Abstract
Hydroxyurea has proven clinical efficacy in patients with sickle cell disease. Potential mechanisms for the beneficial effects include fetal hemoglobin induction and the reduction of cell adhesive properties, inflammation and hypercoagulability. Using a murine model of sickle cell disease in which fetal hemoglobin induction does not occur, we evaluated whether hydroxyurea administration would still yield improvements in hematologic parameters and reduce end-organ damage. Animals given a maximally tolerated dose of hydroxyurea that resulted in significant reductions in the neutrophil and platelet counts showed no improvement in hemolytic anemia and end-organ damage compared to control mice. In contrast, animals having high levels of fetal hemoglobin due to gene transfer with a γ-globin lentiviral vector showed correction of anemia and organ damage. These data suggest that induction of fetal hemoglobin by hydroxyurea is an essential mechanism for its clinical benefits.
Keywords: hydroxyurea, HbF induction, sickle cell disease
Introduction
Sickle cell disease (SCD) is a chronic hemolytic, vaso-occlusive disorder that results in multi-organ complications involving the central nervous system, lungs, kidneys, bones and spleen.1 Patients with hereditary persistence of fetal hemoglobin (HbF) have milder clinical phenotypes.2,3 In the early 1980s, pharmacological agents that increased HbF were identified. First azacytidine4 and, subsequently, hydroxyurea5 were found to increase levels of HbF in both primate models and patients with SCD. A decade later, the Multicenter Study of Hydroxyurea (MSH) demonstrated that hydroxyurea administration to adults with severe SCD phenotypes resulted in a reduction in pain crisis, acute chest syndrome, hospitalizations and transfusion requirements.6 Recent studies suggest that hydroxyurea therapy may also preserve organ function in children.7
The beneficial effects of hydroxyurea are often attributed to the increase in HbF observed in responding patients.8–10 Increased cellular levels of HbF reduce the propensity of deoxygenated HbS to polymerize.11 However, the possibility that the clinical benefit of hydroxyurea is mediated through mechanisms other than Hb induction has been raised. A follow-up MSH study concluded that a reduction in the peripheral blood leukocyte count, but not HbF level, was a significant marker of clinical improvement.12 Furthermore, some reports have suggested that hydroxyurea may impart some of its clinical benefit through a variety of other mechanisms, including reduction of leukocyte count,9,13 alteration of red blood cell volume,13 reduced phosphatidylserine exposure,14 reduction in adhesion receptors,15,16 increased production of nitric oxide,17 increased activity of the cation transport system,18 or reduction of the hypercoagulable state.
To date, no studies have been performed to elucidate the degree of benefit from hydroxyurea derived from HbF induction versus that due to other potential HbF-independent mechanisms. To evaluate whether there are significant clinical benefits from hydroxyurea that are independent of HbF induction, we evaluated the effects of long-term hydroxyurea administration using the adult BERK sickle cell mouse model in which HbF induction is not operative.19 Although these SCD mice demonstrate γ-globin and HbF expression during fetal development, γ-globin gene expression is silenced shortly after birth. This SCD murine model demonstrates a significant sickle phenotype including organ damage, anemia,19 leukocytosis,20 vascular inflammation,21 increased cellular adhesion20 and altered NO bioavailability.22 Here we demonstrate that long-term administration of hydroxyurea in the absence of HbF induction fails to improve anemia or lend protection from organ damage.
Design and Methods
Adult C57BL/6 (wild-type) mice (Jackson Laboratories, Bar Harbor, ME) and Berkley SCD mice (BERK)19 (expressing exclusively human α- and sickle β-globin), were housed, bred, and used for experimentation in the Animal Resource Center at St. Jude Children’s Research Hospital under protocols approved by the Institutional Animal Care and Use Committee. Cohorts of sex and aged-matched SCD mice were generated by transplanting lethally irradiated 12-week old C57BL/6 mice with bone marrow from BERK mice as previously described.23 Transplantation of C57BL/6 mice was used to generate age- and sex-matched cohorts of sickle cell mice without pre-existing organ damage.
