Abstract
Background
Allogeneic stem cell transplantation is associated with a powerful ‘graft-versus-leukemia’ effect that is generally considered to result from an alloreactive T-cell immune response. However, disease remission can also be observed after syngeneic transplantation and we investigated whether a T-cell immune response to cancer-testis antigens can be detected in patients in the post-transplant period.
Design and Methods
The T-cell immune response against cancer-testis antigens was studied in a cohort of 41 patients who underwent allogeneic stem cell transplantation for the management of acute myeloid leukemia or multiple myeloma. The cytokine secretion assay was combined with magnetic selection to allow detection of an interferon-γ-secreting T-cell response to a panel of cancer-testis antigen peptides.
Results
A cancer-testis antigen-specific CD8+ T-cell immune response was observed in the peripheral blood of five patients with an average magnitude of 0.045% of the CD8+ T-cell repertoire. Four of these patients had undergone reduced intensity conditioning transplantation with alemtuzumab for the treatment of acute myeloid leukemia and three remain in long-term remission. T-cell immunity was focused against peptides derived from MAGE proteins and was markedly increased within the bone marrow.
Conclusions
Functional cancer-testis antigen-specific CD8+ T-cell immune responses develop in the early period following reduced intensity allogeneic stem cell transplantation and are preferentially localized to bone marrow. These immune responses are likely to contribute to the cellular basis of the graft-versus-leukemia effect.
Keywords: allogeneic stem cell transplantation, antigen-specific, CD8+ T cell, graft-versus-leukemia effect
Introduction
Allogeneic stem cell transplantation is used widely in the management of hematologic malignancies but the frequency of disease relapse remains a major challenge. The ‘graft-versus-leukemia’ (GvL) effect of allogeneic transplantation results from the recognition of host tumor cells by the donor immune system1–4 and an improved understanding of the cellular targets of this response would facilitate the development of novel immunotherapeutic approaches.
The magnitude of the GvL effect is related to the degree of histoincompatibility between the donor and recipient and this has focused interest on minor histocompatibility antigens as the predominant target for T cells which mediate allogeneic immune responses.5–7 However, it is now appreciated that GvL responses may also be observed in the setting of syngeneic transplantation in which there is no genetic mismatch between donor and recipient8 and this has concentrated attention on the possibility that stem cell transplantation may be associated with immune responses against ‘tumor-specific’ antigens.9
Cancer-testis antigens (CTAg) are proteins whose physiological expression is restricted to germline tissue and are, therefore, not exposed to the systemic immune system so that immunological tolerance is not established. CTAg expression is often observed in malignant cells and may reflect induction of a ‘germline transcription’ profile during cell transformation.10–13 CTAg-specific humoral and cellular immune responses have been reported in patients with a variety of solid tumors14–16 and there is increasing interest in their potential role in hematopoietic malignancies.17–21
CTAg may also represent an important target for the GvL effect of allogeneic transplantation and antibodies to CTAg proteins have been demonstrated in patients who have undergone stem cell transplantation for the treatment of myeloma.21 A T-cell response to the CTAg protein NY-ESO was also observed in one patient in this cohort but this was coincident with disease relapse. In this study we screened 41 patients for the presence of a CD8+ T-cell immune response to CTAg peptides in the post-transplant period and related this to clinical outcome. Such immune responses were observed in five patients and, when present in the early period after transplantation, were associated with long-term disease control.
