Abstract
BH3-only members of the Bcl-2 intracellular protein family, which include Bim, Bmf, Bik, Bad, Bid, Puma, Noxa and Hrk, mediate many developmentally programmed and induced cytotoxic signals. They have key roles in development, tissue homeostasis, immunity and tumor suppression, and compounds mimicking them are promising anti-cancer agents. Their activity is normally constrained by transcriptional and/or diverse post-transcriptional controls. When activated, these death ligands engage pro-survival Bcl-2-like proteins via the BH3 domain, inactivating their function. Bim and Puma bind all the pro-survival proteins, whereas others, such as Noxa and Bad, engage distinct subsets and exhibit complementary killing. Hence, multiple pro-survival proteins must be inactivated to unleash Bax and Bak, which drive apoptosis. Whether certain BH3-only proteins also directly activate Bax/Bak remains controversial.
Introduction
The cell suicide program termed apoptosis removes damaged, infected and superfluous cells. In most circumstances, a cell’s decision whether to live or die rests largely with the Bcl-2 family of interacting proteins [1•,2•]. In mammalian cells, its pro-survival members (Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1) oppose two pro-apoptotic groups: the Bax group and the BH3-only proteins. Members of the Bax group (Bax, Bak and Bok) are structurally similar to Bcl-2 and bear three ‘BH’ (Bcl-2 homology) domains, whereas the BH3-only proteins, which include Bim, Bad, Bid, Bik, Bmf, Puma, Noxa and Hrk, share only the BH3 interaction domain. The BH3-only proteins monitor cellular wellbeing and, when activated by cytotoxic signals, engage pro-survival relatives by inserting the BH3 domain, an amphipathic α helix, into a hydrophobic groove on their surface. This primes the cell for apoptosis, but commitment also requires activation of Bax or Bak [3,4]. Once activated, Bax and Bak form oligomers in intracellular membranes, including the mitochondrial outer membrane. The resulting membrane permeabilization releases pro-apoptotic proteins, such as cytochrome c, that provoke activation of the caspases mediating cell demolition [5].
As reviewed previously [1•,2•,6–8,9•,10], the BH3-only proteins participate in vital biological processes, and their absence contributes to autoimmunity and neoplasia. Here we review recent advances on their regulation and biological roles. Pertinent to cancer therapy, it has become evident that various BH3-only proteins mediate the cytotoxic responses elicited by chemotherapeutic agents, and there is exciting progress towards creating ‘BH3 mimetics’ as novel anti-cancer agents [11••,12••]. The recent discovery of selective association of BH3-only proteins with certain pro-survival relatives has delineated functional subclasses of the pro-survival proteins [13••,14••]. A new function for BH3-only proteins has emerged with discoveries that Noxa [14••] and the novel BH3-bearing ubiquitin ligase Mule/ARF-BP1 [15••] promote Mcl-1 degradation. Finally, we address the vexed issue of whether BH3-only proteins trigger activation of Bax and Bak directly [16,17•,18••] or whether they do so indirectly by inactivating the pro-survival family members that guard Bax and Bak [14••].
BH3-only proteins provide fine control over apoptotic responses
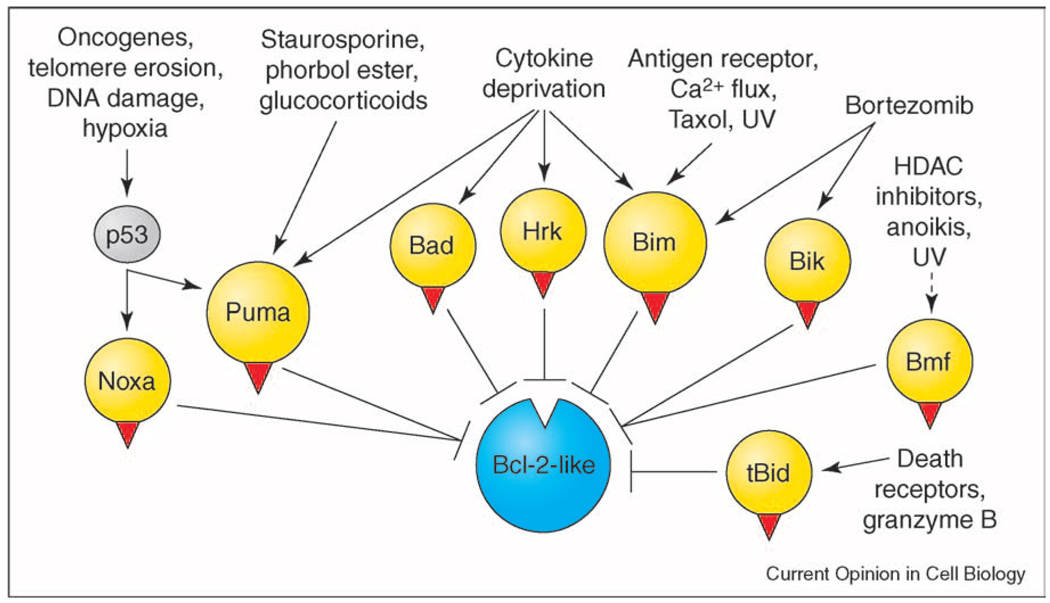
The multiplicity and complex regulation of mammalian BH3-only proteins allows exquisite control over apoptosis. Studies with cells from knockout mice indicate that one or a few BH3-only proteins have a dominant role in the responses to diverse cytotoxic stimuli (Figure 1). They mediate death induced not only by physiological stimuli such as cytokine deprivation or detachment from the cell matrix (a type of cell death termed anoikis) but also by the signals induced by activated oncogenes, DNA damage, chemotherapy drugs and γ-irradiation. Bim and Puma have the most prominent roles (Figure 1), probably because each antagonizes all the pro-survival proteins (see below). Loss of Bim renders lymphocytes refractory to paclitaxel (Taxol), ionomycin and cytokine deprivation [19]. Loss of Puma, unlike Bim, does not provoke cell accumulation during hematopoiesis, but T cells are rendered resistant to phorbol ester (PMA), γ-irradiation, etoposide and cytokine deprivation [20••,21••] and the developing central nervous system is refractory to γ-irradiation [21••]. Moreover, Noxa-deficient transformed fibroblasts are partially resistant to DNA-damage induced apoptosis [20••,22] and Bad-deficient mammary epithelial cells show some resistance to withdrawal of epidermal growth factor [23]. The redundancy of BH3-only function (Figure 1) probably conceals much wider roles.
