocytopenic purpura. Our data shed more light on the pathogenesis of acquired thrombotic thrombocytopenic purpura and provide further rationales for adjunctive immunotherapy.
Keywords: immunoglobulin, thrombotic thrombocytopenic purpura, ADAMTS13, adjunctive immunotherapy
Introduction
Thrombotic thrombocytopenic purpura (TTP), a life-threatening thrombotic microangiopathy, is characterized by profound thrombocytopenia and hemolytic anemia with varying degrees of neurological disturbances and/or renal abnormalities. TTP can be classified into at least three major groups: congenital, idiopathic (or autoimmune) and non-idiopathic (or secondary) TTP. Congenital TTP is caused by constitutional deficiency of plasma ADAMTS13 activity due to compound heterozygous or homozygous mutations in the ADAMTS13 gene.1,2 Idiopathic TTP or acquired autoimmune TTP mainly occurs in previously healthy individuals as a result of production of autoantibodies that bind and neutralize the proteolytic activity of ADAMTS13 and/or accelerate clearance of ADAMTS13 in vivo.3,4 Patients with non-idiopathic or secondary TTP constitute a heterogeneous group secondary to pregnancy, autoimmune diseases, infection, drugs (e.g. cyclosporine A, FK 506, mitomycin, ticlopidine and clopidogrel), and hematopoietic progenitor cell transplantation.2,5 To date, plasma infusion and/or plasma exchange remains the first-line therapy to reduce the mortality rate regardless of the etiology and pathophysiology of the TTP.6
Plasma ADAMTS13 activity in patients with acquired autoimmune TTP is usually severely deficient (less than 5% of normal) and an inhibitor can be demonstrated by functional assays in approximately 50% to 90% of the cases.7 When an enzyme-linked immunosorbent assay (ELISA) is used, autoantibodies against ADAMTS13 are detectable in 97% to 100% of patients.7,8 Most of these autoantibodies are type G immunoglobulins (IgG),4 particularly IgG1 and IgG4 subtypes.9 Type M (IgM) and type A (IgA) isotypes have also been described in some cases.8,10 The severe deficiency of plasma ADAMTS13 activity and the presence of anti-ADAMTS13 autoantibodies are considered to be highly specific for the diagnosis of acquired idiopathic TTP.11,12 Adjunctive immunosuppressive therapies such as cyclophosphamide, cyclosporine and rituximab have been shown to be effective for patients with high-titer inhibitors or for those refractory to routine plasma exchange therapy.13 Persistent anti-ADAMTS13 autoantibodies in patients’ plasma are associated with frequent relapses and poor long-term outcome.14–16
Despite the recognition of the importance of anti-ADAMTS13 IgG in the pathogenesis of acquired TTP, the binding epitopes of IgG autoantibodies have not been well investigated in a large cohort of TTP patients. The results published to date are either from small case series or obtained using non-physiological antigens or detection methods and are not concordant. For example, Klaus et al.,17 using recombinant ADAMTS13 fragments expressed in E. coli and a direct western blotting technique, showed that all 25 patients investigated harbored IgG autoantibodies that were specific toward the spacer domain of ADAMTS13 in addition to various other domains. However, Luken et al.,18 using recombinant ADAMTS13 fragments expressed in insect cells, demonstrated that all seven of their patients’ IgG bound the spacer domain, but rarely the other domains of ADAMTS13. Further studies are, therefore, necessary to strengthen or confirm either one of these previous observations, which may help in the understanding of the pathogenesis of TTP and the design of rational therapies.
To circumvent the potential pitfalls of the two previous studies, we expressed recombinant ADAMTS13 and variants/fragments in human embryonic kidney (HEK)-293 cells and employed a more physiological detection system including immunoprecipitation followed by western blotting to detect the immune complexes. A total of 67 patients with acquired (idiopathic) TTP were prospectively enrolled into the study from three major U.S. medical centers. The results demonstrate that multiple functional domains of ADAMTS13, particularly the Cys-rich and spacer domains, appear to be frequently targeted by anti-ADAMTS13 IgG in patients with acquired (idiopathic) TTP. The presence of anti-ADAMTS13 IgG autoantibodies against the middle and distal C-terminal domains of ADAMTS13 seems to be inversely correlated with the initial platelet counts on admission. These findings suggest a role of multiple C-terminal domains of ADAMTS13 for function in vivo.