Design and Methods
Patients
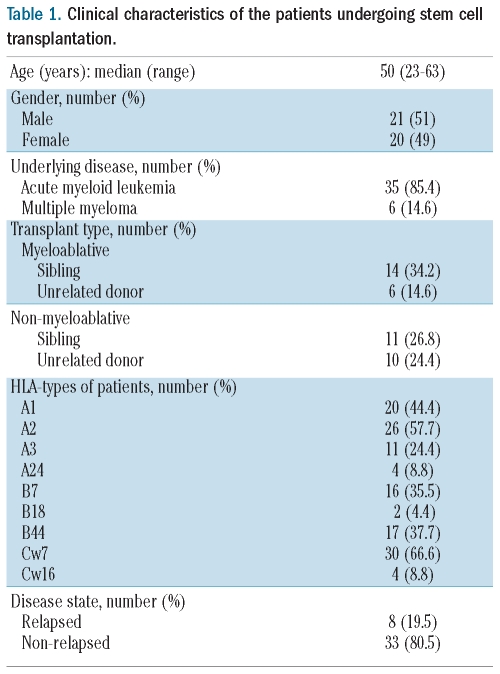
Forty-one patients with a primary diagnosis of acute myeloid leukemia (AML) or multiple myeloma (MM) who were undergoing allogeneic stem cell transplantation were studied. Written informed consent was obtained prior to enrolment in the study and appropriate ethical approval was obtained from the South Birmingham Regional Ethics Committee. Thirty-five patients had a diagnosis of AML, while the remaining six were undergoing treatment for MM. Twenty patients received a myeloablative conditioning regimen consisting of cyclophosphamide (60 mg/kg × 2 days) and 14.4 Gy total body irradiation, and 21 received a reduced intensity conditioning regimen incorporating fludarabine (25 mg/m2 × 5 days), alemtuzumab (140 mg/m2) and melphalan (10 mg × 5 days) (Table 1).
Table 1.
Clinical characteristics of the patients undergoing stem cell transplantation.
Patients’ samples
Heparinized peripheral blood samples of 50 mL were taken prior to the transplant and then at several time points in the post-transplant period. Bone marrow samples were obtained at the time of routine clinical monitoring and mononuclear cells were isolated from all samples by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). All assays were carried out using freshly isolated peripheral blood or bone marrow samples.
Detection of cancer-testis antigen-specific T cells
A panel of 20 peptides (Alta Biosciences, Birmingham, UK) from ten CTAg gene families was chosen on the basis of having been previously identified as T-cell epitopes (as detailed on the website of the Academy of Cancer Immunity) and also shown to have RNA expression in AML and/or MM.22,23 The peptides, their HLA restriction and gene derivation are detailed in the Online Supplementary Table S1. Peptide-specific T cells were identified from peripheral blood mononuclear cells using detection of interferon-gamma (IFN-γ) cytokine secretion and an enrichment assay kit (CSA, Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. Freshly isolated peripheral blood mononuclear cells were seeded into wells of tissue culture plates (Iwaki) in RPMI 1640 media (Invitrogen) supplemented with 10% human serum (H+D Supplies) and L-glutamine (Invitrogen) at a cell density of 1×107/mL and left overnight without stimulation at 37°C in 5% CO2. Peptides were then added either individually or in pools of not more than six at a final concentration of 10 μg/mL. An equivalent volume of dimethylsulfoxide (Sigma, UK) was added to a negative control well which was then used to determine gate positioning for FACS analysis, as shown in Figure 1. Staphylococcal enterotoxin B (Sigma, UK) was used as a positive control at a concentration of 1 μg/mL. Following a 3-h stimulation period, the cells were labeled for 5 min with IFN-γ catch reagent and incubated under continuous rotation for 45 min at 37°C. The cells were then labeled with IFN-γ detection reagent conjugated to a phycocerythrin fluorochrome, followed by anti-phycoerythrin magnetic beads. Magnetic selection was carried out either manually using MS columns (Miltenyi Biotech) for double positive selection, or using the equivalent “posseld” selection program on an autoMACs (Miltenyi Biotech). Pre- and post-selection samples were labeled with CD4-fluorescein isothiocyanate, CD8-phycoerythrin cyanin 5.1 monoclonal antibodies (Beckman Coulter, High Wycombe, UK) and propidium iodide (1 μg/mL, Sigma, UK) to exclude dead cells. Flow cytometric analysis was carried out using either a Beckman Coulter XL-2 flow cytometer (Beckman Coulter, High Wycombe, UK) with WinMDI software for analysis (Scripps Institute, La Jolla, USA) or a BD LSRII with FACS DIVA analysis software (BD Biosciences). The percentage of antigen-specific T cells in the CD4+ or CD8+ pool was calculated using the number of cytokine-secreting cells in either the positively selected fraction or pre-selected samples gated on CD4+ or CD8+ cells within the peripheral blood mononuclear cells. Any IFN-γ-secreting cells detected in the unstimulated negative control were considered to be background and subtracted from the frequency of cells producing IFN-γ following peptide stimulation.