Figure 1.
BH3-only proteins monitor cellular wellbeing. BH3-only proteins are activated by a variety of cellular stresses. Once activated, they initiate apoptosis by binding and neutralizing Bcl-2 pro-survival proteins via their BH3 domain (red triangle). Bid, which is typically activated following caspase cleavage, amplifies the apoptotic response by engaging the Bcl-2 pro-survival proteins. Potentially tBid may also engage Bax (or Bak) (see text).
Diverse modes of regulation
To avoid unwarranted cell death, BH3-only proteins are restrained by multiple mechanisms [1•,2•,7]. Hrk/DP5, Bim, Puma and Noxa are subject to transcriptional control. As reviewed elsewhere [1•,2•,7], post-transcriptional constraints include sequestration of phosphorylated Bad by 14-3-3 proteins and production of Bid as a largely inert protein requiring cleavage by, for example, caspase-8 to generate the active truncated product, tBid (Figure 1). In some cell types, both Bim and Bmf seem to monitor cytoskeletal integrity, Bim being sequestered to microtubule complexes [24] and Bmf to the myosin V motor complex by different dynein light chains [25]. UV-irradiation induces release of both Bim and Bmf by a process that may involve their phosphorylation [26]. Interestingly, Bim but not Bmf is released following paclitaxel treatment, which affects the microtubules, whereas anoikis frees Bmf [25]. In some cells, Bim contributes to anoikis [27]. Its regulation seems to vary with cell type, because much of the Bim in viable hematopoietic cells is associated not with microtubules but with Bcl-2-like proteins on mitochondria [28,29].
Several BH3-only proteins, including Bim, Bad, Bmf and Bik, are phosphorylated, but precisely how this regulates their activity is incompletely understood. Bim phosphorylation has been reviewed recently [30]. Survival signals such as phorbol ester or growth factors provoke Erk1/2 to phosphorylate Bim on one or more serines (including Ser 55, 65 and 100), inducing its degradation via the proteasome [29,31,32]. However, Bim may also be phosphorylated on Ser 65 by JNK following growth factor withdrawal in certain neurons, and this may enhance apoptosis [33]. Intriguingly, following growth factor withdrawal in osteoclasts, Bim ubiquitination is reduced, leading to increased Bim levels and cell death [34].
BH3-only proteins selectively engage Bcl-2 pro-survival proteins
The interaction of BH3-only proteins with pro-survival relatives has been most clearly visualized in the high-resolution 3D structure of a Bim BH3 fragment bound to Bcl-xL [35••]. Four hydrophobic pockets in the Bcl-xL groove accommodate the four conserved hydrophobic BH3 residues on one face of the BH3 ligand, whereas hydrophilic residues along the groove make salt bridges with the hydrophilic face of the BH3. Since the bound Bim BH3 α helix spanned at least 29 residues, with extensive groove contacts, the interaction surface may comprise ~30 residues, although the BH3 core is only 12–16 residues long [35••]. The homologous complex of C. elegans EGL-1 and CED-9 yielded similar conclusions [36].
