Abstract
The study of human erythropoiesis in health and disease requires a robust culture system that consistently and reliably generates large numbers of immature erythroblasts that can be induced to differentiate synchronously. We describe a culture method modified from Leberbauer et al. (2005) and obtain a homogenous population of erythroblasts from peripheral blood mononuclear cells (PBMC) without prior purification of CD34+ cells. This pure population of immature erythroblasts can be expanded to obtain 4×108 erythroblasts from 1×108 PBMC after 13–14 days in culture. Upon synchronized differentiation, high levels of enucleation (80–90%) and low levels of cell death (<10%) are achieved. We compared the yield of erythroblasts obtained from PBMC, CD34+ cells or PBMC depleted of CD34+ cells and show that CD34− cells represent the most significant early erythroid progenitor population. This culture system may be particularly useful for investigating the pathophysiology of anemic patients where only small blood volumes are available.
Keywords: erythroid, erythropoiesis, CD34
Introduction
The detailed study of erythropoiesis requires a culture system that reliably generates homogenous populations of erythroblasts at any given stage of differentiation and in quantities amenable to biochemical analysis. Most methods for culturing human erythroblasts utilize CD34+ cells isolated from peripheral blood mononuclear cells (PBMC) or cord blood as the source of hematopoietic stem cells.1–6 However, the number of cells required to perform large scale biochemical experiments outnumbers the amount of erythroblasts that can generally be cultured from CD34+ cells isolated from the small samples of peripheral blood usually provided. Furthermore, these culture systems suffer from significant spontaneous differentiation resulting in asynchronous cultures, making it difficult to study the cellular events that occur at specific stages of differentiation.
Leberbauer et al. described a culture method which exploited the signal transduction cascades of stress erythropoiesis, achieving a 109-fold expansion of erythroblasts from cord blood cells without prior CD34+ isolation.7 It was not clear from this study whether the high yield of progenitors was primarily from CD34+ progenitor cells or due to a contribution of CD34− cells in cord blood. CD34− hematopoietic stem cells are present in cord blood, peripheral blood and bone marrow8,9 and support hematopoiesis/erythropoiesis.10 We hypothesized that these CD34− cells may make a substantial contribution to erythroid expansion when total PBMC are used as starting material for the in vitro culture of erythroblasts.
Here we have modified a protocol originally described by Leberbauer et al.7 and show that the yield of erythroblasts expanded either from PBMC or from PBMC depleted of CD34+ cells is dramatically higher than from CD34+ cells alone. Importantly, PBMC-derived erythroblasts have similar proliferation and differentiation kinetics to erythroblasts derived from CD34+ cells. We conclude that the bulk of the erythroid potential in peripheral blood resides in the CD34− progenitor cells, a fraction that has until now been largely ignored when culturing erythroblasts.
Design and Methods
Antibodies
Monoclonal antibodies used were BIRMAK3 (CD34), BRIC256 (GPA), BRIC6 (band 3) (IBGRL, Bristol, UK), c-kit-PE (Pharmingen, San Diego, CA, USA), CD71-PE (Pharmingen), CD14-PE (Sigma, Dorset, UK), CD3-PE (Serotec, Kidlington, UK) and CD20-PE (Serotec). Secondary antibodies: rabbit anti-mouse PE conjugated, rabbit anti-mouse IgG2B and IgG1 control antibodies (Dako, Glostrup, Denmark).
Erythroblast expansion and differentiation (3 phase culture system)
Blood was obtained from healthy donors and informed consent was given in accordance with the Declaration of Helsinki. PBMC were isolated by density purification using Percoll (GE Healthcare, Little Chalfont, UK) with density ρ1.077. CD34+ cells were purified using Magnetic Activated Cell Sorting (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s instructions. Cells were cultured as described previously7 but with modifications. PBMC and PBMC depleted of CD34+ cells were seeded at 1×107 cells/ml whereas CD34+ cells were seeded at 1–2×106 cells/mL in expansion medium (ExpM, phase 1), consisting of Stemspan (Stem Cell Technologies, Grenoble, France) supplemented with SCF (cell supernatant equivalent to 100 ng/mL), Epo (2 U/mL, Bristol Royal Infirmary, Bristol, UK), dexamethasone (1 μM, Sigma), IGF-1 (40 ng/mL, R&D systems) and cholesterol-rich lipids (40 μg/mL, Sigma) and ng/mL IL-3 (R&D systems). After 4–5 days, erythroblasts were purified by density purification (Percoll, ρ1.075) to remove lymphocytes and cells were reseeded in expansion medium without IL-3 (phase 2) and kept between 1.5 and 2×106/mL by daily counting and medium changes. Differentiation of erythroblasts: cells were washed three times with PBS and reseeded in Stemspan medium (phase 3) supplemented with Epo (10U/mL, Bristol Royal Infirmary), insulin (10 μg/mL, Sigma), human AB plasma (3%, Sigma), thyroid hormone (T3, 1 μM, Sigma), IGF-1 (40ng/mL, R&D systems) and holotransferrin (0.5mg/mL, Sigma). Cells were kept between 2–4×106 cells/ml by daily counting and medium changes.