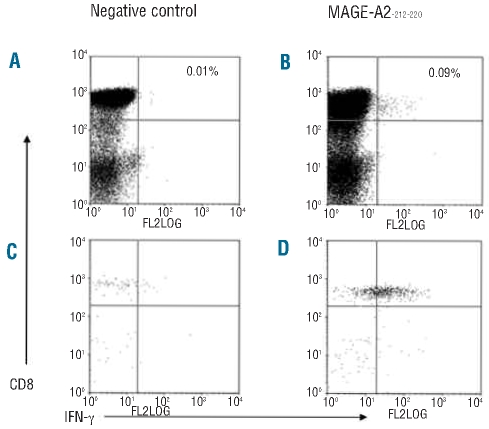
Figure 1.
Flow cytometric enumeration of frequency of CTAg responding CD8+ cells. Following the IFN-γ cytokine secretion assay and enrichment, perioheral blood mononuclear cells incubated with dimethylsulfoxide only during the stimulation period are shown, prior to (A) and following (C) magnetic selection, along with IFN-γ responses to MAGE-A2-212–220 prior to (B) and following (D) magnetic selection.
Results
Cancer-testis antigen-specific CD8+ T-cell responses can be detected in patients following stem cell transplantation
The IFN-γ cytokine secretion assay was used to identify CTAg-specific T-cell responses to a panel of 20 immunodominant peptides derived from ten CTAg genes (Online Supplementary Table S1). Peptides were selected on the basis that they were derived from proteins for which RNA expression had previously been demonstrated in AML and/or MM, and immunogenicity had been previously documented in patients with solid tumor malignancies.24 Peripheral blood lymphocytes were isolated from patients at several time points following transplantation and were stimulated with CTAg peptides appropriate to the HLA genotype of the patient.
Overall, CTAg-specific CD8+ T cells were identified in five patients who were screened at a wide variety of time points in the post-transplant period. The frequency of the CTAg-specific CD8+ T-cell response was variable and ranged between 0.0005% and 0.2% of the total CD8+ T-cell pool. The mean frequency of responses was 0.045%, which equates to approximately 1 in 2000 CD8+ T cells and is comparable to the magnitude of many virus-specific immune responses. Of the 20 patients who received reduced intensity conditioning with alemtuzumab, four had detectable CD8 responses to CTAg peptides, whereas of the 21 who underwent myeloablative conditioning only one had CTAg-specific CD8 (Figure 2).
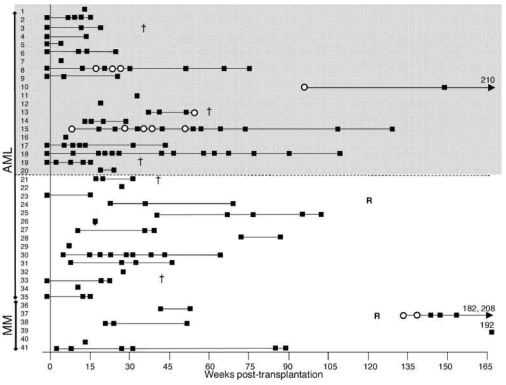
Figure 2.
Summary of the screening of all patients’ samples. The times of all screens are shown in weeks post-transplantation (y-axis). Assays in which no responses were seen are shown as black squares and positive responses are shown as open circles. Samples screened more than 165 weeks post-transplantation are indicated by a number representing the week at which the sample was screened. A cross (†) denotes time of death of patients who died due to relapse, and a letter R shows when patients suffered relapse but were successfully treated and have survived. The gray area shows those patients who received reduced intensity conditioning. Response frequencies in patients 15 and 8 are shown in Figure 3 (A and B, respectively), and patients 37 and 13 are detailed in Figure 4 (A and B, respectively).
The majority of CTAg-specific immune responses could be detected without the requirement for magnetic enrichment, although selection was used to increase the reliable sensitivity of the IFN-γ cytokine secretion assay down to 0.0001%.25 If a CTAg-specific response was only apparent after magnetic selection then the precursor frequency was calculated on the basis of the post-selection sample, although this approach tends to underestimate the true value due to cell loss during selection. An example of flow cytometric analysis before and after magnetic enrichment is shown in Figure 1. These responses could be detected serially in some cases and the absolute magnitude of the response varied at different time points in the post-transplant period.