Since determining whether hydroxyurea could prevent or reduce the development of organ pathology in engrafted sickle cell mice was our primary objective, transplanted SCD mice were injected with hydroxyurea by intraperitoneal route five days per week, starting nine weeks after transplantation, prior to full engraftment by sickle cell marrow and the development of organ damage (Online Supplementary Figure S1). Only mice demonstrating full engraftment (>99% HbS) at 12 weeks after transplantation, three weeks after the initiation of hydroxyurea, continued to be evaluated in this study. Hydroxyurea solution was prepared fresh daily for injection by mixing hydroxyurea (Sigma, St. Louis, MO, USA) with sterile Plasmalyte (Baxter, Deerfield, IL, USA) and filtering through Steriflip (Millipore, Billireca, MA, USA). Control animals received injections with vehicle only (Plasma-Lyte). To compare the HbF-independent effects derived from hydroxyurea to the effects due solely to HbF, we generated BERK sickle cell mice with high levels of HbF by gene transfer as previously described.23
Blood for hematologic analysis was obtained by retroorbital puncture. Complete blood counts (CBC) were obtained using a Forcyte blood count analyzer (Oxford Science, Oxford, CT, USA). Reticulocyte counts and hemoglobin composition, using cellulose acetate gel electrophoresis and high performance liquid chromatography (HPLC), were performed as previously described.23 Water deprivation tests were performed as previously described.23 All animal histology was scored blindly by the Veterinary Pathologist (KLB) at St. Jude Children’s Research Hospital. Pathological scoring of the liver, spleen, and kidneys was adopted from a previously described scoring system.21
Results and Discussion
In our studies, we chose to generate age- and sex-matched SCD animals for our experiments by transplanting lethally irradiated, wild-type C57Bl/6 mice with BERK SCD bone marrow cells, as we did previously.23 This approach accurately reproduces in recipient animals all of the disease manifestations of the donor SCD mice. This strategy facilitated initiating hydroxyurea administration prior to full engraftment of sickle hematopoiesis (Online Supplementary Figure S1) and onset of sickle-associated organ damage. It also permitted maximizing the potential benefits of hydroxyurea by assessing its ability to prevent, rather than reverse, organ pathology. Hydroxyurea treatment did not alter the complete engraftment of SCD hematopoiesis, as judged by cellulose acetate electrophoresis at eight and 12 weeks posttransplantation (Online Supplementary Figure S1). As expected, after 16 weeks of hydroxyurea therapy cellulose acetate gel electrophoresis and HPLC analysis showed only the presence of HbS while HbF was undetectable in both hydroxyurea-treated and control mice (Online Supplementary Figure S2). This confirmed that any hydroxyurea effect observed in this SCD model would be unrelated to the induction of HbF.
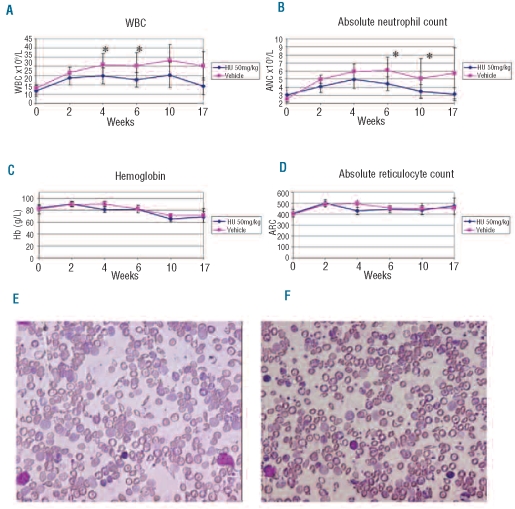
To determine whether hydroxyurea would improve anemia and/or prevent or diminish the development of organ damage in the absence of HbF induction, we first sought to determine a dose that could result in a stable, well-tolerated reduction in neutrophil count, similar to titrating hydroxyurea dosage in human patients with SCD. Therefore hydroxyurea, at doses of 25 mg/kg, 50 mg/kg, and 100 mg/kg, or vehicle was administered five days per week to SCD mice. Mice given 25 mg/kg demonstrated no effect on CBC compared to vehicle-treated controls (data not shown). In contrast, after four weeks of treatment, mice receiving 100 mg/kg of hydroxyurea displayed significant bone marrow suppression, including severe anemia, which led to death or compassionate euthanasia in 4 of the 8 mice (Online Supplementary Table S1). The 4 remaining mice were taken off drug therapy for one week with subsequent blood count recovery and a reduced dose 75 mg/kg was then administered. However, after six weeks at the 75 mg/kg dosage, severe anemia (4.7 g/dL±1.9) again developed in all 4 animals. In contrast, hydroxyurea administered at 50 mg/kg for six weeks (n=8) produced a significant reduction in the total leukocyte (30.4±6.9 vs. 22.7±4.3×109/L, P<0.02) and absolute neutrophil count (ANC; 6.1±0.8 vs. 4.4±1.6×109/L, P<0.02) compared to vehicle-treated controls. At the 50 mg/kg dose, there was no improvement in the Hb level (82±6 vs. 82±1 g/L, P=0.8) or change in the absolute reticulocyte count (4.5±0.3×109/L vs. 4.6±0.3×109/L, P=0.5). These changes in CBC were consistent throughout a total of 17 weeks of hydroxyurea therapy (Figure 1).