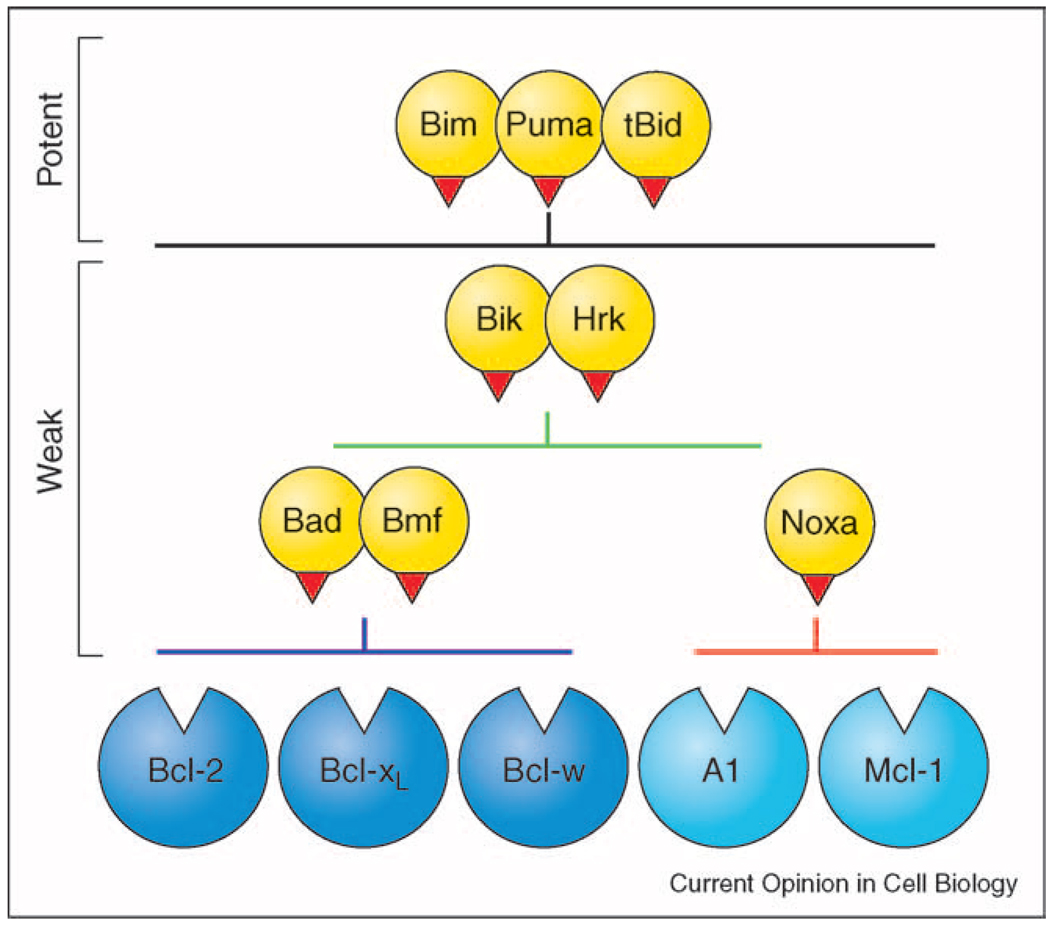
Systematic study of the binding of BH3-only proteins to Bcl-2 pro-survival proteins has revealed that only certain pairs associate inside cells [13••]. Bim and Puma bind all five pro-survival proteins, as does tBid (L Chen and DCS Huang, personal communication), but other BH3-only proteins exhibit marked selectivity (Figure 2). Most strikingly, Bad and Bmf bind only Bcl-2, Bcl-xL and Bcl-w, whereas Noxa binds only Mcl-1 and A1. Insights into binding selectivity are emerging: a Noxa BH3 peptide with only two substitutions bound Bcl-xL and Bcl-w in addition to Mcl-1 [13••], while a single substitution allowed the Bad BH3 to bind all the pro-survival proteins [37]. Determining the basis of selectivity will aid development of BH3 mimetics that target selected pro-survival proteins (see below).
Figure 2.
BH3-only proteins bind promiscuously or selectively to Bcl-2 pro-survival proteins. BH3-only proteins such as Bim, Puma and tBid engage all Bcl-2 pro-survival proteins and thus are potent killers. All other BH3-only proteins bind selectively to subsets of the Bcl-2 pro-survival proteins and hence are weaker killers [13••].
Efficient apoptosis requires neutralization of two subsets of pro-survival proteins
The selectivity of BH3-only proteins for their pro-survival counterparts markedly affects their ability to kill cells (Figure 2). Bim and Puma, which target all pro-survival proteins, are more potent killers than BH3-only proteins that target only a subset [13••]. The complementary binding profiles of Bad and Noxa (X) suggests that apoptosis might require neutralization of two classes of Bcl-2 pro-survival proteins, one comprising Bcl-2, Bcl-xL and Bcl-w and the other Mcl-1 and A-1. Notably, whereas Bad and Noxa alone are weak killers, their combined action, effectively neutralizing Bcl-2, Bcl-xL, Bcl-w and Mcl-1 (Figure 2), potently induces apoptosis [13••].
Mcl-1 and Bcl-xL sequester Bak in healthy cells
Analysis of Bak regulation revealed why efficient killing requires neutralization of two classes of pro-survival proteins [14••]. Mcl-1 had been implicated in Bak regulation by its formation of complexes with Bak [38•] and its degradation early in apoptosis [39•]. However, targeting Mcl-1 via either RNAi [39•] or Noxa overexpression failed to kill cells [13••], implicating additional pro-survival relatives. Indeed, in healthy cells Bak was bound by both Mcl-1 and Bcl-xL (Figure 3) but, significantly, not by Bcl-2, Bcl-w or A1 [14••]. Thus, Bak interacted with one member from each of the two inferred pro-survival Bcl-2 classes [13••]. The interactions required the Bak BH3 domain, which also proved necessary for Bak dimerization and killing activity [14••]. While Noxa displaced Bak from Mcl-1, Bak-mediated killing also required its displacement from Bcl-xL by another BH3-only protein, such as Bad [14••]. These findings suggest that Bak is restrained solely by Mcl-1 and Bcl-xL and induces apoptosis if, and only if, freed from both (Figure 3). Thus, the BH3-only proteins may activate Bak simply by freeing it from the pro-survival proteins designed to guard it.