Patients were recruited to the study at a range of different time points in the post–transplant period (Figure 2) and the association between CTAg-specific immunity and time post-transplant is discussed below.
Cancer-testis antigen-specific CD8+ T-cell responses were observed in two patients with long-term disease-free survival
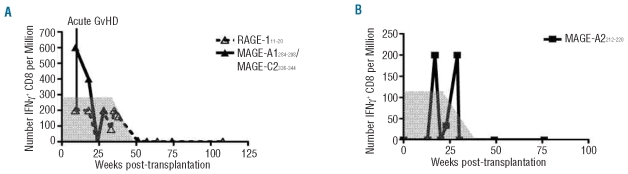
CTAg-specific CD8+ T cells were detected in two patients within the first year following transplantation and both of these had undergone reduced intensity conditioning allografting for AML (Figure 3). AML patient 189A (Figure 3A) had a T-cell response to peptide RAGE-111–20 and also to the peptide pool comprising MAGE-C2336–344/MAGE-A1289–298. These responses were observed at 9 weeks post-transplant which was the first time point of analysis. Of note, the patient had suffered from grade IV graft-versus-host disease (GvHD) of the skin 3 weeks after transplantation and this had been treated with oral prednisolone. The T-cell response to RAGE-111–20 was present at five separate times of analysis until 51 weeks post-transplant although the magnitude of the response was variable and was undetectable on four occasions. Cyclosporine A treatment had been discontinued at 27 weeks post-transplantation and this coincided with a transient increase in the RAGE-specific T-cell response (Figure 3A).
Figure 3.
Responses to CTAg peptides were detected at various times post-transplantation in AML patients The frequencies over time of CTAg-specific T cells in two AML patients who had responses are shown. The gray area indicates prophylactic tapered cyclosporine treatment given during the early weeks following transplantation. Patient 189A (A) suffered grade IV acute graft-versus-host disease during week 7 post-transplantation which was treated with prednisolone. Patient 235A (B) had fluctuating responses between weeks 17 and 29 which were undetectable at week 20 post-transplantation.
CTAg-specific T cells were detected in patient 235A at 17, 23 and 29 weeks after transplantation and this also coincided with tapering of cyclosporine A therapy, which was finally discontinued by 25 weeks. However, at 20 weeks, no response was detected (Figure 3B). This patient was one of 13 also screened prior to beginning conditioning for transplantation, although no pre-transplant responses were detected.
Importantly, patients 189A and 235A have remained in remission since transplantation and now remain disease-free at 52 and 37 months post-transplant, respectively. Overall, there were 14 patients who were screened for CTAg-specific immune responses on two or more occasions within the first year post-transplant and who are alive without relapses. As CTAg-specific T cells were observed in two of these, we were able to demonstrate immunity to this relatively limited CTAg peptide panel in 14.3% of this group. Screening began on an additional AML patient (138A) at 92 weeks post-transplant and a T-cell response was observed against peptides MAGE-C2336–344 and MAGE-A1161–169 but not detected thereafter (data not shown). This patient has also remained relapse-free during follow-up.
Cancer-testis antigen-specific T-cell responses can also be detected at the time of disease relapse
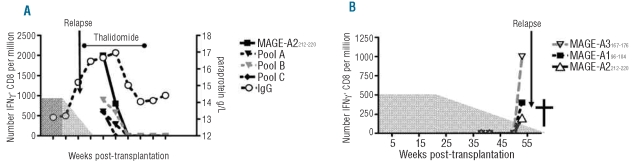
Our previous studies of CTAg-specific immunity in patients with myeloma revealed that CD8+ responses are often observed around the time of disease relapse and probably reflect immune response to increased tumor load.19 A similar pattern was observed in patient 125M with a diagnosis of MM who was initially studied more than 2 years after stem cell transplantation with a myeloablative conditioning regimen. The serum paraprotein level had risen at 124 weeks post-transplant and indicated the onset of disease relapse. The first CTAg analysis was undertaken 10 weeks after this point and revealed a broad CTAg peptide-specific immune response with specificity for peptides in three different peptide pools and included reactivity against the peptide MAGE-A2-212–220. The patient was treated with thalidomide and the next sample was taken at 143 weeks at which time the magnitude of the MAGE-A2212–220 -specific response had fallen by 60% and responses to other peptide pools were not observed. Interestingly, the patient responded well to thalidomide with a reduction in the paraprotein level and no CTAg-specific immune response could be detected in subsequent analyses (Figure 4A).