Figure 1.
Hydroxyurea therapy produced consistent reductions in WBC and ANC without improvement in anemia over 17 weeks. (A) Hydroxyurea at 50mg/kg produced a reduced white blood cell count compared to vehicle treated sickle cell mice. (B) Hydroxyurea at 50 mg/kg produced a reduced absolute neutrophil count compared to vehicle treated sickle cell mice. (C and D) Hydroxyurea at 50 mg/kg produced no improvement in anemia compared to vehicle treated sickle cell mice. P value <0.05 is represented by *. (E) Peripheral blood smear from HU treated sickle cell mouse. (F) Peripheral blood smear from vehicle treated mouse.
Consistent with the persistence of anemia, the serum LDH in the hydroxyurea-treated group remained elevated, similar to control mice (1,424±750 vs. 1,373±505 IU/L, P=0.9), suggesting no improvement in the rate of hemolysis. No functional improvement in renal function was detected as judged by water deprivation tests. There was no difference in the marked reduction in urine concentrating ability in hydroxyurea-treated mice, compared to control mice (Online Supplementary Figure S3). Furthermore, we observed no reductions in the number of sickle cells and anisocytosis on the peripheral blood smears of the hydroxyurea-treated mice relative to control animals (Figure 1 E and F).
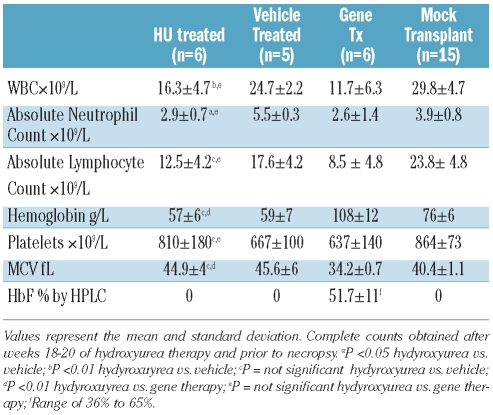
Based on the initial dose-finding study, an additional cohort of SCD mice was then generated and treated with 50 mg/kg hydroxyurea or vehicle five days per week for 20 weeks. Blood counts obtained after ten weeks of hydroxyurea therapy again demonstrated a significant reduction in total leukocyte and absolute neutrophil counts, along with a reduction in platelets, without improvement in the anemia or reticulocytosis (data not shown) and these changes persisted until the termination of the study at 18–20 weeks posttransplantation (Table 1).
Table 1.
Hydroxyurea does not improve the anemia of SCD despite significant reduction in neutrophil count.
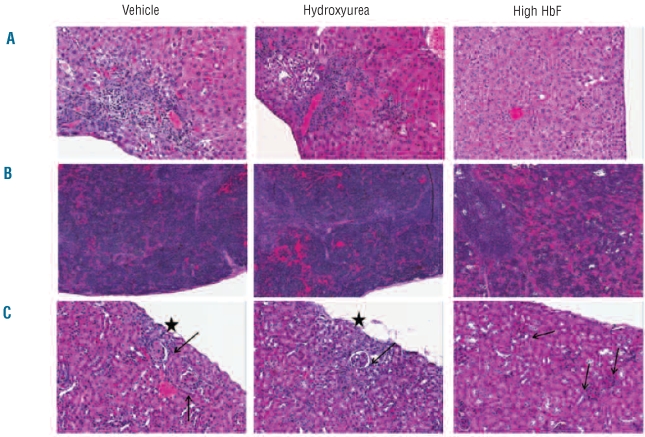
To evaluate whether hydroxyurea could prevent or diminish the development of organ damage, necropsy and pathological analyses of major organs were performed on 6 mice that had received 18–20 weeks of hydroxyurea. Vehicle-treated mice served as controls. Hydroxyurea-treated mice showed no improvement in the severe, multi-organ damage, compared to control mice (Figure 2 and Online Supplementary Table S2). In comparison, 6 SCD mice with high levels of HbF (mean 52%) generated through stem cell gene transfer, but not treated with hydroxyurea, also demonstrated significant reductions in total white blood cell and absolute neutrophil counts, similar to the levels achieved by chronic hydroxyurea administration (hydroxyurea ANC 2.9 vs. gene therapy ANC 2.6 ×109/L). Importantly, and in contrast to the hydroxyurea-treated SCD mice, the SCD mice with high HbF demonstrated a significant correction of the anemia and an absence of significant organ damage with correction of urinary concentrating ability (Figure 2, Online Supplementary Figure S3, Online Supplementary Table S2), as we have observed previously.23
Figure 2.