Design and Methods
Patients
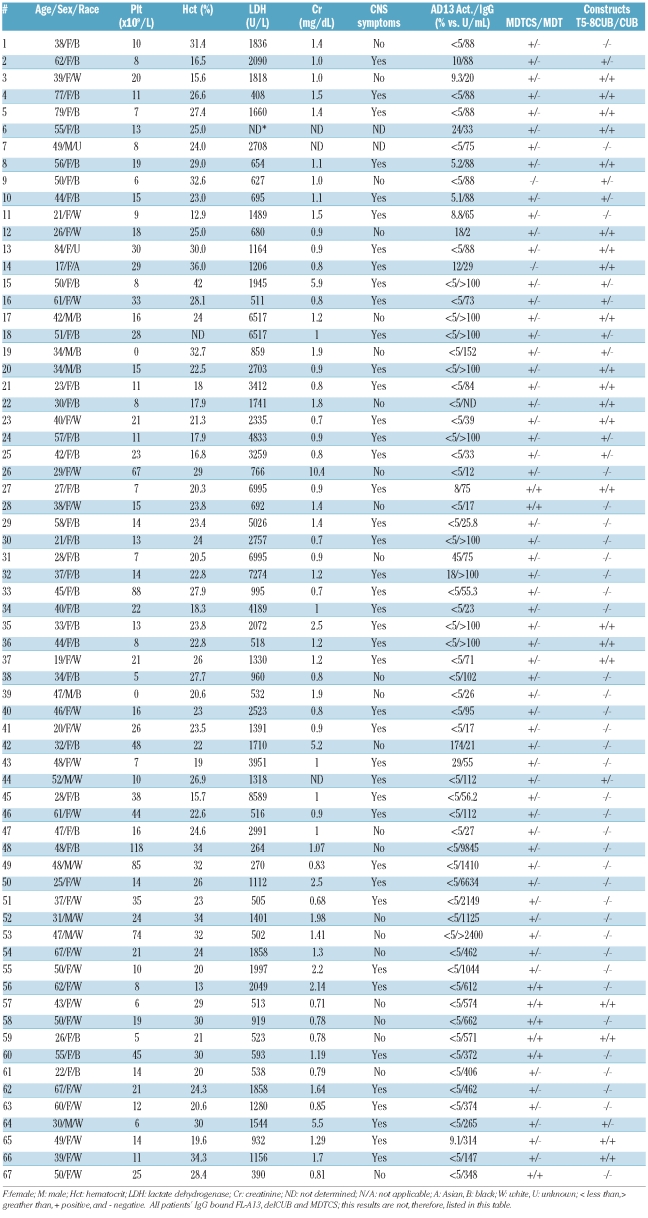
The study protocols were reviewed and approved separately by the Institutional Review Boards at The Children’s Hospital of Philadelphia and The University of Pennsylvania Medical Center, Ohio State University and Northwestern University, respectively. Informed consent to participation in the study was obtained from patients or the patients’ legal guardians at the participating centers. Patients aged from 17 to 84 years old (mean±SD; 43±15 years) with a clinical diagnosis of acquired idiopathic TTP who were receiving plasma exchange therapy and undergoing follow-up were enrolled into the study. Patients with acquired thrombotic microangiopathies secondary to pregnancy, cancer, drugs, hematopoietic progenitor cell transplantation and infections were all excluded from the study. The patients’ demographic data and laboratory values are summarized in Table 1. The diagnostic criteria for acquired (idiopathic) TTP and protocols for sample collection have been described previously.16,19
Table 1.
Demographic and clinical data and antibody binding sites in patients with idiopathic TTP.
Assay for plasma ADAMTS13 activity and autoantibodies against ADAMTS13
FRETS-VWF73 (Peptides International, Louisville, KY, USA)20,21 and GST-VWF73-His peptide plus surface enhanced laser desorption/ionization (SELDI) time-of-flight (TOF) mass spectrometry22–25 were used for determining plasma ADAMTS13 activity as previously described. Normal human plasma was used for calibration. The results obtained from FRETS-vWF73 were well correlated with those from the SELDI-TOF method (r=0.87, n=9). The plasma levels of IgG against ADAMTS13 were determined by TECHNOZYM® ADAMTS-13 INH (Technoclone, Vienna, Austria) or by IMUBIND® ADAMTS13 autoantibodies (American Diagnostics, Stamford, CT, USA). The assays were performed in duplicate according to the manufacturers’ recommendations. In the TECHNOZYM® ADAMTS-13 INH assay, plasma IgG levels less than 12 units/mL were defined as negative; 15–49 units/mL were assigned a score of 1+, those from 50–100 units/mL a score of 2+, and levels greater than 100 units/mL were scored as 3+. In the IMUNBIND® ADAMTS13 autoantibody assay, IgG levels less than 10 units/mL were considered negative, those of 10–49 units/mL given a score of 1+, levels of 50–100 units/mL a score of 2+ and levels greater than 100 units/mL a score of 3+. One arbitrary unit is equivalent to 1 μg of purified anti-ADAMTS13 IgG according to the manufacturer’s recommendation (American Diagnostica, Stamford, CT, USA).