Figure 4.
Responses to CTAg peptides were detected post-transplantation in relapsed patients The frequencies over time of CTAg-specific T cells in relapsed myeloma patient 125M are shown (A). The gray area indicates prophylactic tapered cyclosporine treatment given during the early weeks following transplant. Paraprotein levels are plotted on the right-hand axis. CTAg-specific responses were detected in AML patient 172A shortly before relapse (B).
Patient 172A with a pre-transplant diagnosis of AML was screened from 38 weeks post-transplant and responses were seen at 53 weeks to peptides MAGE-A196–104, MAGE-A2212–220 and MAGE-A3167–176. At 55 weeks the patient was diagnosed with a relapse of AML and died shortly afterwards (Figure 4B).
RAGE-1-specific T cells can be detected at higher frequency within bone marrow than within peripheral blood
Paired blood and bone marrow samples from patient 189A were obtained at 35 and 51 weeks post-transplantation. T cells specific for RAGE-111–20 were detected by the IFN-γ cytokine secretion assay and the frequency was found to be up to seven times higher in bone marrow than in peripheral blood mononuclear cells. In peripheral blood, at both 35 and 51 weeks, 0.0006% of CD8+ T cells were specific for RAGE-111–20, whereas in the bone marrow, the frequencies were 0.0021% at 35 weeks and 0.0042% at 51 weeks (Online Supplementary Figure S1).
The cancer-testis antigen-specific CD8+ T-cell response post-transplantation is preferentially focused on peptides derived from the MAGE family of proteins
Twenty CTAg peptides were used in this study and T-cell responses within patients undergoing stem cell transplantation were detected in response to six of these. Interestingly, responses were focused on peptides derived from the MAGE family of proteins and four patients showed T-cell immune responses against peptide MAGE-A2212–220 (Online Supplementary Figure S2). Overall, CD8+ T-cell responses were observed against peptides derived from the MAGE subfamily proteins A1, A2, A3 and C2, and a strong immune response against RAGE-1 was also observed in one patient.
Discussion
Almost all experimental studies of GvL have focused on minor histocompatibility antigens and the potential role of ‘tumor-associated’ proteins has been relatively ignored. CTAg are important targets in autologous cancer-specific immune responses and have the capacity to contribute to the allogeneic GvL response. The development of a humoral immune response against CTAg proteins has been reported as a frequent event in patients undergoing stem cell transplantation for MM, and T-cell clones against NY-ESO have been reported in one patient at the time of disease relapse.21
We studied CTAg-specific T-cell immunity in a large cohort of patients undergoing allogeneic stem cell transplantation for AML and MM, and observed CTAg-specific CD8+ T cells in five of the 41 patients. While this may seem a relatively low frequency it must be appreciated that the peptide pool only contains a small fraction of the potential immune epitopes from CTAg proteins, and in addition the potential relevance of each peptide is limited by the HLA genotype of the patient. As such the use of a broader range of CTAg peptides would be expected to facilitate the detection of many more CTAg-specific T-cell responses within this group of patients. The magnitude of individual CTAg peptide-specific immune responses averaged 0.045%, or around 1 in 2000 of the CD8+ T-cell repertoire. This is comparable to the level of T-cell response to many viral epitopes26 and indicates that the immunogenicity of these proteins can be quite considerable in this setting. The range of responses varied between 0.0005% and 0.2% of the CD8+ T-cell pool and suggests that individual peptides are likely to reflect either dominant or subdominant epitopes within each patient.