Figure 3.
Displacement model for Bak regulation. The model [14••] proposes that both Mcl-1 and Bcl-xL, but not other pro-survival family members (e.g. Bcl-2), sequester Bak in healthy cells until cytotoxic signals activate a combination of BH3-only proteins that can displace Bak. While Noxa can readily displace Bak from Mcl-1 and promote Mcl-1 degradation, another BH3-only protein that can bind Bcl-xL (e.g. Bad) is also required for Bak liberation [14••]. The Bak BH3 (red triangle) is required both for Bak regulation and for formation of Bak oligomers, but their structure is unknown. Some of the Bak molecules in healthy cells, like cytosolic Bax [40], may have a ‘receptor’ conformation and be in equilibrium with the BH3-exposed ‘ligand’ conformation bound by Mcl-1 and Bcl-xL.
Whether Bax is similarly regulated is uncertain, because in healthy cells Bax is largely a monomeric cytosolic protein whereas Bak resides in complexes on intracellular membranes [1•,2•]. Their virtually equivalent function, however, argues for some similarity in regulation. Pertinently, in some detergents, Bax binds via its BH3 domain to various pro-survival relatives. One might speculate that the small proportion of Bax molecules associated with intracellular membranes in healthy cells represents an active conformer with its BH3 domain exposed. If so, certain Bcl-2 pro-survival proteins might sequester this conformer, as proposed for Bak [14••] (Figure 3).
Noxa and Mule promote Mcl-1 degradation
Intriguingly, Noxa binding to Mcl-1 prompts proteasome-dependent degradation of Mcl-1 [14••]. However, Mcl-1 poly-ubiquitination can be catalyzed by the novel E3 ubiquitin ligase Mule/ARF-BP1, which also specifically binds Mcl-1 via a Bak-like BH3 domain [15••]. Hence, there may be two routes to Mcl-1 degradation, with the Noxa-induced degradation involving another ubiquitin ligase, presumably one lacking a BH3 domain. The respective roles of Noxa and Mule in the constitutive turnover of Mcl-1 and its induced degradation during apoptosis remain to be established.
Models for the activation of Bak and Bax
Although BH3-only proteins require either Bax or Bak for cell killing [3,4], it remains unresolved whether they control the activation of Bax and Bak directly or indirectly [10].
The structural similarity of Bax to pro-survival proteins [35••,40] has encouraged the view that certain BH3-only proteins directly engage Bax. Letai et al. [16] proposed that BH3-only proteins comprised both ‘sensitisers’, which only inactivate the pro-survival proteins, and ‘activators’, which directly engage Bax and Bak. In this model (Figure 4a), the activators, proposed to include tBid and Bim, may normally be sequestered by the pro-survival proteins. Following cytotoxic stimuli, binding of sensitizers (e.g. Bad or Bik) to Bcl-2 displaces tBid (or Bim), allowing it to engage Bax and trigger its oligomerization. This model derives from observations that Bim or Bid BH3 peptides provoked cytochrome c release from isolated mitochondria, whereas a Bad or Bik BH3 peptide could do so only together with limiting amounts of a Bim or Bid BH3 peptide [16]. However, since Bim and Bid engage more pro-survival proteins than Bad and Bik (Figure 2) [13••], these results might instead reflect the extent of inactivation of pro-survival proteins present on the mitochondria.
Figure 4.
Models for how BH3-only proteins activate Bax and Bak. (a) Direct binding model. BH3-only proteins are proposed to constitute both ‘sensitizers’ (e.g. Bad) that bind only to the pro-survival proteins and ’activators’ (e.g. tBid) that can also bind Bax and Bak. The sensitizers are proposed to liberate the activators from Bcl-2-like proteins, so that they can directly engage Bax (or Bak) to induce cell death [16]. (b) Displacement model. BH3-only proteins are proposed to activate Bax and Bak by displacing them from the Bcl-2 pro-survival proteins that sequester their active forms [14••]. For Bak activation, BH3-only proteins must liberate Bak from both Mcl-1 and Bcl-xL [14••] (see Figure 3). It is proposed that an active form of Bax, located on the mitochondrial membrane, must be similarly released from pro-survival guards. The structure of the oligomers of Bax and Bak is unknown.
Such complications were precluded by Kuwana et al, who showed that a Bid or Bim BH3 peptide could cooperate with Bax to permeabilize artificial liposomes in the absence of pro-survival proteins [18••]. Their results, however, do not establish whether the co-operation of Bid or Bim with Bax involves direct binding or whether this is the physiological pathway for Bax activation. Conceivably, tBid might affect the membrane independently of binding Bax, although tBid alone does not usually provoke membrane permeabilization [41]. Evidence for binding of BH3-only proteins to Bax or Bak has been scanty and often inconsistent. With Bim, for example, certain minor isoforms (e.g. BimS), but not the predominant BimEL and BimL, bound Bax in certain detergents [42]. The dephosphorylation of BimEL provoked by cytokine deprivation was reported to allow it to bind Bax [29], but no association of the endogenous proteins was detected in dying cells [28]. Earlier, the absence of tBid in oligomeric Bax complexes led to the suggestion that tBid binds in a ‘hit-and-run’ fashion [43].