Immunoprecipitation plus western blotting
Recombinant ADAMTS13 or variants were prepared as described previously.22,24 Approximately 150 ng of purified recombinant proteins were incubated with 5–20 μL of patients’ plasma or normal human plasma in the presence of 0.1% pro-tease inhibitor cocktail (Sigma, St Louis, MO, USA) at 4°C overnight. Protein A- and protein G-Sepharose 4B resins (20 μL of each) (GE Healthcare, Piscataway, NJ, USA) were then added and incubated at room temperature for 2 h. After being washed three times with phosphate-buffered saline, the antigen-antibody complexes were eluted from the protein A/G-Sepharose 4B resins by heating at 100°C for 5 min with 40 μL of Laemmli’s sample buffer (62.5 mM Tris-HCl, pH 6.8, 25% glycerol, 2% SDS and 0.01% bromophenol blue; Bio-Rad, Hercules, CA, USA) containing 5% β-mercaptoethanol. The proteins were fractionated on an 8% or 4–20% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membrane was incubated with monoclonal anti-V5 IgG (1:5,000) in TBS containing 2.5% non-fat milk and 0.05% Tween 20, followed either by a peroxidase-conjugated goat anti-mouse IgG (1:5,000) (BD Biosciences, Franklin Lakes, NJ, USA) or an IRDye® 800CW-labeled rabbit anti-mouse IgG (LI-COR Bioscience, Lincoln, Nebraska) in TBS containing 2.5% non-fat milk and 0.05% Tween-20. The enhanced chemiluminescent reagents (BD Biosciences)26 or Odyssey infrared imaging system (LI-COR Bioscience, Lincoln, Nebraska)27 was used to determine the bound secondary antibody, as previously described.
Statistical analysis
A scatter plot was obtained using Sigma plot software. The two-tailed Mann-Whitney test was used to determine whether platelet counts or autoantibody titers in one group whose autoantibodies bound to both N-terminal and C-terminal fragments (including TSP1 2–8, TSP1 5–8CUB and/or CUB) were different from those in another group whose autoantibodies only bound to the N-terminal fragments. The U and P values were determined using the Smith’s Statistical Package (SSP) (http://stat-pages.org/javasta2.html).
Results
Demographic and clinical data
The patients’ demographic data and clinical information are all summarized in Table 1. The mean age of all 67 patients with acquired idiopathic TTP was approximately 43 years, with the ages ranging from 17 to 84 years. Female patients predominated, with a female to male ratio of approximately 6 to 1. Just over half of the patients (35/67) were black; the other half were white (29/67), Asian (1/67) or of unknown race (2/67). Forty-three of the 67 patients (64%) had platelet counts less than 20×109/L on admission or at the time of sample collection with the mean platelet count being 22×109/L (range, 0 to 118×109/L). The mean hematocrit was 25 g/dL (range, 12 to 42 g/dL). Serum levels of lactate dehydrogenase were elevated (normal less than 250 U/L) in all patients tested, with the mean±SD value being 2,060±1,966 U/L (range, 254 to 8,589 U/L). Sixteen of 64 (25%) patients were found to have elevated serum creatinine (normal less than 1.5 mg/dL). All patients received plasma exchange therapy and many were treated with additional adjunctive therapies such as corticosteroids. Patients 50, 52, 57, 58, 60, 61, 64 and 65 were given cyclosporine A and patients 2, 5, 8, 10, and 49 were given vincristine. The patients were followed up from 30 days to 3 years to assess their clinical response.
Plasma ADAMTS13 activity and anti-ADAMTS13 immunoglobulins
All the patients’ plasma ADAMTS13 activity and anti-ADAMTS13 IgG titers are summarized in Table 1. The ADAMTS13 activity and inhibitors in patients 1–47 were measured by a FRETS-vWF73 assay, whereas those in patients 48–67 were determined by the SELDI-TOF technique. The results showed that 59/67 (~88%) of patients had plasma ADAMTS13 activity less than 10% of normal, 7/67 patients (~10%) had activity between 10% and 50% of normal, and 1/67 (1.5%) patients had plasma ADAMTS13 activity that was greater than 50% of normal. Anti-ADAMTS13 IgG (greater than 15 units/mL by the TECHNOZYM® standard or greater than 10 units/mL by the IMUBIND® standard) were detected in 64/67 (~96%) patients, with the IgG titers ranging from 17 to 9,845 units/mL. Two patients (12 and 26) negative by ELISA were positive for multiple ADAMTS13 variants by the immunoprecipitation plus western blot technique, suggesting that this latter technique may be more sensitive than commercial ELISA in identifying IgG against ADAMTS13 in TTP patients. The plasma IgG from healthy donors did not bind ADAMTS13 and variants/fragments detectably by immunoprecipitation and western blot (data not shown).