Interestingly, the strength of the CTAg-specific immune responses also varied over time within individual patients and seemed to correlate with clinical events. The reduction in prophylactic immune suppression after stem cell transplantation is often associated with increased allogeneic immune responses and in two patients (189A and 235A) we were able to detect CTAg-specific T cells immediately following cessation of cyclosporine A treatment. The magnitude of CTAg-specific immunity that is required for clinical efficacy is unclear but in CTAg vaccine trials for metastatic melanoma, tumor regression has been demonstrated even though it is not always possible to detect tumor-specific T cells. This has led to the belief that very low numbers of T cells are required for an effective anti-tumor response16,27 and the levels within our cohort of patients are certainly in this range. In the setting of allogeneic transplantation it is well documented that patients can achieve durable remissions on withdrawal of immunosuppression.28
The CTAg-specific immune response in patient 235A was interesting in that it fluctuated quite markedly in magnitude. Two strong positive responses were seen at weeks 17 and 29 post-transplantation but on two occasions between these dates the response was much lower, and in one case undetectable. The reason for this is unclear but is possibly related to biological factors such as T-cell homing and tissue distribution which frequently results in some degree of fluctuation in T-cell responses. However, although the IFN-γ cytokine secretion assay has been validated in our laboratory, we cannot rule out the possibility that the lack of a detectable response on that occasion could have been due to a technical problem with the assay. The IFN-γ secretion assay is a powerful technique but the sensitivity of detection that was required for this study is at the limit of current technology.
Interestingly, all of the AML patients who developed CTAg-specific immune responses had undergone reduced intensity conditioning which includes the use of alemtuzumab. This regimen is strongly immunosuppressive and provides valuable prophylaxis against GvHD. However, the clinical outcome for AML patients after reduced intensity conditioning has been very encouraging29 and it is tempting to speculate that induction of a CTAg-specific immune response could provide one explanation for this.
Although our studies were performed largely on peripheral blood samples, CTAg-specific T cells are only likely to be effective in the control of hematopoietic malignancies if they are able to enter the bone marrow. We found that CTAg-specific CD8+ T cells were selectively recruited to marrow such that their frequency was up to seven-fold higher at this site. We have recently described a unique ‘bone marrow homing’ chemokine receptor profile on CD8+ T cells30 and in future studies it will be important to determine the phenotype of CTAg-specific T cells at these sites. Interestingly, a recent study investigated T-cell immunity to the leukemia-associated antigens WT1 and proteinase-3 in patients with myeloid malignancy and also found higher frequencies in the bone marrow than in peripheral blood.31
The major clinical question must relate to the potential contribution of the CTAg-specific T-cell response to the GvL effect. It seems clear that such responses were induced as a result of the allogeneic transplant as no immune response was observed in the 13 patients who were screened prior to transplantation, and the magnitude of the CTAg-specific population was increased in association with withdrawal of immune suppression in two patients. CTAg-specific T cells have been shown to have the capacity to kill primary tumor cells in vitro32 and it is, therefore, tempting to speculate that such immune responses do indeed contribute to disease control.
However, CTAg-specific T-cell immune responses were also seen in two patients at the time of disease relapse. We have seen this pattern previously in patients with MM and believe it reflects the response of CD8+ T cells to increased availability of CTAg protein from recurrent tumor.18 Interestingly, the allogeneic NY-ESO-specific T-cell response reported by Atanackovic et al. was also observed at the time of relapse of MM.21 Future studies will need to address the phenotype and magnitude of T-cell responses in relation to relapse. Interestingly, the AML patient in our study in whom we detected a CTAg-specific CD8+ response at relapse had very low levels of donor chimerism prior to relapse and it will be important to address whether CTAg-specific immunity at relapse reflects expansion of recipient-derived T cells.
Somewhat to our surprise, the majority of T-cell responses were specific for peptides derived from MAGE proteins. Although it was initially felt that MAGE was not expressed in AML,33 mRNA expression has recently been reported.34 The fact that MAGE expression is highly restricted to germ cells and malignant tissue is likely to dictate that MAGE-specific immune responses are manifest as a GvL response rather than GvHD.35,36
In conclusion we have demonstrated frequent occurrence of CTAg-specific CD8+ T cells in patients following reduced intensity stem cell transplantation. The association between the early detection of such an immune response and prolonged disease remission provides support for the concept that CTAg-specific immunity plays an important role in GvL. Further studies are now indicated to explore the potential for enhancing CTAg-specific immunity in the post-transplant setting.