Intriguingly, both a Bid and a Puma BH3 peptide were reported recently to engage Bax by its N-terminal α helix rather than binding to its hydrophobic groove [17•]. Hence, Bid and Puma might open the N-terminal moiety of Bax, a step in Bax translocation to intracellular membranes, although the structural similarity of Bcl-xL and Bax [35••,40] is difficult to reconcile with a BH3 domain binding to them in such different ways.
Whereas the evidence for binding of BH3-only proteins to Bax/Bak remains controversial, their robust binding to the pro-survival proteins (Figure 2), often with low nanomolar affinity [13••,16,44], is unquestioned. That conclusion favors an alternative model (Figure 4b), derived from evidence that Mcl-1 and Bcl-xL constrain an active conformer of Bak until the binding of BH3-only proteins to them displaces Bak [14••] (Figure 3). Since Noxa alone induced Bak-mediated death of fibroblasts lacking Bcl-xL [14••], free Bak may spontaneously cause apoptosis. If this model proves to hold for Bax, a proportion of membrane-bound Bax molecules must adopt a BH3-exposed ‘ligand’ conformation that can be bound by Bcl-2 pro-survival proteins. When BH3-only proteins displace this conformer from all relevant pro-survival guards, the active Bax could nucleate oligomerization (Figure 4b). Thus, in this displacement model (Figure 4b), death is the default, whereas survival is the default in the direct binding model (Figure 4a) [18••].
Since most data pertinent to the direct model has involved Bax and most evidence pertinent to the indirect model has involved Bak, it remains conceivable that the direct model holds for Bax and the indirect model for Bak.
Physiological and pathological functions of BH3-only proteins
As reviewed further elsewhere [9•], gene disruption is clarifying the physiological functions of BH3-only proteins. Bim-deficient mice have been particularly informative. They revealed that Bim is critical for removing superfluous hematopoietic cells, for apoptosis induced by cytokine deprivation and for prevention of autoimmunity [19]. Bim is crucial not only for eliminating lymphocytes that recognize self-antigens, during the development of both T cells [45] and B cells [46], but also for terminating T cell immune responses [47,48]. Furthermore, the lymphopenia provoked by loss of the Interleukin-7 receptor is alleviated by loss of Bim [49]. Bim also ensures the death of granulocytes [50], osteoclasts [34], mast cells [51] and neurons [52,53] upon cytokine deprivation.
Tissue homeostasis seems to be regulated by the balance between BH3-only proteins and their pro-survival relatives. In a striking illustration of this concept, loss of a single bim allele precluded the degenerative fatal polycystic kidney disease provoked in mice by loss of the bcl-2 gene and restored lymphocyte numbers [54].
Intracellular pathogens often subvert the host apoptotic program, because it limits their spread. Remarkably, Chlamydia, a bacterial parasite of macrophages, blocks apoptosis by targeting every tested BH3-only protein for proteasomal degradation [55,56]. Presumably a chlamydial gene product can bind BH3 domains and route the proteins to the proteasome.
Roles of BH3-only proteins in tumorigenesis
The apoptosis provoked by DNA damage requires p53 and is considered central to p53 tumor suppression function [57]. Two of its transcriptional targets encode Puma and Noxa, which appear to mediate its apoptotic function. Indeed, Puma-deficient lymphocytes proved nearly as refractory to DNA damage as those lacking p53 [20••,21••] whereas Noxa has a more restricted role in fibroblasts [20••,22]. Interestingly, the expression of Noxa, Puma, Bim and Hrk/DP5 is also directly upregulated by the transcription factor E2F1, which is normally constrained by the tumor suppressor pRB but is deregulated in many tumors [58]. Since the apoptosis elicited by E2F1 was reduced by downregulation of Puma or Noxa, their increased expression may represent a barrier to tumorigenesis. Pertinently, Puma expression is markedly reduced during melanoma progression [59].
Just as Bcl-2 and its homologs are well-recognized oncoproteins [1•,60], their BH3-only antagonists can act as tumor suppressors. Notably, leukemogenesis in Eμ-myc transgenic mice was accelerated by loss of even a single bim allele [61•]. Bim may suppress leukemia by mediating Myc-induced apoptosis, as over-expressed Myc reduced levels of Bcl-xL and Bcl-2 while elevating those of Bim [6]. Thus, Bim deficiency may promote oncogenesis by favoring cell survival in the face of active oncogenes. Pertinently, 17% of human mantle cell lymphomas display homozygous deletions of the human BIM locus [62]. Other BH3-only proteins may also restrain neoplasia in specific cell types. RNAi to Puma accelerated Eμ-myc-driven lymphomagenesis [63]. Moreover, some aged Bad-deficient mice develop diffuse large B cell lymphoma [23] and some Bid-deficient animals succumb late in life to a chronic myeloproliferative syndrome that resembles chronic myelomonocytic leukaemia [64].