Binding domains of anti-ADAMTS13 immunoglobulins in patients with idiopathic thrombotic thrombocytopenic purpura
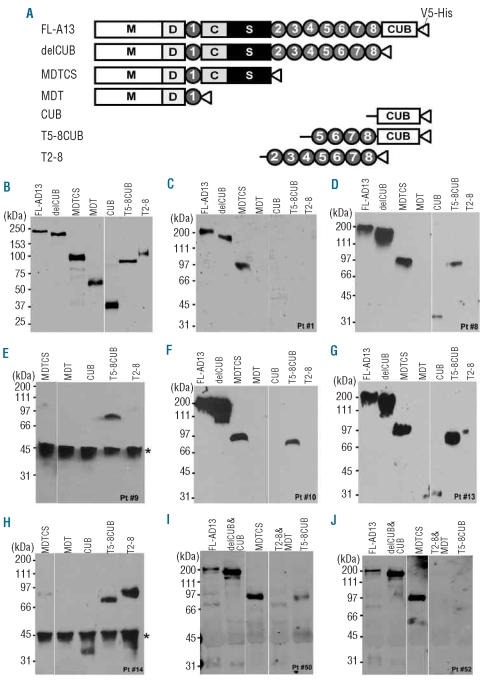
To determine anti-ADAMTS13 IgG binding sites, we prepared a series of truncated ADAMTS13 variants in addition to full-length protein from HEK293 cells (Figure 1A). Most proteins except MDT, TSP1 2-8 and CUB used in this study were affinity-purified to greater than 90% of purity as determined by SDS-PAGE and Coomassie blue staining (data not shown). Western blotting with anti-V5 demonstrated the correct sizes of all recombinant ADAMTS13 and variants/fragments, as expected, with little degradation (Figure 1B). The antigen-antibody reactions were performed in solution by an immunoprecipitation method. The immune complexes were determined by western blotting with highly specific monoclonal anti-V5 IgG as described in the Design and Methods section. The combination of all three (recombinant ADAMTS13/variants expressed in mammalian cells, immunoprecipitation and western blotting) allowed us to detect conformation-sensitive or perhaps discontinuous binding epitopes. Figure 1C through Figure 1J show eight representative autoantibody binding patterns from patients 1, 8, 9, 10, 13, 14, 50, and 52. The IgG from patients 1 (Figure 1C) and 52 (Figure 1J) recognized only the N-terminal fragments up to the spacer domain. The IgG from patients 8 (Figure 1D), 10 (Figure 1F), 13 (Figure 1G) and 50 (Figure 1I) recognized, to various degrees, the more distal C-terminal domains of ADAMTS13. Interestingly, patients 9 (Figure 1E) and 14 (Figure 1H) appeared to have IgG that predominantly targeted the C-terminal fragments of ADAMTS13 with no or weak reactivity toward the N-terminal fragment, MDTCS.
Figure 1.
Identification of anti-ADAMTS13 IgG auto-antibodies by immunoprecipitation and western blotting. (A) The schematic domain representation of the constructs of recombinant ADAMTS13 and variants used in the study. M: metalloprotease domain; D: disintegrin domain; 1–8: 1–8th thrombospondin type 1 repeats; C and S: cys-rich and spacer domains; and CUB: two CUB domains. (B) Purified and partially purified ADAMTS13 and variants (~20–50 ng/lane) were detected by SDS-PAGE and western blotting with anti-V5 (1:5,000) and IDye800-labeled anti-mouse IgG. (C-J) eight representative binding patterns of plasma anti-ADAMTS13 IgG from patients 1, 8, 9, 10, 13, 14, 50 and 52, respectively. In these experiments, ~200 ng of recombinant ADAMTS13 and variants were incubated at 4ºC overnight with 5–10 μL of patients’ plasma. After being washed three times with TBS, the bound ADAMTS13/variant-IgG complexes were pulled down by the mixture of protein-G/protein A Sepharose 4B, and determined by western blot with anti-V5 (1:5,000), followed by either anti-mouse IgG, peroxidase conjugated (1:5,000) and chemiluminescent reagents (C-H) or by IDye800-labeled anti-mouse IgG (1:12,500) and Odyssey imaging detection (panels I and J). The star on the left right side of panels E and H indicates an unknown non-specific protein contaminated in the protein A-Sepharose 4B beads used at this particular time.