HbF expression, but not hydroxyurea therapy, corrects the end-organ damage of SCD. Representative hematoxylin and eosin (H&E)-stained tissue sections from the indicated experimental groups, 20x magnification, (A) Liver: fibrosis, inflammation and collapse of hepatic parenchyma are present in both the vehicle- and hydroxyurea-treated animal groups but not in the gene therapy group. (B) Spleen: excessive extramedullary hematopoiesis in the red pulp of the vehicle and hydroxyurea groups but not in the gene therapy group. (C) Kidney: loss of tubules with fibrosis and inflammation of the interstitium and increased eosinophilic matrix in the glomerular tuft of animals in the vehicle and hydroxyurea groups, but not present in the gene therapy group. The stars denote the foci of cortical collapse and fibrosis and the arrows indicate glomeruli. H&E-stained tissue on glass slides were digitized at 20x magnification utilizing an Aperio ScanScope® XT (Aperio, Vista, CA, USA). The ScanScope® scans slides with a 20x/0.75 Plan Apo objective. Slides were evaluated and images were captured utilizing ImageScope™(Aperio, Vista, CA, USA) viewing software.
Hydroxyurea has proven clinical benefit in patients with SCD and a severe disease phenotype. Historically, the induction of HbF has been considered an important factor in disease amelioration given its anti-sickling properties.8–10 However, hydroxyurea administration causes pleiotropic effects, making it difficult to unequivocally ascribe its beneficial effects to a single or combination of mechanisms. In the current study, we used an SCD murine model to evaluate the clinical benefit of hydroxyurea absent HbF induction. Although we identified a maximally tolerated dose of hydroxyurea that significantly reduced the absolute neutrophil and platelet counts, there was no improvement in the severe anemia and multi-organ damage which occurred despite drug administration for up to five months. In contrast, SCD mice genetically modified to express high levels of HbF showed resolution of anemia and an absence of organ pathology. Interestingly, the neutrophil counts of the two groups of treated mice were similar; demonstrating that simply reducing this parameter using hydroxyurea did not have a discernible clinical impact.
The BERK SCD model is characterized by a well-documented progression of sickle-related organ damage with similarities to human patients.24 By initiating hydroxyurea prior to complete engraftment with sickle hematopoiesis and the development of severe organ damage, the potential to observe effects from drug treatment were optimized. We generated SCD animals having high levels of HbF through the same bone marrow transplant model, therefore providing HbF before the onset of disease pathogenesis. In contrast to mice treated with hydroxyurea, sickle cell mice having high levels of HbF showed correction of anemia and lacked pathological evidence of significant organ damage. These mice also showed correction of the functional impairment in renal concentrating ability, while hydroxyurea-treated mice did not.
One limitation of our study was that hydroxyurea, in the absence of HbF induction, caused a dose-limiting, worsening of anemia in treated mice that precluded achieving a greater reduction in neutrophil count. This suggests that the ability to achieve a lower target neutrophil count, as often occurs in clinical practice, is probably dependent on the improvement in erythropoiesis and red cell lifespan that is provided by higher levels of HbF in responding patients. Thus, while we cannot be certain that a lower neutrophil count would not have resulted in some clinical benefit, it appears that HbF induction may be an important component that allows robust reduction of the neutrophil count without worsening of the anemia.
In summary, previous studies have raised the possibility that the beneficial effect of hydroxyurea may be related to alterations of cell adhesion, membrane properties, nitric oxide and cation transport systems.14–18 In this study, we took advantage of a unique transplant model of murine SCD to assess the ability of hydroxyurea to significantly impact SCD disease pathogenesis in the absence of HbF induction. We conclude that although non-HbF effects may augment those due solely to HbF for patients on therapy, HbF induction appears to be critical to the beneficial effects of hydroxyurea.
Acknowledgments
the authors would like to thank the staff of the Animal Resource Center of St. Jude Children’s Research Hospital for animal care and experimental analysis. We thank Dr. Dennis Jay of the Department of Pathology at St. Jude Children’s Research Hospital for HPLC analysis of hemoglobin.