Implications of BH3-only proteins for cytotoxic therapy
Many anti-cancer agents act through specific BH3-only proteins (Figure 1). For example, histone deacetylase (HDAC) inhibitors, which are showing promise in killing cancer cells, may act by upregulating bmf transcription [65]. Similarly, proteasome inhibitors like bortezomib (Velcade), now in several clinical trials, appear to work by upregulating various BH3-only proteins [66•,67–69]. Thus, bortezomib kills melanoma cells but, surprisingly, not normal melanocytes, and acts in part by inducing Noxa expression in a p53-independent fashion [68,69]. Furthermore, the apoptosis of chronic myeloid leukemia cells elicited by the kinase inhibitor Gleevec (imatinib mesylate) relies in part on Bim [70].
A recent study illustrates how an improved understanding of apoptotic regulation will impact on future treatment [66•]. The sensitivity of carcinoma cells to paclitaxel required Bim, but an activated Ras oncogene rendered the cells refractory to paclitaxel by targeting Bim for proteasomal degradation. Nevertheless, Bim levels and paclitaxel sensitivity were both restored by bortezomib. These results provide a rationale for combining paclitaxel and bortezomib in therapy, particularly in tumors with an activated Ras or Raf [66•]. Similarly, in the killing of some human cancer cell lines, bortezomib exhibits synergy with TRAIL, a ligand for certain death receptors, apparently because of its ability to increase levels of Bik and/or Bim [67]. Thus, the highly empirical combination chemotherapy may soon have a more rational foundation.
The promise of BH3 mimetic anti-cancer drugs
The appeal of BH3 mimetics as a new class of anti-cancer agent [71] is that most tumors have defects in the p53 pathway, precluding induction of the BH3-only proteins Noxa and Puma, and many over-express Bcl-2. A drug that directly targeted pro-survival proteins might therefore be effective. Two approaches to the creation of BH3 mimetics are now showing promise.
One approach uses a modified BH3 peptide [11••]. A 24-mer Bid BH3 peptide, constrained by ‘hydrocarbon stapling’ between two residues on one face of the α helix to maintain its helicity, proved to be protease-resistant and unexpectedly cell-permeant. It potently induced apoptosis in Jurkat T leukemia cells, whereas a peptide with a point mutation that abolished Bcl-xL association was inert. In vivo, the constrained Bid peptide transiently suppressed the growth of a transplanted leukemia [11••]. Although the decrease in tumor burden was modest, this approach may have potential for the development of novel anti-tumor agents.
The second approach involves non-peptidic organic compounds designed to fit into the groove of a pro-survival protein. The recently described BH3 mimetic ABT-737 [12••] behaves like Bad: it targets Bcl-2, Bcl-xL and Bcl-w with high affinity but not Mcl-1 or A1. ABT-737 was effective as a single agent in killing cells from several tumor types and caused regression of certain tumors in mice. The Bad-like selectivity of ABT-737 suggests that it would be most effective as a single agent for treating tumors with low levels of Mcl-1, but it should also show synergy with agents that downregulate or inactivate Mcl-1 (Figure 3).
Conclusions
As the BH3-only proteins have now been implicated in many cytotoxic responses (Figure 1), we surmise that nearly all such signals are funneled through one or more of them. The discovery that Bim, Puma and tBid engage all the pro-survival proteins but that the other death ligands engage only subsets (Figure 2) is helping to resolve how these death ligands flip the apoptotic switch. In the activation of Bak, the BH3-only proteins appear to act indirectly by displacing Bak from its sole guards, Mcl-1 and Bcl-xL (Figure 3). Whether the death ligands activate Bax by directly binding (Figure 4a) or indirectly by displacing it from pro-survival guards (Figure 4b) remains unresolved. Nevertheless, a deeper understanding of how the three factions of the Bcl-2 family interact to control cell death is now emerging. That knowledge is galvanizing the development of BH3 mimetics as a novel approach to cancer therapy.
Update
A non-apoptotic role has now been ascribed to full-length Bid [72,73], and Puma reportedly linked to a putative apoptotic role for cytoplasmic p53 [74].
Intact Bid is thought to be largely inert, but two studies now suggest that it has a non-apoptotic role in the DNA damage response [72,73]. Both conclude that, upon DNA damage, Bid is phosphorylated by the ATM kinase and is needed for S-phase growth arrest. This role does not require its BH3 domain and promotes cell survival, but, paradoxically, one of the studies [73] also reports that Bid contributes to apoptosis after DNA damage.
A conundrum regarding the apoptotic activity of p53 is that its transcriptional target Puma is required for almost all the apoptotic response of lymphocytes to DNA damage [20••,21••], yet some studies have argued that p53 can also directly activate apoptosis from the cytoplasm by engaging either Bcl-2 and Bcl-xL [75] or Bax [76]. To address this conundrum, the new report [74] suggests a complex model: cytoplasmic p53 is sequestered by Bcl-xL until Puma, but not other BH3-only proteins, engages Bcl-xL and releases p53, presumably allowing the freed p53 to engage and activate Bax. Surprisingly, Puma is reported to be unable to kill cells that lack either Bcl-xL or p53 [74]. This conclusion seems at variance, however, with the ability of Puma to kill fibroblasts that lack Bcl-xL or active p53 [14••] and the well-established ability of Puma to mediate several death signals that do not require p53 [20••,21••]. Accordingly, whether cytoplasmic p53 has a physiologically relevant role in apoptosis remains questionable.