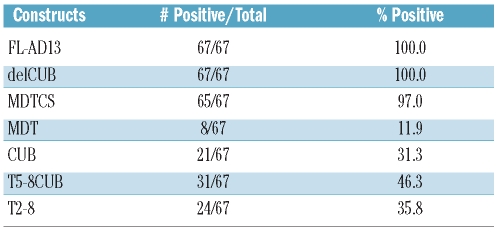
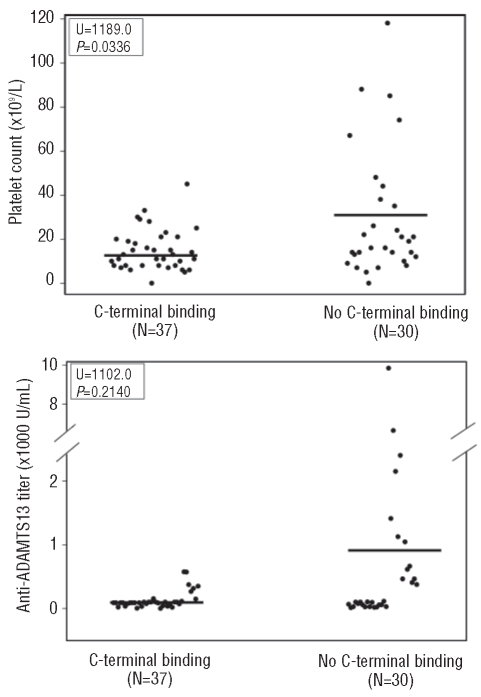
All of the 67 patients with acquired TTP appeared to bind full-length ADAMTS13 (construct FL-AD13) and the variant truncated after the eighth TSP1 repeat (construct delCUB). Nearly all (~97%) of them (the exceptions being patients 9 and 14) recognized the N-terminal half of ADAMTS13 (construct MDTCS). However, when the Cys-rich and spacer domains were further removed from the MDTCS fragment (construct MDT), the anti-ADAMTS13 IgG reactivity dropped to approximately 12% (Tables 1 and 2), suggesting that the Cys-rich and spacer domains of ADAMTS13 contain the major binding sites for autoantibodies against ADAMTS13 in patients with acquired (idiopathic) TTP. Moreover, we found that various C-terminal domains of ADAMTS13 were recognized by the plasma anti-ADAMTS13 IgG in patients with acquired TTP (Table 1). Of the 67 patients, 21 (31%), 24 (36%) and 31 (46%) had IgG that bound the CUB domains (CUB), the TSP1 2-8 repeats (T2-8) and the TSP1 5-8 repeats plus CUB domains (T5-8CUB), respectively (Table 2). As controls, IgG isolated from ten healthy individuals did not bind to any one of the ADAMTS13 proteins detectably under the conditions used for our immunoprecipitation/western blot experiments (data not shown). Platelet counts on admission were lower in the group of patients whose anti-ADAMTS13 IgG reacted with the middle and distal C-terminal domains of ADAMTS13 than in the group of patients whose IgG targeted only the N-terminal fragments up to the spacer domain (U=1189.0, P=0.0336) (Figure 2A). In contrast, there appeared to be a trend toward higher anti-ADAMTS13 IgG titers in patients whose IgG bound only to the N-terminal fragments than in patients whose IgG reacted with both N-terminal and C-terminal fragments. The difference was not, however, statistically significant (U=1102.0, P=0.2140) (Figure 2B). Moreover, no correlation was found between the presence of anti-ADAMTS13 IgG against the TSP1 2-8 and/or CUB domains and the frequency of neurological symptoms (data not shown). These findings suggest that the autoantibodies that bind the N-terminus and C-terminus of ADAMTS13 may synergistically interfere with ADAMTS13 function in vivo.
Table 2.
ADAMTS13 domains recognized by anti-ADAMTS13 IgG in patients with acquired idiopathic TTP
Figure 2.
Correlations between the C-terminal antibody binding and patients’ platelet counts or autoantibody titers. Panel A and panel B show the platelet counts and plasma autoantibody titers, respectively, in one group with anti-ADAMTS13 IgG against the middle and distal C-terminal domains (C-terminal binding) and in another group with IgG binding only the N-terminal fragments up to the spacer domain of ADATMS13 (no C-terminal binding). The differences between the two groups were determined by a two-tailed Mann-Whitney test. P values less than 0.05 were considered to be statistically significant.