Cytospins and hemoglobin assays
Hemoglobin was measured as described.11 Hemoglobin concentration was normalized to cell volume. To analyze cell morphology, 1×105 cells were centrifuged onto slides, and stained with histological dyes and neutral benzidine.12 Images were taken with a charge-coupled device (CCD) camera and processed using Adobe Photoshop 6.0 (Adobe, San Jose, CA, USA).
Flow cytometry
Cells (3×105) were washed in ice-cold PBS and in ice-cold PB (PBS, 4% BSA). Cells were reconstituted in PB supplemented with normal rabbit serum and stained for 1 h at 4°C with specific antibodies (see figure legends). Where appropriate, cells were washed once in ice cold PB and incubated with PE/FITC conjugated anti-mouse secondary antibodies. Fluorescent signals were measured using FACS CantoII-F60 machine (BD Biosciences, Oxford, UK). Data were analyzed using Flowjo 7.2.5 software (Flowjo, Ashland, OR, USA).
Results and Discussion
The yield of erythroblasts expanded from total PBMC or CD34− cells is significantly higher than from CD34+ cells alone
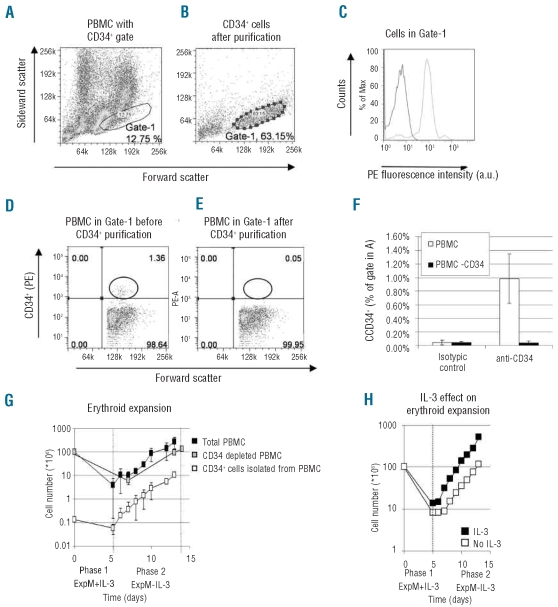
To test the hypothesis that CD34− progenitor cells in PBMC can differentiate into the erythroid lineage and to compare the yield of erythroblast expansion, CD34+ cells, CD34− PBMC and total PBMC were isolated from a constant amount of peripheral blood. Figure 1A–F summarize this purification and isolation. We compared the expansion of erythroblasts from 108 PBMC, 108 CD34− PBMC and 2×105 CD34+ cells isolated from 108 PBMC (Figure 1G and H). Figure 1A shows the forward and side scatter plot of the total PBMC population. The location (Gate-1) of purified CD34+ cells as shown in Figure 1B and C shows that all cells within Gate-1 are CD34+ after CD34+ purification. Figure 1D–F show that about 1% of the total PBMC found in Gate-1 are CD34+ cells and that this population is lost from PBMC after CD34+ purification, yielding PBMC devoid of CD34+ cells (CD34− population, Figure 1E).
Figure 1.