Acknowledgements
We thank our colleagues David Huang, Andreas Strasser, Suzanne Cory, Peter Colman, Mark Hinds, Alan Harris, Philippe Bouillet, Ruth Kluck and Hamsa Puthalakath for valuable discussions. Our research is supported by a NHMRC Program grant (257502), a Specialized Center of Research grant from the Leukemia and Lymphoma Society, and NIH project grants CA80188 and CA43540. SNW holds a fellowship from the Cancer Council of Victoria.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1. Cory S, Huang DCS, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. A useful review on the Bcl-2 family and its role in cancer.
- 2. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. A good recent overview of apoptosis.
- 3.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-xL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 4.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 6.Huang DCS, Strasser A. BH3-only proteins — essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 7.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 8.Bouillet P, Strasser A. BH3-only proteins – evolutionarily conserved pro-apoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–1574. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 9. Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. The most detailed review on the BH3-only proteins, particularly on their physiological roles in the immune system.
- 10.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- 11. Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of Apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. This is the first paper to show that constrained BH3 peptides can induce apoptosis in cancer cell lines and retard the growth of a transplanted leukemia.
- 12. Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. This study shows that it is feasible to develop a non-peptide organic mimic of the BH3 domain and that this compound (ABT-737) has marked anti-tumor activity, validating along with [11••] the search for BH3 mimetic drugs.
- 13. Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DCS. Differential targeting of pro-survival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. This paper is important because it demonstrates that BH3-only proteins bind either promiscuously or selectively to Bcl-2 pro-survival proteins and that Bcl-2 pro-survival proteins of two types must be neutralized for efficient apoptosis (see also [14••]).
- 14. Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Pro-apoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. This is the first paper to show that Bak is sequestered solely by Mcl-1 and Bcl-xL and induces apoptosis if and only if freed from both.
- 15. Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. This study reports the unexpected discovery of a BH3-bearing ubiquitin ligase, denoted Mule/ARF-BP1, that can trigger the degradation of Mcl-1, as does Noxa ([14••]).
- 16.Letai A, Bassik M, Walensky L, Sorcinelli M, Weiler S, Korsmeyer S. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 17. Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first α helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. This paper is interesting because it provides evidence that peptides spanning the BH3 domains of Bid and PUMA bind to the N-terminal region of Bax rather than its groove.
- 18. Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. This paper is important because it establishes that Bid or Bim BH3 peptides can cooperate with Bax to permeabilize artificial liposomes in the absence of pro-survival proteins.
- 19.Bouillet P, Metcalf D, Huang DCS, Tarlinton DM, Kay TWH, Köntgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 20. Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins Puma and Noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. This paper is important because, along with [21••], it shows that Puma and, to a lesser extent, Noxa are central mediators of p53-induced apoptosis and that Puma is also critical for several other non-p53-dependent cytotoxic signals.
- 21. Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–328. doi: 10.1016/s1535-6108(03)00244-7. This study, along with [20••], shows the key role of Puma in mediating essentially the entire p53-induced apoptotic response after DNA damage in lymphocytes.
- 22.Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17:2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puthalakath H, Huang DCS, O’Reilly LA, King SM, Strasser A. The pro-apoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 25.Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DCS, Strasser A. Bmf: a pro-apoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 26.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA. 2003;100:2432–2437. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Swanson RA, Wang M, Hildeman DA, Schaefer BC, Liu X, Suzuki H, Mihara K, Kappler J, Marrack P. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc Natl Acad Sci USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 31.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 32.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 33.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, et al. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38:899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 34.Akiyama T, Bouillet P, Miyazaki T, Kadono Y, Chikuda H, Chung UI, Fukuda A, Hikita A, Seto H, Okada T, et al. Regulation of osteoclast apoptosis by ubiquitylation of proapoptotic BH3-only Bcl-2 family member Bim. EMBO J. 2003;22:6653–6664. doi: 10.1093/emboj/cdg635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. This paper establishes that the Bim BH3 α helix spans at least 29 residues and makes extensive contacts with the groove of Bcl-xL, suggesting that the association requires more than the core 12–16 residues of the BH3 domain.
- 36.Yan N, Gu L, Kokel D, Chai J, Li W, Han A, Chen L, Xue D, Shi Y. Structural, biochemical, and functional analyses of CED-9 recognition by the proapoptotic proteins EGL-1 and CED-4. Mol Cell. 2004;15:999–1006. doi: 10.1016/j.molcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 37.Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DCS, Hinds MG. Solution structure of prosurvival Mcl-1 and characterization of its binding by proapoptotic BH3-only ligands. J Biol Chem. 2005;280:4738–4744. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 38. Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17:2922–2932. doi: 10.1101/gad.1156903. This paper is notable because it was the first to demonstrate that Mcl-1 can directly sequester Bak in healthy cells. See also [15••].
- 39. Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. This study provided the first evidence that Mcl-1 degradation plays a special role early in apoptosis (see also [15••,38•]).