Footnotes
Funding: this work was funded by a grant from Leukaemia Research (04029)
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
AM carried out the experiments, designed the research, and drafted the paper; KP and OCG designed the research and reviewed the paper; JA, MC, FC, GP, PM and CC consented patients and provided clinical information; PAHM designed the research and drafted the paper.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 2.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–50. [PubMed] [Google Scholar]
- 3.Porter DL, Collins RH, Jr, Shpilberg O, Drobyski WR, Connors JM, Sproles A, et al. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant. 1999;5(4):253–61. doi: 10.1053/bbmt.1999.v5.pm10465105. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–62. [PubMed] [Google Scholar]
- 5.Mutis T, Verdijk R, Schrama E, Esendam B, Brand A, Goulmy E. Feasibility of immunotherapy of relapsed leukemia with ex vivo-generated cytotoxic T lymphocytes specific for hematopoietic system-restricted minor histocompatibility antigens. Blood. 1999;93(7):2336–41. [PubMed] [Google Scholar]
- 6.Spaapen R, Mutis T. Targeting haematopoietic-specific minor histocompatibility antigens to distinguish graft-versus-tumour effects from graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21(3):543–57. doi: 10.1016/j.beha.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, Hui KM, Younes HM, Brickner AG. Targeting minor histocompatibility antigens in graft versus tumor or graft versus leukemia responses. Trends Immunol. 2008;29(12):624–32. doi: 10.1016/j.it.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fefer A, Cheever MA, Greenberg PD. Identical-twin (syngeneic) marrow transplantation for hematologic cancers. J Natl Cancer Inst. 1986;76(6):1269–73. [PubMed] [Google Scholar]
- 9.Schetelig J, Kiani A, Schmitz M, Ehninger G, Bornhauser M. T cell-mediated graft-versus-leukemia reactions after allogeneic stem cell transplantation. Cancer Immunol Immunother. 2005;54(11):1043–58. doi: 10.1007/s00262-005-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5(8):615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 11.Old LJ. Cancer is a somatic cell pregnancy. Cancer Immun. 2007;7:19. [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson BJ, Iseli C, Panji S, Zahn-Zabal M, Hide W, Old LJ, et al. Rapid evolution of cancer/testis genes on the X chromosome. BMC Genomics. 2007;8:129. doi: 10.1186/1471-2164-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183(3):725–9. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaugler B, Brouwenstijn N, Vantomme V, Szikora JP, Van der Spek CW, Patard JJ, et al. A new gene coding for an antigen recognized by autologous cytolytic T lymphocytes on a human renal carcinoma. Immunogenetics. 1996;44(5):323–30. doi: 10.1007/BF02602776. [DOI] [PubMed] [Google Scholar]
- 15.Baumgaertner P, Rufer N, Devevre E, Derre L, Rimoldi D, Geldhof C, et al. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer Res. 2006;66(4):1912–6. doi: 10.1158/0008-5472.CAN-05-3793. [DOI] [PubMed] [Google Scholar]
- 16.Lonchay C, van der Bruggen P, Connerotte T, Hanagiri T, Coulie P, Colau D, et al. Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci USA. 2004;101 (Suppl 2):14631–8. doi: 10.1073/pnas.0405743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rhee F, Szmania SM, Zhan F, Gupta SK, Pomtree M, Lin P, et al. NY-ESO-1 is highly expressed in poor-prognosis multiple myeloma and induces spontaneous humoral and cellular immune responses. Blood. 2005;105(10):3939–44. doi: 10.1182/blood-2004-09-3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodyear O, Piper K, Khan N, Starczynski J, Mahendra P, Pratt G, et al. CD8+ T cells specific for cancer germline gene antigens are found in many patients with multiple myeloma, and their frequency correlates with disease burden. Blood. 2005;106(13):4217–24. doi: 10.1182/blood-2005-02-0563. [DOI] [PubMed] [Google Scholar]
