Abstract
In chronic myeloid leukemia (CML), cytogenetic abnormalities found in addition to the t(9;22) translocation may impact the response to therapy. Loss of the Y chromosome is generally overlooked in this context, owing to its relatively frequent occurrence in healthy elderly patients. In this multicenter retrospective study, the outcome after imatinib treatment of 30 CML patients with karyotype showing Y chromosome loss (Y−) was compared to 30 Y+ control males diagnosed and treated at the same time in the same institutions. Y− patients had significantly delayed cytogenetic and molecular responses, lower event-free survival and shorter overall survival than Y+ patients. The negative impact of this abnormality was particularly marked when it occurred in a sub-clone (clonal evolution) rather than in all mitoses. These data indicate that loss of the Y chromosome should be taken into account in the prognostic evaluation of chronic myelogenous leukemia patients.
Keywords: loss, y chromosome, Philadelphia-positive, CML, imatinib
Introduction
Imatinib (Gleevec or Glivec), a competitive inhibitor of the Bcr-Abl tyrosine-kinase, is the standard treatment of chronic myeloid leukemia (CML).1 Despite the outstanding results of imatinib in chronic phase of CML, cases of treatment failure or suboptimal response have been reported.2 Although the validity of prognostic markers is strongly dependant on the therapeutics involved, Sokal score, which was described and used before the tyrosine-kinase inhibitors (TKI) era, remains of interest to predict the outcome of imatinib-treated patients.3 Other elements described as predictors of poor outcome include cytogenetic findings. In addition to the classical Philadelphia chromosome, t(9;22)(q34;q11), 3–5% of subjects have additional karyotype abnormalities in CML cells.3 These may be found in all the cells (Additional Chromosomal Abnormality: ACA) or they may be acquired in a subclone, thus defining a clonal evolution (CE). CE is one of the criteria defining the accelerated phase and is associated with poor prognosis. This statement holds true for imatinib treated patients.4–7 On the other hand, no study clearly demonstrates that the existence of ACA in all cells at diagnosis still carries an adverse prognosis for imatinib-treated patients.2 Loss of the Y chromosome can sometimes be observed in Philadelphia-positive cells, either as ACA or CE. However, the prognostic value of the loss of Y chromosome (Y−) is difficult to assess since it commonly occurs in normal cells of elderly men.8 Here groups of Y− and Y+ patients were compared to investigate evolution and outcome.
Design and Methods
For this retrospective study, cytogenetic data of male patients with CML diagnosed between 1994 and 2007 in 6 participating centers (University Hospitals of Bordeaux, Toulouse, Marseille, Lyon, Limoges, and Nice) were screened for loss of Y chromosome (Y−) as the sole abnormality besides the t(9;22) translocation in at least 3 metaphases, at diagnosis or during evolution. Loss of the Y chromosome could occur at diagnosis or later during treatment (interferon) but only patients with loss of the Y chromosome before imatinib was started were selected. Clinical and routine biological data were gathered for those patients who received imatinib, either in first or later lines of treatment. Evolution data included hematologic, cytogenetic [monitored every six months until complete cytogentic response (CCR)] and molecular (monitored every three months) response, transformation to accelerated or blastic phases, according to the World Health Organization (WHO) criteria. Follow up was censored at bone marrow transplantation (one patient in each group) or when treatment was switched to second generation TKI (5 Y− and 3 Y+ patients). Thirty patients responding to these criteria (Y−) were compared to a group of Y+ patients constituted as follows: each Y− patient was matched to the previous or the subsequent male patient diagnosed with CML in the same center, whoever was closer in time to the case. Controls had no cytogenetic abnormality besides the t(9;22) and were treated with imatinib.
Time to complete cytogenetic response (CCR) or major molecular response (MMR) was defined as the interval between imatinib treatment onset and CCR or MMR. Time to response, outcome of adverse event (acceleration, blastic transformation) and survival were compared using the log rank test analysis (Kaplan-Meier survival curves). Cytogenetics (at least 20 metaphases analyzed after R banding) and qRT-PCR were performed in each center. All centers participate in the French Quality Assessment program and thus obtained conversion factor for expression of BCR-ABL/ABL according to the international standard.