- 40.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 41.Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, Herrmann A, Basanez G. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- 42.Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 44.Hinds MG, Lackmann M, Skea GL, Harrison PJ, Huang DCS, Day CL. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouillet P, Purton JF, Godfrey DI, Zhang L-C, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 46.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreative B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellegrini M, Belz G, Bouillet P, Strasser A. Shut down of an acute T cell immune response to viral infection is mediated by the pro-apoptotic Bcl-2 homology 3-only protein Bim. Proc Natl Acad Sci USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by pro-apoptotic Bcl-2 family member, Bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in Interleukin 7 receptor-deficient mice. J Exp Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 51.Alfredsson J, Puthalakath H, Martin H, Strasser A, Nilsson G. Proapoptotic Bcl-2 family member Bim is involved in the control of mast cell survival and is induced together with Bcl-X(L) upon IgE-receptor activation. Cell Death Differ. 2005;12:136–144. doi: 10.1038/sj.cdd.4401537. [DOI] [PubMed] [Google Scholar]
- 52.Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JM, Strasser A, Johnson EMJ. Induction of Bim, a proapoptotic BH3-only Bcl-2 family member, is critical for neuronal apoptosis. Neuron. 2001;29:615–628. doi: 10.1016/s0896-6273(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 53.Whitfield J, Neame SJ, Paquet L, Bernard O, Ham J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron. 2001;29:629–643. doi: 10.1016/s0896-6273(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 54.Bouillet P, Cory S, Zhang L-C, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency are prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 55.Fischer SF, Vier J, Kirschnek S, Klos A, Hess S, Ying S, Hacker G. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200:905–916. doi: 10.1084/jem.20040402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ying S, Seiffert BM, Hacker G, Fischer SF. Broad degradation of proapoptotic proteins with the conserved Bcl-2 homology domain 3 during infection with Chlamydia trachomatis. Infect Immun. 2005;73:1399–1403. doi: 10.1128/IAI.73.3.1399-1403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 58.Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279:8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 59.Karst AM, Dai DL, Martinka M, Li G. PUMA expression is significantly reduced in human cutaneous melanomas. Oncogene. 2005;24:1111–1116. doi: 10.1038/sj.onc.1208374. [DOI] [PubMed] [Google Scholar]
- 60.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 61. Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukaemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. This paper shows that a BH3-only protein, namely Bim, can act as a tumor suppressor by countering the apoptotic impetus of an active oncogene (Myc) (see also [63]).
- 62.Tagawa H, Karnan S, Suzuki R, Matsuo K, Zhang X, Ota A, Morishima Y, Nakamura S, Seto M. Genome-wide array-based CGH for mantle cell lymphoma: identification of homozygous deletions of the proapoptotic gene BIM. Oncogene. 2005;24:1348–1358. doi: 10.1038/sj.onc.1208300. [DOI] [PubMed] [Google Scholar]
- 63.Hemann MT, Zilfou JT, Zhao Z, Burgess DJ, Hannon GJ, Lowe SW. Suppression of tumorigenesis by the p53 target PUMA. Proc Natl Acad Sci USA. 2004;101:9333–9338. doi: 10.1073/pnas.0403286101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, Kutok JL, Alt FW, Korsmeyer SJ. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Adachi M, Kawamura R, Imai K. Bmf is a possible mediator in histone deacetylase inhibitors FK228 and CBHA-induced apoptosis. Cell Death Differ. 2005 doi: 10.1038/sj.cdd.4401686. in press. [DOI] [PubMed] [Google Scholar]
- 66. Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. This study illustrates how understanding of BH3-only proteins and their regulation may impact on cancer treatment strategies.
- 67.Nikrad M, Johnson T, Puthalalath H, Coultas L, Adams J, Kraft AS. The proteasome inhibitor bortezomib sensitizes cells to killing by death receptor ligand TRAIL via BH3-only proteins Bik and Bim. Mol Cancer Ther. 2005;4:443–449. doi: 10.1158/1535-7163.MCT-04-0260. [DOI] [PubMed] [Google Scholar]
- 68.Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, Bennett F, Pollock PM, Trent JM, Hendrix MJ, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65:6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari AW, Jr, Lowe SW, Soengas MS. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65:6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 70.Kuribara R, Honda H, Matsui H, Shinjyo T, Inukai T, Sugita K, Nakazawa S, Hirai H, Ozawa K, Inaba T. Roles of Bim in apoptosis of normal and Bcr-Abl-expressing hematopoietic progenitor. Mol Cell Biol. 2004;24:6172–6183. doi: 10.1128/MCB.24.14.6172-6183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cory S, Adams JM. Killing cancer cells by flipping the Bcl-2/Bax Switch. Cancer Cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Zinkel SS, Hurov KE, Ong C, Abtahi FM, Gross A, Korsmeyer SJ. A role for proapoptotic BID in the DNA-damage response. Cell. 2005;122:579–591. doi: 10.1016/j.cell.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 73.Kamer I, Sarig R, Zaltsman Y, Niv H, Oberkovitz G, Regev L, Haimovich G, Lerenthal Y, Marcellus RC, Gross A. Proapoptotic BID is an ATM effector in the DNA-damage response. Cell. 2005;122:593–603. doi: 10.1016/j.cell.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 74.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–1735. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 75.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 76.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]