Expansion of erythroblasts from PBMC outperforms expansion from CD34+ cells purified from PBMC. (A and B) Dot plots showing forward and side scatter plots before (A, total PBMC) and after (B) CD34+ isolation. Gate-1 shows area where CD34+ cells reside (see C). (C) CD34 positivity of cells in Gate-1 using an antibody against CD34 (BIRMAK3) compared to an IgG control: shows all cells in Gate-1 are CD34+. (D and E) Dot plots show PBMC present in Gate-1 as FSC against CD34 positivity before (D) and after (E) CD34+ cell-depletion. No CD34+ cells remain after depletion (right plot, black circle). Circle indicates position of CD34+ cells. (A–E) Representative of four independent experiments. (F) Amount of CD34+ cells isolated from PBMC as percentage of cells found in Gate-1 (see B). White bars: PBMC; black bars: PBMC depleted of CD34+ cells. Note absence of CD34+ positive cells after the depletion of CD34+ cells (black bars, CD34+ staining). Standard deviations (SD) represent four independent experiments. (G) Outgrowth of erythroblasts from 1x108 PBMC (black squares), 1x108 PBMC depleted of CD34+ cells (gray squares), 0.2x106 CD34+ cells purified from 1x108 PBMC (white squares). Expansion monitored for 14 days and total cell numbers (in millions) plotted against time in days. SD calculated from four independent experiments. (H) Addition of IL-3 during 1st expansion phase increases outgrowth of erythroblasts from total PBMC: 1x108 PBMC expanded for 13 days in the presence (black squares) or absence (white squares) of IL-3 during 1st culture phase. (G and H) Vertical dotted lines represent media changes for different culture phases (see Design and Methods). Graph represents average of two experiments.
After 13 days of culture, CD34− PBMC yielded 10–12 times more erythroblasts than CD34+ cells purified from the same amount of peripheral blood. Furthermore, erythroblast expansion from total PBMC outperformed that from CD34+ cells by about 20 times (300 million vs. 15 million erythroblasts on day 13, Figure 1G), reflecting the combined contribution of both CD34− and CD34+ populations. The rate of proliferation between CD34+ cells, CD34− PBMC, and total PBMC was similar suggesting that variations in cellular input account for the different yields. Thus CD34+ cells represent a minority cell population amongst the cells in PBMC with the specific capability of differentiating into erythroblasts and hence only using CD34+ cells as starting material ignores a significant portion of erythroid potential within the PBMC. This improvement of erythroblast yield is of importance when using peripheral blood from limited sources.
The cell culture conditions used in phase 2 (Figure 1G and H) are as described by Leberbauer et al.7 However, we found that inclusion of IL-3 during the first 4–5 days causes approximately 4 times more outgrowth of erythroblasts compared to Leberbauer et al.7 This could be a result of increased proliferation and/or a better direction of hematopoietic stem cell differentiation into the myeloid branch of hematopoiesis (Figure 1H).
The dynamics of peripheral blood progenitor cell differentiation into a pure population of erythroblasts
During the first days, the expanding and differentiating CD34− progenitors progress to become CD34+ cells (Online Supplementary Figures S1 and S2), and the percentage of this CD34+ population increases with time (Online Supplementary Figure S1). At day 3 of culture, the CD34+ cells are c-kit+ and CD71low/medium but GPA− which may indicate a mixture of common myeloid and megakaryocyte/erythroid common progenitors (Online Supplementary Figure S2). Interestingly, the CD34− PBMCs were largely devoid of erythroid colony forming ability upon isolation from the peripheral blood. This has been described before in a subset of CD34− hematopoietic stem cells, the side population cells or SP cells.13 However, a gradual increase of erythroid colony formation was observed using our in vitro culture conditions, coinciding with the appearance of CD34+ cells in the CD34− PBMC culture (Online Supplementary Table S1).