Results and Discussion
Characteristics of patients’ groups
For both Y− (n=30) and Y+ groups (n=30), 27 patients were in chronic phase and 3 were in accelerated phase at the onset of imatinib treatment. The method of selecting controls limits possible bias in the management of patients, and the groups were equivalent in terms of age at diagnosis (mean 56.2 years [Standard Error to the Mean, SEM=3.4] vs. 49.0 [SEM=2], P=0.09), age at imatinib start (mean 58.1 years [SEM=3.47] vs. 51.2 [SEM=2.28], P=0.1), previous treatment (Y− group: n=18, 60%; Y+ group: n=16, 53%) and Sokal score (mean 1.007 [SEM=0.13], n=16 with available data vs. 1.002 [SEM=0.13], n=19, P=0.98). For the majority of the Y− group, loss of the Y chromosome was found at CML diagnosis (80%, n=24) and in all the studied mitoses (63%, n=19) but in a subclone, i.e. clonal evolultion, for 11 patients (37%). In 2 patients, this sub-clone appeared during follow up but before imatinib onset.
Loss of Y chromosome has adverse effect on all studied outcome parameters
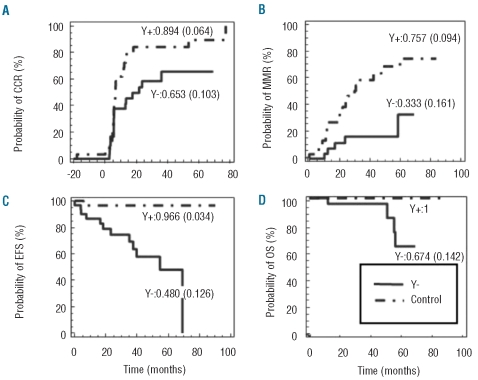
Complete cytogenetic response was achieved by 17 (57%) of the Y− group versus 26 (87%) in Y+ group. Using log rank analysis, the median time to achieve a CCR was significantly higher in the Y− group than in the Y+ group (21.9 vs. 6.7 months, P=0.0276, Figure 1A). Similarly, only 6 out of 29 Y− patients for whom molecular data was available (20%) achieved a major molecular response as compared to 19 out of 30 control patients. Median time to MMR was not reached for -Y patients versus 29.97 months for control (P=0.0023, Figure 1B). This slower cytogenetic/molecular response in patients with loss of the Y chromosome, logically translated into higher frequency of events: 9 patients of the Y− group versus only one of the Y+ group evolved to an accelerated phase (P=0.0014) and only patients of the Y− group (n=4, 13%) suffered a transformation to acute leukemia (P=0.02, Figure 1C). Three of these died, the fourth patient was in remission after allogeneic bone marrow transplantation. A fourth Y− patient also died, whereas none of the Y+ group died during the observation period (overall survival, P=0.013, Figure 1D), indicating that loss of the Y chromosome is indeed an adverse prognostic factor.
Figure 1.
Probability of achieving complete cytogenetic response (CCR, panel A), major molecular response (MMR, panel B), event-free survival (EFS, panel C) and overall survival (OS, panel D) were assessed using log rank test analysis for Y− (continuous line) and control (discontinuous line) patients. For each series the probability at five years (and standard error) are indicated.
Is the adverse prognosis of Y− limited to a subset of patients?
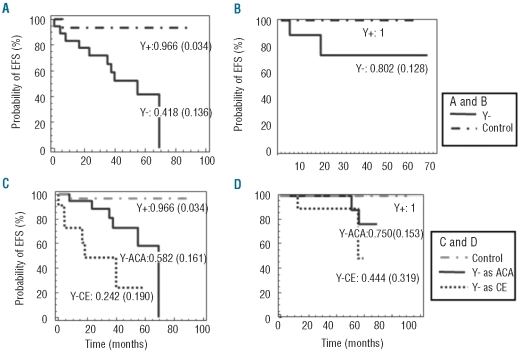
Due to the relative rarity of this anomaly, the study included both de novo patients treated with imatinib as a first-line therapy and previously treated patients (interferon). Considering only those who had received treatments prior to imatinib, Y− patients (n=18) still had longer time to CCR and MMR than those of the Y+ group (n=16), but this difference did not reach statistical significance, probably owing to the size of this subpopulation (23.7 vs. 6.89 months, respectively, P=0.19 for CCR; median not reached (MNR) vs. 47.4 months, P=0.1 for MMR). Acceleration was also more frequent in the Y− group (n=7 vs. n=1 in the Y+ group, P=0.01; Figure 2A). For patients who had received imatinib as first-line therapy (n=12 in the Y− group; n=14 in the Y+ group), the difference in terms of acceleration was not significant (n=2 for Y− vs. n=0 for Y+, Figure 2B), but MMR was significantly delayed in -Y patients (P=0.02). Subgroup analysis reduced the statistical power of the analyses; however, the indicators of poor response to imatinib were more frequent in Y− patients, whether they had received prior treatment or not.