- 19.Goodyear OC, Pratt G, McLarnon A, Cook M, Piper K, Moss P. Differential pattern of CD4+ and CD8+ T-cell immunity to MAGE-A1/A2/A3 in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma. Blood. 2008;112(8):3362–72. doi: 10.1182/blood-2008-04-149393. [DOI] [PubMed] [Google Scholar]
- 20.Pratt G, Goodyear O, Moss P. Immunodeficiency and immunotherapy in multiple myeloma. Br J Haematol. 2007;138(5):563–79. doi: 10.1111/j.1365-2141.2007.06705.x. [DOI] [PubMed] [Google Scholar]
- 21.Atanackovic D, Arfsten J, Cao Y, Gnjatic S, Schnieders F, Bartels K, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109(3):1103–12. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 22.Guinn BA, Gilkes AF, Woodward E, Westwood NB, Mufti GJ, Linch D, et al. Microarray analysis of tumour antigen expression in presentation acute myeloid leukaemia. Biochem Biophys Res Commun. 2005;333(3):703–13. doi: 10.1016/j.bbrc.2005.05.161. [DOI] [PubMed] [Google Scholar]
- 23.van Baren N, Brasseur F, Godelaine D, Hames G, Ferrant A, Lehmann F, et al. Genes encoding tumor-specific antigens are expressed in human myeloma cells. Blood. 1999;94(4):1156–64. [PubMed] [Google Scholar]
- 24.Van Der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065x.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 25.Campbell JD. Detection and enrichment of antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Methods. 2003;31(2):150–9. doi: 10.1016/s1046-2023(03)00125-7. [DOI] [PubMed] [Google Scholar]
- 26.Blake N, Haigh T, Shaka’a G, Croom-Carter D, Rickinson A. The importance of exogenous antigen in priming the human CD8+ T cell response: lessons from the EBV nuclear antigen EBNA1. J Immunol. 2000;165(12):7078–87. doi: 10.4049/jimmunol.165.12.7078. [DOI] [PubMed] [Google Scholar]
- 27.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 28.Weaver CH, Clift RA, Deeg HJ, Storb R, Appelbaum FR, Bensinger W, et al. Effect of graft-versus-host disease prophylaxis on relapse in patients transplanted for acute myeloid leukemia. Bone Marrow Transplant. 1994;14(6):885–93. [PubMed] [Google Scholar]
- 29.Tauro S, Craddock C, Peggs K, Begum G, Mahendra P, Cook G, et al. Allogeneic stem-cell transplantation using a reduced-intensity conditioning regimen has the capacity to produce durable remissions and long-term disease-free survival in patients with high-risk acute myeloid leukemia and myelodysplasia. J Clin Oncol. 2005;23(36):9387–93. doi: 10.1200/JCO.2005.02.0057. [DOI] [PubMed] [Google Scholar]
- 30.Palendira U, Chinn R, Raza W, Piper K, Pratt G, Machado L, et al. Selective accumulation of virus-specific CD8+ T cells with unique homing phenotype within the human bone marrow. Blood. 2008;112(8):3293–302. doi: 10.1182/blood-2008-02-138040. [DOI] [PubMed] [Google Scholar]
- 31.Melenhorst JJ, Scheinberg P, Chattopadhyay PK, Gostick E, Ladell K, Roederer M, et al. High avidity myeloid leukemia-associated antigen-specific CD8+ T cells preferentially reside in the bone marrow. Blood. 2009;113(10):2238–44. doi: 10.1182/blood-2008-04-151969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bluman EM, Coulie PG, Xiaojuan S, Machan J, Lin C, Meitner PA, et al. Lysis of human chondrosarcoma cells by cytolytic T lymphocytes recognizing a MAGE-A3 antigen presented by HLA-A1 molecules. J Orthop Res. 2007;25(5):678–84. doi: 10.1002/jor.20368. [DOI] [PubMed] [Google Scholar]
- 33.Chambost H, van Baren N, Brasseur F, Olive D. MAGE-A genes are not expressed in human leukemias. Leukemia. 2001;15(11):1769–71. doi: 10.1038/sj.leu.2402278. [DOI] [PubMed] [Google Scholar]
- 34.Martinez A, Olarte I, Mergold MA, Gutierrez M, Rozen E, Collazo J, et al. mRNA expression of MAGE-A3 gene in leukemia cells. Leuk Res. 2007;31(1):33–7. doi: 10.1016/j.leukres.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 36.Jungbluth AA, Busam KJ, Kolb D, Iversen K, Coplan K, Chen YT, et al. Expression of MAGE-antigens in normal tissues and cancer. Int J Cancer. 2000;85(4):460–5. [PubMed] [Google Scholar]