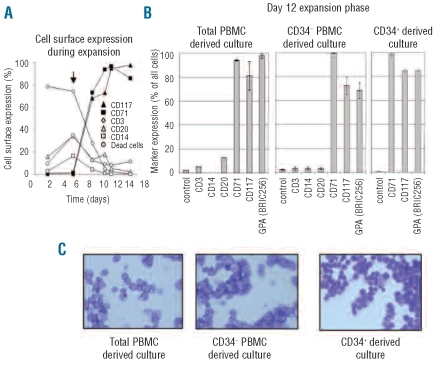
The differentiation of PBMC into an immature erythroblast population takes 5–6 days (Figure 2A) and requires a further density purification step at day 5 to remove lymphocytes and to purify the expanded immature erythroblasts (arrow, Figure 2A). The addition of glucocorticoids in the culture media keeps the erythroblasts immature, as assessed by the continued expression of c-kit, the blast-like morphology and the absence of more differentiated erythroid cells like normoblasts and reticulocytes [Figure 2C and Figure 3D (0 h); the effects of glucocorticoids are more elaborately discussed in Leberbauer et al.]7 After the second Percoll step at day 5, the majority of the cells are CD34−/CD117+/CD71+ and GPA− indicating that these cells are early erythroid progenitors (Online Supplementary Figure S3). These erythroblasts continue to expand for a minimum of 12 days (Figure 1G) with typically less than 10% cell death during the expansion phase (Figure 2A). After 12 days of expansion no significant contamination by other cell lineages such as lymphocytes (CD3), monocytes (CD14) or B cells (CD20) was observed (Figure 2B and C). The majority of cells at day 12 remain immature erythroblasts that are CD117+, CD71+, GPA+, CD34− and band 3−, with no differences observed between erythroblasts derived from CD34+ cells, total PBMC or PBMC depleted of CD34+ cells (Figure 2B and Figure 3E for band 3).
Figure 2.
Pure erythroblast population obtained after 12-day expansion. (A) Cell surface expression of lineage specific markers in PBMC culture as percentage of positive cells against time in days. At indicated times, cell surface expression of CD3 (lymphocytes, white diamonds), CD20 (B cells, white triangles), CD14 (monocytes, white squares), c-kit (erythroblasts, black triangles) and CD71 (erythroblasts, black squares) was assessed by flow cytometry. Numbers of dead cells (white circles) assessed with propidium iodide. Black arrow shows time point of 2nd Percoll density purification to remove lymphocytes. (B) Cell surface expression of lineage markers CD3 (lymphocytes), CD14 (monocytes), CD20 (B cells) and erythroid markers CD117 (c-kit), CD71 (transferrin receptor) and GPA (glycophorin A) as percentage of positive cells against time in days checked at day 12 during expansion of total PBMC (left panel), CD34+ depleted PBMC (middle panel) and CD34+ cells (left panel) by flow cytometry. Presence of lymphocytes, monocytes and B cells was not assayed on CD34+ cultures (these are lost during CD34+ purification protocol). Experiments represent 2–4 independent measurements. (C) Benzidine/Giemsa stained cytospins of respective cultures in (B). Note absence of other hematopoietic lineages and presence of a pure blast population.
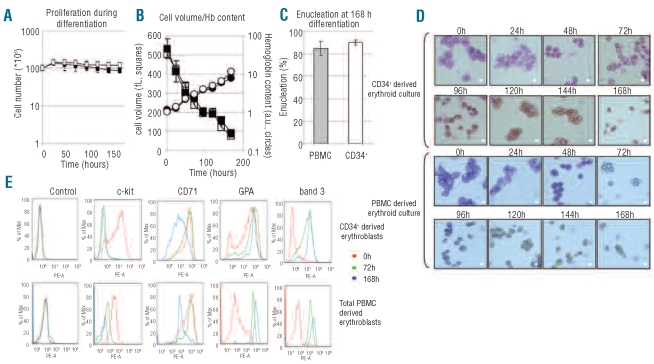
Figure 3.
Differentiation of erythroblasts derived from total PBMC or CD34+ cells is comparable. (A) Proliferation and cell cycle arrest during differentiation of erythroblasts from 1x108 total PBMC (black squares) or 0.2x106 CD34+ cells purified from 1x108 total PBMC (white squares). Cell number in millions plotted against time (h). See Design and Methods for differentiation conditions. (B) Hemoglobinization and cell volume loss during differentiation of erythroblasts expanded from total PBMC (black squares; black circles) or CD34+ cells (white squares; white circles) during erythroblast differentiation (three independent measurements). (C) Comparison of enucleation rates after 168 h differentiation between erythroblasts cultured from total PBMC or CD34+ cells. Error bars indicate SD from at least three independent experiments, counting 125 cells at 168 h in differentiation per cytospin. (D) Cell morphology during erythroblast differentiation is similar between total PBMC and CD34+ derived erythroblasts. At indicated times, aliquots of cell culture were taken, cytospinned and stained with a benzidine/Giemsa stain (see Design and Methods). Scale bars = 9 μm. (E) Cell surface expression of specific erythroid markers over time. Cell surface expression of c-kit, CD71, GPA and band 3 checked on expanding erythroblasts (0 h, red), after 72 h of differentiation (green) and after 168 h of differentiation (blue) for CD34+ derived erythroblasts (top panels) and for erythroblasts derived from total PBMC (bottom panels). Figures representative of three independent experiments.