Footnotes
Funding: this work was supported by training grant T32-CA070089 (JDL) from the National Cancer Institute, the Basic and Translational Research Program for Sickle Cell Disease grant U54HL070590 (REW, DAP) from the National Heart, Lung, and Blood Institute of the NIH, and the American Lebanese Syrian Associated Charities. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
JDL designed research, analyzed and interpreted data, wrote manuscript; TP and KLB performed research; REW designed research and edited manuscript; DAP designed research, analyzed and interpreted data, wrote manuscript.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005;84(6):363–76. doi: 10.1097/01.md.0000189089.45003.52. [DOI] [PubMed] [Google Scholar]
- 2.Herman EC, Jr, Conley CL. Hereditary persistence of fetal hemoglobin. A family study. Am J Med. 1960;29:9–17. doi: 10.1016/0002-9343(60)90003-6. [DOI] [PubMed] [Google Scholar]
- 3.Pembrey ME, Wood WG, Weatherall DJ, Perrine RP. Fetal haemoglobin production and the sickle gene in the oases of Eastern Saudi Arabia. Br J Haematol. 1978;40(3):415–29. doi: 10.1111/j.1365-2141.1978.tb05813.x. [DOI] [PubMed] [Google Scholar]
- 4.DeSimone J, Heller P, Hall L, Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci USA. 1982;79(14):4428–31. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dover GJ, Humphries RK, Moore JG, Ley TJ, Young NS, Charache S, et al. Hydroxyurea induction of hemoglobin F production in sickle cell disease: relationship between cytotoxicity and F cell production. Blood. 1986;67(3):735–8. [PubMed] [Google Scholar]
- 6.Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332(20):1317–22. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 7.Hankins JS, Helton KJ, McCarville MB, Li CS, Wang WC, Ware RE. Preservation of spleen and brain function in children with sickle cell anemia treated with hydroxyurea. Pediatr Blood Cancer. 2008;50(2):293–7. doi: 10.1002/pbc.21271. [DOI] [PubMed] [Google Scholar]
- 8.Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991;325(1):11–6. doi: 10.1056/NEJM199107043250103. [DOI] [PubMed] [Google Scholar]
- 9.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 10.Powars DR, Weiss JN, Chan LS, Schroeder WA. Is there a threshold level of fetal hemoglobin that ameliorates morbidity in sickle cell anemia? Blood. 1984;63(4):921–6. [PubMed] [Google Scholar]
- 11.Bookchin RM, Nagel RL. Interactions between human hemoglobins: sickling and related phenomena. Semin Hematol. 1974;11(4):577–95. [PubMed] [Google Scholar]
- 12.Charache S, Barton FB, Moore RD, Terrin ML, Steinberg MH, Dover GJ, et al. Hydroxyurea and sickle cell anemia. Clinical utility of a myelosuppressive “switching” agent. The Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Medicine (Baltimore) 1996;75(6):300–26. doi: 10.1097/00005792-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman SA, Schultz WH, Davis JS, Pickens CV, Mortier NA, Howard TA, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–45. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 14.Covas DT, de Lucena Angulo I, Vianna Bonini Palma P, Zago MA. Effects of hydroxyurea on the membrane of erythrocytes and platelets in sickle cell anemia. Haematologica. 2004;89(3):273–80. [PubMed] [Google Scholar]
- 15.Styles LA, Lubin B, Vichinsky E, Lawrence S, Hua M, Test S, et al. Decrease of very late activation antigen-4 and CD36 on reticulocytes in sickle cell patients treated with hydroxyurea. Blood. 1997;89(7):2554–9. [PubMed] [Google Scholar]
- 16.Hillery CA, Du MC, Wang WC, Scott JP. Hydroxyurea therapy decreases the in vitro adhesion of sickle erythrocytes to thrombospondin and laminin. Br J Haematol. 2000;109(2):322–7. doi: 10.1046/j.1365-2141.2000.02040.x. [DOI] [PubMed] [Google Scholar]
- 17.Cokic VP, Beleslin-Cokic BB, Tomic M, Stojilkovic SS, Noguchi CT, Schechter AN. Hydroxyurea induces the eNOS-cGMP pathway in endothelial cells. Blood. 2006;108(1):184–91. doi: 10.1182/blood-2005-11-4454. [DOI] [PubMed] [Google Scholar]
- 18.Bridges KR, Barabino GD, Brugnara C, Cho MR, Christoph GW, Dover G, et al. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood. 1996;88(12):4701–10. [PubMed] [Google Scholar]
- 19.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–8. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 20.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS. Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci USA. 2002;99(5):3047–51. doi: 10.1073/pnas.052522799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, et al. Transgenic sickle mice have vascular inflammation. Blood. 2003;101(10):3953–9. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- 22.Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278(6):H1799–806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- 23.Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17(2):245–52. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manci EA, Hillery CA, Bodian CA, Zhang ZG, Lutty GA, Coller BS. Pathology of Berkeley sickle cell mice: similarities and differences with human sickle cell disease. Blood. 2006;107(4):1651–8. doi: 10.1182/blood-2005-07-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]