Discussion
In this study recombinant full-length ADAMTS13 and various variants/fragments of ADAMTS13 expressed and purified from mammalian cells were used to investigate the domains of ADAMTS13 that the anti-ADAMTS13 IgG from patients with acquired idiopathic TTP bind under more physiologically relevant conditions than had been previously explored. Our results demonstrate that nearly all patients (~97%) harbor IgG that specifically target the N-terminal half of ADAMTS13 (i.e. the MDTCS fragment), but that the removal of the Cys-rich and spacer domains markedly reduces the antibody reactivity, suggesting that the Cys-rich and spacer domains contain the major binding sites for anti-ADAMTS13 IgG in these patients. These results are consistent with those previously reported in two smaller series.17,18 It remains to be determined where exactly the binding epitopes of the anti-ADAMTS13 IgG are on the Cys-rich and spacer domain. Limited data from literature suggest that certain regions in the spacer domain, such as amino acid residues Tyr572-Asn579 and Arg657-Gly666, may be frequently targeted by the anti-ADAMTS13 IgG in patients with acquired TTP.28 Our recent data have shown that the amino acid residues Arg659, Arg660, and Tyr661 in the spacer domain are critical for the recognition of von Willebrand factor (vWF) under various conditions.29 Several other studies26,30 have also shown that the spacer domain of ADAMTS13 is required for productive cleavage of various peptidyl substrates such as FRETS-vWF73, GST-vWF73, and multimeric vWF under static/denaturing conditions. The spacer domain has also been shown to be critical for recognition and cleavage of soluble and cell bound multimeric vWF under fluid shear stress.29,31 The anti-ADAMTS13 IgG that bind other N-terminal domains (metalloprotease, disintegrin domain, first TSP1 repeat and Cys-rich domain) are, therefore, expected to cause inhibition of vWF proteolysis as these domains appear to participate in substrate recognition under various conditions.
Moreover, approximately one third to half of patients’ anti-ADAMTS13 IgG target the C-terminal half of ADAMTS13 including the TSP1 2–8 repeats and/or the CUB domains (Tables 1 and 2). It does, however, remain to be determined whether the anti-ADAMTS13 IgG targeted at these middle and distal C-terminal domains of ADAMTS13 are clinically important or not. We found that only very few TTP patients harbor the anti-ADAMTS13 IgG that specifically target these domains without binding to the N-terminal half of ADAMTS13. For instance, patients 9 and 14 had anti-ADAMTS13 IgG that predominantly bound the middle and distal C-terminal fragments (TSP1 5-8 plus CUB, TSP1 2-8 and/or CUB domain). Reactivity with MDTCS was either undetectable (Figure 1E) or weakly detectable (Figure H). Interestingly, patient 9 showed a severe deficiency of plasma ADAMTS13 activity (less than 5%) and patient 14 had moderately reduced plasma ADAMTS13 activity (12%), as determined by the FRETS-vWF73 assay. Both patients achieved a clinical recovery, with increases of plasma ADAMTS13 activity according to the FRETS assay (data not shown), after plasma exchange therapy. These results suggest that the binding of anti-ADAMTS13 IgG predominantly to the C-terminal half of ADAMTS13 alone may also be pathogenic in vivo. Several recent studies including ours have demonstrated that the middle and distal C-terminal domains of intact ADAMTS13 may play a role in the recognition and proteolytic cleavage of native vWF under fluid shear stress.24,32 Moreover, the distal TSP1 repeats bind the endothelial cell surface, which may enhance proteolytic cleavage of newly released ultralarge-vWF on endothelial cells by ADAMTS13 under flow conditions.33 The inverse correlation between anti-ADAMTS13 IgG positivity for the middle and distal C-terminal domains of ADAMTS13 and the patients’ initial platelet counts (Figure 2A) further supports the hypothesis that anti-ADAMTS13 IgG autoantibodies against the more distal C-terminal domains in conjunction with those against the proximal C-terminal domains may have a synergistic effect on ADAMTS13 function in vivo.
It is worth noting that two patients with normal (patient 42) or near normal (patient 31) plasma ADAMTS13 activity according to the FRETS-vWF73 assay and two other patients (patients 12 and 26) with no detectable anti-ADAMTS13 IgG by the ELISA method were positive for anti-ADAMTS13 IgG according to our assay (Table 1), suggesting that the combination of immunoprecipitation and western blotting may be more sensitive than an ELISA or direct western blotting for the identification of the conformation-sensitive autoantibodies. The immobilization of the protein of interest (ADAMTS13 and variants/fragments) on the plastic surface, as in the ELISA assay, or on the nitrocellulose membrane, as in the direct western blot assay previously described,17 may mask some of the potential binding sites or cause conformational changes of the target protein antigens, resulting in potentially false negative results. Our assay may, therefore, help to identify more patients with anti-ADAMTS13 IgG in their plasma. The detection of plasma anti-ADAMTS13 IgG in conjunction with severely deficient ADAMTS13 activity is highly specific for acquired (autoimmune) TTP,11,12 which may warrant adjunctive immunotherapies in addition to standard plasma exchange therapy.