Thus peripheral blood contains a multitude of different progenitor cells that have the capacity to produce erythroblasts and only a portion of these are CD34+, a heterogeneous pool of hematopoietic stem cells in itself.9 Indeed, CD34− cells isolated from cord blood have long-term repopulation abilities in irradiated NOD/SCID mice,10 demonstrating their hematopoietic potential. Furthermore, erythroid outgrowth from CD34− /Lin− cell populations obtained from PBMC has been demonstrated before14 but has never been directly compared to the erythroid potential of CD34+ cells as conducted here.
Terminal differentiation of erythroblasts derived from total PBMC or CD34+ to reticulocytes is comparable
Terminal differentiation is the final stage of erythropoiesis involving enucleation, hemoglobinization, membrane remodeling and removal of unwanted proteins via exosomes. Key erythroid specific membrane proteins are expressed during terminal differentiation alongside globin. To determine whether the differentiation from total PBMC is similar to CD34+, erythroblasts derived from CD34+ cells or from total PBMC were induced to differentiate (Phase 3, see Design and Methods).
Differentiation of erythroblasts is normally accompanied by a short burst in proliferation (24 h) followed by cell cycle arrest, a reduction in cell volume, hemoglobinization and enucleation.7 No differences in these differentiation parameters were found between PBMC or CD34+ derived erythroblasts (Figure 3A–C). In addition, Figure 3D shows that there are no obvious morphological differences during differentiation as both cultures display normal progression from pro-erythroblasts (0 h) to basophllic normoblasts (24–48 h) to polychromatic normoblasts (48–96 h) to orthochromatic normoblasts (96–144 h) to enucleated reticulocytes (120–168 h). Importantly, the cytospins also demonstrate that using glucocorticoids during the expansion phase ensures that differentiation of precursor cells is arrested at the erythroblast stage creating a strikingly homogeneous pool of erythroblasts (Figure 3D, first panels) that remain remarkably synchronous throughout the subsequent stages of differentiation.
Figure 3E shows the cell surface expression of membrane proteins expressed during specific stages of erythropoiesis. No gross differences in cell marker expression profiles and dynamics of c-kit, CD71, GPA and band 3 were observed between PBMC derived and CD34+ derived erythroblasts during differentiation. Furthermore, the expression profiles followed generally accepted dynamics with: i) CD71 cell surface expression, already present on erythroblasts, increasing marginally during differentiation (72 h) but severely decreasing at the end of differentiation; ii) upregulation of GPA and band 3; and iii) downregulation of c-kit, a marker of immature erythroblasts.
In summary, we have demonstrated for the first time that the expansion of erythroblasts from total PBMC or CD34− cells yields significantly more erythroblasts compared to isolating only the CD34+ cells purified from the same amount of PBMC. We have shown that the CD34− cells in our culture system enter a CD34+ phase before progressing through the normal hematopoietic/erythropoiesis pathway to the nascent reticulocyte. Importantly, terminal differentiation of PBMC derived erythroblasts to enucleated reticulocytes is of a similar nature within all the parameters studied here (hemoglobinization, morphology, proliferation and erythroid marker expression). The culture of PBMC described here will prove invaluable for investigating the alterations that occur during blood diseases such as hereditary spherocytosis15 or congenital dyserythropoietic anemia type II (CDAII) where only small amounts of blood may be available for study.
Acknowledgments
the authors would like to thank Rosey Mushens for providing monoclonal antibodies and Marieke von Lindern for providing the SCF producing cell line.
Footnotes
Funding: the work was supported by NHS Blood and Transplant (NHSBT) project grants for EvdA and SP (GD), a BBSRC DTA NHSBT Case Studentship for TJS (AMT), and a NHSBT Wellcome Trust Career Development Fellowship (AMT).