Figure 2.
Event-free survival was assessed for –Y (continuous line) or control (dashed line) patients previously treated (A) or treated de novo with imatinib (B). Event-free survival (C) and overall survival (panel D) were determined in patients with loss of the Y chromosome in all mitoses (ACA, continuous line), or as a clonal evolution (CE, discontinuous line) and in control (dashed line) patients. For each series the probability at five years (and standard error) are indicated.
Since clonal evolution (CE) is considered an acceleration criterion, while additional chromosomal abnormalities (ACA) are only “warning” signs, further subgroup analysis of the Y− patients was performed. There was no significant difference between these two subgroups (ACA n= 19, CE n=11) in terms of CCR or MMR, but median event-free survival (EFS) was significantly longer in patients with -Y occurring as an ACA than CE (69 vs. 18 months, P=0.017). However, for Y+ patients, both overall survival and EFS were significantly longer than for those with loss of Y in all mitoses (ACA): respectively P=0.0001, Figure 2C and P= 0.0029, Figure 2D. The poor prognostic significance of CE has been well documented in CML,7 but losses of the Y chromosome are generally not taken into account in such studies. Besides, the significance of ACA is less clear. Here, both groups had indicators of poor response to imatinib. This confirms that clonal evolutions are bona fide markers of acceleration and indicates that, in this context, loss of the Y chromosome should not be considered differently from other clonal evolutions. Furthermore, in the context of ACA, loss of the Y chromosome is associated with a significantly decreased EFS. It is possible that, depending on which chromosome is involved, not all ACA carry the same prognostic impact, and the most frequent of these abnormalities should be systematically studied separately.
Taken together, this multicenter study shows that in patients treated with imatinib, loss of the Y chromosome, a finding estimated between 1% and 5% of the patients analyzed for this study, impacts the probability and time to obtain cytogenetic and molecular responses, as well as overall survival. This finding raises the question of whether Y− patients should be treated with more aggressive strategies such as those that have been proposed in accelerated phases: higher doses of imatinib mesylate or second generation TKI.
Acknowledgments
we are also grateful to the Groupe Francophone de Cytogénétique Hématologique (GFCH) and the French Intergroup of CML (Fi-LMC) for their collaboration.
Footnotes
Funding: the authors acknowledge funding from La Ligue Contre le Cancer, Comité Aquitaine-Charentes (EL-FXM) and from INCa (to tumorothèque du CHU de Bordeaux).
Authorship and Disclosures
FXM and EL designed the study and wrote the article. EL analyzed the data. All authors contributed to data collection and interpretation, discussed the final results and revised the paper critically for intellectual content. All authors collaborated in the final approval of the version to be published.
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. New Engl J Med. 2006;355(23):2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108(6):1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 3.Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–99. [PubMed] [Google Scholar]
- 4.Cortes J, O’Dwyer ME. Clonal evolution in chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18(3):671–84. doi: 10.1016/j.hoc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 5.O’Dwyer M, Mauro MJ, Kurilik G, Mori M, Balleisen S, Olson S, et al. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood. 2002;100(5):1628–33. doi: 10.1182/blood-2002-03-0777. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed AN, Pemberton P, Zonder J, Schiffer CA. The effect of imatinib mesylate on patients with Philadelphia chromosome-positive chronic myeloid leukemia with secondary chromosomal aberrations. Clin Cancer Res. 2003;9(4):1333–7. [PubMed] [Google Scholar]
- 7.Cortes JE, Talpaz M, Giles F, O’Brien S, Rios MB, Shan J, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101(10):3794–800. doi: 10.1182/blood-2002-09-2790. [DOI] [PubMed] [Google Scholar]
- 8.Pierre R, Hoagland HC. Age-associated aneuploidy: loss of the Y chromosome from human bone marrow cells with aging. Cancer. 1972;30(4):889–94. doi: 10.1002/1097-0142(197210)30:4<889::aid-cncr2820300405>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]