We conclude that multiple domains of ADAMTS13 are targeted by anti-ADAMTS13 IgG in patients with acquired (idiopathic) TTP. Although the Cys-rich and spacer domains of ADAMTS13 contain the major binding epitopes for the anti-ADAMTS13 IgG, the other more distal C-terminal domains are also targeted by anti-ADAMTS13 IgG, which may result in a synergistically detrimental effect on ADAMTS13 function in vivo. Our findings further support the role of various C-terminal domains of ADAMT13 for antithrombotic functions in vivo. This in turn provides the basis for developing new diagnostic and therapeutic strategies for the care of patients with acquired autoimmune TTP.
Acknowledgments
the authors wish to thank Ivy Weiss for analyzing some of the plasma ADAMTS13 activity and inhibitors.
Footnotes
Funding: this study was supported by an American Heart Association-Established Investigator Award, and National Institute of Health (HL-079027 to XLZ and P50HL081012 to Joel Bennett at University of Pennsylvania with XLZ as a co-investigator).
Authorship and Disclosures
XLZ is the principal investigator who takes primary responsibility for the paper. XLZ, HMW, SRC, and CLB recruited the patients. DS, HMW, and EF performed the laboratory work for this study. CGS participated in the statistical analysis, XLZ and DS wrote the paper. CLB, HMW, EF, and HCK helped to revise the manuscript.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413(6855):488–94. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 2.Zheng XL, Sadler JE. Pathogenesis of thrombotic microangiopathies. Annu Rev Path Mech Dis. 2008;3:249–77. doi: 10.1146/annurev.pathmechdis.3.121806.154311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelat SG, Smith AG, Ai J, Zheng XL. Inhibitory autoantibodies against ADAMTS-13 in patients with thrombotic thrombocytopenic purpura bind ADAMTS-13 protease and may accelerate its clearance in vivo. J Thromb Haemost. 2006;4(8):1707–17. doi: 10.1111/j.1538-7836.2006.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai HM, Lian EC. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339(22):1585–94. doi: 10.1056/NEJM199811263392203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moake JL. Thrombotic microangiopathies. N Engl J Med. 2002;347(8):589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 6.Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325(6):398–403. doi: 10.1056/NEJM199108083250605. [DOI] [PubMed] [Google Scholar]
- 7.Tsai HM, Raoufi M, Zhou W, Guinto E, Grafos N, Ranzurmal S, et al. ADAMTS13-binding IgG are present in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2006;95(5):886–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Rieger M, Mannucci PM, Kremer Hovinga JA, Herzog A, Gerstenbauer G, Konetschny C, et al. ADAMTS13 autoantibodies in patients with thrombotic microangiopathies and other immunomediated diseases. Blood. 2005;106(4):1262–7. doi: 10.1182/blood-2004-11-4490. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari S, Mudde GC, Rieger M, Veyradier A, Kremer Hovinga JA, Scheiflinger F. IgG subclass distribution of anti-ADAMTS13 antibodies in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2009;7(10):1703–10. doi: 10.1111/j.1538-7836.2009.03568.x. [DOI] [PubMed] [Google Scholar]
- 10.Scheiflinger F, Knoebl P, Trattner B, Plaimauer B, Mohr G, Dockal M, et al. Non-neutralizing IgM and IgG antibodies to von Willebrand factor-cleaving protease (ADAMTS-13) in a patient with thrombotic thrombocytopenic purpura (TTP) Blood. 2003;102(9):3241–3. doi: 10.1182/blood-2003-05-1616. [DOI] [PubMed] [Google Scholar]
- 11.Tsai HM. Is severe deficiency of ADAMTS-13 specific for thrombotic thrombocytopenic purpura? Yes. J Thromb Haemost. 2003;1(4):625–31. doi: 10.1046/j.1538-7836.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- 12.Veyradier A, Obert B, Houllier A, Meyer D, Girma JP. Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: a study of 111 cases. Blood. 2001;98(6):1765–72. doi: 10.1182/blood.v98.6.1765. [DOI] [PubMed] [Google Scholar]
- 13.Zheng XL, Sadler JE. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome. In: Young NS, Gerson SL, High KA, editors. Clinical Hematology. Philadelphia: Mosby Elsevier; 2006. pp. 802–13. [Google Scholar]
- 14.Coppo P, Wolf M, Veyradier A, Bussel A, Malot S, Millot GA, et al. Prognostic value of inhibitory anti-ADAMTS13 antibodies in adult-acquired thrombotic thrombocytopenic purpura. Br J Haematol. 2006;132(1):66–74. doi: 10.1111/j.1365-2141.2005.05837.x. [DOI] [PubMed] [Google Scholar]