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
AMT and GD were the principle investigators. EvdA, SP and TJS performed the experiments. EvdA, SP, TJS, GD and AMT conceived and designed experiments and wrote the manuscript.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Miharada K, Hiroyama T, Sudo K, Nagasawa T, Nakamura Y. Efficient enucleation of erythroblasts differentiated in vitro from hematopoietic stem and progenitor cells. Nat Biotechnol. 2006;24(10):1255–6. doi: 10.1038/nbt1245. [DOI] [PubMed] [Google Scholar]
- 2.Neildez-Nguyen TM, Wajcman H, Marden MC, Bensidhoum M, Moncollin V, Giarratana MC, et al. Human erythroid cells produced ex vivo at large scale differentiate into red blood cells in vivo. Nat Biotechnol. 2002;20(5):467–72. doi: 10.1038/nbt0502-467. [DOI] [PubMed] [Google Scholar]
- 3.Giarratana MC, Kobari L, Lapillonne H, Chalmers D, Kiger L, Cynober T, et al. Ex vivo generation of fully mature human red blood cells from hematopoietic stem cells. Nat Biotechnol. 2005;23(1):69–74. doi: 10.1038/nbt1047. [DOI] [PubMed] [Google Scholar]
- 4.Fujimi A, Matsunaga T, Kobune M, Kawano Y, Nagaya T, Tanaka I, et al. Ex vivo large-scale generation of human red blood cells from cord blood CD34+ cells by co-culturing with macrophages. Int J Hematol. 2008;87(4):339–50. doi: 10.1007/s12185-008-0062-y. [DOI] [PubMed] [Google Scholar]
- 5.Panzenbock B, Bartunek P, Mapara MY, Zenke M. Growth and differentiation of human stem cell factor/erythropoietin-dependent erythroid progenitor cells in vitro. Blood. 1998;92(10):3658–68. [PubMed] [Google Scholar]
- 6.Mahajan MC, Karmakar S, Newburger PE, Krause DS, Weissman SM. Dynamics of α-globin locus chromatin structure and gene expression during erythroid differentiation of human CD34(+) cells in culture. Exp Hematol. 2009;37(10):1143–56. e3. doi: 10.1016/j.exphem.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leberbauer C, Boulme F, Unfried G, Huber J, Beug H, Mullner EW. Different steroids co-regulate long-term expansion versus terminal differentiation in primary human erythroid progenitors. Blood. 2005;105(1):85–94. doi: 10.1182/blood-2004-03-1002. [DOI] [PubMed] [Google Scholar]
- 8.von Lindern M, Parren-van Amelsvoort M, van Dijk T, Deiner E, van den Akker E, van Emst-de Vries S, et al. Protein kinase C alpha controls erythropoietin receptor signaling. J Biol Chem. 2000;275(44):34719–27. doi: 10.1074/jbc.M007042200. [DOI] [PubMed] [Google Scholar]
- 9.Sonoda Y. Immunophenotype and functional characteristics of human primitive CD34-negative hematopoietic stem cells: the significance of the intra-bone marrow injection. J Autoimmun. 2008;30(3):136–44. doi: 10.1016/j.jaut.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, et al. SCID-repopulating cell activity of human cord blood-derived CD34-cells assured by intra-bone marrow injection. Blood. 2003;101(8):2924–31. doi: 10.1182/blood-2002-09-2782. [DOI] [PubMed] [Google Scholar]
- 11.Bakker WJ, Blazquez-Domingo M, Kolbus A, Besooyen J, Steinlein P, Beug H, et al. FoxO3a regulates erythroid differentiation and induces BTG1, an activator of protein arginine methyl transferase 1. J Cell Biol. 2004;164(2):175–84. doi: 10.1083/jcb.200307056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beug H, Doederlein G, Freudenstein C, Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- 13.Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, et al. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med. 1997;3(12):1337–45. doi: 10.1038/nm1297-1337. [DOI] [PubMed] [Google Scholar]
- 14.Bender JG, Unverzagt KL, Walker DE, Lee W, Van Epps DE, Smith DH, et al. Identification and comparison of CD34-positive cells and their subpopulations from normal peripheral blood and bone marrow using multicolor flow cytometry. Blood. 1991;77(12):2591–6. [PubMed] [Google Scholar]
- 15.van den Akker E, Satchwell TJ, Pellegrin S, Flatt JF, Maigre M, Daniels G, et al. Investigating the key membrane protein changes during in vitro erythropoiesis of protein 4.2 (−) cells (mutations Chartres 1 and 2) Haematologica. 2010 Feb 23; doi: 10.3324/haematol.2009.021063. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]