- 15.Peyvandi F, Lavoretano S, Palla R, Feys HB, Vanhoorelbeke K, Battaglioli T, et al. ADAMTS13 and anti-ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93(2):232–9. doi: 10.3324/haematol.11739. [DOI] [PubMed] [Google Scholar]
- 16.Zheng XL, Richard KM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and non-idiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103(11):4043–9. doi: 10.1182/blood-2003-11-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klaus C, Plaimauer B, Studt JD, Dorner F, Lammle B, Mannucci PM, et al. Epitope mapping of ADAMTS13 autoantibodies in acquired thrombotic thrombocytopenic purpura. Blood. 2004;103(12):4514–9. doi: 10.1182/blood-2003-12-4165. [DOI] [PubMed] [Google Scholar]
- 18.Luken BM, Turenhout EA, Hulstein JJ, Van Mourik JA, Fijnheer R, Voorberg J. The spacer domain of ADAMTS13 contains a major binding site for antibodies in patients with thrombotic thrombocytopenic purpura. Thromb Haemost. 2005;93(2):267–74. doi: 10.1160/TH04-05-0301. [DOI] [PubMed] [Google Scholar]
- 19.Vesely SK, George JN, Lammle B, Studt JD, Alberio L, El-Harake MA, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102(1):60–8. doi: 10.1182/blood-2003-01-0193. [DOI] [PubMed] [Google Scholar]
- 20.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129(1):93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 21.Niiya M, Uemura M, Zheng XW, Pollak E, Dockal M, Scheiflinger F, et al. Increased ADAMTS13 proteolytic activity in rat hepatic stellate cells upon activation in vitro and in vivo. J Thromb Haemost. 2006;4(5):1063–70. doi: 10.1111/j.1538-7836.2006.01893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai J, Smith P, Wang S, Zhang P, Zheng XL. The proximal carboxyl-terminal domains of ADAMTS13 determine substrate specificity and are all required for cleavage of von Willebrand factor. J Biol Chem. 2005;280(33):29428–34. doi: 10.1074/jbc.M505513200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kokame K, Matsumoto M, Fujimura Y, Miyata T. VWF73, a region from D1596 to R1668 of von Willebrand factor, provides a minimal substrate for ADAMTS-13. Blood. 2003;103(2):607–12. doi: 10.1182/blood-2003-08-2861. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP-1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110(6):1887–94. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin M, Cataland S, Bissell M, Wu HM. A rapid test for the diagnosis of thrombotic thrombocytopenic purpura using surface enhanced laser desorption/ionization time-of-flight (SELDI-TOF)-mass spectrometry. J Thromb Haemost. 2006;4(2):333–8. doi: 10.1111/j.1538-7836.2006.01758.x. [DOI] [PubMed] [Google Scholar]
- 26.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278(32):30136–41. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao WJ, Krishnaswamy S, Camire RM, Lenting PJ, Zheng XL. Factor VIII accelerates proteolytic cleavage of von Willebrand factor by ADAMTS13. Proc Natl Acad Sci USA. 2008;105:7416–21. doi: 10.1073/pnas.0801735105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luken BM, Turenhout EA, Kaijen PH, Greuter MJ, Pos W, Van Mourik JA, et al. Amino acid regions 572–579 and 657–666 of the spacer domain of ADAMTS13 provide a common antigenic core required for binding of antibodies in patients with acquired TTP. Thromb Haemost. 2006;96(3):295–301. doi: 10.1160/TH06-03-0135. [DOI] [PubMed] [Google Scholar]
- 29.Jin SY, Skipwith CG, Zheng XL. Amino acid residues Arg(659), Arg(660), and Tyr(661) in the spacer domain of ADAMTS13 are critical for cleavage of von Willebrand factor. Blood. 2010;115(11):2300–10. doi: 10.1182/blood-2009-07-235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao W, Anderson PJ, Majerus EM, Tuley EA, Sadler JE. Exosite interactions contribute to tension-induced cleavage of von Willebrand factor by the antithrombotic ADAMTS13 metalloprotease. Proc Natl Acad Sci USA. 2006;103(50):19099–104. doi: 10.1073/pnas.0607264104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin SY, Skipwith CG, Shang D, Zheng XL. von Willebrand factor cleaved from endothelial cells by ADAMTS13 remains ultralarge in size. J Thromb Haemost. 2009;7(10):1749–52. doi: 10.1111/j.1538-7836.2009.03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zanardelli S, Chion AC, Groot E, Lenting PJ, McKinnon TA, Laffan MA, et al. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood. 2009;114(13):2819–28. doi: 10.1182/blood-2009-05-224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vomund AN, Majerus EM. ADAMTS13 bound to endothelial cells exhibits enhanced cleavage of von Willebrand factor. J Biol Chem. 2009;284(45):30925–32. doi: 10.1074/jbc.M109.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]